Abstract
Remimazolam besylate is an ultra-short-acting benzodiazepine derivative recently approved in Japan for general anaesthesia. However, less attention has been paid to the compatibility of remimazolam with infusion solutions, and the mechanism underlying the incompatibility remains unknown. The patient was a 65-year-old man who underwent a high tibial osteotomy. After the induction of general anaesthesia using remimazolam solution (5 mg/mL), we noticed precipitate completely blocking the lumen of the intravenous tube connected to a Ringer’s acetate Physio140 drip. The mixture of remimazolam solution (5 mg/mL) with Physio140 solution immediately resulted in the formation of substantial precipitate. Nuclear magnetic resonance analysis revealed that the precipitate was remimazolam. Ultraviolet spectrophotometry revealed that the mixture of remimazolam solution with higher ratios of Physio140 resulted in significantly lower solubility, concomitant with an increase in pH. It would be important to consider the remimazolam concentration and infusion solution pH to avoid the production of precipitates.
Keywords: anaesthesia, drug interactions, safety
Background
Remimazolam besylate (Anerem, Mundipharma KK, Tokyo, Japan) is an ultra-short-acting benzodiazepine derivative recently approved in Japan for general anaesthesia.1 Remimazolam has a rapid onset of action like propofol, but also benefits from the existence of an antagonist (flumazenil), similar to other benzodiazepines.2 However, less attention has been paid to the compatibility of remimazolam with infusion solutions, and the mechanism underlying the incompatibility remains unknown. The package insert for Anerem advises that the drug should be reconstituted in normal saline, but not Ringer’s lactate in which the drug is difficult to dissolve.3 Here, we outline a case in which precipitate developed when remimazolam solution dissolved in normal saline was administrated by infusion with Ringer’s acetate, resulting in total occlusion of an intravenous catheter. Furthermore, we investigated the physicochemical properties of the precipitate in vitro.
Case presentation
The patient was a 65-year-old man who underwent a high tibial osteotomy for right knee osteoarthritis. He had a history of smoking and dyslipidaemia, but no medical contraindications to the use of remimazolam. In compliance with the package insert,3 we ensured that remimazolam was dissolved in normal saline and used at infusion rate of 12 mg/kg/hour for induction, and 1 mg/kg/hour for maintenance. In the absence of any recommendation detailing the concentration of remimazolam solution that should be used, we prepared remimazolam at 5 mg/mL. In the operating room, the patient’s vital signs were monitored by sphygmomanometry, electrocardiography, pulse oximetry and electroencephalography (using a Bispectral Index (BIS) monitor (Covidien, Boulder, Colorado, USA)). A Ringer’s acetate Physio140 (Otsuka Pharmaceutical, Tokushima, Japan) drip was commenced intravenously into the right arm. After adequate preoxygenation, remimazolam and fentanyl were administered through the connecting tubing using a three-way stopcock. Rocuronium was then administered. Endotracheal intubation was safely performed, and mechanical ventilation was commenced. After performing a right femoral nerve block, we noticed substantial precipitation in the intravenous tube lumen, occluding intravenous access. Fortunately, the patient’s blood pressure and heart rate were not significantly affected, and his BIS value remained in the 40s. We immediately changed the blocked tubing and used sevoflurane for maintenance of general anaesthesia in place of remimazolam. Surgery was completed without further problems: operative time was 183 min, and anaesthetic time was 250 min. Arousal from anaesthesia was unremarkable, with normal vital signs.
Investigations
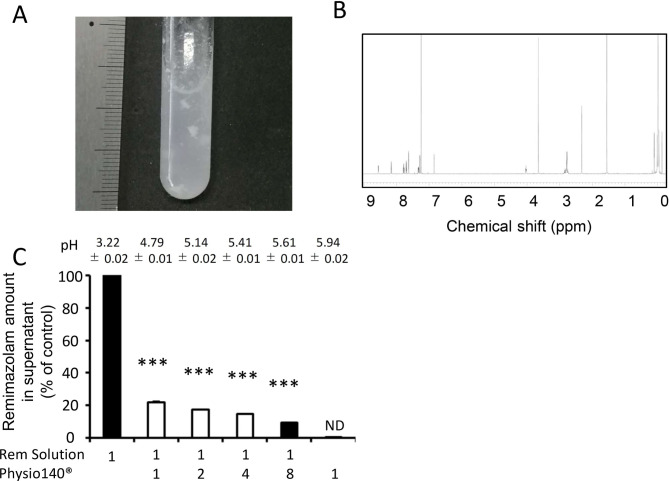
To investigate the precipitate, we performed an in vitro analysis. To replicate the conditions used prior to surgery, we prepared a standard solution of remimazolam at 5 mg/mL using Anerem 50 mg in normal saline. The mixture of remimazolam standard solution with an equal volume of Physio140 immediately resulted in the formation of substantial precipitate (figure 1A). Interestingly, we found that this precipitate was resolubilised by the addition of more Physio140. After washing with distilled water and subsequent drying in the open air, the precipitate was analysed by proton nuclear magnetic resonance (NMR) (JNM–ECA-500II NMR system, JEOL Resonance, Tokyo, Japan). As shown in figure 1B, the spectra of the sample were consistent with that of remimazolam, which indicated that the precipitate was remimazolam.4 Next, mixtures of the remimazolam standard solution and Physio140 were prepared at various ratios (figure 1C). Precipitate developed at remimazolam 5 mg/mL: Physio140 ratios of 1:1 to 1:4. Although precipitate also formed in a 1:8 mixture of the two solutions, it disappeared immediately. The mixtures were filtered through a 0.22 µm polytetrafluoroethylene syringe filter to separate supernatants from precipitate. We measured the concentration of remimazolam in supernatants of these mixtures by using ultraviolet spectrophotometry (UV-1800 spectrometer, Shimadzu, Kyoto, Japan). The absorbance of sample solutions was acquired at 230 nm using an optical path length of 10 mm. The supernatants of mixtures of remimazolam and Physio140 were diluted with purified water to adjust the concentration of remimazolam to 10 µg/mL or less, which ensured the reliability of measurements according to Lambert-Beer’s law. The derived concentrations of remimazolam in the 1:1, 1:2 and 1:4 mixtures with Physio140 were 21.8%±0.7%, 17.4%±0.5% and 14.9%±0.2%, respectively, compared with the standard 5 mg/mL solution control (figure 1C). These results indicate that the concentration of remimazolam in the 1:1, 1:2 and 1:4 mixtures were 1.09, 0.87 and 0.75 mg/mL, respectively. Furthermore, they indicate that, above concentrations of 0.75 mg/mL, the solution of remimazolam in saline mixed with Physio140 would be saturated. Lower saturated concentrations of remimazolam were observed as the pH of the mixture was increased.
Figure 1.
Precipitation produced by the mixture of remimazolam and Ringer’s acetate. (A) Gross appearance of the mixture of remimazolam solution at 5 mg/mL and equal volume of Ringer’s acetate Physio140. (B) Proton nuclear magnetic resonance spectra (500 MHz) of the precipitate. The precipitate was washed with distilled water and dried, and the precipitation was dissolved in CDCl3. The spectra of the sample were δ 8.56 (d, J=4.0 Hz, 1H), 8.16 (d, J=8.0 Hz, 1H), 7.78 (td, J=7.7, 1.7 Hz, 1H), 7.70 (dd, J=8.6, 2.3 Hz, 1H), 7.63 (d, J=2.3 Hz, 1H), 7.34–7.32 (m, 1H), 7.28 (d, J=8.6 Hz, 1H), 6.85 (d, J=1.1 Hz, 1H), 4.04–4.02 (m, 1H), 3.65 (s, 3H), 2.86–2.74 (m, 4H) and 2.32 (s, 3H) using tetramethylsilane as an internal reference, which are consistent with that of remimazolam. (C) Solubility of remimazolam. Concentration of remimazolam was measured by ultraviolet absorption compared with remimazolam at 5 mg/mL. Remimazolam amount in supernatant was calculated as per cent of absorbance of supernatant at 230 nm to those of mixture. Precipitations were developed at a ratio of 1:1 to 1:4 (open), but not 1:8 or standard remimazolam solution at 5 mg/mL (closed). Bars, SD. ***P<0.001 compared with control. ND, not detectable. All experiments were performed at least three times.
Outcome and follow-up
No postoperative complications were observed up to the patient’s discharge from hospital on postoperative day 23.
Discussion
We have detailed a case in which incompatibility of remimazolam with infusion solution resulted in the total occlusion of an intravenous catheter, despite compliance with the product package insert. Interestingly, we found that the precipitate resolubilised after addition of more infusion solution. NMR spectra revealed that the precipitate was remimazolam.
Additional precautions against remimazolam use are described in supplementary information published by the pharmaceutical company.5 This information contains a one-line warning stating that precipitation has been observed when mixing Ringer’s acetate Physio140 with remimazolam solution at 2 mg/mL. It does not describe the physicochemical properties of the precipitate. In this study, using NMR spectroscopy, we found that the precipitate formed was remimazolam. The literature reports that benzodiazepines such as diazepam and midazolam have water solubilities of less than 0.1 mg/mL at neutral pH.6 7 Although remimazolam is soluble up to 10 mg/mL at an acidic pH, it has low water solubility at neutral pH.5 The remimazolam precipitate was resolubilised by adding infusion solution. This phenomenon has also been observed with diazepam.8 These data suggest that the mechanism underlying resolubilisation of the precipitate was an increase in the volume of media, even though the solubility of remimazolam is lower at neutral pH. As shown in figure 1C, the concentration of remimazolam used should be lower than 0.75 mg/mL in an intravenous catheter to avoid formation of precipitates.
Recently, Sasaki et al also reported a case in which incompatibility of remimazolam with Ringer’s acetate resulted in the total occlusion of an intravenous catheter.9 Although they found that higher concentrations (5 mg/mL) of remimazolam and lower infusion rates (150 mL/hour or less) of Ringer’s acetate contribute to precipitate formation, the mechanism underlying the incompatibility were not addressed.
There are several limitations to this study. First, the precise mechanisms underlying production of the precipitate remain unclear. Although we showed, using proton NMR, that remimazolam was detected in the precipitate, we cannot rule out the possibility that other protons, such as calcium ions, are associated with its production. Unlike ceftriaxone, the insoluble calcium salt of which can cause lung and kidney damage,10 remimazolam precipitation is unlikely to cause systemic complications because it would be resolubilised by dilution into in the systemic circulation. Second, this report only investigates the compatibility of remimazolam with a single infusion solution (Physio140). Therefore, anaesthesiologists should check the compatibility of remimazolam solution at the concentration they intend to use with their preferred infusion solution before using remimazolam for the first time.
Learning points.
We present a case in which substantial precipitation of remimazolam in normal saline developed, resulting in complete occlusion of an intravenous catheter.
Anaesthesiologists should pay close attention to the compatibility of remimazolam with infusion solutions in clinical use.
It is important to consider the concentration of remimazolam and the pH of the infusion solution used to avoid the production of precipitates.
Acknowledgments
We thank Mr Toshio Fujimori for preparing the samples. We thank Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Footnotes
Contributors: All authors contributed to the study concept and design. MM was the attending anaesthesiologist during the case and prepared the manuscript. KO and YO performed nuclear magnetic resonance and ultraviolet spectrometry. MY supervised manuscript preparation. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Masui K. Remimazolam besilate, a benzodiazepine, has been Approved for general anesthesia!! J Anesth 2020;34:479–82. 10.1007/s00540-020-02755-1 [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther 2020;42:614–24. 10.1016/j.clinthera.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Mundipharma KK. Anerem® 50 mg for IV injection, Package insert, 2nd version. Tokyo: 2020 (in Japanese). Available: https://mundipharma.co.jp/medical-assets/anerem/anerem-pi.pdf [Accessed 28 Mar 2021].
- 4.Tilbrook GS, Schumacher A, Emmenegger R. United States patent 9512078 B2..
- 5.Mundipharma KK. Anerem® 50 mg for IV injection, interview form, 2nd version. Tokyo: 2020 (in Japanese). Available: https://mundipharma.co.jp/medical-assets/anerem/anerem-if.pdf [Accessed 28 Mar 2021].
- 6.Mason NA, Cline S, Hyneck ML, et al. Factors affecting diazepam infusion: solubility, administration-set composition, and flow rate. Am J Hosp Pharm 1981;38:1449–54. 10.1093/ajhp/38.10.1449 [DOI] [PubMed] [Google Scholar]
- 7.Andersin R. Solubility and acid-base behaviour of midazolam in media of different pH, studied by ultraviolet spectrophotometry with multicomponent software. J Pharm Biomed Anal 1991;9:451–5. 10.1016/0731-7085(91)80246-6 [DOI] [PubMed] [Google Scholar]
- 8.Onuki Y, Hasegawa N, Kida C, et al. Supersaturated state of diazepam injection following dilution with infusion fluid. J Pharm Health Care Sci 2015;1:9. 10.1186/s40780-014-0009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasaki H, Hoshijima H, Mizuta K. Ringer's acetate solution-induced precipitation of remimazolam. Br J Anaesth 2021;126:e87–9. 10.1016/j.bja.2020.11.021 [DOI] [PubMed] [Google Scholar]
- 10.Bradley JS, Wassel RT, Lee L, et al. Intravenous ceftriaxone and calcium in the neonate: assessing the risk for cardiopulmonary adverse events. Pediatrics 2009;123:e609–13. 10.1542/peds.2008-3080 [DOI] [PubMed] [Google Scholar]