Abstract
Background:
Though moderate-to-vigorous intensity physical activity (MVPA) is recommended, limited research exists on sedentary behavior (SED) during pregnancy.
Methods:
We conducted a prospective cohort study to describe objectively-measured patterns of SED and activity during each trimester of pregnancy. Women wore thigh- (activPAL3) and waist-mounted (Actigraph GT3x) activity monitors. SED and activity were compared across trimesters using likelihood ratio tests and described using group-based trajectories. Exploratory analyses associated SED and activity trajectories with adverse pregnancy outcomes (APOs) and excessive gestational weight gain (GWG).
Results:
Pregnant women (n=105; mean (SD) age=31 (5) years; prepregnancy BMI=26.2 (6.6) kg/m2) had mean SED of 9.7, 9.5., and 9.5 hr per day (p=0.062) across trimesters, respectively. Some activities differed across trimesters: standing (increased, p=0.01), stepping (highest in 2nd trimester, p=0.04), steps/day (highest in 2nd trimester, p=0.008), and MVPA (decreased, p<0.001). Prolonged SED (bouts ≥30 minutes) and bouted MVPA (≥10 minutes) were stable (p>0.05). In exploratory analyses, higher SED and lower standing, stepping, and steps/day trajectories were associated with increased odds of APOs (p<0.05). No trajectories were associated with excessive GWG.
Conclusion:
Pregnant women exhibited stable SED of nearly 10 hr per day across pregnancy. Future research evaluating SED across pregnancy and APO risk is warranted.
Keywords: accelerometry, epidemiology, pregnancy, sedentary behavior, sitting/standing
INTRODUCTION
For pregnant women, the American College of Obstetrics and Gynecology1 recommends ≥30 minutes of aerobic exercise on most days of the week and the 2018 Physical Activity Guidelines for Americans2 recommend ≥150 minutes of moderate-intensity aerobic physical activity per week. A recent meta-analyses demonstrated that pregnant women participating in an exercise intervention vs. control had a 38–41% decrease in adverse pregnancy outcomes (APO) such as gestational diabetes, gestational hypertension, and preeclampsia,3 as well as reduced gestational weight gain (GWG). These benefits improve both short- and long-term intergenerational health.4, 5 Despite this, an estimated 3 in 4 pregnant women in the United States do not achieve aerobic activity guidelines.6 These low rates are at least partially attributable to typical but also pregnancy-unique barriers such as lack of time, fatigue, discomfort/pain or medical restriction, and concern for the baby.7
Less is known about sedentary behavior (SED) during pregnancy. SED is defined as time spent awake and in a seated, reclining, or lying posture at low intensity8 and is now considered an independent risk factor for cardiovascular disease and diabetes among general populations.9 Limited evidence also suggests SED is associated with greater weight gain.9–11 Reflecting this evidence, the 2018 Physical Activity Guidelines for Americans added a general recommendation to ‘sit less and move more’.2 This newer framework considers not just time spent in moderate-to-vigorous physical activity (MVPA), which accounts for a small portion of the day, but rather the full waking day that consists mostly of sitting and light-intensity activities (i.e., standing and other low intensity activities).8 Moreover, prolonged SED that occurs in bouts of at least 30 minutes has been implicated as more harmful than shorter bouts of SED.12 Understanding typical SED patterns in pregnant women could be important since associations found between SED and cardiometabolic outcomes in the general population could manifest during pregnancy as outcomes such as APO and GWG. Also, given the low participation rates and barriers to MVPA in pregnancy, reducing SED may be a more achievable alternative.
The limited studies evaluating SED during pregnancy mostly use self-report instruments.13 However, self-reported SED is susceptible to social desirability and recall biases14 and has poor-to-moderate agreement for measuring total duration of SED compared to best practice measurement (e.g., activPAL) during pregnancy.15 In response, a recent ‘call to action’ highlighted the need for objectively-monitored physical activity during pregnancy.16 We echo and extend this recommendation to objectively measure all waking-day activity, including SED, light-intensity activities (e.g., standing and stepping), and MVPA, across pregnancy, in order to provide precise estimates and accurate, translatable clinical and public health recommendations.
The objective of the Monitoring Movement and Health (MoM Health) study was to comprehensively describe patterns of objectively-measured SED and activity (standing, stepping, steps/day, and MVPA) across pregnancy. An additional exploratory aim was to relate these patterns to the risk of APO and excessive GWG.
METHODS
Participants and Setting
This prospective cohort study measured SED and activity using best practice objective methodology, maternal-fetal health and pregnancy outcomes, and determinants of SED across pregnancy. The study was conducted in Pittsburgh, PA from March 2017 through June of 2019. Participants had three study visits that occurred during the first (8–13 weeks), second (20–22 weeks) and third trimesters (32–34 weeks) of pregnancy.
Participants were recruited during their first trimester using media advertisements, information tables, general research registries, and referrals from other research studies or prenatal care providers. Using these methods, we recruited a convenience sample of women responding to our advertisements who were 8–13 weeks pregnant by self-report, between 18–45 years old, and planning to receive prenatal care and give birth at a University of Pittsburgh Medical Center facility. Women were only excluded if they were currently using antihypertensive or glucose-lowering medications, had a serious medical condition or one that severely limited ambulation, or were currently participating in a lifestyle intervention research study. All participants provided informed consent, including for research personnel to abstract prenatal, birth, and postpartum clinical data from their electronic health record. The University of Pittsburgh Institutional Review Board approved all research procedures. The study was registered on clinicaltrials.gov (NCT03084302).
Measurements
Participants self-reported demographic information and medical history. Height was measured with shoes removed using a stadiometer. At each trimester study visit, SED and activity were assessed using two objective monitors. Two monitors were necessary to meet best practice standards for measuring SED and standing time (activPAL3 micro)17 and MVPA (ActiGraph GT3X).18 Participants were instructed to wear both monitors for seven days, complete a monitor wear log noting any nonwear and sleep periods, and return the monitors and log by postage-paid mail.
Time spent in SED,19 standing (upright but otherwise stationary), and stepping (upright and moving), as well as steps/day were assessed using the activPAL3. The device was affixed to the anterior thigh using a waterproof, transparent dressing.17 Participants were instructed to wear the activPAL3 24 hours per day, including while bathing, with removal only when swimming to prevent monitor loss. Event-type data were exported using PALtechnologies software (v.7.2.38); nonwear and sleep time were removed using participant diaries.17, 20 Steps were measured using the activPAL3 due to the 24-hr wear protocol that could maximize data capture.17, 21 Daily time spent in SED (total), prolonged SED (bouts lasting for at least 30 minutes), standing, stepping, steps/day, and overall waking wear time were then quantified for each wear day and averaged across valid days. activPAL3 data were considered valid with ≥4 days that each had ≥10 hours of valid wear time.22, 23
Total and bouted MVPA were assessed using a separate device (ActiGraph GT3X triaxial accelerometer) and wear protocol. Participants were instructed to wear monitors on an elastic waist belt during waking hours only, except during any water activities (e.g., bathing or swimming). Reflecting changing anatomy across pregnancy, pictures were provided to facilitate correct positioning of the monitor at the waist or, as needed, below the gravid abdomen, but always in vertical alignment with the right knee.18 Using 1-minute epochs and ActiLife software v6.12.2, the Choi algorithm was used to define valid wear time and epochs with ≥2690 cpm were summed to quantify daily MVPA.23, 24 Daily minutes of bouted MVPA were calculated as ≥10 continuous min above the same MVPA cutpoint,24 with an allowance for ≤2 min below the threshold.25 MVPA was quantified within each day and averaged. Wear time of ≥10 hours on each of ≥4 days was required to be considered valid.22 We did not evaluate vigorous activity separately due to minimal levels (mean 0.2% of the day).2
After participants gave birth, available electronic health records were abstracted and then independently reviewed by two research personnel. Any disputes were settled by consensus or, if needed, the study’s maternal-fetal medicine physician. Self-reported prepregnancy weight and measured weight at delivery were abstracted from the medical chart and used to calculated GWG. We have validated self-reported prepregnancy weights among women recruited from the same site with r>0.99.26 Excessive GWG was categorized based on 2009 Institute of Medicine Guidelines.27 APO included a physician-diagnosis of gestational hypertension, preeclampsia, gestational diabetes, or fetal growth restriction in the electronic health record and/or a preterm birth (gestational age at delivery <37 weeks) as consistent with American College of Obstetrics and Gynecology definitions.28–30 Hypertensive disorders of pregnancy included gestational hypertension (diagnosed with de novo blood pressure >140/90 mmHg on two occasions) and preeclampsia (diagnosed as elevated blood pressure with evidence of other organ dysfunction). Women with missing prenatal and birth medical records were excluded from the exploratory analysis.
Statistical Analysis
We recruited n=120 women (anticipating 100 completers) to afford 80% power, with two-sided α=0.05, to detect a 0.5 hr difference in SED between any two trimesters as well as an association where a 3-group trajectory explained 8% of the variance in outcomes. Analyses were conducted using Stata v14 (College Station, TX). Continuous variables were evaluated for normality. Participant characteristics were described using means (SD), median (25th and 75th percentile), or numbers (percentages) and compared across SED trajectories using one-way analysis of variance or Fisher’s exact tests.
Due to missed visits, monitor loss, or inadequate wear time, we obtained valid SED, standing, stepping, and steps/day data for 92%, 88%, and 83% and MVPA data for 91%, 87%, and 79% in the first, second, and third trimesters, respectively. Daily estimates were averaged across valid wear days. Percentage of time spent in each activity were calculated by dividing daily averages by wear time. Steps/day were normalized to average wear time in each trimester. Bouted MVPA was found to be right skewed and thus was natural log-transformed for analysis where appropriate. Nested linear mixed models with SED and activity as the dependent variables, trimester modeled as an independent factor variable to allow for nonlinear associations, and covariate adjustment for monitor wear time were compared using likelihood ratio tests to evaluate omnibus differences across trimesters.
As activity patterns across pregnancy were of interest, proportion of time in SED, activity variables, and normalized steps/day were then used to separately construct group-based trajectories across pregnancy trimesters using the Stata traj command.31 Women with at least one valid assessment of activPAL or ActiGraph data were assigned to a trajectory with a missing at random assumption for those missing valid data at up to two time points.31 Optimal trajectories were chosen using the Bayesian Information Criterion, maximum proportion of posterior probabilities >70%, and clinical meaningfulness of trajectory groups.
For the exploratory analyses associating SED and activity trajectories with APO and GWG, unadjusted rates of APO, hypertensive disorders of pregnancy, and excessive GWG were summarized by trajectories and compared using Fisher’s exact tests. Logistic regression models evaluated associations between trajectories and outcomes with covariate adjustment for age, race (black/non-black), education, and prepregnancy BMI. A likelihood ratio test was used to evaluate whether trajectory group explained significant variability in the outcome variable. A final model added concurrent adjustment for SED and MVPA trajectories.
RESULTS
Of 120 women enrolled, nine were excluded due to first trimester miscarriage, congenital abnormality, or twin pregnancy and six were excluded because they lacked valid objective SED data (monitor loss, withdrawal or inadequate wear); the resulting sample size was 105. Demographic and clinical characteristics of women enrolled in MoM Health (Table 1) were similar to all women giving birth during the same period at the University of Pittsburgh Magee Womens Hospital (n=12,775, mean (SD) age of 30 (5) years, 21% black, 41% nulliparous, 27% obese, and 7–8% history of APO).
Table 1.
Participant Characteristics of Pregnant Women in the MoM Health Study, Overall and across SED Trajectories (n=105)
| Overall (n=105) |
Low SED (n=20) |
Medium SED (n=42) |
High SED (n=43) |
p-value | |
|---|---|---|---|---|---|
| Age, years | 31 (5) | 33 (5) | 32 (4) | 30 (5) | 0.086 |
| Race | |||||
| Black | 18 (17%) | 3 (15%) | 4 (10%) | 11 (26%) | 0.136 |
| Non-black | 87 (83%) | 17 (85%) | 38 (90%) | 32 (74%) | |
| Education | |||||
| ≤High School | 10 (10%) | 1 (5%) | 3 (7%) | 6 (14%) | 0.300 |
| Some College or Associates Degree | 22 (21%) | 7 (35%) | 7 (17%) | 8 (19%) | |
| Bachelor’s Degree | 27 (26%) | 5 (25%) | 8 (19%) | 14 (33%) | |
| Graduate Degree | 46 (44%) | 7 (35%) | 24 (57%) | 15 (35%) | |
| Parity | |||||
| Nulliparous | 45 (43%) | 6 (30%) | 16 (38%) | 23 (53%) | 0.154 |
| 1 or more | 60 (57%) | 14 (70%) | 26 (62%) | 20 (47%) | |
| History of APO * | |||||
| Gestational Hypertension | 4 (7%) | 0 (0%) | 1 (4%) | 3 (15%) | 0.267 |
| Preeclampsia | 6 (10%) | 2 (15%) | 3 (12%) | 1 (5%) | 0.646 |
| Gestational Diabetes | 3 (5%) | 1 (7%) | 1 (4%) | 1 (5%) | 1.000 |
| Prepregnancy BMI, kg/m 2 | 26.2 (6.6) | 29.1 (8.5) | 24.6 (5.4) | 26.4 (6.6) | 0.046 |
| Prepregnancy BMI classification | |||||
| Underweight (<18.5 kg/m2) | 5 (5%) | 0 (0%) | 4 (10%) | 1 (2%) | 0.225 |
| Normal (18.5–24.9 kg/m2) | 49 (47%) | 8 (40%) | 20 (48%) | 21 (49%) | |
| Overweight (25.0–29.9 kg/m2) | 26 (25%) | 3 (15%) | 11 (26%) | 12 (28%) | |
| Obese (≥30.0 kg/m2) | 25 (24%) | 9 (45%) | 7 (17%) | 9 (21%) |
Data presented ast mean (SD) or n (%);
Abbreviations: APO, adverse pregnancy outcomes; BMI, body mass index; SED, sedentary behavior
Among 60 parous women (By SED trajectory: low n=14; medium n=26; high n=20)
Participant characteristics are reported overall and across SED trajectories in Table 1. Participant demographics were not different across SED trajectories, with the exception of prepregnancy BMI. Higher SED trajectory was associated, but not perfectly collinear, with higher prolonged SED, lower standing, lower stepping, and lower steps/day trajectories (all p<0.001). However, SED trajectory was independent from MVPA and bouted MVPA trajectories (p>0.3, Supplemental Table 1).
SED and Activity across Trimesters of Pregnancy
Women spent nearly two thirds of their day in SED (Table 2). Though estimates varied little, percentage of time spent in SED differed significantly across trimesters (p=0.048) with the first trimester being the highest and the second trimester being the lowest. Prolonged SED was stable across pregnancy. When not sedentary, women spent about a quarter of their day standing and this estimate was slightly, though significantly, higher in the second and third trimesters whether measured as a duration or a percentage (p<0.02). Women spent just over 10% of their day stepping. Duration and percentage of time spent stepping also differed slightly but significantly across trimesters (p<0.05), where it was highest in the second trimester and lowest in the third trimester. On average, women met the step-based threshold32 and guidelines2 for recommended steps/day and MVPA, respectively. However, both declined meaningfully across pregnancy (p=0.008 for steps/day and p<0.001 for MVPA). Though median bouted MVPA also appeared to decline, there were no statistically significant differences across trimesters (p>0.05).
Table 2.
SED and Activity across Pregnancy Trimesters among Women in the MoM Health Study
| 1st Trimester | 2nd Trimester | 3rd Trimester | p-value * | |
|---|---|---|---|---|
| activPAL3 n = | 102 | 98 | 92 | |
| SED | ||||
| Hours per day | 9.7 (1.6) | 9.5 (1.4) | 9.5 (1.3) | 0.062 |
| % of time | 64.9 (10.2) | 63.0 (9.3) | 63.4 (9.6) | 0.048 |
| Prolonged SED | ||||
| Hours per day | 5.1 (1.9) | 5.0 (1.7) | 5.0 (1.6) | 0.451 |
| % of time | 34.1 (12.6) | 33.4 (11.3) | 33.3 (11.6) | 0.487 |
| Standing | ||||
| Hours per day | 3.7 (1.3) | 3.9 (1.2) | 4.0 (1.3) | 0.014 |
| % of time | 24.4 (8.2) | 26.0 (7.4) | 26.3 (7.9) | 0.011 |
| Stepping | ||||
| Hours per day | 1.6 (0.5) | 1.7 (0.6) | 1.6 (0.5) | 0.044 |
| % of time | 10.7 (3.3) | 11.0 (3.5) | 10.3 (3.1) | 0.042 |
| Steps/day | ||||
| Steps | 7,626 (2,604) | 7,774 (2,693) | 7,159 (2,353) | 0.008 |
| GT3X n = | 101 | 97 | 88 | |
| MVPA | ||||
| Min per week | 239 (108) | 229 (107) | 198 (112) | <0.001 |
| % of time | 3.9 (1.8) | 3.8 (1.8) | 3.2 (1.8) | <0.001 |
| Bouted MVPA | ||||
| Min per week | 51.5 [12.0, 107.6] | 51.6 [14.0, 109.4] | 27.6 [0.0, 95.1] | 0.085 |
| % of time | 0.8 [0.2, 1.8] | 0.9 [0.2, 1.8] | 0.5 [0.0, 1.6] | 0.149 |
Data are presented as mean (SD) or median [25th percentile, 75th percentile].
Abbreviations: MVPA, moderate-to-vigorous intensity physical activity; SED, sedentary behavior
Omnibus differences across trimesters were tested using likilihood ratio tests comparing nested mixed linear models with and without indicator variables for trimester. Models for hours per day of SED, prolonged SED, standing, and stepping, min per week of MVPA and natural log-transformed bouted MVPA, and steps include covariate adjustment for wear time.
SED and Activity Trajectories
Women were divided into three trajectories (low/medium/high) for SED, prolonged SED, standing, stepping time, steps/day, and MVPA. Only two trajectories emerged for bouted MVPA. The proportion of women belonging in each SED and activity group across trimesters and the estimated amount of time spent in each behavior, averaged across trimesters and standardized to an overall average wear time of 15.1 hr per day, are exhibited in Figure 1.
Figure 1. SED (A), Prolonged SED (B), Standing (C), Stepping (D), Steps/day (E), MVPA (F), and Bouted MVPA (G) Trajectories across Pregnancy Trimesters among Women in the MoM Health Study.
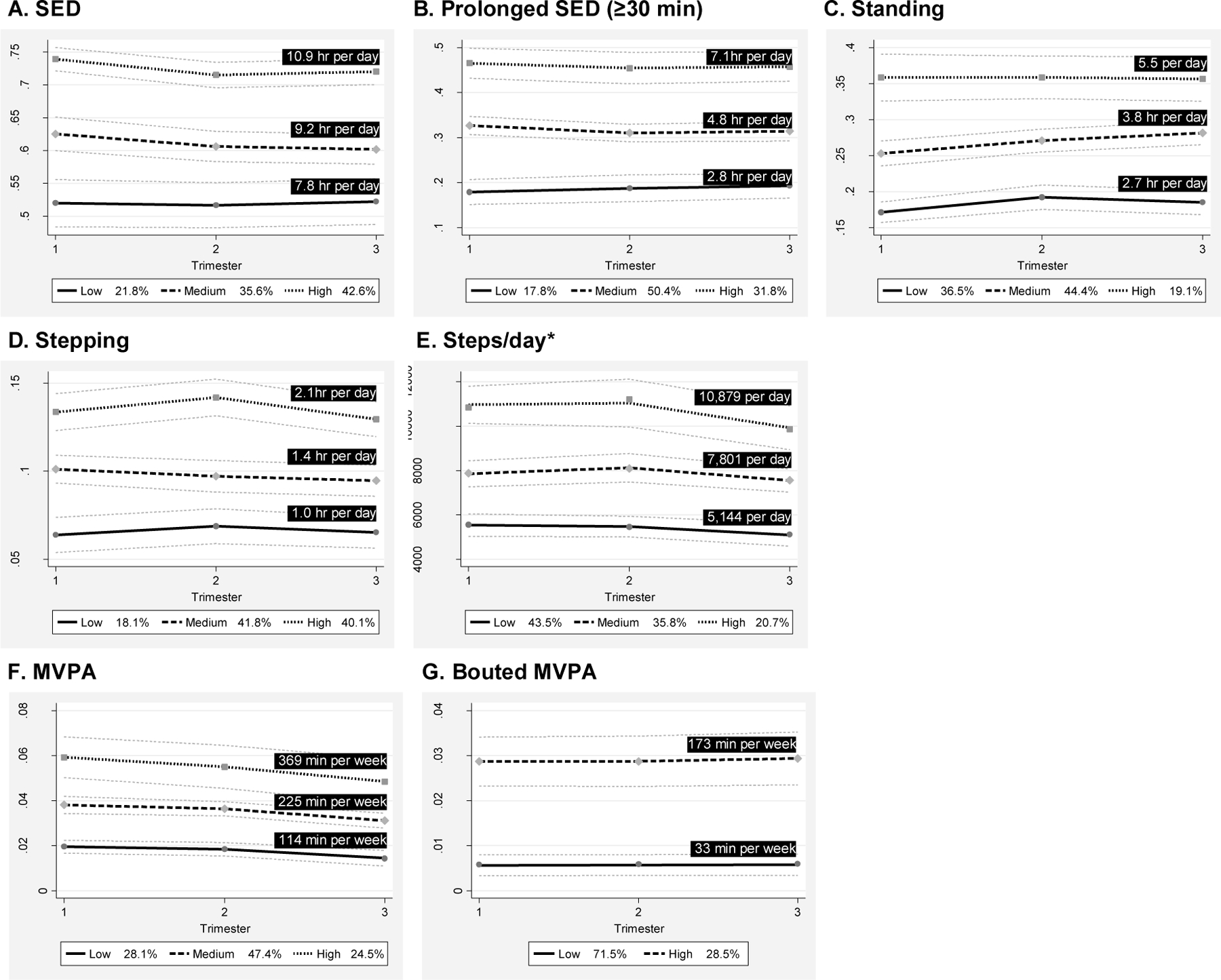
Values represent the average time spent in each beahvior by trajectory, averaged across trimesers and standardized to the overall average wear time (15.1 hr per day).
*Normalized to average wear time
Abbreviations: MVPA, moderate-to-vigorous intensity physical activity; hr, hour; min, minutes; SED, sedentary behavior
Exploratory Associations of SED and Activity Trajectories with Pregnancy Outcomes
Of the 105 women in the SED and activity analysis, 100 women (95%) had available birth records. Of these, 49 women (49%) had excessive gestational weight gain and 19 women had at least one APO (14 had only 1 APO; 5 had ≥1 APO). Among women having an APO, there were 13 cases of hypertensive disorders of pregnancy. Other APO were too rare to consider individually: n=3 had gestational diabetes, n=4 had fetal growth restriction, and n=6 had preterm births.
Unadjusted rates of APO, hypertensive disorders of pregnancy and excessive GWG are presented by all trajectories in Supplemental Table 2. Despite the limited events, overall likelihood ratio tests found that the SED, standing, and stepping trajectories were associated with odds of both overall APO and specifically hypertensive disorders of pregnancy. Steps/day trajectory was associated with odds of overall APO (Table 3). MVPA trajectories were not associated with any outcomes in adjusted models. No trajectories were associated with excessive GWG. Reflecting the small event rates, confidence intervals for odds ratios were wide but significantly differed from the null for odds of APO when comparing high vs. low SED trajectories and medium vs. low steps/day trajectories. Of note, mutual adjustment for SED and MVPA trajectories had a trivial effect on associations (data not shown).
Table 3.
Risk of APO and Excessive GWG SED and Activity Trajectories among Women in the MoM Health Study
| APO (n=19, 19%) OR [95% CI] |
p-value | Hypertensive Disorders of Pregnancy (n=13, 13%) OR [95% CI] |
p-value | Excessive GWG (n=49, 49%) OR [95% CI] |
p-value | |
|---|---|---|---|---|---|---|
| SED Trajectory | ||||||
| Low | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | |||
| Medium | 1.23 [0.18, 8.28] | 0.007 | 0.23 [0.02, 2.91] | 0.006 | 1.22 [0.35, 4.30] | 0.326 |
| High | 6.76 [1.20, 38.14] | 3.59 [0.65, 19.95] | 0.56 [0.15, 2.03] | |||
| Prolonged SED Trajectory | ||||||
| Low | ** | ** | 1.00 [reference] | 0.205 | ||
| Medium | 1.13 [0.33, 3.87] | |||||
| High | 0.43 [0.11, 1.75] | |||||
| Standing Trajectory | ||||||
| Low | 1.00 [reference] | 0.046 | 1.00 [reference] | 0.022 | 1.00 [reference] | |
| Medium | 0.33 [0.10, 1.08] | 0.13 [0.02, 0.69] | 2.24 [0.80, 6.33] | 0.175 | ||
| High | 0.17 [0.03, 0.99] | 0.30 [0.05, 1.70] | 3.02 [0.77, 11.88] | |||
| Stepping Trajectory | ||||||
| Low | 1.00 [reference] | 0.029 | 1.00 [reference] | 0.026 | 1.00 [reference] | |
| Medium | 0.42 [0.10, 1.68] | 0.57 [0.13, 2.61] | 0.79 [0.22, 2.83] | 0.935 | ||
| High | 0.11 [0.02, 0.64] | 0.06 [0.01, 0.74] | 0.82 [0.23, 2.99] | |||
| Steps/day Trajectory | 0.020 | 0.053 | 0.162 | |||
| Low | 1.00 [reference] | 1.00 [reference] | 1.00 [reference] | |||
| Medium | 0.17 [0.05, 0.64] | 0.22 [0.05, 0.96] | 1.56 [0.55, 4.41] | |||
| High | 0.34 [0.05, 1.34] | 0.11 [0.01, 1.24] | 0.47 [0.12, 1.85] | |||
| MVPA Trajectory | ||||||
| Low | 1.00 [reference] | 0.949 | 1.00 [reference] | 0.797 | Ref. | 0.062 |
| Medium | 1.23 [0.32, 4.79] | 1.76 [0.31, 9.85] | 2.66 [0.79, 8.92] | |||
| High | 1.09 [0.22, 5.32] | 1.61 [0.23, 11.2] | 0.67 [0.16, 2.72] | |||
| Bouted MVPA Trajectory | ||||||
| Low | 1.00 [reference] | 0.279 | Ref. | 0.382 | Ref. | 0.382 |
| High | 2.04 [0.56, 7.39] | 1.98 [0.43, 9.07] | 0.78 [0.27, 2.28] |
Models are adjusted for age, race, education, and prepregnancy BMI.
p-values are calculated from a likelihood ratio test comparing models with and without the trajectory variable.
Abbreviations: APO, adverse pregnancy outcomes; GWG, gestational weight gain; MVPA, moderate-to-vigorous intensity physical activity; OR, odds ratio; SED, sedentary behavior
Odds ratios for prolonged SED could not be calculated for APO or hypertensive disorders of pregnancy as there were no events in the low category.
DISCUSSION
Patterns of SED and Activity
Pregnant women, on average, participated in SED for nearly 10 hours per day or about two thirds of their waking time. At the same time, women spent about a quarter of their day standing. Little other research has measured SED, prolonged SED, and standing time across pregnancy, especially using objective monitors able to measure acceleration and distinguish postures (i.e., activPAL3).13 Our report thus provides important data given the error in estimation of total SED duration when measured by self-report or even other objective monitors.15 Our SED averages were comparable to estimates from the National Health and Nutrition Survey (NHANES) (57%)33 and a sample of Dutch women enrolled in clinical trials to reduce GWG (65%).34 These studies, however, measured SED with uniaxial accelerometers which do not distinguish SED from stationary standing. A separate analysis within the same NHANES sample found that pregnant women spent an estimated 10% of their time in prolonged SED (≥30 min),35 well below our estimates of about 33% of the day from an activPAL3 monitor with a 24-hr wear protocol.
Though highly sedentary, a majority (71.9%) of pregnant women in our cohort were in the medium and high trajectories of MVPA that were within the range recommended by the 2018 Physical Activity Guidelines for Americans (150–300 min per week of MVPA).2 In contrast, a minority (28.5%) of pregnant women in our cohort were in the high bouted MVPA trajectory that would have met the former (2008) guidelines which recommended 150–300 min per week of MVPA to be accumulated in bouts of at least 10 minutes.36 Our findings can be compared to objectively-monitored activity data from pregnant women in NHANES.33 Though NHANES used a different accelerometer, cut points, and a population-based sample with a single measurement per participant, pregnant women were found to engage in ~86 (Troiano cutpoints) or ~783 (Schwarz cutpoints) min per week of MVPA. The study among Dutch women using an ActiTrainer uniaxial accelerometer34 reported 168 and 126 min of MVPA per week in the second and third trimesters, respectively. Taken together, the wide variability in objective MVPA estimates – even within studies when using different data reduction methods – precludes concrete conclusions about activity levels in pregnant women. Yet, a pattern does consistently emerge whereby MVPA decreases across pregnancy. For purposes of population surveillance and refining recommendations, we and others36 strongly support the need for research with objective MVPA assessment, clear reporting of methods, and evaluation of associations with pregnancy outcomes.
SED, Activity, APO, and Excessive GWG
Though limited by our small sample and number of APO, exploratory analyses associated higher SED trajectory with increased odds of an APO. This association was robust to covariate adjustment by MVPA trajectory. Coherent effects were observed where being in medium or high trajectories for standing, stepping, and steps/day was also associated with lower odds of an APO. Speculatively, these data could imply that an overall activity pattern of sitting less by standing and moving more could lead to improved pregnancy outcomes, especially among women with high SED.
Yet, our findings differ from the few existing studies finding null associations between SED and the risk of developing APO (hypertensive disorders of pregnancy,37 gestational diabetes,38 or preterm birth39. This could reflect a spurious finding in our small study or important methodological differences in SED measurement and study design. Our study uniquely used an activPAL3 monitor to measure SED during each trimester and our statistical approach used trajectories to consider activity across pregnancy and evaluate potential threshold effects. Taken together, our findings should be interpreted with caution and need confirmation in a larger observational study using best practice methods for SED assessment and analysis.
Our exploratory null findings between MVPA trajectories and odds of APO can be interpreted in the context of a comprehensive statement from the 2018 Physical Activity Guidelines Advisory Committee40 that found ‘limited evidence’ supporting an association between MVPA and hypertensive disorders of pregnancy, though higher MVPA was not associated with an increase in preterm birth and was strongly associated with gestational diabetes. Though a recent meta-analysis reported randomized exercise interventions decreased the risk of hypertensive disorders of pregnancy and GDM by 38–41%,3 observational studies like ours evaluating associations between MVPA and hypertensive disorders of pregnancy often find no association.40 Thus, while our null findings make a limited contribution due to our small sample, these data do underscore the need for larger observational studies with objective monitoring to clarify the reasons for these inconsistent results.
We found no association between SED trajectories and excessive GWG, similar to a previous research.13 We also found nonsignificant associations between activity trajectories and excessive GWG, which contrasts the Physical Activity Guidelines Advisory Committee report that found ‘strong’ evidence supporting that MVPA reduces GWG with an estimated effect size of −1 kg.40 We suspect that our small sample, the small effect of MVPA on GWG, and our dichotomous excessive GWG outcome likely contributed to our null findings.
Strengths and Limitations
Our study is strengthened by use of best-practice methodology for SED and activity assessment, the prospective, longitudinal design with assessment during each trimester, and high follow-up rates using our medical record review methodology to obtain outcomes. Limitations are also present. Most importantly, our small sample size and specifically the exploratory analyses with a limited number of APO events should be interpreted with caution as these factors could lead to reduced power or unstable estimates. Also, our study may suffer from volunteer bias and this limits the external generalizability. Lastly, we did not differentiate between moderate-vigorous and light intensity steps, which is an important consideration for future research.
Conclusions
Pregnant women spent almost two thirds of their day SED and time spent in SED was clinically (if not statistically) stable across pregnancy. At the same time, these women were quite active and a majority met current recommendations for MVPA and steps/day. Yet, MVPA did appear to decline as pregnancy progressed. Our data also provides preliminary evidence that a strategy of stand/move more and sit less might be associated with reduced risk of APO in pregnant women. More observational research and clinical trials testing this strategy are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the participants of the MoM Health Study.
FUNDING
The MoM Health Study was funded by the American Heart Association (17GRNT3340016) with research registry, recruitment, and statistical support from the University of Pittsburgh Clinical and Translational Science Institute (NIH UL1TR000005).
References
- 1.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstetrics and gynecology. Dec 2015;126(6):e135–42. doi: 10.1097/aog.0000000000001214 [DOI] [PubMed] [Google Scholar]
- 2.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. Jama. 2018;320(19):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport MH, Ruchat S-M, Poitras VJ, et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. British Journal of Sports Medicine. 2018;52(21):1367–1375. doi: 10.1136/bjsports-2018-099355 [DOI] [PubMed] [Google Scholar]
- 4.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Journal of the American College of Cardiology. Mar 22 2011;57(12):1404–23. doi: 10.1016/j.jacc.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandi SM, Filion KB, Yoon S, et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation. Feb 19 2019;139(8):1069–1079. doi: 10.1161/circulationaha.118.036748 [DOI] [PubMed] [Google Scholar]
- 6.Hesketh KR, Evenson KR. Prevalence of US pregnant women meeting 2015 ACOG physical activity guidelines. American journal of preventive medicine. 2016;51(3):e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evenson KR, Moos M-K, Carrier K, Siega-Riz AM. Perceived Barriers to Physical Activity Among Pregnant Women. journal article. Maternal and Child Health Journal. May 14 2008;13(3):364. doi: 10.1007/s10995-008-0359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. Jun 10 2017;14(1):75. doi: 10.1186/s12966-017-0525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzmarzyk PT, Powell KE, Jakicic JM, Troiano RP, Piercy K, Tennant B. Sedentary Behavior and Health: Update from the 2018 Physical Activity Guidelines Advisory Committee. Med Sci Sports Exerc. Jun 2019;51(6):1227–1241. doi: 10.1249/mss.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barone Gibbs B, Aaby D, Siddique J, et al. Bidirectional 10-year associations of accelerometer-measured sedentary behavior and activity categories with weight among middle-aged adults. Int J Obes (Lond). Mar 2020;44(3):559–567. doi: 10.1038/s41366-019-0443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadgraft NT, Winkler E, Climie RE, et al. Effects of sedentary behaviour interventions on biomarkers of cardiometabolic risk in adults: systematic review with meta-analyses. Br J Sports Med. Apr 8 2020;doi: 10.1136/bjsports-2019-101154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz KM, Howard VJ, Hutto B, et al. Patterns of Sedentary Behavior and Mortality in U.S. Middle-Aged and Older Adults: A National Cohort Study. Annals of internal medicine. 2017;167(7):465–475. doi: 10.7326/M17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. Mar 16 2017;14(1):32. doi: 10.1186/s12966-017-0485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews CE, Moore SC, George SM, Sampson J, Bowles HR. Improving self-reports of active and sedentary behaviors in large epidemiologic studies. Exercise and sport sciences reviews. 2012;40(3):118–126. doi: 10.1097/JES.0b013e31825b34a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barone Gibbs B, Paley JL, Jones MA, Whitaker KM, Connolly CP, Catov JM. Validity of self-reported and objectively measured sedentary behavior in pregnancy. BMC pregnancy and childbirth. Feb 11 2020;20(1):99. doi: 10.1186/s12884-020-2771-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerin E, Ferraro ZM, Adamo KB, Prud’homme D. The Need to Objectively Measure Physical Activity During Pregnancy: Considerations for Clinical Research and Public Health Impact. Maternal and child health journal. May 2018;22(5):637–641. doi: 10.1007/s10995-018-2475-4 [DOI] [PubMed] [Google Scholar]
- 17.Edwardson CL, Winkler EA, Bodicoat DH, et al. Considerations when using the activPAL monitor in field-based research with adult populations. Journal of sport and health science. 2017;6(2):162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly CP, Coe DP, Kendrick JM, Bassett DR Jr., Thompson DL Accuracy of physical activity monitors in pregnant women. Med Sci Sports Exerc. Jun 2011;43(6):1100–5. doi: 10.1249/MSS.0b013e3182058883 [DOI] [PubMed] [Google Scholar]
- 19.Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN)–Terminology Consensus Project process and outcome. International Journal of Behavioral Nutrition and Physical Activity. 2017;14(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barone Gibbs B, Kline CE. When does sedentary behavior become sleep? A proposed framework for classifying activity during sleep-wake transitions. Int J Behav Nutr Phys Act. Aug 22 2018;15(1):81. doi: 10.1186/s12966-018-0712-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett DR, Toth LP, LaMunion SR, Crouter SE. Step counting: a review of measurement considerations and health-related applications. Sports Med. 2017;47(7):1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. Jan 2012;44(1 Suppl 1):S68–76. doi: 10.1249/MSS.0b013e3182399e5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport. 2011/09/01/ 2011;14(5):411–416. doi: 10.1016/j.jsams.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Troiano RP, Berrigan D, Dodd K, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40 [DOI] [PubMed] [Google Scholar]
- 26.Catov JM, Abatemarco D, Althouse A, Davis EM, Hubel C. Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obesity (Silver Spring). May 2015;23(5):1071–8. doi: 10.1002/oby.21006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACOG Committee opinion no. 548: weight gain during pregnancy. Obstetrics and gynecology. Jan 2013;121(1):210–2. doi:http://10.1097/01.AOG.0000425668.87506.4c [DOI] [PubMed] [Google Scholar]
- 28.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133(1):e1–e25. doi: 10.1097/aog.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 29.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. Feb 2018;131(2):e49–e64. doi: 10.1097/aog.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 30.Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. Oct 2012;120(4):964–73. doi: 10.1097/AOG.0b013e3182723b1b [DOI] [PubMed] [Google Scholar]
- 31.Jones BL, Nagin DS. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociological Methods & Research. 2013/11/01 2013;42(4):608–613. doi: 10.1177/0049124113503141 [DOI] [Google Scholar]
- 32.Tudor-Locke C, Craig CL, Thyfault JP, Spence JC. A step-defined sedentary lifestyle index: <5000 steps/day. Applied Physiology, Nutrition, and Metabolism. 2013/02/01 2012;38(2):100–114. doi: 10.1139/apnm-2012-0235 [DOI] [PubMed] [Google Scholar]
- 33.Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Preventive Medicine. 2011/07/01/ 2011;53(1):39–43. doi: 10.1016/j.ypmed.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 34.Ruifrok AE, Althuizen E, Oostdam N, et al. The relationship of objectively measured physical activity and sedentary behaviour with gestational weight gain and birth weight. Journal of pregnancy. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkins M, Kim Y, Gabriel KP, Rockette-Wagner BJ, Chasan-Taber L. Sedentary behavior patterns in non-pregnant and pregnant women. Preventive Medicine Reports. 2017/06/01/ 2017;6:97–103. doi: 10.1016/j.pmedr.2017.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States. Department of Health and Human Services. 2008 physical activity guidelines for Americans : be active, healthy, and happy! ODPHP publication. U.S. Dept. of Health and Human Services; 2008:ix, 61 p. [Google Scholar]
- 37.Chasan-Taber L, Silveira M, Pekow P, et al. Physical activity, sedentary behavior and risk of hypertensive disorders of pregnancy in Hispanic women. Hypertension in pregnancy. Feb 2015;34(1):1–16. doi: 10.3109/10641955.2014.946616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes L, Bell R, Robson S, Poston L. Association between physical activity in obese pregnant women and pregnancy outcomes: the UPBEAT pilot study. Ann Nutr Metab. 2014;64(3–4):239–46. doi: 10.1159/000365027 [DOI] [PubMed] [Google Scholar]
- 39.Both MI, Overvest MA, Wildhagen MF, Golding J, Wildschut HIJ. The association of daily physical activity and birth outcome: a population-based cohort study. European Journal of Epidemiology. 2010/06/01 2010;25(6):421–429. doi: 10.1007/s10654-010-9458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DIPIETRO L, EVENSON KR, BLOODGOOD B, et al. Benefits of Physical Activity during Pregnancy and Postpartum: An Umbrella Review. Medicine & Science in Sports & Exercise. 2019;51(6):1292–1302. doi: 10.1249/mss.0000000000001941 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


