Introduction
Hyper-immunoglobulin E syndrome (HIES) is a rare immunodeficiency characterized by a high serum IgE level, atopic dermatitis, and recurrent skin and lung infections. The exact prevalence of HIES is unknown, but the overall incidence rate of primary immunodeficiency was reported to be 4.6 per 100,000 person-years.1,2 Genetic variants in STAT3, DOCK8, and PGM3 have been associated with increased HIES risk. Traditionally, the main treatments include long-term prophylactic antibiotics, topical anti-inflammatory agents, and supportive care.3 Recently, biologics have emerged as an additional option for treating HIES with treatment-refractory atopic dermatitis. Dupilumab, a human monoclonal antibody that blocks the shared receptor subunit of IL-4 and IL-13, mitigates the signs and symptoms of atopic dermatitis.4,5 Previously, 2 case reports have demonstrated the substantial improvements of atopic dermatitis following the injection of dupilumab.6,7 Here, we report a rare HIES case whose atopic dermatitis was successfully controlled with a lower dose of dupilumab.
Case description
An 18-year-old male patient presented with erythematous papules, lichenified plaques, xerosis, and hyperpigementation on his face, trunk, and limbs. His mother reported that the symptoms had developed gradually since birth and that they had not responded well to potent topical steroids, topical tacrolimus, or emollients. Since childhood, he had also suffered from retained primary teeth and recurrent skin and respiratory tract infections. His older sister had similar symptoms and died of pneumonia at the age of 5 years. No other family members had the same symptoms. The eczema area and severity index (EASI) score was 60, and the numerical rating scale score was 9 at the first clinic visit. The serum IgE concentration was 55,700 IU/mL (normal range, <100 IU/mL), and the absolute eosinophil count was 1235/μL (normal range, 50-350/μL). Based on the examinations and previous genetic tests, he was diagnosed with HIES.
We administered subcutaneous dupilumab at a loading dose of 600 mg. One month after the loading dose, we commenced subcutaneous injections of 300 mg dupilumab every 2 weeks. On patient request, we reduced the 300 mg dose from every 2 weeks to every 4 weeks from the sixth injection. The patient request was motivated by financial cost rather than any adverse effects from treatment. He continued to receive a monthly injection, and he has received 19 doses of dupilumab in total so far.
Over the first 4 weeks of treatment, the patient was gradually relieved from the pruritus (Fig 1). The erythematous papules, lichenified plaques, and xerosis significantly improved after 10 injections (Fig 2). The improvement was also revealed by the following observations: The EASI score decreased from 60 to 9.3, and the numerical rating scale score from 9 to 2. The serum IgE level decreased from 55,700 IU/mL to 13,800 IU/mL (normal range, <100 IU/mL), and the absolute eosinophil count from 1235/μL to 1142/μL (normal range, 50-350/μL).
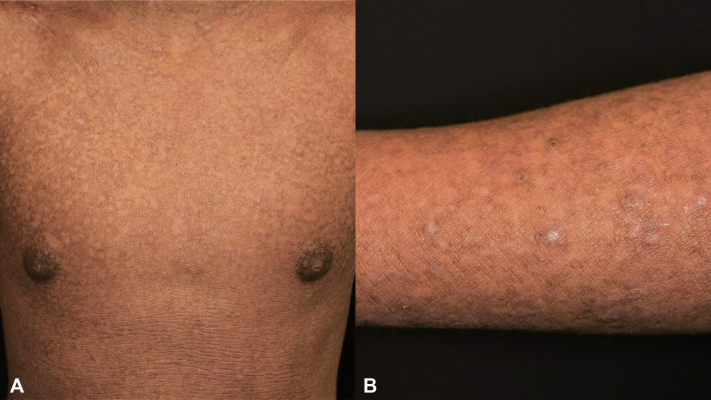
Fig 1.
Four weeks after the beginning of treatment, diffuse xerosis, erythematous papules, and plaques over trunk and forearm were observed.
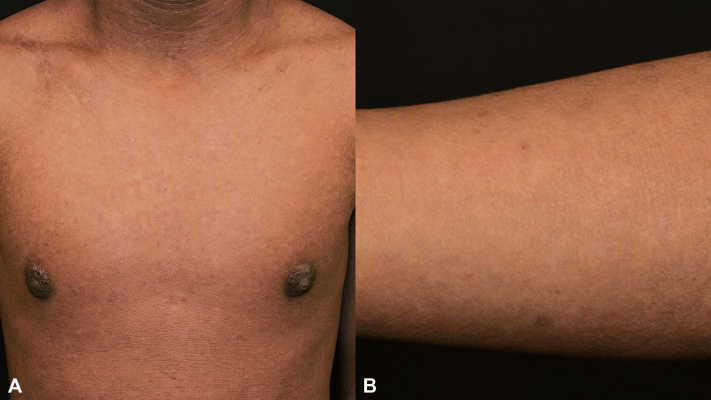
Fig 2.
Ten months after the beginning of treatment, resolution of hyperpigmentation, erythematous papules, and plaques was observed.
However, the patient reported a pruritic eruption on his trunk 17 months after commencement of treatment. The papules resolved quickly with daily administration of 15 mg prednisolone for 3 days.
Common adverse events of dupilumab include nasopharyngitis, upper respiratory tract infections, injection site infections, and conjunctivitis.8 Throughout the treatment, the patient had a carbuncle on his scalp, which developed 10 months after commencement of his treatment. We did not observe other serious adverse events.
Discussion
Two pathways of eczema formation in HIES have been described.9 The first pathway involves STAT3 and ZNF431 and impairs IFN-γ production and IL-10 signal transduction, leading to an unbalanced IL-4 state. The second pathway involves DOCK8 and WASP, which reduces T-cell receptor signaling and causes T regulatory cell dysfunction, resulting in T helper 2-cell expansion.9 Dupilumab's effects on inhibiting signaling of IL-4 and IL-13 and type 2 cytokines may explain the successful treatment in our case.
Two previous case reports described treatment of atopic dermatitis in HIES patients with a loading dose of 600 mg dupilumab, followed by 300 mg dupilumab every other week.6,7 In contrast, our case received a lower dose at a later treatment stage with a 4-week interval of dupilumab injection. In the study by Simpson et al., they compared the treatment efficacy of atopic dermatitis in adolescents with every-2-week and every-4-week regimens. The every-2-week regimen had a higher proportion of patients achieving EASI-75 and was superior in other categorical efficacy end points. The authors pointed out that the every-2-week regimen had higher dupilumab trough concentrations, which were associated with greater efficacy.5 The results may explain the relapse of pruritic papules in our case after months of treatment.
This case demonstrates the feasibility of administering dupilumab in a HIES patient with severe atopic dermatitis. However, long-term effects and safety remain to be further explored in this subset of patients.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: This study was approved by the Institutional ReviewBoard of Chang Gung Medical Foundation (IRB No 202001517B0).
References
- 1.Yong P.F., Freeman A.F., Engelhardt K.R., Holland S., Puck J.M., Grimbacher B. An update on the hyper-IgE syndromes. Arthritis Res Ther. 2012;14(6):228. doi: 10.1186/ar4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joshi A.Y., Iyer V.N., Hagan J.B., St Sauver J.L., Boyce T.G. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin Proc. 2009;84(1):16–22. doi: 10.4065/84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman A.F., Holland S.M. The hyper-IgE syndromes. Immunol Allergy Clin North Am. 2008;28(2):277–291. doi: 10.1016/j.iac.2008.01.005. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson E.L., Bieber T., Guttman-Yassky E. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 5.Simpson E.L., Paller A.S., Siegfried E.C. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: A phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi: 10.1001/jamadermatol.2019.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lévy R., Béziat V., Barbieux C. Efficacy of dupilumab for controlling severe atopic dermatitis in a patient with hyper-IgE syndrome. J Clin Immunol. 2020;40(2):418–420. doi: 10.1007/s10875-020-00751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sogkas G., Hirsch S., Jablonka A., Witte T., Schmidt R.E., Atschekzei F. Dupilumab to treat severe atopic dermatitis in autosomal dominant hyper-IgE syndrome. Clin Immunol. 2020;215:108452. doi: 10.1016/j.clim.2020.108452. [DOI] [PubMed] [Google Scholar]
- 8.Gooderham M.J., Hong H.C., Eshtiaghi P., Papp K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28–S36. doi: 10.1016/j.jaad.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Al-Shaikhly T., Ochs H.D. Hyper IgE syndromes: clinical and molecular characteristics. Immunol Cell Biol. 2019;97(4):368–379. doi: 10.1111/imcb.12209. [DOI] [PubMed] [Google Scholar]