Abstract
Introduction
Numbers of patients undergoing mitral valve repair (MVr) surgery for severe mitral regurgitation have grown and will continue to rise. MVr is routinely performed via median sternotomy; however, there is a move towards less invasive surgical approaches.
There is debate within the clinical and National Health Service (NHS) commissioning community about widespread adoption of minimally invasive MVr surgery in the absence of robust research evidence; implementation requires investment in staff and infrastructure.
The UK Mini Mitral trial will provide definitive evidence comparing patient, NHS and clinical outcomes in adult patients undergoing MVr surgery. It will establish the best surgical approach for MVr, setting a standard against which emerging percutaneous techniques can be measured. Findings will inform optimisation of cost-effective practice.
Methods and analysis
UK Mini Mitral is a multicentre, expertise based randomised controlled trial of minimally invasive thoracoscopically guided right minithoracotomy versus conventional sternotomy for MVr. The trial is taking place in NHS cardiothoracic centres in the UK with established minimally invasive mitral valve surgery programmes. In each centre, consenting and eligible patients are randomised to receive surgery performed by consultant surgeons who meet protocol-defined surgical expertise criteria. Patients are followed for 1 year, and consent to longer term follow-up.
Primary outcome is physical functioning 12 weeks following surgery, measured by change in Short Form Health Survey (SF-36v2) physical functioning scale. Early and 1 year echo data will be reported by a core laboratory. Estimates of key clinical and health economic outcomes will be reported up to 5 years.
The primary economic outcome is cost effectiveness, measured as incremental cost per quality-adjusted life year gained over 52 weeks following index surgery.
Ethics and dissemination
A favourable opinion was given by Wales REC 6 (16/WA/0156). Trial findings will be disseminated to patients, clinicians, commissioning groups and through peer reviewed publication.
Trial registration number
Keywords: cardiac surgery, cardiothoracic surgery, health economics
Strengths and limitations of this study.
Largest randomised controlled trial of minimally invasive cardiac surgery globally.
Strong and ongoing patient and public involvement and international clinical consensus informed trial design.
Robust design, with 90% power, includes randomisation to surgery performed by consultant surgeons with defined expertise beyond the learning curve. Design requires some patients to move between surgeons, which may impact recruitment but assures quality of outcomes and reduces bias.
Multicentre UK trial with blinding of central primary outcome assessor and echocardiogram core laboratory.
Up to 5 year estimates of key clinical and survival outcomes.
Introduction
Mitral regurgitation is most commonly caused by degenerative mitral valve disease, which leads to dilatation of the mitral valve annulus, leaflet prolapse or leaflet restriction. It can also be caused by rheumatic valve disease, or infective endocarditis.
Mitral valve repair (MVr) surgery is the optimal treatment for patients with severe mitral regurgitation caused by degenerative disease; when compared with mitral valve replacement, it carries a lower perioperative mortality, improved survival, better preservation of left ventricular function and lower long-term morbidity and mortality.1–6
MVr surgery for mitral valve regurgitation is frequently performed7 and patient numbers in the UK increased by a third between 2003 and 2012 (from 1549 to 21188 9; the number of patients undergoing isolated MVr surgery more than doubled over the same time (681 in 2003 to 1456 in 2012)).8 9 The rise is expected to continue. Furthermore, international guidance recommends that asymptomatic patients may benefit from early surgery.10
Choice of comparators
Mitral valve surgery is routinely performed via sternotomy which involves dividing the sternum completely, enabling easy access to the heart and cannulation of the great vessels centrally to establish cardiopulmonary bypass. It allows flexibility in myocardial protection strategies and potentially simplifies deairing and haemostasis at the end of the procedure.
Disadvantages of a sternotomy incision include an increase in bleeding because of the size of the incision.11 The mitral valve is posterior to the incision and valve access can be difficult. Wound infections occur in 2%–3% of patients, and significant morbidity, and mortality, can result from this complication.12 13 The sternotomy incision is usually closed using multiple stainless steel wires facilitating immobilisation of the sternum while sternal union occurs. It can take up to 3 months for the sternum to heal completely.12 During this period, the activity of patients is significantly limited to reduce the risk of sternal dehiscence. These limitations can reduce the speed of recovery and limit patients’ ability to return to usual activities. Moreover, limited activity during this period can increase the risk of complications in the recovery period.
Minimally invasive approaches are increasingly used in all surgical specialties. In cardiac surgery, they are routinely used for coronary revascularisation14 aortic valve surgery, aortic root surgery15 and surgery for assist devices.16
Several publications have established the safety of minimally invasive mitral valve surgery.17–22 Outcomes in a cohort of 1000 patients undergoing minimally invasive mitral valve surgery between 2003 and 2011 demonstrated a mortality of 0.7% at 1 year, and 8 year survival of 90%.18 Of those surviving, freedom from reoperation at 8 years was 93%.18 The approach may also significantly reduce morbidity and mortality in high-risk patients including in the elderly.19–21 A propensity matched analysis of 2400 cases from 3 UK centres suggested a reduction of blood transfusion (20.5% vs 14.4%, p=0.005) and median length of hospital stay (7 vs 6 days, p<0.001) when minimally invasive surgery was compared with conventional surgery despite longer procedural times.22
There is emerging evidence from non-NHS healthcare settings that the minimally invasive approach is less costly than conventional sternotomy; with cost savings driven by reduced intensive care and hospital stay as well as reduced need for blood transfusion.23
Current evidence supporting the rationale for the trial
Five published meta-analyses compare minimally invasive with conventional MVr.24–28 These identify only two small randomised controlled trials (RCTs) reporting short-term29 and long-term30 outcomes and base their main conclusions on evidence from observational studies;18 19 23 31–38 these provide no evidence of differences in clinical outcomes. The literature is severely limited by the absence of robust data on comparative outcomes between the two techniques.24–28
The International Society of Minimally Invasive Cardiac surgery published a consensus document on the role of minimally invasive mitral valve surgery in contemporary cardiac surgery practice in 2010.39 This highlights the limitations of available evidence in reaching a consensus, particularly the lack of adequately powered prospective RCTs to establish the comparative effectiveness of the two approaches. Its recommendation was for further prospective RCTs, adequately powered to assess quality of life, complications, efficacy (repair rates) and clinically relevant outcomes, particularly long term survival, patient functionality and freedom from re-operation.39
Less than 5% of patients having mitral valve surgery in the UK currently have a minimally invasive MVr, largely as a result of the lack of clear and definitive evidence. There is consensus in the cardiac surgery community that the optimum surgical approach to treat these patients urgently needs defining. This is particularly pressing as there is emerging evidence relating to the effectiveness of percutaneous MVr with the mitraclip and other devices in degenerative40 and functional mitral regurgitation.41 It is now clear that trials of percutaneous approaches and surgery are needed, but cannot be undertaken until the optimal surgical approach is clearly defined.
Methods and analysis
Study design
UK Mini Mitral is a multicentre, RCT of minimally invasive thoracoscopically-guided right minithoracotomy (intervention) versus conventional sternotomy (control) in patients undergoing MVr. The trial includes an internal pilot to assess the likelihood of meeting recruitment targets.
The trial will answer the questions ‘Are improvements in physical functioning and associated return to usual activities seen in patients who undergo minimally invasive mitral valve surgery compared with conventional surgery?’ and ‘Is minimally invasive mitral valve surgery compared with conventional surgery cost-effective?’
Setting
Three hundred and sixteen patients due to receive MVr surgery will be recruited from UK NHS cardiothoracic surgery units with established minimally invasive and conventional mitral valve services. All centres will be able to accommodate the needs of this trial including established minimally invasive MVr services, research nurse and echocardiographer support, and facilities to carry out trial assessments. Only units in equipoise in the way they manage their patients requiring MVr will participate.
At each centre, individual surgeons perform one type of operation; MVr via thoracoscopically guided right minithoracotomy or MVr via median sternotomy. Before performing surgery within the trial, each surgeon has completed 50 of these; the Trial Steering Committee (TSC) reviews a record of the number of operations for each surgeon, and agrees to their participation, in advance of them doing so. Depending on their allocation, patients can be required to move to another surgeon following randomisation.
Eligibility criteria
Patients are eligible to take part if they are scheduled to have surgery for MVr and fulfil all eligibility criteria:
Inclusion criteria
Adult (≥18 years old at consent) with degenerative mitral valve disease, requiring MVr*.
Written informed consent.
Fit for cardiac surgery and cardiopulmonary bypass.
*Patients requiring concomitant surgery for atrial fibrillation (AF), tricuspid valve repair, and/or patent foramen ovale (PFO) closure are included
Exclusion criteria
Concomitant cardiac surgery other than for AF, tricuspid valve repair and PFO closure.
Requiring mitral valve replacement.
Acute (current) infective endocarditis.
Emergency or salvage surgery.
Only one surgical technique indicated.
Pregnant.**
Currently participating in another interventional clinical trial.
≥ 4 weeks as an inpatient prior to randomisation.
Previous cardiac surgery.
Mobility impairments that would preclude Short Form Health Survey (SF36-v2) completion.
**Female patients between the ages of 18 and 50 will receive a pregnancy test at baseline.
Randomisation
Eligible patients will be randomised in a 1:1 ratio to undergo MVr using minimally invasive thoracoscopically guided right minithoracotomy (intervention under study) or conventional median sternotomy (control arm/usual care) by members of the research team at each centre using a 24-hour, central, secure, web-based randomisation system with concealed allocation (procured from Sealed Envelope).
Randomisation is performed using a minimisation scheme, which adjusts for baseline SF-36v2 physical functioning score, presence or absence of AF and/or PFO, and presence and severity of tricuspid regurgitation.
Trial surgical interventions
The intervention arm will receive MVr via minimally invasive thoracoscopically guided right minithoracotomy. The control group receive MVr via conventional sternotomy.
Intervention arm
The patient is intubated with a single or double lumen endotracheal tube. Cardiopulmonary bypass is established by femoral artery cannulation and venous return achieved from the venae cavae using a single bicaval cannula placed from the femoral vein, or with an additional cannula in the superior venae cava. Transoesophageal echocardiography confirms the optimum location of the venous and arterial cannulas.
A 4–7 cm right lateral minithoracotomy is then used to enter the thorax through the third or fourth intercostal space. A soft-tissue retractor, with or without a small thoracic retractor, is used to spread the ribs. The pericardium is opened 3–4 cm anterior and parallel to the phrenic nerve from the distal ascending aorta to the diaphragm. A video camera is inserted through a 5–10 mm port.
Endoballoon occlusion, or a transthoracic clamp, achieves aortic occlusion. Cardiac arrest is achieved with repeated doses of cardioplegia. The mitral valve is approached through a paraseptal incision and a left atrial retractor is used to expose the mitral valve.
Following the mitral valve procedure, the left atrium is closed, the heart deaired and aortic occlusion removed. Cardiopulmonary bypass is then discontinued and the thoracotomy incision closed once haemostasis has been achieved.
Control arm
The sternum is divided completely (from the collarbone to the bottom of the breastbone) and cardiopulmonary bypass established by siting cannulas in the right atrium and inferior venae cava and ascending aorta.
Cardiac arrest is achieved with cardioplegia and the mitral valve approached via the left atrium. The valve is repaired and assessed intraoperatively by water testing. Once the repair is deemed satisfactory, the atrium is closed, deairing manoeuvres performed, and the aortic cross clamp removed to allow reperfusion of the heart. Cardiopulmonary bypass is discontinued once haemostasis is achieved and the sternum is closed.
Outcomes
The primary outcome is physical functioning and associated return to usual activities measured by change in SF-36v2 physical functioning scale42 12 weeks following index surgery using a 4-week recall period.
The primary economic outcome is cost effectiveness measured in terms of incremental cost per quality-adjusted life year (QALY) gained over 52 weeks following index surgery.
Secondary outcomes post index MVr:
Feasibility and study recruitment, explored by means of a 6-month internal pilot.
Cardiac function; assessed using transthoracic echocardiography (TTE), early (1 day to 12 weeks) and late (52 weeks) by an independent core laboratory.
Mitral valve and mitral valve surgery related events and survival (morbidity and mortality); compared at 52 weeks and up to 5 years using adverse event data and Hospital Episode Statistics and National Institute for Cardiac Outcomes Research data.
Physical functioning and quality of life over 52 weeks; compared using SF-36v2 and EuroQol 5 Dimension 5 Level (EQ-5D-5L).42–45
Levels of physical activity and quality of sleep; quantified using accelerometer measures (GENEActiv46–50) over 52 weeks.
Surgical outcomes quantified over 52 weeks.
Costs, including intervention-specific estimates, of the two operations and their sequalae over 52 weeks.
QALYs, estimated from EQ-5D-5L43–45 and SF-6D (derived from the SF-36v2) over 52 weeks.
Modelled costs and QALYs over the patient lifetime.
Modelled incremental cost per QALY gained over the patient lifetime
Hospital Episode Statistics (HES) data will be explored to understand if it adequately captures mitral valve events.
Sample size
The primary outcome measure is change in physical functioning scale within SF-36v242 from baseline to 12 weeks following index surgery. On the basis of the literature and consulting with cardiac and patient communities, a minimally clinically important difference is 10 points on the scale. UK Mini Mitral is powered to investigate the superiority of thoracoscopically guided right minithoracotomy over conventional sternotomy.
One study31 reports a small variation (SD of 8) in SF-36 physical function scores; this is not seen in other studies32 33 which report a SD of 30. We used the conservative estimate to inform the original sample size calculation:
Assuming alpha of 5% and 90% power, 382 patients (191 in each arm) would be required to detect a minimally clinically important difference of 10 points in the SF-36v242 physical functioning scale at 12 weeks (assuming SD of 30); allowing for attrition, we planned to randomise 400 patients. To assess our assumptions, we performed a blinded sample size re-estimation using baseline SF-36v2 physical functioning scale data from 177 trial patients. Using the re-estimated SD of 26.3 with 90% power, 288 patients are required to detect a 10 point difference in SF-36v2 physical functioning at 12 weeks. Accounting for 10% attrition, 316 patients will be recruited.
Trial procedures
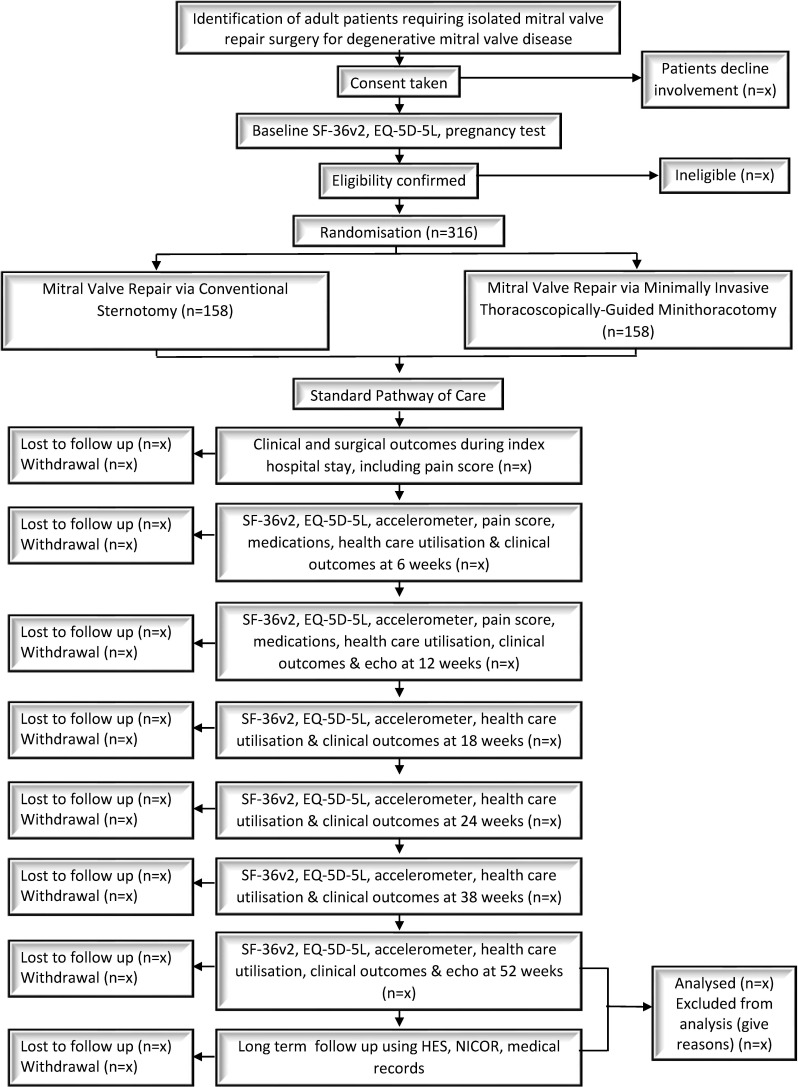
Patients due to undergo isolated MVr surgery at participating centres are identified at the point of referral (elective patients) and from the inpatient waiting list (urgent but non-emergency patients) by the clinical research team and approached about the trial, including given an information sheet and consent form. After discussion, consent is sought, baseline assessments performed and eligibility checked and confirmed by one of the consultant surgeons participating in the trial. Eligible patients are randomised, and their general practitioners informed. A full schedule of events is detailed in table 1, and a participant flow chart provided in figure 1. Patients have assessments at 6, 12, 18, 24, 38 and 52 weeks following index surgery. Longer term follow-up will involve routinely collected data.
Table 1.
UK Mini Mitral Schedule of Events
| Study procedure | Baseline | Day of surgery | Index hospital stay | Follow-up: time is calculated from the day of index surgery | |||||
| −26 weeks to day of surgery | Day 0 | Day 0 until discharge following index surgery | 6 weeks | 12 weeks | 18 weeks | 24 weeks | 38 weeks | 52 weks | |
| Consent | X | ||||||||
| Medical history | X | ||||||||
| Physical examination | X | ||||||||
| Demographics | X | ||||||||
| Concomitant medications | X | X | X | X | |||||
| Pregnancy test | X | ||||||||
| NYHA class | X | X | X | ||||||
| ECHO (TTE) | X | X | X | X | X | ||||
| TOE | X | X | |||||||
| SF-36v2 | X | X | X | X | X | X | X | ||
| EQ-5D-5L | X | X | X | X | X | X | X | ||
| euroSCORE | X | ||||||||
| Accelerometer | X | X | X | X | X | X | X | ||
| Eligibility check | X | ||||||||
| Randomisation | X | ||||||||
| MVr surgery | X | ||||||||
| Postoperative details | X | ||||||||
| Wound Pain score | Xi | X | X | ||||||
| Ward usage and date of discharge | X | ||||||||
| Discharge destination | X | ||||||||
| RBC and other blood product transfusions | X | X | |||||||
| AEs and SAEs | X | X | X | X | X | X | X | X | |
| Reoperation/further surgery | X | X | X | X | X | X | X | X | |
| Health Care Utilisation Questionnaire | X | X | X | X | X | X | |||
| HES data | X | X | X | X | X | X | |||
| NICOR data | X | X | X | X | X | X | |||
| Medical record review | X | X | X | X | X | X | |||
AE, adverse event; ECHO, Echocardiogram; EQ-5D-5L, EuroQol 5 Dimension 5 Level; HES, Hospital Episode Statistics; MVr, mitral valve repair; NICOR, National Institute for Cardiac Outcomes Research; NYHA, New York Heart Association; RBC, Red Blood Celleuor; SAE, serious adverse event; SCORE, European System for Cardiac Operative Risk Evaluation; SF36-v2, Short Form Health Survey; TOE, transoesophageal echocardiography; TTE, transthoracic echocardiography.
Figure 1.
UK Mini Mitral Trial flow diagram. EQ-5D-5L, EuroQol 5 Dimension 5 Level; SF-36v2, Short Form Health Survey.
SF-36v2 and EQ-5D-5L
Physical functioning and overall quality of life are assessed using SF-36v2 and EQ-5D-5L42–45 51 questionnaires administered prior to randomisation. Questionnaire completion is repeated over the telephone at 6, 12, 18, 24, 38 and 52 weeks following index surgery.
Echocardiogram
Cardiac function is assessed via TTE at baseline, post operatively (1 day to 12 weeks) and at 52 weeks. Measurements of left ventricular volumes, dimensions and function, mitral regurgitation severity and estimates of right heart function and pulmonary artery pressure are reported.
Accelerometer
Levels of physical activity and quality of sleep are quantified using accelerometer measures. Accelerometers are worn on patients’ non-dominant wrist nonstop for 7 consecutive days at seven timepoints (baseline, 6, 12, 18, 24, 38 and 52 weeks following index surgery). The restrictions of movement by being an inpatient means accelerometer measures are not possible for inhouse urgent patients and are not to be undertaken.
Statistical analysis
Primary analysis will follow intention to treat principles, with patients analysed according to randomisation, irrespective of surgical intervention received. A full statistical analysis plan will be developed and agreed with the Independent Data Monitoring and Ethics Committee (IDMEC) and TSC prior to the end of data collection.
Outcome data will be analysed at the end of the study; no interim outcome analyses are planned.
Primary analysis of change in physical functioning, measured using SF-36v2 at 12 weeks, will use a general linear model accounting for surgical intervention, baseline scores, valve pathology and concomitant surgery. Robust SE will account for intrapatient correlation due to repeated measures. A similar modelling approach will be used for the final analysis of change in physical functioning at 52 weeks.
Secondary outcomes: continuous outcomes will be analysed using a general linear model, binary outcomes will be analysed using a generalised estimating equation to account for repeated binary data over time, categorical outcomes (with more than two categories) will be analysed using baseline-category logit model, while log-rank test and frailty modelling will be used to analyse time-to-event outcomes. Analysis of all outcomes with repeated measures on the same patient will account for intrapatient correlation. Multiple imputation for secondary outcomes will be used if necessary to sensitise incomplete data. All outcomes will also be described using simple statistics to facilitate interpretation and communication of findings. Secondary analysis will also include hierarchical modelling of patients, surgeons and centres, to provide an estimate of treatment effect at each of these levels.
Each question within the SF-36v2 physical functioning scale will be scored based on the RAND scoring system.42 The average of the per-patient scale scores (assuming each question carries equal weight) will provide the primary outcome for physical functioning at 12 weeks.
Economic analysis
The economic evaluation will include both a within trial economic evaluation to estimate the incremental cost per QALY gained at 52 weeks and a model based economic evaluation to estimate cumulative costs and QALYs and incremental cost per QALY gained over a patient lifetime. The analysis will take the perspective of the UK NHS.
The within trial analysis will consist of a microcosting of the intervention (minimally invasive minithoracotomy) and usual care (conventional sternotomy). The cost of each surgical procedure will be derived from information captured in the case report form and obtained from the team at clinical centres. The subsequent use of NHS services will be captured through patient questionnaires to record use of subsequent primary and secondary care services.
A state transition model will enable extrapolation of costs and outcomes beyond 52 weeks for the predicted lifetime of participants. Data used to populate the model will come from the within trial analysis and external data sources systematically derived from the literature as per best practice guidance.52 External data will help identify health state utilities for events occurring long term and not captured within the trial. This will include HES data, validated using medical records and the National Adult Cardiac Surgical Database, adjudicated by an independent expert panel. The independent expert panel will assess which use of health services is attributable to sequelae from index surgery, or underlying disease condition (eg, surgical revision or complication, worsening mitral disease or infection) and/or which is unrelated.
Trial conduct and governance
The Trial Management Group oversees all day-to-day aspects to ensure that the protocol is adhered to, ensuring patient safety and data integrity; they meet monthly. The IDMEC report to the TSC and provide advice on the ongoing conduct and safety; they meet 6 monthly. The TSC, where independent members are in the majority, provides overall supervision; they meet 6 monthly.
Patient and public involvement
Meetings with cardiac surgeons and cardiologists in the UK and the USA established the need for, and feasibility of, a definitive trial. Ideas were discussed with patient members of the South Cleveland Heart Fund in 2014 and again in 2015. All patients fully supported the trial and informed the primary outcome; their view that returning to usual activities was the best way to compare the two techniques matched that of surgeons and cardiologists. A patient is a member of the TSC and the team have committed to sharing the results of this trial with patients.
Blinding
SF-36v2 and EQ-5D-5L assessments are completed over the telephone by a central blinded assessor.
All echocardiograms are sent to an independent Core Laboratory for assessment by a senior echocardiographer, who reports these without knowledge of the intervention.
Ethics and dissemination
The trial team carefully thought through potential issues and aimed to address these in the trial design. This study does not include patients who will lack capacity, nor minors. This trial is not based in an emergency setting. The trial is conducted in accordance with the protocol, the principles of Good Clinical Practice, and the favourable ethical opinion. All patients provide written informed consent prior to participation, and for their data to be used in future research.
The main issue is a relatively new cardiac procedure, though all cardiac surgery has inherent risks. To mitigate this, surgery will only be performed by highly experienced consultant cardiac surgeons who will have performed at least 50 operations of the same type prior to the start of their involvement. The trial will require the collection and storage of sensitive personal data. All data are handled in accordance with the appropriate legislation. We will only publish deidentified aggregated data.
The results of the trial are expected to fill an important and large gap in the international literature. We expect that the trial results will inform an important revision of national and international. We will publish the findings in peer-reviewed journals, and disseminate results to patients, and the international clinical community.
Supplementary Material
Acknowledgments
We are grateful to the IDMEC, TSC, and the independent expert panel for their support of the trial. We would like to acknowledge the considerable work being undertaken by Principal Investigators and members of the site teams, and at Newcastle Clinical Trials Unit in support of this trial.
Footnotes
Twitter: @VipinZamvar
Contributors: EA and JZ conceived the idea for the trial. EA, RHM, HCH, ASK, GJM, JZ and LV codesigned the trial, secured funding from NIHR HTA and wrote the protocol. EA is chief Investigator. RHM and HCH provide methodological input and oversee NCTU activity. ASK leads statistical aspects and analysis. LV leads health economic aspects and analysis, supported by CF-G. LM manages the trial. RG leads the core laboratory. GJM provides ongoing clinical and academic contributions. JZ, AW, GL, RD, AG, SK, VZ, RP, CL, MD-H, RC, HAV, FC, SS, MC, MB, GN, PPP, OW, PM, BHK, MDP, ADM and DP are surgeons in the trial and who have contributed to ongoing trial design and the Statistical Analysis Plan. This paper was drafted from the approved version of the protocol, version 5.0, 18th June 2020; all authors commented and amended drafts of the paper and approved the final manuscript.
Funding: This work is supported by a National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme (Ref: 14/192/110). The UK Mini Mitral Trial is sponsored by South Tees Hospitals NHS Foundation Trust.
Competing interests: LV was a member of the NIHR Health Technology Assessment Clinical Evaluation and Trials Panel from 2015 to 2018.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1.McNeely CA, Vassileva CM. Long-Term outcomes of mitral valve repair versus replacement for degenerative disease: a systematic review. Curr Cardiol Rev 2015;11:157–62. 10.2174/1573403x10666140827093650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaur P, Kaneko T, McGurk S, et al. Mitral valve repair versus replacement in the elderly: short-term and long-term outcomes. J Thorac Cardiovasc Surg 2014;148:1400–6. 10.1016/j.jtcvs.2014.01.032 [DOI] [PubMed] [Google Scholar]
- 3.Coutinho GF, Correia PM, Antunes MJ. Concomitant aortic and mitral surgery: to replace or repair the mitral valve? J Thorac Cardiovasc Surg 2014;148:1386–92. 10.1016/j.jtcvs.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Candan O, Ozdemir N, Aung SM, et al. Effect of mitral valve repair versus replacement on left ventricular rotational deformation: a study with speckle tracking echocardiography. J Heart Valve Dis 2013;22:651–9. [PubMed] [Google Scholar]
- 5.Markar SR, Sadat U, Edmonds L, et al. Mitral valve repair versus replacement in the elderly population. J Heart Valve Dis 2011;20:265–71. [PubMed] [Google Scholar]
- 6.Chikwe J, Goldstone AB, Passage J, et al. A propensity score-adjusted retrospective comparison of early and mid-term results of mitral valve repair versus replacement in octogenarians. Eur Heart J 2011;32:618–26. 10.1093/eurheartj/ehq331 [DOI] [PubMed] [Google Scholar]
- 7.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro heart survey on valvular heart disease. Eur Heart J 2003;24:1231–43. 10.1016/s0195-668x(03)00201-x [DOI] [PubMed] [Google Scholar]
- 8.Keogh B, Kinsman R. Fifth national adult cardiac surgical database report improving outcomes for patients, 2003The Society of Cardiothoracic Surgeons of Great Britain and Ireland. Available: https://scts.org/_userfiles/resources/5thBlueBook2003.pdf [Google Scholar]
- 9.SCTS Blue Book . Cardiac surgery audit data bluebook, the Society for cardiothoracic surgery (SCTS) blue book.
- 10.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2017;135:e1159–95. 10.1161/CIR.0000000000000503 [DOI] [PubMed] [Google Scholar]
- 11.Kirmani BH, Jones SG, Malaisrie SC, et al. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst Rev 2017;4:CD011793. 10.1002/14651858.CD011793.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culliford AT, Cunningham JN, Zeff RH, et al. Sternal and costochondral infections following open-heart surgery. A review of 2,594 cases. J Thorac Cardiovasc Surg 1976;72:714–26. [PubMed] [Google Scholar]
- 13.Zacharias A, Habib RH. Factors predisposing to median sternotomy complications. deep vs superficial infection. Chest 1996;110:1173–80. 10.1378/chest.110.5.1173 [DOI] [PubMed] [Google Scholar]
- 14.Rabindranauth P, Burns JG, Vessey TT, et al. Minimally invasive coronary artery bypass grafting is associated with improved clinical outcomes. Innovations 2014;9:421–6. 10.1097/IMI.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 15.Shrestha M, Krueger H, Umminger J, et al. Minimally invasive valve sparing aortic root replacement (David procedure) is safe. Ann Cardiothorac Surg 2015;4:148–53. 10.3978/j.issn.2225-319X.2014.08.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojas SV, Avsar M, Khalpey Z, et al. Minimally invasive off-pump left ventricular assist device exchange: anterolateral thoracotomy. Artif Organs 2014;38:539–42. 10.1111/aor.12226 [DOI] [PubMed] [Google Scholar]
- 17.Ko K, de Kroon TL, Post MC, et al. Minimally invasive mitral valve surgery: a systematic safety analysis. Open Heart 2020;7:e001393. 10.1136/openhrt-2020-001393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199–206. 10.1016/j.jtcvs.2012.12.070 [DOI] [PubMed] [Google Scholar]
- 19.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421–6. 10.1097/00000658-199710000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutsch M-A, Krane M, Schneider L, et al. Health-Related quality of life and functional outcome in cardiac surgical patients aged 80 years and older: a prospective single center study. J Card Surg 2014;29:14–21. 10.1111/jocs.12233 [DOI] [PubMed] [Google Scholar]
- 21.Moscarelli M, Fattouch K, Gaudino M, et al. Minimal access versus sternotomy for complex mitral valve repair: a meta-analysis. Ann Thorac Surg 2020;109:737–44. 10.1016/j.athoracsur.2019.07.034 [DOI] [PubMed] [Google Scholar]
- 22.Grant SW, Hickey GL, Modi P, et al. Propensity-matched analysis of minimally invasive approach versus sternotomy for mitral valve surgery. Heart 2019;105:783–9. 10.1136/heartjnl-2018-314049 [DOI] [PubMed] [Google Scholar]
- 23.Grossi EA, Goldman S, Wolfe JA, et al. Minithoracotomy for mitral valve repair improves inpatient and postdischarge economic savings. J Thorac Cardiovasc Surg 2014;148:2818–22. 10.1016/j.jtcvs.2014.08.029 [DOI] [PubMed] [Google Scholar]
- 24.Sündermann SH, Sromicki J, Rodriguez Cetina Biefer H, et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:1989–95. 10.1016/j.jtcvs.2014.01.046 [DOI] [PubMed] [Google Scholar]
- 25.Ding C, Jiang D-ming, Tao K-yu, et al. Anterolateral minithoracotomy versus median sternotomy for mitral valve disease: a meta-analysis. J Zhejiang Univ Sci B 2014;15:522–32. 10.1631/jzus.B1300210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao C, Gupta S, Chandrakumar D, et al. A meta-analysis of minimally invasive versus conventional mitral valve repair for patients with degenerative mitral disease. Ann Cardiothorac Surg 2013;2:693–703. 10.3978/j.issn.2225-319X.2013.11.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modi P, Hassan A, Chitwood WR. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008.;;34:943–52. Nov. 10.1016/j.ejcts.2008.07.057 [DOI] [PubMed] [Google Scholar]
- 28.Cheng DCH, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations 2011;6:84–103. 10.1097/IMI.0b013e3182167feb [DOI] [PubMed] [Google Scholar]
- 29.Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg 2011;142:77–83. Jul. 10.1016/j.jtcvs.2010.08.033 [DOI] [PubMed] [Google Scholar]
- 30.Nasso G, Bonifazi R, Romano V, et al. Three-Year results of repaired Barlow mitral valves via right minithoracotomy versus median sternotomy in a randomized trial. Cardiology 2014;128:97–105. 10.1159/000357263 [DOI] [PubMed] [Google Scholar]
- 31.Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748–56. 10.1016/j.jtcvs.2012.09.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamelas J, Sarria A, Santana O, et al. Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann Thorac Surg 2011;91:79–84. 10.1016/j.athoracsur.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 33.Mazine A, Vistarini N, Ghoneim A, et al. Very high repair rate using minimally invasive surgery for the treatment of degenerative mitral insufficiency. Can J Cardiol 2015;31:744–51. 10.1016/j.cjca.2014.12.029 [DOI] [PubMed] [Google Scholar]
- 34.Holzhey DM, Shi W, Borger MA, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg 2011;91:401–5. 10.1016/j.athoracsur.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 35.Petracek MR, Leacche M, Solenkova N, et al. Minimally invasive mitral valve surgery expands the surgical options for high-risks patients. Ann Surg 2011;254:606–11. 10.1097/SLA.0b013e3182300399 [DOI] [PubMed] [Google Scholar]
- 36.Iribarne A, Easterwood R, Russo MJ, et al. A minimally invasive approach is more cost-effective than a traditional sternotomy approach for mitral valve surgery. J Thorac Cardiovasc Surg 2011;142:1507–14. 10.1016/j.jtcvs.2011.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760–5. 10.1016/j.ejcts.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 38.Yoo JS, Kim JB, Jung S-H, et al. Echocardiographic assessment of mitral durability in the late period following mitral valve repair: minithoracotomy versus conventional sternotomy. J Thorac Cardiovasc Surg 2014;147:1547–52. 10.1016/j.jtcvs.2013.05.042 [DOI] [PubMed] [Google Scholar]
- 39.Falk V, Cheng DCH, Martin J, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the International Society of minimally invasive coronary surgery (ISMICS) 2010. Innovations 2011;6:66–76. 10.1097/imi.0b013e318216be5c [DOI] [PubMed] [Google Scholar]
- 40.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. New England Journal of Medicine 2018;379:2307–18. [DOI] [PubMed] [Google Scholar]
- 41.Feldman T, Foster E, Qureshi M, et al. TCT-788 the Everest II randomized controlled trial (RCT): three year outcomes. J Am Coll Cardiol 2012;60:B229–30. 10.1016/j.jacc.2012.08.833 [DOI] [Google Scholar]
- 42.Ware JE, Kosinski M, Bjorner JB, et al. User’s Manual for the SF-36v2TM Health Survey. 2nd Ed. Lincoln RI: QualityMetric Incorporated, 2007. [Google Scholar]
- 43.van Hout B, Janssen MF, Feng Y-S, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708–15. 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 44.Devlin NJ, Shah KK, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7–22. 10.1002/hec.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esliger DW, Rowlands ANNV, Hurst TL, et al. Validation of the GENEA Accelerometer. Med Sci Sport Exerc 2011;43:1085–93. 10.1249/MSS.0b013e31820513be [DOI] [PubMed] [Google Scholar]
- 47.Pavey T, Gomersall S, Clark B, et al. The validity of the GENEActiv wrist-worn accelerometer for measuring sedentary behaviour in free living. J Sci and Med in Sport 2014;18:e34. 10.1016/j.jsams.2014.11.221 [DOI] [PubMed] [Google Scholar]
- 48.Sirichana W, Moore-Gillon CE, Patel MH. Daily physical activity in COPD: quantification by Tri_Axial Accelerometry. Am J Respir Crit Care Med 2013;187:A1361 https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2013.187.1_MeetingAbstracts.A1361 [Google Scholar]
- 49.Troiano RP, McClain JJ, Brychta RJ, et al. Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014;48:1019–23. 10.1136/bjsports-2014-093546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hildebrand M, VAN Hees VT, Hansen BH, et al. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816–24. 10.1249/MSS.0000000000000289 [DOI] [PubMed] [Google Scholar]
- 51.EQ-5D. Available: https://euroqol.org/
- 52.NICE . Guide to the methods of technology appraisal; 2013. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.