Abstract
Primary age-related tauopathy (PART) is a neurodegenerative entity defined as neurofibrillary degeneration generally restricted to the medial temporal region (Braak stage I–IV) with complete or near absence of diffuse and neuritic plaques. Symptoms range in severity but are generally milder and later in onset than in Alzheimer disease (AD). Recently, an early predilection for neurofibrillary degeneration in the hippocampal CA2 subregion has been demonstrated in PART, whereas AD neuropathologic change (ADNC) typically displays relative sparing of CA2 until later stages. In this study, we utilized a semiquantitative scoring system to evaluate asymmetry of neurofibrillary degeneration between left and right hippocampi in 67 PART cases and 17 ADNC cases. 49% of PART cases demonstrated asymmetric findings in at least one hippocampal subregion, and 79% of the asymmetric cases displayed some degree of CA2 asymmetry. Additionally, 19% of cases revealed a difference in Braak score between the right and left hippocampi. There was a significant difference in CA2 neurofibrillary degeneration (p = 0.0006) and CA2/CA1 ratio (p < 0.0001) when comparing the contralateral sides, but neither right nor left was more consistently affected. These data show the importance of analyzing bilateral hippocampi in the diagnostic evaluation of PART and potentially of other neurodegenerative diseases.
Keywords: Alzheimer disease, Braak, CA1, CA2, Neurofibrillary tangles, Primary age-related tauopathy, Thal
INTRODUCTION
Primary age-related tauopathy (PART) is a relatively recently defined neurodegenerative entity, previously referred to as “tangle-only dementia,” “tangle-predominant senile dementia,” or simply “age-related neurofibrillary degeneration” (1, 2). It has several features in common with Alzheimer disease (AD) including the presence of tau-positive neurofibrillary tangles (NFTs), consisting of paired helical filaments composed of both 3R- and 4R-tau (3, 4). It is unclear if these NFTs progress through the medial temporal lobe in the same sequence as AD neuropathologic change (ADNC), although they have been shown to have a more limited distribution in PART (3, 5–7). PART NFT pathology progresses in an amyloid-independent manner that is in contrast to ADNC and occurs in a somewhat different spatial arrangement than other amyloid-independent tauopathies (8, 9). Although there currently exists debate as to whether PART is a separate entity or should be included as a subset of ADNC (10–12), PART is currently defined as the presence of NFTs with Braak stages I–IV, Thal phase 0–2, and sparse or absent neuritic plaques by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) criteria (3, 5, 13, 14).
More recently, it has been shown that PART also has different genetic risk factors than those for AD, including a statistical association with the MAPT H1 haplotype and lack of association with APOE ε4 (15). Further studies have shown that there are morphological differences between PART and ADNC; for example, PART NFTs seem to have a predilection for CA2 with less severe CA1 and entorhinal cortex tau pathology (16–21) in contrast to ADNC, which typically spares the CA2/3 regions until later in the disease course (16, 22, 23). In addition, NFTs rarely progress to neocortical stages (Braak V or VI) in the absence of β-amyloid plaques that are characteristic of AD (9), and PART cases frequently lack evidence of both β-amyloid plaques and soluble β-amyloid (15).
Clinically, PART cohorts tend to include a higher percentage of female patients and the “oldest old,” and PART cases demonstrate significantly less severe cognitive decline compared with patients with ADNC, although individuals along the full spectrum of cognitive function including normal cognition, mild cognitive impairment, and dementia are typically found in these cohorts (3, 19, 24). As in ADNC, severe tau pathology in PART is more likely to be associated with more severe clinical symptoms (3, 25), although large-scale studies of patients with PART suggest that the CA2 NFT pathology is more related to patient age, and Braak stages in these patients correlate poorly with cognitive decline (measured by Mini-Mental State Examination [MMSE] and Clinical Dementia Rating [CDR]), which suggests that the CA2 pathology may be more a function of aging than a clinical disease process, or perhaps a specific clinical correlate of CA2 involvement is yet to be identified (21). PART has also recently been linked to clinical depression, although it is unclear if depression early in life is a risk factor for PART or if PART pathology may result in depressive symptoms (26).
While many medical centers perform only unilateral evaluation of hippocampi in the diagnosis and workup of neurodegenerative diseases, a certain degree of asymmetry between right and left neocortical and hippocampal pathology in AD (particularly in early Braak stages) has been reported in several studies over the decades (27–30), and more recently in other neurodegenerative diseases including argyrophilic grain disease (AGD) and frontotemporal lobe degeneration (FTLD) (31–35). Review of these cases suggests that tau asymmetry may be more common in cases with low β-amyloid (low Thal phase and CERAD plaque score) (32), suggesting that asymmetry may be more pronounced in PART cases. Herein, we examine the bilateral hippocampi of 67 PART cases and 17 ADNC cases to evaluate the degree of hippocampal NFT asymmetry using a semiquantitative scoring system in each subregion of the bilateral hippocampi (entorhinal cortex, dentate gyrus, and CA1–CA4) (21).
MATERIALS AND METHODS
Case Selection
Cases from State University of New York (SUNY) Upstate Medical University, Syracuse, NY and Mayo Clinic Tissue Registry, Rochester, MN from 2011 to 2019 were reviewed by 2 board-certified neuropathologists (J.M.W. and T.E.R.) blinded to any previous neurologic diagnoses for evidence of phospho-tau, β-amyloid, α-synuclein, and TDP-43. In total, we identified 67 cases of definite or possible PART (defined as Braak stage I–IV, Thal phase 0–2, and absent or sparse neuritic plaques by CERAD criteria) with bilateral hippocampal sampling at hippocampal level 7 (36), irrespective of available clinical information. This included 35 cases of definite PART (Braak I–IV, Thal 0, CERAD 0) and 32 cases of possible PART (Braak I–IV, Thal 1–2, CERAD 0–1) (Table 1) (3). The PART cases included here had a mean age of 79.6 ± 6.1 years old (range 70–94 years). We identified an additional 17 bilateral ADNC cases with a mean age of 75.3 ± 7.5 years old (range 65–91 years). The study was granted an Institutional Review Board exemption from the Institutional Privacy Office at SUNY Upstate Medical University and was certified by HIPAA review.
TABLE 1.
Semiquantitative Assessment of Bilateral PART and AD Cases
| Case # | Entorhinal Tau |
CA1 Tau |
CA2 Tau |
CA3 Tau |
CA4 Tau |
Dentate Tau |
CA2/CA1 Ratio |
Braak Score |
CERAD | Thal | CAA | Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | L | R | L | R | |||||
| 1 | 2 | 1 | 2 | 0.5 | 2 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | III | I | 0 | 0 | 0 | PART |
| 2 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0.5 | 0 | 0.5 | 0 | 0 | 2 | 1 | III | III | 0 | 0 | 0 | PART |
| 3 | 2 | 1 | 2 | 1 | 2 | 0 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 1 | 0 | III | II | 0 | 0 | 0 | PART |
| 4 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 1 | 0 | III | III | 0 | 0 | 0 | PART |
| 5 | 1 | 1 | 0 | 2 | 0.5 | 0 | 0.5 | 0 | 0 | 0 | 0 | 2 | III | III | 0 | 0 | 0 | PART | ||
| 6 | 2 | 1 | 2 | 1 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1.5 | 1 | III | III | 0 | 0 | 0 | PART |
| 7 | 2 | 2 | 3 | 3 | 1 | 2 | 0 | 0.5 | 0.5 | 1 | 0 | 0 | 0.33 | 0.67 | IV | IV | 0 | 0 | 0 | PART |
| 8 | 2 | 3 | 2 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0.25 | 0.5 | IV | IV | 0 | 0 | 0 | PART |
| 9 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0.5 | 0 | 0.5 | 0 | 0 | 0.5 | 0.5 | IV | IV | 0 | 0 | 0 | PART |
| 10 | 2 | 2 | 1 | 1 | 3 | 3 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 | 3 | 3 | III | III | 0 | 0 | 0 | PART |
| 11 | 2 | 3 | 3 | 3 | 2 | 1 | 0 | 0.5 | 1 | 0.5 | 0.5 | 0 | 0.67 | 0.33 | IV | IV | 0 | 0 | 0 | PART |
| 12 | 2 | 2 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0.5 | 0.5 | III | III | 0 | 0 | 0 | PART |
| 13 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0.5 | 0.5 | 0 | 0 | 1 | 2 | III | III | 0 | 0 | 0 | PART |
| 14 | 1 | 1 | 2 | 2 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0.5 | 0.5 | 0.5 | IV | IV | 0 | 0 | 0 | PART |
| 15 | 2 | 2 | 1 | 1 | 2 | 3 | 1 | 1 | 0.5 | 1 | 0 | 0 | 2 | 3 | III | III | 0 | 0 | 0 | PART |
| 16 | 1 | 1 | 2 | 1 | 2 | 0.5 | 1 | 0.5 | 1 | 0 | 0.5 | 0 | 1 | 0.5 | IV | III | 0 | 0 | 0 | PART |
| 17 | 1 | 1 | 0.5 | 1 | 1 | 2 | 0 | 0.5 | 0 | 0.5 | 0.5 | 0.5 | 2 | 2 | II | III | 0 | 0 | 0 | PART |
| 18 | 2 | 2 | 3 | 3 | 2 | 3 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0.5 | 0.67 | 1 | IV | IV | 0 | 0 | 0 | PART |
| 19 | 1 | 0.5 | 2 | 0.5 | 2 | 0 | 0.5 | 0 | 1 | 0 | 0.5 | 0 | 1 | 0 | IV | I | 0 | 0 | 0 | PART |
| 20 | 0.5 | 2 | 0.5 | 1 | 0 | 1 | 0 | 0.5 | 0 | 0.5 | 0 | 0.5 | 0 | 1 | I | III | 0 | 0 | 0 | PART |
| 21 | 2 | 1 | 1 | 0.5 | 2 | 0.5 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | III | II | 0 | 0 | 0 | PART |
| 22 | 1 | 1 | 0.5 | 0.5 | 2 | 2 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 4 | 4 | II | II | 0 | 0 | 0 | PART |
| 23 | 1 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 1 | 1 | II | II | 0 | 0 | 0 | PART |
| 24 | 1 | 1 | 0.5 | 0.5 | 0 | 2 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | II | II | 0 | 0 | 0 | PART |
| 25 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0.5 | 0 | 0.5 | 0 | 0 | 0 | 0 | II | II | 0 | 0 | 0 | PART |
| 26 | 2 | 2 | 1 | 1 | 0.5 | 0 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0 | 0.5 | 0 | II | II | 0 | 0 | 0 | PART |
| 27 | 1 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 1 | I | I | 0 | 0 | 0 | PART |
| 28 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0 | 2 | 1 | I | I | 0 | 0 | 0 | PART |
| 29 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0 | 0.5 | 0.5 | 0.5 | 0 | 1 | 2 | I | I | 0 | 0 | 0 | PART |
| 30 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | I | I | 0 | 0 | 0 | PART |
| 31 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 0 | I | I | 0 | 0 | 0 | PART |
| 32 | 1 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | I | I | 0 | 0 | 0 | PART |
| 33 | 0.5 | 0.5 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | I | I | 0 | 0 | 0 | PART |
| 34 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | I | I | 0 | 0 | 0 | PART |
| 35 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0 | 1 | 1 | I | I | 0 | 0 | 0 | PART |
| 36 | 2 | 2 | 2 | 2 | 1 | 3 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | 0.5 | 1.5 | IV | IV | 0 | 1 | 0 | PART |
| 37 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | IV | IV | 0 | 1 | 0 | PART |
| 38 | 2 | 2 | 2 | 2 | 1 | 2 | 0.5 | 0 | 0.5 | 0 | 0 | 0 | 0.5 | 1 | IV | IV | 0 | 1 | 0 | PART |
| 39 | 2 | 2 | 2 | 2 | 1 | 1 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | IV | IV | 0 | 1 | 0 | PART |
| 40 | 1 | 1 | 0.5 | 2 | 0 | 2 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 1 | II | III | 0 | 1 | 0 | PART |
| 41 | 2 | 2 | 3 | 3 | 2 | 2 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0.5 | 0.67 | 0.67 | IV | IV | 0 | 1 | 0 | PART |
| 42 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0.5 | 1 | 1 | IV | IV | 0 | 2 | 0 | PART |
| 43 | 1 | 1 | 2 | 2 | 3 | 3 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 | 1.5 | 1.5 | IV | IV | 0 | 1 | 0 | PART |
| 44 | 2 | 1 | 2 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | III | II | 0 | 2 | 1 | PART |
| 45 | 2 | 1 | 1 | 1 | 2 | 3 | 0.5 | 0.5 | 0.5 | 3 | 0 | 0 | 2 | 3 | III | III | 0 | 2 | 0 | PART |
| 46 | 1 | 2 | 3 | 2 | 2 | 2 | 1 | 0.5 | 1 | 0.5 | 1 | 1 | 0.67 | 1 | III | III | 0 | 2 | 0 | PART |
| 47 | 1 | 2 | 0.5 | 1 | 0.5 | 1 | 0 | 0 | 0 | 0.5 | 0 | 0 | 1 | 1 | II | III | 0 | 2 | 0 | PART |
| 48 | 1 | 0.5 | 1 | 2 | 3 | 3 | 0.5 | 2 | 1 | 3 | 0.5 | 2 | 3 | 1.5 | III | III | 0 | 2 | 0 | PART |
| 49 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 0.5 | 0.5 | 0 | 0 | 0.5 | 1 | 2 | III | III | 0 | 1 | 0 | PART |
| 50 | 0.5 | 0.5 | 1 | 0.5 | 2 | 1 | 0 | 0 | 0.5 | 0 | 0 | 0 | 2 | 2 | II | II | 0 | 1 | 0 | PART |
| 51 | 0.5 | 0.5 | 0.5 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | I | II | 0 | 1 | 0 | PART |
| 52 | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0.5 | III | III | 0 | 1 | 0 | PART |
| 53 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | I | I | 0 | 1 | 1 | PART |
| 54 | 1 | 1 | 0.5 | 0.5 | 1 | 0 | 0 | 0 | 0.5 | 0 | 0.5 | 0 | 2 | 0 | II | II | 0 | 2 | 0 | PART |
| 55 | 3 | 3 | 2 | 2 | 2 | 2 | 0.5 | 0.5 | 1 | 0.5 | 0 | 0 | 1 | 1 | IV | IV | 1 | 1 | 1 | PART |
| 56 | 3 | 3 | 1 | 2 | 2 | 2 | 0.5 | 0 | 1 | 0.5 | 0 | 0 | 2 | 1 | IV | IV | 1 | 1 | 0 | PART |
| 57 | 2 | 2 | 2 | 2 | 3 | 3 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 1.5 | 1.5 | IV | IV | 1 | 2 | 1 | PART |
| 58 | 2 | 2 | 2 | 2 | 3 | 3 | 0.5 | 0.5 | 0.5 | 0.5 | 0 | 0.5 | 1.5 | 1.5 | IV | IV | 1 | 1 | 1 | PART |
| 59 | 1 | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | III | III | 1 | 2 | 0 | PART |
| 60 | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1.5 | 1.5 | IV | IV | 1 | 1 | 0 | PART |
| 61 | 3 | 3 | 2 | 2 | 3 | 3 | 0 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 1.5 | 1.5 | IV | IV | 1 | 1 | 1 | PART |
| 62 | 2 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0.5 | 0.5 | 1 | III | II | 0 | 2 | 0 | PART |
| 63 | 1 | 1 | 2 | 2 | 3 | 3 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 1.5 | 1.5 | III | III | 1 | 2 | 1 | PART |
| 64 | 1 | 1 | 1 | 1 | 1 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | II | II | 1 | 1 | 0 | PART |
| 65 | 1 | 0.5 | 1 | 0.5 | 2 | 1 | 0.5 | 0 | 0.5 | 0.5 | 0 | 0 | 2 | 2 | II | I | 1 | 1 | 0 | PART |
| 66 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | I | I | 1 | 1 | 0 | PART |
| 67 | 0.5 | 0.5 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | I | I | 0 | 2 | 0 | PART |
| 68 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 0.5 | 2 | 1 | 2 | 1 | 0.33 | 0.33 | VI | VI | 3 | 5 | 0 | ADNC |
| 69 | 3 | 3 | 2 | 2 | 2 | 2 | 0.5 | 0 | 1 | 0.5 | 0 | 0 | 1 | 1 | V | V | 2 | 4 | 1 | ADNC |
| 70 | 3 | 3 | 3 | 3 | 0.5 | 1 | 1 | 0.5 | 1 | 1 | 0.5 | 1 | 0.17 | 0.33 | V | V | 3 | 4 | 1 | ADNC |
| 71 | 3 | 3 | 3 | 3 | 1 | 2 | 0.5 | 1 | 1 | 1 | 1 | 1 | 0.33 | 0.67 | VI | VI | 3 | 5 | 0 | ADNC |
| 72 | 3 | 3 | 3 | 3 | 0.5 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 | 0.17 | 0.33 | V | V | 2 | 4 | 0 | ADNC |
| 73 | 3 | 3 | 3 | 3 | 1 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 0.33 | 0.17 | VI | VI | 3 | 5 | 1 | ADNC |
| 73 | 3 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0.67 | 0.17 | VI | VI | 2 | 5 | 0 | ADNC |
| 74 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 0.33 | 0.33 | V | V | 3 | 5 | 1 | ADNC |
| 75 | 3 | 3 | 3 | 3 | 1 | 1 | 0.5 | 1 | 0.5 | 0.5 | 2 | 2 | 0.33 | 0.33 | VI | VI | 3 | 5 | 1 | ADNC |
| 76 | 3 | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.33 | 0.33 | VI | VI | 3 | 4 | 0 | ADNC |
| 77 | 3 | 3 | 3 | 3 | 1 | 1 | 0.5 | 1 | 1 | 1 | 0.5 | 0.5 | 0.33 | 0.33 | V | V | 3 | 5 | 0 | ADNC |
| 78 | 3 | 3 | 3 | 3 | 2 | 2 | 0.5 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.67 | 0.67 | IV | IV | 2 | 4 | 0 | ADNC |
| 79 | 2 | 2 | 2 | 2 | 2 | 2 | 0.5 | 0.5 | 0 | 0.5 | 0 | 0 | 1 | 1 | V | V | 1 | 3 | 0 | ADNC |
| 80 | 1 | 3 | 3 | 3 | 2 | 2 | 1 | 1 | 2 | 2 | 0.5 | 1 | 0.67 | 0.67 | IV | IV | 3 | 4 | 0 | ADNC |
| 81 | 2 | 3 | 3 | 3 | 2 | 2 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0.67 | 0.67 | V | V | 3 | 4 | 0 | ADNC |
| 82 | 1 | 2 | 0.5 | 0.5 | 0 | 1 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 2 | II | III | 1 | 3 | 0 | ADNC |
| 83 | 0.5 | 0.5 | 3 | 3 | 3 | 3 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | IV | IV | 2 | 3 | 1 | ADNC |
Note: Underlined = ≥1 semiquantitative score/Braak score greater than the contralateral counterpart; italic = ≤1 semiquantitative score/Braak score greater than the contralateral counterpart.
Immunohistochemistry
Four-µm-thick sections of formalin-fixed, paraffin-embedded tissue underwent heat-induced epitope retrieval using CC1 (Ventana, Tucson, AZ), followed by Bielschowsky Silver Stain (Abcam, Cambridge, United Kingdom) on hippocampal, frontal, and neocortical sections; phospho-tau (p-tau) (AT8; Thermo Fisher Scientific, Waltham, MA) on hippocampal and neocortical sections; β-amyloid (Covance, Inc., Princeton, NJ) on hippocampal, neocortical, cerebellar, and midbrain sections, α-synuclein (Santa Cruz Biotechnology, Santa Cruz, CA) on hippocampal, neocortical, cingulate, midbrain, and medulla sections; and TDP-43 (Sigma-Aldrich, Inc., St. Louis, MO) on hippocampal, neocortical, and cingulate sections, on a Ventana Benchmark Ultra automated stainer, using Ventana UltraView Universal DAB Detection kits, according to the manufacturer protocols. All cases were stained with the same set of antibodies for consistency.
Other Concurrent Pathologies
TDP-43 and α-synuclein were performed on every hippocampal section to evaluate for concurrent limbic-predominant age-related TDP-43 encephalopathy (LATE) or Lewy body disease (LBD) affecting CA1 or CA2 pathology. The cases were also evaluated for evidence of chronic traumatic encephalopathy (CTE) to exclude additional p-tau pathology in the hippocampi.
The pathologic diagnoses included “pure” PART in 45 cases (one of which had a pituitary adenoma and one had metastatic lung adenocarcinoma in the left cerebellum), 8 had large or multifocal ischemic or hemorrhagic infarcts, and 15 had other concurrent neurodegenerative diseases: 4 brainstem-predominant LBD, 4 AGD, 3 age-related p-tau astrogliopathy (ARTAG), 1 possible CTE, 1 multiple system atrophy, and 1 TDP-43-positive frontotemporal dementia/amyotrophic lateral sclerosis (FTLD-TDP-43/ALS).
The ADNC cohort included 5 cases with “pure” AD pathology and 12 cases with additional pathologies, including 3 instances of diffuse neocortical stage Lewy body disease, 1 instance of brainstem-predominant Lewy body disease, 6 cases with ischemic or hemorrhagic insults, 1 case with hippocampal sclerosis, 1 case with evidence of prior trauma, and 3 cases with tumors including meningioma (2 cases) and pituitary adenoma (1 case).
Semiquantitative Scoring
In all cases, bilateral hippocampal regions (entorhinal cortex, CA1, CA2, CA3, CA4, and dentate gyrus) were individually examined by J.M.W. and T.E.R. for density of NFTs and p-tau-positive dystrophic neurites with Bielschowsky silver stains and p-tau immunohistochemical stains. Braak stage, CERAD score, and Thal phase were also evaluated bilaterally according to previously established criteria (5, 14, 37). A score of 0.5 was given for single or “rare” tangles, 1 for “mild” p-tau pathology, 2 for “moderate” p-tau pathology, and 3 for “severe” p-tau pathology (Supplementary DataFig. S1) (21). Overall differences in tau burden between sides (labeled as “higher tau burden” and “lower tau burden”) were determined by summing the semiquantitative tau scores for each region evaluated on each side. The CA2/CA1 ratio was calculated for each side in each case evaluated.
Statistical Analysis
Differences in mean p-tau burden in hippocampal subregions were calculated using Analysis of Variance (ANOVA) (21), and differences between subregions in PART and ADNC were calculated using the Mann-Whitney U test (17). Proportion of cases in each group with asymmetry was calculated using the Fisher exact test. All statistical calculations were performed with GraphPad Prism version 8.4 (GraphPad, La Jolla, CA).
RESULTS
Comparison of PART and ADNC
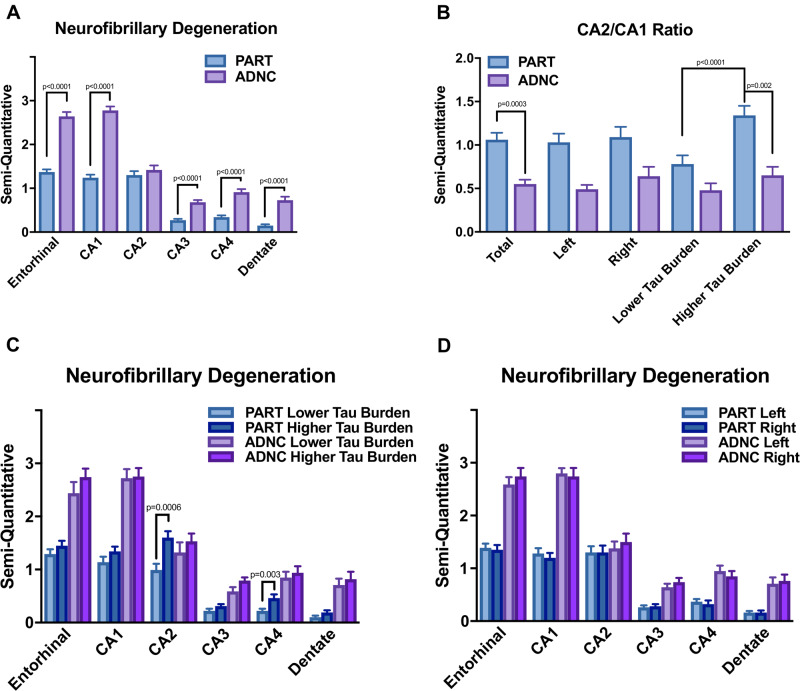
CA2 pathology severity was equal to or greater than CA1 pathology in 63% of PART hippocampi examined, but only 18% of ADNC hippocampi displayed this pattern (Table 1). There was significantly less neurofibrillary degeneration identified in the entorhinal region, CA1, CA3, CA4, and dentate gyrus in the PART cases compared with ADNC cases (p < 0.0001), but CA2 pathology in PART cases was statistically equivalent to CA2 pathology in ADNC cases (p = 0.4705) (Fig. 1A). The average semiquantitative CA2 tau-positive neurofibrillary pathology in the PART cohort was statistically equal to the pathology in CA1, and there was relatively less p-tau staining in the entorhinal cortex. This is in contrast to ADNC, in which there is relative CA2–CA4 sparing and more severe p-tau pathology in CA1 and the entorhinal cortex. There was a significant difference in CA2/CA1 ratios between PART and ADNC in both overall hippocampi (p = 0.0003), similar to previous reports (16, 17, 21), and in the sides with more significant overall p-tau burden (p = 0.002) (Fig. 1B).
FIGURE 1.
Bar graphs demonstrating a significantly higher level of p-tau-positive neurofibrillary degeneration between ADNC and PART in the entorhinal, CA1, CA3, and CA4 regions of the hippocampi (p < 0.0001 in all cases), but no significant difference was found between ADNC and PART in overall CA2 p-tau burden (p = 0.4705) (A). There is a significant difference in total CA2/CA1 ratio between total PART and ADNC cases (p = 0.0003), between the PART and ADNC cases on the side with the more severe p-tau burden (p = 0.002), and between the sides with less and more severe p-tau burden within the PART group (p < 0.0001) (B). There was a significant difference in p-tau pathology only in the CA2 region (p = 0.0006) and CA4 region (p = 0.0034) of PART cases between the sides with more and less severe p-tau pathology (C), however, there is no significant difference in p-tau-positive neurofibrillary degeneration between right and left sides in any region in either disease process (D).
Asymmetry in the PART Cohort
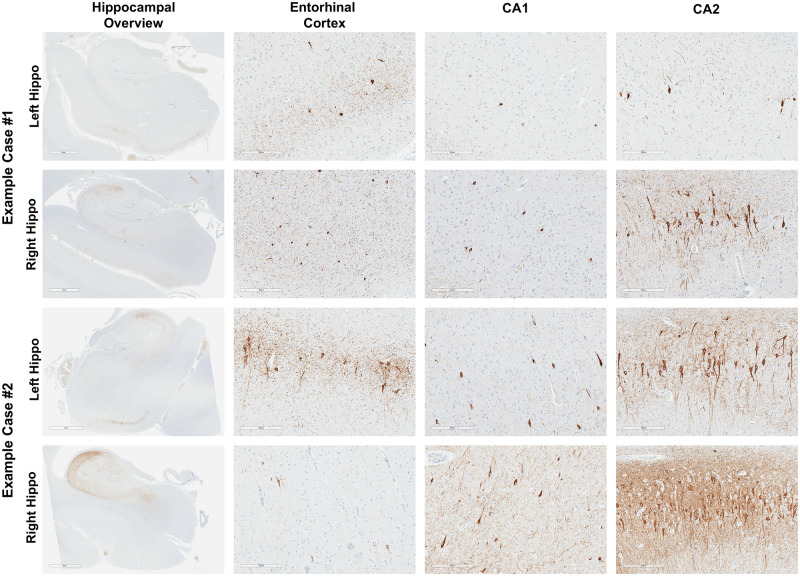
We evaluated bilateral hippocampi in both PART and ADNC cases. Within the PART cohort, 49% of cases (33/67) displayed asymmetry in at least one hippocampal subregion (defined as a difference of at least one by semiquantitative analysis), and 19% of cases (13/67) revealed asymmetry in Braak stage between left and right hippocampi (Table 2). The asymmetry occurred most frequently and was most severe in the CA2 subregion of the hippocampus (Fig. 2; Supplementary DataFig. S2). There was a significant difference in CA2/CA1 ratio in hippocampal sides with greater p-tau burden compared with hippocampal sides with less p-tau burden (p < 0.0001) (Fig. 1B) and a significant difference in p-tau-positive pathology in the CA2 (p = 0.0006) and CA4 (p = 0.0034) regions of PART cases between the side with greatest and least p-tau burden (Fig. 1C), but no quantifiable asymmetry was consistently seen between left and right side in PART or ADNC in any hippocampal region (Fig. 1D). Asymmetry of β-amyloid was not seen in the PART cases, although this is likely due in part to the infrequency of diffuse or neuritic plaques in these cases.
TABLE 2.
Overview of PART and ADNC Asymmetry
| Diagnosis | n | Mean Age; Range (Years) | Total Asymmetric Cases | Total Asymmetric Hippocampal Regions | Cases With Asymmetric Braak Scores | Hippocampal Regions With Asymmetry |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entorhinal | CA1 | CA2 | CA3 | CA4 | Dentate | ||||||
| PART | 67 | 79.6; 70–94 | 49.3% (33/67) | 14.0% (56/401) | 19.4% (13/67) | 19.7% (13/66) | 16.4% (11/67) | 38.8% (26/67) | 1.5% (1/67) | 6.0% (4/67) | 1.5% (1/67) |
| ADNC | 17 | 75.3; 65–91 | 35.3% (6/17) | 7.8% (8/102) | 5.9% (1/17) | 23.5% (4/17) | 0% | 11.8% (2/17) | 0% | 5.9% (1/17) | 5.9% (1/17) |
FIGURE 2.
p-Tau (AT8) staining of bilateral hippocampi from 2 representative cases of PART, with hippocampal overview and higher magnification of entorhinal cortex, CA1, and CA2 hippocampal, demonstrating variable asymmetry in CA2 (example case #1) and entorhinal cortex, CA1, and CA2 (example case #2). Scale bars for hippocampal overview panels = 4 mm, all other scale bars = 300 µm.
There was a trend toward a greater percentage of PART cases with asymmetry in at least one subregion (49%) compared with the ADNC group (35%), and a trend toward a greater percentage of PART cases with asymmetrical Braak stages (19%) compared with the ADNC group (6%). ADNC cases were more frequently asymmetrical in the entorhinal region, while the majority of PART cases with asymmetry had asymmetrical findings in the CA2 subregion (79% of the PART cases with asymmetry) (Table 1).
These findings highlight significant asymmetry in tau-positive NFT pathology in PART cases, primarily in the CA2 subregion, that does not depend on a specific laterality, and this asymmetry was not observed as frequently in cases with ADNC.
DISCUSSION
This is the first study to evaluate a cohort of PART cases for hippocampal asymmetry, although asymmetrical pathology in the hippocampus and neocortex has been investigated in other neurodegenerative diseases, with varying clinical implications (27–29, 31–35). Similar to previous studies (16, 17, 21), we demonstrate that the average level of CA2 pathology is statistically indistinguishable in PART compared with ADNC, while the NFT pathology in entorhinal, CA1, CA3, CA4, and dentate gyrus is significantly lower in PART compared with ADNC (Fig. 1A), and the CA2/CA1 ratio is significantly higher in PART compared with ADNC (Fig. 1B). In addition, we demonstrate that the CA2/CA1 ratio is significantly higher in the side with higher overall p-tau pathology burden in PART cases but not ADNC cases (Fig. 1B), and the overall level of CA2 and CA4 pathology is higher in the side with increased overall p-tau pathology in PART, but not in any other CA subregion in PART or ADNC (Fig. 1C). This difference is not consistently associated with either side (Fig. 1D). Asymmetry in at least one hippocampal subregion of at least one degree by our semiquantitative score system was identified in 33/67 PART cases (49%), and 13/67 PART cases displayed asymmetry in Braak stage (19%), compared with ADNC, in which 35% of cases (6 of 17 asymmetric cases) demonstrated asymmetry in at least one hippocampal subregion, and 6% (1/17) displayed asymmetrical Braak stages. The ADNC case with asymmetrical Braak stages had low p-tau burden bilaterally, similar to previous observations (23).
Given the propensity for Lewy body disease to preferentially affect the CA2 subregion (38, 39) and LATE pathology to affect the CA1/subiculum (40–42), each case was screened for these disorders with additional stains for α-synuclein and TDP-43 to rule out these potential confounding factors. The PART cohort included no cases with either additional diagnosis, although there were 4 cases with brainstem predominant Lewy body disease. The ADNC cohort had multiple instances of concurrent limbic/neocortical Lewy body disease and hippocampal sclerosis, and those had no significant increase in CA2 p-tau pathology compared with other cases. One case of possible CTE was also identified among the 67 PART cases.
PART as a disease entity is generally believed to display milder symptoms on average than AD, corresponding to the more limited Braak stage (3), although there are cases with severe memory deficits and dementia, especially in older patients in these cohorts, and has been associated with depression (26). The CA2 hippocampal subfield receives projections primarily from the entorhinal cortex and projects to the ventral CA1 neurons, as well as forming a reciprocal circuit with the lateral and medial septum, circuitry thought to be involved with social cognition (43) including encoding names and faces (44). In addition, animal models have hinted at roles for CA2 in social memory (45–47). However, in disorders such as LBD where CA2 is affected, it has been demonstrated that the degree of pathology in CA1 is more correlated with cognitive decline than the degree of CA2 pathology (48).
AGD is a 4R-tauopathy that, similar to PART, may occur in the absence of β-amyloid, has a predilection for the CA2 subregion, tends to affect older individuals than classic AD, and may result in dementia in the more advanced stages (49–51). Studies have shown histologic asymmetry in the majority of AGD cases, and patients with a greater pathologic burden on the left side had more significant dementia than those with more right-sided pathology, which is hypothesized to be due to more prominent impairment in the dominant hemisphere in these cases (23). Similarly, primary progressive aphasia (PPA), a clinical syndrome that can result from multiple underlying pathologies including ADNC and FTLD, among other entities (34), tends to result in several different variants of language impairments including logopenic and semantic aphasia (52). As with AGD, the symptoms of PPA seem to be related to an asymmetric neurodegeneration in the dominant hemisphere (33–35).
While PART is a purely neuropathologic diagnosis that does not require the presence of any specific clinical features, a limitation of the current study is that the majority of these cases were collected from community cohorts without detailed neurology and neuropsychology data (CDR, MMSE, or other verified dementia history), and so we were unable to correlate the CA2 pathology or CA2 asymmetry in PART cases with any definitive clinical symptoms. Previous work has suggested that the excess CA2 neurofibrillary degeneration burden present in PART cases may be a function of age and may not correlate well with CDR or MMSE results (21). It is possible that the asymmetry found in these cases may serve as a source of cognitive reserve in which the side with lower p-tau pathology may compensate to some degree for the side with greater p-tau pathology, and this may partially explain the lack of correlation between hippocampal pathology and clinical symptoms found in some studies that utilized unilateral hippocampi only. In the future, however, prospective studies to investigate the clinical and pathologic correlations of asymmetry in this disease and to identify any potential symptomatic differences between increased p-tau burden in the dominant and nondominant hemispheres should prove enlightening. Despite the lack of comprehensive clinical information in this set of cases, this study suggests that there are a significant number of PART cases that display asymmetry, and findings from other related neurodegenerative disease states suggest that asymmetry may indeed be clinically relevant.
The finding that PART is more frequently asymmetric than ADNC, and observations from previous data on cases with Alzheimer-type pathology (23) demonstrating that p-tau pathology appears more asymmetric in cases with lower Thal phase and CERAD scores, suggest that p-tau asymmetry may be more pronounced in cases without β-amyloid or with minimal concurrent β-amyloid deposition. It should be noted, however, that β-amyloid pathology has also been found to be asymmetric in a subset of ADNC cases (23).
Current standard workup for neurodegenerative diseases at most institutions includes only unilateral histologic examination of the hippocampus and neocortex with retention of the opposite hemibrain for nonmorphologic research analyses; this study demonstrates that unilateral examination of some brain regions may mislead the examiner in judging the severity of disease and has potential implications for the staging of PART and ADNC. For more accurate neurolopathogical diagnoses, and a more complete assessment of incidence and severity, a recommendation for bilateral tissue examination is supported by this study. In addition, these findings, along with the more general finding that PART displays an early selective vulnerability of the CA2 hippocampal subfield for neurofibrillary degeneration (16, 17, 21), and molecular studies indicating divergent underlying genetic risk factors (3, 7, 9, 10, 15, 25), support the hypothesis that PART is a neuropathologically distinct entity from AD.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge support from The Barker Brain Bank, Reed Precision Medicine Funds, and The Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases at the Joe and Teresa Long School of Medicine, University of Texas Health San Antonio. In addition, the authors would like to thank all of the brain donors for allowing this work to be possible.
K.F.B. is supported in part by a grant from the National Institute on Aging (NIA) (R01 AG062348). S.S. is supported in part by grants from the NIA (R01 AG054076 and U01 AG052409). J.F.C. is supported by grants from the National Institutes of Health (NIH) (R01 NS095252 and R01 AG054008), Alzheimer’s Association (NIRG-15-363188), and the Tau Consortium.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Bancher C, Jellinger KA.. Neurofibrillary tangle predominant form of senile dementia of Alzheimer type: A rare subtype in very old subjects. Acta Neuropathol 1994;88:565–70 [DOI] [PubMed] [Google Scholar]
- 2. Jellinger KA, Attems J.. Neurofibrillary tangle-predominant dementia: Comparison with classical Alzheimer disease. Acta Neuropathol 2007;113:107–17 [DOI] [PubMed] [Google Scholar]
- 3. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duyckaerts C, Delatour B, Potier MC.. Classification and basic pathology of Alzheimer disease. Acta Neuropathol 2009;118:5–36 [DOI] [PubMed] [Google Scholar]
- 5. Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 6. Braak H, Braak E, Bohl J.. Staging of Alzheimer-related cortical destruction. Eur Neurol 1993;33:403–8 [DOI] [PubMed] [Google Scholar]
- 7. Nelson PT, Abner EL, Schmitt FA, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol 2009;68:774–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irwin DJ. Tauopathies as clinicopathological entities. Parkinsonism Relat Disord 2016;22(Suppl 1):S29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crary JF. Primary age-related tauopathy and the amyloid cascade hypothesis: The exception that proves the rule? J Neurol Neuromed 2016;1:53–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jellinger KA, Alafuzoff I, Attems J, et al. PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 2015;129:757–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braak H, Del Tredici K.. Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta Neuropathol 2014;128:767–72 [DOI] [PubMed] [Google Scholar]
- 12. Duyckaerts C, Braak H, Brion JP, et al. PART is part of Alzheimer disease. Acta Neuropathol 2015;129:749–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: A commentary. Neurobiol Aging 1997;18:S91–4 [DOI] [PubMed] [Google Scholar]
- 14. Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800 [DOI] [PubMed] [Google Scholar]
- 15. Santa-Maria I, Haggiagi A, Liu X, et al. The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol 2012;124:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walker J, Richardson T, Farrell K, et al. Quantification and distribution of neuropathologic changes in primary age-related tauopathy. J Neuropathol Exp Neurol 2018;77:484. [Google Scholar]
- 17. Jellinger KA. Different patterns of hippocampal tau pathology in Alzheimer's disease and PART. Acta Neuropathol 2018;136:811–3 [DOI] [PubMed] [Google Scholar]
- 18. von Gunten A, Kovari E, Rivara CB, et al. Stereologic analysis of hippocampal Alzheimer's disease pathology in the oldest-old: Evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol 2005;193:198–206 [DOI] [PubMed] [Google Scholar]
- 19. von Gunten A, Ebbing K, Imhof A, et al. Brain aging in the oldest-old. Curr Gerontol Geriatr Res 2010;2010:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haroutunian V, Hoffman LB, Beeri MS.. Is there a neuropathology difference between mild cognitive impairment and dementia? Dialog Clin Neurosci 2009;11:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walker JM, Richardson TE, Farrell K, et al. Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol 2021;80:102–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: A retrospective study. Lancet Neurol 2011;10:785–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milenkovic I, Petrov T, Kovacs GG.. Patterns of hippocampal tau pathology differentiate neurodegenerative dementias. Dement Geriatr Cogn Disord 2014;38:375–88 [DOI] [PubMed] [Google Scholar]
- 24. Jellinger KA, Bancher C.. Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol 1998;8:367–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ikeda K, Akiyama H, Arai T, et al. Clinical aspects of ‘senile dementia of the tangle type’—a subset of dementia in the senium separable from late-onset Alzheimer's disease. Dement Geriatr Cogn Disord 1999;10:6–11 [DOI] [PubMed] [Google Scholar]
- 26. Besser LM, Crary JF, Mock C, et al. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology 2017;89:1707–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilcock GK, Esiri MM.. Asymmetry of pathology in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1987;50:1384–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janota I, Mountjoy CQ.. Asymmetry of pathology in Alzheimer's disease. J Neurol Neurosurg Psychiatry 1988;51:1011–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moossy J, Zubenko GS, Martinez AJ, et al. Lateralization of brain morphologic and cholinergic abnormalities in Alzheimer's disease. Arch Neurol 1989;46:639–42 [DOI] [PubMed] [Google Scholar]
- 30. Braak H, Del Tredici K.. Neuroanatomy and Pathology of Sporadic Alzheimer’s Disease. 1st ed.Berlin: Springer Nature; 2015. [PubMed] [Google Scholar]
- 31. Adachi T, Saito Y, Hatsuta H, et al. Neuropathological asymmetry in argyrophilic grain disease. J Neuropathol Exp Neurol 2010;69:737–44 [DOI] [PubMed] [Google Scholar]
- 32. Stefanits H, Budka H, Kovacs GG.. Asymmetry of neurodegenerative disease-related pathologies: A cautionary note. Acta Neuropathol 2012;123:449–52 [DOI] [PubMed] [Google Scholar]
- 33. Rogalski E, Cobia D, Martersteck A, et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 2014;83:1184–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain 2014;137:1176–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim G, Vahedi S, Gefen T, et al. Asymmetric TDP pathology in primary progressive aphasia with right hemisphere language dominance. Neurology 2018;90:e396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duvernoy H, Cattin F, Risold P-Y.. Sectional anatomy and magnetic resonance imaging. In: Duvernoy H, Cattin F, Risold P-Y, ed. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. 4th ed.Berlin: Springer-Verlag; 2013:129. [Google Scholar]
- 37. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–86. [DOI] [PubMed] [Google Scholar]
- 38. Dickson DW, Feany MB, Yen SH, et al. Cytoskeletal pathology in non-Alzheimer degenerative dementia: New lesions in diffuse Lewy body disease, Pick's disease, and corticobasal degeneration. J Neural Transm Suppl 1996;47:31–46 [DOI] [PubMed] [Google Scholar]
- 39. Pang CC, Kiecker C, O'Brien JT, et al. Ammon's horn 2 (CA2) of the hippocampus: A long-known region with a new potential role in neurodegeneration. Neuroscientist 2019;25:167–80 [DOI] [PubMed] [Google Scholar]
- 40. Smith VD, Bachstetter AD, Ighodaro E, et al. Overlapping but distinct TDP-43 and tau pathologic patterns in aged hippocampi. Brain Pathol 2018;28:264–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol 2013;126:161–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hatanpaa KJ, Raisanen JM, Herndon E, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: Differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol 2014;73:136–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piskorowski RA, Chevaleyre V.. Memory circuits: CA2. Curr Opin Neurobiol 2018;52:54–9 [DOI] [PubMed] [Google Scholar]
- 44. Zeineh MM, Engel SA, Thompson PM, et al. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 2003;299:577–80 [DOI] [PubMed] [Google Scholar]
- 45. Langston RF, Stevenson CH, Wilson CL, et al. The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res 2010;215:275–91 [DOI] [PubMed] [Google Scholar]
- 46. Hitti FL, Siegelbaum SA.. The hippocampal CA2 region is essential for social memory. Nature 2014;508:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stevenson EL, Caldwell HK.. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci 2014;40:3294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adamowicz DH, Roy S, Salmon DP, et al. Hippocampal alpha-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J Neurosci 2017;37:1675–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Braak H, Braak E.. Argyrophilic grains: Characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett 1987;76:124–7 [DOI] [PubMed] [Google Scholar]
- 50. Braak H, Braak E.. Argyrophilic grain disease: Frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm (Vienna) 1998;105:801–19 [DOI] [PubMed] [Google Scholar]
- 51. Tolnay M, Clavaguera F.. Argyrophilic grain disease: A late-onset dementia with distinctive features among tauopathies. Neuropathology 2004;24:269–83 [DOI] [PubMed] [Google Scholar]
- 52. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998;51:1546–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.