Abstract
Despite the dramatic increase in antimicrobial resistance, there is a dearth of antibiotics in development and few pharmaceutical companies working in the field. Further, any new antibiotics are likely to have a short shelf life. Ab-based interventions offer alternatives that are not likely to be circumvented by the widely prevalent antibiotic resistance genes. Bovine colostrum (BC)—the first milk after parturition, rich in nutrients and immune components—promotes gut integrity and modulates the gut microbiome. We developed a hyperimmune BC (HBC) enriched in Abs to a highly conserved LOS core region of Gram-negative bacteria by immunizing pregnant cows with a vaccine comprised of detoxified LOS from Escherichia coli O111 Rc (J5) mutant non-covalently complexed to group B meningococcal outer membrane protein (J5dLOS/OMP). This vaccine generated robust levels of anti-J5 LOS Ab in the colostrum. When given orally to neutropenic rats challenged orally with Pseudomonas aeruginosa, administration of HBC improved survival compared to non-immune rats, while both BC preparations improved survival compared to PBS controls. Elevated circulating endotoxin levels correlated with mortality. HBC and to a lesser extent non-immune BC reduced bacterial burden from the liver, lung, and spleen. We conclude that HBC and to a lesser extent BC may be effective supplements that improve outcome from lethal gut-derived disseminated infection and may reduce transmission of Gram-negative bacilli from the gastrointestinal tract.
Keywords: Antibody, antimicrobial resistance, bovine colostrum, endotoxin, Pseudomonas aeruginosa
Introduction
The emergence and increasing incidence of antibiotic resistance genes among bacterial pathogens is recognized to be a major public health threat in food, farm animals, and human populations alike.1 Excessive use of antibiotics for medicinal purposes in human and veterinary medicine and in animal feed lots likely contributes selection pressures on pathogens, which promotes the acquisition, expression, and dissemination of antibiotic resistance genes.2–5 The recent spread of multidrug-resistant (MDR) bacteria with transferable resistance genes against “last resort” antibiotics such colistin, fluoroquinolones, and carbapenems has raised the specter of a future post-antibiotic era.5–7
The World Health Organization (WHO), United Nations, and Centers for Disease Control and Prevention each observed that if the current trend in antimicrobial resistance continues, 10 million people worldwide would die of infections, potentially affecting $100 trillion of economic output by 2050.8–10 Governments and public–private partnerships (e.g., CARB-X) have responded by providing incentives to develop new antibiotics, as large pharmaceutical companies have ceased antibiotic development programs and small companies focusing on antibiotics have gone out of business even following regulatory approval of their drugs.11,12 Clearly, additional approaches are urgently required.
Possible approaches to regain an upper hand against MDR bacterial pathogens include: (a) developing entirely new classes of chemotherapeutic agents to keep pace with the rapid evolution and dissemination of antibiotic resistance genes, while (b) developing non-antibiotic alternative strategies to complement or even replace antibiotic therapy for MDR pathogens. Finding novel antibiotics to defend against MDR pathogens will be challenging. Despite years of investigation in microbial genomics and advances in combinatorial chemistry, new discoveries have been successful in improving already existing classes of antibiotics. However, finding entirely new broad-spectrum microbial targets for the next generation of antibiotics has proven elusive thus far.13,14 With thousands of antimicrobial resistance genes widely prevalent, any new antibiotic is likely to have a relatively short shelf life.15
The development of vaccines directed against MDR Gram-negative pathogens may prevent the acquisition and transmission of these bacteria and not be subject to antimicrobial resistance mechanisms.10 Vaccines against healthcare-associated Gram-negative bacilli (GNB) such as Klebsiella, Escherichia coli, and Pseudomonas infections and the Gram-positive anaerobic organism Clostridioides difficile are currently under development.16–18 The active induction or passive administration of vaccine-induced Abs may prevent the colonization and block subsequent mucosal invasion by GNB. One innovative approach to reduce bacterial colonization and infection via the gut mucosa is to administer bovine colostrum (BC) enriched in Abs to bacterial pathogens orally. Using pathogen-specific vaccines, hyperimmune BC (HBC) has been produced for the prevention and/or treatment of infections with Shigella flexneri, enterotoxigenic E. coli (travelers’ diarrhea), cryptosporidium, and C. difficile, among many infections.19–23 BC also contains high concentrations of oligosaccharides, which limit attachment of bacteria in the gut, thereby lowering colonization rates of potential pathogens.24,25
Since each GNB species has multiple clinically relevant serotypes, however, such a strategy will require multivalent vaccines, and many are in development. In contrast, a vaccine directed against highly conserved epitopes in the LPS in the cell envelope of most Gram-negative bacteria may provide broad coverage. Braude et al. were first to develop a bacterial antiserum designed to prevent endotoxic shock in humans.26,27 The initial vaccine preparation was a mutant strain of E. coli O111, which did not express the complete LPS outer core structure owing to a mutation at the Rc position within the core glycolipid of LPS.28 This strain was designated as E. coli J5. The Abs raised in response to the heat-killed bacterial challenge were then collected from healthy human volunteers. The resulting human plasma was used to produce an immune serum (J5 antisera) for use in septic patients with GNB sepsis.29 The whole cell J5 bacterium vaccine has been formulated and used for the prevention of bovine mastitis for decades in veterinary medicine.30
Initial clinical trials with J5 antisera or similarly anti-LPS-enriched plasma preparations given as passive immunotherapy for septic patients caused by Gram-negative pathogens significantly improved outcomes.29,31,32 Subsequent clinical trials failed to show that passive administration of anti-core glycolipid Abs improved survival, but these studies did not ensure adequate levels of circulating Abs.33 In contrast, active immunization with a core glycolipid vaccine is likely to elicit longer-lasting Abs. The vaccine formulation now under investigation is a unique therapeutic consisting of the LPS from the original E. coli J5 bacterium detoxified by alkali treatment and non-covalently linked with the outer membrane protein of group B Neisseria meningitidis to optimize conformational epitopes and provide adjuvant immunogenicity.34,35 This vaccine is known as E. coli J5 detoxified LPS/Neisseria meningitidis group B outer membrane protein and will subsequently be referred to as J5dLOS/OMP. In two separate Phase I studies, it has been shown to be safe, well tolerated, and immunogenic.36,37
We have previously demonstrated that immunizing pregnant dairy cows with this novel core glycolipid anti-LPS vaccine could generate a robust Ab response in the plasma and colostrum following parturition, but we did not examine its functional activity.38 The current studies were undertaken to determine if orally administered anti-endotoxin HBC can be protective from intestinal colonization and infection with the common, opportunistic, Gram-negative bacterial pathogen Pseudomonas aeruginosa. In the presence of chemotherapy-induced severe neutropenia, rats become acutely susceptible to Pseudomonas bacteremia and mortality during or shortly after the duration of severe neutropenia.39
Methods
Reagents
All chemicals and reagents used in these experiments were purchased from Sigma–Aldrich (St. Louis, MO) unless otherwise specifically stated.
Animal care and the neutropenic rat model of infection
Female albino specific-pathogen-free Sprague Dawley rats (125–150 g) were used in these experiments and were purchased from Charles River Laboratories (Wilmington, MA). The animals were housed in an IACUC-approved university-affiliated facility under BSL-2 conditions. The experimental protocol was approved by the Warren Alpert Medical School of Brown University Institutional Animal Care Committee before any experiments were undertaken. Rats were allowed to adjust to the laboratory conditions for at least 7 d before beginning any experiments. Animals were housed in environmentally isolated cages and maintained at a constant ambient temperature and humidity on a 12 h day/night cycle. Animals were provided with an ad libitum supply of commercial rodent chow and distilled water. The details of the neutropenic rat model are described further in our earlier work.39
Three groups of rats were rendered neutropenic with cyclophosphamide given intraperitoneally at 100 mg/kg at time 0 and a second dose at 50 mg/kg 72 h later. A single dose of moxifloxacin 10 mg/kg was given intramuscularly to overcome colonization resistance before oral challenge with P. aeruginosa 12:4:4 (Fisher-Devlin Immuno-type 6). The bacterial challenge was given at a dose of 107 CFU in 2 ml by gastric lavage on d 0, 2, and 4. The investigators were blinded to the treatment assignment until the end of the experiments. One group of rats received the Ab-enriched BC (n = 13). A second group received non-immune colostrum (n = 13), while a third group served as a control group and was given PBS (n = 4). Orogastric feeding of the colostrum or PBS at a volume of 2 ml/d began on d 3 and continued for the next 6 d (last dose on d 8). The study objective was to determine if the HBC was effective in limiting endotoxin release into the circulation and in improving survival in these septic animals. The endpoints were the level and frequency of P. aeruginosa bacteremia, systemic LPS levels, clinical signs of sepsis (fever, lethargy, mass loss), and survival. LPS was measured in heat-treated plasma using the quantitative limulus amebocyte lysate (LAL) assays using standard methods. Quantitative bacteriology of liver, lung, and spleen homogenates were performed by plating serial 1:10 dilutions on Pseudomonas-specific agar.
Bovine immunization
Four Holstein cows selected based on their expectation to give birth within 3 mo were immunized as previously described.38 Cows were immunized subcutaneously on d 0, 16, 41, and 58 with J5dLOS/OMP vaccine given with the adjuvant Emulsigen–DR (MVP Adjuvants). None of the cows had previously received any coliform-containing vaccine. The cows developed high titers of systemic Ab against this conserved core region of LPS, which was then secreted into the colostrum that was obtained within the first d of delivery. The BC from one cow that contained a high titer of anti-J5dLOS polyclonal Abs (70,320 ELISA units/ml) directed against core glycolipid structure of LPS following immunization with the J5dLOS/OMP vaccine was selected for further study.38
Recognition of P. aeruginosa by J5 antisera
J5 antisera
A New Zealand white rabbit was immunized with the same lot of J5dLOS/OMP vaccine used to immunize the cows.38 The initial inoculation was with 20 μg of the J5dLOS/OMP vaccine with 0.125 ml Hiltonol (polyI/polyC) adjuvant (OncovirR), a TLR3 agonist, with boosts at 14 and 28 d. The rabbit was bled at d 42.
Bacterial preparations
The LPS was prepared from the following PA strains: PAO1, a laboratory-adapted strain having a O5 serotype, PA14 and PA BE-2, both clinical isolates from the collection of one of us (R.E.), and PA IATS serotypes O1, O6, and O11, originally obtained from Dr. Joseph Lam (Guelph, Ontario, Canada). They were grown in lysogenic broth (LB) supplemented with 1 mM MgCl2 at 37°C and rotated at 250 rpm. Briefly, following hot/phenol water extraction, contaminating nucleic acids and proteins were removed by digestion with RNase A, DNase I, and proteinase K followed by dialysis. LPS samples were then subjected to extractions to remove residual phospholipids and lipoproteins.40–42 Lysates were prepared from these latter three strains as well as from Staphylococcus aureus USA300 (negative control) and E. coli O111, J5 mutant from overnight cultures. Bacterial pellets were re-suspended in LPS lysis buffer (0.1 M Tris HCl, 2% SDS, 10% glycerol, and 4% 2-mercaptoethanol, pH 8), boiled at 100°C, and treated with proteinase K, as previously described.43
Western blots
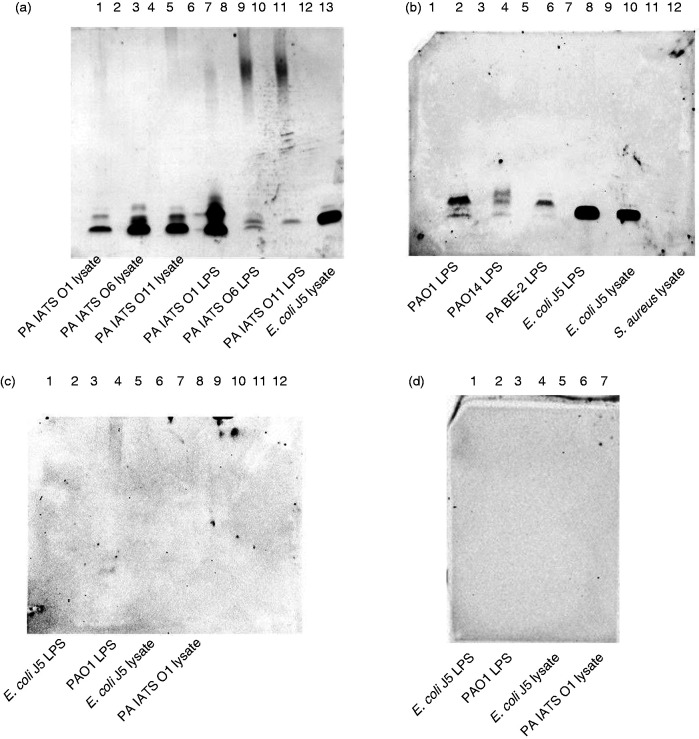
Bacterial lysates and LPS from multiple PA strains and E. coli O111 J5 mutant were run on Novex precast Tricine polyacrylamide gels (10–20%), as previously described, using a discontinuous buffer system with a cathode buffer (0.1 M Tris, 0.1 M Tricine, 0.1% SDS, pH 8.25) in the upper chamber and anode buffer (0.2 M Tris, pH 8.9) in the lower chamber.44 The gel was run at approximately 100–150 V until the loading dye reached the bottom of the gel. The gel was transferred onto a mini PVDF membrane (Bio-Rad, Hercules, CA; cat. # 170-4275) in transfer buffer and run at 1.3 A, 25 V, for 13 min.44 A S. aureus lysate was used as an irrelevant Ag control. The J5 rabbit antisera was added at a dilution of 1:5000, followed by goat anti-rabbit IgG-HRP at 1:20,000 (Figure 4a and b). Pre-immune sera from the same rabbit were run against J5 and PAO1 LPS and lysates (Figure 4c) and goat anti-rabbit IgG-HRP at 1:20,000 in the absence of primary Ab (Figure 4d).
Figure 4.
Western blot binding of anti-J5dLOS/OMP rabbit sera to P. aeruginosa LPS and bacterial lysates. (a) J5-immune sera was added onto P. aeruginosa IATS O1 lysate (lane 1); P. aeruginosa IATS O6 lysate (lane 3); P. aeruginosa IATS O11 (lane 5); P. aeruginosa IATS O1 LPS (lane 7); P. aeruginosa IATS O6 LPS (lane 9); P. aeruginosa IATS O11 LPS (lane 11); E. coli J5 lysate (lane 13). (b) J5-immune sera was added onto P. aeruginosa PAO1 (IATS O5) LPS (lane 2); P. aeruginosa O14 LPS (lane 4); P. aeruginosa BE-2 LPS (lane 6); E. coli J5 LPS (lane 8); E. coli J5 lysate (lane 10); and S. aureus USA 300 lysate (lane 12). (c) Pre-immune sera was blotted onto E. coli J5 LPS (lane 2); P. aeruginosa PAO1 (IATS O5) LPS (lane 4); E. coli J5 lysate (lane 6); and P. aeruginosa IATS O1 lysate (lane 8). (d) Anti-rabbit IgG-HRP (i.e., secondary Ab only) was added onto E. coli J5 LPS (lane 1); P. aeruginosa PAO1 (IATS O5) LPS (lane 3); E. coli J5 lysate (lane5); and P. aeruginosa IATS O1 lysate (lane 7).
Statistical analysis
Survival outcomes were compared by Kaplan–Meier survival plots using the log rank test. Differences between two groups were analyzed by a Mann–Whitney U-test for quantitative microbiology of microbiology measures in tissue samples and endotoxin determinations. Numeric data are presented as median values with 25–75th percentile values, and P values with a two-sided value of < 0.05 were considered significant.
Results
Effects of immune and non-immune BC on survival
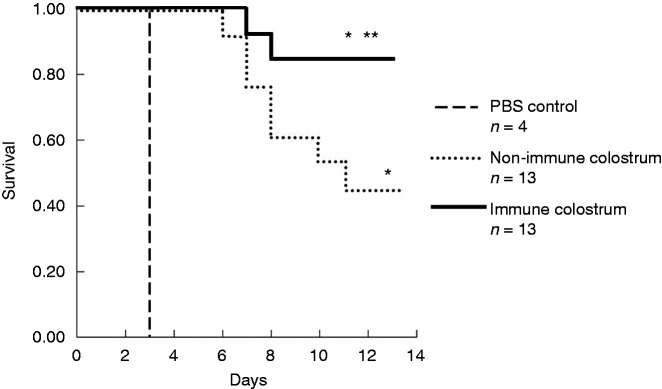
Rats randomized to receive BC and non-immune BC had greater survival (17/30) compared to those that received PBS (0/4; P < 0.001). Rats that were treated with the J5 HBC had a greater survival after P. aeruginosa challenge than did the rats randomized to the non-immune BC (11/13 vs. 6/13; P < 0.0459). The Kaplan–Meier survival plots are depicted in Figure 1.
Figure 1.
Kaplan–Meier survival plot comparing outcomes of the three groups of animals used in these experiments. Animals receiving either hyperimmune bovine colostrum (HBC) or non-immune colostrum had improved survival compared to those receiving PBS (17/30 vs. 0/4; *P < 0.001). Survival was significantly greater in the HBC-treated group compared to the non-immune colostrum groups (11/13 vs. 6/13; **P < 0.0459).
Effects on bacterial burdens from the P. aeruginosa challenge strain within organs
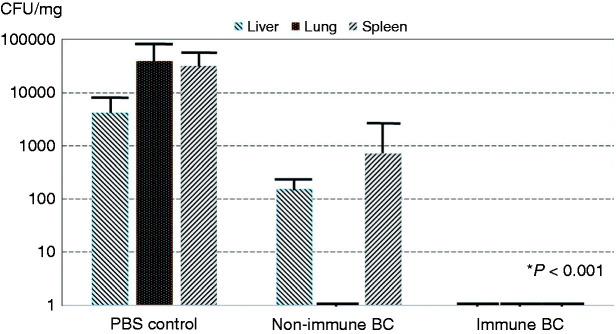
All lethally infected and surviving animals underwent necropsy at the end of the 10 d experiment and quantitative assessment of colony counts (CFU/mg tissue) in liver, lung, and splenic tissues. The microbial burden in the liver, lung, and spleen (expressed in median CFU Pseudomonas/mg tissue) was several orders of magnitude higher in the PBS control group (4200 CFU/mg in liver; 39,677 CFU/mg lung; 32,000 CFU/mg spleen) versus the non-immune BC-treated group (159 CFU/mg liver; 0 CFU/mg lung; 719 CFU/mg spleen) and HBC-treated group (median level of 0 CFU/mg in each of the organs; P < 0.001; Figure 2). No difference in bacterial burden was observed between the immune and non-immune colostrum-treated groups.
Figure 2.
Quantitative microbial colony counts from the liver, lung, and spleen at necropsy. The results are displayed as CFU/mg tissue sample in the three treatment groups with the challenge strain of P. aeruginosa. The organ bacterial burden in the three tissues in animals treated with immune bovine colostrum (BC) is less than that in PBS control animals (P < 0.001). Although the animals treated with the immune BC had bacteria in twofold fewer spleens than those treated with non-immune BC, this did not achieve statistical significance.
Plasma endotoxin levels
Circulating endotoxin levels in the plasma of each rat were measured at d 6, 2 d after the last orogastric dose of P. aeruginosa, when animals first appeared ill. We observed elevated LAL levels in animals in the HBC and BC groups who eventually succumbed versus those that survived (median of non-survivors = 2.68 ng/ml vs. 0.17 ng/ml for survivors; P = 0.0051 two-tailed Mann–Whitney U-test). All animals (two in the HBC and two in the BC groups) with LAL values of 2.5 ng/ml died, while two animals in the BC group died despite LAL levels < 0.5 ng/ml (Figure 3).
Figure 3.
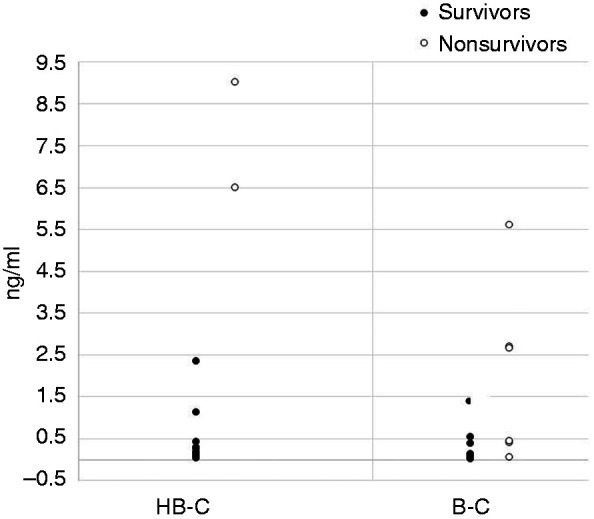
LAL levels in the blood of animals obtained at d 6. The LAL levels (ng/ml) are shown for animals treated with HBC and BC. Non-survivors in each group ae represented as open symbols. All animals in the control (PBS) treatment group succumbed by d 3.
Recognition of P. aeruginosa by J5 antisera
Western blots
The J5 antisera bound to low molecular mass moieties in the lysates and their respective LPS preparations of three different P. aeruginosa IATS O serotype strains (PA IATS O1, PA IATS O6, and PA IATS O11) at similar mobilities as the lysate from E. coli J5 (Figure 4a). The J5 sera also bound to the LPS derived from the laboratory strain PAO1 (O5 serotype) as well as to the two clinical isolates, PA14 and PA BE-2, also at a gel mobility similar to that of the E. coli J5 LPS (Figure 4b). The J5 antisera did not bind to S. aureus USA300 lysate (negative control; Figure 4b, lane 12). The pre-immune rabbit antisera did not bind to either the J5 LOS or lysate (Figure 4c), and there was no binding in the absence of primary (anti-J5) Ab (Figure 4d). Of potential interest, while the J5 antisera appeared to recognize a shared band in each of the preparations, there also seemed to be heterogenous binding as well.
Discussion
In the present study, we report that administration of both non-immune BC and J5 HBC improved survival in neutropenic rats lethally challenged with a heterologous pathogen, P. aeruginosa. However, animals that received the J5 HBC had a better survival than those that were administered the non-immune BC. Since we did not include a hyperimmune colostrum raised against an irrelevant Ag, however, we cannot dismiss the possibility that the protection observed with the J5 HBC might have been attributable to the effect of nonspecific Ag stimulation. The survival results were correlated with the bacterial organ burden. At their moribund state (72 h), control rats had >104 CFU/g tissue in the lung and spleen, while those treated with non-immune BC had ∼30-fold fewer CFU/g tissue. Rats that received the HBC had no detectable bacteria when they were euthanized at 14 d. Thus, the J5 HBC promoted the clearance of P. aeruginosa. Elevated endotoxin levels at d 6 correlated with overall survival. The median LAL level in the HBC group was lower than that of the BC group (0.26 vs. 0.41 ng/ml), but this was not significant.
BC, the first milk after parturition in lactating mammals, provides essential nutrients and nonspecific immune factors, including immunoglobulins, which provide passive immunity until newborn immunity is established.25,45 In contrast to humans, maternal immunoglobulin in cattle does not cross the placenta, and therefore calves are entirely dependent on colostrum for Abs. Bovine IgG1, the main milk Ab, survives transit through the gut, remains active in the intestinal tract, and may replace secretory IgA. Immunoglobulin concentrations are nearly 100-fold higher in colostrum than mature milk. BC also strengthens host defenses with antimicrobial peptides, lactoferrin, and cytokines, including IL-17.46 It also contains high amounts of TGF-β, which has anti-inflammatory properties, regulates tissue repair, and is essential in the induction of regulatory T cells. BC has recently been found to contain microRNAs that also have immune regulation potential. With high amounts of growth factors, BC promotes the maturation and integrity of the gastrointestinal (GI) tract and helps establish the microbial composition of the GI tract by promoting the growth of beneficial microflora and protecting against the adherence of pathogens. Thus, BC provides immune and nutritional support, promotes gut integrity, and enhances gut microbiome development These components of BC diminish on succeeding days after delivery and are not present in mature milk.
Animals that received the hyperimmune BC showed decreased organ bacterial burden, suggesting that the J5 IgG promoted the uptake and clearance of the P. aeruginosa. Published studies have reported that the core structures of P. aeruginosa and Enterobacteriaceae are chemically different. Yet, we were unable to find studies that examined whether they were immunologically different.47 In the current report, we find that J5 antisera does bind to a low molecular mass moiety in multiple serotypes and clinical isolates of P. aeruginosa that migrate similarly to J5 LPS (Figure 4). The basis for this cross-reactivity needs to be more clearly defined using defined core mutants of P. aeruginosa.
Our initial studies with a J5-affinity-purified IgG that lacked any anti-Pseudomonas LPS IgG showed highly significant protection against lethal infection caused by this same challenge strain of PA.35 The ability of passively administered J5-specific IgG Ab to protect against PA infection strongly suggests that the protection was both Ab mediated and J5 Ag specific. Since then, we have reported that in preclinical studies, J5 Ab protects against a wide range of GNB, including Pseudomonas, Klebsiella, and E. coli, and in multiple animal models of infection when administered passively or elicited actively.34,35,39,48–51 Further, in a large blinded randomly controlled clinical study, Ziegler et al. demonstrated a significant protective effect of J5 immune sera, where PA was the second leading cause of GNB bacteremia (44 total infections).29 In summary, there are considerable data suggesting that J5 Ab can recognize both P. aeruginosa and Enterobacteriaceae core oligosaccharides, despite their structural differences.
BC has achieved GRAS (“generally regarded as safe”) status. There is a growing literature on the use of HBC generated by the immunization of pregnant dairy cows with various vaccines before delivery for the treatment of infections in humans.19–23 Ben Ya’acov et al. immunized cows with a heat-killed vaccine comprised of a mixture of enteropathogenic E. coli (Imm124ER) and showed that multiple oral daily doses of the HBC that contained IgG and IgA Abs specific for multiple ETEC Ags ameliorated the loss of weight in a murine model of chemically induced (trinitrobenzene sulfonate) colitis.52,53 In this study, however, the titer of LPS-specific Abs within the colostrum was not determined, and functional activity beyond reducing weight loss in the colitis model was not assessed. Consequently, it is not clear if the protective capacity of Imm124ER colostrum is limited to ETEC strains or if it could be more broadly cross-react with invasive strains of E. coli or heterologous GNB.52
In contrast to these studies, we generated Abs to a broad spectrum of GNB and showed that while the non-immune BC provided protection compared to controls, the J5 HBC conferred greater protection against infection with a heterologous pathogen and was accompanied by a reduction in bacterial organ burden. HBC with Abs directed at a highly conserved epitope(s) in the LPS of GNB could be an important component of treatment of those conditions characterized by a “leaky” gut and/or translocation of endotoxin into the circulation, which may lead to systemic inflammation. For example, endotoxemia during coronary artery bypass surgery is associated with increased morbidity, and this is less likely if the patient has preexisting Abs against endotoxin.54–56 In our study, we demonstrate that treatment with J5 HBC reduces endotoxemia and mortality. The fact that the J5 HBC further improved survival and reduced organ bacterial load compared to non-immune BC also suggests that the anti-LPS Abs contributed by facilitating bacterial clearance. Since the non-immune BC also reduced mortality, it is likely that the BC promoted reduced gut permeability, but we did not assess that mechanism directly. A broadly cross-reactive HBC also might also be useful to prevent the transmission of GNB pathogens that colonize the GI tract of patients at risk of infection, including MDR GNB, such as those in a nursing home or in long-term care facilities. Given the prevalence of MDR bacteria, BC enriched in Abs to bacteria associated with healthcare-associated infections merits further investigation.
Acknowledgments
We thank Dr. Yuanyuan Liang of the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine for her statistical analyses.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publications of this article: Drs Cross and Opal hold an issued patent on this J5dLOS/OMP vaccine.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The original work to immunize the cows was funded by the Maryland Proof-of-Concepts Alliance, University of Maryland, College Park Agreement Z855809, CFDA 12.431. Funding was also provided by NIH/NIAID grant AI135561.
ORCID iD: Alan S Cross https://orcid.org/0000-0003-4362-9936
References
- 1.CDC. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC, 2019. [Google Scholar]
- 2.Kuipers A, Koops WJ, Wemmenhove H. Antibiotic use in dairy herds in the Netherlands from 2005 to 2012. J Dairy Sci 2016; 99: 1632–1649. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy D. Time to deal with antibiotics. Science 2013; 342: 777. [DOI] [PubMed] [Google Scholar]
- 4.D’Costa VM, King CE, Kalan L, et al. Antibiotic resistance is ancient. Nature 2011; 477: 457–461. [DOI] [PubMed] [Google Scholar]
- 5.Yin W, Li H, Shen Y, et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 2017; 8: e00543–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhullar K, Waglechner N, Pawlowski A, et al. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One 2012; 7: e34953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opal SM. Non-antibiotic treatments for bacterial diseases in an era of progressive antibiotic resistance. Crit Care 2016; 20: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization, 2014. [Google Scholar]
- 9. United Nations meeting on antimicrobial resistance. Bull World Health Organ 2016; 94: 638–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on Antimicrobial Resistance, December 2014.
- 11.Brown ED. Is the GAIN Act a turning point in new antibiotic discovery? Can J Microbiol 2013; 59: 153–156. [DOI] [PubMed] [Google Scholar]
- 12.Langreth R. Deadly superbugs win as Wall Street flees makers of antibiotics. Bloomberg News, 17 June 2019.
- 13.Opal SM, Pop-Vicas A. Molecular mechanisms of antibiotic resistance in bacteria. In: Bennett JE, Dolin R, Blaser M. (eds) Principles and practice of infectious diseases. 8th ed. Philadelphia, PA: Elsevier, 2014, pp.235–251. [Google Scholar]
- 14.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med 2013; 368:299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nesme J, Cécillon S, Delmont TO, et al. Large-scale metagenomic-based study of antibiotic resistance in the environment. Curr Biol 2014; 24: 1096–1100. [DOI] [PubMed] [Google Scholar]
- 16.Hegerle N, Choi M, Sinclair J, et al. Development of a broad spectrum glycoconjugate vaccine to prevent wound and disseminated infections with Klebsiella pneumoniae and Pseudomonas aeruginosa. PLoS One 2018; 13: e0203143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frenck RW, Jr, Ervin J, Chu L, et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a Phase 2 randomised controlled trial. Lancet 2019; 19: 631–640. [DOI] [PubMed] [Google Scholar]
- 18.Feldman MF, Mayer Bridwell AE, Scott NE, et al. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae. Proc Natl Acad Sci U S A 2019; 116: 18655–18663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sponseller JK, Steele JA, Schmidt DJ, et al. Hyperimmune bovine colostrum as a novel therapy to combat Clostridium difficile infection. J Infect Dis 2015; 211: 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savarino SJ, McKenzie R, Tribble DR, et al. Hyperimmune bovine colostral anti-CS17 antibodies protect against enterotoxigenic Escherichia coli diarrhea in a randomized, doubled blind, placebo-controlled human infection model. J. Infect Dis 2019; 220: 505–513. [DOI] [PubMed] [Google Scholar]
- 21.Otto W, Najnigier B, Stelmasiak T, et al. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand J Gastroenterol 2011; 46: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tacket CO, Binion SB, Bostwick E, et al. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg 1992; 47: 276–283. [DOI] [PubMed] [Google Scholar]
- 23.Okhuysen PC, Chappell CL, Crabb J, et al. Prophylactic effect of bovine anti-cryptosporidium hyperimmune colostrum immunoglobulin in healthy volunteers challenged with Cryptosporidium parvum. Clin Infect Dis 1998; 26: 1324–1329. [DOI] [PubMed] [Google Scholar]
- 24.Fuhrer A, Sprenger N, Kurakevich E, et al. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med 2010; 207: 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struff WG, Sprotte G. Bovine colostrum as a biologic in clinical medicine: a review – part II: clinical studies. Int J Clin Pharmacol Ther 2008; 46: 211–225. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler EJ, Douglas H, Braude AI. Human antiserum for prevention of the local Shwartzman reaction and death from bacterial lipopolysaccharides. J Clin Invest 1973; 52: 3236–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler EJ, Douglas H, Sherman JE, et al. Treatment of E. coli and Klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol 1973; 111: 433–438. [PubMed] [Google Scholar]
- 28.Elbein AD, Heath EC. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. I. The biochemical properties of a uridine diphosphate galactose 4-epimeraseless mutant. J Biol Chem 1965; 240: 1919–1925. [PubMed] [Google Scholar]
- 29.Ziegler EJ, McCutchan JA, Fierer J, et al. Treatment of Gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med 1982; 307: 1225–1230. [DOI] [PubMed] [Google Scholar]
- 30.Hogan JS, Weiss WP, Smith KL, et al. Effects of an Escherichia coli J5 vaccine on mild clinical coliform mastitis. J Dairy Sci 1995; 78: 285–290. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner J-D, Glauser MP, McCutchan JA, et al. Prevention of Gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet 1985; 2: 59–63. [DOI] [PubMed] [Google Scholar]
- 32.Schedel I, Dreikhausen U, Nentwig B, et al. Treatment of Gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med 1991; 19: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 33.Cross AS, Opal SM, Bhattacharjee AK, et al. Immunotherapy of sepsis: flawed concept or faulty implementation? Vaccine 1999; 17: S13–S21. [DOI] [PubMed] [Google Scholar]
- 34.Bhattacharjee A, Opal SM, Taylor R, et al. A non-covalent complex vaccine prepared with detoxified Escherichia coli J5 (Rc chemotype) lipopolysaccharide and Neisseria meningitidis group B outer membrane protein produces protective antibodies against Gram-negative bacteremia. J Infect Dis 1996; 173: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharjee AK, Opal SM, Palardy JE, et al. Affinity-purified Escherichia coli J5 lipopolysaccharide-specific IgG protects neutropenic rats against Gram-negative bacterial sepsis. J Infect Dis 1994; 170: 622–629. [DOI] [PubMed] [Google Scholar]
- 36.Cross AS, Opal SM, Palardy JE, et al. Phase I study of detoxified Escherichia coli J5 lipopolysaccharide (J5dLOS)/group B meningococcal outer membrane protein (OMP) complex vaccine in human subjects. Vaccine 2003; 21: 4576–4588. [DOI] [PubMed] [Google Scholar]
- 37.Cross AS, Greenberg N, Billington M, et al. Phase 1 testing of detoxified LPS/group B meningococcal outer membrane protein vaccine with and without synthetic CPG 7909 adjuvant for the prevention and treatment of sepsis. Vaccine 2015; 33: 6719–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross A, Karreman HJ, Zhang L, et al. Immunization of cows with novel core glycolipid vaccine induces anti-endotoxin antibodies in bovine colostrum. Vaccine 2014; 32: 6107–6114. [DOI] [PubMed] [Google Scholar]
- 39.Collins HH, Cross AS, Dobek A, et al. Oral ciprofloxacin and a monoclonal antibody to lipopolysaccharide protect leukopenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis 1989; 159: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 40.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. In: Whistler RL. (ed) Methods in carbohydrate chemistry general polysaccharides. Vol 5. New York: Academic Press, 1965, pp.83–91. [Google Scholar]
- 41.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957; 226: 497–509. [PubMed] [Google Scholar]
- 42.Hirschfeld M, Ma Y, Weis JH, et al. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol 2000; 165: 618–622. [DOI] [PubMed] [Google Scholar]
- 43.Davis MR, Jr, Goldberg JB. Purification and visualization of lipopolysaccharide from Gram-negative bacteria by hot aqueous-phenol extraction. J Vis Exp 2012; 63: e3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin SE, Melander RJ, Brackett CM, et al. Small molecule potentiation of Gram-positive selective antibiotics against Acinetobacter baumannii. ACS Infect Dis 2019; 5: 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr 2010; 156: S3–S7. [DOI] [PubMed] [Google Scholar]
- 46.Duranti S, Mancabelli L, Mancino W, et al. Exploring the effects of COLOSTRONONI on the mammalian gut microbiota composition. PLoS One 2019; 14: e0217609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivera M, McGroarty EJ. Analysis of a common-antigen lipopolysaccharide from Pseudomonas aeruginosa. J Bacteriol 1989; 171: 2244–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Opal SM, Palardy JE, Chen WH, et al. Active immunization with a detoxified endotoxin vaccine protects against lethal polymicrobial sepsis: its use with CpG adjuvant and potential mechanisms. J Infect Dis 2005; 192: 2074–2080. [DOI] [PubMed] [Google Scholar]
- 49.Cross AS, Opal SM, Warren HS, et al. Active immunization with a detoxified Escherichia coli J5 lipopolysaccharide group B meningococcal outer membrane protein complex vaccine protects animals from experimental sepsis. J Infect Dis 2001; 183: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 50.Neely AN, Bhattacharjee AK, Babcock GF, et al. Differential effects of two different routes of immunization on protection against Gram negative sepsis by a detoxified Escherichia coli J5 lipopolysaccharide group B meningococcal outer membrane protein complex vaccine a burned mouse model. J Burn Care Rehabil 2002; 23: 333–340. [DOI] [PubMed] [Google Scholar]
- 51.Chen WH, Kang TJ, Bhattacharjee AK, et al. Intranasal administration of a detoxified endotoxin vaccine protects mice against heterologous Gram-negative bacterial pneumonia. Innate Immun 2008; 14: 269–278. [DOI] [PubMed] [Google Scholar]
- 52.Ben Ya’acov A, Lichtenstein Y, Zolotarov L, et al. The gut microbiome as a target for regulatory T cell-based immunotherapy: induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol 2015; 15: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adar T, Ben Ya’acov A, Lalazar G, et al. Oral administration of immunoglobulin G-enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cells. Clin Exp Immunol 2012; 167: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein DJ, Briet F, Nisenbaum R, et al. Endotoxemia related to cardiopulmonary bypass is associated with increased risk of infection after cardiac surgery: a prospective observational study. Crit Care 2011; 15: R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bölke E, Orth K, Jehle PM, et al. Enteral application of an immunoglobulin-enriched colostrum milk preparation for reducing endotoxin translocation and acute phase response in patients undergoing coronary bypass surgery – a randomized placebo-controlled pilot trial. Wien Klin Wochenschr 2002; 114: 923–928. [PubMed] [Google Scholar]
- 56.Bennett-Guerrero E, Ayuso L, Hamilton-Davies C, et al. Relationship of preoperative antiendotoxin core antibodies and adverse outcomes following cardiac surgery. JAMA 1997; 277: 646–650. [PubMed] [Google Scholar]