Abstract
NK cells are the major lymphocyte subset of the innate immune system that mediates antiviral and anti-tumor responses. It is well established that they develop mechanisms to distinguish self from non-self during the process of NK cell education. Unlike T and B cells, natural killer cells lack clonotypic receptors and are activated after recognizing their target via germline-encoded receptors through natural cytotoxicity, cytokine stimulation, and Ab-dependent cellular cytotoxicity. Subsequently, they utilize cytotoxic granules, death receptor ligands, and cytokines to perform their effector functions. In this review, we provide a general overview of human NK cells, as opposed to murine NK cells, discussing their ontogeny, maturation, receptor diversity, types of responses, and effector functions. Furthermore, we also describe recent advances in human NK cell biology, including tissue-resident NK cell populations, NK cell memory, and novel approaches used to target NK cells in cancer immunotherapy.
Keywords: Natural killer, development, education, receptors, activation, functions
Human NK cells – from development to effector functions
NK cells are innate lymphocytes that belong to the rapidly expanding family of innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans.1–3 However, in contrast with these conventional NK (cNK) cells in terms of their phenotype, function, and ontogeny, tissue-resident NK (trNK) cells have been observed in organs such as the lungs, liver, kidneys, lymph nodes, thymus, and the uterus.4–6 Human NK cells can be primarily classified into two subtypes that arise during development: CD3−CD56bright CD16− cells and CD3−CD56dim CD16+ cells, with the latter being functionally more mature than the former. CD56dim cells are highly cytotoxic, whereas CD56bright cells are more specialized to secrete cytokines.7 NK cells were discovered in the mid-1970s by Kiessling and Herberman through independent studies.8,9 On observing that these cells were able to spontaneously kill syngeneic and allogeneic tumor cells without the requirement of prior sensitization or affinity maturation, they were named “natural killers”.2,10,11 Since then, they have gained enormous interest from researchers due to their successful utilization in immunotherapy. This review aims to summarize the established and emerging knowledge to broaden our current understanding of human NK cells.
Development and functional maturation of human NK cells
Stages in human NK cell development
Human NK cells were originally thought to develop solely in the bone marrow (BM). Nonetheless, recent findings suggest that they also develop in extramedullary sites such as secondary lymphoid tissues, liver, and the uterus, etc.12 Within human BM they originate from Lin- CD34+ CD133+ CD244+ multipotent hematopoietic stem cells (HSCs) that commit to the lymphoid lineage to become CD45RA+ CD133+ lymphoid primed multipotent progenitors (LMPPs).13 LMPPs differentiate into common lymphoid progenitors (CLP) marked by CD38+ CD7+ CD10+ CD127+ that have the potential to become Pro-B, Pre-T, or ILCs, apart from CD7+, CD127+ (IL-7Rα+), CD122+ (IL-2Rβ+), CD117+ (c-Kit+), and IL-1R1low Stage 1 NK progenitors (NKPs).14,15 Thus, the irreversible fate of the decision of CLPs into the NK cell lineage is defined by the up-regulation of CD122.16,17 NKPs transition into Stage 2 Pre-NK cells marked by the expression of CD7+ CD127+ and down-regulation of CD3ε. Observations from recent studies have further split Stage 2 Pre-NK cells into Stage 2a and 2b, based on whether they lack or express IL-1R1, respectively.18 The acquisition of activating receptors such as NKG2D(CD314), NKp46(CD335), NKp30(CD337), and CD161 marks the transition from Stage 2b Pre-NK cells into Stage 3 immature NK cells (iNK) cells.13 iNK cells develop into Stage 4 CD56bright NK cells which are divided into substages 4a and 4b, with the latter distinct from the former by the expression of NKp80.19 CD56 bright NK cells eventually develop into Stage 5 CD56dim mature NK (mNK) cells by the gradual up-regulation of CD94/NKG2C and CD16(FcγRIII), and by the down-regulation of CD56, c-Kit(CD117), and CD94/NKG2A.20 Ultimately, the terminally mature Stage 6 NK cells will be marked by the expression of CD57+ and killer cell immunoglobulin-like receptors (KIR+/CD158+) (Appendix Table 1).21–24
Factors influencing human NK Cell development
The expression of cytokine receptors, such as c-Kit (CD117), CD127, and CD122, defining various developmental stages, reflects the significance of cytokines in fine-tuning human NK cell development.25,26 In controlling human NK cell development, critical components from these signaling pathways can act as either upstream regulators or downstream targets of transcription factors (TFs) belonging to transcriptional regulatory networks.27,28 The early commitment of human HSCs into LMPPs and CLPs is regulated by IL-3, IL-7, and membrane-bound SCF/KL and Flt3L/Flk2 that interact with c-Kit and Flt3, respectively.13,25 The primary TF involved in this is Notch1.13 However, besides acting synergistically to promote CD34+ cell proliferation, Flt3L and SCF have been reported to enhance IL-15-mediated development and maturation of human NKPs by inducing the expression of CD122 and/or IL-15Rα (CD215).29–31 Despite all this, research evidence suggests that both IL-15 and c-Kit might not be essential for human NK cell lineage commitment.31,32 Subsequently, the commitment to human NKPs is controlled by RUNX3 and ID2. Indeed, RUNX3 has been shown to enhance the expression of NK cell receptors, such as KIR and NKp46. E4BP4 and EST1 promote the transition to iNK cells. Next, the gradual transitioning to human CD56bright cells is regulated by the transcription factors GATA2 and EOMES.13 In parallel, it is worth mentioning that IL-7 is considered indispensable for the survival of human CD56bright NK cells.33 Finally, T-bet promotes them to become Stage 5 CD56dim cells. Interestingly, Notch1 has also been shown to increase KIR expression in enhancing human NK cell cytotoxicity.13
Educating human NK cells to distinguish self from non-self
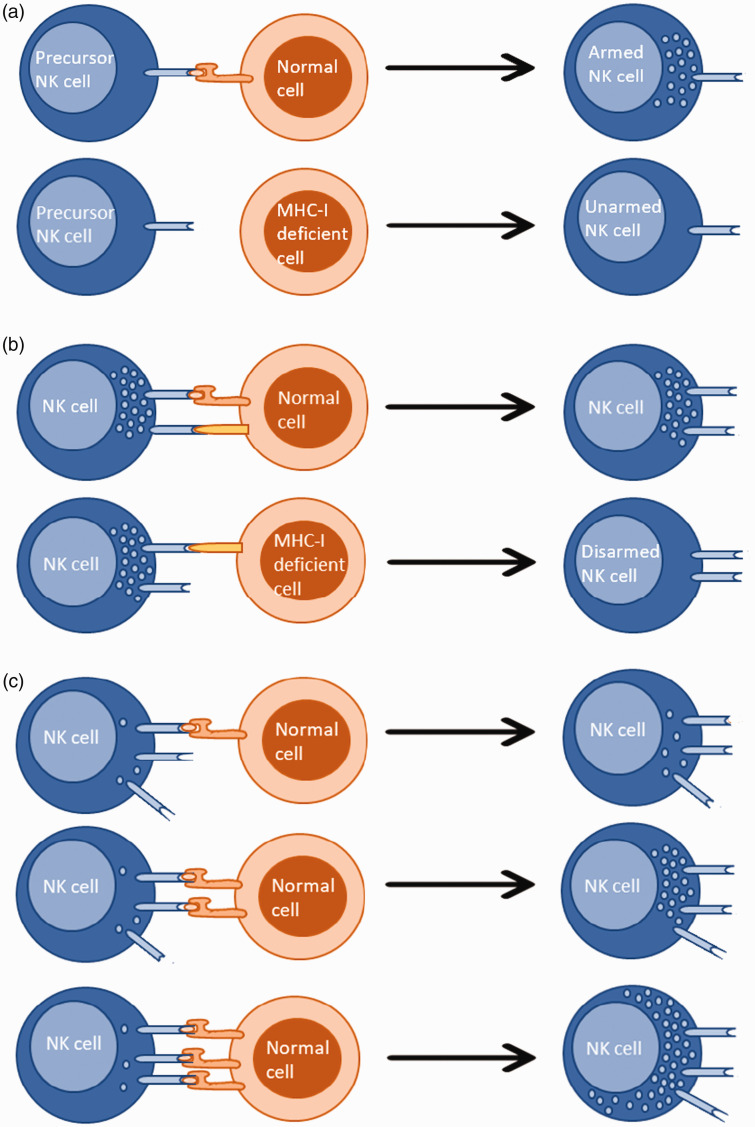
Human NK cells undergo education within the BM to avoid inadvertent attacks against self. However, this process is not exclusively restricted to the BM and is known to occur continuously throughout their life, adjusted by the local environment and changes to it.82 There have been numerous models proposed to explain human NK cell self-tolerance, the best-known so far being the arming and disarming models. According to the arming model, inhibitory signaling through the engagement of a MHC class I molecule by its corresponding KIR inhibitory receptor (functionally analogous to Ly49 in mice) promotes functional maturation of human precursor NK cells but not mature NK cells. Whereas, in the disarming model, both precursor and mature human NK cells that receive unopposed positive signaling via activating receptors are rendered hyporesponsive. Yet, the exact mechanisms underlying these observations have still not been fully defined.34 Supporting these models, it has been shown that increasing inhibitory receptor signaling, compared with activating receptor signaling, results in elevated responsiveness of human NK cells and vice versa.35 Moreover, stronger inhibitory receptor-ligand interactions demonstrating greater efficacy in educating human NK cells suggests that both quantitative and qualitative aspects of the interaction are important.36 Furthermore, human NK cells in possession of highly variable functional copies of KIR genes were shown to be functionally more competent, suggesting that the diversity among inhibitory receptors is positively correlated to human NK cell activity. Interestingly, studies have revealed that, compared with degranulation, the production of IFN-γ requires stronger interactions.36 These observations demonstrate that human NK cell education is not an ‘on and off’ procedure but something that can be fine-tuned. In fact, this is known as the ‘Rheostat’ model, which is widely accepted for its role in training NK cell function and marries together concepts of disarming and licensing (Figure 1).36,81
Appendix Figure 1.
Different models explaining NK cell education. (A) Precursor NK cells develop into armed NK cells after recognition of self MHC-I. The inability to recognize self MHC-I results in a loss of their effector potential. (B) Precursor and/or mature NK cells maintain their effector potential as long as they recognize self MHC-I. The inability to do so leads to disarming. (C) NK cell education is a modulated process and not an ‘on and off’ procedure. NK cell effector potential increases with the increased number of inhibitory receptor-self MHC-I contacts.
Functional competency observed in human NK cell education may also be attributed to the confinement of activating receptors towards the immunological synapse (IS) during cell-cell contact.37,43 However, He et al. have demonstrated that human NK cell education can take place even via non-classical MHC and non-MHC ligands.36
Human NK cell target recognition and activation
Human NK cell receptors
Human NK cells lack clonotypic receptors. Therefore their activity is believed to be controlled by a sophisticated array of germline-encoded activating and inhibitory receptors, and thus by a very delicate balance between inhibitory and activating stimuli.38 The ability of human NK cells to integrate these different signals remains decisive in their activation, granting them the ability to precisely recognize and destroy stressed cells whilst guaranteeing self-tolerance.39 Human NK cell receptors are transmembrane proteins formed by an extracellular ligand-binding portion and an intracellular cytoplasmic tail. If the receptor is inhibitory, the intracellular cytoplasmic tail contains an immunoreceptor tyrosine-based inhibition motif capable of direct stimulation of protein phosphatases, whereas if it is an activating receptor it will indirectly stimulate protein kinases by recruiting adaptor proteins containing immunoreceptor tyrosine-based activation motifs.40 KIRs and CD94/NKG2A(CD94/CD159a) are two major classes of inhibitory receptors expressed by human NK cells. KIR recognizes HLA class I, whereas the leukocyte Ig-like receptor 1 (LIRl)/Ig-like transcript 2 (ILT2) receptor recognizes both classical and non-classical human leukocyte Ag (HLA) class I molecules and are encoded by genes on the leukocyte receptor complex on chromosome 19q13.4.40 Instead, CD94/NKG2A, a heterodimeric C-type lectin receptor, recognizes non-classical MHC class Ib molecules such as HLA-E and is encoded by genes on the NK gene complex (NKC) on chromosome 12p13. However, activating forms of KIRs and LIRs have also been identified.40 The natural cytotoxicity receptors (NCR) NKp46 (NCR1), NKp30 (NCR3), and NKp44 (NCR2), together with NKG2D, a type II transmembrane and C-type lectin-like type II homodimeric receptor, are considered as activating receptors involved in human NK cell-mediated lysis.41,42 NKG2C is another activating receptor that binds HLA-E as NKG2A and NKG2E but with a lower affinity. Additionally, 2B4, NTB-A, DNAM-1, CD59, and NKp80 are co-receptors that amplify activating signals. Moreover, TLRs are also present. Finally, the presence of FcγIII, that recognizes the Fc portion of IgG Abs (CD16), enables Ab-dependent cellular cytotoxicity (ADCC) (Appendix Table 2).41,42
Owing to the stochastic nature of the expression of human NK cell receptors, there is significant diversity in the array of activating and inhibitory receptors among autologous NK cells and this must have paved the way for their functional diversity, with some being more capable of eliminating a specific type of threat.43 Furthermore, it is tempting to think that some receptors, such as inhibitory receptors toward MHCs, are expressed by all human NK cells due to their fundamental role in the prevention of autoreactivity.39
Human NK cell target cell contact
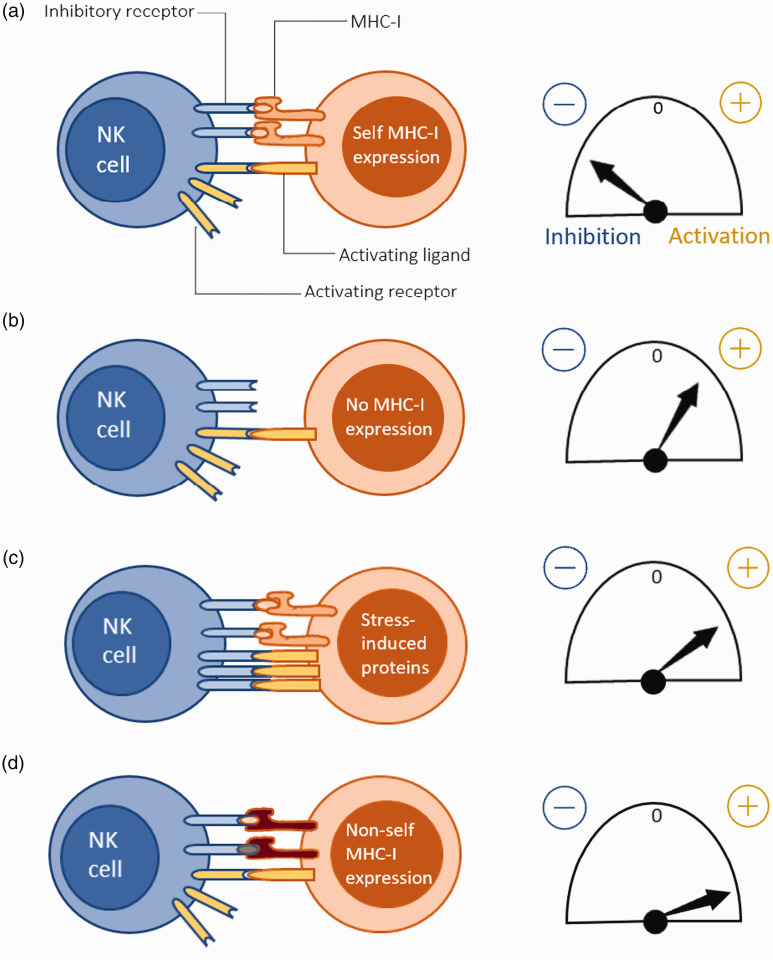
Different scenarios of human NK cell signal integration can be described. Recognition of normal self occurs when inhibitory and activating signals balance out or when there is a greater tendency towards inhibition, and the human NK cell is inhibited. Instead, if the human NK cell recognizes a non-self MHC-I, it gets activated. One occasion where this is manifested is when NK cells reject transplanted donor tissues expressing allogeneic MHC-I.26,43 By contrast, recognition of missing self occurs when inhibitory signals are lacking, usually when the target cell has lost MHC-I expression, for example, due to a tumor or viral infection. However, as mentioned previously, this process is more complicated than a mere lack of self MHC and thus needs to be sustained by other unknown stimuli. Another possibility is activation by recognizing an induced/altered self in case activating ligands are up-regulated in the target cell because of stress or infection (Figure 2).26,43
Appendix Figure 2.
Mechanisms of NK cell target recognition and their corresponding activation thresholds. (A) ‘Normal self’: Increased autologous MHC-I recognition by the NK cell leading to a net inhibition. (B) ‘Missing self’: Net activation of NK cell due to the lack of inhibitory signaling from inhibitory receptor-self MHC-I contact. (C) ‘Altered self’: Activating ligands are induced in the target cell due to stress, possibly caused by an underlying infection, and results in a net activation of the NK cell. (D) ‘Non-self’: NK cell activation when the target cell expresses non-self MHC-I, for example during an allogeneic transplant.
Stimulation and polarization of the human NK cell response by cytokines
Apart from intrinsic regulation by transcription factors, signaling mediated by cytokine cocktails from the microenvironment in which human NK cells reside also influences the type of effector function they perform.44 Accordingly, it has been shown that prior stimulation with a cocktail consisting of IL-2, IL-15, and IL-18 amplifies human NK cell responses upon secondary stimulation, in a process referred to as ‘priming’.45 Indeed, three main human NK cell polarization states have been defined in humans: cytotoxic, regulatory, and tolerant NK cells.44 IL-12, IL-18, and IL-21 have been shown to enhance human NK cell cytotoxicity, i.e. they promote the NKcytotoxic phenotype. In fact, human NK cells produce high levels of IFN-γ in response to stimulation with IL-12. Instead, IL-18 not only promotes the production of IFN-γ but also up-regulates the expression of CD25 in human NK cells.45 Interestingly, stimulation using a combination of IL-12 and IL-18 has been shown to reverse human NK cell anergy.46 Similarly, stimulation of exhausted human NK cells with IL-21 has been shown to retrieve their cytotoxicity, triggering the release of IFN-γ, TNF-α, and certain chemokines that recruit both naïve and activated T cells.47 Thus, cytokine treatment of human NK cells could be an extremely effective approach in NK cell immunotherapy. IGF-1 is another signaling molecule that enhances human NK cell cytotoxicity by promoting the synthesis of perforin. By contrast, IL-10 and TGF-β are known to promote the acquisition of the CD27- CD11b- NKtolerant phenotype. This subset of human NK cells is of particular importance in suppressing inflammation in chronic pathogenic environments or immune-tolerant organs such as the liver.26 Similarly, IL-15 and TGF-β act to promote the differentiation towards the CD56bright CD27- NKregulatory phenotype. Regulatory decidual NK cells that are involved in immune tolerance in the first trimester of human pregnancy belong to this subtype.26
Ab-dependent cellular cytotoxicity
Human NK cells possess Fc receptors that bind Abs in their invariant (Fc) region. This triggers a signaling cascade causing their activation and, as a result, release lytic compounds that eliminate cells that have been opsonized by Abs.48 Though the predominant activating Fc receptor among human NK cells is the low-affinity FcγRIIIA/CD16a that binds the Fc domain of IgG, an inhibitory FcγRIIC/CD32c receptor has also been observed on NK cells of some individuals.49,50 However, upon binding to CD16a, only polyvalent (but not monomeric) Ag-Ab complexes can initiate signal transduction upon the clustering and subsequent phosphorylation of CD3ζ leading to cellular activation.50
Mechanisms of human NK cell action
The following section evaluates (at least) three major mechanisms by which human NK cells perform their effector function, i.e. the cytotoxic elimination of their target.51,52
The predominant mechanism by which human NK cells kill their target is by the release of lysosomal-related organelles known as lytic granules, in a process referred to as ‘degranulation’.53,54 The synthesis of cytotoxic granules in human NK cells takes place during development and maturation.55 Recent evidence suggests that two freestanding pools of secretory lysosomes may exist for FasL and perforin, granulysin and granzyme (lytic granules). It is doubted whether their disjointed recruitment takes place from two different routes that originate either from a common source, such as a multivesicular body (MVB), or from distinct storage compartments.55,56 Nonetheless, lytic granules lead to two distinct cytotoxic pathways in the target cells, which include perforin-mediated osmotic lysis (necrosis) and granzyme (a group of serine proteases) induced apoptotic cell death.57 Degranulation, despite being a highly regulated process, initiates with human NK cell-target immunological synapse (IS) formation.53 Moreover, the observation of mitochondrial reorganization towards the target cell IS, supposedly to compensate for any energy lost, suggests that the stability of the human NK cell-target conjugate is dependent on ATP.54 Receptor-ligand interactions being constantly broken and re-established makes the IS a dynamic structure.54 Besides this, the availability of a new target in close proximity to a target-conjugated human NK cell induces mechanisms that result in its detachment, ultimately leading to an increased speed of killing in human NK cells due to integrated signals from both the previous and current targets. This is often referred to as ‘kinetic priming’.54 The spatial and temporal integration of signals at the IS leads to cytoskeletal rearrangement-assisted microtubule-organizing center (MTOC) polarization in an attempt to cause lytic granule convergence.55,58,59 Therefore, directional degranulation allows focused release of granule content within a complex tissue while preventing the destruction of the plethora of surrounding healthy cells. However, in tumor microenvironments, induction of diffuse degranulation could be an effective therapeutic approach, not only because tumor cells would vastly outnumber the infiltrating NK cells, but also because the kinetics and efficiency of human NK cell-mediated killing are independent of the three-dimensional orientation of the point of degranulation.57 Upon activation by a single target, a human NK cell releases about a tenth of its total lytic granule reserve (which includes > 200 ≈2–4). Despite the first few degranulation events (2--4) being adequate for target cell killing, human NK cells continue to degranulate at the IS until active detachment takes place.53
Mechanisms of target cell death induced by the engagement of death receptors (DR) by their cognate ligands have been well characterized. Indeed, after stimulation human NK cells express death receptor ligands such as TNF-α, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and FasL/CD178 that trigger the extrinsic apoptotic pathway by binding to TNFR1, TRAIL receptor (TRAIL-R) i.e. DR4/TRAIL-R1 & DR5/TRAIL-R2, and Fas/CD95/APO-1 on their targets, respectively.52,60,61 Ligand binding leads to DR oligomerization to recruit adaptor proteins that will recruit and process initiator caspases to trigger apoptotic cell death directly via effector caspases or indirectly via the intrinsic mitochondrial pathway. However, the inability to activate caspase 8 leads to oligomerized mixed lineage kinase domain-like protein-mediated necroptosis.62
Several authors have emphasized the fact that CD56bright NK cells constitute a minority (≈ 10%) of peripheral blood (PB)-NK cells but are the major subset of human NK cells responsible for the production of cytokines. Thus, further supported by the low frequency of expression of CD16 among human CD56bright NK cells, they are regarded as being poorly cytotoxic with mostly immunoregulatory functions.7,63–65 However, it has been shown that, with aging, there is a decline in the CD56bright subset and the CD56dim cell subset dominates. Hence, the role of human CD56bright cells in initiating T and B cell-mediated adaptive responses is impaired.11,158 The reason for human CD56bright NK cells being more potent producers of cytokines may be attributed to differences at the level of the signaling cascade, as both subsets (CD56bright and CD56dim) of human NK cells have reported expressing identical levels of activating and inhibitory receptors.7,10 Among the most prominently released cytokines are IFN-γ and TNF-α, and to a lesser extent the release of IL-5, IL-10, IL-13, certain growth factors like IL-3, G-CSF, GM-CSF, as well as chemokines such as CCL2/MCP-1, CXCL8/IL-8, CXCL10/IP-10, XCL1/lymphotactin, CCL1, CCL3/MIP-1α, CCL4/MIP-1β, and CCL5/RANTES have been reported.7,10,66–69 Interestingly, the release of these factors is sequential, with chemokines preceding pro-inflammatory cytokines, and is dependent upon the chronology and strength of stimulation.7,10 Human NK cell cytokine secretion can be both beneficial and disadvantageous. For example, Jewett et al. have reported that human NK cells exhibit ‘split anergy’, a process that terminates in a selective loss of cytotoxicity but increased cytokine secretion upon interaction with cancer stem cells (undifferentiated tumors). This led to IFNγ-mediated tumor differentiation with reduced proliferative capacity, but also resulted in human NK cell inactivation owing to increased tumor resistance.68 A large number of existing studies in the broader literature have examined the relevance of cytokines to human NK cell effector functions. For instance, IFN-γ effectively activates Th1 cells, not only by inducing the production of chemokines to recruit Ag-presenting cells (APC) but also by inducing ample expression of molecules and chaperones involved in Ag presentation.65,69,70 Furthermore, it facilitates both the acidification of the phagosome as well as its rapid fusion with lysosomes and intensifies the generation of both ROS and RNS by accelerating the synthesis of the key enzymes involved.69,71 Indeed, it promotes apoptosis by simultaneously increasing the number of proapoptotic factors and by inducing the synthesis of antiviral mediators.69,72 Moreover, it enhances opsonic uptake of extracellular pathogens via receptor-mediated phagocytosis by inducing the up-regulation of complement proteins and FcR.69 Similarly, TNF-α stimulates B cell proliferation that leads to Ag-non-specific Ig production, elicits cytotoxic and anti-proliferative effects against various types of tumor cells, and promotes host defense against intracellular pathogens through inhibition, induction of cell death and granuloma formation. Additionally, it mediates contact-dependent endothelial activation, leading to the production of procoagulants, adhesion molecules, and pro-inflammatory cytokines, which seem to be manifestations of microvascular inflammation.73 However, unlike INF-γ, TNF-α has been shown to exist as either transmembrane TNF-α (mTNF-α) or soluble TNF-α (sTNF-α).74 As a matter of fact, TNF-α converting enzyme (TACE) on human NK cells is capable of proteolytically cleaving mTNF to release sTNF.75 Indeed, supporting outside in (reverse) signaling, increased human NK cell cytotoxicity has been observed upon pre-stimulation of transmembrane TNF-α with soluble TNF-R1.64,73
Diverse array of functions
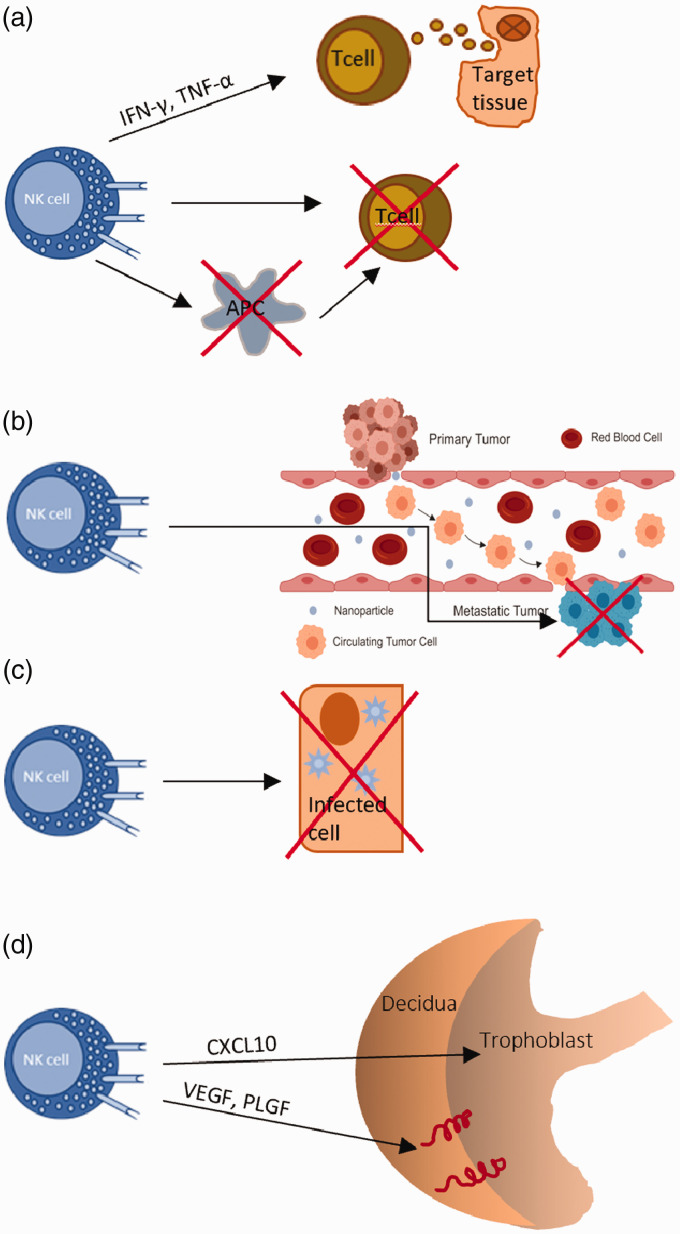
As the largest lymphocyte population of the innate immune system, human NK cells are known to be involved in a wide variety of biological roles, though much is yet to be unveiled. In particular, human NK cells are known to suppress graft versus host disease (GvHD), but recent controversial evidence suggests that under certain circumstances they can also promote GvHD.76 However, as shown in vitro using both human and mice cells, NK cells can prevent alloreactive T cell-mediated GvHD development, either by the direct and selective depletion of stressed conventional T (not Treg) cells that have up-regulated stress ligands, such as NKG2D, or indirectly by killing off APCs (Figure 3A).76
Appendix Figure 3.
Functions of NK cells. (A) NK cells suppress GvHD by directly killing T cells or by indirectly killing APCs. Moreover, NK cells can also promote the development of GvHD through the secretion of pro-inflammatory cytokines. (B) NK cells destroy metastatic cells. (C) NK cells destroy infected or stressed cells that display up-regulated activating ligands. (D) uNK cells drive the migration of the trophoblast during pregnancy through the secretion of chemokines. They secrete angiogenic growth factors to promote the remodeling of decidual spiral arteries during the early stages of pregnancy.
Furthermore, in a model proposed by Simonetta et al. to explain this controversy, they demonstrate that secretion of pro-inflammatory cytokines by activated human NK cells can indirectly induce GvHD by stimulating alloreactive T cells, and that this prevails over their anti-GvHD cytotoxic effect. In this case, the timing of adoptive NK cell transfer is also clinically relevant in determining whether it will induce or protect from GvHD: the earlier the transfer, the better the outcome.76 Apart from this, an inverse correlation between the human NK cell count and metastasis suggests that human NK cells are also involved in the control of metastasis. This was further backed by the observation of up-regulated human NK cell activation receptors and augmented cytotoxicity being linked to better cancer prognosis. Normal and malignant cells express multiple ligands for human NK inhibitory receptors (NKIRs) and are thus able to evade human NK cell attack. By contrast, potentially metastatic cancer cells up-regulate human NK activating receptors coupled with the down-regulation of NKIRs, leading to the antimetastatic effects of human NK cells.77 Moreover, human NK cells can act against viruses and other pathogens. Human NK cells can easily detect virus-infected cells, not only because of the increased expression of host-derived stress-induced ligands but also because of the loss of self MHC class I. Their activation and subsequent release of IFN-γ leads to the elevated production of antiviral mediators such as dsRNA-dependent protein kinase (PKR) and IFN regulatory factors in the target cell. Instead, according to recent in vivo and in vitro studies, the response against intracellular pathogens involves the granzyme-mediated inactivation of key enzymes involved in the defense against oxidative stress, which leads to the production of ROS and disrupts their essential metabolic pathways. Similarly, human NK cell killing of fungi has been observed in vivo.78 Using animal models it has been shown that impaired human NK cell functionality leads to the accumulation of senescent cells in tissues, and thus suggests that human NK cells are crucial in maintaining tissue homeostasis. This is further confirmed by observing that human NK cells up-regulate several activating receptors such as NKG2D and DNAX accessory molecule-1(DNAM-1) upon interaction with stressed cells.11 Furthermore, human NK cells exert regulatory functions on the immune system. For example, they are involved in the maturation of DCs, direct activation of adaptive immune cells by secreting IFN-γ, and killing of immature DCs and overstimulated Mϕs. It has also been suggested that human NK cells could cause vascular injury due to their expression of receptors for endothelial cell adhesion molecules that can be activated by endothelial membrane ligands such as CX3CL1.79 As reported previously, a subset of human uterine CD56bright NK cells (uNK) has been shown to play an active role in controlling trophoblast invasion and vascular remodeling.80 Besides this, there had been much debate on whether human uNK cells possess memory. However, a better-equipped placental vascularization and trophoblast invasion in subsequent pregnancies are highly suggestive of such a phenotype.3,80 CD56bright CD16− uNKs constitute the majority (70–80%) of uNK cells and reshape spiral arteries through the secretion of proangiogenic factors like VEGF, PLGF, and NKG5, and are also involved in directing trophoblast migration and invasion through the secretion of cytokines such as GM-CSF, CSF-1, CXCL12, CXCL10, IL-2, LIF, TNF-α and INF-γ. By contrast, a minor subset of CD56dim CD16+ uNK cells displays cytotoxicity towards the extravillous trophoblast (EVT) and autologous endometrial cells. Indeed, a study by Giuliani et al. found that women who had a higher population of cytotoxic CD56dim CD16+ uNK cells were at a higher risk of infertility and related disorders (Figure 3).80,82
Human NK cells in distinct tissue compartments
Most of our current knowledge on human NK cells has been gathered using peripheral blood mononuclear cells or conventional NK (cNK) cells. However, recently there seems to be a growing interest among researchers to characterize the phenotype and behavior of trNK cells.83 The observation of NCRs that also recognize induced-self ligands and non-self Ags in human trNKs suggest that trNKs have similar mechanisms of activation as cNKs.84 However, human trNKs are known to be developmentally, functionally, and immunophenotypically distinct from cNKs. The expression of different types and levels of KIRs compared with cNKs seems to be suggestive of this functional divergence.5,85 In addition, human trNKs require unique transcription factors such as Hobit, observed in human liver-resident NK cells, that guide them towards tissue residency by repressing genes required for tissue egression.86 Other factors responsible for the dichotomy include the up-regulation of either CD69; a type II C-lectin membrane receptor that also acts as an activation-associated marker, CXCR6; a chemokine receptor, CD103; an integrin subunit that forms αEβ7 and binds to the cell adhesion molecule E-cadherin, or CD49a; an α1β1 integrin receptor α-subunit that binds to collagen IV, all of which are known to be key markers of tissue retention.87,89 However, the types of cytokines and cells that drive the differentiation of human NKPs towards tissue retention has been poorly defined and is left over to be investigated. Interestingly, a study conducted by Salzberger et al. demonstrated that in contrast to human peripheral blood CD56bright NKs, CD56bright CXCR6+ trNKs expressed higher levels of the amino acid transporter CD98 but lower levels of Glut1. This suggests possible differences in their metabolism, of which the functional relevance is not yet clear.88 Human trNK subsets that have so far gained the attention of researchers mainly include those which reside in the gut, liver, thymus, uterus, lymph nodes, and the kidney, even though they have been observed in many other tissues. Nonetheless, each subset has been shown to follow distinct developmental pathways depending on their resident tissue compartment.89
Recently, several studies have begun to unveil the clinical relevance of these distinct human trNK subsets. For example, NK cells make up 10–20% of lymphocytes in the human lung, and out of this only 20% accounts for lung resident CD49a+CD69+CD103+ CD16− trNKs.5,93 It has been shown that they facilitate CD8+T cytotoxic and CD4+ T h1 responses against influenza-infected epithelial cells through IFN-γ secretion after being rendered hyperresponsive.90,91,93 The remaining majority is known to transition between the lung and peripheral blood and has the CD56dimCD16− mature phenotype.5 Moreover, a subset of innate-like NKs is involved in promoting regeneration of the human tracheal epithelium by secreting IL-22.90,92 In the human liver, NK cells account for 50% of the total lymphocyte count and comprise hepatic CD56bright CCR5+ CXCR6+ CD69+ trNKs, transient cNKs, and memory-like NKs.94,95 This high percentage itself shows the extent of their contribution towards detoxification, which is undoubtedly one of the major functions of the liver. Moreover, they promote hepatic tolerance and homeostasis by interacting with hepatocyte HLA-E through the NKG2A inhibitory receptor to prime DCs via the release of TGF-β, eventually resulting in the expansion of CD4+CD25+ Treg cells.96 Such a role is further confirmed by finding that human hepatic trNKs can limit fibrosis by killing hepatic stellate cell-derived myofibroblasts.97 In the human lymph nodes, where communication between innate and adaptive immunity takes place, NK cells are comprised of thymus-derived NKs, circulating NKs, and a unique subset of lymphoid trNKs that develop within the lymph node.98,99 Here, human NKs are known to interact with DCs and release IFNγ to drive the differentiation of IFNγ-producing CD4+Th1. Indeed, the addition of an Ab blocking IFNγ was able to impair NK-dependent Th1 responses.100 As lymph nodes usually act as the first site of tumor metastasis, mechanisms could be developed to enhance lymph nodal tissue retention of human NKs to improve prognosis in early stages of lymph node metastasis.101,102 NK cells account for approximately 25% of the total lymphocytes in the human kidney. These NK cells are mainly CD56bright but it is still doubted whether they act as transient circulating NK cells or whether they are permanently resident.103 Indeed, Law et al. have recently reported the observation of CD69+ CD56bright NKs in human kidney biopsies from patients with various renal pathologies, which seems to be indicative of tissue residency, but also observed that these NKs were CD117+, which was absent in human trNKs observed in the uterus, spleen, lymph nodes and the BM. Further studies will be required to clarify this issue.104 Several studies have also described that human kidney NKs contribute to renal injury. This is said to be mediated by an interplay between tubular epithelial cells and kidney NKs.105 Although the existence of human thymic NKs was known for a long time, their function is largely unknown, even today.106 Nonetheless, their functions have been speculated using data from murine studies. In fact, human thymic NKs could be involved in the regulation of thymic T cell development, possibly by clearing negatively selected T cells. Moreover, it has been suggested that they have the potential to regulate the extent of MHC class I expression on stromal cells.5 However, these affirmations require confirmation through human studies. By contrast, numerous studies have been conducted on human uNK cells. It is well established that human uNK cells account for ≥ 70% of all lymphocytes in the first trimester of pregnancy and their majority is characterized by a CD94+ NKG2A+ CD103+ CD49a+ CD16− phenotype.107–109 Before becoming less granular, they peak in numbers during the first trimester and progressively diminish in frequency during gestation.110 In the human uterus, we have already described in the previous section how uNKs contribute to spiral artery remodeling, an essential process for a healthy pregnancy, and we also discussed the different uNK subsets. Indeed, they secrete matrix metalloproteinases (MMPs) to directly control decidua-associated vascular remodeling. Besides this, they regulate trophoblast mediated-vascular remodeling by a balance between extravillous trophoblast (EVT) pro-invasive factors (GM-CSF, CCL1, XCL1) and anti-invasive factors (TGF-β).111–114 Recently, Bonaccorsi et al. discussed the presence of trNKs in human carotid atherosclerotic plaques. The authors have shown that these trNKs contribute to disease progression and increase the instability of the plaque via the release of IFNγ which induced MMP activity. Hereby, we see that we are discovering novel roles of human NK cells in tissue compartments, which hopefully in the future could pave the way for human NKs to be targeted for treating diseases other than cancer.115
Human NK cell memory
NK cells have historically been defined by nonspecific innate killing of tumor and virus-infected cells.116 However, in the past decade, ample studies have demonstrated that they can elicit memory-like responses. Some of the earliest evidence of NK cell memory came from a mouse model of cytomegalovirus infection (CMV) where, like T cells, Ly49H+ (a receptor that recognizes virally-encoded m157 on infected cells) murine NKs were capable of rapid responses to a secondary challenge imposed by the same haptens.117 Similar to what has been observed in mice, a subset of human NKs bearing the human analog of murine Ly49H, the MHC class I binding activating receptor CD94/NKG2C, has been shown to undergo massive clonal expansion following human CMV reactivation in immunosuppressed transplant patients.118 It is worth noting that surface markers lack specificity for the identification of these adaptive NK cells. However, previous studies have suggested that the loss of certain intracellular adapter signaling molecules such as FcεR1γ, DAB2, SYK, and/or EAT-2 and the retention of others such as CD3ζ, ZAP-70, and SAP provides such specificity. Also, human adaptive NK cells are characterized by the down-regulation of transcription factors PLZF and IKZF2.119–121 This has been attributed to the methylation of their promoters. In fact, human adaptive NK cells, which are also characterized by the absence of IFN-γ secretion following stimulation by IL-12 and IL-18, are owing to the methylation of promoters of their respective receptors, which in turn are known to be under the control of PLZF.121 Human adaptive NKs were also hyporesponsive to autologous T cell-mediated stimulation. Despite all this, it has been observed that CD16 ligation was able to induce IFN-γ secretion and vigorous proliferation of these NKs, owing to the hypomethylated state of the IFN-γ promoter.119–121 Moreover, Liu et al. have shown that the synergistic interaction between CD2 (responsible for the recruitment of CD16 to the IS) and CD16 in human CMV(HCMV) seropositive patients resulted in adaptive NK activation (but not cNK activation) leading to both the increased secretion of IFN-γ and TNF-α, as well as enhanced Ab-mediated responses. Conversely, CD2 mediated co-stimulation had not been observed in HCMV seronegative patients. This suggests that CD2 co-stimulation plays an important role in adaptive NK responses which requires further investigation.122,123 Also, the cytotoxic capacity of human adaptive NK cells compared with cNKs is something that requires further evidence to be clarified.85 Interestingly, other forms of NK memory have been described. For example, Zhang et al. have described Ab-dependent memory-like NK (g- NK) cells distinguished by FcRγ deficiency; they had high expression of FcR but lacked the intracellular γ signaling chain. These g- NKs were observed at low frequency in all individuals but expanded in HCMV seropositive patients, though were not limited to them.120,124 Indeed, they elicited enhanced responses superior to those cNKs seen upon FcR-mediated Ab-dependent recognition of antiviral Abs during either HSV-1 or HCMV infections, regardless of prior antigenic exposure. Furthermore, g- NKs stably persisted in the long term in plasma of healthy individuals.120,124 Similarly, another form termed cytokine-induced memory NKs have been described by Cooper et al. These cytokine-activated human NKs have been shown to persist for 7–22 d following adoptive transfer to a naïve host and were capable of secreting significantly elevated levels of IFN-γ upon restimulation, along with cytotoxicity like that observed in naïve NKs. However, prior sensitization with cytokines alone seems unlikely to explain Ag-specific responses, raising the question of whether there had been other factors that made this possible. Interestingly, similar cytokine-induced memory NKs were also observed after vaccination against influenza in humans.125,126 Recently, Brillantes et al. have shown that human NKs can elicit memory and memory-like responses towards a wide variety of microbial pathogens.127 However, what is still unclear is the mechanisms that NKs deploy to mount memory responses. This is because there is poor evidence showing that human NKs increase the frequency of cells with a specific receptor to decrease repertoire diversity, as normally happens in adaptive immunity.117 A work by Horowitz et al. has revealed that 6,000–30,000 unique subsets of NKs are present in each individual owing to receptor assortment to generate different combinations.129 In turn, the clones of specific subsets responsive to distinct Ags could possibly expand. However, whether this leads to a more mature repertoire favoring cytokine-secreting cells over direct killing is to be determined.116 The discovery of memory in the human NK compartment makes us wonder whether it could be harnessed by vaccination. This could be particularly effective in HIV infections where CD4+T cells get rapidly depleted as it provides an alternative where B and T cells cannot be harnessed.127,128
Targeting human NK cells in cancer immunotherapy
Within the past few years, NK-based immunotherapy has become a convincing approach for treating several malignancies.130 NK-based immunotherapy is now preferred over chimeric Ag receptor (CAR) T cell therapy owing to safety issues and other challenges that have arisen with the use of CAR T cell therapy, including toxicities, finding targets specific to abnormal cells, and overcoming the immunosuppressive tumor microenvironment.131,132 Furthermore, NK-based therapies need not be patient-specific as in the case of CAR T cell therapy.133 Adoptive immunotherapy has paved the way for overcoming the inhibition of human NK cells, which originally resulted in reduced levels of activating receptors and consequently impaired their tumoricidal activity. This is explained by NK cell-based adoptive immunotherapy frequently involving ex vivo expansion and activation of human NK cells to amplify both their numbers and enhance tumor killing.130,134 One strategy that could be adopted to do this is to culture and expand human NK cells using cytokines such as IL-2, IL-12, IL-15, IL-18, and IL-21.135 Alternatively, feeder cells, which are division restricted and cannot thus proliferate, are used to enhance human NK proliferation and activation.134,136 However, according to Parkhurst et al., although the transfer of autologous NKs led to high levels of circulating NKs, it did not lead to tumor regression in vitro unless reactivated with IL-2.137,138 This could partly be due to inhibitory KIR-mediated recognition of self MHC-class I on tumor cell targets and the inability to ensure the pure expansion of human NK cells.138 In this context, Abs could be targeted towards inhibitory KIRs or miRNA could be used to stop the expression of inhibitory KIR to induce a ‘missing self’ condition.23 Nonetheless, these cells could mediate ADCC without in vitro reactivation with IL-2 due to retention of CD16 expression. Thus, mAb therapy, coupled with adoptive autologous NK transfer, seems to be an attractive solution.137 Furthermore, to solve this researchers also began to investigate the possibility of using human allogeneic NKs that have proven themselves to be effective by inducing potent anti-tumor activity and, unlike T cells, it is less likely to cause GvHD.139,140 But even if allogeneic NK adoptive immunotherapy has proven to be effective against hematological malignancies, the heterogeneity of solid tumors, accompanied by the hypoxic and TGF-β, indoleamine 2,3-dioxygenase (IDO), IL-4, and prostaglandin E2 (PGE2) abundant tumor microenvironment have made this challenging.141–145 Thus, approaches to improve their action against solid tumors include combination therapies with immunomodulatory agents, monoclonal Abs, cytokines, checkpoint inhibitors, and genetic engineering to enhance infiltration into solid tumors.146 Besides using cytokines and feeder cells, an alternative strategy used to overcome the limited quantity of primary NK cells available for NK-based immunotherapy involves the use of NK cell lines such as NK-92, NKL, YT, NK3.3, and NK-YS (note that only NK-92 is currently approved for clinical studies in patients), all of which were expanded clones from patients with malignant leukemia, except for NK3.3 that has been obtained from a healthy donor.147,148 Irradiation of NK cells obtained from patients with malignancies prior to infusion has been deemed necessary to prevent excessive proliferation in vivo that may result from malignant transformation. Furthermore, there are concerns regarding toxicity arising from the frequent use of IL-2 for maintaining NK-92 cells. Also, some human NK cell lines lack activating receptors and therefore they need to be genetically incorporated to make them tumoricidal.149 Apart from these, alternatives sources that are widely available include umbilical cord blood, and human embryonic and pluripotent stem cells (PSCs).150,151 The human induced-PSCs (iPSCs) have no risk of becoming malignant and can be genetically engineered easily using both viral vectors and clustered regularly interspaced short palindromic repeats (CRISPR) technology.152–154 Finally, CAR NK cells are now under consideration due to lower in vivo side effects such as neurotoxicity, GvHD, and cytokine release syndrome.155 Moreover, their short life makes them low risk compared with CAR T cells. The basic structure of the CAR NK cell signaling domain is much like that of the CAR T cell and is comprised of extracellular, transmembrane, and intracellular signaling domains. Indeed, the intracellular domain includes CD3ζ along with costimulatory molecules such as CD244 (2B4), CD28, CD137, and NKG2D, whereas the scFv region of the extracellular domain contains the heavy and light chains.138 Interestingly, beyond targeting tumor Ags, Parihar et al. demonstrated both in vitro and in a mouse xenograft model, that CAR NK cells could be effectively targeted against human myeloid-derived suppressor cells, highlighting the possibility of using them to eliminate immunosuppressive cells within the tumor microenvironment.156,157
Concluding remarks and future outlook
Previous research on human NK cells has undoubtedly made us understand that they have enormous potential to be harnessed for disease therapy, including but not limited to cancer. Despite challenges such as sustaining in vivo survival, difficulty obtaining large quantities, and counteracting tumor immune-evasion, we believe that further elucidation of NK cell immunity with a human-centered approach will help overcome these challenges and eventually strengthen their utilization in immunotherapy.
Appendix Table 1.
Human NK cell surface markers expressed during distinct developmental stages.
| HSC | LMPP | CLP | NKPStage 1 | Pre-NKStage 2a | Pre-NK Stage 2b | iNK Stage 3 | CD56brightStage 4a | CD56brightStage 4b | CD56dimStage 5 | CD56dimStage 6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lin | – | – | – | – | – | – | – | – | – | – | – |
| CD34 | + | low | + | + | + | + | +/− | – | – | – | – |
| c-Kit | + | low | + | + | – | + | + | +/low | low/− | low/− | – |
| CD244 | – | + | + | + | + | + | + | + | + | + | + |
| CD45RA | – | + | + | + | + | + | + | +/− | +/− | – | – |
| CD127 | – | – | + | + | + | + | +/− | – | – | – | – |
| CD7 | – | – | + | + | + | + | + | + | + | + | + |
| CD10 | – | – | + | + | +/− | – | – | – | – | – | – |
| ILR1 | – | – | – | low | – | + | + | +/low | low/− | low/− | low/− |
| CD122 | – | – | – | + | – | + | + | + | + | + | + |
| HLA-DR | – | – | – | + | + | + | – | – | – | – | – |
| NKp46 | – | – | – | – | – | – | +/− | + | + | + | + |
| NKp30 | – | – | – | – | – | – | +/− | + | + | + | + |
| CD161 | – | – | – | – | – | – | +/− | + | + | + | + |
| NKG2D | – | – | – | – | – | – | +/− | + | + | + | + |
| NKG2A | – | – | – | – | – | – | – | + | + | low/− | low/− |
| NKp80 | – | – | – | – | – | – | – | – | + | + | + |
| CD56 | – | – | – | – | – | low/− | low/− | ++ | ++ | low | low |
| CD16 | – | – | – | – | – | – | – | – | – | + | + |
| KIR | – | – | – | – | – | – | – | – | – | +/− | + |
| NKG2C | – | – | – | – | – | – | – | – | – | + | + |
| CD57 | – | – | – | – | – | – | – | – | – | – | + |
Appendix Table 2.
A list of some common human NK cell receptors and their corresponding ligands.
| Receptor | Ligand |
|---|---|
| Non-HLA-specific receptors | |
| Co-receptors | |
| CD59 | LFA-2 (CD2) |
| NTB-A (CD352) | NTB-A (CD352) |
| NKp80 | AICL (activation-induced C-type lectin) |
| DNAM-1 (226) | Nectin-2 (CD112), PVR (CD155) |
| 2B4 (244) | CD48 |
| Activating | |
| NKp30 (CD337) | B7-H6, BAG6/BAT3, HCMV-pp65, heparin sulfate |
| NKp44 (CD336) | 21spe-MLL5-Nidogen-1, viral HA, proliferating nuclear cell Ag (PCNA) |
| NKp46 (CD335) | CFP (complement factor P)/properdin, viral HA and HN, PfEMP1, heparin sulfate |
| NKG2D (CD314) | MIC-A, MIC-B, ULBP 1-6 |
| FcγRIII (CD16) | IgG |
| Inhibitory | |
| PD-1 (CD279) | PD-L1 (CD274), PD-L2 (CD273) |
| Siglec-7 (CD328) | Ganglioside DSGb5 |
| IRP60 (CD300a) | Phosphatidylethanolamine, phosphatidylserine, Pseudorabide virus, α-Herpesvirus |
| Tactile (CD96) | PVR (CCD155) |
| IL1R8 | IL-37 |
| TIGIT | PVR (CD155) |
| TIM-3 | Gal-9, PtdSer, HMGB1, CEACAM1 |
| HLA-specific receptors | |
| Activating | |
| KIR2DS1 | HLA-C2 |
| KIR2DS2/3 | unknown |
| KIR2DL4 | HLA-G |
| KIR2DS4 | HLA-A*11 and some HLA-C |
| KIR2DS5 | HLA-C2 (variable) |
| KIR3DS1 | HLA-Bw4, HLA-F |
| NKG2C | HLA-E |
| Inhibitory | |
| NKG2A | HLA-E |
| KIR2DL1 | HLA-C2 |
| KIR2DL2/3 | HLA-C1, HLA-C2, few HLA-Bb |
| KIR2DL5 | unknown |
| KIR3DL1 | HLA-A-Bw4, HLA-B-Bw4 |
| KIR3DL2 | HLA-A*03, HLA-A*11 |
| ILT2/LIR-1 | Different MHC-I alleles |
| LAG-3 (CD223) | MHC-II |
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Arosh Shavinda Perera Molligoda Arachchige https://orcid.org/0000-0002-3875-0267
References
- 1.Langers I, Renoux VM, Thiry M, et al. Natural killer cells: role in local tumor growth and metastasis. Biologics 2012; 6: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng H, Zhigang T. Natural killer cell memory: Progress and implications. Front Immunol 2017; 8: 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sojka DK, Yang L, Yokoyama WM. Uterine natural killer cells. Front Immunol 2019; 10: 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlazzo G, Thomas D, Lin SLet al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol 2004; 172: 1455–1462. [DOI] [PubMed] [Google Scholar]
- 5.Hashemi E, Malarkannan S. Tissue-resident NK cells: Development, maturation, and clinical relevance. Cancers 2020; 12: 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquardt N, Kekäläinen E, Chen Pet al. Unique transcriptional and protein-expression signature in human lung tissue-resident NK cells. Nat Commun 2019; 10: 3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauriat C, Long EO, Ljunggren HG, et al. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010; 115: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiessling R, Klein E, Wigzell H. ‘Natural’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975; 5: 112–117. [DOI] [PubMed] [Google Scholar]
- 9.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer 1975; 16: 216–229. [DOI] [PubMed] [Google Scholar]
- 10.Marischen L, Englert A, Schmitt ALet al. Human NK cells adapt their immune response towards increasing multiplicities of infection of Aspergillus fumigatus. BMC Immunol 2018; 19: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kale A, Sharma A, Stolzing A, et al. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing 2020; 17: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scoville SD, Freud AG, Caligiuri MAet al. Modeling human natural killer cell development in the era of innate lymphoid cells. Front Immunol 2017; 8: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Malarkannan S. Transcriptional regulation of natural killer cell development and functions. Cancers 2020; 12: 1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renoux VM, Zriwil A, Peitzsch C, et al. Identification of a human natural killer cell lineage-restricted progenitor in fetal and adult tissues. Immunity 2015; 43: 394–407. [DOI] [PubMed] [Google Scholar]
- 15.Scoville SD, Freud AG, Caligiuri MA. Cellular pathways in the development of human and murine innate lymphoid cells. Curr Opin Immunol 2018; 56: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol 2013; 34: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mace EM, Hsu AP, Monaco-Shawver Let al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56 (bright) subset. Blood 2013; 121: 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scoville SD, Mundy-Bosse BL, Zhang MHet al. A progenitor cell expressing transcription factor RORgammat generates all human innate lymphoid cell subsets. Immunity 2016; 44: 1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metkar SS, Marchioretto M, Antonini Vet al. Perforin oligomers form arcs in cellular membranes: a locus for intracellular delivery of granzymes. Cell Death Differ 2015; 22: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Vito C, Mikulak J, Mavilio D. On the way to become a natural killer cell. Front Immunol 2019; 10: 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kared H, Martelli S, Tan SW, et al. Adaptive NKG2C(+)CD57(+) natural killer cell and Tim-3 expression during viral infections. Front Immunol 2018; 9: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins PL, Cella M, Porter SIet al. Gene regulatory programs conferring phenotypic identities to human NK cells. Cell 2019; 176: 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pesce S, Squillario M, Greppi M, et al. New miRNA signature heralds human NK cell subsets at different maturation steps: involvement of miR-146a-5p in the regulation of KIR expression. Front Immunol 2018; 9: 2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs R, Hintzen G, Kemper Aet al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001; 31: 3121–3127. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Tian Z, Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol 2017; 8: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel AM, Yang C, Thakar MSet al. Natural killer cells: Development, maturation, and clinical utilization. Front Immunol 2018; 9: 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang M, Li D, Chang Zet al. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J Exp Med 2015; 212: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JC. Transcriptional control of NK cells. Nat Killer Cells 2016; 395: 1–36. [DOI] [PubMed] [Google Scholar]
- 29.Yu HX, Fehniger TA, Fuchshuber P, et al. Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood 1998; 92: 3647–3657. [PubMed] [Google Scholar]
- 30.Colucci F, Di Santo JP. The receptor tyrosine kinase c-kit provides a critical signal for survival, expansion, and maturation of mouse natural killer cells. Blood 2000; 95: 984–991. [PubMed] [Google Scholar]
- 31.Benson DM. Stem cell factor and interleukin-2/15 combine to enhance MAPK-mediated proliferation of human natural killer cells. Blood 2009; 113: 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vosshenrich CA, Ranson T, Samson SI, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol 2005; 174: 1213–1221. [DOI] [PubMed] [Google Scholar]
- 33.Michaud A, Dardari R, Charrier Eet al. IL-7 enhances survival of human CD56bright NK cells. Journal of immunotherapy (Hagerstown, Md: 1997) 2010; 33(4): 382–390 [DOI] [PubMed]
- 34.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol 2006; 6: 520–531. [DOI] [PubMed] [Google Scholar]
- 35.Frutoso M, Mortier E. NK cell hyporesponsiveness: more is not always better. Int J Mol Sci 2019; 20: 4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He Y, Tian Z. NK cell education via nonclassical MHC and non-MHC ligands. Cell Mol Immunol 2017; 14: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadad U, Thauland TJ, Martinez OM, et al. NKp46 clusters at the immune synapse and regulates NK cell polarization. Front Immunol 2015; 6: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long EO, Kim HS, Liu D, et al. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 2013; 31: 227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molgora M, Supino D, Mavilio D, et al. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand J Immunol 2018; 88: e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrillo-Bustamante P, Kesmir C, De Boer RJ. The evolution of natural killer cell receptors. Immunogenetics 2016; 68: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biassoni R. Natural killer cell receptors. In: Sigalov AB (eds) Multichain immune recognition receptor signaling. advances in experimental medicine and biology 2008, vol 640. Springer, New York, NY. [PubMed]
- 42.Sivori S, Vacca P, Del Zotto G, et al. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol 2019; 16: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol 2011; 32: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu BQ, Tian ZG, Wei HM. Subsets of human natural killer cells and their regulatory effects. Immunology 2014; 141: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stegmann KA, De Souza JB, Riley EM. IL-18-induced expression of high affinity IL-2R on murine NK cells is essential for NK-cell IFN-gamma production during murine Plasmodium yoelii infection. Eur J Immunol 2015; 45: 3431–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardolino M, Azimi CS, Iannello Aet al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest 2014; 124: 4781–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo H, Jeon I, Kim BSet al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun 2017; 8: 15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forthal DN, Finzi A. Antibody-dependent cellular cytotoxicity in HIV infection. AIDS (London, England) 2018; 32: 2439–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanseviero E. NK cell-Fc receptors advance tumor immunotherapy. J Clin Med 2019; 8: 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai HS, Griffin N, Bolyard C, et al. The Fc domain of immunoglobulin is sufficient to bridge NK cells with virally infected cells. Immunity 2017; 47: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gradzien M, Rapak A. Effect of natural compounds on NK cell activation. J Immunol Res 2018; 2018: 4868417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinvalet D. Mitochondria entry of cytotoxic proteases: a New insight Into the granzyme B cell death pathway. Oxid Med Cell 2019; 2019: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gwalani LA, Orange JS. Single degranulations in natural killer cells can mediate target cell killing. J Immunol 2018; 200: 3231–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Netter P, Anft M, Watzl Cet al. Termination of the activating NK cell immunological synapse is an active and regulated process. J Immunol 2017; 199: 2528–2535. [DOI] [PubMed] [Google Scholar]
- 55.Kabanova A, Zurli V, Baldari CT. Signals controlling lytic granule polarization at the cytotoxic immune synapse. Front Immunol 2018; 9: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lettau M, Kabelitz D, Janssen O. Lysosome-related effector vesicles in T lymphocytes and NK cells. Scand J Immunol 2015; 82: 235–243. [DOI] [PubMed] [Google Scholar]
- 57.Hsu HT, Mace EM, Carisey AFet al. NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J Cell Biol 2016; 215: 875–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mace EM, Dongre P, Hsu HTet al. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol and Cell Biol 2014; 92: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertrand F, Müller S, Roh K, et al. An initial and rapid step of lytic granule secretion precedes M.T.O.C. polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci USA 2013; 110: 6073–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mocarski ES, Kaiser WJ, Livingston-Rosanoff D, et al. True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. J Immunol 2014; 192: 2019–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon S, Kim T, Choi I. Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med 2015; 47: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zwirner NW, Ziblat A. Regulation of NK cell activation and effector functions by the IL-12 family of cytokines: the case of IL-27. Front Immunol 2017; 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parodi M, Raggi F, Cangelosi Det al. Hypoxia modifies the transcriptome of human NK cells, modulates their immunoregulatory profile, and influences NK cell subset migration. Front Immunol 2018; 9: 2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas R, Yang X. NK-DC crosstalk in immunity to microbial infection. J Immunol Res 2016; 2016: 6374379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frederick A, Bobanga ID, Rauhe Pet al. CCL3 augments tumor rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFNγ. OncoImmunology 2018; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agudelo O, Bueno J, Villa Aet al. High IFN-gamma and TNF production by peripheral NK cells of Colombian patients with different clinical presentation of Plasmodium falciparum. Malar J 2012; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bui VT. Augmented IFN-γ and TNF-α induced by probiotic bacteria in NK cells mediate differentiation of stem-like tumors leading to inhibition of tumor growth and reduction in inflammatory cytokine release; regulation by IL-10. Front Immunol 2015; 6: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jewett A.A, Kos J, Kaur Ket al. Natural killer cells: diverse functions in tumor immunity and defects in pre-neoplastic and neoplastic stages of tumorigenesis. Mol Ther Oncolytics 2019; 16: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-γ): exploring its implications in infectious disease. BioMol Concepts 2018; 9: 64–79. [DOI] [PubMed] [Google Scholar]
- 70.Mah AY, Cooper MA. Metabolic regulation of natural killer cell IFN-γ production. Crit Rev Immunol 2016; 36: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo SJ, Chowdhury IH, Szczesny B, et al. Macrophages promote oxidative metabolism to drive nitric oxide generation in response to Trypanosoma cruzi. Infect Immun 2016; 84: 3527–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui F, Qu D, Sun R, et al. NK cell-produced IFN-γ regulates cell growth and apoptosis of colorectal cancer by regulating IL-15. Exp Ther Med 2020; 19: 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horiuchi T, Mitoma H, Harashima S, et al. Transmembrane TNFα: structure, function and interaction with anti-TNF agents. Rheumatology 2010; 49: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qu Y, Zhao G, Li H. Forward and reverse signaling mediated by transmembrane tumor necrosis factor-alpha and TNF receptor 2: potential roles in an immunosuppressive tumor microenvironment. Front Immunol 2017; 8: 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma N, Trinidad CV, Trembath APet al. NKG2D signaling between human NK cells enhances TACE-mediated TNF-α release. J Immunol 2017; 199: 2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simonetta F., Alvarez M., Negrin RS. Natural killer cells in graft-versus-host-disease after allogeneic hematopoietic cell transplantation. Front Immunol 2017; 8: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.López-Soto A, Gonzalez S, Smyth MJ, et al. Control of metastasis by NK cells. Cancer Cell 2017; 32: 135–154. [DOI] [PubMed] [Google Scholar]
- 78.Belizário JE, Neyra JM, Setúbal Destro Rodrigues MF. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. Innate Immun 2018; 24: 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol 2008; 9: 503–510. [DOI] [PubMed] [Google Scholar]
- 80.Jabrane-Ferrat N. Features of human decidual NK cells in healthy pregnancy and during viral infection. Front Immunol 2019; 10: 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol 2009; 30: 143–149. [DOI] [PubMed] [Google Scholar]
- 82.Giuliani E, Parkin KL, Lessey BA, et al. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014; 72: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sojka DK, Plougastel-Douglas B, Yang L, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife 2014; 3: e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burrows K, Chiaranunt P, Ngai L, et al. Rapid isolation of mouse ILCs from murine intestinal tissues. Method Enzymol 2020; 631: 305–327. [DOI] [PubMed] [Google Scholar]
- 85.Freud AG, Mundy-Bosse BL, Yu J, et al. The broad spectrum of human natural killer cell diversity. Immunity 2017; 47: 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mackay LK, Minnich M, Kragten NA, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016; 352: 459–463. [DOI] [PubMed] [Google Scholar]
- 87.Cibrian D, Sánchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur J Immunol 2017; 47: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salzberger W, Martrus G, Bachmann Ket al. Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71. PLoS One 2018; 13: e0201170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dogra P, Rancan C, Ma W, et al. Tissue determinants of human NK cell development, function, and residence. Cell 2020; 180: 749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar P, Thakar MS, Ouyang Wet al. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol 2013; 6: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scharenberg M, Vangeti S, Kekäläinen Eet al. Influenza A virus infection induces hyperresponsiveness in human lung tissue-resident and peripheral blood NK cells. Front Immunol 2019; 10: 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar P, Rajasekaran K, Palmer JM, et al. IL-22: An evolutionary missing-link authenticating the role of the immune system in tissue regeneration. J Cancer 2013; 4: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marquardt N, Kekäläinen E, Chen Pet al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(-)CD56(dim) cells. J Allergy Clin Immunol 2017; 139: 1321–1330. [DOI] [PubMed] [Google Scholar]
- 94.Podhorzer A, Machicote A, Belén Set al. Intrahepatic and peripheral blood phenotypes of natural killer and T cells: differential surface expression of killer cell immunoglobulin-like receptors. Immunology 2018; 154: 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mikulak J, Bruni E, Oriolo Fet al. Hepatic natural killer cells: organ-specific sentinels of liver immune homeostasis and physiopathology. Front Immunol 2019; 10: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jinushi M, Takehara T, Tatsumi Tet al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology 2007; 120: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fasbender F, Widera A, Hengstler JGet al. Natural killer cells and liver fibrosis. Front Immunol 2016; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kastenmüller W, Torabi-Parizi P, Subramanian Net al. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 2012; 150: 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Freud AG, Becknell B, Roychowdhury Set al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 2005; 22: 295–304. [DOI] [PubMed] [Google Scholar]
- 100.Morandi B, Bougras G, Muller WAet al. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol 2006; 36: 2394–2400. [DOI] [PubMed] [Google Scholar]
- 101.Chandrasekaran S, Chan MF, Li J, et al. Supernatural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2016; 77: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 2006; 5: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrega P, Bonaccorsi I, Di Carlo Eet al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol 2014; 192: 3805–3815. [DOI] [PubMed] [Google Scholar]
- 104.Law B, Wilkinson R, Wang Xet al. Interferon-γ production by tubulointerstitial human CD56bright natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int 2017; 92: 79–88. [DOI] [PubMed] [Google Scholar]
- 105.Cantoni C, Granata S, Bruschi Met al. Recent advances in the role of natural killer cells in acute kidney injury. Front Immunol 2020; 11: 1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodewald HR, Moingeon P, Lucich JL, et al. A population of early fetal thymocytes expressing Fc gamma RII/III contains precursors of T lymphocytes and natural killer cells. Cell 1992; 69: 139–150. [DOI] [PubMed] [Google Scholar]
- 107.King A, Balendran N, Wooding Pet al. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol 1991; 1: 169–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koopman LA, Kopcow HD, Rybalov Bet al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 2003; 198: 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.King A, Hiby SE, Verma S, et al. Uterine NK cells and trophoblast HLA class I molecules. Am J Reprod Immunol 1997; 37: 459–462. [DOI] [PubMed] [Google Scholar]
- 110.Williams PJ, Searle RF, Robson SC, et al. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol 2009; 82: 24–31. [DOI] [PubMed] [Google Scholar]
- 111.Robson A, Harris LK, Innes BAet al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012; 26: 4876–4885. [DOI] [PubMed] [Google Scholar]
- 112.Smith SD, Dunk CE, Aplin JDet al. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Path 2009; 174: 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lash GE, Otun HA, Innes BAet al. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Human Reproduction 2010; 25: 1137–1145. [DOI] [PubMed] [Google Scholar]
- 114.Gaynor LM, Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol 2017; 8: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bonaccorsi I, Spinelli D, Cantoni Cet al. Symptomatic carotid atherosclerotic plaques are associated with increased infiltration of natural killer (NK) cells and higher serum levels of NK activating receptor ligands. Front Immunol 2019; 10: 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paust S, Blish CA, Reeves RK. Redefining memory: building the case for adaptive NK cells. J Virol 2017; 91: e00169- 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457: 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Foley B, Cooley S, Verneris MRet al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 2012; 189: 5082–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hwang I, Zhang T, Scott JM, et al. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int Immunol 2012; 24: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee J, Zhang T, Hwang Iet al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015; 42: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schlums H, Cichocki F, Tesi B, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015; 42: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu LL, Landskron J, Ask EHet al. Critical role of CD2 Co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep 2016; 15: 1088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rölle A, Halenius A, Ewen EM, et al. CD2-CD58 interactions are pivotal for the activation and function of adaptive natural killer cells in human cytomegalovirus infection. Eur J Immunol 2016; 46: 2420–5. [DOI] [PubMed] [Google Scholar]
- 124.Zhang T, Scott JM, Hwang I, et al. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRγ deficiency J Immunol 2013; 190: 1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cooper MA, Elliott JM, Keyel PA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci 2009; 106: 1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goodier MR, Rodriguez-Galan A, Lusa Cet al. Influenza vaccination generates cytokine-induced memory-like NK cells: impact of human cytomegalovirus infection. J Immunol 2016; 197: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brillantes M, Beaulieu AM. Memory and memory-like NK cell responses to microbial pathogens. Front Cell Infect Microbiol 2020; 10: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun JC, Lanier LL. Is there natural killer cell memory and can it be harnessed by vaccination? NK cell memory and immunization strategies against infectious diseases and cancer. CSH Perspect Biol 2018; 10: a029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Horowitz A, Strauss-Albee DM, Leipold Met al. Genetic and environmental determinants of human NK cell diversity revealed by mass spectrometry. Sci Transl Med 2013; 5: pp. 208ra145 DOI: 10.1126/scitranslmed.3006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meyer-Monard S, Passweg J, Siegler Uet al. Clinical-grade purification of natural killer cells in haploidentical hematopoietic stem cell transplantation. Transfusion 2009; 49: 362–371. [DOI] [PubMed] [Google Scholar]
- 131.Lamers CH, Sleijfer S, van Steenbergen Set al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013; 21: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharma P, Hu-Lieskovan S, Wargo JAet al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Basar R, Daher M, Rezvani K. Next-generation cell therapies: the emerging role of CAR-NK cells. Blood Adv 2020; 4: 5868–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Granzin M, Wagner J, Köhl Uet al. Shaping of natural killer cell antitumor activity by ex vivo cultivation. Front Immunol 2017; 8: 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Denman CJ, Senyukov VV, Somanchi SSet al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 2012; 7: e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bae DS, Lee JK. Development of NK cell expansion methods using feeder cells from human myelogenous leukemia cell line. Blood research 2014; 49: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parkhurst MR, Riley JP, Dudley ME, et al. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011; 17: 6287–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shin MH. NK cell-based immunotherapies in cancer. Immune Netw 2020; 20: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295: 2097–2100. [DOI] [PubMed] [Google Scholar]
- 140.Shah NN, Baird K, Delbrook CPet al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T cell-depleted stem cell transplantation. Blood 2015; 25: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chambers AM, Lupo KB, Matosevic S. Tumor microenvironment-induced immunometabolic reprogramming of natural killer cells. Front Immunol 2018; 9: 2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Castriconi R, Cantoni C, Della Chiesa Met al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA 2003; 100: 4120–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marcenaro E, et al. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J Immunol 2005; 174: 3992–3998. [DOI] [PubMed] [Google Scholar]
- 144.Pietra G, Manzini C, Rivara S, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72: 1407–1415. [DOI] [PubMed] [Google Scholar]
- 145.Balsamo M, Vermi W, Parodi M, et al. Melanoma cells become resistant to NK-cell-mediated killing when exposed to NK-cell numbers compatible with NK-cell infiltration in the tumor. Eur J Immunol 2012; 42: 1833–1842 [DOI] [PubMed] [Google Scholar]
- 146.Habif G, Crinier A, André Pet al. Targeting natural killer cells in solid tumors. Cell Mol Immunol 2019; 16: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng M, Zhang J, Jiang Wet al. Natural killer cell lines in tumor immunotherapy. Front Med 2012; 6: 56–66. [DOI] [PubMed] [Google Scholar]
- 148.Kornbluth J, Flomenberg N, Dupont B. Cell surface phenotype of a cloned line of human natural killer cells. J Immunol 1982; 129: 2831–2837. [PubMed] [Google Scholar]
- 149.Jochems C, Hodge JW, Fantini Met al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016; 7: 86359–86373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nham T, Poznanski SM, Fan IYet al. Ex vivo-expanded natural killer cells derived from long-term cryopreserved cord blood are cytotoxic against primary breast cancer cells. J Immuno 2018; 41: 64–72. [DOI] [PubMed] [Google Scholar]
- 151.Spanholtz J, Tordoir M, Eissens D, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PLoS One 2010; 5: e9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hermanson DL, Bendzick L, Pribyl L, et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells 2016; 34: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Giudice A, Trounson A. Genetic modification of human embryonic stem cells for derivation of target cells. Cell Stem Cell 2008; 2: 422–433. [DOI] [PubMed] [Google Scholar]
- 154.Xie F, Ye L, Chang JC, et al. Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res 2014; 24: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hartmann J, Schüßler-Lenz M, Bondanza Aet al. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med 2017; 9: 1183–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parihar R, Rivas C, Huynh M, et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res 2019; 7: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang P, Zhao S, Wu Cet al. Effects of CSF1R-targeted chimeric antigen receptor-modified NK92MI & T cells on tumor-associated macrophages. Immunotherapy 2018; 10: 935–949. [DOI] [PubMed] [Google Scholar]
- 158.Chidrawar SM, Khan N, Chan YL, et al. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 2006; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]