Abstract
Neutralizing tumour necrosis factor (TNF) antibodies have been widely used to treat inflammatory bowel disease (IBD) in the clinical practice. In this review, the principal biomarker analysis revealed that faecal calprotectin, C-reactive protein, serum or mucosal concentrations of anti-TNF monoclonal antibodies (mAbs) and antibodies to anti-TNF mAbs are commonly used as current biomarkers in the evaluation of anti-TNF therapeutic efficacy. However, mucosal cytokine transcripts. microRNAs, proteomics and faecal and mucosal gut microbiota profile and mucosal histological features are reported to be novel candidates of biomarkers with high clinical utility in the evaluation of anti-TNF therapeutic efficacy in patients with IBD. Therefore, a robust validation of novel promising biomarkers and comparison studies between current used and novel biomarkers are urgently required to improve their value in the evaluation of therapeutic efficacy and optimization of personalized medicine and identification of IBD candidates for anti-TNF therapy in future clinical practice.
Keywords: Biomarker, Inflammatory bowel disease, Ulcerative colitis, Crohn's disease, Anti-TNF therapy
1. Introduction
It is widely known that inflammatory bowel disease (IBD), principal types include Crohn's disease (CD) and ulcerative colitis (UC), is a group of chronic inflammatory disorders that mainly affect the gastrointestinal tract. Although the aetiology for IBD remains to be fully identified, accumulative evidence has strongly suggested that environmental factors, genetic predisposition, and dysregulated immune response are strongly associated with the development of IBD [[1], [2], [3]].
Clinically, the goals of IBD treatment are to induce and maintain disease remission and mucosal healing, and then improve the patient's quality of life. Tumour necrosis factor (TNF)-α is a proinflammatory cytokine and plays an essential role in the induction and maintenance of inflammation in the intestine [4]. As a result of this, block of TNF signal by anti-TNF monoclonal antibodies (mAbs) becomes a main therapeutic strategy for severe steroid refractory or dependant IBD patients today [4]. The evaluation of therapeutic efficacy in IBD patients with anti-TNF mAbs is extremely important for optimise therapeutic strategy [5]. Clinically, there is currently no single “gold standard” approach to assess anti-TNF therapeutic efficacy in patients with either CD or UC. Instead, it is made based on a combination of disease activity index, endoscopic observation with histological examination and biomarkers in IBD patients with anti-TNF treatment [6]. In addition, laboratory biomarkers provide a reproducibly quantifiable tool for the evaluation of disease status and therapeutic efficacy in IBD patients. Therefore, the discovery of reliable biomarkers would be extremely useful in the clinical practice and adds a great help to assess therapeutic efficacy, minimize side-effects, and optimize personalized medicine and strategy for identifying IBD patients who will benefit from anti-TNF therapy [7,8].
In this study, we conducted a review that focus on current and novel biomarkers in the context of biological therapies against TNF- in patients with IBD.
2. A brief looking back the role of TNF-alpha in the pathogenesis of IBD
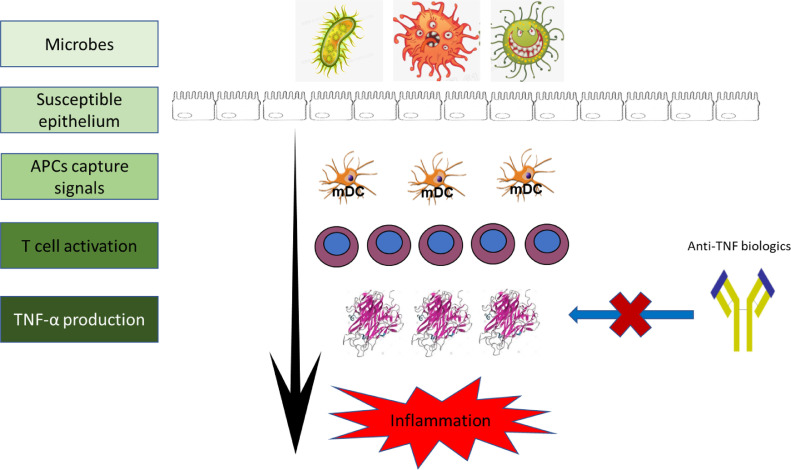
TNF-α is a pleotropic cytokine with a potential proinflammatory capacity and has been recognized as a triggering cytokine in the induction of colorectal inflammation and involved in the pathogenesis of IBD [4] (see Fig. 1). Today, anti-TNF mAbs have been widely introduced to treat severe steroid refractory or dependant IBD patients in clinical practice. Clinical evidence showed that anti-TNF therapy result in an improved clinical success including a rapid disease remission, a high rate of mucosal healing, and improved quality of life in ~ 60% of IBD patients. However, clinical studies reported that 20~40% of IBD patients did not respond to anti-TNF-α induction therapy, and ~50% of primary response IBD patients may subsequently loss of response to anti-TNF-α maintaining therapy after induction period [9]. Moreover, anti-TNF-α bioagents are very expensive and have several site effects. Therefore, how to evaluate therapeutic response and identify IBD patients who will benefit from anti-TNF therapy becomes important [8,9].
Fig. 1.
Schematic summary of TNF-α’s role and anti-TNF therapy in the pathogenesis of IBD.
Current data suggested that TNF-α plays a central role in the pathogenesis of IBD, and block of TNF-α signal by anti-TNF mAbs could significantly suppress inflammation and improve mucosal healing.
3. Current commonly used biomarkers in the evaluation of anti-TNF therapeutic response in patients with IBD
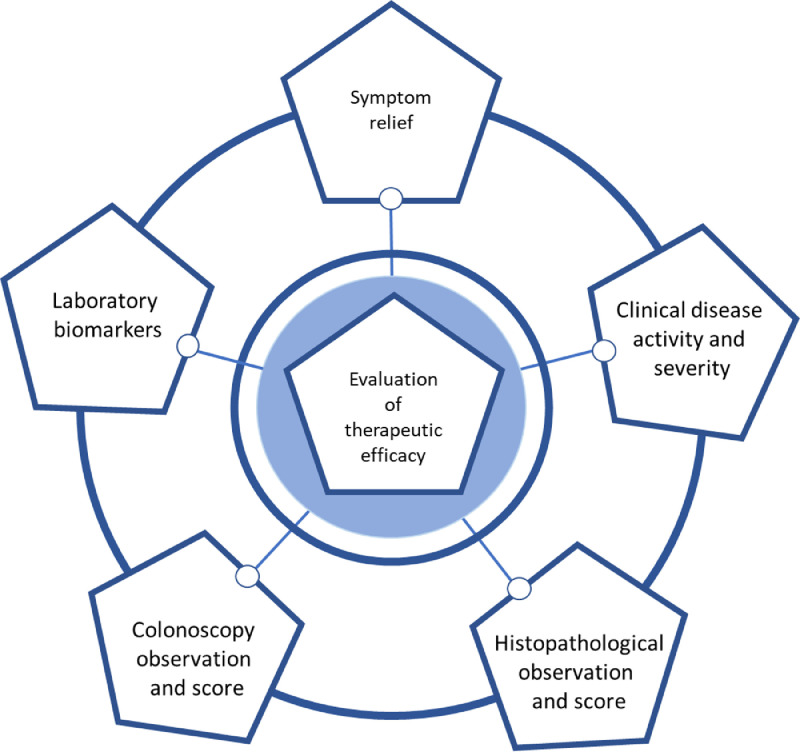
Researchers have previously identified multiple biomarkers such as faecal calprotectin (FC), C-reactive protein (CRP), levels of anti-TNF-α agent level and anti-drug antibody to provide reproducible, quantitative tools to assess disease severity and status, and therapeutic efficacy in patients with IBD (see Fig. 2).
Fig. 2.
Schematic summary of the current approaches used in the evaluation of different therapeutic efficacy in patients with IBD.
Current approaches used in the evaluation of anti-TNF therapy in patients with IBD are based on multiple clinical parameters e.g., symptom relief, changes of severity evaluated by clinical disease activity and severity index, colonoscopy evaluation with histological examination and laboratory biomarkers.
3.1. FC
Clinical results revealed that significantly reduced FC level might predict disease remission and mucosal healing in patients with IBD who received various therapies including anti-TNF mAbs [10]. Studies showed that the levels of FC were well correlated with endoscopic scores of disease activity in IBD patients who received anti-TNF mAbs [11,12]. Further studies reported that a high level of pre-treatment baseline FC could predicate a high rate of nonresponse [13] and a lower rate of clinical remission or mucosal healing in IBD patients undergoing anti-TNF therapy [14,15]. Colombel et al. showed that FC concentration is a reliable biomarker to predict treatment failure and decision to escalate in CD patients with adalimumab treatment [16]. Increased CRP concentration could show a slightly lower predicating significance than FC concentration. Increased concentration of FC could significantly influence the decision to escalate after CD patients received adalimumab treatment at different week-points [16].
3.2. Serum levels of CRP
The importance of CRP in anti-TNF induction therapy has been intensively studied. Two clinical studies reported that a high baseline concentration of CRP was associated with a reduced rate of clinical remission and response to anti-TNF mAbs in steroid refractory or moderate-to-severe UC patients [17,18]. Normalization of CRP levels at week 14 could predict rates of maintained response or remission in CD patients with IFX maintenance therapy [19]. Acute severe ulcerative colitis (ASUC) is a clinical emergency condition that needs a prompt therapeutic intervention [20]. Choy et al. reported that the CRP/albumin ratio following IFX salvage could predict the therapeutic response and identify who at high risk of colectomy in patients with ASUC [21].
3.3. Serum levels of anti-TNF mAbs
The effective level of anti-TNF mAbs can be reflected in the level of drugs in the blood. In a systematic review with meta-analysis, Moore et al. [22] found that a trough threshold of IFX during maintenance (> 2 µg/ml) was associated with a greater probability of remission and mucosal healing in IBD patients with IFX therapy [22]. Similarly, a high trough serum level of adalimumab could predicate a better long-term clinical outcome in CD patients [23,24]. In contrast to above findings, Hinojosa et al. [25] reported that low serum levels of adalimumab were correlated with loss of clinical and endoscopic response and low rate of mucosal healing in IBD patients with either adalimumab induction or maintenance therapies.
3.4. Anti-drug antibody
Considerable evidence has shown that the administration of anti-TNF mAbs into human body may induce the production of anti-drug antibody, and levels of anti-drug antibody are associated with both short and long-term response to anti-TNF mAbs in IBD patients [26,27].
Casteele et al. [28] revealed that a cut-off anti-drug antibody value <3.15 U/mL was correlated with disease remission in CD patients with IFX therapy. Moreover, Brandse et al. [29] underlined that higher serum concentrations of anti-drug antibody in IBD patients with anti-TNF therapy may predicate a low quality of life and more complications. In addition, Kharlamovaa et al. showed that measuring drug and anti-drug antibody concentrations in IBD patients with anti-TNF treatment could help to identify anti-drug antibody positive IBD patients, which could lead to a more personalized and efficient treatment regime [30]. The drug-tolerant assay method enabled to detect anti-drug antibody earlier and regardless of drug level at time of sampling [30]. Further studies showed that the detective rate of anti-drug antibody in the drug-tolerant assay was much higher than that in the drug-sensitive assay (63% vs. 21%) [31], which allows a better follow-up of anti-drug antibody concentration changes and observation of true transient versus persistent anti-drug antibody in IBD patients with anti-TNF therapy.
Current biomarkers used in the evaluation of anti-TNF therapeutic efficacy in IBD patients are summarized in Table 1 and Fig. 2.
Table 1.
Significance of commonly used current biomarkers in the evaluation of anti-TNF therapeutic efficacy in patients with IBD in the clinical practice.
| Biomarker | Decreased or normalized in CD |
Decreased or normalized in UC |
||||
|---|---|---|---|---|---|---|
| Response | Remission | Mucosal healing | Response | Remission | Mucosal healing | |
| Faecal calprotectin | + [13] | + [79] | + [11] | + [79] | + [79] | + [12] |
| CRP | + [75, 80] | + [81] | + [82] | + [83] | + [19] | + [82] |
| Serum levels of anti-TNF drugs | + [22, 25, 84, 85] | + [[22], [23], [24], 28] | + [22, 25] | + [86] | + [86] | + [87] |
| Anti-drug antibody | + [88, 89] | + [28, 90] | + [89] | + [88] | + [91] | + [89] |
(reference ID].
4. Novel putative biomarkers
The specificity of current biomarkers likes CRP are relatively low; in addition, the sensitivity of these biomarkers between patients with CD and UC are different, which limits their clinical values in the evaluation of anti-TNF therapeutic efficacy in patients with IBD. Moreover, the expression levels of those biomarkers in IBD patients with anti-TNF therapy are not always consistent with changes of disease activity and the rate of mucosal healing after treatment. Therefore, development and identification of novel biomarkers to promote personalised treatment of IBD patients with anti-TNF therapy is needed. Which could better identify patients whose IBD is refractory to the anti-TNF therapy, help clinicians to prevent unnecessary anti-TNF-α therapy, accelerate the process of providing effective for each patient.
4.1. Mucosal transcript landscape
4.1.1. TNF-α
Anti-TNF mAbs could significantly reduce the expression level of TNF-α and suppress the inflammation, therefore the changed mucosal TNF-α transcript level might be used as a promising biomarker to assess the anti-TNF efficacy. More recently, we have validated a correlation between mucosal TNF-α transcript levels and response to anti-TNF mAbs in UC [7]. Validation data demonstrated that baseline mucosal TNF-α transcript level was a better biomarker with a high-test reliability for the prediction of clinical outcomes than other clinical parameters e.g., calprotectin, the UC disease activity index (UCDAI) score, Mayo endoscopic score in patients with UC [7].
Consequently, we evaluated whether the mucosal levels of TNF-α transcript after IFX treatment in patients with UC could predicate the therapeutic response. We found that the mucosal TNF-α transcript level, in responders to IFX therapy, was significantly reduced, in which a reduced mucosal TNF-α transcript level was well correlated to the disease remission and mucosal healing rate examined by colonoscopy observation in both UC and CD responders with anti-TNF introduction therapy [32–34]. Furthermore, normalization of mucosal TNF-α transcript levels could predicate a good long-term disease remission in UC responders with discontinuing anti-TNF therapy [35].
4.1.2. IL-17A
TH17 signature cytokine IL-17A has been repeatedly shown to be a valuable candidate biomarker to assess anti-TNF efficacy in patients with IBD. Our data showed that UC patients with a higher mucosal IL-17A transcript level tended to have a higher rate of disease remission in response to three IFX infusions, showing a predicate value in UC patients received IFX induction therapy [36]. Nevertheless, decreased mucosal IL-17A transcript level was associated with complete mucosal healing defined by colonoscopy examination in CD patients received adalimumab treatment [33], and normalized mucosal IL-17A transcript level after IFX therapy appeared to predicate a long-term disease remission in patients with CD [34].
4.1.3. IL-7 receptor (IL-7R)
Belarif et al. [37] reported that increased mucosal IL-7R transcript levels were associated with non-responders of severe CD and UC patients to either immunosuppressive/corticosteroid, anti-TNF, or anti-α4β7 therapies. High expression of both IL7R and IL-7R signalling signature in the colon before treatment was closely associated with nonresponse rate to anti-TNF therapy in patients with IBD. Their findings suggest that an overexpression of mucosal IL-7R correlated strongly to failure of anti-TNF therapy in patients with CD and UC. Its value as a biomarker candidate remains to be validated.
4.1.4. Cytokine oncostatin M (OSM) and its receptor (OSMR)
Cytokine OSM belongs to IL-6 subfamily and can be produced by several types of immune and stromal cells. More recently, Kim et al. [38] reported that high mucosal levels of both OSM and its receptor OSMR were strongly associated with the inflammation degree and disease severity in patients with IBD. Furthermore, anti-TNF-resistant IBD mice lacking OSM developed less inflammation and colitis than wild-type mice [38]. Genetic deletion or pharmacological blockade of OSM could significantly attenuate the process of colitis. In patients with IBD, a high expression level of OSM was associated with the failure of anti-TNF (IFX and golimumab) therapies [38]. Therefore, OSM might be considered as a biomarker candidate in the evaluation of anti-TNF therapeutic efficacy in patients with IBD [39].
4.1.5. Other cytokines
Our data demonstrated that in addition to decreased TNF mRNA level, IFX therapy in patients with UC also induced a decreased mRNA levels of interferon (IFN)-γ in inflamed mucosa [32]. Mavragani et al. [40] have reported that levels of type I and II IFN genes in the serum could predict the response to IFX in patients with IBD. Responders tended to have significant decreased level of both types of interferon [40]. Their studies confirmed that both Types I and II IFN gene can be the candidates of biomarker for IFX therapy.
4.2. Triggering receptor expressed on myeloid cells 1 (TREM1)
Studies showed that TREM-1 was involved in the development of inflammation and anti-TNFα responsiveness mechanism, and levels of TREM-1 in peripheral blood and inflamed mucosa were a potential biomarker of anti-TNF therapeutic efficacy in patients with IBD [41,42]. Gaujoux et al. [42] reported baseline serum TREM-1 (sTREM1) concentration may predicate anti-TNF response with very high accuracy in patients with CD, responders had a higher level of TREM-1 than non-responders. In inflamed mucosa, TREM1 expression level was also decreased in IFX responders [42]. However, Verstockt et al. observed a opposite connection between sTREM-1 level and mucosal healing. CD patients with anti-TNF therapy (adalimumab and infliximab) who achieved mucosal healing after 6 months tended to have a lower baseline sTREM-1 levels than those non-responders [41]. Recently, they further showed that low sTREM1 level could predict anti-TNF response in both CD and UC [43]. Such discrepancy between two results might potentially be attributed to differences in several aspects e.g., sample size, definition standards of response and different ethnicity [41]. Thus, whether the expression level of sTREM-1 could be an accurate biomarker for the evaluation of anti-TNF response remains to be validated in well-designed studies with large sample size.
4.3. Faecal and mucosal microbiota profile
Faecal microbiota was a rich source of biomarkers that were associated with intestinal inflammation [44]. Magnusson et al. [45] reported that the gut microbiota profile between responders and non-responders was different. Responders tended to have a lower dysbiosis indexes and a higher abundance of Faecalibacterium prausnitzii than non-responders at baseline. In addition, an increased abundance of Faecalibacterium prausnitzii was observed in responders during the IFX/adalimumab induction therapy [45]. Therefore, this finding might discriminate responders from non-responders at baseline. The metabolomic analyses of faecal samples showed that metabolite exchange was significantly associated with later clinical remission in patients with IBD who received anti-TNF therapy [44]. Vatn and European colleagues [46] reported that differences were observed for some bacterial markers, but they did not find a statistical significance between responders and non-responders in IBD patients with anti-TNF therapy. Dovrolis et al. [47] found that the populations of several bacterial genera in IBD patients were dramatically decreased after IFX therapy regardless of response. Their study further revealed that IFX treatment could significantly impact the gut microbial composition and the inflamed tissue transcriptome in IBD patients. In which, enterotypes at baseline correlates with transcriptome changes and could differentiate IFX responders versus non-responders and predicts anti-TNF response in patients with IBD [47]. Other studies confirmed that altered gut microbiota profile could reflect the rate of biotherapy response in patients with CD [48]. More recently, Wang et al. [49] examine the changed faecal microbiota profile in paediatric CD patients with anti-TNF therapy. They found that IFX in paediatric CDs could increase the bile salt hydrolases-producing bacteria, higher abundances of Methylobacterium, Sphingomonas, Staphylococcus, and Streptococcus were associated with a higher rate of sustained response in these patients with IFX therapy [49]. In a systematic review with 8 anti-TNF studies in patients with IBD, Estevinho et al. [50] indicated that the microbiomes of faecal or colon samples taken from IBD patients, in response to anti-TNF therapies, showed a decreased abundance of Escherichia and Enterococcus bacteria, but increased abundance of short-chain fatty acid-producing bacteria. In addition, higher microbial diversity at baseline or throughout treatment was associated with response to anti-TNF therapy [50].
4.4. MicroRNAs
MicroRNAs (miRNAs) are small, non-coding RNAs and are involved in the proinflammatory cytokine production and inflammation process as seen in IBD. Thus, circulating and faecal miRNAs have been considered as novel biomarker candidates that predict therapeutic response in patients with IBD [51]. For example, Batra et al. validated that the expression of seven miRNAs showed in remarkable changes after treatment in responders but not in non-responders in a small cohort of paediatric IBD with diverse treatments including anti-TNF mAbs [52]. However, a Greek group evaluated the association of miRNA polymorphisms with anti-TNF treatment response [53] did not detect any correlations between studied miRNA (miR-146 rs2910164, miR-196a rs11614913, miR-221 rs113054794 and miR-224 rs188519172) polymorphisms and patients’ response to anti-TNF mAbs in 107 CDs. Therefore, the profile of serum or mucosa miRNAs as promise biomarkers in analysing the therapeutic response to anti-TNF mAbs in IBD patients remains to be investigated in future clinical practice.
4.5. Proteomics
The interaction between environmental factors and susceptible genes will inevitably result in a significant alteration of proteome profile in both serum and inflamed mucosa of IBD, which has been recently considered as the potential diagnostic and differentiate biomarkers for IBD [54]. For instance, Meuwis et al. reported that 4 serum proteins (platelet aggregation factor 4, haptoglobin a2, fibrinopeptide A, and myeloid-related protein 8) were associated with acute-phase inflammation and tended to be high sensitivity and specificity diagnostic biomarkers in patients with IBD [55]. Zhang et al. [56] reported that serum proteomic profiling may have a diagnostic value in differentiating IBD from intestinal tuberculosis. Starr et al. [57] showed that a panel of five proteins as candidate biomarkers that could distinguish patients with paediatric IBD from controls, and a panel of 12 proteins that enable differentiation between CD and UC [57]. Furthermore, Drobin et al. [58] have identified that 13 serum proteins were associated with cytokine signalling, immune-metabolic regulation, and immune cell activation in IBD patients.
Changed inflammation-related proteomic profiling could also be biomarker candidates in the evaluation of therapeutic response in IBD. Medina-Medina et al. [59] identified that 17 proteins regulated by acetylation had a predicting value for primary response to anti-TNF therapy and 4 proteins were potential biomarkers of loss of response in 54 CD patients. D'Haens et al. [60] recently developed a validation test to identify remission in CD patients with biologicals, based on serum levels of 13 proteins (ANG1, ANG2, CRP, SAA1, IL-7, EMMPRIN, MMP1, MMP2, MMP3, MMP9, TGFA, CEACAM1, and VCAM1) combined with the endoscopic healing index analysis. Their validation results showed that this test could accurately identify remission in CD patients, which were comparable to measurement of FC and higher than measurement of serum CRP [60]. Pierre et al. [61] identified that protein biomarker candidates could be distinctly classified as biomarkers associated with the risk of short-term and biomarkers associated with mid/long-term relapse in CD patients with IFX biological treatment, which related to different pathophysiological processes. Their results might help the clinicians to select the protein biomarker candidates for the evaluation of short-term and mid/long-term relapse in response to anti-TNF mAbs. However, Telesco et al. [62] reported that the specificity of a panel of 13 candidate biomarkers at gene levels in predicating mucosal healing response to golimumab treatment in patients with UC was low and not sufficient for the clinical utility.
4.6. Genomic
Susceptibility loci in IBD can be identified [63], the value of different genomic biomarkers such as functional polymorphisms in the relevant genes encoding in predicating response to TNF therapy in patients with IBD has been evaluated [64,65]. Since the target of anti-TNF mAbs is on TNF, the functional polymorphisms for TNF and TNF receptor superfamily have attracted much attention and researched [63,64]. Studies revealed that polymorphisms in cytokine pathways (IL-1B, IL-6, IFN-gamma, TNFRSF1A, NLRP3, IL1RN, IL-18, and JAK2), the NFκB pathway (TLR2, TLR4 and NFKBIA) were closely associated with response to anti-TNF therapy in patients with IBD [64,66]. Polymorphisms in encoding many other genes such as HLA-DQA1*05 have also been reported to be associated with response to anti-TNF therapy [67].
Recently, the development of genomic-relevant serological antibodies in patients with IBD has been reported. Degenhardti et al. [68] showed that the tested serological antibodies anti-GP2 IgA and IgG were associated with CD and had a high discriminatory capability for CD versus UC. In addition, a genome wide association study of antibody expression in their cohort could identify distinct loci associated with levels of anti-GP2 isoform beta IgG and IgA, many of which are known susceptibility loci for IBD [68]. However, their significance in the evaluation of anti-TNF efficacy in CD verse UC has not been studied. Finally, biomarkers might also play a key role in differentiating paediatric- from adult-onset IBD. For example, genome-wide association study (GWAS) has revealed the single-nucleotide polymorphism differences in the polygenic architecture between paediatric- and adult-onset IBD [69]. Which could be the potential genetic biomarkers to study the role and significance of accumulated rare and deleterious variants in affected paediatric- and adult-onset.
4.7. Other new biomarkers
The therapeutic response to anti-TNF mAbs may also induce changes of mucosal histology. We found that densities of T lymphocytes and macrophages were significantly decreased in UC patients with healed mucosa after IFX introduction treatment [32]. Li et al. [70] reported that both UC and CD patients with IFX therapy showed a sustained decreased population of regulatory T cells (Tregs) in peripheral blood and downregulated expression level of FoxP3 (a marker for Tregs) transcript in inflamed mucosa, particularly in UC and CD responders. However, as many immune cell and new subsets of lymphocytes such as TH9 and TH22 have been identified in the colorectal mucosa [71], their exact role in the pathogenesis of IBD and therapeutics remains unclear.
More recently, Osterman et al. [72] reported that ileal microvillar length has a significance to predict the therapeutic response to ustekinumab (mAbs to IL-12 and IL-23) and Vedolizumab (mAbs to integrin) in patients with CD. However, the predicative value of ileal microvillar length in IBD patients with anti-TNF mAbs remains to be investigated. Andreou et al. [73] showed that serum B-cell activating factor (BAF) concentration at baseline in CD response to IFX was higher than CD non-response to IFX. Which was associated with its polymorphisms. Importantly, serum BAF concentration in CD responders was remarkably reduced after IFX [73]. Their findings suggest that serum BAF might have a predicating significance for anti-TNF therapy. Reports indicated that low serum albumin levels could predicate a worse response to IFX or golimumab in patients with UC [18,74].
For the convenience for readers, we have summarized selected novel biomarkers in Table 2.
Table 2.
Novel putative biomarkers in the evaluation of anti-TNF therapeutic efficacy in patients with IBD.
| Biomarker | Decreased or normalized in CD |
Decreased or normalized in UC |
||||
|---|---|---|---|---|---|---|
| Response | Remission | Mucosal healing | Response | Remission | Mucosal healing | |
| Mucosal transcripts | ||||||
| TNF-α | + [33, 34] | + [33, 34] | + [33, 34] | + [35] | + [35] | + [35] |
| IL-17A | + [34] | + [34] | + [34] | + [33, 36] | + [33, 36] | + [33, 36] |
| OSM | + [38, 39] | + [38, 39] | + [38, 39] | + [38, 39] | ||
| IL-7R | + [37] | + [37] | ||||
| TREM1 | + [41, 43] | + [41, 43] | ||||
| miRNAs | + [52, 53] | + [52] | ||||
| Faecal and mucosal microbiota profile | + [44, 46, 48, 49] | + [44], [45], [46] | ||||
| Proteomics | + [59, 76] | |||||
| Genomic | + [63, 64] | + [63, 64] | ||||
TNF-α: tumour necrosis factor-α; IL-17A: interleukin-17A; miRNAS: MicroRNAs; OSM: Oncostatin M; IL-7R: interleukin-7 receptor; TREM1: Triggering receptor expressed on myeloid cells 1.
(reference ID).
5. Combination, validation and comparison studies between current and novel biomarker in the evaluation of anti-TNF therapeutic response in IBD
The use and significance of different biomarkers in the clinical practice might be varied. Some biomarkers such as genomic, RNA and protein levels, and FC concentration have been frequently used to assess pre-treatment non-response to anti-TNF mAbs in patients with CD [16,65]. However, the level of drugs and ADA are often applied to the monitoring of anti-TNF therapy. Mucosal TNF transcript is a biomarker that not only help in evaluation of therapeutic response, but also help to identify the candidates who should prior to the administration and personation of anti-TNF mAbs in patients with UC [7]. Moreover, the accuracy and power of individual biomarker in the evaluation of therapeutic response in patients with IBD might be low. One of strategies to improve the power of biomarkers in clinic is to use the combination of different biomarkers in the evaluation of anti-TNF therapeutic response in patients with IBD [6]. Roblin et al. [75] reported that CRP levels, together with IFX trough levels and anti-drug antibody levels were associated with the rate of loss response to infliximab in patients with IBD. However, how to optimally group these different biomarkers for clinical use is a particular future task.
Vatn et al. [46] have compared faecal microbiota profile with other inflammatory biomarkers such as CRP and faecal calprotectin in patients with IBD, they observed a strong correlation between the degree of dysbiosis and the faecal calprotectin levels. Alterations in microbiota composition have previously been associated with response to anti-TNF therapy [45]. However, they could not validate such correlation between changed microbiota and anti-TNF therapeutic response [46]. More recently, our group have validated the value of mucosal TNF transcript as a reliable biomarker in the evaluation of anti-TNF therapeutic response in patients with UC [7]. We were able to show that baseline mucosal TNF-α transcript level was a better biomarker than other clinical parameters e.g., calprotectin, the UC disease activity index (UCDAI) score, Mayo endoscopic score in patients with UC [7]. Meuwis et al. [76] validated that changed serum proteins could predict the therapeutic response to anti-TNF in a small cohort of CD patients. Haberman et al. validated that a subset of severity genes and changed microbiota profile are associated with response to anti-TNF mAbs in adult UCs [77]. However, the sensitivity and specificity of most of them require validation studies on a larger cohort of patients (see Summarized in Fig. 3). Therefore, validation of current and novel biomarkers in well-designed studies with large patient size should be considered in the future. In addition, whether one biomarker is better than another biomarker in reflecting disease activity change is important for its use in the evaluation of therapeutic response in patients with IBD. To the best of our knowledge, high-quality comparison studies between commonly used current and novel biomarkers are waiting to be conducted. These works are extremely important for the selection and development of ideal biomarkers, optimization of personal medicine and strategy for identifying IBD patients who will benefit from anti-TNF therapy in the future clinical practice.
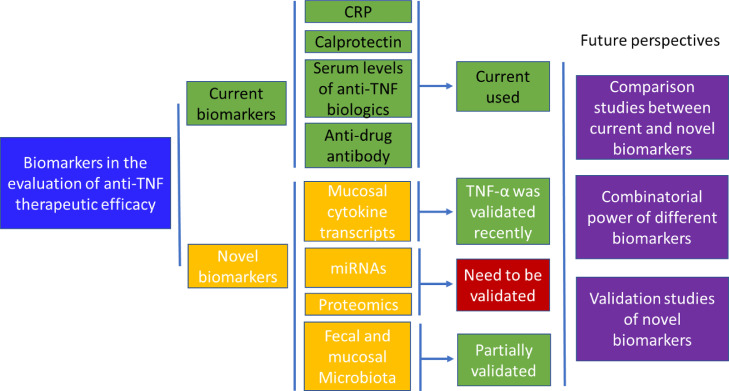
Fig. 3.
Schematic summary of the statues of current and novel laboratory biomarkers used in the evaluation of anti-TNF therapy in patients with IBD
Many laboratory factors have been used as biomarkers in the evaluation of anti-TNF therapeutic response. However, the sensitivity and specificity of most of them have not been well validated in clinical studies with a larger sample size. Future comparison studies between current and novel biomarkers and the combination power of different biomarkers in clinic in the evaluation of anti-TNF therapeutic response in patients with IBD are waiting to be conducted.
6. Limitations of this review
Due to the high number of biomarkers and publications in biomarker research field, we focused this review only on commonly used and selected novel biomarkers for the evaluation of anti-TNF therapeutic efficacy in patients with IBD. We could have missed some promising novel biomarkers. Future reviews could be extended to include these biomarkers in the evaluation of anti-TNF efficacy when the data is sufficient. Furthermore, the accuracy and potential value for these novel biomarkers in the evaluation of response to anti-TNF therapy in IBD patients need to be validated in large sample size cohorts of IBD patients. Finally, because of sample size, anti-TNF mAbs and methodological heterogeneity of included studies, we did not conduct a formal assessment of quality and risk of bias of included studies and a meta-analysis for individual biomarkers was not carried out in this review.
7. Conclusions
Anti-TNF strategy has been widely used and shows a significant benefit in the management of severe steroid refractory or dependant IBD patients. How to find reliable laboratory biomarkers may significantly help the evaluation, improvement and personalization of anti-TNF therapy in the clinical practice. Most biomarkers seem to reflect a generalized inflammation, are not IBD specific. Therefore, their usage and clinical significance in IBD patients with different types of anti-TNF mAbs are non-different.
Many new biomarkers are reported. However, most of them reflect a generalized inflammation, are not CD or UC specific. Therefore, the inflammatory biomarkers usually cannot differentiate CD and UC. One of the main works in the future is to validate these novel biomarkers in well-designed studies with large size of IBD patients. Furthermore, comparison studies between current and novel biomarkers are necessary, which may help the clinicians to select reliable biomarkers, better evaluate efficacy and personalize medicine of anti-TNF therapy in the future clinical practice.
8. Outstanding questions
The importance of biomarkers in the evaluation of therapeutic efficacy in patients with IBD has been intensively studied. Current analysis provides new insights and challenges to optimize personal anti-TNF strategy (precision medicine) in patients with IBD.
8.1. Optimize the use of different biomarker for clinical practice
Can we classify the laboratory biomarkers into categories such as surveillance of disease activity, monitoring therapeutic response and predicating prognosis [78]?
8.2. Biomarkers in the candidate selection prior to for the administration of anti-TNF mAbs
Some biomarkers such as mucosal TNF transcript could predicate the response rate to anti-TNF mAbs [34]. Should we measure the levels of the biomarker before we decide to prescribe anti-TNF mAbs to a patient? Should those patients with a high level of the biomarker be in the priority of consideration for anti-TNF therapy?
8.3. Optimizing dose and duration of anti-TNF mAbs
The levels of some biomarkers such as FC and serum CRP could predicate the failure rate in IBD patients with anti-TNF therapy [16]. How to identify a correlation between the dose/duration of anti-TNF mAbs and the biomarker levels?
To improve the accuracy and power of individual biomarker in the clinic, one of strategies is to combinate different biomarkers in the evaluation of anti-TNF therapeutic response in patients with IBD. However, how to optimally group different biomarkers for clinical use remains to be investigated.
8.4. Search strategy and selection criteria
A literature research was electronically carried out in academic databases PubMed, MEDLINE and Google scholar by the authors using the search terms “biomarker”, “inflammatory bowel diseases”, “ulcerative colitis”, “Crohn's disease”, “anti-TNF treatment”, “efficacy”, “predicators”, “C-reactive protein”, “calprotectin”, "infliximab level", "anti-infliximab”, "adalimumab level" and "anti-adalimumab". After screening the abstracts, relevant publications from appropriate papers added as additional literature sources.
We used the following eligibility criteria to include publications in this systematic review (2): (1) article written in English and full-text available; (2) only studies performed in humans; (3) individual patient information; (4) studies only published as abstract or not in English were excluded.
The electronic literature search resulted in a total of 2651 abstracts (2641 in English from MEDLINE, PUBMED and Google scholar, 10 full-text publications in English by cross-reference search), latest search date: 31st of May 2020. Of which 91 full-text publications were finally included for analysis after excluding publications not meeting the selection criteria.
Contributors
GC had the idea for this systematic review and performed the electronic search for literatures, QF, ZL and RG performed literature selection, data extraction and analysis. JF joined the data analysis and discussion. All the listed authors contributed to this manuscript in writing and final approvement.
Declaration of Competing Interest
The authors declare no conflicts of interests.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (Program No. 81071969) and the Medical Research Program, Northern Norway Regional Health Authority (Helse Nord RHF), Norway (Program No. SFP-44-04). The funders did not play any role in paper design, data collection, data analysis, interpretation, writing of the paper. We apologize for not being able to cite all the excellent studies and reviews due to space limitations.
References
- 1.Atreya R., Neurath M.F. IBD pathogenesis in 2014: molecular pathways controlling barrier function in IBD. Nat Rev Gastroenterol Hepatol. 2015;12(2):67–68. doi: 10.1038/nrgastro.2014.201. [DOI] [PubMed] [Google Scholar]
- 2.Cui G., Yuan A. A systematic review of epidemiology and risk factors associated with Chinese inflammatory Bowel disease. Front Med. 2018;5:183. doi: 10.3389/fmed.2018.00183. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthakrishnan A.N. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 4.Aardoom M.A., Veereman G., de Ridder L. A review on the use of anti-TNF in children and adolescents with inflammatory Bowel disease. Int J Mol Sci. 2019;20(10):2529. doi: 10.3390/ijms20102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopetuso L.R., Gerardi V., Papa V., Scaldaferri F., Rapaccini G.L., Gasbarrini A. Can we predict the efficacy of anti-TNF-α agents? Int J Mol Sci. 2017;18(9) doi: 10.3390/ijms18091973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dragoni G., Innocenti T., Galli A. Biomarkers of inflammation in inflammatory Bowel disease: how long before abandoning single-marker approaches? Dig Dis. 2020 doi: 10.1159/000511641. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Florholmen J.R., Johnsen K.M., Meyer R., Olsen T., Moe O.K., Tandberg P. Discovery and validation of mucosal TNF expression combined with histological score - a biomarker for personalized treatment in ulcerative colitis. BMC Gastroenterol. 2020;20(1):321. doi: 10.1186/s12876-020-01447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atreya R., Neurath M.F., Siegmund B. Personalizing treatment in IBD: hype or reality in 2020? can we predict response to anti-TNF? Front Med. 2020;7:517. doi: 10.3389/fmed.2020.00517. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisbert J.P., Chaparro M. Predictors of primary response to biologic treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in patients with inflammatory Bowel disease: from basic science to clinical practice. J Crohn's Colitis. 2020;14(5):694–709. doi: 10.1093/ecco-jcc/jjz195. [DOI] [PubMed] [Google Scholar]
- 10.Liu F., Lee S.A., Riordan S.M., Zhang L., Zhu L. Global studies of using fecal biomarkers in predicting relapse in inflammatory Bowel disease. Front Med. 2020;7 doi: 10.3389/fmed.2020.580803. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sipponen T., Savilahti E., Karkkainen P., Kolho K.L., Nuutinen H., Turunen U. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008;14(10):1392–1398. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen V., Roseth A., Ahmad T., Skar V., Moum B. Fecal calprotectin: a reliable predictor of mucosal healing after treatment for active ulcerative colitis. Gastroenterol Res Pract. 2017;2017 doi: 10.1155/2017/2098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltran B., Iborra M., Saez-Gonzalez E., Marques-Minana M.R., Moret I., Cerrillo E. Fecal calprotectin pretreatment and induction infliximab levels for prediction of primary nonresponse to infliximab therapy in Crohn's disease. Dig Dis. 2019;37(2):108–115. doi: 10.1159/000492626. [DOI] [PubMed] [Google Scholar]
- 14.Bertani L., Blandizzi C., Mumolo M.G., Ceccarelli L., Albano E., Tapete G. Fecal calprotectin predicts mucosal healing in patients with ulcerative colitis treated with biological therapies: a prospective study. Clin Transl Gastroenterol. 2020;11(5):e00174. doi: 10.14309/ctg.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumolo M.G., Bertani L., Ceccarelli L., Laino G., Di Fluri G., Albano E. From bench to bedside: fecal calprotectin in inflammatory Bowel diseases clinical setting. World J Gastroenterol. 2018;24(33):3681–3694. doi: 10.3748/wjg.v24.i33.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombel J.F., Panaccione R., Bossuyt P., Lukas M., Baert F., Vanasek T. Effect of tight control management on Crohn's disease (CALM): a multicenter, randomized, controlled phase 3 trial. Lancet. 2018;390(10114):2779–2789. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 17.Reinisch W., Sandborn W.J., Hommes D.W., D'Haens G., Hanauer S., Schreiber S. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomized controlled trial. Gut. 2011;60(6):780–787. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 18.Detrez I., Dreesen E., Van Stappen T., de Vries A., Brouwers E., Van Assche G. Variability in golimumab exposure: a 'real-life' observational study in active ulcerative colitis. J Crohns Colitis. 2016;10(5):575–581. doi: 10.1093/ecco-jcc/jjv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinisch W., Wang Y., Oddens B.J., Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35(5):568–576. doi: 10.1111/j.1365-2036.2011.04987.x. [DOI] [PubMed] [Google Scholar]
- 20.Seah D., De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43(4):482–513. doi: 10.1111/apt.13491. [DOI] [PubMed] [Google Scholar]
- 21.Choy M.C., Seah D., Gorelik A., An Y.K., Chen C.Y., Macrae F.A. Predicting response after infliximab salvage in acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018;33(7):1347–1352. doi: 10.1111/jgh.14072. [DOI] [PubMed] [Google Scholar]
- 22.Moore C., Corbett G., Moss A.C. Systematic Review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory Bowel disease. J Crohn's Colitis. 2016;10(5):619–625. doi: 10.1093/ecco-jcc/jjw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodini G., Giannini E.G., Savarino V., Del Nero L., Pellegatta G., De Maria C. Adalimumab trough serum levels and anti-adalimumab antibodies in the long-term clinical outcome of patients with Crohn's disease. Scand J Gastroenterol. 2016;51(9):1081–1086. doi: 10.3109/00365521.2016.1157894. [DOI] [PubMed] [Google Scholar]
- 24.Verstockt B., Moors G., Bian S., Van Stappen T., Van Assche G., Vermeire S. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn's disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48(7):731–739. doi: 10.1111/apt.14943. [DOI] [PubMed] [Google Scholar]
- 25.Hinojosa J., Munoz F., Martinez-Romero G.J. Relationship between serum adalimumab levels and clinical outcome in the treatment of inflammatory Bowel disease. Dig Dis. 2019:1–7. doi: 10.1159/000499870. [DOI] [PubMed] [Google Scholar]
- 26.Barre A., Colombel J.F., Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47(7):896–905. doi: 10.1111/apt.14550. [DOI] [PubMed] [Google Scholar]
- 27.Frederiksen M.T., Ainsworth M.A., Brynskov J., Thomsen O.O., Bendtzen K., Steenholdt C. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis. 2014;20(10):1714–1721. doi: 10.1097/MIB.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 28.Vande Casteele N., Khanna R., Levesque B.G., Stitt L., Zou G.Y., Singh S. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut. 2015;64(10):1539–1545. doi: 10.1136/gutjnl-2014-307883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandse J.F., Vos L.M., Jansen J., Schakel T., Ponsioen C.I., van den Brink G.R. Serum concentration of anti-TNF antibodies, adverse effects and quality of life in patients with inflammatory Bowel disease in remission on maintenance treatment. J Crohns Colitis. 2015;9(11):973–981. doi: 10.1093/ecco-jcc/jjv116. [DOI] [PubMed] [Google Scholar]
- 30.Kharlamova N., Hermanrud C., Dunn N., Ryner M., Hambardzumyan K., Vivar Pomiano N. Drug tolerant anti-drug antibody assay for infliximab treatment in clinical practice identifies positive cases earlier. Front Immunol. 2020;11:1365. doi: 10.3389/fimmu.2020.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Stappen T., Vande Casteele N., Van Assche G., Ferrante M., Vermeire S., Gils A. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67(5):818–826. doi: 10.1136/gutjnl-2016-313071. [DOI] [PubMed] [Google Scholar]
- 32.Olsen T., Cui G., Goll R., Husebekk A., Florholmen J. Infliximab therapy decreases the levels of TNF-alpha and IFN-gamma mRNA in colonic mucosa of ulcerative colitis. Scand J Gastroenterol. 2009;44(6):727–735. doi: 10.1080/00365520902803507. [DOI] [PubMed] [Google Scholar]
- 33.Rismo R., Olsen T., Ciu G., Paulssen E.J., Christiansen I., Florholmen J. The effect of adalimumab for induction of endoscopic healing and normalization of mucosal cytokine gene expression in Crohn's disease. Scand J Gastroenterol. 2012;47(10):1200–1210. doi: 10.3109/00365521.2012.711853. [DOI] [PubMed] [Google Scholar]
- 34.Rismo R., Olsen T., Cui G., Paulssen E.J., Christiansen I., Johnsen K. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn's disease. Scand J Gastroenterol. 2013;48(3):311–319. doi: 10.3109/00365521.2012.758773. [DOI] [PubMed] [Google Scholar]
- 35.Olsen T., Rismo R., Gundersen M.D., Paulssen E.J., Johnsen K., Kvamme J.M. Normalization of mucosal tumor necrosis factor-alpha: a new criterion for discontinuing infliximab therapy in ulcerative colitis. Cytokine. 2016;79:90–95. doi: 10.1016/j.cyto.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Rismo R., Olsen T., Cui G., Christiansen I., Florholmen J., Goll R. Mucosal cytokine gene expression profiles as biomarkers of response to infliximab in ulcerative colitis. Scand J Gastroenterol. 2012;47(5):538–547. doi: 10.3109/00365521.2012.667146. [DOI] [PubMed] [Google Scholar]
- 37.Belarif L., Danger R., Kermarrec L., Nerriere-Daguin V., Pengam S., Durand T. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J Clin Invest. 2019;129(5):1910–1925. doi: 10.1172/JCI121668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim W.M., Kaser A., Blumberg R.S. A role for oncostatin M in inflammatory bowel disease. Nat Med. 2017;23(5):535–536. doi: 10.1038/nm.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstockt S., Verstockt B., Vermeire S. Oncostatin M as a new diagnostic, prognostic and therapeutic target in inflammatory bowel disease (IBD) Expert Opin Ther Targets. 2019;23(11):943–954. doi: 10.1080/14728222.2019.1677608. [DOI] [PubMed] [Google Scholar]
- 40.Mavragani C.P., Nezos A., Dovrolis N., Andreou N.P., Legaki E., Sechi L.A. Types I and II interferon signatures can predict the response to anti-TNF agents in inflammatory Bowel disease patients: involvement of the microbiota. Inflamm Bowel Dis. 2020;26(10):1543–1553. doi: 10.1093/ibd/izaa216. [DOI] [PubMed] [Google Scholar]
- 41.Verstockt B., Verstockt S., Blevi H., Cleynen I., de Bruyn M., Van Assche G. TREM-1, the ideal predictive biomarker for endoscopic healing in anti-TNF-treated Crohn's disease patients? Gut. 2019;68(8):1531–1533. doi: 10.1136/gutjnl-2018-316845. [DOI] [PubMed] [Google Scholar]
- 42.Gaujoux R., Starosvetsky E., Maimon N., Vallania F., Bar-Yoseph H., Pressman S. Cell-centered meta-analysis reveals baseline predictors of anti-TNFalpha non-response in biopsy and blood of patients with IBD. Gut. 2019;68(4):604–614. doi: 10.1136/gutjnl-2017-315494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verstockt B., Verstockt S., Dehairs J., Ballet V., Blevi H., Wollants W.J. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMedicine. 2019;40:733–742. doi: 10.1016/j.ebiom.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aden K., Rehman A., Waschina S., Pan W.H., Walker A., Lucio M. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory Bowel diseases. Gastroenterology. 2019;157(5):1279–1292. doi: 10.1053/j.gastro.2019.07.025. e11. [DOI] [PubMed] [Google Scholar]
- 45.Magnusson M.K., Strid H., Sapnara M., Lasson A., Bajor A., Ung K.A. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis. 2016;10(8):943–952. doi: 10.1093/ecco-jcc/jjw051. [DOI] [PubMed] [Google Scholar]
- 46.Vatn S., Carstens A., Kristoffersen A.B., Bergemalm D., Casen C., Moen A.E.F. Faecal microbiota signatures of IBD and their relation to diagnosis, disease phenotype, inflammation, treatment escalation and anti-TNF response in a European Multicentre Study (IBD-Character) Scand J Gastroenterol. 2020;55(10):1146–1156. doi: 10.1080/00365521.2020.1803396. [DOI] [PubMed] [Google Scholar]
- 47.Dovrolis N., Michalopoulos G., Theodoropoulos G.E., Arvanitidis K., Kolios G., Sechi L.A. The interplay between mucosal microbiota composition and host gene-expression is linked with infliximab response in inflammatory Bowel diseases. Microorganisms. 2020;8(3) doi: 10.3390/microorganisms8030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doherty M.K., Ding T., Koumpouras C., Telesco S.E., Monast C., Das A. Fecal microbiota signatures are associated with response to ustekinumab therapy among Crohn's disease patients. MBio. 2018;9(2) doi: 10.1128/mBio.02120-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Gao X., Zhang X., Xiao F., Hu H., Li X. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn's disease. Gut Microbes. 2021;13(1):1–18. doi: 10.1080/19490976.2020.1865708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Estevinho M.M., Rocha C., Correia L., Lago P., Ministro P., Portela F. Features of fecal and colon microbiomes associate with responses to biologic therapies for inflammatory Bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2020;18(5):1054–1069. doi: 10.1016/j.cgh.2019.08.063. [DOI] [PubMed] [Google Scholar]
- 51.Iborra M., Moret I., Beltrán B. MicroRNAs as novel biomarkers in IBD: characterization and current status. Biochem Anal Biochem. 2015;4(4) [Google Scholar]
- 52.Batra S.K., Heier C.R., Diaz-Calderon L., Tully C.B., Fiorillo A.A., van den Anker J. Serum miRNAs are pharmacodynamic biomarkers associated with therapeutic response in pediatric inflammatory Bowel disease. Inflamm Bowel Dis. 2020;26(10):1597–1606. doi: 10.1093/ibd/izaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papaconstantinou I., Kapizioni C., Legaki E., Xourgia E., Karamanolis G., Gklavas A. Association of miR-146 rs2910164, miR-196a rs11614913, miR-221 rs113054794 and miR-224 rs188519172 polymorphisms with anti-TNF treatment response in a Greek population with Crohn's disease. World J Gastrointest Pharmacol Ther. 2017;8(4):193–200. doi: 10.4292/wjgpt.v8.i4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks J., Watson A., Korcsmaros T. Omics approaches to identify potential biomarkers of inflammatory diseases in the focal adhesion complex. Genom Proteom Bioinform. 2017;15(2):101–109. doi: 10.1016/j.gpb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meuwis M.A., Fillet M., Geurts P., de Seny D., Lutteri L., Chapelle J.P. Biomarker discovery for inflammatory bowel disease, using proteomic serum profiling. Biochem Pharmacol. 2007;73(9):1422–1433. doi: 10.1016/j.bcp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Zhang F., Xu C., Ning L., Hu F., Shan G., Chen H. Exploration of serum proteomic profiling and diagnostic model that differentiate Crohn's disease and intestinal tuberculosis. PLoS ONE. 2016;11(12) doi: 10.1371/journal.pone.0167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starr A.E., Deeke S.A., Ning Z., Chiang C.K., Zhang X., Mottawea W. Proteomic analysis of ascending colon biopsies from a pediatric inflammatory bowel disease inception cohort identifies protein biomarkers that differentiate Crohn's disease from UC. Gut. 2017;66(9):1573–1583. doi: 10.1136/gutjnl-2015-310705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drobin K., Assadi G., Hong M.G., Andersson E., Fredolini C., Forsstrom B. Targeted analysis of serum proteins encoded at known inflammatory Bowel disease risk loci. Inflamm Bowel Dis. 2019;25(2):306–316. doi: 10.1093/ibd/izy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medina-Medina R., Iglesias-Flores E., Benítez J.M., Marín-Pedrosa S., Salgueiro I., Ferrín G. P008 Proteomic markers of response to anti-TNF drugs in patients with Crohn's disease. J Crohn's Colitis. 2019;13(Supplement_1):S090. S. [Google Scholar]
- 60.D'Haens G., Kelly O., Battat R., Silverberg M.S., Laharie D., Louis E. Development and validation of a test to monitor endoscopic activity in patients with Crohn's disease based on serum levels of proteins. Gastroenterology. 2020;158(3):515–526. doi: 10.1053/j.gastro.2019.10.034. e10. [DOI] [PubMed] [Google Scholar]
- 61.Pierre N., Baiwir D., Huynh-Thu V.A., Mazzucchelli G., Smargiasso N., De Pauw E. Discovery of biomarker candidates associated with the risk of short-term and mid/long-term relapse after infliximab withdrawal in Crohn's patients: a proteomics-based study. Gut. 2020;(0):1–8. doi: 10.1136/gutjnl-2020-322100. [DOI] [PubMed] [Google Scholar]
- 62.Telesco S.E., Brodmerkel C., Zhang H., Kim L.L., Johanns J., Mazumder A. Gene expression signature for prediction of golimumab response in a phase 2a Open-label trial of patients with ulcerative colitis. Gastroenterology. 2018;155(4):1008–1011. doi: 10.1053/j.gastro.2018.06.077. e8. [DOI] [PubMed] [Google Scholar]
- 63.Wang M.H., Friton J.J., Raffals L.E., Leighton J.A., Pasha S.F., Picco M.F. Novel genetic risk variants can predict anti-TNF agent response in patients with inflammatory Bowel disease. J Crohns Colitis. 2019;13(8):1036–1043. doi: 10.1093/ecco-jcc/jjz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bek S., Nielsen J.V., Bojesen A.B., Franke A., Bank S., Vogel U. Systematic review: genetic biomarkers associated with anti-TNF treatment response in inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;44(6):554–567. doi: 10.1111/apt.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gole B., Potocnik U. Pre-treatment biomarkers of anti-tumor necrosis factor therapy response in Crohn's disease-a systematic review and gene ontology analysis. Cells. 2019;8(6) doi: 10.3390/cells8060515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bank S., Julsgaard M., Abed O.K., Burisch J., Broder Brodersen J., Pedersen N.K. Polymorphisms in the NFkB, TNF-alpha, IL-1β, and IL-18 pathways are associated with response to anti-TNF therapy in Danish patients with inflammatory Bowel disease. Aliment Pharmacol Ther. 2019;49(7):890–903. doi: 10.1111/apt.15187. [DOI] [PubMed] [Google Scholar]
- 67.Sazonovs A., Kennedy N.A., Moutsianas L., Heap G.A., Rice D.L., Reppell M. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn's disease. Gastroenterology. 2020;158(1):189–199. doi: 10.1053/j.gastro.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 68.Degenhardt F., Dirmeier A., Lopez R., Lang S., Kunst C., Roggenbuck D. Serologic anti-GP2 antibodies are associated with genetic polymorphisms, fibrostenosis, and need for surgical resection in Crohn's disease. Inflamm Bowel Dis. 2016;22(11):2648–2657. doi: 10.1097/MIB.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ostrowski J., Paziewska A., Lazowska I., Ambrozkiewicz F., Goryca K., Kulecka M. Genetic architecture differences between pediatric and adult-onset inflammatory Bowel diseases in the Polish population. Sci Rep. 2016;6:39831. doi: 10.1038/srep39831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z., Arijs I., De Hertogh G., Vermeire S., Noman M., Bullens D. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16(8):1299–1310. doi: 10.1002/ibd.21229. [DOI] [PubMed] [Google Scholar]
- 71.Cui G. TH9, TH17, and TH22 cell subsets and their main cytokine products in the pathogenesis of colorectal cancer. Front Oncol. 2019;9:1002. doi: 10.3389/fonc.2019.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osterman M.T., VanDussen K.L., Gordon I.O., Davis E.M., Li K., Simpson K. Epithelial cell biomarkers are predictive of response to biologic agents in Crohn's disease. Inflamm Bowel Dis. 2020 doi: 10.1093/ibd/izaa251. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andreou N.P., Legaki E., Dovrolis N., Boyanov N., Georgiou K., Gkouskou K. B-cell activating factor (BAFF) expression is associated with Crohn's disease and can serve as a potential prognostic indicator of disease response to Infliximab treatment. Dig Liver Dis. 2020 doi: 10.1016/j.dld.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 74.Fasanmade A.A., Adedokun O.J., Olson A., Strauss R., Davis H.M. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(5):297–308. doi: 10.5414/cpp48297. [DOI] [PubMed] [Google Scholar]
- 75.Roblin X., Marotte H., Leclerc M., Del Tedesco E., Phelip J.M., Peyrin-Biroulet L. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015;9(7):525–531. doi: 10.1093/ecco-jcc/jjv061. [DOI] [PubMed] [Google Scholar]
- 76.Meuwis M.A., Fillet M., Lutteri L., Maree R., Geurts P., de Seny D. Proteomics for prediction and characterization of response to infliximab in Crohn's disease: a pilot study. Clin Biochem. 2008;41(12):960–967. doi: 10.1016/j.clinbiochem.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 77.Haberman Y., Karns R., Dexheimer P.J., Schirmer M., Somekh J., Jurickova I. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat Commun. 2019;10(1):38. doi: 10.1038/s41467-018-07841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen P., Zhou G., Lin J., Li L., Zeng Z., Chen M. Serum biomarkers for inflammatory Bowel disease. Front Med. 2020;7:123. doi: 10.3389/fmed.2020.00123. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molander P., af Bjorkesten C.G., Mustonen H., Haapamaki J., Vauhkonen M., Kolho K.L. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFα blocking agents. Inflamm Bowel Dis. 2012;18(11):2011–2017. doi: 10.1002/ibd.22863. [DOI] [PubMed] [Google Scholar]
- 80.Magro F., Rodrigues-Pinto E., Santos-Antunes J., Vilas-Boas F., Lopes S., Nunes A. High C-reactive protein in Crohn's disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. 2014;8(2):129–136. doi: 10.1016/j.crohns.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Shiga H., Abe I., Onodera M., Moroi R., Kuroha M., Kanazawa Y. Serum C-reactive protein and albumin are useful biomarkers for tight control management of Crohn's disease in. Jpn Sci Rep. 2020;10(1):511. doi: 10.1038/s41598-020-57508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beigel F., Deml M., Schnitzler F., Breiteneicher S., Goke B., Ochsenkuhn T. Rate and predictors of mucosal healing in patients with inflammatory bowel disease treated with anti-TNF-alpha antibodies. PLoS ONE. 2014;9(6):e99293. doi: 10.1371/journal.pone.0099293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Louis E., Vermeire S., Rutgeerts P., De Vos M., Van Gossum A., Pescatore P. A positive response to infliximab in Crohn disease: association with a higher systemic inflammation before treatment but not with -308 TNF gene polymorphism. Scand J Gastroenterol. 2002;37(7):818–824. [PubMed] [Google Scholar]
- 84.Bortlik M., Duricova D., Malickova K., Machkova N., Bouzkova E., Hrdlicka L. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn's disease. J Crohns Colitis. 2013;7(9):736–743. doi: 10.1016/j.crohns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Cornillie F., Hanauer S.B., Diamond R.H., Wang J., Tang K.L., Xu Z. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63(11):1721–1727. doi: 10.1136/gutjnl-2012-304094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yarur A.J., Jain A., Sussman D.A., Barkin J.S., Quintero M.A., Princen F. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016;65(2):249–255. doi: 10.1136/gutjnl-2014-308099. [DOI] [PubMed] [Google Scholar]
- 87.Ungar B., Levy I., Yavne Y., Yavzori M., Picard O., Fudim E. Optimizing Anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory Bowel diseases. Clin Gastroenterol Hepatol. 2016;14(4):550–557. doi: 10.1016/j.cgh.2015.10.025. e2. [DOI] [PubMed] [Google Scholar]
- 88.Vande Casteele N., Gils A., Singh S., Ohrmund L., Hauenstein S., Rutgeerts P. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108(6):962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 89.Pallagi-Kunstar E., Farkas K., Szepes Z., Nagy F., Szucs M., Kui R. Utility of serum TNF-α, infliximab trough level, and antibody titers in inflammatory Bowel disease. World J Gastroenterol. 2014;20(17):5031–5035. doi: 10.3748/wjg.v20.i17.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazor Y., Almog R., Kopylov U., Ben Hur D., Blatt A., Dahan A. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther. 2014;40(6):620–628. doi: 10.1111/apt.12869. [DOI] [PubMed] [Google Scholar]