Abstract
Novel or unexpected sounds that deviate from an otherwise repetitive sequence of the same sound cause behavioural distraction. Recent work has suggested that distraction also occurs during reading as fixation durations increased when a deviant sound was presented at the fixation onset of words. The present study tested the hypothesis that this increase in fixation durations occurs due to saccadic inhibition. This was done by manipulating the temporal onset of sounds relative to the fixation onset of words in the text. If novel sounds cause saccadic inhibition, they should be more distracting when presented during the second half of fixations when saccade programming usually takes place. Participants read single sentences and heard a 120 ms sound when they fixated five target words in the sentence. On most occasions (p = .9), the same sine wave tone was presented (“standard”), while on the remaining occasions (p = .1) a new sound was presented (“novel”). Critically, sounds were played, on average, either during the first half of the fixation (0 ms delay) or during the second half of the fixation (120 ms delay). Consistent with the saccadic inhibition hypothesis (SIH), novel sounds led to longer fixation durations in the 120 ms compared to the 0 ms delay condition. However, novel sounds did not generally influence the execution of the subsequent saccade. These results suggest that unexpected sounds have a rapid influence on saccade planning, but not saccade execution.
Keywords: Novelty distraction, eye-movements, reading, saccadic inhibition, auditory distraction
Novel or unexpected sounds that occur in an otherwise repetitive sequence of the same sound cause cognitive distraction that is observed in both neurophysiological and behavioural responses (Berti, 2012; Escera et al., 2000; Horváth et al., 2008; Parmentier, 2014; Parmentier et al., 2019; Schröger, 1996). The detrimental effect of unexpected sounds on behavioural performance has been reported using visual, auditory and crossmodal categorization tasks, two-alternative forced-choice tasks, Go/ NoGo, visual matching, and serial recall tasks (Bendixen et al., 2010; Berti & Schröger, 2001; Hughes et al., 2005; Li et al., 2013; Ljungberg et al., 2012; Ljungberg & Parmentier, 2012; Pacheco-Unguetti & Parmentier, 2016; Parmentier, 2016; Röer et al., 2015, 2018). Interestingly, recent research has suggested that unexpected sounds may also lead to longer fixation durations during everyday tasks such as reading and scene viewing (Graupner et al., 2007; Vasilev, Parmentier, et al., 2019). In the present study, we tested the hypothesis that the increase in fixation durations during reading is due to inhibition of saccade planning. In addition, we examined whether novel sounds affect the execution of reading saccades.
Distraction by auditory novelty
Novel sounds are distracting not because of their low frequency of occurrence, but because they violate sensory predictions (Bubic et al., 2009; Parmentier et al., 2011; Schröger et al., 2007), even when the stimuli and responses in the primary task are predictable (Parmentier & Gallego, 2020). Novelty distraction is associated with specific electrophysiological responses: (a) a mismatch negativity (MMN) component, reflecting an early detection of auditory change in the brain (Näätänen et al., 1978, 2007); (b) a P3a component, reflecting the involuntary orientation of attention away from the main task (Berti, 2012; Escera et al., 2000; Schröger & Wolff, 1998); and (c) a re-orientation negativity (RON) component, interpreted as the re-orientation of attention to the main task (Berti & Schröger, 2001; Horváth et al., 2008; Schröger et al., 2000). Hence, novelty distraction is typically viewed as the outcome of an involuntary orienting response (Sokolov, 1963) towards the novel sound. The shift of attention to and away from the novel sound, as well as the processing aftermath of its involuntary semantic analysis, have been argued to be important determinants of the reduction in main task performance (Parmentier et al., 2008, 2014; Schröger, 1996).
Interestingly, recent evidence has suggested that novel sounds may also lead to general motor inhibition (Dutra et al., 2018; Wessel, 2017; Wessel & Aron, 2013, 2017). For example, Wessel and Aron (2013) found that novel sounds led to a reduction in cortico-spinal excitability some 150 ms after their presentation, which was interpreted as evidence for global inhibition of the motor system. This reduction in cortico-spinal excitability appears to be directly related to motor planning as it was positively correlated with action stopping in a Go/NoGo task (Dutra et al., 2018). These results suggest that unexpected novel sounds may trigger a transient and rapid inhibition of motor responses. Such inhibition may occur earlier in time than the attention orienting response (Wessel & Aron, 2017) and stop ongoing processes to enable a more rapid and effective analysis of unexpected sounds (Wessel, 2017).
While novelty distraction is typically measured from response times using forced-choice categorization tasks, recent evidence has suggested that deviant sounds may also affect oculomotor control (Graupner et al., 2007; Vasilev et al., 2019; Widmann et al., 2014; see also Marois & Vachon, 2018; Wetzel et al., 2016 for pupil dilation responses). For example, Graupner et al. (2007) presented visual and auditory distractors at every fifth fixation in a scene viewing task. Participants were presented with 17 standard distractors, 16 standard distractors, and 1 deviant distractor, or no distractors. Graupner et al. (2007) found that the deviant sound led to an increase in fixation durations, which was due to a reduction in the proportion of terminated fixations. This reduction occurred at two distinct time intervals: first at around 90 ms and then at around 150 ms after the sound’s onset.
In addition, Widmann et al. (2014) studied the categorisation of sounds using microsaccades, which are miniature eye-movements that occur about 1–2 times per second (Engbert, 2006). Widmann et al. (2014) found a significant difference between standard and target sounds starting at 142–148 ms, and between distractors (intensity/ pitch deviants) and target sounds starting at 148–196 ms after sound onset. After factoring in neural transmission and motor delays, they argued that microsaccades can show the categorisation of target vs. non-target sounds some 80–100 ms after sound onset.
Furthermore, Vasilev et al. (2019) presented a 50 ms sound when readers fixated five target words in a sentence. Participants either heard five standard sounds (a sine wave) or four standard and one deviant sound (a burst of white noise). The authors found that the deviant sound led to an increase in fixation durations immediately after its presentation. A time-course analysis revealed that the deviant sound began to affect fixation durations some 180 ms following the sound’s onset. Because the increase in fixation durations originated relatively late in the fixation duration distribution and did not appear to be related to the lexical processing of words in the sentence, Vasilev et al. (2019) hypothesised that this delay stemmed from the disrupted programming of the next saccade. In summary, these results suggest that unexpected sounds can affect oculomotor control during everyday tasks such as scene viewing and reading.
Eye-movement control during reading
During reading, the eyes alternate between short periods of relative stability (i.e., fixations) and quick, ballistic movements (i.e., saccades). While fixations allow readers to uptake high-resolution visual information from the current word, saccades bring their eyes to unexplored parts of the text. There is now a large body of evidence showing that fixation durations during reading are sensitive to different linguistic properties of words, such as their lexical frequency (Inhoff & Rayner, 1986; Rayner & Duffy, 1986; Schilling et al., 1998) or predictability given the preceding context (Balota et al., 1985; Kliegl et al., 2006; Rayner et al., 2011; Rayner & Well, 1996; see Rayner, 1998, 2009 for a review). This suggests that fixation durations are influenced by the cognitive processing of the text.
The average fixation duration during reading is about 240 ms (Reichle & Reingold, 2013). In that time, readers have to extract linguistic information from the fixated word to recognise it and then plan a saccade to the next word. There is evidence that word processing starts soon after fixation onset. For example, visual input during the first 60 ms appears to be crucial for word recognition as lexical processing occurs even if the word disappears afterwards (Liversedge et al., 2004; Rayner et al., 2003). In addition, linguistic variables typically have a quick influence on fixation durations. For instance, survival analyses have shown that lexical frequency, predictability, lexical ambiguity, and preview validity start to influence fixation durations between 120 and 140 ms from fixation onset (Reingold & Sheridan, 2014). Moreover, neurophysiological evidence has shown that lexical processing typically occurs around 127–172 ms after fixation onset on average (Reichle & Reingold, 2013). However, because some parafoveal pre-processing usually occurs before words are fixated, lexical processing often starts even earlier than that (Reichle & Reingold, 2013). Therefore, lexical processing of the fixated word typically begins prior to the planning of the next saccade.
This idea is illustrated well in serial-attention models of eye-movement control such as E-Z Reader (Reichle et al., 1998, 2009). In this model, word recognition starts with a 50 ms visual processing stage that reflects the time needed for visual information from the retina to reach the cortex (Pollatsek et al., 2006; see also Foxe & Simpson, 2002). This is then followed by two attention-dependent lexical processing stages —familiarity check (L1) and lexical access (L2). L1 roughly corresponds to the recognition of the orthographic wordform and is used to estimate the difficulty of accessing the meaning of the word. L2, on the other hand, reflects the actual act of word identification (i.e., recognising and retrieving the word’s meaning from memory; Reichle et al., 2003). Once L1 is completed, the programming of the saccade to the next word is initiated because completion of the second stage (L2) is imminent. The programming of the next saccade also occurs in two stages: (a) a labile stage (M1) in which the current saccade plan can be cancelled by another programme and (b) a non-labile stage (M2) in which the current saccade plan can no longer be cancelled. Once lexical processing of the fixated word is completed, attention shifts covertly to the next word in anticipation of the eye-movement.
Parallel-attention models such as SWIFT (Engbert et al., 2002, 2005) differ from E-Z Reader in that attention is allocated to more than one word at a time, but they also share a number of core assumptions. For instance, lexical processing in SWIFT also occurs in two stages, as does the programming of the next saccade. However, contrary to E-Z Reader, the trigger to start planning the next saccade in SWIFT is not determined by the completion of some initial lexical processing, but is instead based on a random saccadic timer with a pre-defined mean (Engbert et al., 2005). Nevertheless, this timer can be inhibited foveally by the processing difficulty of the fixated word, such that less frequent (i.e., more difficult) words will prolong fixation durations. This effectively delays the onset of the next saccade to allow for lexical processing of less frequent words to occur. Such delay mechanism could only work if some lexical processing occurs early enough to allow the difficulty of the fixated word to be estimated and the programming of the next saccade to be delayed as a consequence. Therefore, in both models, lexical processing of the fixated word must generally start early on in the fixation, whereas saccade planning by necessity occurs towards the end of fixations.
Neural control of saccades
The execution of saccades occurs immediately after their planning. However, it is currently not known whether saccade execution can be perturbed by novel sounds. Saccades begin with an acceleration phase that lasts until their peak velocity is reached, after which they start to decelerate. The duration and peak velocity of saccades increases non-linearly with greater saccadic amplitude (often called the “main sequence”; Bahill et al., 1975). Reading saccades usually have an amplitude of ~ 2º (7–9 letters; Rayner, 2009) and last for about 20–40 ms (Bouma & De Voogd, 1974; Pollatsek et al., 2006). Saccade execution is controlled by brainstem burst neurons, which receive their main input from the superior colliculus (SC) (Sparks, 2002; Watanabe & Munoz, 2011). Importantly, saccadic parameters are coupled to the activity of excitatory burst neurons (EBNs) in the brainstem. More specifically, peak saccade velocity is correlated with the maximum firing rate of EBNs, saccade duration is correlated with their burst duration, and the number of spikes in the burst is correlated with saccade amplitude (Fuchs et al., 1985; Galley, 1989; Sparks, 2002). Therefore, as the execution of saccades depends on the firing of brainstem neurons, any inhibition of these neurons should also be reflected in slower saccade velocities and longer saccade durations.
Present study
Unexpected sounds lead to an immediate increase in fixation durations during reading (Vasilev, Parmentier et al., 2019), which may occur due to global transient inhibition of motor responses (Wessel & Aron, 2013, 2017). Therefore, the increase in fixation durations may be due to saccadic inhibition during the planning stages of the next saccade. We will refer to this explanation as the saccadic inhibition hypothesis (SIH). This hypothesis is supported by the finding that deviance distraction occurs late in fixation duration distributions and does not appear to be modulated by the lexical frequency of words (Vasilev, Parmentier et al., 2019). However, this evidence is only indirect in nature. Here, we set out to test the SIH more directly by manipulating the timing of auditory distractors (standard versus novel) relative to the fixation onset of words.
As already mentioned, lexical processing of words starts soon after fixation onset, whereas programming of the next saccade by necessity occurs towards the end of fixations. Therefore, if the SIH is correct, novel sounds should be more distracting when played temporarily closer to the end of fixation (i.e., when saccade programming takes places). To test this hypothesis, we manipulated the temporal onset of sounds relative to the fixation onset of words. A 120 ms sound was played when participants fixated five target words in a sentence. The same sine wave tone (“standard”) was played on most occasions (p = .9), while a new environmental sound (“novel”) was played on the remaining occasions (p = .1). Critically, and orthogonally to this sound manipulation, the sound’s onset either coincided with the fixation’s onset (0 ms delay) or followed it by 120 ms (120 ms delay). Because the average reading fixation is about 240 ms long (Reichle & Reingold, 2013), this timing manipulation ensured that the sound occurred, on average, either during the first half or the second half of the fixation. Therefore, under the SIH, novel sounds should lead to significantly longer fixation durations than standard sounds in the 120 ms delay condition compared to the 0 ms delay condition.
A secondary goal of our study was to examine the unexplored issue of whether novel sounds may affect not only the programming, but also the execution of the next saccade. This is a relevant question given both the temporal overlap between saccade planning and execution, and the anatomical overlap between structures thought to be implicated in the temporary suppression of motor activity and structures controlling the execution of saccades. Wessel and Aron (2013) have argued that unexpected events lead to a global inhibition of the motor system, which may be modulated by the subthalamic nucleus (STN). The STN contains visual-motor neurons (Fawcett et al., 2005; Matsumura et al., 1992) and receives direct projections from the cerebral cortex (Ma & Geyer, 2017). Furthermore, the STN may be involved in maintaining a sustained fixation before goal-directed saccades by suppressing neurons in the SC via an indirect route through the substantia nigra pars reticulata (SNr) (Hikosaka et al., 2000). Of interest, the SC also controls the execution of saccades in the brainstem (Sparks, 2002; Watanabe & Munoz, 2011). Therefore, it is possible, but yet unknown, that the motor inhibition thought to follow unexpected sounds may also affect the firing of EBNs, which is related to the peak velocity of saccades (Fuchs et al., 1985). If novel sounds affect the execution of saccades, then we should observe a reduction in their velocity and an increase in their duration. This should particularly be the case in the 120 ms delay condition, where the novel sound is played temporarily closer to the execution of the next saccade.
Hypotheses
H1: Consistent with previous results (Vasilev, Parmentier et al., 2019), novel sounds should result in longer fixation durations compared to standard sounds (i.e., main effect of Sound).
H2: Consistent with the SIH, novel sounds should be more distracting when played during the second half of fixation when the next saccade is usually being programmed (i.e., Sound x Delay interaction).
H3.1: If novel sounds also affect the execution of the next saccade, they should result in a decrease in saccade velocity and an increase in saccade durations. In addition, these effects should be stronger in the 120 ms compared to the 0 ms delay condition because the former is temporally closer to the execution of the next saccade (H3.2).
Method
Participants
Sixty-four undergraduate students from Bournemouth University (53 female) participated for course credits. Their average age was 19.3 years (range: 18–31 years; SD = 2.12 years).1 Participants were native English speakers who reported normal or corrected-to-normal vision, normal hearing, and no prior diagnosis of reading disorders. Participants were naïve as to the purpose of the experiment. The study was approved by the Bournemouth University Research Ethics Committee and all participants provided informed written consent.
Materials and design
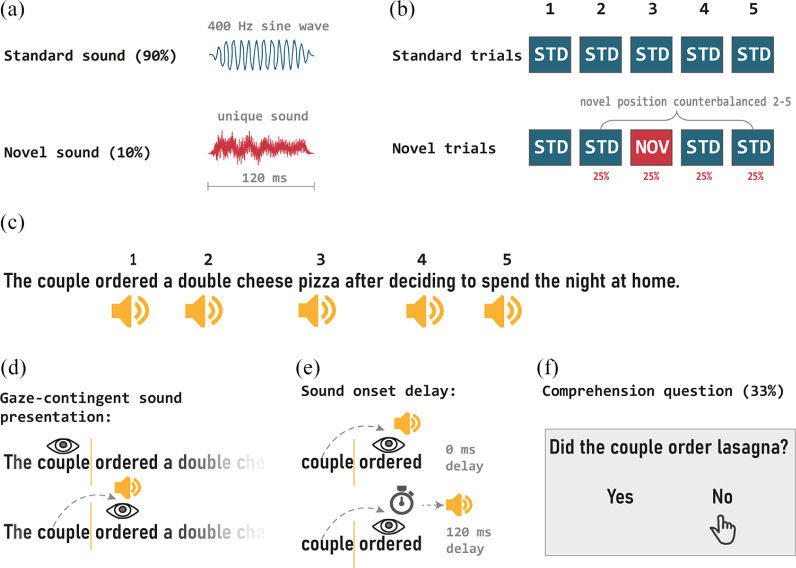
The method is illustrated in Figure 1. The reading stimuli consisted of 120 English sentences that were taken from Vasilev et al. (2019). In each sentence, there were five target words on which the sounds were presented using a gaze-contingent manipulation (see Inhoff et al., 2002). The target words were always the 3rd, 5th, 7th, 9th, and 11th word in the sentence. Each target word was followed by one non-target word on which no sounds were played. This ensured that the sounds were not played too quickly after one another. The target words were 6.75 letters long on average (SD = 1.89 letters; range: 3–13 letters). Short function words were avoided as targets to increase the likelihood that the target words would be fixated during first-pass reading.
Figure 1.
Diagrammatic illustration of the task’s key characteristics. (a) Standard and novel sounds used in the experiment. (b) Presentation of sounds in standard and novel trials. Standard trials contained five standard sounds, while novel sounds contained four standard and one novel sound (played equiprobably at positions 2–5). (c) Example sentence with the position of words on which the five sounds were played. (d) Gaze-contingent sound presentation in the experiment. Sounds were played when participants crossed an invisible boundary (Rayner, 1975; denoted by a vertical orange line) located before the five target words. (e) Sound onset delay manipulation. Sounds were played either immediately (0 ms delay) or with a 120 ms delay after crossing the boundary. The delay was the same for all sounds played in a given sentence. (f) Example of a comprehension question (presented after 33% of sentences).
Sixty novel sounds were taken from Andrés et al. (2006). The sounds were originally 200 ms long but were compressed to 120 ms (without alteration in pitch) for the purpose of the present experiment. The novel sounds consisted of different environmental sounds (e.g., sound of a drill, telephone ringing, engine, etc.). The standard sound was a 120 ms sinewave tone with a frequency of 400 Hz, with 10 ms fade-in and fade-out ramps. All sounds were monoaural, with a sampling rate of 44100 Hz, a bit depth of 16 bit. A silence condition was not included since (a) we previously showed that the presentation of five gaze-contingent standard sounds does not affect reading behaviour compared to silent reading (Vasilev, Parmentier et al., 2019); and (b) because it was not relevant to our objective, which was to determine whether the timing of the novel sounds influences oculomotor control.
The study had a 2 x 2 within-subject design, with sound type (standard vs. novel) and sound onset delay (0 vs. 120 ms) as the factors. Each participant saw 30 trials per condition. There were two types of trials that corresponded to the two sound conditions. In standard trials (which represented 50% of all trials), the standard sound was played on each of the five target words in the sentence. In contrast, in novel trials (remaining 50% of the trials), one novel and four standard sounds were played. Novel trials always started with a standard sound on Target Word 1 to reactive the representation of the standard sound at the beginning of the trial, as this representation may weaken or become “dormant” due to the pause between trials (e.g., Cowan et al., 1993; for a review, see Winkler & Schröger, 2015). The novel sound was then presented on one of the four remaining Target Words (2 through 5) with equal probability across the experiment. Novel sounds made up 10% of all sounds played during the experiment. In each sound condition, half of the sounds were played with no delay (i.e., 0 ms) and the other half were played with 120 ms delay after the target word was fixated. The delay was always the same for the five sounds played in each sentence. The assignment of conditions to sentences was counter-balanced across participants with a Latin-square design. The experiment always started with three standard trials to establish the sinewave tone as the standard sound. The remaining trials were then presented in a different pseudo-random order for each participant.
Apparatus
Participants’ eye-movements were recorded with an Eyelink 1000 eye-tracker at 1000 Hz. Viewing was binocular, but only the right eye was recorded. Participants’ head was stabilised with a chin-and-forehead rest to reduce head-movements. The stimuli were displayed on a Cambridge Research Systems LCD ++ monitor (resolution: 1920 x 1080 pixels; refresh rate: 120 Hz). The sound stimuli were played on a Creative Labs Sound Blaster X-Fi SB0770 sound card and were presented binaurally through Bose QuietComfort 25 noise-cancelling headphones at a 65 dB(A) SPL.
The experiment was programmed in Matlab R2014a (MathWorks, 2014) using the Psychophysics Toolbox v.3.0.11 (Brainard, 1997; Pelli, 1997) and Eyelink libraries (Cornelissen et al., 2002). The sound stimuli were played using the low-latency mode of the Psychophysics Toolbox (output latency was 14 ms). The sentences were formatted in a monospaced Courier New 18pt. font and appeared on a single line in the middle of the screen. The sentences were presented with a 50-pixel offset from the left side of the screen and appeared as black text over white background. The letter width was 14 pixels and the eye-to-screen distance was 80 cm. At this distance, each letter subtended ~0.34º of visual angle horizontally. The experiment was run on a PC in a Windows 7 64-bit environment.
Procedure
Participants were tested individually in a session that lasted about 40 min. Before the start of the experiment, a 3-point horizontal calibration was performed. Calibration accuracy was then monitored with a drift check presented before each trial and participants were recalibrated whenever necessary (the error was kept at < 0.3º throughout the experiment). All beeps during calibration and drift check were turned off. Participants were instructed to ignore any sounds they may hear and to read the sentences for comprehension. The experiment started with six practice items presented in silence. Each trial began with a black gaze box that was centred at the first letter in the sentence. Once a stable fixation inside the gaze box was detected, the box disappeared and the sentence was presented on the screen.
The gaze-contingent sound presentation was implemented by placing an invisible boundary (Rayner, 1975) before each of the five target words (for more details on this technique, see Eiter & Inhoff, 2010; Inhoff et al., 2002, 2004). Once the gaze position of the eye crossed an invisible boundary, the sound was presented. Depending on the sound onset condition, the sound was played either immediately after crossing the boundary (0 ms delay) or 120 ms after crossing the boundary (120 ms delay). This typically happened as participants made a forward saccade towards the target word. A third of the sentences were followed by a Yes/No comprehension question (see Figure 1f). Participants pressed the left button of the mouse to terminate the trial and to answer the comprehension questions. The questions were used to ensure that participants were reading for comprehension and were not a variable of main interest in the analyses.
Data analysis
The analysis focused on the immediate effect of the sound, which was measured on the first fixation and first saccade after it was played. We did not restrict the analysis only to cases where the Target Word was fixated during first-pass reading, but also included cases where it was skipped and another word was fixated. This was because our hypothesis predicted a general inhibition of saccade planning that should occur regardless of which word is fixated. However, the results did not change when restricting the analysis only to cases where the target word was fixated (see the online Supplementary Material A).
The fixation and saccade data were analysed only for sounds played at Target Word locations 2–5 since no novel sounds occurred on Target Word 1. Because the standard sound was presented much more frequently than the novel sounds, only one standard sound was sampled per trial. This sound was picked using the design matrix that was used to counter-balance the presentation of novel sounds. This ensured that a balanced dataset was formed, which had an equal number of standard and novel sounds that were presented equally often on Target Words 2–5. In the fixation data, we analysed only the first fixation duration during which the sound was played. This was because Vasilev et al. (2019) established that the first fixation was the source of the sound deviance effect. To measure the effect of novel sounds on the execution of the next saccade after the playing the sound, a few measures were used: saccade duration, saccade amplitude, peak and average saccade velocity.
The data were analysed with (Generalised) Linear Mixed Models (GLMM[s]) using the lme4 package v.1.1-21 (Bates et al., 2014) in the R software v.3.6.2 (R Core Team, 2019). The fixed factors in the model were sound type (standard vs. novel), sound onset delay (0 vs. 120 ms delay), and their interaction. Random intercepts were added for both participants and items (Baayen et al., 2008). In addition, we tried adding random slopes for sound type and sound onset delay (Barr et al., 2013). However, the models converged only with a random slope for sound onset delay (subjects) for the fixation duration and saccade velocity analyses, and sound type (subjects) for the saccade duration and amplitude analyses. Fixation durations, saccade durations, and saccade amplitude were log-transformed in the models as this improved the distribution of residuals. However, the results did not change when using the untransformed values. Treatment contrast coding was used for both the sound type (baseline: standard) and sound onset delay (baseline: 0 ms delay) conditions. The results were considered statistically significant if the |t|- and |z|-values were ⩾ 1.96. In addition, Bayesian LMMs were used to calculate Bayes Factors (BF10) to quantify the evidence in support of the null and the alternative hypothesis (Dienes, 2014). Full details are provided in the online Supplementary Material B. While BF10 > 1 indicate support for the alternative hypothesis, BF10 < 1 indicate evidence for the null hypothesis.
In addition, a survival analysis was done to determine the time course of novelty distraction by estimating the earliest point in time when the effect could be reliably detected. This was done using the confidence interval divergence point analysis (CI-DPA; Reingold & Sheridan, 2014, 2018). This is a survival analysis technique that helps determine the earliest point in time where the distributions of the two sound conditions begin to significantly diverge from one another. The analysis was done with 10 000 bootstrap iterations following the method described in Reingold and Sheridan (2018).
Results
All participants exhibited a comprehension accuracy score greater than 84.6%, thus indicating they had no problems understanding the sentences. Participants’ mean comprehension accuracy was 95.7% in the standard (SD = 20.3%) and 95% in the novel sound condition (SD = 21.7%). There was no significant difference between the sound conditions, z = -1.01. When asked after the experiment, only four participants had some awareness that the timing of the sounds depended on their eye-movements.2 During the data pre-processing, 6.02% of trials were removed due to eye blinks. In addition, 5.47% of trials were excluded due to boundary “hooks”3 and a further 12.19% of trials were excluded because the trigger to play the sound was not sent to the soundcard before the onset of the next fixation.4 Finally, trials with fixation durations shorter than 80 ms or longer than 1000 ms (0.31%), peak saccade velocity greater than 1000 º/s (0.06%), or saccades with amplitude greater than 15º (0.03%) were discarded as outliers. This left 75.91% of the data for analysis (5,825 trials). The distribution of trials per condition was: 1,474 (standard – 0 ms delay), 1,475 (novel – 0 ms delay), 1,452 (standard – 120 ms delay), and 1,424 (novel – 120 ms delay). There was no significant difference in the number of trials per condition, χ2(1) = 0.128, p = .72. First-pass skipping of the target occurred on 10.4% of trials, but these trials were retained as mentioned above.
First fixation duration
Descriptive statistics for the first fixation duration during which the sound was played are presented in Table 1 and are illustrated in Figure 2. The results from the LMM analysis are presented in Table 2. Consistent with H1, there was a main effect of Sound, indicating that novel sounds led to longer fixation durations compared to the standard sound (d = 0.159). While there was no main effect of Onset Delay, the interaction between Sound and Onset Delay was significant. This interaction supports H2 in showing that novel sounds yielded more distraction in the 120 ms delay condition (d = 0.233) than in the 0 ms delay condition (d = 0.09), which can be clearly seen in Figure 2. The BF10 results provided converging evidence to the same conclusion. While the data favoured the null hypothesis of no difference for Onset Delay, it favoured the alternative hypothesis of a true difference for both the main effect of Sound and the interaction between Sound and Onset Delay.
Table 1.
Mean descriptive statistics for the first fixation duration during which the sound is played and the first saccade immediately after playing the sound (SDs in parentheses).
| Sound | Onset delay | First fixation duration (ms) | Next saccade after playing the sound | |||
|---|---|---|---|---|---|---|
| Saccade duration (ms) | Saccade amplitude (º) | Peak saccade velocity (º/s) | Average saccade velocity (º/s) | |||
| Novel | 0 ms | 242 (79) | 21.5 (6.5) | 2.7 (1.29) | 225 (75.7) | 118 (30.2) |
| Standard | 0 ms | 235 (75.6) | 21.3 (6.2) | 2.7 (1.28) | 221 (74.6) | 117 (30.2) |
| Novel | 120 ms | 255 (89.7) | 22 (6.8) | 2.84 (1.36) | 232 (78) | 121 (30.8) |
| Standard | 120 ms | 235 (78.6) | 21.5 (6.2) | 2.73 (1.22) | 223 (72.5) | 119 (29.1) |
SD: standard deviation.
1º = 2.9 letters.
Figure 2.
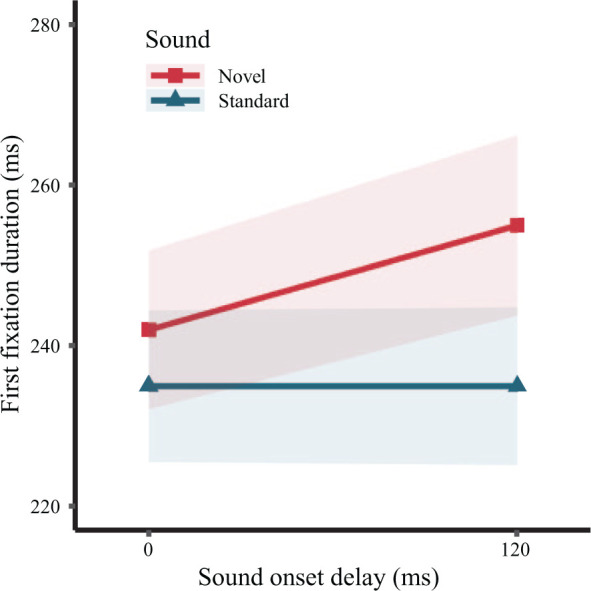
Duration of the first fixation during which the sound is played. Shading indicates ± 1 SE.
Table 2.
LMM results for the first fixation duration during which the sound is played.
| Fixed effects | Estimate | Std. Error | t value | BF10 |
|---|---|---|---|---|
| Intercept | 5.413 | 0.014 | 373.86 | |
| Sound (novel vs standard) | 0.033 | 0.011 | 3.108 | 24.07 |
| Delay (120 vs 0 ms) | –0.002 | 0.011 | –0.145 | 0.137 |
| Sound x delay | 0.042 | 0.015 | 2.792 | 7.88 |
| Random effects | Variance | SD | Corr. | |
| Intercept (item) | 0.00113 | 0.0336 | ||
| Intercept (subjects) | 0.00927 | 0.0963 | ||
| Delay (subjects) | 0.00113 | 0.0336 | –0.09 | |
| Residual | 0.08107 | 0.2847 |
LMM: linear mixed models; BF: Bayes factor; SD: standard deviation.
BF10: Bayes factor (values > 1 indicate evidence for the alternative hypothesis; values < 1 indicate evidence for the null hypothesis). Statistically significant t-values (⩾ 1.96) and BF10s meeting the 3/0.33 threshold (approx. corresponding to the 0.05 alpha level) are formatted in bold.
A simple-effects analysis (Lenth et al., 2019) of the interaction with Tukey p-value adjustments confirmed that the novelty distraction effect (novel - standard difference) was significant in both the 0 ms (b = -0.033, SE = 0.011, t = -3.107, p = .0102) and the 120 ms delay condition (b = -0.074, SE = 0.011, t = −6.992, p < .0001). However, novel sounds were more distracting in the 120 ms than in the 0 ms delay condition (b = -0.040, SE = 0.011, t = -3.513, p = .0031), whereas the standard sound did not differ between the two delay conditions (b = 0.002, SE = 0.011, t = 0.145, p = .9989). In summary, novel sounds were more distracting when played with a 120 ms delay.
One possibility is that novel sounds may have affected word processing beyond the first fixation. If participants were more likely to make additional first-pass fixations on the target word, this may indicate lexical processing difficulty. To examine this, we did a post hoc analysis of first-pass re-fixation probability on the target word. The results (presented in the Supplementary Material C) indicated no significant differences in this measure. This suggests that the effect of novel sounds was largely constrained to the first fixation during which they are played.
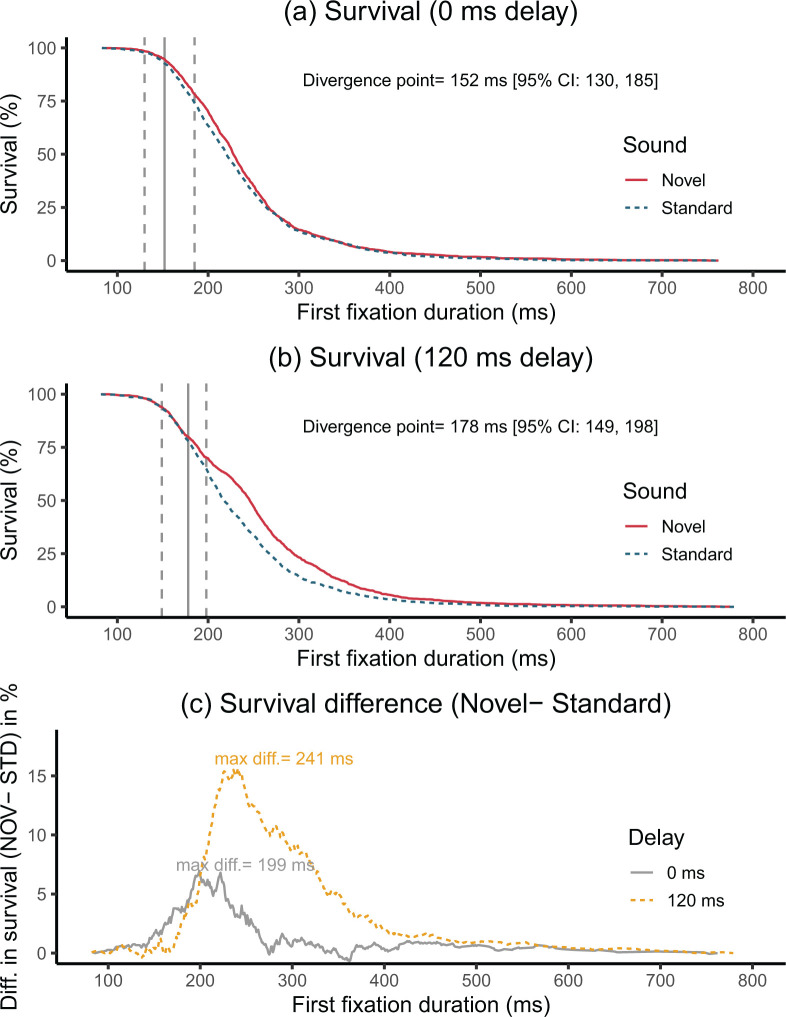
To determine the earliest point in time when the novel sound started to significantly influence fixation durations, the CI-DPA analysis (Reingold & Sheridan, 2018) was used with the first fixation data. The results are illustrated in Figure 3. In the 0 ms delay condition, the distributions of the two sound conditions began to significantly diverge at 152 ms (95% CI = [130, 185]. In contrast, the two distributions in the 120 ms onset delay condition started to diverge at 178 ms (95% CI = [149, 198]) (58 ms post the sound onset). Therefore, while the divergence point for the 120 ms delay condition occurred some 26 ms later, the novel sounds in both conditions had a relatively quick influence on fixation durations. Nevertheless, as Figure 3c shows, the strongest reduction in terminated fixations between the novel and the standard sound did not occur until 199 ms in 0 ms delay condition and 241 ms in the 120 ms delay condition (199 and 121 ms post the sound onset, respectively). Therefore, while the difference was detectible statistically early on, it did not reach its greatest magnitude immediately in the distribution of fixation durations.
Figure 3.
Results from the divergence point analysis of the first fixation duration during which the sound is played (a, b) and difference in survival between the novel and standard conditions (c). In panels (a, b), the survival curves are plotted for the two sound conditions, denoting the percentage of “surviving” (i.e., remaining) fixations at each duration bin. The divergence point is illustrated by a vertical solid line and the 95% confidence interval (CI) is illustrated by vertical dotted lines. Panel (c) shows the difference in “survival” curves between the standard and novel conditions, along with the time point of maximum difference (i.e., when the effect was strongest in the distribution of fixations).
Saccade execution
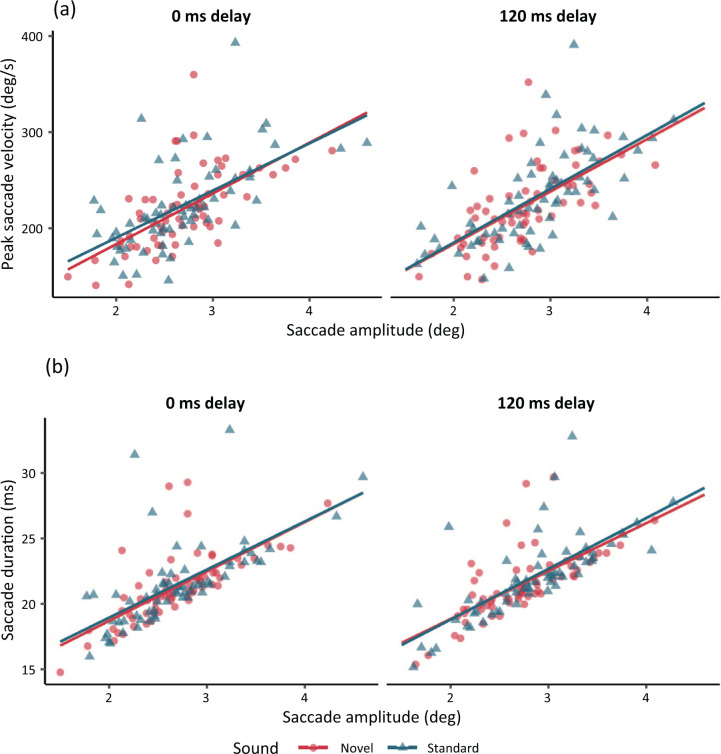
The descriptive statistics for saccade measures are shown in Table 1 and the LMM results are presented in Tables 3 and 4. Contrary to H3.1 and H3.2, there were no significant differences between the two sound conditions and no interactions with sound Onset Delay. As Figure 4 shows, novel sounds had no influence on the main sequence of the next saccade. The BF10 results showed substantial evidence in favour of the null hypothesis in all but two cases: the main effect of Sound in peak saccade velocity and the Sound x Onset Delay interaction in saccade amplitude. However, even in those two cases, the data still supported the null over the alternative hypothesis. It should be noted that the frequentist model showed a hint of a main effect of Sound in peak saccade velocity (t = 1.89), which was opposite to predictions (i.e., peak velocity increasing in the novel compared to the standard sound). However, the Bayesian model still weakly favoured the null hypothesis of no difference (BF10 = 0.462). Therefore, while the support for the null was not strong, there was no evidence to support the alternative either. Finally, a post hoc analysis indicated that the results were generally not modulated by whether participants made an intra-word or an inter-word saccade (see the online Supplementary Material A). In summary, the results suggest that, once triggered, the execution of a saccade was generally not perturbed by the recent presentation of a novel sound.
Table 3.
LMM results for the duration and amplitude of the next saccade after playing the sound.
| Fixed effects | Saccade duration | Saccade amplitude | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | t value | BF10 | Estimate | Std. Error | t value | BF10 | |
| Intercept | 3.013 | 0.016 | 189.77 | 0.865 | 0.025 | 34.001 | ||
| Sound (novel vs standard) | 0.005 | 0.011 | 0.472 | 0.062 | -0.006 | 0.02 | -0.279 | 0.140 |
| Delay (120 vs 0 ms) | 0.012 | 0.011 | 1.072 | 0.096 | 0.021 | 0.018 | 1.152 | 0.252 |
| Sound x delay | 0.016 | 0.015 | 1.059 | 0.129 | 0.035 | 0.026 | 1.359 | 0.433 |
| Random effects | Variance | SD | Corr. | Variance | SD | Corr. | ||
| Intercept (item) | 0.00063 | 0.02520 | 0.00244 | 0.04937 | ||||
| Intercept (subjects) | 0.01209 | 0.10997 | 0.02935 | 0.17131 | ||||
| Sound (subjects) | 0.00053 | 0.02308 | 0.62 | 0.00528 | 0.07267 | 0.15 | ||
| Residual | 0.08451 | 0.29071 | 0.24474 | 0.49471 | ||||
LMM: linear mixed models; BF: Bayes factor; SD: standard deviation.
BF10: Bayes factor (values > 1 indicate evidence for the alternative hypothesis; values < 1 indicate evidence for the null hypothesis). Statistically significant t-values (⩾1.96) and BF10s meeting the 3/ 0.33 threshold (approx. corresponding to the 0.05 alpha level) are formatted in bold.
Table 4.
LMM results for the peak and average saccade velocity of the next saccade after playing the sound.
| Fixed effects | Peak saccade velocity | Average saccade velocity | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | t value | BF10 | Estimate | Std. Error | t value | BF10 | |
| Intercept | 219.92 | 5.495 | 40.02 | 117.103 | 1.785 | 65.604 | ||
| Sound (novel vs standard) | 4.382 | 2.319 | 1.89 | 0.462 | 0.646 | 1.005 | 0.643 | 0.041 |
| Delay (120 vs 0 ms) | 3.196 | 2.59 | 1.234 | 0.190 | 1.326 | 1.049 | 1.264 | 0.076 |
| Sound x delay | 4.488 | 3.301 | 1.36 | 0.272 | 1.869 | 1.43 | 1.307 | 0.114 |
| Random effects | Variance | SD | Corr. | Variance | SD | Corr. | ||
| Intercept (item) | 49.47 | 7.033 | 10.364 | 3.219 | ||||
| Intercept (subjects) | 1733.38 | 41.634 | 165.983 | 12.883 | ||||
| Delay (subjects) | 81.09 | 9.005 | –0.02 | 5.177 | 2.275 | –0.31 | ||
| Residual | 3932.9 | 62.713 | 740.827 | 27.218 | ||||
LMM: linear mixed models; BF: Bayes factor; SD: standard deviation.
BF10: Bayes factor (values > 1 indicate evidence for the alternative hypothesis; values < 1 indicate evidence for the null hypothesis). Statistically significant t-values (⩾ 1.96) and BF10s meeting the 3/ 0.33 threshold (approx. corresponding to the 0.05 alpha level) are formatted in bold.
Figure 4.
A scatterplot of peak saccade velocity and saccade amplitude (a) and saccade duration and saccade amplitude (b) in the two onset delay conditions (“main sequence”; Bahill et al., 1975). Each data point shows the mean value for novel (circle shape) and standard sounds (triangle shape) for each subject. Lines show the regression slopes.
Discussion
The present study tested whether the increase in fixation durations during reading in response to unexpected sounds reflects a perturbation of the planning of the next saccade. In addition, it examined whether novels sounds affect not only the planning, but also the execution of the next saccade. Consistent with previous results (Vasilev, Parmentier et al., 2019), novel sounds led to longer first fixation durations compared to standard sounds immediately after their presentation. Although the magnitude of the effect was small, it was comparable to that of other auditory distraction effects during reading (Vasilev et al., 2018; but see also Hyönä & Ekholm, 2016; Yan et al., 2018). Critically, however, novel sounds were more distracting when presented with a 120 ms delay compared to a 0 ms delay. Because the sound in the 120 ms delay condition was played temporarily closer to when readers usually plan their next saccade, the present results support the SIH, which stated that the observed distraction is due to the transient inhibition of saccade planning.
Interestingly, however, saccade execution parameters such as amplitude, duration, and velocity remained unaffected by novel sounds in both delay conditions. The Bayes Factor analyses favoured the null hypothesis of no difference over the alternative hypothesis, and the evidence for the null was substantial (Jeffreys, 1961) in all but two cases. This suggests that novel sounds affect only the planning but not the execution of saccades (at least when the sound is played during a fixation). Therefore, while novelty distraction was rapid enough to delay the onset of the next saccade, its effect was short-lived and did not affect the movement of the eyes during a saccade once this movement was triggered. In this sense, the present results suggest that novel sounds do not lead to a general inhibition of all oculomotor behaviour but, rather, to a transient one that affects only saccade programming (at least when the sounds are presented up to 120 ms into the fixation).
The greater distraction in first fixations durations in the 120 ms delay condition is consistent with the notion that unexpected sounds lead to a general and temporary inhibition of motor responses (Dutra et al., 2018; Wessel, 2017; Wessel & Aron, 2013). However, such inhibition would be expected to affect all oculomotor behaviour, including the execution of saccades. Because saccadic variables such as peak velocity are assumed to be closely correlated with the firing of brainstem neurons (e.g., Di Stasi et al., 2013; Fuchs et al., 1985), our results suggest that novel sounds do not affect the neural circuits that control the movement of the eyes during a saccade. While this result may appear at odds with Wessel and Aron’s (2013) global motor suppression account, this suppression could be very time-sensitive and disappear by the time the eyes are in motion. In fact, Wessel and Aron (2013) reported that inhibition of motor-evoked potentials was present 150 ms after the sound onset, but had already disappeared 25 ms later. Therefore, the inhibition in the present experiment may be too transient to affect the execution of the next saccade.
A stronger test of whether novel sounds inhibit saccade execution would be to play them during a saccade. However, as most saccades are considerably shorter than the 60 to 150 ms needed for the effect to occur, the influence of novel sounds on saccade execution may be better studied in smooth pursuit tasks where observers have to continuously follow a moving object with their eyes (e.g., Lisberger et al., 1987; Robinson, 1965). Of course, one can also try to adjust the timing of the sounds in the current paradigm to maximise the chance that the strongest inhibition occurs exactly during the saccade. However, as reading saccades are very short, this critical point can be easily missed. As such, simple saccade tasks where there is strong experimental control over when the next saccade occurs may be preferable.
The time-course of novelty distraction in first fixation durations revealed that novel sounds first began to have an effect some 150 ms after fixation onset in the 0 ms delay condition, and some 180 ms after fixation onset in the 120 ms delay condition. The 150 ms onset in the former condition is in line with Wessel and Aron’s (2013) findings. Interestingly, the distraction effect in the 120 ms delay condition first started some 60 ms after the sound’s onset (i.e., 180–120 = 60 ms). Therefore, novel sounds exerted a faster effect on fixation durations when the sound was played, on average, during the second half of fixations. This rapid effect echoes Graupner et al.’s (2007) results where an auditory deviant caused a “first” saccadic inhibition some 90 ms after sound onset (the sounds in their study were played 100 ms after fixation onset, making it similar to the 120 ms delay condition in our study). In addition, the early onset in the 120 ms condition is generally consistent with Widmann et al.’s (2014) finding that microsaccadic inhibition originating some 80–100 ms post the sound onset may indicate the fast categorisation of auditory sounds.
Nevertheless, it is important to emphasise that the divergence point analysis indicates only the first point in time where a difference in the fixation duration distributions can be detected. In fact, as Figure 3c shows, the maximum difference between the two sound conditions did not occur until ~ 200 ms after the sound presentation in the 0 ms delay condition and ~ 120 ms after the sound presentation in the 120 ms delay condition (i.e., 240–120 = 120 ms). Therefore, despite the early onset, the effect needed some 50–60 ms to reach its maximum magnitude, and fixations continued to be affected well beyond the initial divergence point.
The earlier onset of the effect in the 120 ms delay condition may occur because novel sounds usually coincided with the critical period during which saccade planning takes place. Because very few fixations terminated within the first 100 ms from the sound’s onset in the 0 ms delay condition, such early inhibition may not be seen in that condition.5 Therefore, the overall pattern of inhibition was somewhat similar across the two delay conditions, but it was much stronger and started earlier in the 120 ms delay condition. Thus, while novelty distraction was present in both delay conditions, the magnitude of the effect was greater in the 120 ms condition because it affected a larger proportion of fixations.
What is the purpose of this temporary inhibition of eye-movement control by novel sounds? It may represent an adaptive response in the face of an unexpected contextual change where readers delay their oculomotor plans to react to something unexpected (i.e., auditory novelty pointing towards potential danger). Unexpected sounds are arguably surprising events (e.g., Parmentier et al., 2019; Wessel et al., 2016). As such, this “freezing” of oculomotor plans prior to goal-directed saccades may allow readers time to process this contextual change and select an appropriate response (see also Wessel, 2017, 2018). Generally speaking, sounds violating sensory prediction can be regarded as one instance of an unexpected change in our immediate environment, which brings some uncertainty and calls for a reappraisal of current actions plans. In line with this contention, novel sounds have been shown to render the repetition of one’s behaviour more difficult to facilitate the adoption of a different action plan (Bendixen & Schröger, 2008; Parmentier, 2016; Roeber et al., 2005, 2009). Interestingly, there is some evidence that saccadic inhibition can also occur in response to visual deviants (e.g., Godijn & Kramer, 2008; Graupner et al., 2007). Therefore, one interesting question for future research would be to explore whether this inhibitory response is specific to auditory stimuli, or if it reflects a more general response to unexpected contextual changes, which also extends to the visual domain.
Currently, it is not clear whether the increase in fixation durations simply reflects a neural inhibition that slows down the neural firing leading up to a saccade or if the current saccade plan is cancelled to plan a different one. Computational models of reading (e.g., Engbert et al., 2005; Reichle et al., 1998) assume that saccade planning occurs in two stages: one cancellable and another non-cancellable. This proposition is based on Becker and Jürgens’ (1979) findings that saccades can be programmed in parallel and modified if a new target is presented sufficiently early in the planning stages. In the context of our study, the next saccade plan may be reset or cancelled due to the detection of auditory novelty and, perhaps, the following reappraisal of action plans, before the saccade is re-programmed. Alternatively, saccade plans may stay the same, but simply take longer to programme because they are interrupted by a transient global neuro-motor inhibition. Future research might possibly adjudicate between these two explanations by using the double-step paradigm (e.g., Becker, 1972), where a target jumps to a new location prior to the execution of a saccade towards it. If novel sounds affect saccadic plans, then they should also influence the parallel planning of saccades in response to double-step stimuli.
Given that the maximum inhibition in the 120 ms delay condition occurred close to when the “average” fixation duration in the experiment was ending and the next saccade was starting (~ 240 ms; see Figure 3c), it is perhaps surprising that novel sounds did not affect saccade execution. In the E-Z Reader framework, the observed inhibition was well within the last 25–50 ms of the fixation, corresponding to the non-labile stage where the direction and distance of the next saccade is communicated to the motor system (Reichle et al., 2003, 2009). However, the subsequent movement of the eyes during the saccade was not affected. We speculate that this may occur because the inhibitory effect is very time-sensitive and may affect behaviour only within a very narrow window of time when the novel sound is first detected (~ 25 ms in Wessel & Aron, 2013). In addition, saccades are very quick ballistic movements- their duration and velocity are not under voluntary control (Leigh & Zee, 1999) and they are usually too short to be influenced by perceptual feedback (Optican & Pretegiani, 2017). In this sense, there may be little adaptive value in inhibiting a saccadic movement once it has already started because the individual will have a limited ability to act upon the unexpected stimulus during the saccade. As this is the first study to look at this topic, more evidence is required to reach a firmer conclusion.
Finally, while the present data is consistent with the SIH, it does not prove that novelty distraction is completely independent of language processing. For instance, the experimental manipulation is not sensitive to higher-level syntactic or integration processes that may occur after readers have left the fixated word. While the available data suggest that distraction is mostly constrained to the current fixation (Vasilev, Parmentier et al., 2019), further research is needed to better understand if unexpected sounds can have any effect at all on language processing. Nevertheless, we anticipate that the present results will be useful in stimulating further research on how unexpected sounds may affect complex everyday tasks such as reading.
In summary, the present study showed that novel, task-irrelevant sounds exert greater distraction in a reading task when they occur temporarily close to the end of fixations, thus suggesting that they disrupt the planning of the next saccade. At the same time, novel sounds do not appear to influence the execution of the next saccade. This suggests that their inhibition is short-lived, and the distractive impact of the novel sounds generally does not extend to subsequent oculomotor behaviour. These results provide further evidence that unexpected sounds can lead to a rapid inhibition of oculomotor planning in everyday tasks such as reading.
Supplemental Material
Supplemental material, sj-docx-1-qjp-10.1177_1747021820982267 for Distraction by auditory novelty during reading: Evidence for disruption in saccade planning, but not saccade execution by Martin R Vasilev, Fabrice BR Parmentier and Julie A Kirkby in Quarterly Journal of Experimental Psychology
Acknowledgments
We thank Rachel Sauvarin for her help with some of the data collection. We are also grateful to 3 anonymous reviewers for their valuable comments on a previous version of this manuscript. Data files, analysis scripts and materials are available at: https://doi.org/10.17605/OSF.IO/Q8HJ9.
Four more participants were tested but replaced: one due to equipment failure and three due to tracking problems caused by wearing glasses or contact lenses.
Three participants were aware that the sound presentation depended on their reading speed, although they did not know that the sounds were played only on certain words in the sentence. The fourth participant was aware that the sounds are played on specific words, but they were not certain which the exact words in the sentence were.
A “hook” occurs when participant’s gaze drifts across the invisible boundary, thus triggering it, but then crosses it back and the next fixation occurs left of the boundary.
This was done to exclude cases where the sound would be played too late relative to fixation onset. Due to a sound output latency of 14 ms in our system, this ensured that the maximum possible onset of the sound would be 14 ms after fixation onset. However, because the trigger to play the sound was usually sent during the preceding saccade, the sound output latency relative to fixation onset was usually much less (M = 4.28 ms in the 0 ms condition; M = 123.6 ms in the 120 ms delay condition [the additional 120 ms are due to the experimental delay manipulation]).
Following the typical convention in reading research, fixations shorter than 80 ms were discarded before analysis. However, the results from the survival analysis did not change when these fixations were retained in the data (the divergence point in the 0 ms delay condition was then 150 ms 95% CI = [122, 184].
Footnotes
Authors’ note: These data were presented at the 2020 EPS meeting (8–10 January) in London, UK.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Martin Vasilev was supported by a doctoral and a post-doctoral fellowship from Bournemouth University. Fabrice Parmentier was supported by a research grant from the Spanish Ministry of Science, Innovation and Universities (PSI2014-54261 -P), the Spanish State Agency for Research (AEI), and the European Regional Development Fund (FEDER).
ORCID iD: Martin R Vasilev  https://orcid.org/0000-0003-1944-8828
https://orcid.org/0000-0003-1944-8828
Data accessibility statement:


The data and materials from the present experiment are publicly available at the Open Science Framework website: https://osf.io/dscjy and https://osf.io/z769x
Supplementary material: The Supplementary Material is available at: qjep.sagepub.com
References
- Andrés P., Parmentier F. B. R., Escera C. (2006). The effect of age on involuntary capture of attention by irrelevant sounds: A test of the frontal hypothesis of aging. Neuropsychologia, 44(12), 2564–2568. 10.1016/j.neuropsychologia.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Baayen H., Davidson D. J., Bates D. M. (2008). Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language, 59(4), 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Bahill A. T., Clark M. R., Stark L. (1975). The main sequence, a tool for studying human eye movements. Mathematical Biosciences, 24(3–4), 191–204. 10.1016/0025-5564(75)90075-9 [DOI] [Google Scholar]
- Balota D. A., Pollatsek A., Rayner K. (1985). The interaction of contextual constraints and parafoveal visual information in reading. Cognitive Psychology, 17(3), 364–390. 10.1016/0010-0285(85)90013-1 [DOI] [PubMed] [Google Scholar]
- Barr D. J., Levy R., Scheepers C., Tily H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. 10.1016/j.jml.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. M., Machler M., Bolker B. M., Walker S. C. (2014). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Becker W. (1972). The control of eye movements in the saccadic system. Bibliotheca Ophthalmologica: Supplementa Ad Ophthalmologica, 82, 233–243. http://www.ncbi.nlm.nih.gov/pubmed/4568575 [PubMed] [Google Scholar]
- Becker W., Jürgens R. (1979). An analysis of the saccadic system by means of double step stimuli. Vision Research, 19(9), 967–983. 10.1016/0042-6989(79)90222-0 [DOI] [PubMed] [Google Scholar]
- Bendixen A., Grimm S., Deouell L. Y., Wetzel N., Mädebach A., Schröger E. (2010). The time-course of auditory and visual distraction effects in a new crossmodal paradigm. Neuropsychologia, 48(7), 2130–2139. 10.1016/j.neuropsychologia.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Bendixen A., Schröger E. (2008). Memory trace formation for abstract auditory features and its consequences in different attentional contexts. Biological Psychology, 78(3), 231–241. 10.1016/j.biopsycho.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Berti S. (2012). Automatic processing of rare versus novel auditory stimuli reveal different mechanisms of auditory change detection. NeuroReport, 23(7), 441–446. 10.1097/WNR.0b013e32835308b5 [DOI] [PubMed] [Google Scholar]
- Berti S., Schröger E. (2001). A comparison of auditory and visual distraction effects: Behavioral and event-related indices. Cognitive Brain Research, 10(3), 265–273. 10.1016/S0926-6410(00)00044-6 [DOI] [PubMed] [Google Scholar]
- Bouma H., De Voogd A. H. (1974). On the control of eye saccades in reading. Vision Research, 14(4), 273–284. 10.1016/0042-6989(74)90077-7 [DOI] [PubMed] [Google Scholar]
- Brainard D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. 10.1163/156856897X00357 [DOI] [PubMed] [Google Scholar]
- Bubic A., von Cramon D. Y., Jacobsen T., Schröger E., Schubotz R. I. (2009). Violation of expectation: Neural correlates reflect bases of prediction. Journal of Cognitive Neuroscience, 21(1), 155–168. 10.1162/jocn.2009.21013 [DOI] [PubMed] [Google Scholar]
- Cornelissen F. W., Peters E. M., Palmer J. (2002). The Eyelink Toolbox: Eye tracking with MATLAB and the Psychophysics Toolbox. Behavior Research Methods, Instruments, & Computers, 34(4), 613–617. 10.3758/BF03195489 [DOI] [PubMed] [Google Scholar]
- Cowan N., Winkler I., Teder W., Näätänen R. (1993). Memory prerequisites of mismatch negativity in the auditory event-related potential (ERP). Journal of Experimental Psychology: Learning, Memory, and Cognition, 19(4), 909–921. 10.1037/0278-7393.19.4.909 [DOI] [PubMed] [Google Scholar]
- Dienes Z. (2014). Using Bayes to get the most out of non-significant results. Frontiers in Psychology, 5, Article 781. 10.3389/fpsyg.2014.00781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi L. L., Catena A., Cañas J. J., Macknik S. L., Martinez-Conde S. (2013). Saccadic velocity as an arousal index in naturalistic tasks. Neuroscience and Biobehavioral Reviews, 37(5), 968–975. 10.1016/j.neubiorev.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Dutra I. C., Waller D. A., Wessel J. R. (2018). Perceptual surprise improves action stopping by nonselectively suppressing motor activity via a neural mechanism for motor inhibition. Journal of Neuroscience, 38(6), 1482–1492. 10.1523/JNEUROSCI.3091-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiter B. M., Inhoff A. W. (2010). Visual word recognition during reading is followed by subvocal articulation. Journal of Experimental Psychology: Learning Memory and Cognition, 36(2), 457–470. 10.1037/a0018278 [DOI] [PubMed] [Google Scholar]
- Engbert R. (2006). Microsaccades: A microcosm for research on oculomotor control, attention, and visual perception. Progress in Brain Research, 154, 177–192. 10.1016/S0079-6123(06)54009-9 [DOI] [PubMed] [Google Scholar]
- Engbert R., Longtin A., Kliegl R. (2002). A dynamical model of saccade generation in reading based on spatially distributed lexical processing. Vision Research, 42(5), 621–636. 10.1016/S0042-6989(01)00301-7 [DOI] [PubMed] [Google Scholar]
- Engbert R., Nuthmann A., Richter E. M., Kliegl R. (2005). SWIFT: A dynamical model of saccade generation during reading. Psychological Review, 112(4), 777–813. 10.1037/0033-295X.112.4.777 [DOI] [PubMed] [Google Scholar]
- Escera C., Alho K., Schröger E., Winkler I. (2000). Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiology and Neurotology, 5(3–4), 151–166. 10.1159/000013877 [DOI] [PubMed] [Google Scholar]
- Fawcett A. P., Dostrovsky J. O., Lozano A. M., Hutchison W. D. (2005). Eye movement-related responses of neurons in human subthalamic nucleus. Experimental Brain Research, 162(3), 357–365. 10.1007/s00221-004-2184-7 [DOI] [PubMed] [Google Scholar]
- Foxe J. J., Simpson G. V. (2002). Flow of activation from V1 to frontal cortex in humans: A framework for defining “early” visual processing. Experimental Brain Research, 142(1), 139–150. 10.1007/s00221-001-0906-7 [DOI] [PubMed] [Google Scholar]
- Fuchs A. F., Kaneko C. R. S., Scudder C. A. (1985). Brainstem control of saccadic eye movements. Annual Review of Neuroscience, 8(1), 307–337. 10.1146/annurev.ne.08.030185.001515 [DOI] [PubMed] [Google Scholar]
- Galley N. (1989). Saccadic eye movement velocity as an indicator of (de)activation. A review and some speculations. Journal of Psychophysiology, 3(3), 229–244. [Google Scholar]
- Godijn R., Kramer A. (2008). Oculomotor capture by surprising onsets. Visual Cognition, 16(2–3), 279–289. 10.1080/13506280701437295 [DOI] [Google Scholar]
- Graupner S. T., Velichkovsky B. M., Pannasch S., Marx J. (2007). Surprise, surprise: Two distinct components in the visually evoked distractor effect. Psychophysiology, 44(2), 251–261. 10.1111/j.1469-8986.2007.00504.x [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Takikawa Y., Kawagoe R. (2000). Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews, 80(3), 953–978. 10.1152/physrev.2000.80.3.953 [DOI] [PubMed] [Google Scholar]
- Horváth J., Winkler I., Bendixen A. (2008). Do N1/MMN, P3a, and RON form a strongly coupled chain reflecting the three stages of auditory distraction? Biological Psychology, 79(2), 139–147. 10.1016/j.biopsycho.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Hughes R. W., Vachon F., Jones D. M. (2005). Auditory attentional capture during serial recall: Violations at encoding of an algorithm-based neural model? Journal of Experimental Psychology-learning Memory and Cognition, 31(4), 736–749. 10.1037/0278-7393.31.4.736 [DOI] [PubMed] [Google Scholar]
- Hyönä J., Ekholm M. (2016). Background speech effects on sentence processing during reading: An eye movement study. PLOS ONE, 11(3), Article e0152133. 10.1371/journal.pone.0152133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhoff A. W., Connine C., Eiter B., Radach R., Heller D. (2004). Phonological representation of words in working memory during sentence reading. Psychonomic Bulletin & Review, 11(2), 320–325. 10.3758/BF03196577 [DOI] [PubMed] [Google Scholar]
- Inhoff A. W., Connine C., Radach R. (2002). A contingent speech technique in eye movement research on reading. Behavior Research Methods, Instruments, & Computers, 34(4), 471–480. 10.3758/BF03195476 [DOI] [PubMed] [Google Scholar]
- Inhoff A. W., Rayner K. (1986). Parafoveal word processing during eye fixations in reading: Effects of word frequency. Perception & Psychophysics, 40(6), 431–439. 10.3758/BF03208203 [DOI] [PubMed] [Google Scholar]
- Jeffreys H. (1961). Theory of probability (3rd ed.). Oxford University Press. [Google Scholar]
- Kliegl R., Nuthmann A., Engbert R. (2006). Tracking the mind during reading: The influence of past, present, and future words on fixation durations. Journal of Experimental Psychology. General, 135(1), 12–35. 10.1037/0096-3445.135.1.12 [DOI] [PubMed] [Google Scholar]
- Leigh R. J., Zee D. S. (1999). The neurology of eye movements (3rd ed.). Oxford University Press. [Google Scholar]
- Lenth R., Singmann H., Love J., Buerkner P., Herve M. (2019). emmeans: Estimated marginal means, aka least-squares means (R package version 1.4.2). https://cran.r-project.org/package=emmeans
- Li B., Parmentier F. B. R., Zhang M. (2013). Behavioral distraction by auditory deviance is mediated by the sound’s informational value: Evidence from an auditory discrimination task. Experimental Psychology, 60(4), 260–268. 10.1027/1618-3169/a000196 [DOI] [PubMed] [Google Scholar]
- Lisberger S. G., Morris E. J., Tychsen L. (1987). Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annual Review of Neuroscience, 10(1), 97–129. 10.1146/annurev.ne.10.030187.000525 [DOI] [PubMed] [Google Scholar]
- Liversedge S. P., Rayner K., White S. J., Vergilino-Perez D., Findlay J. M., Kentridge R. W. (2004). Eye movements when reading disappearing text: Is there a gap effect in reading? Vision Research, 44, 1013–1024. 10.1016/j.visres.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Ljungberg J. K., Parmentier F. B. R. (2012). The impact of intonation and valence on objective and subjective attention capture by auditory alarms. Human Factors, 54(5), 826–837. 10.1177/0018720812438613 [DOI] [PubMed] [Google Scholar]
- Ljungberg J. K., Parmentier F. B. R., Leiva A., Vega N. (2012). The informational constraints of behavioral distraction by unexpected sounds: The role of event information. Journal of Experimental Psychology: Learning Memory and Cognition, 38(5), 1461–1468. 10.1037/a0028149 [DOI] [PubMed] [Google Scholar]
- Ma T. P., Geyer H. L. (2017). The basal nuclei. In Haines D. E., Mihailoff G. A. (Eds.), Fundamental neuroscience for basic and clinical applications (5th ed., pp. 377–393). Elsevier. 10.1016/B978-0-323-39632-5.00026-8 [DOI] [Google Scholar]
- Marois A., Vachon F. (2018). Can pupillometry index auditory attentional capture in contexts of active visual processing? Journal of Cognitive Psychology, 30(4), 484–502. 10.1080/20445911.2018.1470518 [DOI] [Google Scholar]
- MathWorks. (2014). Matlab R2014a [Computer software], https://www.mathworks.com/products/matlab.html
- Matsumura M., Kojima J., Gardiner T. W., Hikosaka O. (1992). Visual and oculomotor functions of monkey subthalamic nucleus. Journal of Neurophysiology, 67(6), 1615–1632. 10.1152/jn.1992.67.6.1615 [DOI] [PubMed] [Google Scholar]
- Näätänen R., Gaillard A. W. K., Mäntysalo S. (1978). Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica, 42(4), 313–329. 10.1016/0001-6918(78)90006-9 [DOI] [PubMed] [Google Scholar]
- Näätänen R., Paavilainen P., Rinne T., Alho K. (2007). The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology, 118(12), 2544–2590. 10.1016/j.clinph.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Optican L. M., Pretegiani E. (2017). What stops a saccade? Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1718), 20160194. 10.1098/rstb.2016.0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Unguetti A. P., Parmentier F. B. R. (2016). Happiness increases distraction by auditory deviant stimuli. British Journal of Psychology (London, England : 1953), 107(3), 419–433. 10.1111/bjop.12148 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R. (2014). The cognitive determinants of behavioral distraction by deviant auditory stimuli: A review. Psychological Research, 78(3), 321–338. 10.1007/s00426-013-0534-4 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R. (2016). Deviant sounds yield distraction irrespective of the sounds’ informational value. Journal of Experimental Psychology: Human Perception and Performance, 42(6), 837–846. 10.1037/xhp0000195 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Elford G., Escera C., Andrés P., Miguel I. S. (2008). The cognitive locus of distraction by acoustic novelty in the cross-modal oddball task. Cognition, 106(1), 408–432. 10.1016/j.cognition.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Elsley J. V., Andrés P., Barceló F. (2011). Why are auditory novels distracting? Contrasting the roles of novelty, violation of expectation and stimulus change. Cognition, 119(3), 374–380. 10.1016/j.cognition.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Gallego L. (2020). Is deviance distraction immune to the prior sequential learning of stimuli and responses? Psychonomic Bulletin & Review, 27, 490–497. 10.3758/s13423-020-01717-8 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Turner J., Perez L. (2014). A dual contribution to the involuntary semantic processing of unexpected spoken words. Journal of Experimental Psychology: General, 143(1), 38–45. 10.1037/a0031550 [DOI] [PubMed] [Google Scholar]
- Parmentier F. B. R., Vasilev M. R., Andrés P. (2019). Surprise as an explanation to auditory novelty distraction and post-error slowing. Journal of Experimental Psychology: General, 148(1), 192–200. 10.1037/xge0000497 [DOI] [PubMed] [Google Scholar]
- Pelli D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442. 10.1163/156856897X00366 [DOI] [PubMed] [Google Scholar]
- Pollatsek A., Reichle E. D., Rayner K. (2006). Tests of the E-Z Reader model: Exploring the interface between cognition and eye-movement control. Cognitive Psychology, 52(1), 1–56. 10.1016/j.cogpsych.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Rayner K. (1975). The perceptual span and peripheral cues in reading. Cognitive Psychology, 81(7), 65–81. 10.1016/0010-0285(75)90005-5 [DOI] [Google Scholar]
- Rayner K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124(3), 372–422. 10.1037/0033-2909.124.3.372 [DOI] [PubMed] [Google Scholar]
- Rayner K. (2009). Eye movements and attention in reading, scene perception, and visual search. Quarterly Journal of Experimental Psychology, 62(8), 1457–1506. 10.1080/17470210902816461 [DOI] [PubMed] [Google Scholar]
- Rayner K., Duffy S. A. (1986). Lexical complexity and fixation times in reading: Effects of word frequency, verb complexity, and lexical ambiguity. Memory & Cognition, 14(3), 191–201. 10.3758/BF03197692 [DOI] [PubMed] [Google Scholar]
- Rayner K., Liversedge S. P., White S. J., Vergilino-Perez D. (2003). Reading disappearing text: Cognitive control of eye movements. Psychological Science, 14(4), 385–388. 10.1111/1467-9280.24483 [DOI] [PubMed] [Google Scholar]
- Rayner K., Slattery T. J., Drieghe D., Liversedge S. P. (2011). Eye movements and word skipping during reading: Effects of word length and predictability. Journal of Experimental Psychology: Human Perception and Performance, 37(2), 514–528. 10.1037/a0020990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K., Well A. D. (1996). Effects of contextual constraint on eye movements in reading: A further examination. Psychonomic Bulletin & Review, 3(4), 504–509. 10.3758/BF03214555 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r-project.org/ [Google Scholar]
- Reichle E. D., Pollatsek A., Fisher D. L., Rayner K. (1998). Toward a model of eye movement control in reading. Psychological Review, 105(1), 125–157. 10.1037/0033-295X.105.1.125 [DOI] [PubMed] [Google Scholar]
- Reichle E. D., Rayner K., Pollatsek A. (2003). The E-Z reader model of eye-movement control in reading: Comparisons to other models. The Behavioral and Brain Sciences, 26(4), 445–476. 10.1017/S0140525X03430100 [DOI] [PubMed] [Google Scholar]
- Reichle E. D., Reingold E. M. (2013). Neurophysiological constraints on the eye-mind link. Frontiers in Human Neuroscience, 7, Article 361. 10.3389/fnhum.2013.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle E. D., Warren T., McConnell K. (2009). Using E-Z Reader to model the effects of higher level language processing on eye movements during reading. Psychonomic Bulletin & Review, 16(1), 1–21. 10.3758/PBR.16.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold E. M., Sheridan H. (2014). Estimating the divergence point: A novel distributional analysis procedure for determining the onset of the influence of experimental variables. Frontiers in Psychology, 5, Article 1432. 10.3389/fpsyg.2014.01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold E. M., Sheridan H. (2018). On using distributional analysis techniques for determining the onset of the influence of experimental variables [Special issue]. Quarterly Journal of Experimental Psychology, 71(1), 260–271. 10.1080/17470218.2017.1310262 [DOI] [PubMed] [Google Scholar]
- Robinson D. A. (1965). The mechanics of human smooth pursuit eye movement. The Journal of Physiology, 180(3), 569–591. 10.1113/jphysiol.1965.sp007718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeber U., Berti S., Müller D., Widmann A., Schröger E. (2009). Disentangling effects of auditory distraction and of stimulus-response sequence. Psychophysiology, 46(2), 425–438. 10.1111/j.1469-8986.2008.00766.x [DOI] [PubMed] [Google Scholar]
- Roeber U., Berti S., Widmann A., Schröger E. (2005). Response repetition vs. response change modulates behavioral and electrophysiological effects of distraction. Cognitive Brain Research, 22(3), 451–456. 10.1016/j.cogbrainres.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Röer J. P., Bell R., Körner U., Buchner A. (2018). Equivalent auditory distraction in children and adults. Journal of Experimental Child Psychology, 172, 41–58. 10.1016/j.jecp.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Röer J. P., Bell R., Marsh J. E., Buchner A. (2015). Age equivalence in auditory distraction by changing and deviant speech sounds. Psychology and Aging, 30(4), 849–855. 10.1037/pag0000055 [DOI] [PubMed] [Google Scholar]
- Schilling H. E. H., Rayner K., Chumbley J. I. (1998). Comparing naming, lexical decision, and eye fixation times: Word frequency effects and individual differences. Memory & Cognition, 26(6), 1270–1281. 10.3758/BF03201199 [DOI] [PubMed] [Google Scholar]
- Schröger E. (1996). A neural mechanism for involuntary attention shifts to changes in auditory stimulation. Journal of Cognitive Neuroscience, 8(6), 527–539. 10.1162/jocn.1996.8.6.527 [DOI] [PubMed] [Google Scholar]
- Schröger E., Bendixen A., Trujillo-Barreto N. J., Roeber U. (2007). Processing of abstract rule violations in audition. PLOS ONE, 2(11), Article e1131. 10.1371/journal.pone.0001131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E., Giard M. H., Wolff C. (2000). Auditory distraction: Event-related potential and behavioral indices. Clinical Neurophysiology, 111(8), 1450–1460. 10.1016/S1388-2457(00)00337-0 [DOI] [PubMed] [Google Scholar]
- Schröger E., Wolff C. (1998). Behavioral and electrophysiological effects of task-irrelevant sound change: A new distraction paradigm. Cognitive Brain Research, 7(1), 71–87. 10.1016/S0926-6410(98)00013-5 [DOI] [PubMed] [Google Scholar]
- Sokolov E. N. (1963). Higher nervous functions: The orienting reflex. Annual Review of Physiology, 25(1), 545–580. 10.1146/annurev.ph.25.030163.002553 [DOI] [PubMed] [Google Scholar]
- Sparks D. L. (2002). The brainstem control of saccadic eye movements. Nature Reviews Neuroscience, 3(12), 952–964. 10.1038/nrn986 [DOI] [PubMed] [Google Scholar]
- Vasilev M. R., Kirkby J. A., Angele B. (2018). Auditory distraction during reading: A Bayesian meta-analysis of a continuing controversy. Perspectives on Psychological Science, 13(5), 567–597. 10.1177/1745691617747398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilev M. R., Parmentier F. B., Angele B., Kirkby J. A. (2019). Distraction by deviant sounds during reading: An eye-movement study. Quarterly Journal of Experimental Psychology, 72(7), 1863–1875. 10.1177/1747021818820816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Munoz D. P. (2011). Probing basal ganglia functions by saccade eye movements. European Journal of Neuroscience, 33(11), 2070–2090. 10.1111/j.1460-9568.2011.07691.x [DOI] [PubMed] [Google Scholar]
- Wessel J. R. (2017). Perceptual surprise aides inhibitory motor control. Journal of Experimental Psychology: Human Perception and Performance, 43(9), 1585–1593. 10.1037/xhp0000452 [DOI] [PubMed] [Google Scholar]
- Wessel J. R. (2018). Surprise: A more realistic framework for studying action stopping? Trends in Cognitive Sciences, 22(9), 741–744. 10.1016/j.tics.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J. R., Aron A. R. (2013). Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. Journal of Neuroscience, 33(47), 18481–18491. 10.1523/JNEUROSCI.3456-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J. R., Aron A. R. (2017). On the globality of motor suppression: Unexpected events and their influence on behavior and cognition. Neuron, 93(2), 259–280. 10.1016/j.neuron.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J. R., Jenkinson N., Brittain J. S., Voets S. H. E. M., Aziz T. Z., Aron A. R. (2016). Surprise disrupts cognition via a fronto-basal ganglia suppressive mechanism. Nature Communications, 7, 11195. 10.1038/ncomms11195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel N., Buttelmann D., Schieler A., Widmann A. (2016). Infant and adult pupil dilation in response to unexpected sounds. Developmental Psychobiology, 58(3), 382–392. 10.1002/dev.21377 [DOI] [PubMed] [Google Scholar]
- Widmann A., Engbert R., Schröger E. (2014). Microsaccadic responses indicate fast categorization of sounds: A novel approach to study auditory cognition. Journal of Neuroscience, 34(33), 11152–11158. 10.1523/JNEUROSCI.1568-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I., Schröger E. (2015). Auditory perceptual objects as generative models: Setting the stage for communication by sound. Brain and Language, 148, 1–22. 10.1016/j.bandl.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Yan G., Meng Z., Liu N., He L., Paterson K. B. (2018). Effects of irrelevant background speech on eye movements during reading. Quarterly Journal of Experimental Psychology, 71(6), 1270–1275. 10.1080/17470218.2017.1339718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-qjp-10.1177_1747021820982267 for Distraction by auditory novelty during reading: Evidence for disruption in saccade planning, but not saccade execution by Martin R Vasilev, Fabrice BR Parmentier and Julie A Kirkby in Quarterly Journal of Experimental Psychology