Abstract
The routine analysis of low molecular weight analytes by mass spectrometry is often complicated by the lability of the analyte’s functional groups and/or the lack of moieties that can be easily charged. If a molecule is too labile this precludes analysis by techniques such as electron ionisation or chemical ionisation as the analyte will undergo thermal decomposition prior to ionisation as well as spontaneous fragmentation during the ionisation process. If the analyte has a low propensity to form ions in electrospray ionisation (i.e., lacks acidic or basic sites) then often no analyte related ions are observed. In this paper, the robustness and versatility of the established method of atmospheric pressure chemical ionisation is demonstrated for the analysis of low molecular weight analytes. The utility of the technique is demonstrated through the analysis of 30 reference standards of varying functionality, and further by the analysis of 75 synthetic samples which were problematic when analysed by electron or electrospray ionisation. The resulting spectra are dominated by intact molecular species ([M+H]+ and M+ in positive ion mode and [M − H]− and [M + Cl]− in negative ion mode) along with logical neutral losses reminiscent of what you might expect from the analyte’s structure (losses of H2O from alcohols or CO from aldehydes etc). This paper presents atmospheric pressure chemical ionisation as an essential tool for broadening the chemical space of successful analyses for any routine mass spectrometry service laboratory of facility.
Keywords: APCI, low molecular weight, labile, routine analysis
Introduction
Atmospheric pressure chemical ionisation (APCI)1 mass spectrometry (MS) is well established for the intact analysis of polar analytes with masses up to approximately 1500 Da, such as lipids,2 steroids3 and fatty acids.4 Ionisation in APCI occurs in the vapour phase via a corona discharge stimulating a complex set of ion-molecule reactions involving the solvents, atmospheric water vapour, nitrogen gas and the analyte. This process can generate ions in positive-ion mode via protonation to produce [M+H]+ and in some instances through redox reactions generating the radical cation M·+.5 In the negative-ion mode, ionisation is dominated by deprotonation leading to the formation of [M − H]−. The exact processes that occur depend on the chemistry of the analyte and the nature of the carrier solvents.
One area of chemical space which is placing increasing analytical demands on routine MS service laboratories is the analysis of low molecular weight (LMW) labile organic molecules. For the purposes of this study, LMW is defined as being below 1000 Da. The main issues are that this class of molecule, which encompass a broad range of synthetic targets, are often too thermally labile for successful ionisation as intact species by electron ionisation (EI) or chemical ionisation (CI) or have insufficient functionality to undergo electrospray ionisation (ESI) resulting in poor quality spectra often with no recognisable intact analyte related ions. The choice between APCI and ESI is largely dependent on the analyte’s polarity and the presence of appropriate moieties for ionisation and has been the subject of much study – see for example the work by Thurman et al.6, Thomaidis et al.7 or Tian et al.8
Recently published studies conducted by our group demonstrate our ongoing development of improved methodologies for the analysis of LMW species ranging from involatile organic analytes,9 metal salts10 and LMW polymers.11 This study demonstrates the highly beneficial application of APCI-MS for the routine analysis of a broad range of thermally labile LMW analytes. The initial part of this study was to test the utility of APCI-MS by analysing a wide range of chemical standards. Most of these standards can be successfully studied by either EI or ESI, although the spectra are not always of the highest quality (see supplementary information). The purpose of studying them here is to demonstrate one of the major advantages of APCI-MS, that is crossing a very wide-ranging sample space of polarity, which is not possible with either EI or ESI. The results obtained with APCI-MS are compared to those obtained by EI or ESI. The main part of the study then consists of an analysis of 75 synthetic LMW compounds that failed to produce usable spectra by EI or ESI. In both parts of the study, a full description and rationalisation of the types of ions observed is performed. All analyses are performed at ultra-high resolution on Orbitrap based instrumentation.
Experimental
Chemicals and solvents
The chemical standards were sourced from several vendors either as microanalytical standards or chemical reference standards. See Table S1 in the supplementary information for more information. All solvents were HPLC gradient grade, obtained from Fisher Scientific (Hemel Hempstead, UK). Solutions of the chemical standards were prepared at 1 mg/mL in DCM. Synthetic samples were either provided as 1 mg/mL solutions in DCM or as solids. For the solids, solutions were prepared as for the standards. APCI and ESI analyses were performed on 25-fold diluted samples in MeOH/DCM 50% and EI analysis were performed on 33-fold diluted samples in DCM.
Being a technician led routine service, all samples are made up in the same solution conditions, unless it is specified by the supplier that the analyte is especially reactive or unstable in the standard solvents. In those cases, the sample is made up specifically in unreactive solvents (e.g., THF or Toluene) and run manually. MeOH/DCM (50%) is chosen as our standard solvent for mass spectrometry as it encompasses a broad range of analyte polarities and is fairly unreactive. APCI is tolerant to a range of solvents (polar and non-polar), however in this study, all analyses were performed in our standard solvent system.
Instrumentation
APCI-MS analyses were performed on an Orbitrap Elite mass spectrometer equipped with an APCI source (Thermo Fisher Scientific, Hemel Hempstead, UK) at a resolution of 120,000 and m/z range from 50 to 800. The source parameters were: vapouriser temperature 400 °C, capillary temperature 275 °C, discharge voltage 3.35 kV, discharge current 5μA, sheath gas flow 35, auxiliary gas flow 10, sweep gas flow 5 (arbitrary units). Tuning and calibration of the Orbitrap was performed in positive or negative ESI mode (as appropriate) using the built-in tuning and calibration routines and the appropriate Pierce LTQ ESI Calibration Solution (Applied Biosystems, Warrington, UK). Spectra were achieved by manually summing the scans with the most intense intact molecular species and subtracting a similar number of scans that had no intact analyte peaks. This has the effect of subtracting out solvent peaks, solvent-based impurities, carry over from previous samples and also any decomposition products as these undesirable signals all tend to be more intense in scans where intact molecular ion species were at lower intensity.
EI analyses were performed by GC-MS on a GC QExactive (Orbitrap) fitted with a Trace 1310 GC system (Thermo Fisher Scientific, Runcorn, UK). The Orbitrap was run in EI mode at an electron energy of 70 eV, resolution of 160,000 and m/z range from 50 to 800. The source temperature was 200 °C, transfer line was 250 °C and the transfer capillary was 200 °C. The column was a TraceGOLD low polarity silarylene column (TG-5SILMS, Thermo Scientific, Hemel Hempstead, UK) with the following dimensions: 30 m x 0.25 mm with 0.25 μm film thickness. The column oven temperature was ramped from 50 to 280 °C over 15 minutes with the inlet set to 200 °C. 1 μL of analyte solution was injected onto the column. Spectra were achieved by manually summing across the relevant GC peak and subtracting approximately 30 seconds of background signal.
ESI analyses were performed on an Orbitrap Elite mass spectrometer equipped with a HESI source (Thermo Fisher Scientific, Hemel Hempstead, UK) at a resolution of 120,000 and m/z range from 50 to 800. ESI was performed at a source voltage of 3 kV, source temperature of 350 °C and a capillary temperature of 275 °C. Calibration was as described for APCI above.
Results and discussion
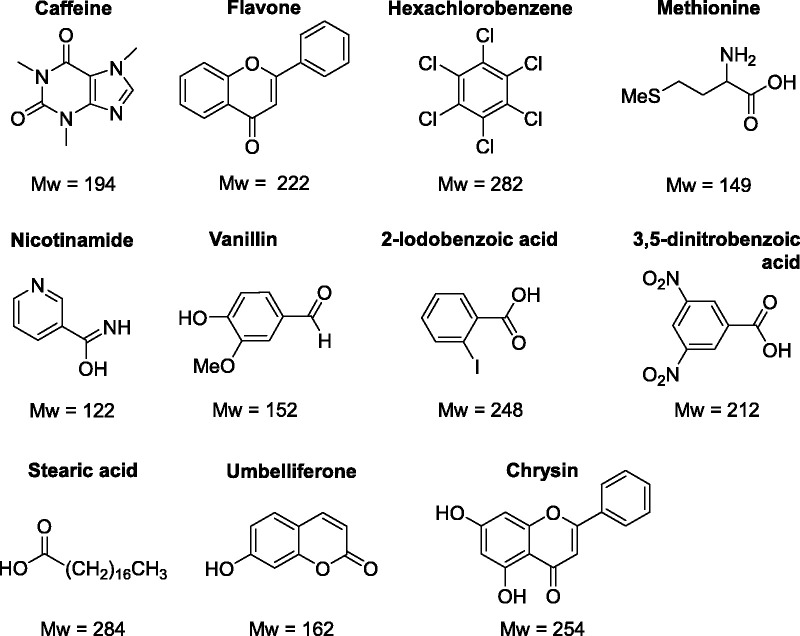
To test the efficacy and applicability of APCI-MS for the analyses of LMW compounds a study of the ionisation of chemical standards was performed using 30 compounds covering a wide range of chemical types, complexity, and functionalities. Figure 1 shows the identities, structures and molecular weights of the key chemical standards analysed. The supplementary information contains the mass spectra of the whole study allowing a full comparison of APCI with EI and ESI, a summary of which is contained in Table 1. Figure 2 shows selected results from analyses performed in the positive ion mode, whereas Figure 3 shows selected results from analyses performed in the negative ion mode. The implications of the results are discussed below.
Figure 1.
The identity, chemical structures and molecular weighs (Mw) of the standards analysed in this study.
Table 1.
The ions, relative intensities (RI) and identities observed in the analysis of the chemical standards by EI, ESI and APCI mass spectrometry. Intensities are quoted to the nearest 5% where 100% intensity is the base peak. Spectrum quality colour code: Green indicates a spectrum with an identifiable intact molecular species at or close to 100% RI. Amber indicates a spectrum with an identifiable intact molecular species observed at lower RI or a spectrum with a high baseline or with significant fragmentation. Red indicates that either the sample was not appropriate for the technique, or that no identifiable intact molecular species were observed.
| Sample | EI |
ESI |
APCI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ion | RI | ID | Ion | RI | ID | Ion | RI | ID | |
|
2-Iodobenzoic acid |
n/a |
n/a |
n/a |
247127 |
100%70% |
[M-H]- I- |
283247127 |
15%100%90% |
[M+Cl]- [M-H]- I- |
|
2,2,2-Trifluoro-N-phenylacetamide |
1891209277 |
100%50%45%20% |
M+[M-CF3]+ [120-CO]+ [92-CH3]+ |
212 |
100% |
[M+Na]+ |
190 |
100% |
[M+H]+ |
|
3-Trifluoromethylbenzoic acid |
n/a |
n/a |
n/a |
189145 |
100%45% |
[M-H]- [189-CO2]- |
225189145 |
5%100%5% |
[M+Cl]- [M-H]- [189-CO2]- |
|
3,5-Dinitro benzoicacid |
n/a |
n/a |
n/a |
211167137 |
100%45%10% |
[M-H]- [211-CO2]- Frag |
211195167 |
100%5%60% |
[M-H]- [211-O]- [211-CO2]- |
|
4-Bromobenzoic acid |
n/a |
n/a |
n/a |
199 |
100% |
[M-H]- |
235199 |
10%100% |
[M+Cl]- [M-H]- |
|
Acetanilide |
1359366 |
15%100%10% |
M+[M-CH2CO]+ Frag |
15813694 |
100%80%5% |
[M+Na]+ [M+H]+ [136-CH2CO]+ |
13694 |
100%15% |
[M+H]+ [136-CH2CO]+ |
|
Arachidic acid |
n/a |
n/a |
n/a |
347311 |
100%65% |
[M+Cl]- [M-H]- |
347 |
100% |
[M+Cl]- |
|
Asparagine |
n/a |
n/a |
n/a |
167131113 |
20%100%5% |
[M+Cl]- [M-H]- [131-H2O]- |
167131113 |
15%100%20% |
[M+Cl]- [M-H]- [131-H2O]- |
|
Aspartic acid |
n/a |
n/a |
n/a |
168132 |
35%100% |
[M+Cl]- [M-H]- |
132 |
100% |
[M-H]- |
|
Atropine |
n/a |
n/a |
n/a |
290 |
100% |
[M+H]+ |
290260142124 |
100%25%10%30% |
[M+H]+ [290-CH2O]+ FragFrag |
|
Caffeine |
1941651098267 |
100%5%10%10%10% |
M+[M-HCO]+ FragFragFrag |
217195 |
15%100% |
[M+Na]+ [M+H]+ |
195 |
100% |
[M+H]+ |
|
(+)-carvone |
150135108938254 |
20%5%30%30%100%45% |
M+[M-CH3]+ [M-C3H6]+ FragFragFrag |
n/a |
n/a |
n/a |
151123109 |
90%40%100% |
[M+H]+ [151-CO]+ [151-C3H6]+ |
|
Chrysin (positive) |
n/a |
n/a |
n/a |
277255 |
65%100% |
[M+Na]+ [M+H]+ |
n/a |
n/a |
n/a |
| Chrysin (negative) | n/a | n/a | n/a | 289253 | 10%100% | [M+Cl]- [M-H]- | 289253 | 5%100% | [M+Cl]- [M-H]- |
|
Cyclohexanone 2,4-dinitrophenylhydrozone |
n/a |
n/a |
n/a |
301279153123 |
100%5%25%35% |
[M+Cl]- [M-H]- FragFrag |
279 |
1005 |
[M+H]+ |
|
Dibenzyl disulphide |
2461819165 |
5%35%100%10% |
M+FragC7H7+Frag |
n/a |
n/a |
n/a |
24791 |
80%100% |
[M+H]+ C7H7+ |
|
Diphenyl |
15412876 |
100%5%5% |
M+[M-C2H2]+ C6H6+ |
n/a |
n/a |
n/a |
154 |
100% |
M+ |
|
Flavone |
22219416512092 |
100%75%20%25%40% |
M+[M-CO]+ FragFragFrag |
245223 |
50%100% |
[M+Na]+ [M+H]+ |
223 |
100% |
[M+H]+ |
|
Hexachlorobenzene |
284249214177142 |
100%30%25%15%25% |
M+[M-Cl]+ [249-Cl]+ [214-Cl]+ [177-Cl]+ |
n/a |
n/a |
n/a |
284 |
100% |
M+ |
|
Isatin |
14711992 |
20%100%60% |
M+[M-CO]+Frag |
170148 |
100%15% |
[M+Na]+[M+H]+ |
148 |
100% |
[M+H]+ |
|
Methionine |
n/a |
n/a |
n/a |
172150133 |
65%100%35% |
[M+Na]+[M+H]+[150-NH3]+ |
150133104 |
100%50%20% |
[M+H]+[150-NH3]+[150-CH2O2]+ |
|
Naphthalene |
128102 |
100%10% |
M+[M-C2H2]+ |
n/a |
n/a |
n/a |
128 |
100% |
M+ |
|
Nicotinamide |
n/a |
n/a |
n/a |
145123 |
35%100% |
[M+Na]+[M+H]+ |
123105 |
100%25% |
[M+H]+[123-H2O]+ |
|
Phenacetin |
17913710980 |
70%90%100%20% |
M+[M-C2H2O]+[137-C2H4]+Frag |
202180 |
100%70% |
[M+Na]+[M+H]+ |
180 |
100% |
[M+H]+ |
|
Pseudoephedrine |
n/a |
n/a |
n/a |
188166148118 |
15%30%100%15% |
[M+Na]+[M+H]+[166-H2O]+Frag |
166151123109 |
100%70%30%90% |
[M+H]+[166-CH3]+C8H11O+C7H9O+ |
|
Sinapinic acid |
n/a |
n/a |
n/a |
223 |
100% |
[M-H]- |
223208193179 |
100%50%15%35% |
[M+H]+[223-CH3]+[223-CH2O]+[223-CO2]+ |
|
Stearic acid |
n/a |
n/a |
n/a |
319283 |
100%45% |
[M+Cl]-[M-H]- |
319 |
100% |
[M+Cl]- |
| Sulphamethazine | n/a | n/a | n/a | 317301279 | 10%100%65% | [M + K]+[M+Na]+[M+H]+ | 279 | 100% | [M+H]+ |
|
Tocopherol nicotinate |
n/a |
n/a |
n/a |
558536469441 |
60%30%35%100% |
[M+Na]+[M+H]+FragFrag |
536 |
1005 |
[M+H]+ |
|
Umbelliferone |
16213410578 |
80%100%15%20% |
M+[M-CO]+FragFrag |
197161 |
25%100% |
[M+Cl]-[M-H]- |
197161 |
5%100% |
[M+Cl]-[M-H]- |
| Vanillin | 15112310981 | 100%5%10%15% | M+[M-CO]+[123-CH2]+Frag | 175153 | 65%100% | [M+Na]+[M+H]+ | 153125 | 100%10% | [M+H]+[153-CO]+ |
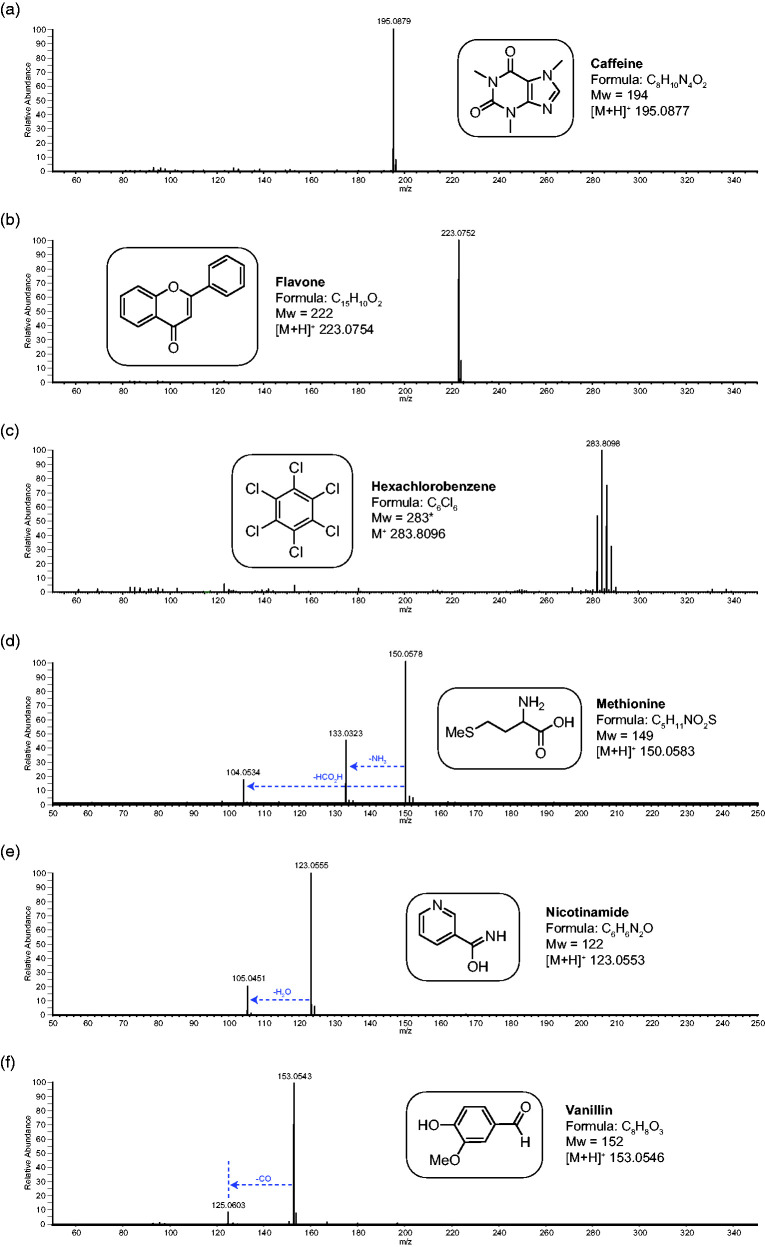
Figure 2.
The positive ion APCI mass spectra of a range of chemical standards. Spectrum (a) is caffeine (Mw 194), (b) is flavone (Mw 222), (c) is hexachlorobenzene (Mw 283), (d) is methionine (Mw 149), (e) is nicotinamide (Mw 122) and (f) is vanillin (Mw 152). *For hexachlorobenzene, the most abundant isotope of the isotope distribution is being considered. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
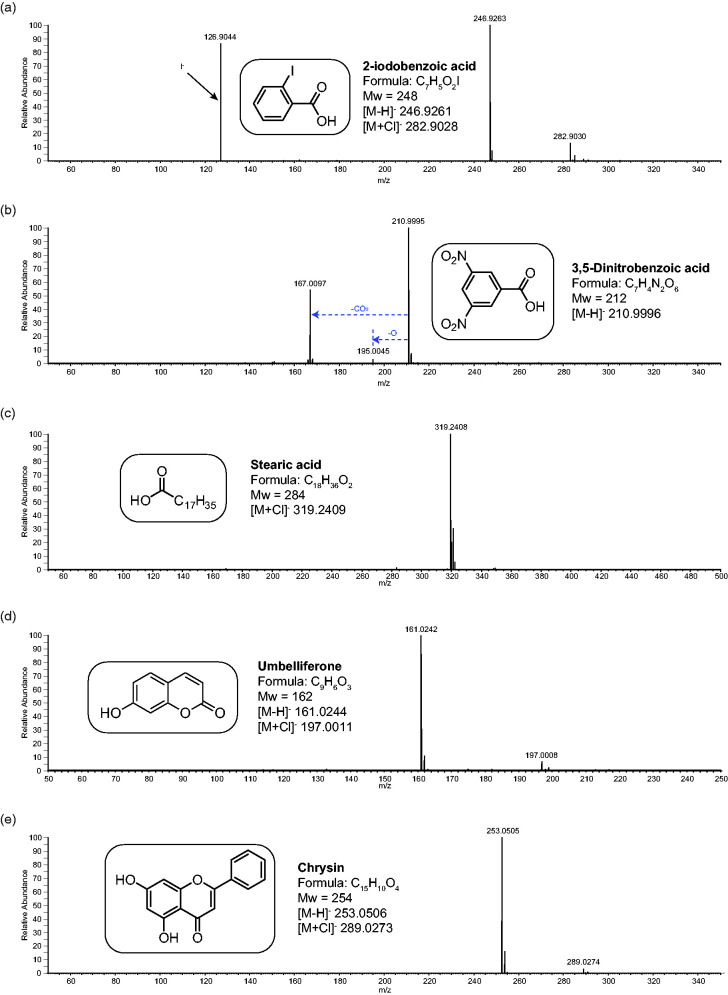
Figure 3.
The negative ion APCI mass spectra of a range of chemical standards. Spectrum (a) is 2-Iodobenzoic acid (Mw 248), (b) dinitrobenzoic acid (Mw 222), (c) is stearic acid (Mw 284), (d) is umbelliferone (Mw 162) and (e) is chrysin (Mw 254). The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
As can be seen from Figure 2 and Table 1, nearly all the analytes in the positive ion mode study generated intense [M+H]+ ions and in the vast majority of cases at 100% relative intensity (RI). In the few cases where the [M+H]+ was not the base peak, it was observed at high intensity except for the case of dibenzyl disulphide where the tropylium ion (C7H7+ m/z 91) was the base peak. With diphenyl, hexachlorobenzene and naphthalene, the M·+ was observed instead of [M+H]+. This is not surprising as these are hydrocarbons or halohydrocarbons with no heteroatoms to protonate.
In the study of the test analytes, very little (or no) fragmentation was observed in most cases. When fragmentation was observed, it was usually of simple neutral losses. For example, with the amino acid methionine (Figure 2(d)) the fragmentation was for the loss of ammonia at 45% RI and loss of formic acid at 20% RI. These are expected losses for this compound.12 With the hydroxy amide, nicotinamide (Figure 2(e)), the molecule fragments via loss of water and for the aldehyde, vanillin (Figure 2(f)), loss of carbon monoxide is observed. These are both the expected neutral losses for these two compounds and are observed at less than 20% RI.13,14
As can be seen in Figure 3 and Table 1, the analysis of carboxylic acids and phenolic compounds by negative ion APCI-MS was also very successfully applied. Generation of anions by deprotonation, however, was not the only ionisation route observed. In several cases, anion formation was by way of formation of the chloride adduct [M+Cl]−. This is a commonly observed low-intensity process in negative ion ESI analysis15 but was not expected in APCI and probably resulted from the use of DCM as the diluent and/or primary solvent.16 This suggests that APCI is a gentler ionisation method than generally expected and can favour adduct formation rather than deprotonation even with acids. Examination of the pKa of the acids (see Table 2) does suggest that there is a pKa dependence on the ionisation process with potential for some preformation of ions, the lower pKa acids favouring deprotonation. With the phenolic compounds, however, chloride adduct formation was only observed at very low RI, despite these having a much higher pKa than the carboxylic acids.
Table 2.
The pKa values of the acidic and phenolic standards analysed along with the molecular ion types observed.
| Compound | pKa | Intact ion type(s) observed. |
|---|---|---|
| 2-Iodobenzoic acid | 2.85 | [M-H]- (100) [M+Cl]- (15) |
| 3-Trifluoromethyl benzoic acid | 3.77 | [M-H]- (100) [M+Cl]- (5) |
| 4-Bromobenzoic acid | 3.97 | [M-H]- (100) [M+Cl]- (15) |
| Arachidic acid | 4.95 | [M+Cl]- (100) |
| Asparagine | 2.02 | [M-H]- (100) [M+Cl]- (10) |
| Aspartic acid | 1.88 | [M-H]- (100) |
| Chrysin | 6.64 | [M-H]- (100) [M+Cl]- (5) |
| Dinitro benzoic acid | 2.77 | [M-H]- (100) |
| Meta trifluoromethyl benzoic acid | 3.77 | [M-H]- (100) [M+Cl]- (5) |
| sinapinic acid | 3.61 | [M-H]- (100) |
| stearic acid | 4.95 | [M+Cl]- (100) |
| Umbelliferone | 7.84 | [M-H]- (100) [M+Cl]- (5) |
With 2-iodobenzoic acid (Figure 3(a)), deprotonation is the most abundant ionisation route, with a low relative intensity chloride adduct formation. There is also an intense ion for I- at m/z 127. With dinitro benzoic acid (Figure 3(b)), deprotonation again totally dominates the ionisation, but some fragmentation is also observed, especially decarboxylation [M-H-CO2]− at m/z 167. With stearic acid (Figure 3(c)), ionisation is dominated by chloride adduction. Stearic acid has the highest pKa of the acids studied at 4.95 so this is not entirely unexpected. For both of the phenolic compounds, umbelliferone and chrysin (Figures 3(d) and (e) respectively), deprotonation was highly dominant, with the chloride adduct being formed at approximately 5% RI.
The applicability APCI-MS is demonstrated here as an ionisation technique for LMW standards. One of the main aims of this study, however, is to compare the results obtained from APCI to those of the more commonly applied EI and ESI. Supplementary information Figures S1 to S4 contain all the spectra for the analyses of the standards in APCI, ESI, and EI. For the analysis of the acids and phenolics, EI is not applicable and so only ESI and APCI can be compared. The results are very similar for both techniques with a trade-off between deprotonation and chloride adduct formation and decarboxylation also being commonly observed. The exception is aspartic acid which gave very poor results by ESI. For the non-polar and hydrocarbon analytes ESI is not applicable and so only EI and APCI can be compared. In both techniques, molecular ion (M+) generation is the most common ionisation route, however the APCI spectra where beneficially almost totally devoid of any fragment ions. For the rest of the analytes, EI failed on more than 50%. ESI also struggled with several of the analytes, for example carvone, pseudoephedrine and tocopherol nicotinate where either no usable spectra were obtained, or the spectra were dominated by fragment ions. APCI gave superior spectra for all these analytes.
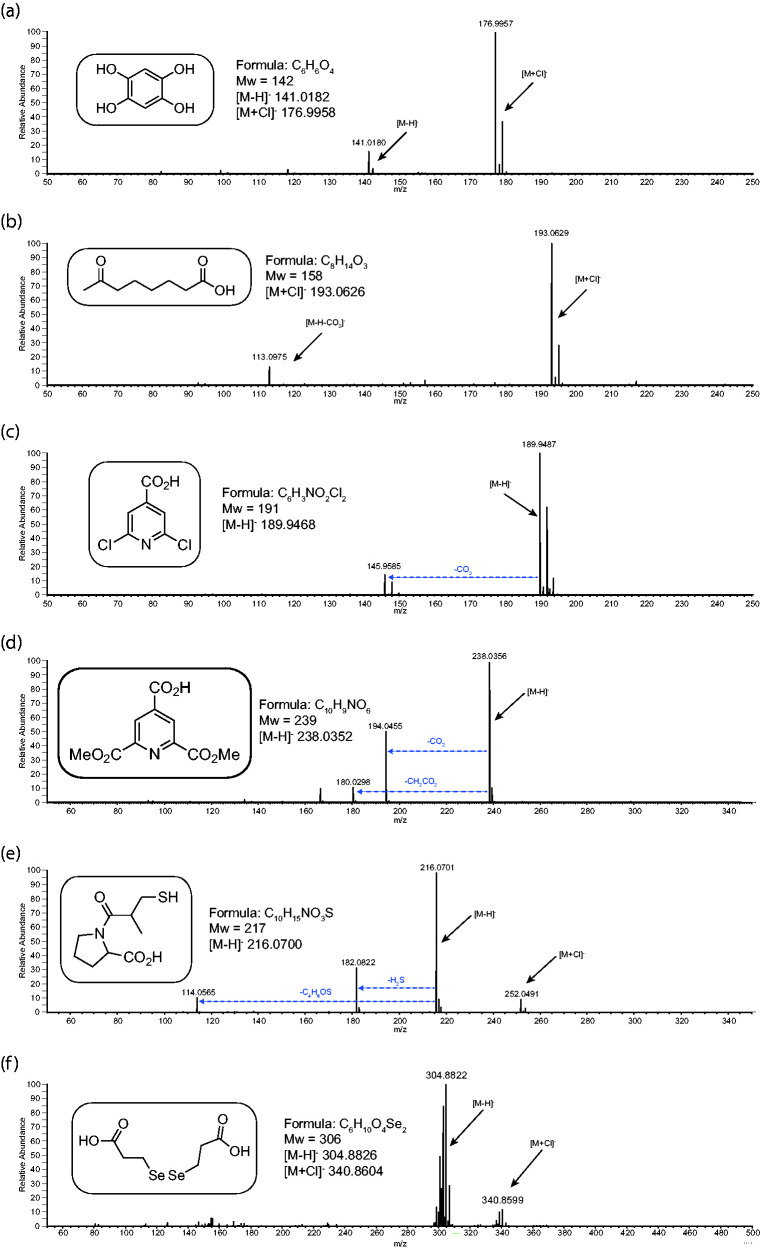
The main purpose of this study, though, is to test the application of APCI-MS to a wide range of labile, synthetic LMW analytes. In this part of the study, 75 analytes were analysed from the synthetic chemistry laboratories at the School of Chemistry, University of Bristol. All these analytes had previously been analysed by NMR and shown to be the correct structure and of high purity. Most of them had also been tested by either GC-MS or ESI-MS and had failed to give molecular ions of adequate quality or intensity for accurate-mass confirmation – in about 50% of the cases no identifiable intact molecular species were generated. All results of this study are presented in the supplementary information, with a selected few shown in Figures 4 to 9 to highlight the type of spectra obtained. The implications of the results are discussed below.
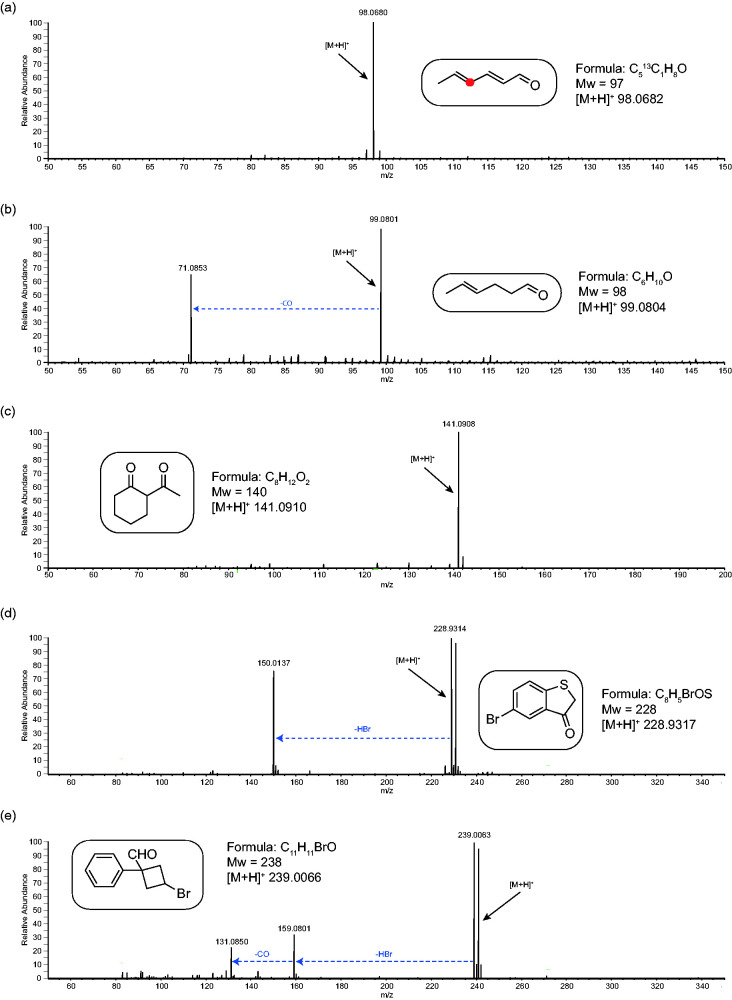
Figure 4.
The positive ion APCI mass spectra of five synthetic aldehydes and ketones. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
Figure 5.
The positive ion APCI mass spectra of four synthetic alcohols and amines. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
Figure 6.
The positive ion APCI mass spectra of four synthetic esters and ethers. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
Figure 7.
The positive ion APCI mass spectra of six synthetic cyclic and heterocyclic compounds. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
Figure 8.
The positive ion APCI mass spectra of six synthetic multifunctional compounds. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
Figure 9.
The negative ion APCI mass spectra of six synthetic acids and phenolic compounds. The structures, formulae and molecular weight information is included in the inserts. Any fragment ions are also identified.
Figure 4 shows the APCI-MS analysis of five synthetic aldehydes and ketones. All five show intense [M+H]+ ions as the base peak. In spectrum (a) the analyte is conjugated, and this prevents fragmentation from occurring. In spectrum (b), the analyte is structurally similar to (a) but is not fully conjugated, and this allows the expected loss of (CO, mass 28) to occur at high intensity. For the diketone shown in spectrum (c), there was no fragmentation observed. In contrast, in spectrum (d), the loss of hydrogen bromide (HBr, mass 80) is now observed, but due to the heterocyclic nature of the analyte, no further losses are observed. In spectrum (e) the first neutral loss is HBr which is then followed by loss of CO. The loss of HBr occurs first due to the Br− being a better leaving group resulting in no loss of CO directly from the protonated molecular ion.
The main implication of these results is that for aldehydes, if the loss of CO can occur, then it most often will. However, if a better leaving group exists, then that will generate a neutral loss that occurs first - for example, the loss of HBr in spectrum (e). For ketones, there is no loss of CO, but other neutral losses can occur depending on functional group or presence of heteroatoms like Br (for example). More examples can be found in the supplementary information (Figure S5) and similar trends are observed.
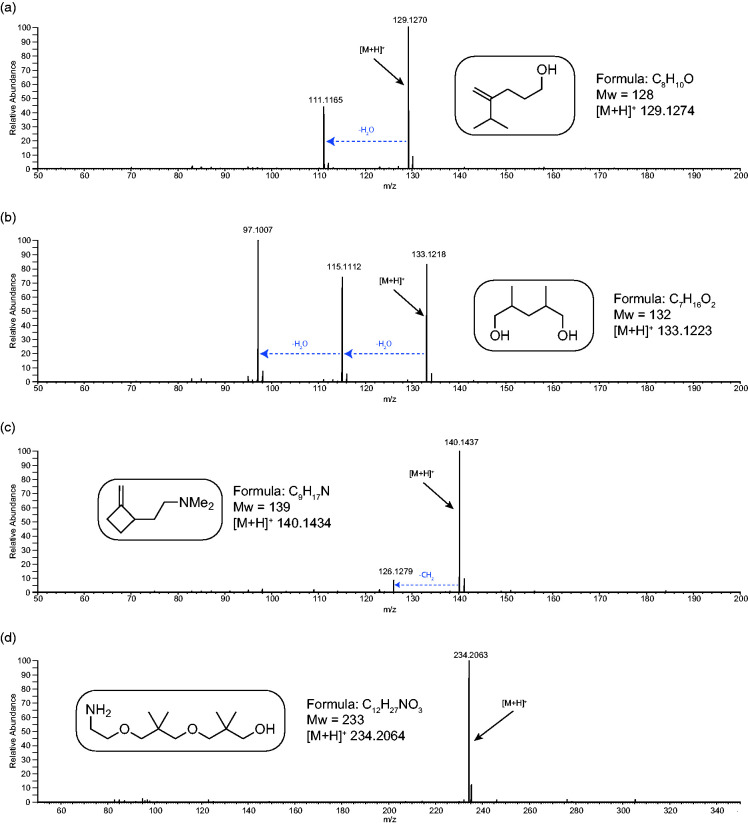
Figure 5 shows the APCI-MS analysis of four synthetic alcohols and amines. All spectra show intense [M+H]+ ions along with some expected neutral losses. For spectrum (a) there is one loss of water and for spectrum (b) - a diol - there are two losses of water. These are in line with expectations for the analysis of alcohols. For the tertiary amine in spectrum (c) there is an unexpected loss of CH2 (mass 14) – although this occurs at low intensity, it is significant. For spectrum (d) there is no fragmentation. Figure S6 shows some further examples where similar trends are observed.
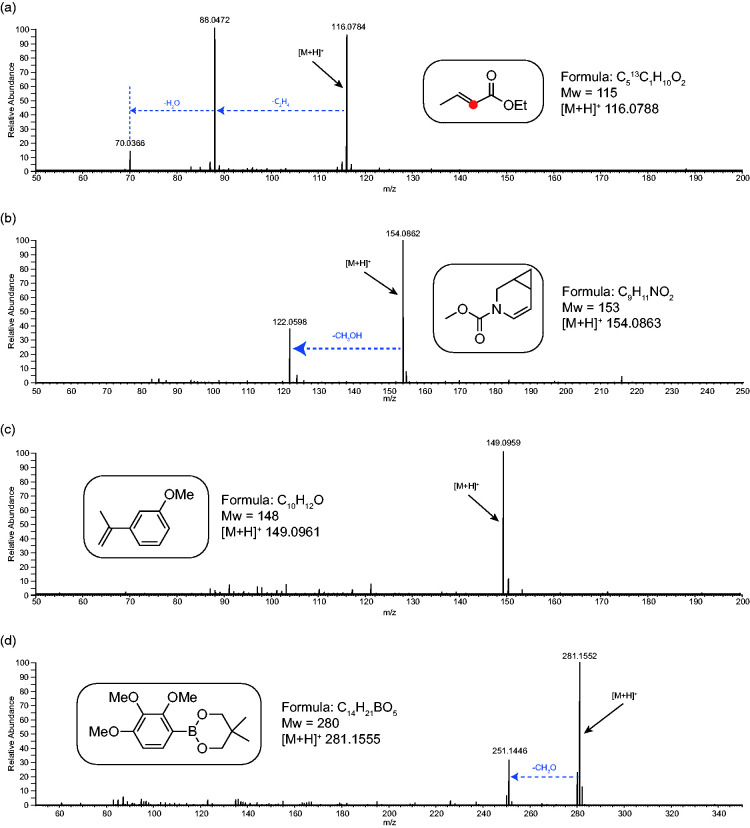
Figure 6 shows the APCI-MS of four synthetic esters and ethers. All spectra show intense [M+H]+ ions along with some neutral losses. Spectrum (a), an ethyl ester, shows an intense loss of ethene (mass 28) followed by a loss of water as opposed to the expected loss of ethanol. The losses of two neutrals is more entropically favoured and, in this case, would be due to charge-remote fragmentation indicative of the protonation site being the carbonyl oxygen of the ester moiety. Spectrum (b), a methyl ester, shows the expected loss of methanol (mass 32). In spectrum (c), an aromatic methoxy ester, no fragmentation is observed and spectrum (d), a loss of formaldehyde (mass 30) is observed. Further examples showing similar trends are shown in Figure S7.
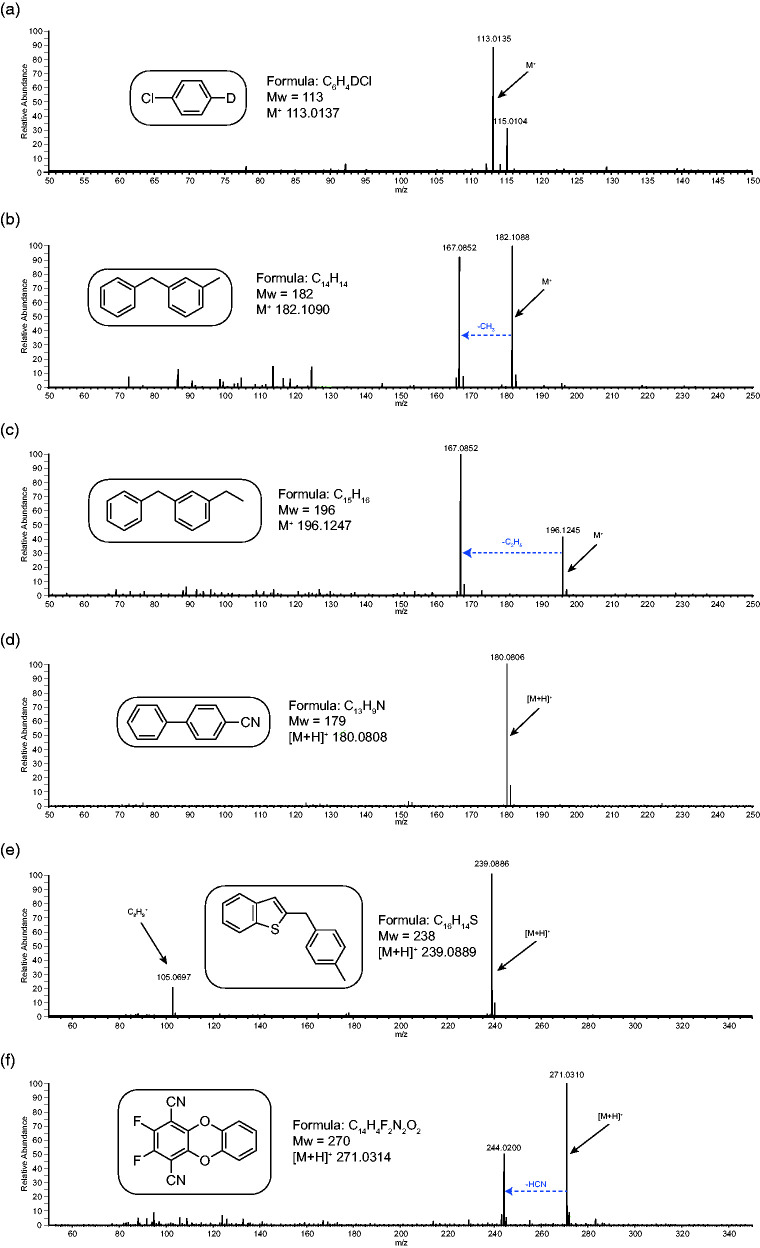
Figure 7 shows the APCI-MS of a range of cyclic and heterocyclic compounds. This class of analyte tend to be one of the most problematic for analysis by ESI and EI. For ESI they tend to give no signal at all and for EI, spectra tend to be dominated by intense aromatic rearrangements preventing routine formula confirmation. For APCI-MS, the ready generation of intact molecular species is demonstrated here for all analytes tested. Figure 7 spectra (a), (b) and (c) all show the generation of the radical molecular ion M·+. This seems to be a common observation with hydrocarbons, although some of the larger ones and the only alkyne studied did generate [M+H]+ instead (see Figure S8). In spectrum (a) the M·+ is the only peak observed. For (b) and (c) (structural analogues) alongside the M·+, there is one fragment ion due to the loss of the alkyl substituent as a radical generating the same carbocation at m/z 167. This route is more abundant in spectrum (c) (the radical molecular ion is reduced in intensity) due to the increased stability of the ethyl radical as opposed to a methyl radical for spectrum (b). Spectra (d), (e) and (f) all show the generation of [M+H]+ and for (d) it is the only ion observed. For spectrum (e) there is also an expected peak for the methyl tropylium cation (C8H9+) at m/z 105. In spectrum (f), the only fragment ion is due to the loss of HCN (mass 27). Supplementary information Figure S8 shows APCI-MS spectra for a range of other cyclic and heterocyclic compounds and similar trends for the generation of intact M·+ and [M+H]+ ions are observed along with the occasional simple neutral loss.
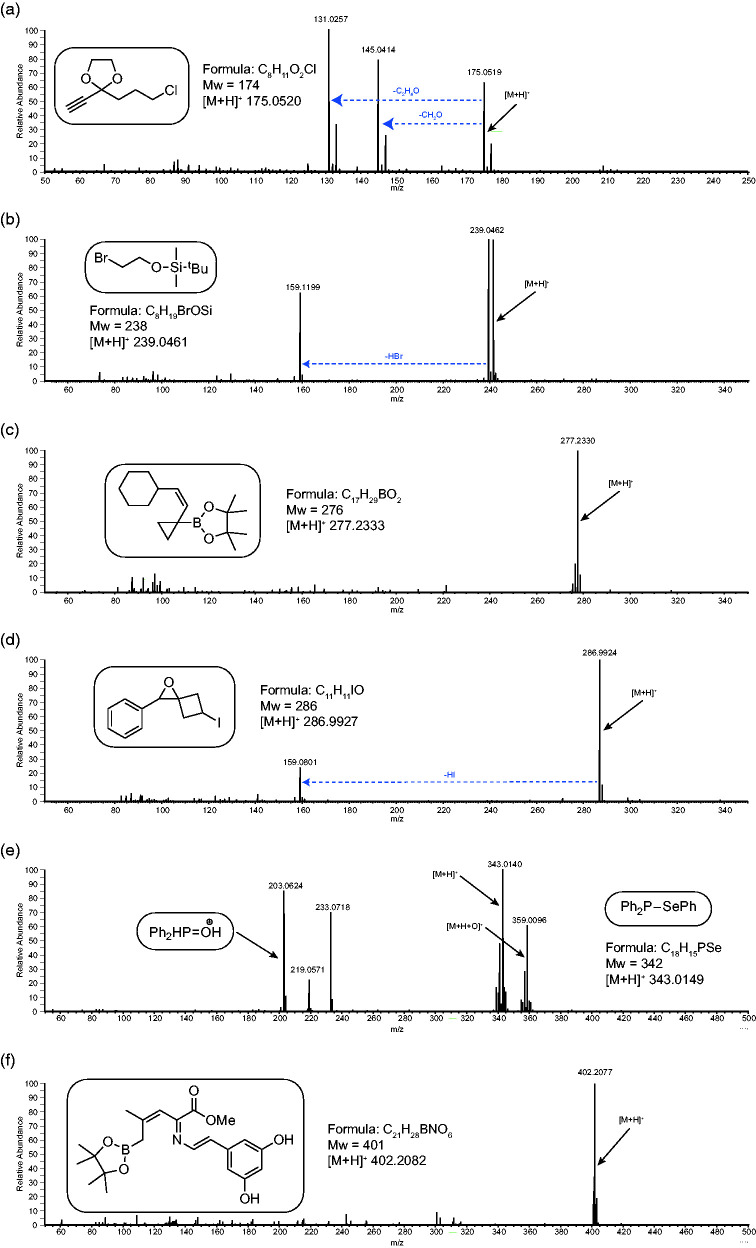
Figure 8 shows spectra for a range of more complex, multifunctional analytes. These all contain highly labile functional groups which are readily lost during EI and are hard to analyse routinely by ESI. In all spectra, an intense [M+H]+ is observed along with some fragmentation. In spectrum (a) the acetal can fragment by two competing pathways either by loss of formaldehyde CH2O (mass 30) or acetaldehyde C2H4O (mass 44) leaving the corresponding methoxy or hydroxy cation. This was unexpected and it seems interesting that both fragmentation routes seem more stable than simple protonation. In spectra (b) and (d), the only fragmentation is for loss of HBr and HI (mass 128) respectively. This type of fragmentation is commonly observed with halogenated analytes and there are several more examples in the supplementary information. For spectra (c) and (f), the only ions observed are the intact molecular species. In spectrum (e), the phosphine selenide, shows [M+H]+ as the base peak, which, due to the reactivity of the P(III) and its ease of oxidation, is virtually impossible to achieve by any other MS technique. Even here there is an intense peak for the oxidation product at [M+O+H]+ m/z 359. The spectrum also contains peaks for the oxidised phosphine fragment ions at m/z 203, 219, 233 which are assumed to be the result of the reaction of the analyte with oxygen and methanol in the APCI plasma. These can be significantly reduced by swapping to less protic solvents (THF for example) but to the detriment of [M+H]+ formation (data not shown). Figure S9 contains several more examples.
Figure 9 shows the negative ion APCI-MS of 6 synthetic acid and phenolic analytes. In all examples you can see the competition in ionisation between deprotonation to generate [M-H]−, chloride adduct formation to generate [M+Cl]− and decarboxylation to generate [M-H-CO2]−. This is similar to the study with the chemical standards discussed above and is almost certainly down to the pKa values of the various analytes and the relative stabilities of the carbanion fragments. For example, in spectra (c) and (d) intense fragment ions result from decarboxylation. This is presumably due to the resulting carbanion being aromatic. In spectra (e) and (f) no loss of CO2 is observed at all. In the case of (e), loss of hydrogen sulphide (H2S) or the thiol ketone sidechain occurs in preference to any other fragmentation.
Conclusions
In conclusion, in this study, the application of APCI-MS for the routine analysis of a wide range of labile LMW analytes has been demonstrated. This class of compound is increasingly being produced by synthetic chemists as they are seen as important building blocks towards more complex synthetic targets. The demand placed on routine MS service laboratories is that the synthetic chemist requires rapid, reliable, and high-quality results for publication. APCI is a readily available and easy-to-use technique. Sample preparation is also straightforward, with APCI being tolerant to a wide range of solvents. APCI is also fully compatible with both normal and reverse-phase HPLC. The spectra produced in this study tend to be dominated by intact molecular ions with only simple neutral losses observed, the loss of water from alcohols or carbon monoxide from aldehydes for example. This is very similar behaviour to that observed with CI, however, unlike CI, APCI-MS spectra can be recorded on a wide range of instruments with the added benefits of tandem MS (opening up the possibility of structural studies) and liquid chromatography systems (allowing the analysis of specific components of a mixture). Due to the sustained high-temperatures experienced by the analyte during CI-MS, it is not particularly applicable to thermally labile analytes, whereas this study clearly demonstrates the versatility and efficacy of APCI-MS across a very wide range of thermally labile analytes and polarity ranges.
The increasing demand for the routine analysis of LMW analytes and the fact that APCI-MS is so easily applied to this important class of compounds, with such excellent results (as included in this study) means that APCI-MS should be offered as a routine tool alongside EI-MS, ESI-MS and MALDI-MS in all routine MS service facilities.
Supplemental Material
Supplemental material, sj-pdf-1-ems-10.1177_14690667211005055 for Atmospheric pressure chemical ionisation mass spectrometry for the routine analysis of low molecular weight analytes by Paul J Gates in European Journal of Mass Spectrometry
Acknowledgements
The author would like to acknowledge the Synthetic Chemistry laboratories at the University of Bristol, School Chemistry for keeping up a continuous supply of often interesting and challenging samples for the MS service to analyse. I would also like to thank Dr Christopher Arthur for his helpful feedback during the production of this paper.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Paul J Gates https://orcid.org/0000-0001-8619-7745
Supplemental material: Supplemental material for this article is available online.
References
- 1.Carroll DI, Dzidic I, Stillwell RN, et al. Atmospheric pressure ionization mass spectrometry: corona discharge ion source for use in liquid chromatograph-mass spectrometer-computer analytical system. Anal Chem 1975; 47: 2369–2373. [Google Scholar]
- 2.Byrdwell WC. Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids. Lipids 2001; 36: 327–346. [DOI] [PubMed] [Google Scholar]
- 3.Yee-Chung MA, Hee-Yong KA. Determination of steroids by liquid chromatography/mass spectrometry. J Am Soc Mass Spectrom 1997; 8: 1010–1020. [Google Scholar]
- 4.Rězanka TJ. Analysis of polyunsaturated fatty acids using high performance liquid chromatography – atmospheric pressure chemical ionization mass spectrometry. High Resolut Chrom 2000; 23: 338–342. [DOI] [PubMed] [Google Scholar]
- 5.Holčapek M, Lísa M, Volná K, et al. Occurrence of radical molecular ions in atmospheric pressure chemical ionization mass spectra of heterocyclic compounds. J Mass Spectrom 2007; 42: 1645–1648. [DOI] [PubMed] [Google Scholar]
- 6.Thurman EM, Ferrer I, Barceló D. Choosing between atmospheric pressure chemical ionization and electrospray ionization interfaces for the HPLC/MS analysis of pesticides. Anal Chem 2001; 73: 5441–5449. [DOI] [PubMed] [Google Scholar]
- 7.Maragou NC, Thomaidis NS, Koupparis MA. Optimization and comparison of ESI and APCI LC-MS/MS methods: a case study of irgarol 1051, diuron, and their degradation products in environmental samples. J Am Soc Mass Spectrom 2011; 22: 1826–1838. [DOI] [PubMed] [Google Scholar]
- 8.Wang R, Zhang L, Zhang Z, et al. Comparison of ESI- and APCI-LC-MS/MS methods: a case study of levonorgestrel in human plasma. J Pharm Anal 2016; 6: 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren AD, Conway U, Arthur CJ, et al. Investigation of colloidal graphite as a matrix for matrix-assisted laser desorption/ionisation mass spectrometry of low molecular weight analytes. J Mass Spectrom 2016; 51: 491–503. [DOI] [PubMed] [Google Scholar]
- 10.Warren AD, Gates PJ. Flavone as a novel matrix for the matrix-assisted laser desorption/ionisation analysis of lanthanide and transition metal salts. J Mass Spectrom 2020; 55: e4609. [DOI] [PubMed] [Google Scholar]
- 11.Conway U, Warren AD, Arthur CJ, et al. A study of the application of graphite MALDI to the analysis of short-chain polyethylene glycols. Polym Chem 2021; 12: 439–448. [Google Scholar]
- 12.Choi S-S, Song MJ, Kim O-B, et al. Fragmentation patterns of protonated amino acids formed by atmospheric pressure chemical ionization. Rapid Commun Mass Spectrom 2013; 27: 143–151. [DOI] [PubMed] [Google Scholar]
- 13.Berdyshev EV. Mass spectrometry of fatty aldehydes. Biochim Biophys Acta 2011; 1811: 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neta P, Simón-Manso Y, Liang Y, et al. Loss of H2 and CO from protonated aldehydes in electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 2014; 28: 1871–1882. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Cole RB. Formation and decompositions of chloride adduct ions, [M+Cl]–, in negative ion electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 2000; 11: 932–941. [DOI] [PubMed] [Google Scholar]
- 16.McEwen CN, Larsen BS. Ionization mechanisms related to negative ion APPI, APCI, and DART. J Am Soc Mass Spectrom 2009; 20: 1518–1521. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ems-10.1177_14690667211005055 for Atmospheric pressure chemical ionisation mass spectrometry for the routine analysis of low molecular weight analytes by Paul J Gates in European Journal of Mass Spectrometry