Abstract
Sepsis is a dysregulated hyperinflammatory disease caused by infection. Sepsis leads to multiple organ dysfunction syndrome (MODS), which is associated with high rates of mortality. The cecal ligation and puncture (CLP) model has been widely used in animals and has become the gold-standard method of replicating features of sepsis in humans. Despite several studies and modified CLP protocols, there are still open questions regarding the multifactorial determinants of its reproducibility and medical significance. In our protocol, which is also aimed at mimicking the sepsis observed in clinical practice, male Wistar rats are submitted to CLP with adequate fluid resuscitation (0.15 M NaCl, 25 ml/kg BW i.p.) immediately after surgery. At 6 h after CLP, additional fluid therapy (0.15 M NaCl, 25 ml/kg BW s.c.) and antibiotic therapy with imipenem-cilastatin (single dose of 14 mg/kg BW s.c.) are administered. The timing of the fluid and antibiotic therapy correspond to the initial care given when patients are admitted to the intensive care unit. This model of sepsis provides a useful platform for simulating human sepsis and could lay the groundwork for the development of new treatments.
Keywords: Sepsis, Rats, Cecal ligation and puncture (CLP), Organ dysfunction, Animal Model, Acute Kidney Injury
Background
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection ( Singer et al., 2016 ). It is a major public health problem worldwide and is the leading cause of multiple organ dysfunction syndrome (MODS), which is associated with high in-hospital mortality (Torio and Moore, 2016; Kellum et al., 2019 ). When sepsis was first defined in 1991, the focus was on the systemic inflammatory response (ACCP/ SCCMCC et al., 1992 ). Since then, studies of pro- and anti-inflammatory molecules have advanced and have begun to reveal that the host adaptive responses go beyond immunological phenomena ( Singer et al., 2016 ). In the early or acute phase, sepsis is characterized by a hyperinflammatory host response accompanied by pro- and anti-inflammatory processes that lead to cell death by apoptosis. In the late or immunosuppressive phase, it is characterized by depletion of immune cells ( Hotchkiss et al., 2013 ). In the early phase, many physiological systems are compromised, hemodynamic and cardiopulmonary alterations being the most clinical significant symptoms ( Rello et al., 2017 ). Despite the use of several modern treatment strategies, including fluid and antimicrobial therapy, the mortality rates associated with sepsis remain high. Therefore, experimental animal models have been developed apace with the evolution of the knowledge of sepsis and advances in its treatment. The establishment of an animal model that replicates the features of sepsis in humans is essential for understanding the pathogenic mechanisms and for developing new treatment strategies. Various experimental rodent models have been described, including intraperitoneal or intravenous infusion of purified endotoxin (lipopolysaccharide), intravascular infusion of certain bacterial species, the abscess model, and the cecal ligation and puncture (CLP) model ( Baker et al., 1983 ; Parker et al., 2001 ; Fink, 2014).
The CLP model is a polymicrobial model of bacterial peritonitis that is considered the gold-standard method of replicating features of sepsis in humans, which is easily reproducible, and can be adapted to mimic specific clinical events. It has been widely used as a research tool for more than 30 years ( Wichterman et al., 1980 ). The use of this model produces a hyperdynamic circulatory state, mitochondrial dysfunction, and endothelial alterations, as well as having acute renal, pulmonary, and cardiac effects. It also stimulates receptor molecules present in immune cells, such as Toll-like receptors (TLRs), and cytokine production, as observed in pre-clinical studies ( Rodrigues et al., 2012 ; Moreira et al., 2014 ; Cóndor et al., 2016 ; Capcha et al., 2020 ). The CLP model can be modified to produce varying degrees of severity, depending on the cecum ligation site and the number of punctures made ( Singleton et al., 2003 ).
In our version of the CLP model, antibiotics and crystalloid solution are administered at 6 h after the procedure, in accordance with intensive care unit (ICU) guidelines for the restoration of blood volume and pressure ( Singer et al., 2016 ). Although early recognition of sepsis is particularly important for appropriate patient management, as well as to achieve favorable outcomes, not all patients have timely access to treatment. Therefore, the time to the start of treatment in our model is intended to simulate the delay that occurs when patients have limited access to health care. In this rat model, 5-day mortality is typically 30-60%.
Here, we describe the CLP model in detail in order to disseminate the methodology, as well as to contribute to a better understanding of the mechanisms involved in the development of sepsis.
Materials and Reagents
Gauze pads (Dentalcremer)
16 G needles (Becton Dickinson, catalog number: 305198)
21 G needles (Becton Dickinson, catalog number: 305165)
Razor blades (no specific brand)
Liquid soap (no specific brand)
Syringes (5 ml and 10 ml)
Operator protective equipment: surgical gloves and face mask
Mononylon 3.0 suture (Ethicon, Johnson & Johnson Medical Device Companies)
Mononylon 4.0 suture (Ethicon, Johnson & Johnson Medical Device Companies)
Male Wistar rats (200-300 g), obtained from institutional animal facility
Isoflurane 100% (Cristália, Isoforine®), stored at room temperature
Imipenem-cilastatin (Merck Sharp & Dohme Corp, Tienam®), stored at 4 °C
Tramadol hydrochloride 50 mg/ml
Ketamine (BioChimico, cloridrato de cetamina), stored at room temperature
Xylazine 2% (Syntec, xilazin), stored at room temperature
Alcohol 70% (Rioquimica, rialcool 70), stored at room temperature
Sterile saline solution 0.9% wt/vol (Fresenius Medical Care Ltda)
Povidone-iodine (Rioquimica, riodeinetopico), stored at room temperature
Equipment
-
Surgical instruments: dissection scissors, microdissection scissors, straight surgical forceps, straight anatomical forceps, and needle holder (IncolInstrumentosCirúrgicos)
Note: Sterilize these instruments by autoclaving before use.
Baby bed warmer (Sunbeam Heating Pad, catalog number: 000756-500-000U)
Isoflurane vaporizer (Harvard Apparatus, 75-0239 INT, catalog number: 1073/216)
Procedure
-
Preoperative settings
Design the study and experiments in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Weigh animals to verify that all weights are within the 200-300 g range and use the individual weights to determine the dose of antibiotic and the amount of fluid resuscitation to be used, as well as the doses of ketamine and xylazine in case anesthesia is necessary.
Clean and disinfect the operating table with disinfectant wipes or alcohol.
-
Anesthesia
-
Anesthetize the animals with isoflurane (0.25-2.5%/kg BW gas inhalation) or with ketamine (80-100 mg/kg BW i.p.) with xylazine (5-15 mg per kg body weight i.p.) for general tests, such as cardiovascular, pulmonary or renal studies.
Note: Commonly, rats weighing around 300 g present more tolerance to anesthetics, therefore we suggest increasing the dose according to the ranges mentioned above.
Verify the effect of the anesthesia by pinching the tail and a toe. Typically, the extremities will be unresponsive within 1-2 min after administration of the anesthetic agent(s). In our experience, isoflurane can be used for all procedures.
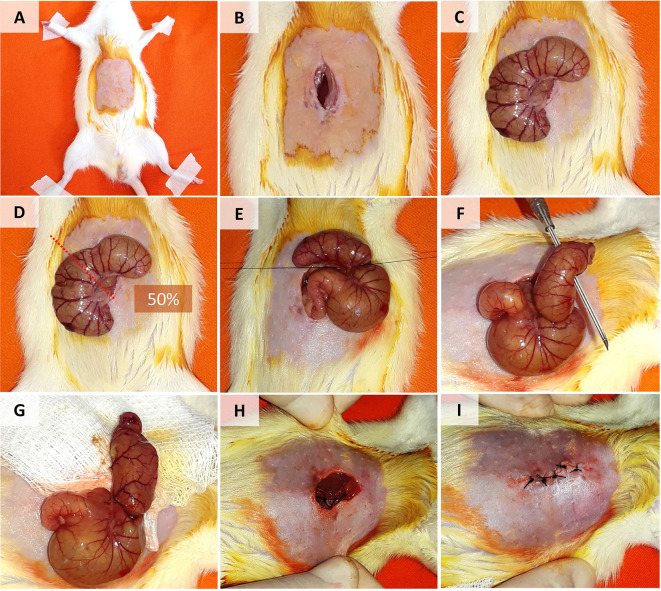
Shave the lower abdominal region using liquid soap and razor blades. Disinfect the region with gauze, alcohol, and povidone-iodine (Figure 1A).
-
Place rats in the supine position on a flat surface (Figures 1A-1I).
Note: In case of training or some reasons where the surgery take much time, we recommend using surgical eye drops to protect rat eyes.
-
-
Cecal ligation and puncture
Make a longitudinal incision (3-4 cm long) in the disinfected region with scissors and tweezers (Figure 1B).
Dissect the muscle fascia from the abdominal musculature and carefully make a 3- to 4-cm incision (Figure 1B).
-
Find the cecum, using anatomical tweezers to expose it (i.e., bring it out of the abdominal cavity). In many cases, the cecum is located on the left side of the abdomen (Figure 1C).
Note: Expose only the cecum and leave the bowels inside peritoneal cavity, taking care to do not damage the mesenteric blood vessels (Figure 1C).
Sham group: After completing the Step C3, return the cecum to the abdominal cavity, then close the abdominal musculature and skin as indicated in the Steps C8 and C9.
-
Use thin tweezers to cross the cecal mesentery with nonabsorbable silk suture 3.0 to ligate the cecum (1.5-cm from the cecal tip or 50% of the cecum; Figure 1D).
Note: Keep the cecal contents near the cecal tip and keep the intestinal passage (through the ileocecal valve) free (Figure 1E).
Puncture the distal cecum twice with a 16 G needle. The perforations should traverse the cecum (Figure 1F).
-
Squeeze the punctured distal cecum in order to extract a small amount of feces (approximately 2 drops) and distribute it around the cecum and peritoneal cavity (Figure 1G).
Note: Extract the same amount of feces and distribute it in the same regions in all animals. Use tweezers to distribute it.
Return the cecum to the abdominal cavity carefully (i.e., without extracting more feces on the abdomen).
Close the abdominal musculature by simple suturing. Before completing the closure, administer fluid resuscitation with pre-warmed saline solution 0.9% or 0.15 M NaCl at 37 °C (25 ml/kg BW i.p.), as illustrated in Figure 1H.
-
Close the abdominal skin with simple sutures (Figure 1I).
Note: Ideally all these steps should be performed within 10 min, controlled from the first surgical step (Video 1).
-
Postoperative care
After abdominal closure, inject tramadol hydrochloride 50 mg/ml (dose: 5 mg/kg i.m., every 8 h) for postoperative analgesia and continue as necessary.
-
Return rats immediately to pre-warmed cages with a baby bed warmer (25 °C) below each cage.
Note: Food and water should be made available.
-
At 6 h after the procedure (sham or CLP), under anesthesia with isoflurane, administer imipenem-cilastatin (14 mg/kg BW in saline solution s.c.). Then inject saline solution 0.9% at 25 °C (25 ml/kg BW in saline solution s.c.).
Note: Subcutaneous administration (s.c.) for saline solution should be distributed in different abdominal regions, we suggest at the least 5 places. Be careful and avoid administering high volume of solution in a specific zone
Return rats immediately to cages in a temperature-controlled environment (20 °C), on a 12/12-h light/dark cycle, with ad libitum access to food and water. The animals should be monitored every 6 h, and outcomes should be evaluated at 24 h or 48 h.
Figure 1. Cecal Ligation and Puncture Model in Rats.
A. Disinfection and shaving of the lower abdominal region. B. Longitudinal incision in the skin and muscle fascia. C. Cecum exposure. D-E. Cecal ligation (in the mid-cecum). F. Puncture of the distal cecum with a 16 G needle. G. Squeezing and distribution of feces around the cecum and peritoneal cavity. H-I. Closure of the abdominal muscle fascia and skin.
Video 1. Cecal Ligation and Puncture Model in Rats.
This video was made at University of São Paulo (USP) according to guidelines from the “USP” on Animal Care and approved by the Research Ethics Committee at University of São Paulo School of Medicine under protocol # 378 603.
Notes
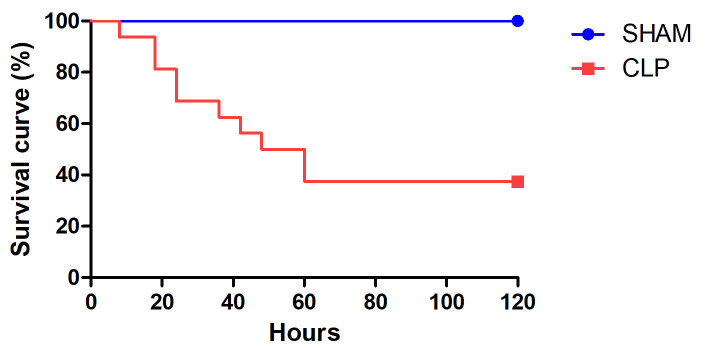
This rat CLP protocol mirrors the clinical conditions seen in patients with sepsis. Despite the fact that fluid and antimicrobial therapy are administered at 6 h after sepsis induction, the rats develop MODS and the overall mortality rate is 30-60% (Figure 2). The results are reproducible, as has been demonstrated in pre-clinical studies ( Rodrigues et al., 2012 ; Moreira et al., 2014 ; Cóndor et al., 2016 ; Capcha et al., 2020 ). However, deviation from the protocol, especially in the cecal ligation or cecal puncture steps, can result in some variability. Volume resuscitation is extremely important to maintain the central blood volume of the rats. The fluid resuscitation is performed in order to mimic the treatment given to patients in the early (hyperdynamic) phase of sepsis. It is also important that the saline solution be pre-warmed (to 37 °C) to avoid iatrogenic hypothermia, which is likely to affect the outcome of the CLP procedure. Precautions should be taken to avoid cecal bleeding and ileocecal obstruction, which could cause the rats to stop drinking water and go into hypovolemic shock.
Figure 2. Survival curve in rats.
Survival rates in rats induced to sepsis by cecal ligation and puncture (CLP, n = 12) or submitted to a sham procedure (n = 16), with administration of fluid and antimicrobial therapy at 6 h after the procedure.
In patients with sepsis, fluids such as saline or crystalloid solution (i.e., Ringer’s lactate solution) are administered in accordance with established guidelines ( Singer et al., 2016 ). Although the total volume administered varies depending on the condition of each patient, it should be sufficient to restore normal blood volume and pressure, thus ensuring organic perfusion. In clinical practice, the initial antimicrobial should be a broad-spectrum antibiotic and should be re-evaluated after the microbiology results are known. Early antimicrobial therapy and focused control are associated with favorable outcomes ( Rello et al., 2017 ). At our institution, we use imipenem-cilastatin, a broad-spectrum antibiotic, as the first-line treatment for patients with sepsis. As in patients, all of these points should be borne in mind in experimental models of sepsis, in order to induce MODS while controlling the disease slightly.
In our pre-clinical studies ( Rodrigues et al., 2012 ; Moreira et al., 2014 ; Cóndor et al., 2016 ; Capcha et al., 2020 ), MODS was observed at 24 h after CLP. Studies employing our version of the CLP protocol have reported that the main effects are renal, cardiovascular, and pulmonary dysfunction ( Rodrigues et al., 2012 ; Moreira et al., 2014 ; Cóndor et al., 2016 ; Capcha et al., 2020 ). Such studies have also reported sepsis-induced acute kidney injury, as identified by evaluating inulin clearance, tubular transport, urinary osmolality, and mitochondrial morphology, as well as glomerular dysfunction, tubular lesion (urine concentrating defect and reduced fractional excretion of electrolytes), and inflammation ( Rodrigues et al., 2012 ; Moreira et al., 2014 ; Cóndor et al., 2016 ). Our version of the CLP protocol also promotes pulmonary inflammation, as demonstrated by greater macrophage infiltration and expression of TLR4 on the surface of macrophages ( Cóndor et al., 2016 ; Capcha et al., 2020 ).
After CLP, there is a significant increase in the serum levels of cytokines, leading to damage of the microvascular endothelium. It has also been demonstrated that CLP leads to downregulation of the expression of endothelial nitric oxide synthase, Slit2, and Robo4, proteins that are responsible for maintaining the integrity of the endothelial barrier ( Moreira et al., 2014 ). In addition, there is considerable evidence that the procedure results in cardiac injury, manifesting as diastolic dysfunction, a reduction in the left ventricular end-diastolic diameter and pressure, and sustained increase in heart rate ( Moreira et al., 2014 ). Sepsis can reduce renal expression of Klotho, which has been be associated with worse outcomes ( Cóndor et al., 2016 ). Sepsis is characterized by a hyperinflammatory mechanism, TLR4/nuclear factor-kappa B signaling being one of the mechanisms most often defined as a parameter to evaluate treatment outcomes. Furthermore, overexpression of pro-inflammatory cytokines in the kidney, spleen, heart, and serum has been reported in animals induced to sepsis. As a consequence of this inflammatory disorder, an apoptotic mechanism involving BAX and Bcl-2 expression, together with terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling-positive cells, has also been reported ( Rodrigues et al., 2012 ; Moreira et al., 2014 ; Cóndor et al., 2016 ). Our version of the CLP protocol can elicit these major mechanisms of sepsis.
The CLP model can be adapted to the needs/goals of each laboratory. In our version of the CLP protocol, the endpoint of MODS is achieved, as evidenced by the mortality rate. This novel CLP protocol was established in order to mimic the conditions seen in ICU patients with sepsis. This protocol, involving controlled MODS, has proven to be reproducible across studies if all of the technical parameters are carefully managed.
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 2010/19012-0). JMCC is the recipient of a FAPESP grant (Grant no. 2015/21308-9). LA is the recipient of a grant from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development; Grant no. 301193/2016-9).
The present protocol was adapted from Wichterman et al. (1980).
Competing interests
The authors declare that they have no conflicts of interest or competing interests.
Ethics
The Research Ethics Committee of the University of São Paulo School of Medicine Hospital das Clínicas, in the city of São Paulo, Brazil, has approved the use of this protocol (Reference no. 378 603). All procedures are in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. ACCP/SCCMCC(1992). American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20(6): 864-874. [PubMed] [Google Scholar]
- 2. Baker C. C., Chaudry I. H., Gaines H. O. and Baue A. E.(1983). Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery 94(2): 331-335. [PubMed] [Google Scholar]
- 3. Capcha J. M. C., Rodrigues C. E., Moreira R. d. S., Silveira M. D., Dourado P., Dos Santos F., Irigoyen M. C., Jensen L., Garnica M. R., Noronha I. L., Andrade L. and Gomes S. A.(2020). Wharton's jelly-derived mesenchymal stem cells attenuate sepsis-induced organ injury partially via cholinergic anti-inflammatory pathway activation. Am J Physiol Regul Integr Comp Physiol 318(1): R135-R147. [DOI] [PubMed] [Google Scholar]
- 4. Cóndor J. M., Rodrigues C. E., Sousa Moreira R., Canale D., Volpini R. A., Shimizu M. H., Camara N. O., Noronha Ide L. and Andrade L.(2016). Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction. Stem Cells Transl Med 5(8): 1048-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fink M. P.(2014). Animal models of sepsis. Virulence 5(1): 143-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hotchkiss R. S., Monneret G. and Payen D.(2013). Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13(12): 862-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kellum J. A., Wen X., de Caestecker M. P. and Hukriede N. A.(2019). Sepsis-Associated Acute Kidney Injury: A Problem Deserving of New Solutions. Nephron 143(3): 174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moreira R. S., Irigoyen M., Sanches T. R., Volpini R. A., Camara N. O., Malheiros D. M., Shimizu M. H., Seguro A. C. and Andrade L.(2014). Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol RegulIntegr Comp Physiol 307(5): R514-524. [DOI] [PubMed] [Google Scholar]
- 9. National Research Council(US). (2011). Committee for the Update of the Guide for the Care and Use of L. Guide for the Care and Use of Laboratory Animals[Online]. 8th edition. [Google Scholar]
- 10. Parker S. J. and Watkins P. E.(2001). Experimental models of gram-negative sepsis. Br J Surg 88(1): 22-30. [DOI] [PubMed] [Google Scholar]
- 11. Rello J., Valenzuela-Sanchez F., Ruiz-Rodriguez M. and Moyano S.(2017). Sepsis: A Review of Advances in Management. Adv Ther 34(11): 2393-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodrigues C. E., Sanches T. R., Volpini R. A., Shimizu M. H. M., Kuriki P. S., Camara N. O. S., Seguro A. C. and Andrade L.(2012). Effects of continuous erythropoietin receptor activator in sepsis-induced acute kidney injury and multi-organ dysfunction. PloS one 7(1): e29893-e29893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., Hotchkiss R. S., Levy M. M., Marshall J. C., Martin G. S., Opal S. M., Rubenfeld G. D., van der Poll T., Vincent J. L. and Angus D. C.(2016). The Third International Consensus Definitions for Sepsis and Septic Shock(Sepsis-3). JAMA 315(8): 801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singleton K. D. and Wischmeyer P. E.(2003). Distance of cecum ligated influences mortality, tumor necrosis factor-alpha and interleukin-6 expression following cecal ligation and puncture in the rat. Eur Surg Res 35(6): 486-491. [DOI] [PubMed] [Google Scholar]
- 15. Torio C. M. and Moore B. J.(2016). National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. Statistical Brief#204. In: Healthcare Cost and Utilization Project(HCUP) Statistical Briefs[Internet]. Rockville(MD): Agency for Healthcare Research and Quality(US).
- 16. Wichterman K. A., Baue A. E. and Chaudry I. H.(1980). Sepsis and septic shock--a review of laboratory models and a proposal.J Surg Res 29(2): 189-201. [DOI] [PubMed] [Google Scholar]