Abstract
Adenomyosis is a non-malignant uterine disorder in which endometrial tissue exists within and grows into the myometrium. Animal models have generated limited insight into the still-unclear pathogenesis of adenomyosis, provided a platform for preclinical screening of many drugs and compounds with potential as therapeutics, and elucidated mechanisms underlying the pain and fertility issues that occur in many women with the disease. Spontaneous adenomyosis has been studied in non-human primates, primarily in the form of case reports. Adenomyosis is routinely experimentally induced in mice through methods such as neonatal tamoxifen exposure, pituitary engraftment, and human tissue xenotransplantation. Several studies have also reported hormonal or environmental toxicant exposures that give rise to murine adenomyosis, and genetically engineered models have been created that recapitulate the human-like condition, most notably involving alteration of β-catenin expression. This review describes the animal models for adenomyosis and their contributions to our understanding of the factors underpinning the development of symptoms. Animal models represent a unique opportunity for understanding the molecular basis of adenomyosis and developing efficacious treatment options for affected women. Herein, we assess their different potentials and limitations with regard to identification of new therapeutic interventions and reflect on future directions for research and drug validation.
Keywords: Adenomyosis, animal model, infertility, non-human primates, rodents
Introduction
Adenomyosis is a common uterine condition that often appears alongside other uterine disorders such as endometriosis and leiomyomas and is characterized by lesions in the myometrium consisting of endometrium-like glands and stroma [1]. Though as many as one third of affected women are asymptomatic, frequent symptoms include abnormal or heavy uterine bleeding, dysmenorrhea, and pelvic pain, and there are strong associations with subfertility and pregnancy complications [1,2]. The only highly effective treatment for adenomyosis is hysterectomy; however, more conservative symptom-targeting treatments using nonsteroidal anti-inflammatory drugs, hormonal therapies, and minor surgeries are often first line treatments [2,3] (and Cope et al and Chen et al, this issue). Though it is widely accepted that adenomyosis begins with the invasion of endometrial tissue into the junctional zone of the myometrium, the etiology is still unclear [1,4]. Several theories have been suggested to explain the origin of this condition, such as abnormal angiogenesis in the junctional zone, myofibroblastic or Müllerian metaplasia, tissue remodeling in response to wound healing, and epithelial-mesenchymal transition (EMT) of endometrial cells resulting in the gain of invasive properties [2,4] (and Antero et al and Zhai et al, this issue). Whatever the true explanation of the pathogenesis, several studies indicate that in spite of their ectopic location, adenomyotic cells are relatively similar to endometrial cells at the molecular level even though there are important differences [5–7].
Animal models have been useful for the study of adenomyosis, in particular regarding analysis of pathogenesis and development of therapeutics. Study of spontaneous adenomyosis in non-human primates have been done primarily in rhesus macaques and baboons, and spontaneous cases occur in a variety of non-primate species such as horses, dogs, cats, rabbits, rats, and mice [8,9]. Cases of spontaneous adenomyosis afford the opportunity to study the natural development of adenomyosis, which is particularly beneficial in non-human primates with very similar reproductive biology to humans. However, inducing the disease in laboratory animals via hormone exposure or genetic alteration allows more precise manipulation of experimental design, more consistent development of disease conditions, and a greater efficiency regarding cost and time. Each of these models has been successfully utilized in the past and has a unique potential to lead to a better understanding of adenomyosis pathogenesis and treatment.
Non-human Primate Models of Adenomyosis
Information related to the incidence of spontaneous adenomyosis amongst animal species is limited, but unlike endometriosis which is present in women and menstruating non-human primates, adenomyosis has been reported in several nonmenstruating animal species [9]. In the context of animal models for adenomyosis, various mouse models have provided significant insight into the pathophysiology of the disease, and these data are extensively described in the subsequent section of this review.
Adenomyosis in non-human primates has been mostly described in case reports (see Table 1 in [10]; [11]). Although still limited, studies in the baboon and rhesus macaque have been more extensive, and spontaneous adenomyosis has been reported in both macaques and baboons [10]. In baboons, adenomyosis is associated with primary infertility [12]. In this study adenomyosis was also strongly associated with the presence of endometriosis, but when endometriosis was excluded when histopathology at necropsy did not show the presence of coexisting endometriosis, it was evident that adenomyosis was the primary cause associated with infertility. Spontaneous adenomyosis has also been reported in both free ranging and captive macaques [13,14].
Table 1.
Major experimental mouse models of adenomyosis
| Model | Molecular defects | Symptoms exhibited | Treatments tested |
|---|---|---|---|
| Pituitary engraftment | Hyperprolactinemia [33,42], increased MMPs [45], increased P4 [50] | Subfertility [50] | MMP inhibitors [45,90], danazol [72–74], GnRH antagonist [73], SPRMs [78–80], fulvestrant [73], rhein [87], angiogenesis inhibitor [91], probucol [92] |
| Tamoxifen treatment | Increased NGF [36,59,62,63], decreased scribble [66], increased collagens [62], platelet-induced EMT [65] | Hyperalgesia [82–86,88,93], subfertility [76,100] | GnRH agonist [76], l-THP [82], andrographolide [82], EGCG [83,84], resveratrol [85], leonurine [86], VPA [82,86,88], antiplatelet therapy [93] |
| β-catenin activation | E2-induced EMT [26] | Infertility [99] | N/A |
| Xenotransplantation | E2/VEGF-induced angiogenesis [38], E2-induced EMT [40,41] | N/A | ANXA2 inhibitor [41], raloxifene [40], photodynamic therapy [39] |
Abbreviations: ANXA2, annexin A2; EGCG, epigallocatechin-3-gallate; EMT, epithelial-mesenchymal transition; E2, estradiol; l-THP, levo-tetrahydropalmatine; MMP, matrix metalloproteinase; NGF, nerve growth factor; P4, progesterone; SPRM, selective progesterone receptor modulator; VEGF, vascular endothelial growth factor; VPA, valproic acid
With the advancement of imaging methodologies, the diagnosis of adenomyosis has improved; however, it remains a poorly understood disease. Furthermore, in some patients, adenomyosis coexists with other gynecological conditions, such as endometriosis and uterine fibroids, which complicates the clinical diagnosis of the disease (reviewed in [15]) (and Upson et al, this issue). The pathogenic mechanisms involved in the development of adenomyosis need to be fully elucidated, but recent studies have shown that sex steroid hormone receptors, inflammatory molecules, extracellular matrix enzymes, growth factors, and neoangiogenic factors play a major roles [16,17] (and Zhai et al, this issue).
Given the difficulty in establishing a definitive diagnosis, especially at early stages of adenomyosis development, a natural animal model, such as the non-human primate, would greatly facilitate our knowledge on the early events associated with the initiation of the pathogenesis. Such a model would also permit studying the impact of the disease on endometrial function which may contribute to adenomyosis-associated infertility. Since spontaneous adenomyosis occurs in both baboons and rhesus monkeys, they may serve good experimental models to study adenomyosis. This concept is further validated by the extensive insights gained regarding the pathophysiology of endometriosis using these two non-human primate models [18–23]. As with the induction of endometriosis, the baboon has multiple advantages as a potential non-human primate model for studying the pathophysiology of adenomyosis. The baboon is larger than the macaque and is amenable to multiple blood draws and laparoscopic surgeries [19,24]. In addition, due to the anatomy of the baboon cervix, trans-cervical aspiration of the endometrium is easily facilitated, which is of significance in the development of a model since hysterotomy was weakly associated with development of adenomyosis in the baboon [12].
Future study of adenomyosis in non-human primates may provide an experimental model for deductive research. Although an induced model of adenomyosis has not been developed to date in the baboon, Barrier et al., [10] have suggested a number of potential methodologies that could be utilized. The use of non-invasive imaging modalities such as MRI and vaginal ultrasound may provide sufficient sensitivity and specificity to identify animals in a colony that could be used to follow the disease and evaluate responses to therapies. A single report using the pigtail macaque describes the utility of using MRI and an anti-estrogenic compound for the treatment of spontaneous adenomyosis [25]. For the induction of adenomyosis in baboons, Barrier et al., [10] have suggested that this non-human primate might be particularly useful for invasive experimental studies by trans-cervical aspiration and intra-myometrial injection of aspirant. Another possibility would be to obtain endometrial basal and stromal cells by trans-cervical aspiration and to modify genes suspected to play a role in pathogenesis of adenomyosis. Our studies in which constitutive activation of β-catenin in the murine uterus leads to development of adenomyosis [26] and other transgenic models [27,28] suggest that similar approaches in the baboon model may be feasible. Although an experimental adenomyosis model in non-human primates has still to be developed, these ideas provide future possibilities for adenomyosis research.
Mouse Models of Adenomyosis
Some of the earliest studies of reporting data on adenomyosis in mouse models took advantage of high rates of spontaneous adenomyosis in two inbred SHN and SLN strains, and several other strains have been reported to regularly develop adenomyosis naturally, such as CD-1, SMXA, GR/A, and C3H/He [9,29–31]. Early on, the discovery was made that inhibition of pituitary function suppressed natural adenomyosis development and that intrauterine pituitary transplantation caused adenomyosis development [32,33]. This pituitary engraftment model has been used frequently as a model for induced adenomyosis in mice. Mice also develop adenomyosis after treatment regimens with estrogenic compounds or progesterone (P4) [34,35]. Neonatal treatment with tamoxifen, a selective estrogen receptor modulator (SERM), reliably produces adenomyotic lesions in mice, and this model has been used extensively for examining adenomyosis pathogenesis and drug screening [1,36]. Though limited in number, a few studies using genetically engineered mice have also reported adenomyosis development, providing insight into genetic abnormalities that may underlie adenomyosis development, such as increased β-catenin activation [26,37]. Finally, several studies have utilized xenotransplantation of human adenomyotic lesions into immunodeficient mice to monitor development or treatment of human lesions in an in vivo system [38–41]. The following sections will describe what has been learned about the pathogenesis of adenomyosis in mouse models, the therapeutic effects of several proposed treatments using these mouse models, and the degree to which these models recapitulate the symptoms of pain and infertility often observed in women with adenomyosis (Table 1).
Pathogenesis
HORMONE EXPOSURE
Clues to the causes and pathogenesis of adenomyosis can be gleaned from the mechanisms of disease development in mice. Numerous studies have demonstrated an enhancement of adenomyosis development by systemic exposure to various hormonal agents. In the pituitary transplantation model, most commonly using the SHN strain but also effective in others, pituitary isografts in one horn of the uterus cause adenomyosis by inducing hyperprolactinemia [33,42]. The hyperprolactinemic state leads to the invasion of endometrial stromal tissue into the myometrium followed by penetration of glands throughout the invaded area, which coincides with overall loosening and disruption of the myometrial layer and disintegration of individual muscle cells [43]. It is possible that the surgical process of implanting the pituitary graft may cause mechanical disruption that aids in the invasion of endometrial tissue, but it is not sufficient to drive adenomyosis development as demonstrated by sham controls. At least to some degree, generalized hyperprolactinemia is involved in lesion development in these mice since the uterine horn contralateral to the implanted graft develops adenomyotic lesions, though not to the same degree as the grafted horn, and pituitary grafts under the renal capsule also effectively produce this result [9,44].
One possible molecular mechanism that has been suggested for adenomyosis development based on this model involves upregulation of uterine matrix metalloproteinases (MMPs) [45]. In addition to increased uterine MMP-14 expression, isolated stromal cells from these mice exhibited increased invasiveness that could be abrogated with a MMP inhibitor [45]. Thus, elevated prolactin levels may lead to the increased ability of endometrial cells to invade the myometrium through degradation of the extracellular matrix with MMPs.
It has been noted in particular in the pituitary graft model that endometrial invasion often occurs along vasculature, hinting to the possible involvement of abnormal angiogenesis in this adenomyosis model [8]. Interestingly, some follicle-stimulating hormone (FSH) receptor haploinsufficent mice were noted to develop adenomyosis as well as abnormal vascularity and angiogenesis [46]. More specifically, abnormal vascularity included prominent small vessels or dilated vessels in the endometrium, some of which progressed to masses, and they also exhibited increased angiogenesis in the stroma [46]. These changes were accompanied by increases in luteinizing hormone receptor and progesterone receptor A (PR-A) to B (PR-B) ratio, which may contribute to the uterine pathology [46]. Furthermore, in xenotransplantation mouse models of adenomyosis, estradiol (E2) and vascular endothelial growth factor-induced angiogenesis was shown to be necessary for lesion implantation [38], and Annexin A2 was identified as a mediator of E2-induced pro-angiogenesis [41]. Each of these findings points to the possibility that abnormal angiogenesis may contribute to the development of adenomyosis lesions.
The causal attribution of adenomyosis development to an overabundance of prolactin in the pituitary engraftment model is supported by the findings that temporary inhibition of prolactin secretion suppresses spontaneous adenomyosis development [32] and that prolactin receptor mRNA is increased in the uteri of mice with induced disease [47]. Additionally, adenomyosis also occurs in mice after induction of hyperprolactinemia via dopamine antagonists [48] or dopamine receptor gene knockout [49]. However, a detailed analysis of the broader hormonal state of mice with pituitary graft-induced adenomyosis revealed that elevated prolactin levels were accompanied by increased P4 as well [50]. Moreover, pituitary grafting does not produce adenomyotic lesions in ovariectomized mice unless E2 and P4 are continuously replaced exogenously [33]. Therefore, the effect of elevated prolactin levels on the development of adenomyosis is reliant on other hormones as well.
Several studies have documented the sufficiency of prolonged P4 or P4 analog treatment to produce or heighten the severity of adenomyosis in SHN and BALB/c mice [51–54], which is interestingly at odds with the common clinical treatment of adenomyosis using a progestin-containing intrauterine device [3]. This demonstrates the importance of tight hormonal regulation to uterine health. Reports have also demonstrated that adenomyosis develops after exposure to estrogenic agents prenatally or in adulthood either alone or in combination with P4 [9,34,35,54–56]. Furthermore, chemical pollutants with known estrogenic effects have demonstrated the ability to precipitate adenomyosis development after prenatal, neonatal, or even transgenerational exposure [57,58]. Overall, though it is clear that systemic exposure to various hormonal agents causes or enhances murine adenomyosis development, studies to date have not been detailed or consistent enough to determine the exact mechanism of the affect. Disruption of the hormonal state through a variety of interventions produces similar results, and strain-specificity and concurrent pathologies complicate the interpretation.
ESTROGEN RECEPTOR MODULATION
Neonatal dosing with the SERM tamoxifen reliably leads to adenomyosis development in adult CD-1 or ICR mice [36,59]. Of the similar compounds toremifene and raloxifene, treatment with the former but not the latter produces similar rates of adenomyosis, presumably because tamoxifen and toremifene act as E2 agonists in the uterus in contrast to raloxifene [9,36]. This interpretation is complicated by the fact that treatment with E2 alone does not cause adenomyosis development, even using the same treatment protocol in the same strain of mice, implying that adenomyosis-causing effects of these SERMs are not solely from estrogenic activity [36,60] but perhaps involve other signaling pathways affected by estrogen receptor (ESR1/2).
In contrast to the pituitary engraftment model, the tamoxifen treatment method for inducing adenomyosis is more easily defined and controlled at the molecular level, and it does not require surgical manipulation that may affect lesion development. Another notable procedural difference between these models is that pituitary grafts are normally implanted around 4–8 weeks of age, whereas tamoxifen dosing occurs in the days subsequent to birth [36,43,50]. Thus the tamoxifen model allows earlier developmental changes to occur with defects becoming evident as early as 5–6 days of age [36,60]. Similar to pituitary graft-induced adenomyosis mice, tamoxifen-treated mice displayed disorganized myometrial smooth muscle bundles rather than uniform concentric muscular layers [36,43,60]. Authors studying both models have postulated that myometrial changes occur first, making room for invasion of endometrial cells [36,43]. One piece of evidence supporting this hypothesis is that disruption in smooth muscle is more widespread than areas of invading glands [36]. However, tamoxifen treatment reportedly does not produce adenomyosis in the C57/BL6J mouse even though myometrial loosening still occurs [61]. This finding indicates that the myometrial effects of tamoxifen alone are not sufficient for adenomyosis development but that other strain-specific effects are required, potentially involving the invasive character of the endometrium.
Molecular dysregulation resulting from tamoxifen-induced adenomyosis includes well-documented upregulation of nerve growth factor (NGF), which decreases during in vitro myogenic differentiation [36,59,62,63]. This is a possible molecular mechanism by which myometrial cells could resist differentiation and remain receptive to invasion by the endometrium.
EPITHELIAL-MESENCHYMAL TRANSITION
Another line of evidence gleaned from mouse models regarding the molecular underpinnings of adenomyosis pathogenesis involves EMT-mediated invasion of adenomyotic lesions into the myometrium. The process of EMT allows normally adherent and polarized epithelial cells to become individual migratory cells able to invade the extracellular matrix [64]. Transforming growth factor (TGF)-β/Smad signaling, associated with EMT, has been shown to be increased in invading adenomyotic cells [62,65]. A mechanism for E2-induced loss of epithelial cell polarity has also been proposed based on data showing a decrease of the polarity-related protein scribble in tamoxifen-induced murine adenomyosis [66]. EMT is also associated with increased collagen production leading to fibrosis, and collagens have been found to be upregulated in murine adenomyosis tissue [62]. Moreover, support for the importance of E2-induced EMT in adenomyotic lesion growth has been validated by data from mice with human adenomyosis xenotransplants [40,41].
Further evidence for the involvement of EMT in adenomyosis development is revealed by increases of β-catenin, COUP-TFII, and vimentin as well as a decrease of E-cadherin in epithelial cells of human adenomyosis (Figure 1A) [26]. β-catenin is both an essential component of the E-cadherin–catenin unit necessary to maintain epithelial identity and an integral member of the canonical wingless-type MMTV integration site family member (WNT) signaling pathway [26,37]. Mutations of the Wnt/β-catenin pathway members in cancer result in aberrant activation of their target genes, including those encoding for activators of EMT [67]. Constitutive stabilization of β-catenin in the mouse uterus leads to adenomyosis lesion formation accompanied by decreased epithelial E-cadherin expression and increases of COUP-TFII, vimentin, Snail, and Zinc Finger E-Box Binding Homeobox 1 (ZEB1) in some epithelial cells, which are hallmarks of EMT (Figure 1B) [26]. This effect has also been shown to be E2-dependent, potentially implicating β-catenin in E2-induced EMT noted in other models of adenomyosis [26].
Figure 1.
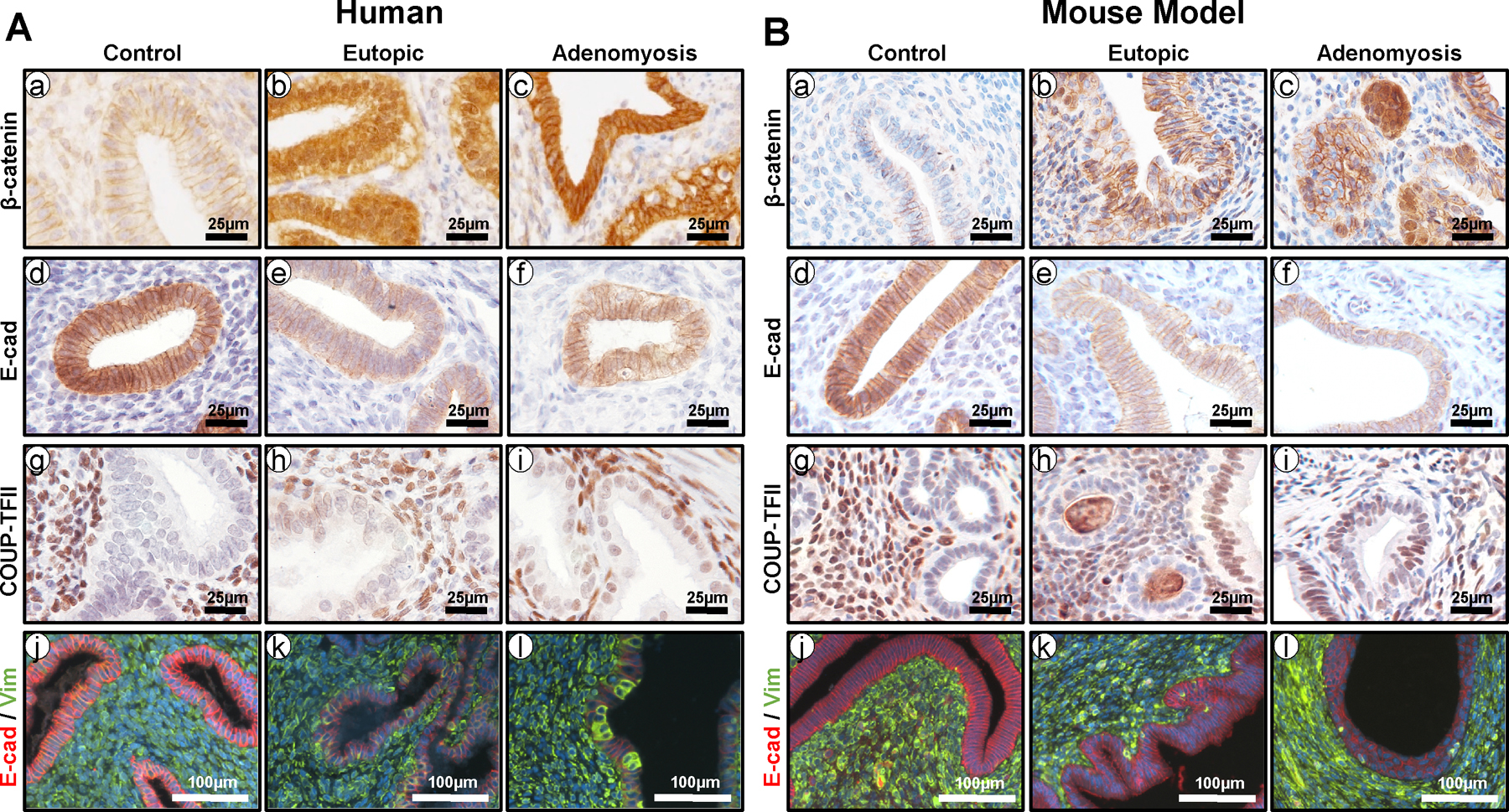
The role of aberrant β-catenin activation in EMT and adenomyosis. (A) Representative immunostaining images showing the levels of β-catenin (a-c), E-cadherin (d-f), COUP-TFII (g-i), and E-cadherin/vimentin (red/green; j-l) in endometrium from control women (a, d, g, and j) and eutopic endometrium (b, e, h, and k) and adenomyosis lesions (c, f, i, and l) from women with adenomyosis. (B) Representative immunostaining images showing the levels of β-catenin (a-c), E-cadherin (d-f), COUP-TFII (g-i), and E-cadherin/vimentin (red/green; j-l) in endometrium from control mice (a, d, g, and j) and eutopic endometrium (b, e, h, and k) and adenomyosis lesions (c, f, i, and l) from mice with β-catenin activation (Pgrcre/+Ctnnb1f(ex3)/+).
MESENCHYMAL AND IMMUNE CELL ROLES
Interestingly, mesenchymal-specific stabilization of β-catenin in the mouse uterus was also reported to result in occasional cases of adenomyosis, implying that adenomyosis-causing β-catenin activity in the uterus is not entirely limited to its epithelial role [37]. Though the alternative mechanism is not clear, other research has shown stromal β-catenin to be important for normal endometrial gland development via WNT signaling [68]. Other evidence for a mesenchymal origin of uterine defects leading to adenomyosis includes the uterine-specific forkhead box L2 (Foxl2) knockout mouse, which displays a hypertrophic, disorganized inner myometrial layer characteristic of adenomyosis potentially due to dysregulated WNT signaling, although no actual adenomyosis has been reported in this mouse [27]. In addition, mesenchymal-specific deletion of dicer, which codes for a riboendonuclease required for microRNA biosynthesis, resulted in abnormal gland formation and adenomyosis accompanied by ectopic expression of WNT signaling molecules [28].
Though not a major area of research in mouse models of adenomyosis, increased levels of immune cells have been noted in lesions of both the pituitary graft and tamoxifen-dosing models [36,69]. Increased eosinophils, as noted in one study, may be involved in tissue remodeling around adenomyotic lesions [36], while the pattern of increased monocyte-lineage cells in another study suggests that they may be involved in cytokine secretion that could lead to endometrial cell growth [69].
In view of all the observations made in mouse models of adenomyosis, it is difficult to favor any one theory of pathogenesis. A considerable number of molecular-based studies support the importance of EMT in promoting the invasiveness of endometrial cells. However, evidence that myometrial loosening and degradation proceeds endometrial invasion cannot be discounted. More detailed study is necessary to pinpoint the origin of adenomyosis pathology, although it is certainly possible that multiple explanations may be true, either in cooperation or acting independently.
Treatment
Therapies that affect steroid hormone levels or downstream signaling such as progestins, gonadotropin-releasing hormone (GnRH) agonists, SERMs, and selective progesterone receptor modulators (SPRMs) are commonly used or experimentally tried medical treatments for gynecological diseases such as endometriosis and adenomyosis [2,3,70,71]. Several studies in mouse models of adenomyosis have supported the efficacy of these treatments for adenomyosis or provided insight into their mechanisms of action.
Subcutaneous injection of danazol, an androgenic progestin, reduced spontaneous and pituitary graft-induced adenomyosis occurrence along with PRL levels in SHN mice [72,73]. Danazol has been used for treatment of adenomyosis, but it is often avoided due to severe androgenic side effects [3,74,75]. However, danazol administered via intrauterine contraceptive device (IUCD) effectively reduced pituitary graft-induced adenomyosis lesions in ICR mice while maintaining lower and more stable plasma levels than oral danazol administration, showing that this delivery mechanism may be effective while reducing side effects [74].
GnRH agonists are sometimes effective in treating E2-driven gynecologic diseases by downregulating the pituitary through negative feedback mechanisms to decrease overall ovarian hormone levels [70]. In a study where danazol only reduced adenomyosis occurrence in pituitary graft-induced adenomyosis model SHN mice, the GnRH antagonist cetrorelix entirely prevented it [73]. Furthermore, transcriptomic analysis of endometrial tissue from mice with tamoxifen-induced adenomyosis treated with a GnRH agonist revealed that treatment significantly altered the differential expression of 359 genes mainly related to the cell cycle, inflammation, and metabolic and proliferation-related signaling pathways, including some regulated by E2 [76]. These results support the idea that GnRH agonists are effective in treating adenomyosis by preventing E2-induced cell proliferation.
SPRMs and SERMs act in tissue-specific ways as either agonists or antagonists of their target hormone receptors [77]. SHN mice with pituitary graft-induced adenomyosis that received the antiprogestin SPRM mifepristone in food, experienced significantly decreased incidence of adenomyosis [78]. Similarly treatment with progestogenic SPRMs CP8816 and CP8863 reduced disease development, possibly by downregulating E2-induced epithelial cell proliferation [79,80]. On the other hand, ESR1/2 antagonist fulvestrant also successfully prevented adenomyosis occurrence in the pituitary graft mouse model, with only one treated mouse developing any disease [73]. Moreover, raloxifene, a SERM without uterine estrogenic activity, prevented establishment of disease in a xenotransplantation model of adenomyosis by preventing E2-induced EMT [40]. While the specific downstream molecular consequences of these treatments are not yet clear, each downregulates hormonal activity in specific ways to prevent adenomyosis development in mouse models.
In addition to standard pharmacological agents, several studies have examined the efficacy of treatment with medicinal herbs and food additives in mouse models of adenomyosis. Neonatal administration of monosodium glutamate, a common food additive, significantly decreased the incidence of spontaneously occurring adenomyosis in SHN mice [81]. A series of recent studies used the tamoxifen-induced adenomyosis model in ICR mice to test the effects of several compounds on myometrial infiltration as well as adenomyosis-associated generalized hyperalgesia and uterine hyperactivity. Levo-tetrahydropalmatine (l-THP) and andrographolide, Chinese medicinal herbs [82], epigallocatechin-3-gallate (EGCG), a catechin found in green tea [83,84], and resveratrol, a polyphenol isolated from the skin of red grapes [85], each reduced myometrial infiltration and uterine hyperactivity in adenomyosis mice versus controls. Additionally, hyperalgesia as measured by reaction time to noxious stimuli was restored from the approximately 30–40% observed in untreated adenomyosis mice compared to controls to around 70–80% and higher in treated mice, depending on the dose [82,83,85]. Notably epigallocatechin-3-gallate is known to possess antiangiogenic activity [83], and resveratrol suppresses EMT [85]. In addition, leonurine, a plant-based alkaloid traditionally used to treat dysmenorrhea in China, partially reduced myometrial infiltration and significantly reduced associated hyperalgesia in this same model system [86]. Furthermore, rhein, a rhubarb derivative, mitigated adenomyosis through inhibition of nuclear factor-κβ (NF- κβ) and β-catenin signaling in pituitary graft-induced adenomyosis in ICR mice [87].
Several experimental treatments and biological process-specific inhibitors have also been tried using mouse models of induced adenomyosis which give insight into some of the processes necessary for disease development. Multiple studies have reported that valproic acid (VPA), a histone deacetylase inhibitor (HDI), significantly reduced adenomyosis-associated hyperalgesia and also suppressed myometrial infiltration, though not always to a level of statistical significance, in tamoxifen-induced adenomyosis model ICR mice [82,86,88]. This effect could involve demethylation of the PR-B promoter, which has been shown to be hypermethylated in adenomyotic cells in vitro and restored to normal by treatment with a HDI and a demethylation agent [89].
MMP secretion is necessary for the invasion of endometrial lesions through extracellular matrix and is upregulated in adenomyosis mouse models [45]. In SHN mice with pituitary graft-induced adenomyosis, a high dose or low dose of ONO-4817, a MMP inhibitor, decreased adenomyosis incidence to 9% or 46% of controls, respectively [90]. ONO-4817 treatment also decreased lesion progression to 63% the invasiveness grade of untreated controls and decreased the in vitro invasiveness of stromal cells to about half that of controls [90]. In a similar model, treatment with angiogenesis inhibitor TNP-470 decreased adenomyosis incidence in conjunction with decreased endometrial blood vessel growth [91]. Moreover, the hypocholesterolemic agent probucol, which protects vasculature from oxidative stress, also reduced incidence of adenomyosis development [92].
Recent evidence has shown that platelet activation may be a cause of EMT in adenomyosis through increased TGF-β/Smad signaling [65]. Remarkably, antiplatelet therapy in ICR mice with tamoxifen-induced adenomyosis using antibody-based platelet depletion or ozagrel, a small molecule antiplatelet drug, dose-dependently counteracted myometrial infiltration and improved adenomyosis-related hyperalgesia, uterine contractility, corticosterone levels and expression of several molecular markers known to be involved in adenomyosis [93]. Finally, photodynamic therapy, which generates reactive oxygen species to induce targeted cell death, was shown to effectively destroy xenotransplanted adenomyotic tissue in nude mice [39]. These results show that the strategy of targeting known adenomyosis-related processes such as MMP secretion, EMT and angiogenesis holds promise. These studies also demonstrate the benefits of murine adenomyosis models for testing experimental therapies at the preclinical stage to simultaneously test the efficacy of treatment and the molecular mechanisms of action.
Pain
Although adenomyosis is asymptomatic in up to one third of women with the disease, severe, persistent pelvic pain is a commonly reported symptom, and there is a correlation between the spread of adenomyosis throughout the myometrium and pelvic pain [2,3,94]. Adenomyosis-associated pain cannot be studied directly in mouse models, so general increased sensitivity to pain (hyperalgesia) is often used as a substitute measure, with the response time of mice to gradually increasing temperature indicating changes in central sensitization. Though not ideal substitutes for analyzing adenomyosis-associated pelvic pain, such experiments are reasonable given the possible association between adenomyosis and neuropathic pain or sensory gain [95].
ICR mice with tamoxifen-induced adenomyosis have been utilized extensively to study adenomyosis-related pain due to the well-established increase in generalized hyperalgesia in this model [82–86,88,93]. Treatment with anti-platelet therapy or a variety of drugs such as VPA, P4, l-THP, andrographolide, leonurine, EGCG, or resveratrol has been demonstrated to alleviate this adenomyosis-related pain sensitivity [82–86,88,93]. Though the mechanisms of action are not completely clear, one possible explanation of may be a restoration of dysregulated NFκB signaling, which is known to be involved in neuropathic pain and is common in this mouse model [82–86,93]. It is also possible that the observed hyperalgesia in these mice is due to an indirectly observed loss of GABAergic inhibition, a suppressor of neuropathic pain, which is reversible with several of these treatments including EGCG, resveratrol, and anti-platelet therapy [83–85,93]. Moreover, in this same model, NGF-β and its receptors, which are involved in producing pain and release of inflammatory factors, were upregulated in the uterus and/or dorsal root ganglia [59]. Notably, this effect increased with age alongside disease progression and is thought to be involved in both the pathogenesis and pain severity of human adenomyosis [2,59]. Though admittedly all these data were gleaned from experiments in one mouse model and only involves generalized pain sensitivity, they still represent a valuable contribution to the limited understanding of adenomyosis-related pain and possible remedial therapies. Future utilization of mouse models of menstruation or alternative methods of disease induction more closely aligned with human reproductive system biology could offer opportunities to study adenomyosis dysmenorrhea and lead to a more clinically relevant understanding of adenomyosis pain.
Infertility
Although adenomyosis has historically been linked to multiparity rather than subfertility, recent improvements in diagnostic techniques have led to an increased association with infertility [96]. Several theories have been proposed for a causal mechanisms of reduced fertility due to adenomyosis, mainly within the categories of abnormal utero-tubal transport and altered endometrial function and receptivity to implantation [96]. Though limited in detail, a few studies with mouse models have contributed to understanding the interplay between adenomyosis and infertility and poor reproductive performance.
Pituitary graft-induced adenomyosis significantly affected the percentage of successful pregnancies one month after induction in one study although the magnitude of the effect was limited, and differences were only significant when three pituitaries were grafted rather than the single pituitary which is normally used in this model [50]. The chronically elevated P4 levels characteristic of this model may explain the negative effect on fertility, implying that induction of adenomyosis in mice through prolonged, ongoing hormonal disruption may not be an effective model for studying fertility defects relevant to human adenomyosis because of the negative effects of altered systemic hormone levels which may mask other more localized causes of infertility.
Direct prenatal or indirect transgenerational exposure to the environmental toxicant dioxin, which has known estrogenic effects, led to both decreased fertility and development of adenomyosis in C57BL/6 mice [58,97]. Decreased fertility in these mice was accompanied by decreased endometrial progesterone receptor (PGR) and estrogen receptor α (ESR1) expression and increased estrogen receptor β (ESR2) expression, similar to the pattern reported in some women with adenomyosis or endometriosis [58,70,97,98].
Mice with adenomyosis caused by uterine-specific β-catenin stabilization were infertile due to a decidualization defect in spite of normal ovarian function and steroid hormone levels, implicating E2-induced cell proliferation and EMT through β-catenin activity in the endometrium as causes of both adenomyosis and infertility in these mice [26,99]. Genetically engineered mice that developed adenomyosis or other associated myometrial defects due to dysregulated WNT signaling (Foxl2 and dicer conditional knockouts) also displayed uterine-based fertility defects [27,28]. These findings indicate a possible role for adenomyosis with a mesenchymal origin in causing infertility, but it is impossible to isolate adenomyosis as an independent cause based on these studies.
Two studies report that tamoxifen-induced adenomyosis led to significantly decreased number of pups per litter, though total litter number was not significantly changed [76,100]. In these experiments, GnRH agonist therapy remedied subfertility caused by induction of adenomyosis likely through reduction of E2-induced endometrial cell proliferation and restoration of P4-induced endometrial receptivity markers [76,100]. These findings comport well with several adenomyosis patient case studies in which GnRH agonist therapy appears to have benefited endometrial receptivity [3].
As in humans, it is difficult to define a direct causative relationship between adenomyosis development and fertility problems in mouse models due to frequently co-occurring reproductive tract dysfunctions. However, taken together the findings from several adenomyosis mouse models, it is reasonable to conclude that adenomyosis induction in mice disrupts fertility primarily through an increase in estrogenic activity and a decrease of P4-mediated receptivity in the endometrium.
Other Animal Models of Adenomyosis
Experimental use of animal models for adenomyosis outside of non-human primates and mice has been very sparse; reports in other animals have mainly been limited to case reports of spontaneous disease occurrence. For example, naturally arising cases have been reported in horses [2,4], cows [101], dogs [8,9,101–103], cats [8,9,101,103], rabbits [8,9], rats [8,9], and even guinea pig [104]. The length of this list reveals the ubiquitous nature of natural adenomyosis development throughout mammalian species; however, such spontaneous cases are rarely reported and not generally associated with any symptoms. This may imply a lack of biological importance in these species, or it may simply be due to a lack of comprehensive studies.
Similar to mice, rabbits and guinea pigs subjected to long-term estrogenic exposure develop adenomyosis [105,106], though no reports of further experimentation using these models are apparent. Finally, induction of hyperprolactinemia is sufficient to drive adenomyosis in rats through comparable methods as those used in mice. Both administration of fluoxetine, a selective serotonin reuptake inhibitor that causes an increase in prolactin secretion, and pituitary isografting can be used to induce adenomyosis, though again, neither of these rat models appears to have been used for substantial further experimentation [106,107]. Nevertheless, these reports substantiate the broader biological applicability of the mouse findings that demonstrate hormonal dysregulation leading to adenomyosis and provide non-mouse options for further study of adenomyosis using induced animal models.
Conclusion
Despite its common occurrence, concrete understanding of adenomyosis pathogenesis and reliable non-surgical treatment options are insufficient. Research using animal models, particularly non-human primates and mice, have supported the clinical associations between adenomyosis, infertility, and pelvic pain. The descriptions of adenomyosis in non-human primates have been limited primarily to case reports [10] with the exception of more detailed studies on adenomyosis-associated infertility in baboons [12] and estrogenic effects and treatments with anti-estrogens in macaques [13,14,25]. Given the spontaneous development of adenomyosis in a number of different non-human primate species and the possibility of inducing the disease in baboons and macaques, advances in imaging technology and molecular biology could be used experimentally to better understand the pathophysiology of this understudied gynecological pathology in primates.
Molecular studies with induced adenomyosis in mice have also provided evidence for the involvement of generalized steroid hormone dysregulation, MMP or NGF upregulation, E2-induced angiogenesis or EMT, and dysregulation of mesenchymal WNT signaling in the pathogenesis of adenomyosis. Findings in mouse models have also supported the efficacy of drugs that modulate hormonal pathways, several medicinal herbs and compounds, and experimental treatments like antiplatelet and photodynamic therapies to suppress the biological progression and some of the symptoms associated with adenomyosis.
The contribution made by animal models to the current understanding of adenomyosis is substantial, but the significant limitations are also important to consider. The large amount of resources necessary and ethical considerations have limited the breadth and experimental detail of adenomyosis study in non-human primates, and reproductive biological differences between rodents and humans both limit the interpretation of mouse findings and preclude the possibility of studying menorrhagia and dysmenorrhea, two of the most common symptoms of adenomyosis in humans [2]. Induced disease models also carry the inherent risk of confounding variables such as surgical, developmental, and systemic hormonal effects, depending on the method and controls used. Furthermore, there is still a profound lack of mechanistic knowledge about exactly how induction of adenomyosis occurs, which necessitates caution in making comparisons with the human disease. Though it is promising that both nonhuman primate and mouse models recapitulate the fertility challenges associated with human adenomyosis pathology to some degree, research of adenomyosis-related pain is almost entirely limited to one mouse model and necessarily focuses on generalized pain sensitivity rather than localized pelvic pain. Increased study in non-human primates or in mouse models of menstruation offer the opportunity to study more clinically relevant features of adenomyosis pathology.
One benefit, as it were, to the relative lack of comprehensive study of adenomyosis with animal models compared to other gynecologic diseases is the obvious potential they hold for future research. To best facilitate novel clinically translatable findings, development of a method for experimentally inducing adenomyosis in non-human primates is critical [10]. Additionally, standardization of induction protocols and outcome measures in rodent models are vital given the well-documented effects of strain and intervention timing and dosage. This is particularly relevant for use in preclinical drug screening. As has been previously noted, there is also a need for outcome measures in animal studies to match clinical priorities since histological disease progression does not necessarily correlate with symptoms in women [95]. Moreover, increased use of in vivo transcriptomic and cistromic analyses in animal models would facilitate the identification of molecular pathways involved in disease pathogenesis or as therapeutic targets, and new genetic models would help pinpoint the molecular basis of disease development. Finally, increased integration of translational human studies with research in animal models would leverage the unique benefits of both systems and result in the best chance at new clinically relevant knowledge and treatment options to combat adenomyosis in women suffering from its effects.
Acknowledgments
Research reported in this publication was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD084478 to J.W.J. and T32HD087166 to R.M.M, MSU AgBio Research, and Michigan State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The Authors declare that there is no conflict of interest.
References
- 1.Yen CF, Huang SJ, Lee CL et al. Molecular Characteristics of the Endometrium in Uterine Adenomyosis and Its Biochemical Microenvironment. Reprod Sci 2017; 24: 1346–1361 [DOI] [PubMed] [Google Scholar]
- 2.Aleksandrovych V, Basta P, Gil K. Current facts constituting an understanding of the nature of adenomyosis. Adv Clin Exp Med 2019; 28: 839–846 [DOI] [PubMed] [Google Scholar]
- 3.Levgur M Therapeutic options for adenomyosis: a review. Arch Gynecol Obstet 2007; 276: 1–15 [DOI] [PubMed] [Google Scholar]
- 4.Koike N, Tsunemi T, Uekuri C et al. Pathogenesis and malignant transformation of adenomyosis (review). Oncol Rep 2013; 29: 861–867 [DOI] [PubMed] [Google Scholar]
- 5.Maier V, Holl M, Dietze R et al. Adenomyotic glands are highly related to endometrial glands. Reprod Biomed Online 2019; 10.1016/j.rbmo.2019.11.007: [DOI] [PubMed]
- 6.Dior UP, Nisbet D, Fung JN et al. The Association of Sonographic Evidence of Adenomyosis with Severe Endometriosis and Gene Expression in Eutopic Endometrium. J Minim Invasive Gynecol 2019; 26: 941–948 [DOI] [PubMed] [Google Scholar]
- 7.Hever A, Roth RB, Hevezi PA et al. Molecular characterization of human adenomyosis. Mol Hum Reprod 2006; 12: 737–748 [DOI] [PubMed] [Google Scholar]
- 8.Habiba M The Animal Model of Adenomyosis. In, Uterine Adenomyosis: Springer; 2016: 123–127 [Google Scholar]
- 9.Greaves P, White IN. Experimental adenomyosis. Best Pract Res Clin Obstet Gynaecol 2006; 20: 503–510 [DOI] [PubMed] [Google Scholar]
- 10.Barrier BF, Allison J, Hubbard GB et al. Spontaneous adenomyosis in the chimpanzee (Pan troglodytes): a first report and review of the primate literature: case report. Human reproduction 2007; 22: 1714–1717 [DOI] [PubMed] [Google Scholar]
- 11.Graham KJ, Hulst FA, Vogelnest L et al. Uterine adenomyosis in an orang-utan (Pongo abelii/pygmaeus). Aust Vet J 2009; 87: 66–69 [DOI] [PubMed] [Google Scholar]
- 12.Barrier BF, Malinowski MJ, Dick EJ Jr. et al. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril 2004; 82 Suppl 3: 1091–1094 [DOI] [PubMed] [Google Scholar]
- 13.DiGiacomo RF. Gynecologic pathology in the rhesus monkey (Macaca mulatta). II. Findings in laboratory and free-ranging monkeys. Vet Pathol 1977; 14: 539–546 [DOI] [PubMed] [Google Scholar]
- 14.Baskin GB, Smith SM, Marx PA. Endometrial hyperplasia, polyps, and adenomyosis associated with unopposed estrogen in rhesus monkeys (Macaca mulatta). Vet Pathol 2002; 39: 572–575 [DOI] [PubMed] [Google Scholar]
- 15.Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res 2019; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrarelli P, Yen CF, Funghi L et al. Expression of Inflammatory and Neurogenic Mediators in Adenomyosis. Reprod Sci 2017; 24: 369–375 [DOI] [PubMed] [Google Scholar]
- 17.Vannuccini S, Tosti C, Carmona F et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online 2017; 35: 592–601 [DOI] [PubMed] [Google Scholar]
- 18.Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod 2009; 15: 577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Hooghe TM, Kyama CM, Chai D et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci 2009; 16: 152–161 [DOI] [PubMed] [Google Scholar]
- 20.Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med 2010; 28: 75–80 [DOI] [PubMed] [Google Scholar]
- 21.Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol 2006; 4 Suppl 1: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moses AS, Taratula OR, Lee H et al. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020; 16: e1906936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slayden OD. Translational In Vivo Models for Women’s Health: The Nonhuman Primate Endometrium--A Predictive Model for Assessing Steroid Receptor Modulators. Handb Exp Pharmacol 2016; 232: 191–202 [DOI] [PubMed] [Google Scholar]
- 24.Fazleabas AT, Brudney A, Gurates B et al. A modified baboon model for endometriosis. Ann N Y Acad Sci 2002; 955: 308–317; discussion 340–302, 396–406 [DOI] [PubMed] [Google Scholar]
- 25.Waterton JC, Breen SA, Dukes M et al. A case of adenomyosis in a pigtailed monkey diagnosed by magnetic resonance imaging and treated with the novel pure antiestrogen, ICI 182,780. Lab Anim Sci 1993; 43: 247–251 [PubMed] [Google Scholar]
- 26.Oh SJ, Shin JH, Kim TH et al. beta-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J Pathol 2013; 231: 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellessort B, Bachelot A, Heude E et al. Role of Foxl2 in uterine maturation and function. Hum Mol Genet 2015; 24: 3092–3103 [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez G, Behringer RR. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev 2009; 76: 678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagasawa H, Kusakawa S. Relationship between incidence and onset age of mammary tumours and uterine adenomyosis in four strains of mice: comparison with the findings of 40 generations previously. In Vivo 2001; 15: 345–349 [PubMed] [Google Scholar]
- 30.Kida H [Histological analysis of spontaneous adenomyosis-like changes in recombinant inbred mouse uterus (SMXA mouse)--a novel animal model for adenomyosis]. Nihon Sanka Fujinka Gakkai Zasshi 1994; 46: 323–330 [PubMed] [Google Scholar]
- 31.Nagasawa H, Naito T. Enhanced potentials for mammary tumourigenesis and uterine adenomyosis in (SLN x C3H/He)F1 virgin mice. Lab Anim 1992; 26: 23–24 [DOI] [PubMed] [Google Scholar]
- 32.Nagasawa H, Mori T. Stimulation of mammary tumorigenesis and suppression of uterine adenomyosis by temporary inhibition of pituitary prolactin secretion during youth in mice (41492). Proc Soc Exp Biol Med 1982; 171: 164–167 [DOI] [PubMed] [Google Scholar]
- 33.Mori T, Nagasawa H, Takahashi S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci 1981; 29: 1277–1282 [DOI] [PubMed] [Google Scholar]
- 34.Walker BE. Ovariectomy of adult mice exposed prenatally to diethylstilbestrol. Cancer Lett 1987; 34: 115–120 [DOI] [PubMed] [Google Scholar]
- 35.Koike E, Yasuda Y, Shiota M et al. Exposure to ethinyl estradiol prenatally and/or after sexual maturity induces endometriotic and precancerous lesions in uteri and ovaries of mice. Congenit Anom (Kyoto) 2013; 53: 9–17 [DOI] [PubMed] [Google Scholar]
- 36.Parrott E, Butterworth M, Green A et al. Adenomyosis--a result of disordered stromal differentiation. Am J Pathol 2001; 159: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanwar PS, Lee HJ, Zhang L et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod 2009; 81: 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang TS, Chen YJ, Chou TY et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med 2014; 18: 1358–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki-Kakisaka H, Murakami T, Hirano T et al. Selective accumulation of PpIX and photodynamic effect after aminolevulinic acid treatment of human adenomyosis xenografts in nude mice. Fertil Steril 2008; 90: 1523–1527 [DOI] [PubMed] [Google Scholar]
- 40.Chen YJ, Li HY, Huang CH et al. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J Pathol 2010; 222: 261–270 [DOI] [PubMed] [Google Scholar]
- 41.Zhou S, Yi T, Liu R et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol Cell Proteomics 2012; 11: M112 017988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koujyo T, Hatakeyama S, Yamada H et al. Induction of endometriosis and adenomyosis by transvaginal pituitary transplantation in mice with and without natural killer cell activity. American journal of reproductive immunology 1998; 40: 441–446 [DOI] [PubMed] [Google Scholar]
- 43.Mori T, Ohta Y, Nagasawa H. Ultrastructural changes in uterine myometrium of mice with experimentally-induced adenomyosis. Experientia 1984; 40: 1385–1387 [DOI] [PubMed] [Google Scholar]
- 44.Mori T, Nagasawa H. Mechanisms of development of prolactin-induced adenomyosis in mice. Acta Anat (Basel) 1983; 116: 46–54 [DOI] [PubMed] [Google Scholar]
- 45.Matsuda M, Sasabe H, Adachi Y et al. Increased invasion activity of endometrial stromal cells and elevated expression of matrix metalloproteinase messenger RNA in the uterine tissues of mice with experimentally induced adenomyosis. Am J Obstet Gynecol 2001; 185: 1374–1380 [DOI] [PubMed] [Google Scholar]
- 46.Danilovich N, Roy I, Sairam MR. Emergence of uterine pathology during accelerated biological aging in FSH receptor-haploinsufficient mice. Endocrinology 2002; 143: 3618–3627 [DOI] [PubMed] [Google Scholar]
- 47.Yamashita M, Matsuda M, Mori T. Increased expression of prolactin receptor mRNA in adenomyotic uterus in mice. Life Sci 1997; 60: 1437–1446 [DOI] [PubMed] [Google Scholar]
- 48.Singtripop T, Mori T, Park MK et al. Development of uterine adenomyosis after treatment with dopamine antagonists in mice. Life Sci 1991; 49: 201–206 [DOI] [PubMed] [Google Scholar]
- 49.Kelly MA, Rubinstein M, Asa SL et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 1997; 19: 103–113 [DOI] [PubMed] [Google Scholar]
- 50.Huseby RA, Soares MJ, Talamantes F. Ectopic pituitary grafts in mice: hormone levels, effects on fertility, and the development of adenomyosis uteri, prolactinomas, and mammary carcinomas. Endocrinology 1985; 116: 1440–1448 [DOI] [PubMed] [Google Scholar]
- 51.Lipschutz A, Iglesias R, Panasevich VI et al. Pathological changes induced in the uterus of mice with the prolonged administration of progesterone and 19-nor-contraceptives. Br J Cancer 1967; 21: 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagasawa H, Aoki M, Mori T. Effects of different schedules of progesterone treatment on mammary tumorigenesis and uterine adenomyosis in SHN virgin mice. Life Sci 1987; 40: 2597–2602 [DOI] [PubMed] [Google Scholar]
- 53.Nagasawa H, Aoki M, Sakagami N et al. Medroxyprogesterone acetate enhances spontaneous mammary tumorigenesis and uterine adenomyosis in mice. Breast Cancer Res Treat 1988; 12: 59–66 [DOI] [PubMed] [Google Scholar]
- 54.Ostrander PL, Mills KT, Bern HA. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. J Natl Cancer Inst 1985; 74: 121–135 [PubMed] [Google Scholar]
- 55.Bennett LM, McAllister KA, Malphurs J et al. Mice heterozygous for a Brca1 or Brca2 mutation display distinct mammary gland and ovarian phenotypes in response to diethylstilbestrol. Cancer Res 2000; 60: 3461–3469 [PubMed] [Google Scholar]
- 56.Walker BE. Tumors of female offspring of mice exposed prenatally to diethylstilbestrol. J Natl Cancer Inst 1984; 73: 133–140 [PubMed] [Google Scholar]
- 57.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 2007; 24: 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruner-Tran KL, Duleba AJ, Taylor HS et al. Developmental Toxicant Exposure Is Associated with Transgenerational Adenomyosis in a Murine Model. Biol Reprod 2016; 95: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Zhang SF, Zou SE et al. Accumulation of nerve growth factor and its receptors in the uterus and dorsal root ganglia in a mouse model of adenomyosis. Reprod Biol Endocrinol 2011; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehasseb MK, Bell SC, Habiba MA. The effects of tamoxifen and estradiol on myometrial differentiation and organization during early uterine development in the CD1 mouse. Reproduction 2009; 138: 341–350 [DOI] [PubMed] [Google Scholar]
- 61.Mehasseb MK, Bell SC, Habiba MA. Neonatal administration of tamoxifen causes disruption of myometrial development but not adenomyosis in the C57/BL6J mouse. Reproduction 2010; 139: 1067–1075 [DOI] [PubMed] [Google Scholar]
- 62.Green AR, Styles JA, Parrott EL et al. Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp Toxicol Pathol 2005; 56: 255–263 [DOI] [PubMed] [Google Scholar]
- 63.Green AR, Edwards RE, Greaves P et al. Comparison of the effect of oestradiol, tamoxifen and raloxifene on nerve growth factor-alpha expression in specific neonatal mouse uterine cell types using laser capture microdissection. J Mol Endocrinol 2003; 30: 1–11 [DOI] [PubMed] [Google Scholar]
- 64.Acloque H, Adams MS, Fishwick K et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009; 119: 1438–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen M, Liu X, Zhang H et al. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Human reproduction 2016; 31: 355–369 [DOI] [PubMed] [Google Scholar]
- 66.Jin Z, Liu H, Xu C. Estrogen degrades Scribble in endometrial epithelial cells through E3 ubiquitin ligase HECW1 in the development of diffuse adenomyosisdagger. Biol Reprod 2020; 102: 376–387 [DOI] [PubMed] [Google Scholar]
- 67.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 2009; 28: 151–166 [DOI] [PubMed] [Google Scholar]
- 68.Arango NA, Szotek PP, Manganaro TF et al. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005; 288: 276–283 [DOI] [PubMed] [Google Scholar]
- 69.Kawahara R, Matsuda M, Mori T. Increase in the number of integrinbeta1-immunoreactive monocyte-lineage cells in experimentally-induced adenomyosis in mice. Life Sci 2003; 73: 907–916 [DOI] [PubMed] [Google Scholar]
- 70.Marquardt RM, Kim TH, Shin JH et al. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int J Mol Sci 2019; 20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Che X, Wang J, He J et al. The new application of mifepristone in the relief of adenomyosis-caused dysmenorrhea. Int J Med Sci 2020; 17: 224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singtripop T, Mori T, Sakamoto S et al. Suppression of the development of uterine adenomyosis by danazol treatment in mice. Life Sci 1992; 51: 1119–1125 [DOI] [PubMed] [Google Scholar]
- 73.Otto C, Schkoldow J, Krahl E et al. Use of a murine endometriosis interna model for the characterization of compounds that effectively treat human endometriosis. Exp Ther Med 2012; 3: 410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, Yuan H, Deng L et al. Evaluation of the efficacy of a danazol-loaded intrauterine contraceptive device on adenomyosis in an ICR mouse model. Human reproduction 2008; 23: 2024–2030 [DOI] [PubMed] [Google Scholar]
- 75.Selak V, Farquhar C, Prentice A et al. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev 2001; 10.1002/14651858.CD000068: CD000068 [DOI] [PubMed]
- 76.Guo S, Lu X, Gu R et al. Transcriptome analysis of endometrial tissues following GnRH agonist treatment in a mouse adenomyosis model. Drug Des Devel Ther 2017; 11: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferrero S, Evangelisti G, Barra F. Current and emerging treatment options for endometriosis. Expert Opin Pharmacother 2018; 19: 1109–1125 [DOI] [PubMed] [Google Scholar]
- 78.Zhou YF, Matsuda M, Mori T et al. Effects of mifepristone (RU486) treatment on the development of uterine adenomyosis induced by pituitary grafting in mice. Life Sci 2000; 67: 2713–2720 [DOI] [PubMed] [Google Scholar]
- 79.Mori T, Kurata Y, Tabata Y et al. Priming effects of novel nonsteroidal progesterone receptor modulators CP8816 and CP8863 on the development of adenomyosis in the mouse uterus. Life Sci 2002; 71: 527–535 [DOI] [PubMed] [Google Scholar]
- 80.Kurata Y, Tabata Y, Shinei R et al. Endocrinological properties of two novel nonsteroidal progesterone receptor modulators, CP8816 and CP8863. J Pharmacol Exp Ther 2005; 313: 916–920 [DOI] [PubMed] [Google Scholar]
- 81.Nagasawa H, Noguchi Y, Mori T et al. Suppression of normal and preneoplastic mammary growth and uterine adenomyosis with reduced growth hormone level in SHN mice given monosodium glutamate neonatally. Eur J Cancer Clin Oncol 1985; 21: 1547–1551 [DOI] [PubMed] [Google Scholar]
- 82.Mao X, Wang Y, Carter AV et al. The retardation of myometrial infiltration, reduction of uterine contractility, and alleviation of generalized hyperalgesia in mice with induced adenomyosis by levo-tetrahydropalmatine (l-THP) and andrographolide. Reprod Sci 2011; 18: 1025–1037 [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Zhu B, Zhang H et al. Epigallocatechin-3-gallate reduces myometrial infiltration, uterine hyperactivity, and stress levels and alleviates generalized hyperalgesia in mice induced with adenomyosis. Reprod Sci 2013; 20: 1478–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y, Zhu B, Zhang H et al. Possible Loss of GABAergic Inhibition in Mice With Induced Adenomyosis and Treatment With Epigallocatechin-3-Gallate Attenuates the Loss With Improved Hyperalgesia. Reprod Sci 2014; 21: 869–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu B, Chen Y, Zhang H et al. Resveratrol Reduces Myometrial Infiltration, Uterine Hyperactivity, and Stress Levels and Alleviates Generalized Hyperalgesia in Mice With Induced Adenomyosis. Reprod Sci 2015; 22: 1336–1349 [DOI] [PubMed] [Google Scholar]
- 86.Nie J, Liu X. Leonurine Attenuates Hyperalgesia in Mice with Induced Adenomyosis. Med Sci Monit 2017; 23: 1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feng T, Wei S, Wang Y et al. Rhein ameliorates adenomyosis by inhibiting NF-kappaB and beta-Catenin signaling pathway. Biomed Pharmacother 2017; 94: 231–237 [DOI] [PubMed] [Google Scholar]
- 88.Liu X, Guo SW. Valproic acid alleviates generalized hyperalgesia in mice with induced adenomyosis. J Obstet Gynaecol Res 2011; 37: 696–708 [DOI] [PubMed] [Google Scholar]
- 89.Jichan N, Xishi L, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci 2010; 17: 995–1005 [DOI] [PubMed] [Google Scholar]
- 90.Mori T, Yamasaki S, Masui F et al. Suppression of the development of experimentally induced uterine adenomyosis by a novel matrix metalloproteinase inhibitor, ONO-4817, in mice. Exp Biol Med (Maywood) 2001; 226: 429–433 [DOI] [PubMed] [Google Scholar]
- 91.Zhou YF, Mori T, Kudo H et al. Effects of angiogenesis inhibitor TNP-470 on the development of uterine adenomyosis in mice. Fertil Steril 2003; 80 Suppl 2: 788–794 [DOI] [PubMed] [Google Scholar]
- 92.Zhou YF, Mori T, Nagasawa H et al. Probucol, a hypocholesterolemic agent, prevents the development of uterine adenomyosis induced by pituitary grafting in mice. Anticancer Res 2004; 24: 2209–2212 [PubMed] [Google Scholar]
- 93.Zhu B, Chen Y, Shen X et al. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol 2016; 14: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sammour A, Pirwany I, Usubutun A et al. Correlations between extent and spread of adenomyosis and clinical symptoms. Gynecol Obstet Invest 2002; 54: 213–216 [DOI] [PubMed] [Google Scholar]
- 95.Guo S-W, Habiba M. Improving the Preclinical Mouse Efficacy Studies of Adenomyosis. In, Uterine Adenomyosis: Springer; 2016: 129–139 [Google Scholar]
- 96.Harada T, Khine YM, Kaponis A et al. The Impact of Adenomyosis on Women’s Fertility. Obstet Gynecol Surv 2016; 71: 557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol 2011; 31: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mehasseb MK, Panchal R, Taylor AH et al. Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil Steril 2011; 95: 2228–2235, 2235 e2221 [DOI] [PubMed] [Google Scholar]
- 99.Jeong JW, Lee HS, Franco HL et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009; 28: 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo S, Li Z, Yan L et al. GnRH agonist improves pregnancy outcome in mice with induced adenomyosis by restoring endometrial receptivity. Drug Des Devel Ther 2018; 12: 1621–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McEntee K, Nielsen SW. Tumours of the female genital tract. Bull World Health Organ 1976; 53: 217–226 [PMC free article] [PubMed] [Google Scholar]
- 102.Stocklin-Gautschi NM, Guscetti F, Reichler IM et al. Identification of focal adenomyosis as a uterine lesion in two dogs. J Small Anim Pract 2001; 42: 413–416 [DOI] [PubMed] [Google Scholar]
- 103.Gelberg HB, McEntee K. Pathology of the canine and feline uterine tube. Vet Pathol 1986; 23: 770–775 [DOI] [PubMed] [Google Scholar]
- 104.Baldi A, Lanza A, Menicagli F et al. Histological and Immunohistochemical Characterization of a Case of Endometriosis in a Guinea Pig (Cavia tschudii). Case Rep Vet Med 2017; 2017: 4594510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meissner WA, Sommers SC, Sherman G. Endometrial hyperplasia, endometrial carcinoma, and endometriosis produced experimentally by estrogen. Cancer 1957; 10: 500–509 [DOI] [PubMed] [Google Scholar]
- 106.Mori T, Kyokuwa M, Nagasawa H. Animal model of uterine adenomyosis: induction of the lesion in rats by ectopic pituitary isografting. Lab Anim Sci 1998; 48: 64–68 [PubMed] [Google Scholar]
- 107.Sengupta P, Sharma A, Mazumdar G et al. The possible role of fluoxetine in adenomyosis: an animal experiment with clinical correlations. J Clin Diagn Res 2013; 7: 1530–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]


