SUMMARY
Background:
Staphylococcus intermedius is a very rare human pathogen. There are only 16 cases in the literature that have described S. intermedius as a cause of infection in humans. Most of these cases have been described in association with exposure to animals, mostly dogs. However, this pathogen can cause infection in healthy individuals even without exposure to animals.
Methods:
All previous cases of S. intermedius infection included in our literature review were found using a PubMed search (1960–November 2009) of the English-language medical literature applying the terms ‘Staphylococcus intermedius’, ‘abscess’, ‘infection’, ‘humans’. The references cited in these articles were examined to identify additional reports.
Results:
We describe the first case of skin abscesses caused by S. intermedius in an immunocompetent patient who used intravenous cocaine after coating his syringes with his saliva. We also summarize the literature regarding infections caused by S. intermedius in humans.
Conclusions:
This case illustrates for the first time that S. intermedius can cause skin abscesses in humans after direct inoculation of this pathogen into the skin and soft tissues. Clinicians should be aware of the fact that although the vast majority of infections from coagulase-positive Staphylococcus infections are secondary to Staphylococcus aureus, S. intermedius is also a potential pathogen in humans.
Keywords: Staphylococcus intermedius, Skin abscess, Saliva, Intravenous drug use
1. Introduction
Staphylococcus intermedius is a coagulase-positive Staphylococcus that is a very rare cause of infection in humans, despite being pathogenic in animals.1 We describe, for the first time, a case of skin abscesses that were caused by S. intermedius in an intravenous drug user and we summarize the available literature on infections caused by S. intermedius in humans.
2. Case report
A 43-year-old male with a history of chronic hepatitis C infection and opioid dependence presented with chills after developing two skin abscesses in the right forearm two days after injecting intravenous cocaine. The patient denied any exposure to animals. He had the habit of licking the syringes prior to injecting cocaine ‘to prevent clotting’. He was afebrile with a temperature of 36.5 °C, hemodynamically stable, and he had two abscesses in the forearm, one 2 × 3 cm and the other 2 × 2 cm (Figure 1). Pertinent laboratory investigations revealed a white blood cell count of 5.1 × 109/l with 70% polymorphonuclear cells. The patient underwent incision and drainage of the two abscesses. Gram staining of the pus revealed Gram-positive cocci in clusters, and vancomycin 1 g intravenously every 12 h was administered empirically. Coagulase-positive staphylococci grew in the culture of the pus and further testing identified the organism as S. intermedius. More specifically, white glistening microbial colonies were isolated on blood and MacConkey agar. The colonies were coagulase-, pyrrolidonyl arylamidase-, and o-nitrophenyl-β-d-galactopyranoside-positive. The isolate was identified correctly as S. intermedius after analysis with the ID32 Staph system using the API database. 16S rRNA gene sequence analysis confirmed the presence of this rare human pathogen. Sensitivity testing revealed that the isolate was susceptible to oxacillin, ampicillin/sulbactam, cefazolin, levofloxacin, clindamycin, doxycycline, gentamicin, trimethoprim–sulfamethoxazole, and vancomycin. The patient was further treated with 875 mg of oral amoxicillin–clavulanic acid twice daily for a total of two weeks with resolution of the abscesses. Unfortunately, cultures of saliva and nasal/oropharyngeal cultures were not performed in our case, since the patient was discharged prior to final identification of the pathogen.
Figure 1.
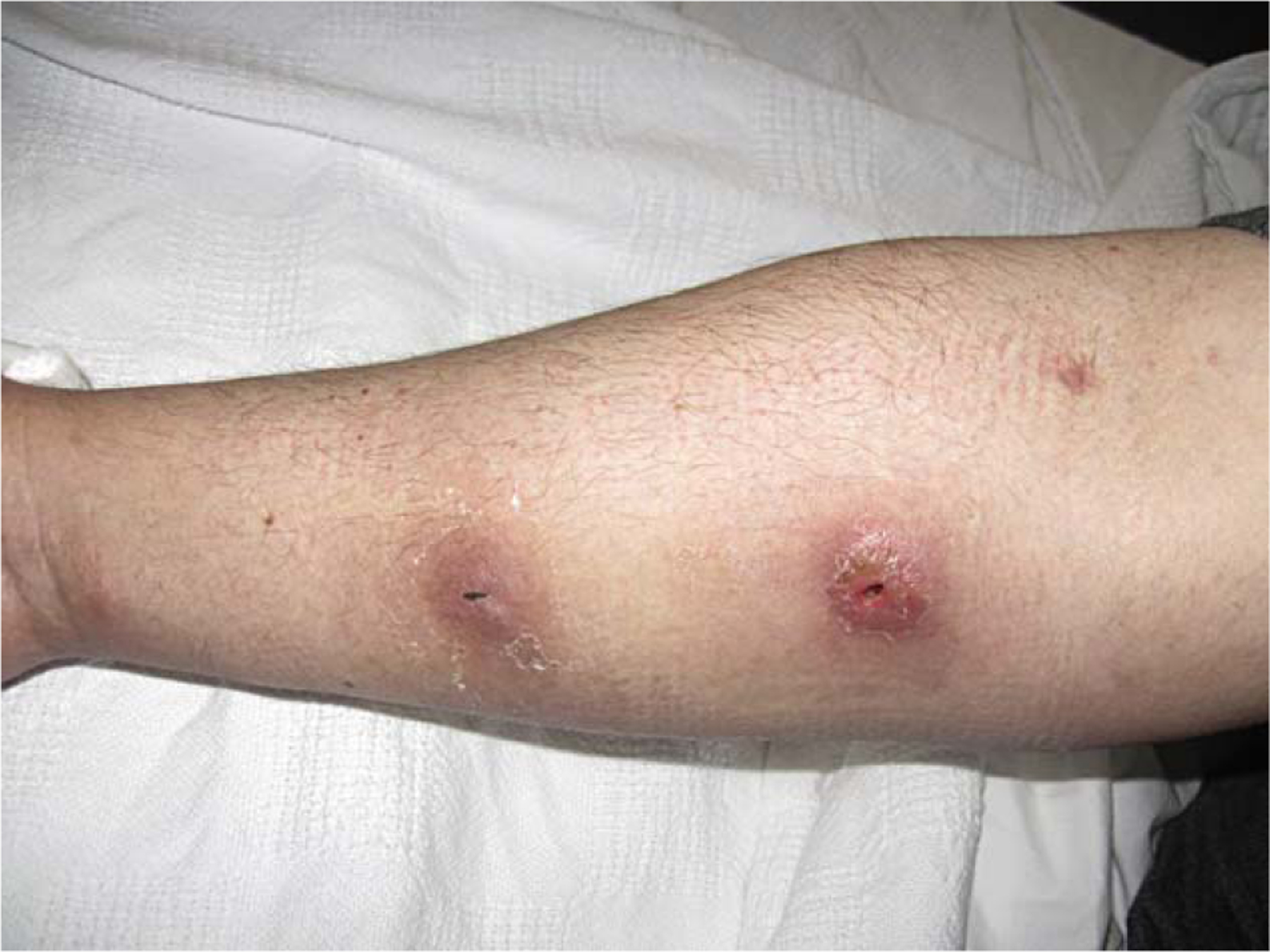
Two abscesses in the forearm (one 2 × 3 cm and the other 2 × 2 cm) that developed two days after intravenous cocaine use.
3. Discussion
Staphylococcus intermedius is a coagulase-positive Staphylococcus and a zoonotic organism that can be isolated from dogs, pigeons, minks, cats, horses, foxes, raccoons, goats, and gray squirrels.1 Talan et al. calculated the prevalence of this microorganism in dogs as 39%.2 Although this bacterium is pathogenic in animals, it has been identified very rarely as a cause of infection in humans. Human carriage of S. intermedius is uncommon (less than 20%), based on culture of saliva and S. intermedius anti-DNAse antibodies.3–7 Previous isolations of S. intermedius from humans have been described in either case reports1,8,9 or in the context of studies on populations with an increased risk of acquiring S. intermedius infections (veterinarians and patients bitten by dogs).2,5,8,10 In the latter population, a higher prevalence of this species may be assumed, since risk factors for acquiring or harboring S. intermedius are occupational exposure to pets (e.g., veterinarians) and bites from certain animals, including dogs.2,5,8,10 However S. intermedius can also be a pathogen in humans, as our case indicates. Thus, we summarize the available scientific evidence regarding the role of S. intermedius as a pathogen in humans.
All previous cases included in our literature review were found using a PubMed search (1960–November 2009) of the English-language medical literature applying the terms ‘Staphylococcus intermedius’, ‘abscess’, ‘infection’, ‘humans’. The references cited in these articles were examined to identify additional reports.
S. intermedius has been described as a human pathogen in 16 cases in the literature.1,8,9,11–18 One case of infectious endocarditis caused by S. intermedius in a patient with HIV infection was reported in the Spanish literature and was not included in our analysis.9 Thus, we summarize the data from 15 cases of S. intermedius infections in humans (Table 1).1,8,11–18 The most frequent clinical presentations are wound infections.8,11,12,15 In 1989, Talan et al. described S. intermedius as a human pathogen for the first time in three cases of dog-bite-induced soft tissue infection, which were treated with penicillin and amoxicillin–clavulanate. In humans, it is recognized as an invasive zoonotic pathogen and has been isolated from 18% of canine-inflicted wounds.8 Cellulitis and a malodorous discharge are common, while regional adenopathy, fever, and lymphangitis occur in only 20% of cases.10 The true incidence of S. intermedius in human wound infections is probably underestimated, because coagulase-positive staphylococci are often misclassified as Staphylococcus aureus.1
Table 1.
Staphylococcus intermedius infections in humans (described in the English-language literaturea)
| Author, year [Ref.] | Age, years | Sex | Underlying conditions | Type of infection | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Kempker, 2009 [18] | 28 | F | Had trans-sphenoidal endoscopic tumor removal 5 weeks prior complicated by a CSF leak. Canine exposure | Sinusitis | Bilateral sphenoidotomy with the removal of infected fat grafts, vancomycin and then linezolid for a total of 6 weeks | Complete recovery |
| Atalay, 2005 [16] | 4 | M | None | Brain abscess | Stereotactic drainage of abscess, vancomycin 40 mg/kg/day for 8 weeks | Partial recovery |
| Kikuchi, 2004 [17] | 51 | F | Had radical mastoidectomy for chronic otitis media with cholesteatoma. Canine exposure | Mastoiditis | Local cleaning with saline washes, ofloxacin ear drops | Complete recovery |
| Pottumarthy, 2004 [15] | 60 | F | Breast cancer under chemotherapy | Nail bed infection | NR | NR |
| Pottumarthy, 2004 [15] | 37 | M | None | Leg wound infection | NR | NR |
| Tanner, 2000 [14] | 38 | F | Canine exposure | Otitis | Topical antibiotics neomycin and polymyxin B | Complete recovery (approximately 4 days) |
| Gerstadt, 1999 [13] | 73 | M | Diabetes mellitus type II | Hospital- acquired pneumonia | Vancomycin | Complete recovery |
| Vandenesch, 1995 [1] | 63 | M | Metastatic non-small cell lung carcinoma, splenectomy. Pet exposure (cat) | Catheter-related bacteremia | Amoxicillin–clavulanate and ciprofloxacin for 10 days | Complete recovery |
| Lee, 1994 [8] | NR NR 13 |
NR NR NR |
Pet exposure (dogs) | Wound infections | NR | NR |
| Barnham, 1992 [12] | 78 | M | Pet exposure (dog) | Wound infection | Hydrogen peroxide lavage and a course of amoxicillin–clavulanate | Complete recovery |
| Talan, 1989 [11] | 45 | M | Pet exposure | Wound infection | Amoxicillin–clavulanate (for 10 days) | Complete recovery |
| Talan, 1989 [11] | 20 | M | Pet exposure | Cellulitis | Penicillin (5 days) | Complete recovery |
| Talan, 1989 [11] | 34 | F | Pet exposure | Wound infection | Penicillin (5 days) | Complete recovery |
M, male; F, female; NR, Not reported; CSF, cerebrospinal fluid.
A case of endocarditis caused by Staphylococcus intermedius (Llorca et al, 1992) described in the Spanish literature is not included in the table.
In addition, single cases of endocarditis,9 catheter-related bacteremia,1 pneumonia,13 ear infection,14 mastoiditis,17 sinusitis,18 nail bed infection,15 and brain abscess16 have been reported. Because S. intermedius may also possess enterotoxins,19–21 this species has been considered in addition to S. aureus as the etiologic agent of staphylococcal food poisoning.20,22,23
S. intermedius infections have been described in both immunosuppressed (3/15 cases, 20%)1,13,15 and immunocompetent patients (12/15, 80%).8,11,12,14–18 Based on 13 cases with available data, the average age of patients was 44 years (range 4–78 years), and 7/12 (58.3%) were males and 5/12 (41.7%) were females.
From 11 cases with available data on antimicrobial susceptibility of human isolates of S. intermedius,1,11–13,15–18 penicillin resistance was documented in seven cases (63.6%),11–13,15,16,18 methicillin resistance in three cases (27.3%),13,16,18 cefazolin resistance in three cases (27.3%),13,16,18 resistance to doxycycline in five cases (45.5%),1,12,13,15,18 and resistance to clindamycin in two cases (18.2%).13,16 Resistance to levofloxacin and co-trimoxazole and intermediate susceptibility to gentamicin have also been reported in one case.18 While S. intermedius appears to be more susceptible to penicillin than S. aureus, it may also be resistant to penicillin. Hoskins et al. found that β-lactamase was produced in as many as 55% of S. intermedius isolates.24
From 10 cases with available data on treatment,1,11–14,16–18 antibiotics that have been used for the treatment of S. intermedius infection, based on susceptibility results and site of infection, include topical antibiotics (polymyxin, neomycin, ofloxacin), penicillin, amoxicillin–clavulanic acid, vancomycin, and linezolid (Table 1). The duration of treatment varied from five days for wound infections11 to eight weeks in a case of brain abscess.16 Of cases with data available on outcome, a complete recovery was observed in nine cases1,11–14,17,18 and partial recovery in one case16 of S. intermedius infection.1,11–14,16–18
Identification of S. intermedius is difficult, since S. intermedius may be misclassified as S. aureus unless specific biochemical tests are done.14 To clearly distinguish between S. intermedius and S. aureus, differences in biochemical reactions must be used. The API database is a system that uses specific biochemical reactions to differentiate between the various coagulase-positive staphylococci and is the primary method used in routine medical practice for this purpose. In a comparison of the two organisms, Talan et al. found that in contrast to S. aureus isolates, none of the S. intermedius isolates had a positive acetoin reaction and all S. intermedius isolates, but no S. aureus isolates, had a positive β-galactosidase reaction.2 S. intermedius is coagulase-, pyrrolidonyl arylamidase-, and o-nitrophenyl-β-d-galactopyranoside-positive and hydrolyses urea.15 It grows well on blood and MacConkey agars incubated under aerobic conditions at 35 °C.15 Exact molecular identification is performed by 16S rRNA gene sequencing PCR analysis.15 The very low frequency of coagulase-positive staphylococci other than S. aureus in human specimens does not justify the efficient but expensive identification of all coagulase-positive staphylococci using commercially available galleries. However, specific biochemical tests and PCR of the 16S rDNA gene could be an effective alternative in detecting S. intermedius in human infections where zoonosis is suspected.14
We assume that the patient in our case was a healthy carrier of S. intermedius in his saliva, and as a result of coating the syringes with saliva prior to injection of cocaine, development of abscesses secondary to S. intermedius occurred. Only one case of brain abscess caused by this pathogen has been described in humans.16 This is the first case of a skin abscess caused by S. intermedius in humans. Ways of transmission other than bites have been described. Clinicians should be aware of the potential risk of the development of infections from unusual pathogens isolated in human saliva,7 as we have shown in this case. S. intermedius is a coagulase-positive Staphylococcus that can cause similar infections to S. aureus, and astute acumen and detailed history are paramount to diagnose infections caused by this pathogen.
4. Conclusions
In summary, this case illustrates for the first time that S. intermedius can cause skin abscesses in humans after direct inoculation of the pathogen into the skin and soft tissues. Clinicians should be aware of the fact that although the vast majority of infections from coagulase-positive Staphylococcus infections are secondary to S. aureus, S. intermedius can also be a potential pathogen in humans. Infections from this pathogen can occur both in immunocompetent and immunocompromised patients and thus a detailed history to identify risk factors for this infection (dog exposure, veterinary occupation, exposure to saliva) is important.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Vandenesch F, Celard M, Arpin D, Bes M, Greenland T, Etienne J. Catheter-related bacteremia associated with coagulase-positive Staphylococcus intermedius. J Clin Microbiol 1995;33:2508–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talan DA, Staatz D, Staatz A, Goldstein EJ, Singer K, Overturf GD. Staphylococcus intermedius in canine gingiva and canine-inflicted human wound infections: laboratory characterization of a newly recognized zoonotic pathogen. J Clin Microbiol 1989;27:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoie S, Fossum K. Antibodies to staphylococcal DNases in sera from different animal species, including humans. J Clin Microbiol 1989;27:2444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahoudeau I, Delabranche X, Prevost G, Monteil H, Piemont Y. Frequency of isolation of Staphylococcus intermedius from humans. J Clin Microbiol 1997;35:2153–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talan DA, Staatz D, Staatz A, Overturf GD. Frequency of Staphylococcus intermedius as human nasopharyngeal flora. J Clin Microbiol 1989;27:2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamura Y, Hou XG, Sultana F, Hirose K, Miyake M, Shu SE, et al. Distribution of Staphylococcus species among human clinical specimens and emended description of Staphylococcus caprae. J Clin Microbiol 1998;36(7):2038–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK. Occurrence of staphylococci in the oral cavities of healthy adults and nasal oral trafficking of the bacteria. J Med Microbiol 2008;57(Pt 1):95–9. [DOI] [PubMed] [Google Scholar]
- 8.Lee J Staphylococcus intermedius isolated from dog-bite wounds. J Infect 1994;29:105. [DOI] [PubMed] [Google Scholar]
- 9.Llorca I, Gago S, Sanmartin J, Sanchez R. [Infectious endocarditis caused by Staphylococcus intermedius in a patient infected with HIV]. Enferm Infecc Microbiol Clin 1992;10:317–8. [PubMed] [Google Scholar]
- 10.Goldstein EJ. Bite wounds and infection. Clin Infect Dis 1992;14:633–8. [DOI] [PubMed] [Google Scholar]
- 11.Talan DA, Goldstein EJ, Staatz D, Overturf GD. Staphylococcus intermedius: clinical presentation of a new human dog bite pathogen. Ann Emerg Med 1989;18:410–3. [DOI] [PubMed] [Google Scholar]
- 12.Barnham M, Holmes B. Isolation of CDC group M-5 and Staphylococcus intermedius from infected dog bites. J Infect 1992;25:332–4. [DOI] [PubMed] [Google Scholar]
- 13.Gerstadt K, Daly JS, Mitchell M, Wessolossky M, Cheeseman SH. Methicillin-resistant Staphylococcus intermedius pneumonia following coronary artery bypass grafting. Clin Infect Dis 1999;29:218–9. [DOI] [PubMed] [Google Scholar]
- 14.Tanner MA, Everett CL, Youvan DC. Molecular phylogenetic evidence for noninvasive zoonotic transmission of Staphylococcus intermedius from a canine pet to a human. J Clin Microbiol 2000;38:1628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pottumarthy S, Schapiro JM, Prentice JL, Houze YB, Swanzy SR, Fang FC, et al. Clinical isolates of Staphylococcus intermedius masquerading as methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2004;42(12):5881–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atalay B, Ergin F, Cekinmez M, Caner H, Altinors N. Brain abscess caused by Staphylococcus intermedius. Acta Neurochir (Wien) 2005;147:347–8. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi K, Karasawa T, Piao C, Itoda I, Hidai H, Yamaura H, et al. Molecular confirmation of transmission route of Staphylococcus intermedius in mastoid cavity infection from dog saliva. J Infect Chemother 2004;10(1):46–8. [DOI] [PubMed] [Google Scholar]
- 18.Kempker R, Mangalat D, Kongphet-Tran T, Eaton M. Beware of the pet dog: a case of Staphylococcus intermedius infection. Am J Med Sci 2009;338:425–7. [DOI] [PubMed] [Google Scholar]
- 19.Almazan J, de la FR, Gomez-Lucia E, Suarez G. Enterotoxin production by strains of Staphylococcus intermedius and Staphylococcus aureus isolated from dog infections. Zentralbl Bakteriol Mikrobiol Hyg [A] 1987;264:29–32. [DOI] [PubMed] [Google Scholar]
- 20.Becker K, Harmsen D, Mellmann A, Meier C, Schumann P, Peters G, et al. Development and evaluation of a quality-controlled ribosomal sequence database for 16S ribosomal DNA-based identification of Staphylococcus species. J Clin Microbiol 2004;42(11):4988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards VM, Deringer JR, Callantine SD, Deobald CF, Berger PH, Kapur V, et al. Characterization of the canine type C enterotoxin produced by Staphylococcus intermedius pyoderma isolates. Infect Immun 1997;65(6):2346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker K, Keller B, von EC, Bruck M, Lubritz G, Etienne J, et al. Enterotoxigenic potential of Staphylococcus intermedius. Appl Environ Microbiol 2001;67(12): 5551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khambaty FM, Bennett RW, Shah DB. Application of pulsed-field gel electrophoresis to the epidemiological characterization of Staphylococcus intermedius implicated in a food-related outbreak. Epidemiol Infect 1994;113: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoskins JD, Newman SS, Roy AF, Foil CS, Cox HU. Detection of beta-lactamase produced by Staphylococcus intermedius. Am J Vet Res 1985;46:1526–8. [PubMed] [Google Scholar]


