Abstract
Background
Pneumatosis intestinalis (PI) is a rare condition usually occurring among adults who have undergone solid organ transplant and are taking steroid therapy. The coronavirus disease 2019 (COVID-19) virus uses angiotensin-converting enzyme 2 in gastrointestinal epithelium as a receptor for entry process. Due to the steroid intake, the COVID-19 virus is present in the patient's gastrointestinal tract for extended period of time. It may therefore increase the possibility of PI in such patients. It is usually asymptomatic, with a clinical spectrum ranging from indolent to life-threatening. Unfortunately, there are no algorithms concerning diagnosis and treatment of PI.
Aim of study
The aim of this study is to highlight the problem of PI induced by COVID-19, especially in high-risk groups such as solid organs recipients.
Conclusion
On the basis of the presented case of a severe course of COVID-19–induced PI, we conclude that laparotomy with bowel resection can be a feasible and a safe option for treatment.
Background
Pneumatosis intestinalis (PI) is a condition in which the presence of gas is identified within the wall of the intestines. It is a very rare condition, usually diagnosed in newborns and small children. Among adults it is only occasionally seen and, when present, is associated with conditions including infectious diseases (cytomegalovirus, human immunodeficiency virus, Mycobacterium tuberculosis), pulmonary diseases (chronic obstructive pulmonary disease, pulmonary fibrosis), and gastrointestinal diseases (inflammatory bowel disease, toxic megacolon) [1,2]. Patients who have undergone immunosuppression after solid organ transplantation, especially with glucocorticoids, are predisposed to PI [3]. Despite having been described for the first time in 1730 by DuVernoi in an autopsy report, there are no conclusive guidelines for diagnosing and treating PI [1,4]. In most cases it is asymptomatic and diagnosed accidentally during imaging studies such as a computed tomography scan [5], [6], [7], [8]. The symptoms can vary from mild abdominal pain, nausea, and vomiting to features of peritonitis due to bowel perforation [[1], [2], [3], [4],7,9,10,11,13,14]. The most common treatment is conservative: nihil per os, total parenteral nutrition, empirical antibiotics, and hyperbaric oxygen. In a few reported cases the treatment of choice was surgical because of peritonitis symptoms [3,7,10,12,15].
Since the first outbreak in December 2019 of COVID-19, a viral infection that primarily affects the respiratory system, more reports have appeared on gastrointestinal manifestations. It has been proven that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses angiotensin-converting enzyme 2 (ACE2) as a receptor for its entry process. Due to high ACE2 expression in respiratory, myocardial, and gastrointestinal epithelial cells, the virus can give rise to a variety of symptoms at these sites [5,11,12,15]. The most common gastrointestinal (GI) tract symptoms include mild abdominal pain and diarrhea [7,11,13]. Additionally, COVID-19 viral RNA has been detected for a longer duration of time in the feces of patients taking glucocorticoids compared with patients that are not, suggesting these drugs prolong the virus's presence in the GI tract, potentially increasing the risk of local complications.
Case Description
A 39-year-old man was admitted to the emergency room with fever, pain in the upper abdomen, nausea, and vomiting that started a day before. The patient had undergone a kidney transplant a year before due to kidney failure of unknown origin and was taking immunosuppressants (tacrolimus, methylprednisolone, and mycophenolate mofetil). Moreover, the patient was taking acenocoumarol because of a mechanical aortic valve replacement with repair of an ascending aortic aneurysm type 2 and implantable cardioverter defibrillator implantation undergone 2 years ago as secondary cardiac arrest prevention. On his admission and upon examination, the patient's abdomen was bloated and painful but without muscle guarding.
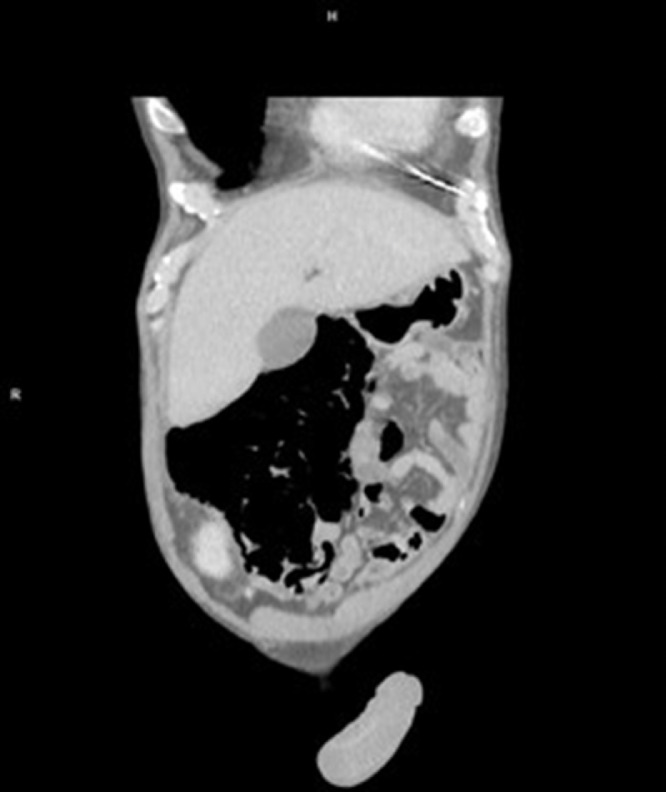
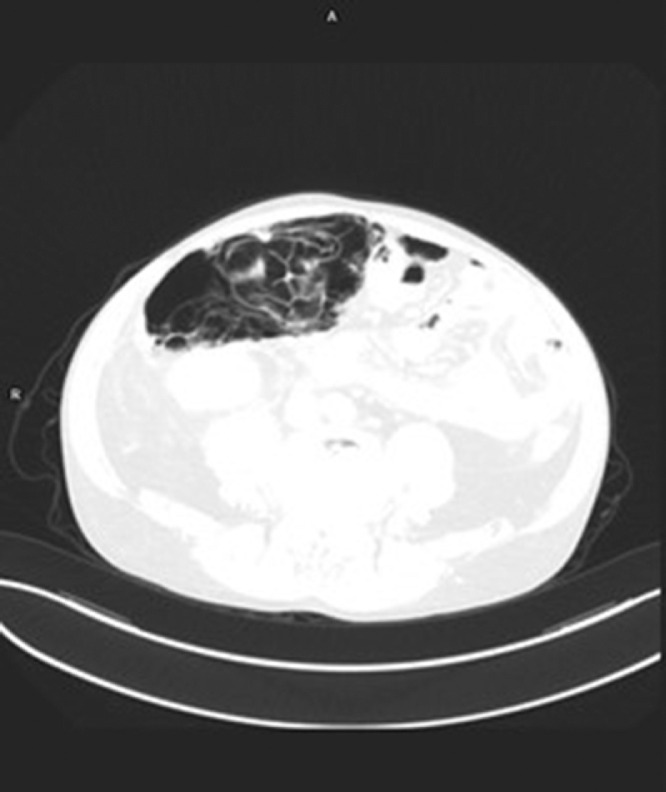
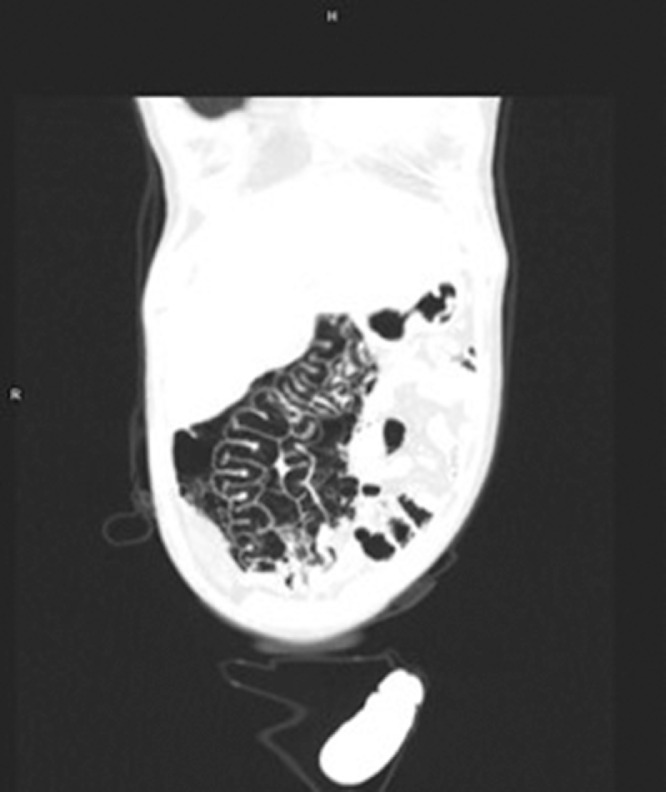
In the laboratory findings, a urinary tract infection was diagnosed with elevated inflammatory markers (C reactive protein 125 mg/L, white blood cells 22.47 × 109/L, procalcitonin 11.16 ng/mL), elevated creatinine (1.61 mg/dL) and bilirubin (1.83 mg/dL) without elevated liver or pancreatic enzymes. An apparent large intestine necrosis and perforation appeared on a computed tomography scan (Fig. 1 -3 ). According to the protocol, a nasopharyngeal swab was performed; the patient tested positive for COVID-19 in polymerase chain reaction test.
Fig 2.
Coronal computed tomography image showing extensive pneumatosis intestinalis and bowel wall thickening.
Fig 1.
Computed tomography image showing extensive pneumatosis intestinalis and bowel wall thickening.
Fig 3.
Coronal computed tomography image showing extensive pneumatosis intestinalis and bowel wall thickening.
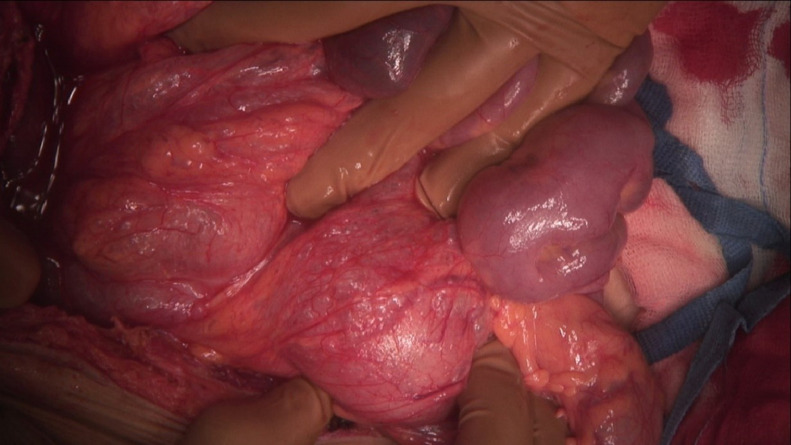
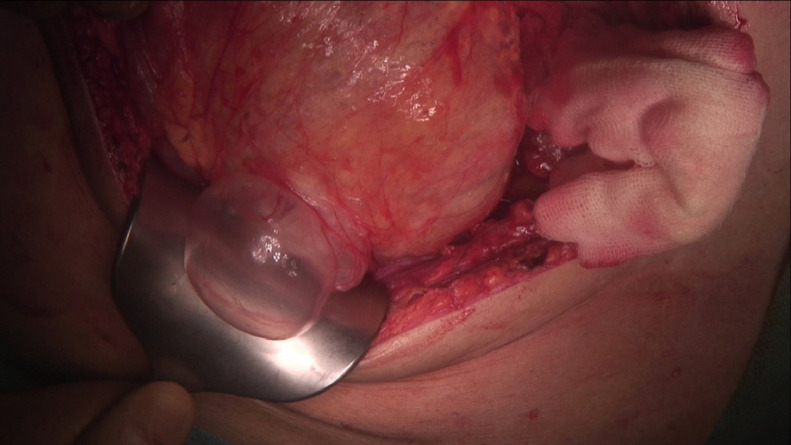
Because of radiological findings and the patient's symptoms, the decision was made to perform an explorative laparotomy. Intraoperatively, a pneumatosis of the right side of the colon and its mesentery was found without frank necrosis (Fig. 4 -5 ). The patient underwent a right hemicolectomy with side-to-side entero-intestinal anastomosis. After treatment in the COVID unit, which included a course of antibiotics, oxygen therapy, deep vein thrombosis prophylaxis, antihemorrhagic treatment, and transfusion of blood products, a decrease in inflammatory markers and stabilization of morphology was observed. After the surgery, the patient did not experience any abdominal symptoms. A colonoscopy was performed during his stay in the COVID unit, and no signs of pneumatosis or necrosis were found. The patient did not develop respiratory insufficiency. After 26 days he was discharged from the hospital.
Fig 4.
Intraoperative picture.
Fig 5.
Intraoperative picture.
Discussion
PI is a very rare condition among adults. It is defined as presence of gas in the wall of the GI tract. Pneumoperitoneum, pneumoretroperitoneum, or gas inclusions can be present in the portal vein [5], [6], [7], [8]. The pathophysiology of PI is still unknown, but a variety of theories have been proposed: the mechanical theory (increase of intraluminal pressure causing mechanical damage), pulmonary theory (alveolar rupture due to chronic lung disease causing release of gas along the aorta and mesenteric arteries and into the intestinal wall), and bacterial theory (aerogenic bacteria penetrating the mucosal barrier and producing gas) [1,3,4,6]. We have listed the diseases and conditions that most strongly predispose patients to PI using the acronym “TRAPInG” (Fig 6 ):
Transplantation (eg, bone marrow, solid organs, graft vs host)
Related to medications (eg, corticosteroids, chemotherapeutic agents)
Autoimmune (eg, lupus)
Pulmonary (eg, asthma, chronic obstructive pulmonary disease)
Infectious (eg, human immunodeficiency virus, cytomegalovirus, COVID-19)
Iatrogenic (eg, endoscopy, barotrauma)
Idiopathic
Gastrointestinal (inflammatory bowel disease, colitis, diverticulitis)
Fig 6.
TRAPInG acronym, based on Khalid et al. [1]
Despite the fact that this condition has been observed since the 18th century, there are still no clear guidelines concerning its diagnosis and treatment [1,4]. We should keep the possibility of PI in mind in patients who have undergone solid organ transplant, present abdominal symptoms, and have been diagnosed with COVID-19 infection.
PI is usually asymptomatic, with a clinical spectrum raging from benign to life-threatening. The most common symptoms include mild abdominal pain, nausea, vomiting, and diarrhea [[1], [2], [3], [4],7,9,10,11,13,14]. The life-threatening forms are related to mesenteric ischemia, bowel obstruction, and necrosis [4,6,10]. A therapeutic approach should be chosen based on the symptoms, physical examination of the patient, and laboratory and radiological findings. If there are no indicators of a life-threatening form of PI, caregivers should choose a conservative approach—NPO status, total parenteral nutrition, fluid and electrolyte supplementation, empirical antibiotics, hyperbaric oxygen, and observation [[1], [2], [3], [4],7,10,15].
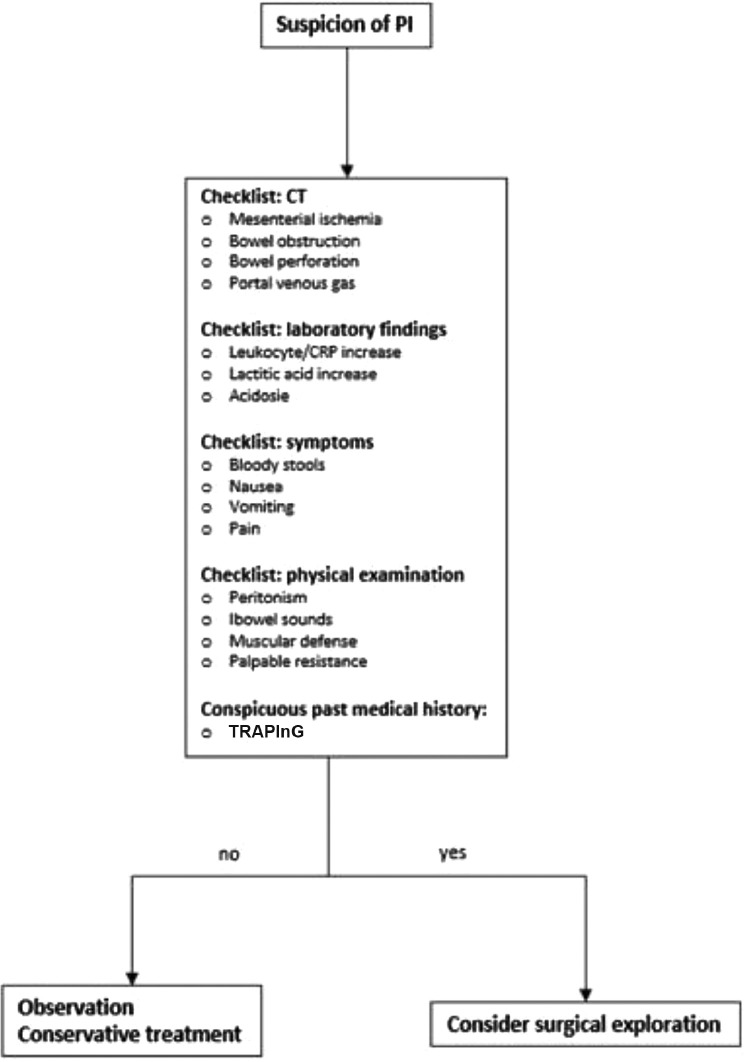
A nonsurgical approach is usually sufficient, although in some cases such as the one described in our study—in which the patient was undergoing immunosuppression treatment, had PI and infection with high inflammatory markers, and had a fever—a surgical approach is necessary. On the basis of our case, we recommend a decision-making algorithm presented by Khalil at al. (Fig 7 ) [1].
Fig 7.
Treatment algorithm of PI, based on Khalid et al. [1] PI, pneumatosis intestinalis; CT, computed tomography.
Conclusion
Because of the possibility of COVID-19 infiltration through the ACE2 receptors in the GI tract, we should keep the possibility of PI in mind, especially in patients such as solid organ recipients who are taking glucocorticoids. Despite the difficulty of diagnosis and uncertainty of treatment algorithms, PI can usually be treated by a conservative approach, but in cases of severe PI, laparotomy and colon resection can be a safe option for COVID-19–induced PI.
Acknowledgments
The authors wish to thank Dr. Maciej Wilczyński for intraoperative photographic documentation and Dr. Bartosz Baścik for radiological documentation.
References
- 1.Khalil PN, Huber-Wagner S, Ladurner R, Kleespics A, Siebeck M, Mutschler W, et al. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231–239. doi: 10.1186/2047-783X-14-6-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu LL, Yang YS, Dou Y, Sen Liu Q. A systematic analysis of pneumatosis cystoids intestinalis. World J Gastroenterol. 2013;19:4973–4978. doi: 10.3748/wjg.v19.i30.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello P, Johnson S, Ramos Mercado A, Hussein S. Pneumatosis intestinalis in a patient with COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemma V, Mistrot D, Row D, Gagliano RA, Bremner RM, Walia R, et al. Pneumatosis intestinalis in solid organ transplant recipients. J Thorac Dis. 2018;10:1984–1997. doi: 10.21037/jtd.2018.02.52. Vol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297:E207–E215. doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan W, Ramadan HK-A. COVID-19 and pneumatosis intestinalis: an early sign of intestinal ischemia [e-pub ahead of print]. Dig Liver Dis. doi:10.1016/j.dld.2020.10.036, accessed February 16, 2021. [DOI] [PMC free article] [PubMed]
- 7.Wong K, Kim DH, Khanijo S, Melamud A, Zaidi G. Pneumatosis intestinalis in COVID-19: case series. Cureus. 2020;12:e10991. doi: 10.7759/cureus.10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lui K, Wilson MP, Low G. Abdominal imaging findings in patients with SARS-CoV-2 infection: a scoping review. Abdom Radiol (NY) 2020:1–7. doi: 10.1007/s00261-020-02739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakshmanan S, Toubia N. Pneumatosis intestinalis in COVID-19 [e-pub ahead of print]. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2020.05.048, accessed February 16, 2021. [DOI] [PMC free article] [PubMed]
- 10.Kielty J, Duggan WP, O'Dwyer M. Extensive pneumatosis intestinalis and portal venous gas mimicking mesenteric ischaemia in a patient with SARS-CoV-2. Ann R Coll Surg Engl. 2020;102:E145–E147. doi: 10.1308/rcsann.2020.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meini S, Zini C, Passaleva MT, Frullini A, Fusco F, Carpi R, et al. Pneumatosis intestinalis in COVID-19. BMJ Open Gastroenterol. 2020;7 doi: 10.1136/bmjgast-2020-000434. [DOI] [PMC free article] [PubMed] [Google Scholar]