Abstract
Background.
The incidence of parathyroid carcinoma is reported to be rising. There is minimal data on prognostic variables associated with cancer-specific survival. The objectives of this study were to evaluate the trends in incidence and assess prognostic factors.
Methods.
A retrospective review of the SEER database between 1973 and 2014 was performed, identifying 520 patients with parathyroid carcinoma. Population-adjusted incidence rates were calculated in 4-year intervals. A Cochrane-Armitage test was performed to analyze changes in trend in incidence, tumor size, and extent of disease. Age, year of diagnosis, race, gender, extent of disease, surgical resection, treatment with radiation, tumor size, and lymph node status were assessed using Mantel-Cox log rank test. Multivariate analysis was performed by Cox regression analysis.
Results.
The incidence of parathyroid carcinoma has been increasing since 1974 from 2 to 11 cases per 10 million people but has since stabilized at 11 cases per 10 million people since 2001. The increasing incidence was attributed to locoregional disease and tumor size < 3 cm. The presence of metastatic disease [hazard ratio (HR) 111.4, 95% confidence interval (CI) 20.6–601.8, p < 0.0001) and tumor size > 3 cm (HR 5.6, 95% CI 1.5–21.2, p = 0.011] were associated with worse cancer-specific survival by univariate and multivariate analyses.
Conclusions.
The incidence of parathyroid carcinoma has remained stable over the past decade. Tumor size < 3 cm and regional disease have increased in incidence. Patients with metastatic disease and tumors > 3 cm have worse cancer-specific survival. These findings can be incorporated in the development of a staging system for parathyroid carcinoma.
Parathyroid carcinoma, a rare disease, has been reported to have an incidence of approximately 1 per 10,000,000 persons per year and with rising incidence.1 Patients may present with signs and symptoms of hyperparathyroidism, including fatigue, bone pain, joint pain, constipation, anorexia, weight loss, renal insufficiency, nephrolithiasis, and osteoporosis.2 On laboratory evaluation, a markedly elevated serum calcium greater than 14 mg/dL and a parathyroid hormone (PTH) level at least twice the upper limit of normal are common in parathyroid carcinoma.3 However, these are nonspecific findings, and parathyroid carcinoma is typically diagnosed by intraoperative recognition of disease and postoperative histologic examination of the resected tissue.4 Preoperative imaging with neck ultrasonography and parathyroid scintigraphy may help with localization but typically do not distinguish between benign and malignant disease.5
Recently, it has been suggested that the incidence of parathyroid carcinoma has been increasing over the past four decades. Lee and colleagues reported the incidence of parathyroid carcinoma had increased by 60% (from 3.58 to 5.73 cases per 10 million patients).6 They hypothesized that the change in NIH Consensus guidelines for parathyroidectomy for asymptomatic hyperparathyroidism (2002) may have resulted in greater identification of patients with asymptomatic parathyroid cancer via increased serum calcium screening.7 It is unknown whether this trend has continued since that analysis.
Several studies have been performed to evaluate prognostic factors associated with survival in parathyroid carcinoma. Characteristics, such as patient age at diagnosis, male sex, positive nodal status, and primary tumor size, have previously been associated with worse overall survival.4–7 However due to the rarity of disease, it has been difficult to reach consensus regarding which factors should be included in a staging system. In addition, because parathyroid carcinoma is an indolent disease, the utilization of overall survival as a primary endpoint may be confounded by the patient’s anticipated future lifespan (based on age) and current comorbidities. Prognostic factors have not been previously characterized with respect to cancer-specific survival. Therefore, the purpose of this study was to evaluate the changes in the incidence of parathyroid carcinoma and to assess prognostic factors associated with cancer-specific survival to build a foundation for a staging system.
METHODS
The National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) was used for this study. SEER began prospective collection in 1973 of cancer incidence and survival data over select U.S. counties and has since expanded to include 20 different population-based registries. These registries encompass approximately 28% of the U.S. population. They record data, including age, gender, race, primary tumor site, tumor histology, extent of disease, treatment, overall survival, and disease-specific survival.
The SEER database was queried for all cases of parathyroid cancer from 1973 through 2013. Patients were identified using ICD-10-CM diagnosis code C75.0 (malignant neoplasm of parathyroid gland) and confirmation of carcinoma by positive histology. Patients were excluded if they were coded with ICD-10 codes consistent with metastatic disease originating from other organ sites. Incidence was tabulated from individual SEER county-level reporting. This event rate was adjusted for the inclusion of additional counties in 1974, 1978, 1980, 1990, and 2000 as the national database was modified to better represent the U.S. population. Incidence is reported per 10 million people per 4-year intervals, given the relative rarity of disease. Cochrane-Armitage trend analyses were performed to assess for changes in incidence of parathyroid carcinoma, changes in incidence with respect to tumor size, and changes in incidence with respect to extent of disease over time with GraphPad (GraphPad Software, Inc., La Jolla, CA).
The primary outcome was disease-specific survival. To assess prognostic factors, univariate analysis was performed assessing age at diagnosis, year of diagnosis, race, gender, tumor grade, extent of disease (local, regional, and metastatic disease), extent of surgical resection (parathyroidectomy, radical resection), administration of radiation therapy, and lymph node status. Prognostic factors were compared by the log-rank (Mantel-Cox) test. Tumor size was assessed in a stepwise fashion to determine a prognostic size. A multivariate Cox Proportional Hazard regression analysis was performed to assess which prognostic factors were independently associated with cancer-specific survival. Univariate and multivariate analyses were performed with SPSS software (IBM, Armonk, NY). A two-tailed p value < 0.05 was considered significant.
RESULTS
A total of 520 patients were identified with parathyroid carcinoma. The median age of diagnosis was 56.5 (range 20–87) years. There was no difference in age at diagnosis by gender. Gender distribution was nearly equal. These patients were predominantly white (n = 392, 75.4%). Most patients presented with either local (66.9%, n = 348) or regional and locally invasive disease (30.6%, n = 159). Only 12 patients initially presented with metastatic disease. Nearly 47% of patients had tumors < 4.0 cm in size. Most patients with reported lymph node status had negative lymph nodes at resection (97.3%, n = 289). Surgical resection entailed either parathyroidectomy or en bloc radical resection. Most patients (78.5%, n = 408) underwent parathyroidectomy, 9.2% (n = 48) underwent en bloc resection, and 9% (n = 47) refused surgery. Radiation therapy was given to 45 patients (8.7%; Table 1). The overall cancer-specific mortality was 10.6% with a median follow-up of 4.5 years (n = 42).
TABLE 1.
Patient characteristics and treatment for parathyroid carcinoma (1973–2013)
| Characteristic | Number (n) | % |
|---|---|---|
| Gender | ||
| Male | 267 | 51.3 |
| Female | 253 | 48.7 |
| Race | ||
| White | 392 | 75.4 |
| Black | 84 | 16.2 |
| Other | 44 | 8.5 |
| Age (year) | ||
| < 45 | 120 | 23.1 |
| 45–59 | 199 | 38.3 |
| 60–69 | 109 | 21.0 |
| 70–79 | 66 | 12.7 |
| 80+ | 15 | 2.9 |
| Extent of disease | ||
| Local disease | 348 | 66.9 |
| Regional disease | 159 | 30.6 |
| Metastatic disease | 12 | 2.3 |
| Unstaged | 1 | 0.2 |
| LN status | ||
| Negative | 289 | 55.6 |
| Positive | 9 | 1.7 |
| Not reported | 222 | 42.7 |
| Size of tumor (cm) | ||
| 0–2.9 | 176 | 33.8 |
| ≥3 | 133 | 25.6 |
| Unknown | 211 | 40.6 |
| Grade | ||
| Well-differentiated | 48 | 9.3 |
| Moderately differentiated | 3 | 0.6 |
| Poorly differentiated | 2 | 0.4 |
| Undifferentiated | 463 | 89.7 |
| Treatment* | ||
| No | 19 | 3.7 |
| Yes | 501 | 96.3 |
| Surgerya | ||
| No definitive treatment | 47 | 9.0 |
| Parathyroidectomy | 408 | 78.5 |
| En bloc radical resection | 48 | 9.2 |
| Other | 17 | 3.3 |
| Radiation | ||
| No | 475 | 91.3 |
| Yes | 45 | 8.7 |
Nineteen patients received no surgical, chemotherapy, or radiotherapy
Patients who did not undergo surgical resection were diagnosed by polypectomy, biopsy, or autopsy at death
The initial incidence of parathyroid carcinoma was 2 cases per 10 million people in from 1974 to 1977. The annual overall incidence rose from 2 to 11 cases per 10 million (p < 0.0001) between 1973 and 2001 but has remained stable between 10 to 13 cases per 10 million since 2001 (Fig. 1). A subgroup analysis of significant prognostic factors showed that there was a trend towards increasing incidence of tumors between 0 and 3 cm (Fig. 2a, p = 0.07) and a trend towards increasing incidence of regional disease at the time of initial operation (Fig. 2b, p = 0.05). There was no significant change in the incidence of tumors ≥ 3 cm, local or metastatic disease at initial presentation.
FIG. 1.
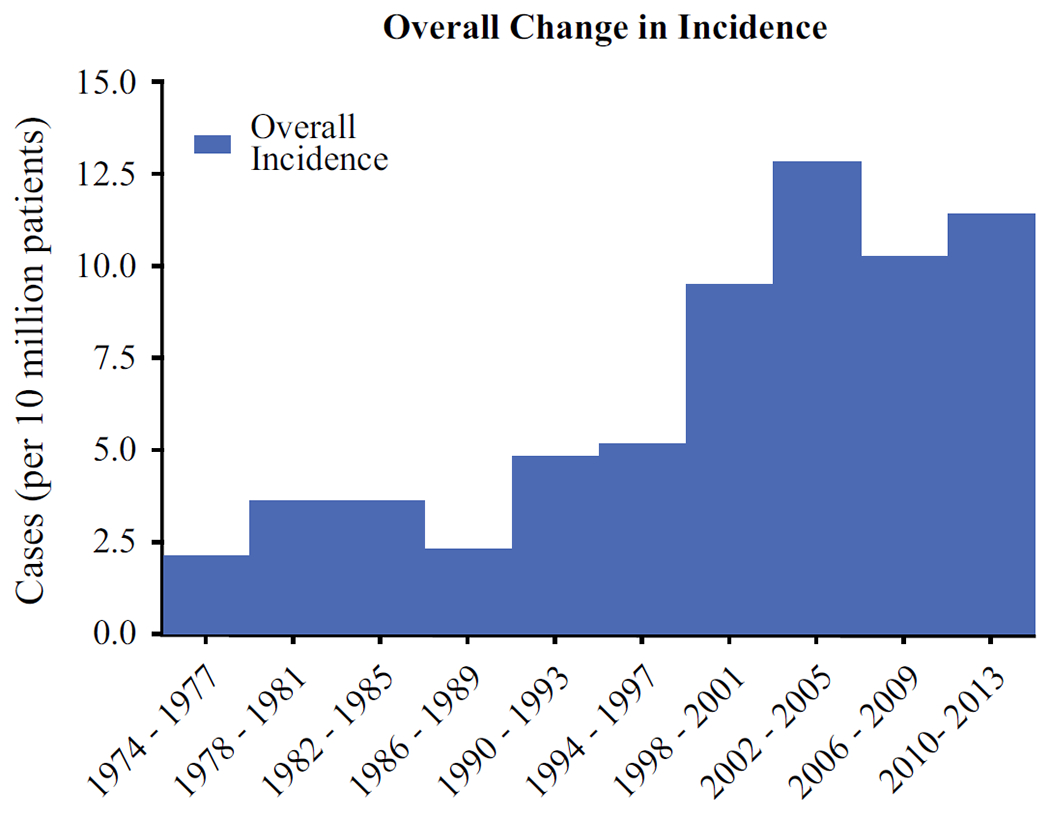
The change in overall incidence of parathyroid carcinoma cases assessed by 4 year intervals showed an increasing incidence until 2001 (p < 0.0001) but has remained stable between 10 to 13 cases per 10 million
FIG. 2.
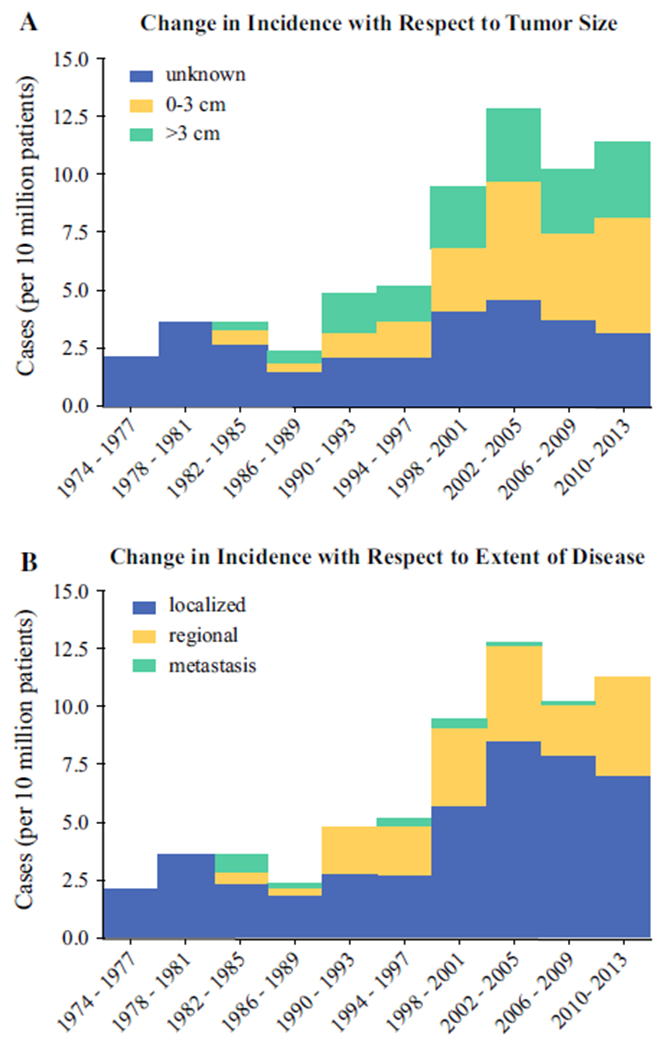
The change in incidence of parathyroid carcinoma cases assessed by 4-year intervals stratified by a tumor size and b extent of disease. Green bars highlight factors that were found to be poor prognostic factors by multivariate analysis
Median disease-specific survival was 75 months for all patients with parathyroid carcinoma. The median survival for patients with local (noninvasive) or regional disease (including locally invasive and lymph node positive disease) was not reached compared with a median survival of only 2.5 months for patients with metastatic disease (Fig. 3b, p < 0.0001). Prognostic factors associated with prolonged disease-specific survival were analyzed by univariate analysis. Tumor size > 3 cm and extent of disease were associated with worse cancer-specific survival. There was no association of age at diagnosis, year of diagnosis, race, gender, tumor grade, lymph node status, extent of surgery, or use of radiation with cancer-specific survival. On multivariate analysis, the presence of metastatic disease at diagnosis [HR 111.4, 95% confidence interval (CI) 20.6–601.8, p < 0.0001] and tumor size greater than or equal to 3 cm (HR 5.6, 95% CI 1.5–21.2, p = 0.011) were significantly associated with worse cancer-specific survival. However, no significant association with cancer-specific survival was found with age, race, tumor grade, type of surgical resection, treatment with radiation, or lymph node status (Table 2).
FIG. 3.
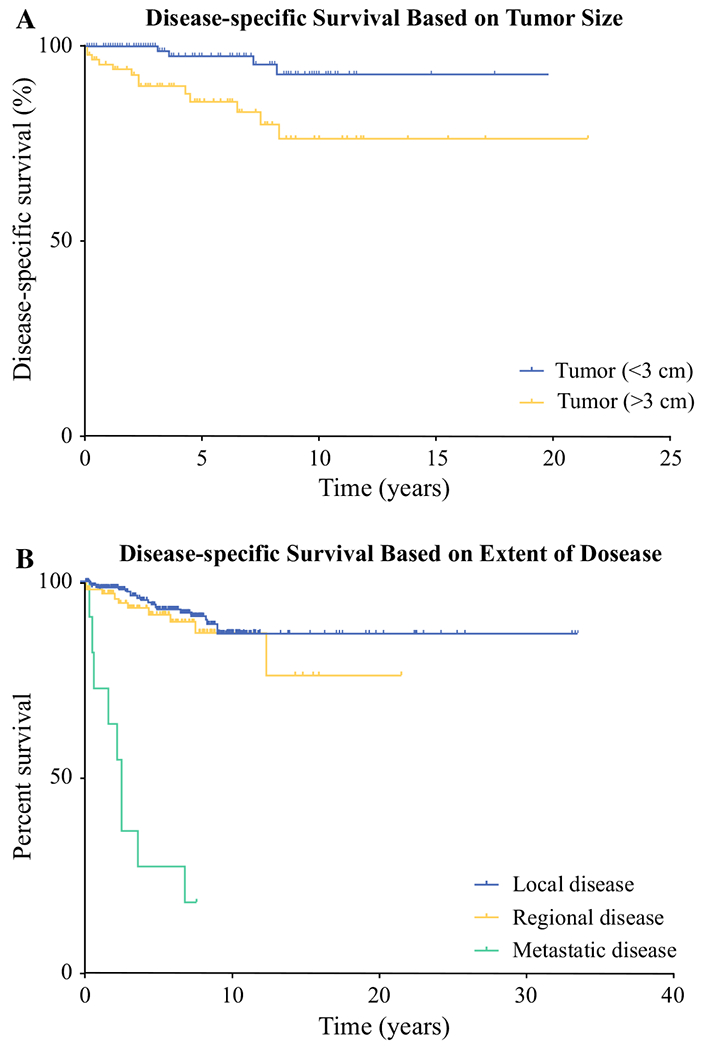
Comparison of a tumor size and b extent of disease by disease-specific survival. The green curves highlight factors that were found to be poor prognostic factors by multivariate analysis
TABLE 2.
Prognostic factors associated with disease-specific survival (multivariate analysis)
| Variable | HR (95% CI) | p value |
|---|---|---|
| Age at diagnosis (year) | ||
| < 45 | Reference | |
| 45–59 | 0.46 (0.07–3.26) | 0.439 |
| 60–69 | 0.70 (0.10–4.87) | 0.721 |
| 70–79 | 1.39 (0.15–13.07) | 0.772 |
| 80+ | 0 (0–1.77e170) | 0.967 |
| Race | ||
| White | Reference | |
| Black | 0.90 (0.16–4.85) | 0.906 |
| Other | 0.72 (0.06–8.95) | 0.797 |
| Tumor size (cm) | ||
| < 3 | Reference | |
| ≥3 | 5.60 (1.50–21.20) | 0.012 |
| Lymph nodes | ||
| Negative | Reference | |
| Positive | 19.86 (2.01–196.71) | 0.307 |
| Extent of disease | ||
| Local disease | Reference | |
| Regional disease | 1.54 (0.32–7.38) | 0.588 |
| Metastatic disease | 104.45 (9.89–1103) | 0.0001 |
| Surgery | ||
| No | Reference | |
| Yes | 0.72 (0.17–3.01) | 0.962 |
| Extent of resection | ||
| Parathyroidectomy | Reference | |
| En bloc resection | 0.72 (0.17–3.01) | 0.648 |
| Unknown | 2.04 (0.21–19.74) | 0.539 |
| Radiation | ||
| No | Reference | |
| Yes | 1.59 (0.44–5.85) | 0.848 |
| Grade | ||
| Well-differentiated | Reference | |
| Moderately differentiated | 6.23 (0.21–183.28) | 0.289 |
| Poorly differentiated | 4.40 (0.22–89.79) | 0.335 |
| Undifferentiated | 10.53 (0.34–325.84) | 0.179 |
DISCUSSION
The incidence of parathyroid carcinoma has been increasing since the inception of the SEER registry in 1973. This study showed that the incidence has plateaued at 11 cases per 10 million people since 2001. In addition, a subgroup analysis showed that patients with tumors between 0 and 3 cm and patients with locoregional disease were responsible for the increasing incidence of parathyroid carcinoma. Furthermore, an initial presentation with metastatic disease and a tumor size greater than 3 cm were independently associated with worse disease-specific survival by univariate and multivariate analysis.
Lee and colleagues initially reported that the incidence of parathyroid cancer had increased significantly from 3.58 to 5.73 per 10 million people in a 16-year period from 1988 to 2003.6 They attributed the increase in incidence to increased screening of serum hypercalcemia leading to a higher rate of diagnosis of primary hyperparathyroidism and subsequent parathyroidectomy. Recent changes in recommendations for parathyroidectomy may have had an impact on the overall diagnosis of parathyroid carcinoma.8,9 For example, in 2002, NIH Consensus guidelines for management of asymptomatic hyperparathyroidism lowered the threshold for referral for parathyroidectomy to 1.0 mg/dL above the upper limit of normal.10 Because parathyroid carcinoma is typically diagnosed incidentally by histology, it is logical that an increase in parathyroidectomies may be associated with a higher rate of incidental diagnosis of parathyroid carcinoma. The timing of the guidelines changes mirror the increased incidence of parathyroid carcinoma in our data, where the incidence from 1994 to 1997 was 5 cases per 10 million persons and increased to 10 cases per 10 million persons during 1998–2001 and 13 cases per 10 million persons during 2002–2005. Incidence did not continue to increase in subsequent years and has instead remained stable. Furthermore, a subgroup analysis of prognostic factors showed that the increasing incidence is primarily due to smaller tumors (< 3 cm) and regional disease (locally invasive and lymph node positive disease). This suggests that the previously observed increase in incidence might be due to improved screening and referral to surgeons for parathyroidectomy.
Prior reports of 5-year overall survival range from 78.3 to 82.3%.7,10 In this analysis, the disease-specific survival was 89.4% (median survival 75 months). Disease-specific survival was utilized as an outcome measure instead of overall survival due to the indolent nature of parathyroid carcinoma, which is reflected by the diagnosis in older patients (median age at diagnosis ranges from 53 to 57).1,10 In addition, the median survival from diagnosis is estimated around 14.3 years, which indicates that death from other factors may be a confounder.10 In this study, 82 patients were censored due to death from other causes, including cardiac disease, complications from diabetes mellitus, and renal disease. The inclusion of these patients would have lowered the observed 5-year survival rate closer to what has been reported in other studies but would have been less accurate.
Primary tumor size 3 cm or larger was found to be independently associated with worse cancer-specific survival in patients with parathyroid carcinoma. Prior studies examining prognostic factors associated with survival have had mixed conclusions. Asare and Hsu identified tumor size to be associated with a small increased risk of death, with the latter reporting tumor size > 3 cm associated with worse disease-specific survival.7,11 Other cohorts have observed no association between tumor size and survival. An analysis of 37 patients treated at the University of California, San Francisco Medical Center, as well as a multicenter study conducted by the Spanish Association of Surgery (62 patients) concluded that tumor size did not have prognostic value.10,12 The latter two studies are limited by small sample size and were likely underpowered to detect the prognostic value of tumor size. In a larger study evaluating 286 cases of parathyroid carcinoma from the National Cancer Database, tumor size was not significantly associated with survival.13 However, this study utilized overall survival relative to the survival probabilities for Caucasian-Americans in 1980. This is not a common outcome measure in descriptive cancer studies and is difficult to interpret Although prior studies have not corroborated the prognostic value of tumor size, our observation is in one of the largest cohorts of patients with parathyroid carcinoma associated with disease-specific survival.
The presence of metastatic disease at diagnosis was found to be a poor prognostic factor, which is consistent with prior studies. Harari and colleagues showed that the presence of either regional or distant metastatic disease in a cohort of 37 patients has been associated with increased mortality.10 This study was limited by reporting on patients treated at a single, tertiary-care referral center over a 43-year period, during which standards for preoperative imaging, histologic diagnosis, and postoperative adjuvant therapy changed significantly. Furthermore, the study did not evaluate for cancer-specific survival. Lee and colleagues also found an association between metastatic disease at diagnosis and poorer overall survival.6 In this study, the presence of metastatic disease was prognostic and associated with disease-specific survival. These findings suggest that metastatic disease at the time of diagnosis should be included in a parathyroid carcinoma staging system.
Positive lymph nodes were not associated with worse disease-specific survival. This was not unanticipated, because lymph node involvement has been of unclear prognostic value for survival in recent literature. Previous analyses of SEER database did not associate positive lymph node status as a prognostic indicator of worse overall survival.5,11 However, lymph node status is not recorded in a large subset of patients in this database. Additionally, for those patients with lymph node status recorded, the frequency of metastatic lymph nodes is small, ranging from 6 to 10.5%.6,11 Some studies have shown a trend toward worse overall or disease-specific survival for positive lymph node status but have failed to reach statistical significance.1,2 This could be due to inadequate sample size. Due to the rarity of parathyroid carcinoma, the infrequency of patients with metastatic disease involving lymph nodes, and the lack of complete information in national databases, strong conclusions regarding the prognostic value of lymph node status cannot be made.
The extent of resection was not a significant factor associated with disease-specific survival. Although current recommendations suggest en bloc surgical resection with microscopically negative margins, this is not supported by our findings or the literature.4 Young and colleagues compared overall survival for patients who underwent either parathyroidectomy alone, en bloc resection, or parathyroidectomy followed by delayed thyroid resection, and found no improvement in survival for patients undergoing more extensive surgery.14 In a larger study of 733 patients with parathyroid carcinoma, concomitant resection of adjacent organs was found not to improve overall survival.7 The lack of en bloc resection may reflect that in clinical practice, parathyroid carcinoma is only recognized with a histological diagnosis. Because there are no effective treatments for metastatic parathyroid carcinoma, an en bloc resection may represent the best chance for cure and should be performed.
There are several limitations to this study. First, this analysis is limited by the quality of prospective data reported in the registry. Tumor size and nodal status were not routinely collected before 1988 and continue to be collected inconsistently. This may reduce the ability to detect whether size and nodal status are associated with disease-specific survival. Additionally, while patients were identified by coding as being confirmed by positive histology, parathyroid cancer can be challenging to diagnose since histologic criteria can be nonspecific. Thus, appropriate diagnosis of parathyroid carcinoma is reliant on the accuracy of the diagnosing pathologist and is not completely controlled in this analysis. The registry also lacks preoperative calcium levels and PTH levels to identify biochemical prognostic factors. Furthermore, Cinacalcet, a calcimimetic approved by the FDA in 2004 for patients with parathyroid carcinoma, may have altered the cancer-specific survival of patients. The impact of this drug’s present application is currently unclear as pharmacologic intervention is not information currently collected by the SEER database.
The incidence of parathyroid carcinoma has been previously reported to be increasing since 1973. In this study, the trend of increasing incidence has leveled off since 2001. For the first time, prognostic factors were assessed and associated with cancer-specific survival in a large cancer registry. Given the dearth of information available on prognosis and cancer-specific survival, this information could be helpful in implementing new staging guidelines for parathyroid carcinoma.
Acknowledgments
FUNDING This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
REFERENCES
- 1.Sadler C, Gow KW, Beierle EA, et al. Parathyroid carcinoma in more than 1,000 patients: a population-level analysis. Surgery. 2014;156(6):1622–30. [DOI] [PubMed] [Google Scholar]
- 2.Owen RP, Silver CE, Pellitteri PK, et al. Parathyroid carcinoma: a review. Head Neck. 2011;33:429–36. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute (2017). Parathyroid Cancer Treatment. https://www.cancer.gov/types/parathyroid/hp/parathyroid-treatment-pdq. Accessed 5 Feb 2017.
- 4.Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol. 2012;13(1):11–23. [DOI] [PubMed] [Google Scholar]
- 5.Hara H, Igarashi A, Yano Y, et al. Ultrasonographic features of parathyroid carcinoma. Endocr J. 2001;48(2):213–7. [DOI] [PubMed] [Google Scholar]
- 6.Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 2007;109(9):1736–41. [DOI] [PubMed] [Google Scholar]
- 7.Asare EA, Sturgeon C, Winchester DJ, et al. Parathyroid carcinoma: an update on treatment outcomes and prognostic factors from The National Cancer Data Base (NCDB). Ann Surg Oncol. 2015;22(12):3990–5. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–68. [DOI] [PubMed] [Google Scholar]
- 9.Bilezikian JP, Khan AA, Potts JT, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Third International Workshop. J Clin Endocrinol Metab. 2009;94(2):335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harari A, Waring A, Fernandez-Ranvier G, et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab. 2011;96(12):3679–86. [DOI] [PubMed] [Google Scholar]
- 11.Hsu KT, Sippel RS, Chen H, Schneider DF. Is central lymph node dissection necessary for parathyroid carcinoma? Surgery. 2014;156 (6):1336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villar-del-Moral J, Jimenez-Garcia A, Salvador-Egea P, et al. Prognostic factors and staging systems in parathyroid carcinoma: a multicenter cohort study. Surgery. 2014;156(5):1132–44. [DOI] [PubMed] [Google Scholar]
- 13.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985–1995: a National Cancer Data Base Report. Cancer. 1999;86:538–44. [DOI] [PubMed] [Google Scholar]
- 14.Young S, Wu JX, Li N, Yeh MW, Livhits MJ. More extensive surgery may not improve survival over parathyroidectomy alone in parathyroid carcinoma. Ann Surg Oncol. 2016;23(9):2898–904. [DOI] [PubMed] [Google Scholar]


