Abstract
Patients with type 2 diabetes are at increased cardiovascular risk. Until recently, reductions in HbA1c and the use of specific glucose-lowering agents have not had a clear, reproducible benefit in reducing the incidence of cardiovascular disease. However, over the past 5 years, members of two categories of diabetes medications, sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists, have been associated with improved rates of major adverse cardiovascular events when used in high-risk type 2 diabetes patients. Importantly, these effects are not necessarily linked to these agents’ effects on HbA1c. Sodium–glucose cotransporter 2 inhibitors have also been associated with reductions in heart failure hospitalization, a benefit that appears to extend to individuals without diabetes with established heart failure. Cardiovascular specialists should become familiar with these emerging data and be prepared to implement corresponding strategies in their practice to improve the cardiovascular outcomes of their patients. Recent clinical trial data and the changing landscape of corresponding professional guidelines are reviewed. Practical recommendations for safe prescribing of these anti-diabetes drugs are provided.
Keywords: Type 2 diabetes, glucose-lowering therapy, cardiovascular disease, heart failure
Patients with cardiovascular disease (CVD) and type 2 diabetes (T2D) suffer very high rates of cardiovascular (CV) morbidity and mortality.[1] Traditional paradigms for their treatments focus on the management of blood pressure and hyperlipidaemia by primary care physicians and/or cardiologists, and glucose control by primary care physicians and/or endocrinologists. As the ultimate goal of caring for these patients is to improve clinical outcomes, there has been a recent focus on alignment of goals, especially after the realisation that improvements in glucose management alone do not necessarily translate into the avoidance of adverse CV events.[2] One reason for these efforts was a mandate by the Food and Drug Administration (FDA) in 2008 to require an assessment of CV outcomes predominantly for the purpose of ensuring safety prior to approval of new glucose-lowering therapies for T2D. This requirement has resulted in the discovery of several novel agents to have significant benefits in the risk of major adverse CV events (MACE), a goal that had been previously largely elusive with older, traditional antihyperglycaemic medications.[3]
The greatly expanded therapeutic armamentarium for the treatment of patients with CVD and T2D has also stimulated a re-examination of the conventional roles of various medical specialties in their management. In a patient-centred model of care, all clinicians taking care of these high-risk patients should be comfortable prescribing therapies that improve outcomes – even if they have traditionally existed within disciplines. The goal of this review is to provide a brief history of the development of therapies for T2D, in the context of attempting to improve CV outcomes. We then provide a practical guide to the management of these patients that integrates the best available contemporary understanding and results from clinical trials, allowing the reader to be comfortable with the use of diabetes therapies that can now be used to address CVD risk.
Drug Development for Type 2 Diabetes: Focus on CVD
There has been a long track record in cardiology of therapies that improve surrogate measures of disease, but do not necessarily translate to better patient outcomes.[4] As a result, the threshold for therapeutic approval of cardiac medications by regulatory agencies has generally required a large, randomised controlled trial with clear, demonstrable benefits of actual clinical events. Until recently, however, this was not the case in drug development for diabetes medications. There was an assumption that tight glucose control, as reflected by improved HbA1c, would lead not only to reductions in microvascular outcomes, such as retinopathy and diabetic kidney disease, but would also reduce macrovascular complications – despite a lack of definitive support for the latter. In fact, the ACCORD trial revealed increased CV mortality for patients assigned to a more intensive glucose-lowering strategy.[5] Other studies, such as ADVANCE and VADT, demonstrated no improvement in CV outcomes with more stringent glucose control.[6,7] These and other data have called into question the utility of the surrogate measure of HbA1c for CV risk.[8]
Despite long-standing controversies regarding the link between glucose control and CV disease, it had always been presumed that glucose-lowering therapies would at least not increase adverse CV events. Indeed, prior to 2008, approval of T2D therapies was largely contingent on evidence of glycaemic efficacy based on HbA1c reductions alone. An inflection point came when two meta-analyses raised concerns about the potential for increased CV risk with the thiazolidinedione rosiglitazone and an investigational dual peroxisome proliferator-activated receptor alpha/gamma agonist.[9,10] Although the overall CV safety of rosiglitazone was later mostly proved by a randomised clinical trial, concerns about diabetes drug safety stemming from these retrospective data sets persisted. In response, the FDA issued a guidance statement to the pharmaceutical industry in 2008, outlining expectations for the development of new antihyperglycaemic agents for T2D. This document essentially required all such therapies to demonstrate, at the minimum, CV safety when compared with a placebo.[3] Similar requirements were subsequently issued by the European Medicines Agency.
Rather than suppress innovation, as was once feared, many new therapies have since been evaluated under this new guidance with one or more dedicated CV safety studies. The results from these trials have provided unprecedented information about the CV impact of therapies tailored to target one of the most important risk factors for coronary artery disease and heart failure (HF).
In the decade since the FDA guidance was issued, there have been 25 long-term prospective clinical trials involving >200,000 participants. While some are yet to report, the available data have already transformed the management of patients with T2D and CVD. Trials showing reductions in MACE with two newer classes, sodium–glucose cotransporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, have in fact prompted a paradigm shift beyond a focus on glucose control alone towards more comprehensive CV risk reduction.[11] In accordance with these insights, there has been a call for cardiologists to play a more active role in prescribing medications for diabetes management, especially since their mechanism of benefit appears beyond merely lowering glucose.[12] Furthermore, these findings have streamlined the recommendations for therapies, as improvement in such hard outcomes has surpassed lowering of glycaemia as a therapeutic goal.
Modes of Action of Commonly Used Glucose-lowering Medications
Pharmacological advances in recent years have led to several mechanistically distinct therapies for T2D. The most commonly used classes of antihyperglycaemic agents can be broadly subdivided into those that increase insulin supply, improve the body’s response to insulin (insulin sensitisers), enhance incretin levels or promote urinary excretion of glucose (Figure 1).[13]
Figure 1: The Most Common Glucose-lowering Drug Classes Used in Type 2 Diabetes and Their Sites of Action.
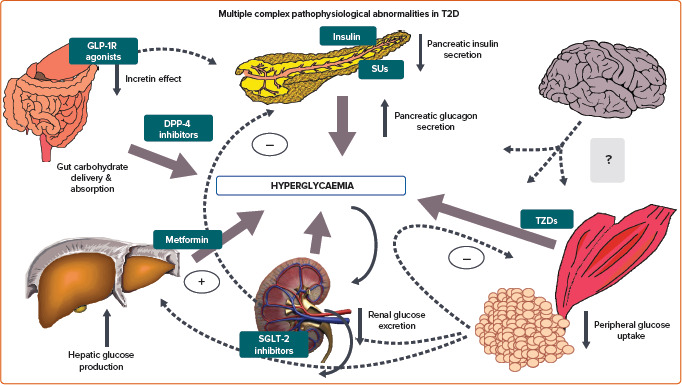
DPP-4 = dipeptidyl peptidase-4; T2D = type 2 diabetes; GLP-1R = glucagon-like peptide-1 receptor; SGLT-2 = sodium–glucose cotransporter 2; SUs = sulfonylureas; TZD = thiazolidinedione.
Sulfonylureas work by stimulating insulin secretion and require residual beta cell function. The sulfonylurea receptor is a component of the adenosine triphosphate-sensitive potassium channel in the pancreatic beta cells. The triphosphate-sensitive potassium channel regulates insulin release from pancreatic beta cells. Sulfonylurea binding inhibits these channels, altering cell resting potential, leading to calcium influx, thereby stimulating insulin secretion. The net effect is increased beta cell responsiveness, promoting greater insulin release at any blood glucose concentration, with the consequent risk of hypoglycaemia.
Metformin, a biguanide, works mainly in the liver to reduce hepatic glucose production, and may be considered a liver insulin sensitiser. The precise mode of action at a cellular level remains controversial, but most likely involves alterations in mitochondrial respiration. When renal function is severely reduced, metformin accumulates in the plasma, and may predispose to lactic acidosis.
Thiazolidinediones reduce insulin resistance by binding to the peroxisome proliferator-activated receptor gamma nuclear receptor, which is found at the highest concentrations in adipocytes, promoting adipocyte differentiation, reducing hepatic fat accumulation and increasing fatty acid storage in peripheral, rather than central, locations. Glucose uptake into skeletal muscle increases and circulating insulin levels decrease with the use of thiazolidinediones, indicating a reduction in insulin resistance.
Pioglitazone has been shown to reduce MACE in high-risk T2D patients, as a secondary outcome in the PROactive study, and in insulin-resistant stroke patients without diabetes in the IRIS trial.[14,15] These drugs result in weight gain and oedema, and increase the risk of HF.
The three newest glucose-lowering drug categories include dipeptidyl peptidase-4 (DPP-4) inhibitors, GLP-1 receptor agonists and SGLT2 inhibitors. DPP-4 inhibitors reduce the degradation of the incretins – GLP-1 and glucose-dependent insulinotropic peptide – thus increasing insulin release, especially in the postprandial state, and suppressing glucagon release as well.[16] They are generally well tolerated. GLP-1 receptor agonists act directly on this pathway, and as more powerful agents, not only beneficially modulate pancreatic insulin and glucagon secretion, but also slow gastric emptying and decrease appetite, the latter probably through central mechanisms.[17] Major side-effects pertain to the gastrointestinal system. SGLT-2 inhibitors lower blood glucose by selectively inhibiting a cotransporter expressed in the proximal convoluted tubule of the nephron. This blocks glucose reabsorption, thereby reducing the renal threshold for glucose and leading to increased urinary glucose excretion.[18] Their major side-effects are genitourinary in nature.
Results from Cardiovascular Outcomes Trials
As noted, since the FDA guidance statement for CV safety with glucose-lowering therapies, several large, industry-sponsored, placebo-controlled trials have tested the safety and efficacy of these latter three groups of medications (Table 1). Most participants had established T2D and either overt CVD or were at high CVD risk owing to multiple concomitant risk factors.
Table 1: Overview of Cardiovascular Outcome Trials with Glucose-lowering Therapies.
| Agent | Trial | Population | n | Median Follow-up (Years) | MACE Outcome, HR [95% CI] |
|---|---|---|---|---|---|
| Dipeptidyl Peptidase-4 Inhibitors | |||||
| Saxagliptin | SAVOR | Age ≥40 years with ASCVD; ≥55 years men or ≥60 years women with ≥1 CV risk factor | 16,492 | 2.1 | HR 1.00 [0.89–1.12] |
| Alogliptin | EXAMINE | Age ≥18 years with recent ACS | 5,380 | 1.5 | HR 0.96 [≤1.16] |
| Sitagliptin | TECOS | Age ≥50 years with ASCVD | 14,671 | 3.0 | HR 0.98 [0.88–1.09] |
| Linagliptin | CARMELINA | History of ASCVD and micro- or macro-albuminuria | 6,979 | 2.2 | HR 1.02 [0.89–1.17] |
| Linagliptin | CAROLINA* | ASCVD; ≥2 CV risk factors; age ≥70 years and microvascular complications | 6,042 | 6.3 | HR 0.98 [0.84–1.14] |
| Glucagon-like Peptide-1 Receptor Agonists | |||||
| Lixisenatide | ELIXA | Age ≥30 years with ASCVD | 6,068 | 2.1 | HR 1.02 [0.89–1.17] |
| Liraglutide | LEADER | Age ≥50 years with ASCVD; age ≥60 years with ≥1 CV risk factor | 9,340 | 3.8 | HR 0.87 [0.78–0.97] |
| Semaglutide | SUSTAIN-6 | Age ≥50 years with ASCVD; CHF or stage 3–5 CKD; age ≥60 years with ≥1 CV risk factor | 3,297 | 2 | HR 0.74 [0.58–0.95] |
| Semaglutide (oral) | PIONEER-6 | Age ≥50 years with ASCVD or CKD; ≥60 years with ≥1 CV risk factor | 3,183 | 1.3 | HR 0.79 [0.57–1.11] |
| Exenatide XR | EXSCEL | 73% had ASCVD | 14,752 | 3.2 | HR 0.91 [0.83–1.00] |
| Albiglutide† | Harmony Outcomes | Age ≥40 years with ASCVD | 9,463 | 1.6 | HR 0.78 [0.68–0.90] |
| Dulaglitide | REWIND | Age ≥50 years with ASCVD, age ≥55 years with ASCVD or 1 CV risk factor; age ≥60 years with ≥2 CV risk factors | 9,901 | 5.4 | HR 0.88 [0.79–0.99] |
| Sodium–Glucose Cotransporter 2 Inhibitors | |||||
| Empagliflozin | EMPA-REG OUTCOME | Age ≥18 years with ASCVD | 7,020 | 3.1 | HR 0.86 [0.744–0.99] |
| Canagliflozin | CANVAS | Age ≥30 years with ASCVD; age ≥50 years with ≥2 risk factors | 10,142 | 3.6‡ | HR 0.86 [0.75–0.97] |
| Canagliflozin | CREDENCE | eGFR 30–90 and albumin:creatinine ratio >300 | 4,401 | 2.6 | HR 0.70 [0.59–0.82] |
| Dapagliflozin | DECLARE | Age ≥40 years with. ASCVD; age ≥55 years men with ≥2 CV risk factors; age ≥60 years women with ≥1 CV risk factor | 17,160 | 4.2 | HR 0.93 [0.84–1.03] |
| Dapagliflozin | DAPA-HF | NYHA class II–IV HF with LVEF ≤40% | 4,744 | 1.5 | HR 0.74 [0.65–0.85] |
| Ertugliflozin | VERTIS-CV | Age ≥40 years with ASCVD | 9,463 | 1.6 | HR 0.95 [0.85–1.11] |
*Compared with glimepiride; all other trials are compared with placebo. †Removed from the market by the manufacturer due to poor penetration. ‡Data expressed as the mean. ACS = acute coronary syndrome; ASCVD = atherosclerotic cardiovascular disease; CHF = congestive heart failure; CKD = chronic kidney disease; CV = cardiovascular; eGFR = estimated glomerular filtration rate; HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Dipeptidyl Peptidase-4 Inhibitors
Several DPP-4 inhibitor trials have been completed, showing neither inferiority nor superiority compared with a placebo with respect to MACE risk. Of note, however, saxagliptin was associated with an increased incidence of hospitalisation for HF in the SAVOR–TIMI 53 trial.[19]
There was also a non-statistically significant increase in hospitalisations for HF with alogliptin in the EXAMINE trial.[20] Such trends for HF were noted in neither the TECOS nor CARMELINA, large CV outcome trials involving two additional DPP-4 inhibitors versus a placebo, but also, neither was able to establish any CV superiority.[21,22] Finally, the CAROLINA demonstrated no differences in CV risk for linagliptin versus glimepiride in the only active comparator CV outcome trial for a DPP-4 inhibitor.[23]
As a result of these studies, DPP-4 inhibitors are deemed to be generally safe for use in high-risk CV patients, perhaps with the exception of saxagliptin and potentially alogliptin with regard to possible HF risk. However, this class clearly does not prevent CV events in high-risk patients with T2D.
Glucagon-like Peptide-1 Receptor Agonists
Several GLP-1 receptor agonists have demonstrated benefits for the reduction of adverse CV events among patients with T2D. Seven CV outcomes studies evaluating GLP-1 receptor agonists have been reported to date, with several showing superior CV outcomes.
The LEADER trial randomised 9,340 patients with T2D and high CV risk (the majority with established CVD) to liraglutide or a placebo in addition to standard of care.[24] After a median follow-up duration of 3.8 years, participants in the liraglutide group experienced a significantly reduced risk versus placebo for the primary composite three-point MACE outcome (CV death, non-fatal myocardial infarction or non-fatal stroke). Although CV mortality was also reduced, no reduction in HF events were observed.
The SUSTAIN-6 trial enrolled 3,297 patients using similar eligibility criteria and the same primary composite endpoint as LEADER. Although SUSTAIN-6 was not powered for superiority, semaglutide significantly reduced the risk of MACE in a consistent manner.[25] A separate trial examining oral as opposed to injectable semaglutide, PIONEER 6, randomised 3,183 high-risk T2D patients; rates of CV outcomes were similar in either arm of the trial, although both the secondary outcomes of CV and all-cause mortality appeared to be significantly reduced.[25]
The EXSCEL trial enrolled 14,752 subjects, with most having overt CVD, and showed a directionally lower risk of outcomes for exenatide compared with a placebo, but this difference did not reach statistical significance.[26]
Lixisenatide, which was tested in 6,068 patients with acute coronary syndrome in the ELIXA trial, showed no signal for any reduction in MACE.[27]
The HARMONY Outcomes randomised 9,463 participants with CVD, finding albiglutide to be superior to a placebo with respect to MACE.[28] Despite these findings, the drug’s manufacturer has withdrawn it from the worldwide market due to disappointing sales.
Finally, the REWIND trial involving 9,901 patients, most of whom had CV risk factors, but not established CVD, showed that weekly injections of dulaglutide improved CV outcomes in patients with type 2 diabetes regardless of prior CV events, with an overall effect size similar to that observed in other GLP-1 receptor agonist CV outcomes trials.[29]
A recently published meta-analysis included 56,004 participants and pooled data from ELIXA (lixisenatide), LEADER (liraglutide), SUSTAIN-6 (semaglutide), EXSCEL (exenatide), Harmony Outcomes (albiglutide), REWIND (dulaglutide) and PIONEER 6 (oral semaglutide).[30] Overall, GLP-1 receptor agonist treatment reduced MACE by 12% (HR 0.88; 95% CI [0.82–0.94]; p<0.0001), with no evidence of treatment heterogeneity across the subgroups examined. The drug class also appeared to broadly improve renal outcomes, reducing a composite of new-onset macroalbuminuria, decline in the glomerular filtration rate, progression to end-stage kidney disease or death attributable to kidney causes by 17% (HR 0.83; 95% CI [0.78–0.89]; p<0·0001). It should be noted, however, that in contrast to SGLT-2 inhibitors (see below), this renal composite endpoint is driven mainly by a reduction in macroalbuminuria, with no clear effect to decrease the decline in glomerular filtration rates. The authors appropriately concluded that treatment with GLP-1 receptor agonists (as a class) have beneficial effects on CV and, potentially, kidney outcomes in patients with T2D.[30]
Sodium–Glucose Cotransporter 2 Inhibitors
SGLT-2 inhibitors were the first glucose-lowering drug category to show a reduction in MACE among T2D patients at high CV risk. The EMPA-REG OUTCOME trial randomised 7,020 patients with T2D and established CVD to empagliflozin versus a placebo; those receiving empagliflozin had a lower rate of the primary composite three-point MACE than did patients receiving placebo.[31] Notably, the primary driver of this risk reduction was a striking 38% reduction in the risk of CV death. Moreover, the drug was associated with a 35% reduction in the risk of HF hospitalisation. In a prespecified secondary outcome, empaglifozin use was associated with a lower risk of progression of kidney disease, including both macroalbuminuria and ‘harder’ renal outcomes, such as a doubling of the serum creatinine level and initiation of renal replacement therapy.[32]
Following the EMPA-REG OUTCOME were results from the CANVAS program, which integrated findings from two placebo-controlled trials, and included 10,142 subjects with T2D and either known CVD or age >50 years, and at least two additional CV risk factors.[33] The rate of the MACE primary outcome was significantly lower with canagliflozin than with a placebo, and there was also similar evidence of benefit in regard to both HF hospitalisations and in the progression of kidney disease. The MACE benefit appeared to be concentrated among patients with established CVD, although the HF and renal benefits appeared to be shared by even those with CV risk factors alone.
Finally, the DECLARE–TIMI 58 trial randomised a lower-risk population – those with T2D who had or were at risk for atherosclerotic CVD – to receive either dapagliflozin or a placebo.[34] While there were no significant differences in the MACE dual primary outcome, a significant decrease in the risk of the second dual primary outcome, CV death or hospitalisation for HF was observed. Furthermore, as with the prior trials, there was a signal of benefit for various renal outcomes.
The final T2D-CV outcome trial involving an SGLT-2 inhibitor, ertugliflozin, was VERTIS CV, including 8,246 participants with T2D and established CVD. In contrast to the earlier studies, VERTIS CV did not meet its primary efficacy outcome.[35] While ertugliflozin was non-inferior to a placebo for MACE, the HRs for the composite (HR 0.97; 95% CI [0.85–1.11]) and each of its components was statistically neutral. However, HF hospitalisations were less frequent in the active therapy arm (HR 0.70; 95% CI [0.54–0.90]).
Findings from the first three CV outcome trials of SGLT-2 inhibitors were consolidated in a meta-analysis of 34,322 patients that summarised these drugs to have a moderate impact on MACE among patients with established CVD, but striking reductions in HF hospitalisations and progression of kidney disease regardless of the presence of overt CVD or HF at baseline.[36]
Moreover, observational data from large international studies of insurance claims, registries and electronic medical records from a broad T2D population observed in real-world practice have reinforced findings from the clinical trials.[37,38] For example, CVD-REAL compared a propensity-matched cohort of patients receiving other oral medications for T2D. Those receiving SGLT-2 inhibitors had a 51% lower associated risk of all-cause mortality and a 39% lower associated risk of hospitalisation for HF. Comparable results were found in the even larger CVD-REAL 2 study, with a 49% lower risk of all-cause mortality, 36% lower risk of hospitalisation for HF, and lower risks of MI and stroke.
Another real-world data set comes from the EMPRISE study in the US, in which the initiation of empagliflozin was associated with at 50% lower risk of HF hospitalisation compared with patients initiating therapy with sitagliptin (a DPP-4 inhibitor).[39] These findings buttress the benefits of SGLT-2 inhibitors in patients with T2D, as demonstrated in randomised clinical trials.
Finally, since a major benefit of SGLT-2 inhibitors was thought to be in patients with HF, several clinical trials are ongoing to test this hypothesis. The first of these, DAPA-HF, randomised 4,744 patients with left ventricular ejection fraction ≤40% and symptomatic HF to dapagliflozin versus a placebo. The primary outcome was a composite of worsening HF (hospitalisation or an urgent visit resulting in IV therapy for HF) or CV death, occurring significantly less in the dapagliflozin arm (HR 0.74; 95% CI [0.65–0.85]; p<0.001).[40] Importantly, the benefit did not appear to be dependent on whether the patient had diabetes or not. It is anticipated that SGLT-2 inhibitors will play a major role in the treatment of HF in the near future.[41]
Other dedicated HF studies with SGLT-2 inhibitors are underway or soon to report. These include EMPEROR-Reduced, EMPEROR-Preserved and DELIVER.
Prescribing Glucose-lowering Therapies for CVD
Currently, we have robust data demonstrating the benefits of GLP-1 receptor agonists and SGLT-2 inhibitors for patients with T2D and coexisting CV disease. According to the most current T2D treatment guidelines from the American Diabetes Association and the European Association for the Study of Diabetes, metformin is first-line therapy for these patients, mainly due to extensive clinical experience and comparably low cost.[42] Then, additional therapies are added, dictated initially by coexisting CV and/or kidney disease. If atherosclerotic CVD predominates the clinical picture, either a GLP-1 receptor agonist or a SGLT-2 inhibitor shown to improve CV outcomes should be used. If, in contrast, HF or CKD predominate, then a SGLT-2 inhibitor would be favoured, with a GLP-1 receptor agonist used if an SGLT-2 inhibitor were contraindicated; for example, if kidney dysfunction is too advanced. In addition, because the effects on glycaemia are unlikely to be driving the outcomes benefits of these agents, adding either GLP-1 receptor agonists and SGLT-2 inhibitors are now to be considered irrespective of HbA1c – a radical departure from older glucocentric approaches.
The latest guidelines from the European Society of Cardiology are similar, but go a step further, proposing that for appropriate patients with or at high risk for CVD, beginning with an SGLT-2 inhibitor or a GLP-1 receptor agonist in lieu of metformin may be more prudent.[43] Both American Diabetes Association and the European Association for the Study of Diabetes and European Society of Cardiology guidelines now identify a broader group of patients eligible for CV risk reduction with these newer drug categories, no longer restricting them to those with overt CVD. Both sets of guidelines are reasonable, with the American Diabetes Association and the European Association for the Study of Diabetes consensus report still endorsing metformin as foundation therapy – a concept that is not necessarily any longer evidence-based.
Dosing and clinical considerations for these therapies are shown in Table 2. In cases of equipoise, other distinguishing features of these agents should dictate which one is initiated first. SGLT-2 inhibitors, for example, would seem to be preferred in the setting of HF or mild-to-moderate diabetic kidney disease. The available routes of administration are perhaps among the most evident differences – oral for SGLT-2 inhibitors and subcutaneous for most GLP-1 receptor agonists, although the first oral GLP-1 receptor agonist (semaglutide) recently became available. Cost may also vary substantially based on local market availability, health system or payer formularies and individual health insurance coverage.
Table 2: Prescribing Information for Glucagon-like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors (Based on US Labels).
| Generic Name | Brand Name | Doses | Schedule | Additional Information |
|---|---|---|---|---|
| GLP-1R agonists (all injectables except Rybelsus) | ||||
| Exenatide | Byetta | 5, 10 µg | Twice daily | 32 G pen needles supplied separately; prefilled, single-use pen |
| Exenatide extended release | Bydureon | 2 mg | Weekly | 23 G, hidden needles pre-attached; prefilled autoinjector device |
| Dulaglutide | Trulicity | 0.75, 1.5 mg | Weekly | 29 G hidden needles pre-attached; prefilled autoinjector device |
| Liraglutide | Victoza | 0.6, 1.2, 1.8 mg | Daily | Indicated to reduce the risk of MACE in T2D with established CVD; 32-G pen needles supplied separately; prefilled, multi-dose pen |
| Lixisenatide | Adlyxin | 10, 20 µg | Daily | ≤8 mm pen needles supplied separately; prefilled, single-use pen |
| Semaglutide (injection) | Ozempic | 0.25, 0.5 mg | Weekly | 32 G 4 mm pen needles included; prefilled, single-use pen |
| Semaglutide (oral) | Rybelsus | 3, 7, 14 mg | Daily | Only oral GLP-1RA available |
| SGLT-2 inhibitors (all oral tablets, most available in fixed-dose combinations with metformin) | ||||
| Canagliflozin | Invokana | 100, 300 mg | Daily | Indicated to reduce risk of MACE in T2D and established CVD; also indicated to reduce risk of progression of kidney disease, CV death and HF hospitalisation in T2D and diabetic kidney disease with macroalbuminuria |
| Dapagliflozin | Farxiga | 5, 10 mg | Daily | Indicated to reduce risk of HF hospitalisation in T2D with established CVD or multiple CV risk factors |
| Empagliflozin | Jardiance | 10, 25 mg | Daily | Indicated to reduce risk of CV death in T2D with established CVD |
| Ertugliflozin | Steglatro | 5, 15 mg | Daily | CV outcomes trial (VERTIS-CV; NCT01986881) |
CV = cardiovascular; CVD = cardiovascular disease; GLP-1R = glucagon-like peptide-1 receptor; GLP-1RA = glucagon-like peptide-1 receptor agonist; HF = heart failure; MACE = major adverse cardiovascular events; SGLT-2 = sodium–glucose cotransporter 2; T2D = type 2 diabetes.
Side-effects and Practical Considerations
Most GLP-1 receptor agonists appear to benefit patients in whom atherosclerotic heart disease predominates, but they may be associated with transient nausea and even vomiting, especially when initiating therapy or uptitrating the dose. The subcutaneous route of administration of most GLP-1 receptor agonists dictates that the individual characteristics of each injection pen should also be considered to match patient preferences. Multi-dose pens, for example, are available with liraglutide, semaglutide and exenatide extended release; these agents also use identical needles to insulin pen therapy, and therefore may already be more familiar to insulin users. However, for insulin-naïve patients, the manual dexterity required to attach and remove pen needles for GLP-1 receptor agonist injectable devices before and after use requires direct education.
For clinicians prescribing these therapies, extensive use of the teach-back technique during an office visit, preferably by experienced nurses or diabetes educators, to confirm a patient or caregiver’s ability to safely inject the medication may be necessary. Additionally, separate prescriptions for pen needles must be sent to the outpatient pharmacy if using liraglutide, exenatide and lixisenatide. Only injectable semaglutide contains pen needles in its original packaging. Dulaglitide or exenatide extended release contain built-in needles supplied in an autoinjector pen, which may be favourable for ease of use or patients for whom visible needles may be problematic. Patients must be coached regarding low initial dosing and gradual uptitration to mitigate gastrointestinal side-effects. We avoid these drugs in patients with complex pancreato-biliary disease, prior history of pancreatitis (per label, although no risk has been demonstrated in clinical trials), gastroparesis, prior bariatric surgeries and multiple or significant baseline gastrointestinal symptoms.
An oral formulation of semaglutide recently became available. Because it uses a unique gastric absorption enhancer, this formulation must be taken on an empty stomach with a certain volume of water and with the subsequent avoidance of food or other medications for at least 30 minutes. However, this agent has not yet been demonstrated to reduce CV events.
SGLT-2 inhibitors are also preferred in the context of atherosclerotic CVD, but appear to be particularly beneficial in cases of HF and/or mild-to-moderate kidney disease. They are known to increase the risk of genital mycotic infections, although the latter are usually easily treatable with topical creams. Other considerations include polyuria (which may be specifically problematic in older men with prostatic disease) and the potential for reductions in volume, causing adverse events – those with HF taking loop diuretics may need their dose reduced, particularly if already volume contracted.
Caution is required when prescribing these agents (particularly canagliflozin) to patients with a history of prior amputations, significant peripheral artery disease, or active lower extremity soft tissue ulcers or infections. Rarely, diabetic ketoacidosis may occur (often euglycaemic, which may mask the initial presentation), but more commonly when these agents are used off-label in those with type 1 diabetes and, possibly, in very insulin-deficient, lean and more fragile T2D patients. Practical advice for the safe prescribing of these agents is listed in Table 3.
Table 3: Practical Advice for the Safe Use of Glucagon-Like Peptide-1 Receptor Agonists and Sodium–Glucose Cotransporter 2 Inhibitors.
| Class | Recommendations |
|---|---|
| GLP-1 receptor agonists |
|
| SGLT-2 inhibitors |
|
*Consider the risks/benefits during heart failure admission and may be continued, depending on the clinical circumstances. eGFR = estimated glomerular filtration rate GI = gastrointestinal; GLP-1R = glucagon-like peptide-1 receptor; PAD = peripheral artery disease; SGLT-2 = sodium–glucose cotransporter 2.
Conclusion
The requirement for testing glucose-lowering therapies developed for T2D in large CV outcomes trials has led to the identification of two medication classes conferring CV outcomes benefit –SGLT-2 inhibitors GLP-1 receptor agonists. These findings have shifted our clinical focus from reduction of HbA1c levels to reducing the risk of MACE, CV death and HF hospitalisations.
The new emphasis on clinical outcomes promotes more patient-centred care over traditional disease-based care, where physicians across several specialties may now collaborate with patients to reduce morbidity and mortality with therapies that were once considered only in the purview of endocrine specialists. Since most patients with CVD and diabetes are seen by cardiologists, this specialty should become engaged in this process – either by discussing evidence-based glucose-lowering therapies with patients and their referring physicians or, potentially, by even taking ownership and prescribing them themselves.
With increasing communication between specialties and a focus on implementation science, such as embedding user-friendly disease treatment algorithms within the electronic health record, we anticipate that the impressive benefits of these therapies seen in clinical trials will translate to widespread clinical benefits for patients in real-world settings.
References
- 1.Scherer PE,, Hill JA. Obesity, diabetes, and cardiovascular diseases: a compendium. Circ Res. 2016;118:1703–5. doi: 10.1161/CIRCRESAHA.116.308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel RB,, Al Rifai M,, McEvoy JW, Implications of specialist density for diabetes care in the United States. JAMA Cardiol. 2019. pp. 1174–5. [DOI] [PMC free article] [PubMed]
- 3.McGuire DK,, Marx N,, Johansen OE, et al. FDA guidance on antihyperglyacemic therapies for type 2 diabetes: one decade later. Diabetes Obes Metab. 2019;21:1073–8. doi: 10.1111/dom.13645. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub WS,, Luscher TF,, Pocock S. The perils of surrogate endpoints. Eur Heart J. 2015;36:2212–8. doi: 10.1093/eurheartj/ehv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC,, Miller ME,, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duckworth W,, Abraira C,, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 7.Group AC,, Patel A,, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Lipska KJ,, Krumholz HM. Ishemoglobin A1c the right outcome for studies of diabetes? JAMA. 2017;317:1017–18. doi: 10.1001/jama.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissen SE,, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE,, Tuzcu EM,, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. 9. Cardiovascular disease and risk management: standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(Suppl 1):S86–104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 12.Das SR,, Everett BM,, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–23. doi: 10.1016/j.jacc.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meneses MJ,, Silva BM,, Sousa M, et al. Antidiabetic drugs: mechanisms of action and potential outcomes on cellular metabolism. Curr Pharm Des. 2015;21:3606–20. doi: 10.2174/1381612821666150710145753. [DOI] [PubMed] [Google Scholar]
- 14.Dormandy JA,, Charbonnel B,, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 15.Kernan WN,, Viscoli CM,, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–31. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornberry NA,, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab. 2009;23:479–86. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–56. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 18.van Baar MJB,, van Ruiten CC,, Muskiet MHA, et al. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41:1543–56. doi: 10.2337/dc18-0588. [DOI] [PubMed] [Google Scholar]
- 19.Scirica BM,, Bhatt DL,, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 20.White WB,, Cannon CP,, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 21.Green JB,, Bethel MA,, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J,, Perkovic V,, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenstock J,, Kahn SE,, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155–66. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correia LC,, Latado A,, Porzsolt F. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:1798. doi: 10.1056/NEJMc1611289. [DOI] [PubMed] [Google Scholar]
- 25.Husain M,, Birkenfeld AL,, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 26.Holman RR,, Bethel MA,, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer MA,, Claggett B,, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez AF,, Green JB,, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 29.Gerstein HC,, Colhoun HM,, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen SL,, Rorth R,, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 31.Zinman B,, Wanner C,, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 32.Wanner C,, Inzucchi SE,, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 33.Neal B,, Perkovic V,, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 34.Wiviott SD,, Raz I,, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 35.Cannon CP,, McGuire DK,, Cherney D, Results of the eValuation of ERTugliflozin EffIcacy and Safety CardioVascular Outcomes Trial (VERTIS CV). Presented at 80th American Diabetes Association Scientific Sessions, 16 June 2020
- 36.Zelniker TA,, Wiviott SD,, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 37.Kosiborod M,, Cavender MA,, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249–59. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosiborod M,, Lam CSP,, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–39. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Patorno E,, Pawar A,, Franklin JM, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139:2822–30. doi: 10.1161/CIRCULATIONAHA.118.039177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMurray JJV,, Solomon SD,, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 41.Lam CSP,, Chandramouli C,, Ahooja V et al. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019;8:e013389. doi: 10.1161/JAHA.119.013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buse JB,, Wexler DJ,, Tsapas A, et al. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:221–8. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 43.Cosentino F,, Grant PJ,, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]


