Abstract
Background
SARS--CoV-2 has emerged as a global threat since its onset in December 2019. India has also been severely affected by the dreadful Corona Virus and is currently battling one of the worst pandemics of history. WHO and the world medical fraternity are putting their efforts to materialize a treatment or vaccine for this novel virus. A randomized open label parallel group study was designed in a Lucknow based level 2 COVID hospital to evaluate the efficacy of Ayurvedic interventions in the management of asymptomatic and mild COVID 19 patients.
Objective
To evaluate the efficacy of Ayurveda in the management of mildly affected COVID-19 patients.
Materials and methods
The current trial was an open label randomized 10-day study. Total 120 asymptomatic and/or mild COVID-19 positive patients fulfilling inclusion criteria were randomly grouped into three. RT-PCR of all the patients were done on 5th, 7th and on 10th day respectively. The observations were noted and results were analyzed statistically. Kruskal–Wallis test and Wilcoxon Sign rank test were used for data analysis where applicable.
Results
Improvement in symptoms, enhancement in Agni and recovery from COVID infection was observed. The results obtained were encouraging and showed better viral clearance and control of symptom progression in the patients placed on Ayurvedic medications.
Conclusion
The promising results in the study showed that an approach involving Ayurveda can be helpful for the management of the mild COVID-19 patients. Ayurveda can be used to limit community spread and check disease progression to a more appalling state.
Trial registration
Trial was registered with Clinical Trials Registry- India (CTRI registration number: CTRI/2020/06/025800.
Keywords: Covid-19, Ayurveda, Randomized trial, Immunity, Agni
Graphical abstract
1. Introduction
SARS-COV-2 has emerged as a new health threat for the entire world as virus has spread to almost every single country. Having its root in Wuhan, in China, it has quickly spread far and wide, posing new challenges in front of whole medical fraternity, which is still in search of devising a vaccine or suitable medicine.
The current pandemic was declared a public health emergency of International concern on 30th January 2020 and a pandemic on 11th March, 2020 by WHO [1]. As of now, more than 41 million cases of COVID-19 have been reported resulting in more than 1.1 million, worldwide [2]. In some countries, even a second wave of the virus is being speculated.
The novel corona virus is primarily spread between people during close contact, most often via small droplets produced by coughing, sneezing, and talking. Less commonly, people may get infected by touching a contaminated surface. Speech generated droplets may remain airborne for tens of minutes. It is most contagious during the first three days after the onset of symptoms, although spread is possible before symptoms appear and also from people who do not show symptoms [3].
Common symptoms include fever, dry cough, fatigue, shortness of breath, anosmia and tastelessness [4,5]. Complications may include pneumonia and acute respiratory distress syndrome. The time from exposure to onset of symptoms is typically around five days but may range from two to fourteen days [6].
The pandemic has caused global social and economic disruption worldwide including the largest global recession since the great depression [7]. It has led to cancellation or postponement of sporting, religious, political, and cultural events, widespread supply shortages exacerbated by panic buying [8].
WHO has set two main goals to contain effects of COVID-19 to a minimum [9]. First is to prioritize and accelerate innovative research to help to contain the spread of the epidemic and facilitate care for those affected. The second objective is to learn from current global pandemic’s response to prepare better for next unforeseen epidemic.
Emphasis has also been laid on early detection of cases through contact tracing and high testing as many asymptomatic cases go unnoticed which act as a reservoir of infection. Early diagnosis is therefore important to contain spread and limit the community spread.
The guidelines by the Health ministry for social distancing and proper hygiene and sanitary methods implementation are already in place for the public as preventive measures. More stringent measures at treatment centers for the management of active COVID patients are still in progress.
At this point when the world needs a major breakthrough for an appropriate line of management, it needs to be seen whether any significant leads can be procured through the intervention of Ayurveda. Ministry of AYUSH in India has propagated use of traditional Indian systems of medicine including Ayurveda for prophylactic use as well as an alternative system of medicine to provide an effective and holistic approach towards COVID management.
On the basis of clinical presentation, the disease resembles sannipataja jwara, one of the types of fever mentioned [10]. Besides, it also bears an analogy with Rajayakshma (shosha) [11] and dushta pratishyaya [12] due to agnimandya. The overall effect of corona virus infection manifests in the form of impaired digestion and hampered immune activities (Oja kshaya). The patients with an associated comorbidity may present with gradual deterioration with difficulty in breathing and eventually this may lead to death.
The primary objective of this study was to evaluate the efficacy of Ayurvedic medicines in COVID-19, to analyze these results and compare it with the active control group. The study also aimed at the assessment of the progression of disease in active COVID-19 patients through this intervention. In this study, an Ayurvedic management on the lines to reinstate digestion and eventually augment bodies’ immunity was planned. Besides, the drugs selected for the study also had proven effects on the upper respiratory tract function enhancement. As per Ayurveda classics, all the diseases have said to be originated due to mandagni [13] (∼Weak digestive aptitude) thereby leading to insufficient immunity to deal with mildest of the infections, thus leading to onset of disease. The disease chiefly appears to affect the pranavaha srotasa (∼respiratory tract), Ojas (∼immunity) and agni (∼digestive capacity).
2. Materials and methods
Total 120 COVID-19 positive subjects presenting with milder symptoms or no symptoms were included in the study. Patient classification and inclusion was based on the Clinical severity and assessment parameters provided by Ministry of Health and Family welfare, Government of India in their Clinical management Protocol: COVID-19 [14].
2.1. Ethical approval and trial registration
The study on approval from Hospital Institutional ethics committee (IEC) was registered with Clinical trial registry (CTRI/2020/06/025800).
2.2. Trial site
Source of patients was isolation ward of Lokbandhu Rajnarayan Combined Hospital, Lucknow which was upgraded as a Level II COVID 19 management center by Uttar Pradesh Government.
2.3. Study design
The trial was a 10 day randomized, open label, multiple arm interventional study. 120 enrolled patients were randomly distributed in three groups. Two groups were placed on Ayurvedic drug intervention while the third group was Control group.
2.4. Inclusion criteria
Patients aged above 25 years and below 60 years were included in the study. Patients tested positive for COVID 19 virus, asymptomatic or with uncomplicated upper respiratory tract infection, having mild symptoms like fever, cough, sore throat, nasal congestion, malaise and headache were included. Those willing to give informed consent were incorporated in the research work.
2.5. Exclusion criteria
Patients below 25 years and above 60 years of age were excluded from the study. Patients presenting with increased severity of disease or having severe difficulty in breathing and/or those having serious co-morbidities were not included. Patients not willing to give informed consent or unwilling to consume Ayurveda drugs were also excluded from the study.
2.6. Consent
A verbal consent was taken from the patients registered for trial telephonically as patients were in isolation wards and only duty doctors and nursing staff were allowed to stay in there with patients. Written consent was avoided due to a risk of spread of infection.
2.7. Treatment groups
Patients were randomly grouped into Group A, Group B and Group C keeping 40 patients in each group (Table 1).
Table 1.
Grouping of the patients, interventions.
| Number of patients | Trial Drug | Dosage | Duration | |
|---|---|---|---|---|
| Group A | 40 | Vyaghryadi Kashaya | 50 mL twice a day with 250 mg of Pippali powder empty stomach morning and evening. | 10 days |
| Samshamani vati | 2 tablets 500 mg twice daily | |||
| Group B | 40 | Fine powder of Shunthi (Zingiber officinale Rosc.) | 2 gm twice daily with warm water after meals. | 10 days |
| Paste of Rasona (Allium sativum L.) kalka | 1 gm once daily with warm water. | |||
| Group C | 40 | Tab Vitamin C | 500 mg twice a day | 10 days |
| Tab Paracetamol | Dose of 500 mg SOS |
2.8. Randomization
120 Patients were randomly placed in three groups on the basis of Computer generated randomization.
2.9. Intervention drugs
The interventional raw drugs were procured from a certified Ayurvedic drug dealer, their standard quality was ascertained by Dr. Rajeev Vilas, Associate professor at Department of RSBK, State Ayurvedic College, Lucknow. Samshamani vati [15], Shunthi and Pippali powders were obtained from a GMP certified company. The preparation of the medicine was done as per the norms and standards of Ayurvedic medicine preparation (∼Kwatha kalpana) [16].
2.10. Preparation of drugs
Vyaghryadi Kashaya [17] (∼decoction) was prepared by boiling equal parts (about 17 g of each) of Kantakari (Solanum xanthocarpum Schrad, & Wendl.), Shunthi (Zingiber officinale Rosc.) and Guduchi (Tinospora cordifolia Thrunb.) in 200 mL potable water, reducing to 50 mL and straining through a clean cotton fabric. This 50 mL is a single dose was prescribed to the patient by adding 250 mg of powdered Pippali (Piper longum L.).
2.11. Advise to patients
Patients were advised to follow a healthy routine of sleeping and awakening early, advised to perform Pranayama (breathing exercises) during the period of their stay, which was ensured by the doctors and nurses on duty in the Isolation wards. On discharge too, they were asked to follow isolation at home for seven days, maintain physical distancing, proper hygiene and continue the similar dietary regimen and lifestyle.
2.12. Diet
Patients in all the three groups were placed on a specific diet regimen and advised with proper pathya (∼things to be done and consumed) and apathya (∼things to be avoided) during the treatment regimen and afterwards. They were provided diet comprising of green gram and green vegetables along with seasonal fruits like water melon. Food items like curd, potatoes, black gram, cheese and those prepared from Maida (∼refined flour), which are heavy to digest were not prescribed in their diet regimen. They were advised to take warm water and saline gargling and refrain from cold water. Besides, all the patients were advised to take at least one onion (Allium cepa L.) in their diet along with their meals.
2.13. RT-PCR
Real time PCR which is considered Gold standard for COVID-19 was the investigation of choice in this study. Nasal and throat swab samples were collected on pre-decided days (on 5th, 7th and on 10th day) by pathologists and samples were sent to microbiology unit of King George Medical University, Lucknow for testing.
2.14. Assessment criteria
The positively tested patients were assessed clinically for symptoms after admission in Isolation ward. They were graded using standard Gradation criteria for assessment before and after the study. The symptoms included fever, sore throat, cough, dyspnea, running nose, generalized weakness, headache, irritability, nausea/vomiting, diarrhea, loss of taste and loss of sense of smell on a scale of 0–5 as per the Assessment guidelines of CCRAS, New Delhi [18].
Continuous monitoring of the patients was done for vital parameters like temperature, respiratory rate and blood pressure, pulse rate and oxygen saturation.
The observations were noted and the results obtained were analyzed using Wilcoxon Signed Rank test and Kruskal Wallis’ test for data accordingly. The results obtained after study were compared within the group and in between the groups for statistical significance (Table 1).
3. Results
3.1. Demographic data
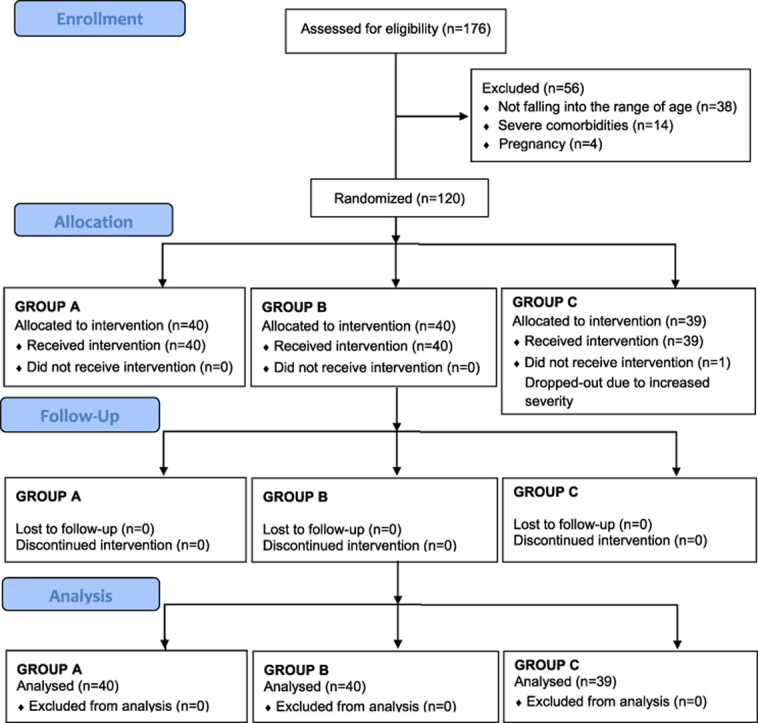
The patients tested positive for COVID-19 were admitted in the isolation ward of Lokbandhu Hospital with level II facility. Out of 176 assessed patients, 120 were included in the trial after their approval and randomly distributed in three groups. 87 patients were male and 33 were females. 77 patients were in the age group of 25–40 years, 16 were in the age group of 40–50 years while 27 were between 50 and 60 years (Table 2).
Table 2.
Demographic data.
| Age Group |
25–40 years |
40–50 years |
50–60 years |
|||
|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | Male | Female |
| Group A | 18 | 06 | 06 | 02 | 03 | 05 |
| Group B | 22 | 07 | 01 | 01 | 07 | 02 |
| Group C | 18 | 06 | 04 | 02 | 08 | 02 |
| Total | 58 | 19 | 11 | 05 | 18 | 09 |
3.2. Classification on the basis of symptoms
All the three groups were assessed on the basis of the symptoms present in the patients. 47 patients out of 120 were COVID positive without any symptoms of disease on admission while 73 patients were having mild symptoms of disease. Number of asymptomatic patients in Group A was 14, 15 in Group B and 18 in Group C (Table 3). Their gradation was done before and after the treatment regimen of ten days (Table 4). Patients with severe symptoms or comorbidities like diabetes, COPD, cardiac patients and pregnant women were not included in the study.
Table 3.
Classification of patients on the basis of symptoms.
| Asymptomatic | Symptomatic | |
|---|---|---|
| Group A | 14 | 26 |
| Group B | 15 | 25 |
| Group C | 18 | 22 |
| Total | 47 | 73 |
Table 4.
Changes observed in symptoms after Treatment.
| Clinical Symptoms | Group A |
Group B |
Group C |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BTa | ATb | P valuec | BTa | ATb | P valuec | BTa | ATb | P valuec | ||
| Fever | 0.45 | 0 | <0.001 | 0.375 | 0.025 | 0.002 | 0.125 | 0.025 | 0.046 | |
| Sore throat | 0.975 | 0.05 | <0.001 | 0.4 | 0.1 | 0.003 | 0.3 | 0.15 | 0.014 | |
| Cough | 0.775 | 0.05 | <0.001 | 0.375 | 0.05 | <0.001 | 0.425 | 0.3 | 0.025 | |
| Dyspnea (difficulty in breathing) | 0.15 | 0 | 0.034 | 0.225 | 0.075 | 0.014 | 0 | 0 | 1.000 | |
| Running Nose | 0.225 | 0 | 0.007 | 0.3 | 0.13 | 0.052 | 0.1 | 0.03 | 0.083 | |
| General weakness | 0.325 | 0.2 | 0.025 | 0.275 | 0.075 | 0.25 | 0.011 | 0.15 | 0.046 | |
| Headache | 0.075 | 0 | 0.083 | 0 | 0 | 1.0 | 0.08 | 0.05 | 0317 | |
| Irritability | 0.31 | 0 | 0.001 | 0.475 | 0.1 | 0.001 | 0.25 | 0.175 | 0.083 | |
| Nausea/vomiting | 0 | 0 | 1 | 0.15 | 0.075 | 0 | 0.063 | 0 | 0.063 | |
| Diarrhea | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Loss of Taste | 0.35 | 0.1 | 0.023 | 0.225 | 0.05 | 0.02 | 0.08 | 0.1 | 0.705 | |
| Loss of Smell | 0.25 | 0.025 | 0.007 | 0.1 | 0.05 | 0.157 | 0.18 | 0.125 | 0.317 | |
BT before treatment.
AT After treatment.
Wilcoxon Signed Rank test.
3.3. Effect on symptoms
Least progression of symptoms, average early recovery with earlier viral clearance was seen in Groups A and B in comparison to Group C. Maximum relief in fever, cough, sore throat and irritability was observed in Group A than Groups B and C, which was statistically significant. In few patients of Group C loss of proper taste and smell was more prominent. Group B patients showed improvement in the abnormal sensation of taste and general weakness which was statistically non-significant, though.
3.4. Effect on Real time PCR
An important criteria of assessment in these patients was the RT-PCR test for which the patients were assessed on 5th, 7th and 10th days respectively in all the three groups. Patients were discharged after two consecutive RT-PCR tests for COVID -19 were negative.
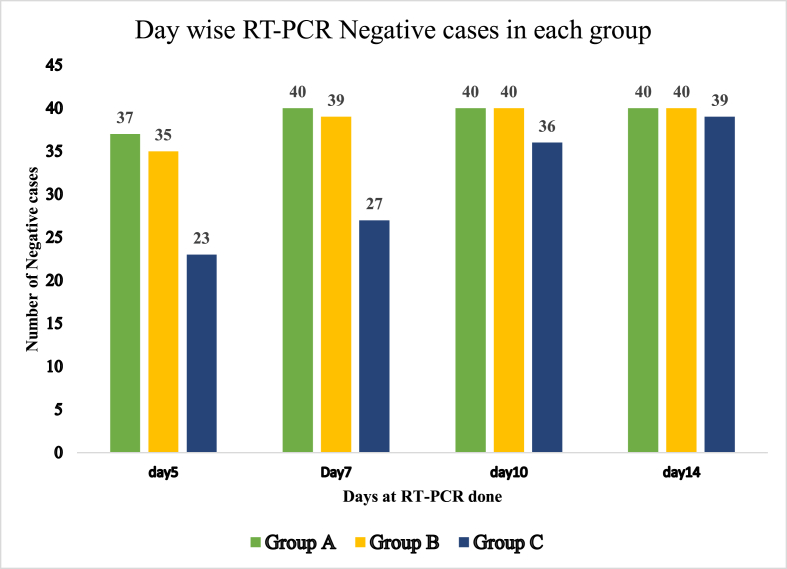
In Group A, RT-PCR became negative in 37 out of 40 patients on 5th day. Remaining three tested negative on the 7th day. All the patients were discharged within 10 days of admission in isolation ward (Table 3). The number of negative cases in Group B and C on 5th day were 35 and 23 respectively. All the patients recovered on 7th day in Group A, while 39 recovered in Group B and 27 in Group C. This infers that the time of viral clearance was minimum in Group A followed by Group B, while it was maximum in Group C.
On day 5, percentage of the patients tested negative in group A was 92.5% while that in group B was 87.5% and 57.75% in group C. On day 7, 100% patients in group A and 97.5% in group B tested negative, while the recovered percentage in group C was 72.5%. By 10th day all patients were negative in groups A and B, while 36 were negative in group C (Table 5, Fig. 1).
Table 5.
Day-wise Negative tested COVID-19 patients in three groups.
| Day 0 | Negative cases on Day 5 | Negative cases on Day 7 | Negative cases on Day 10 | Negative Cases on Day 14 | Test of Significancea | |
|---|---|---|---|---|---|---|
| Group A | 40 Positive cases | 37 | 40 | 40 | 40 | P < 0.001 |
| Group B | 40 Positive cases | 35 | 39 | 40 | 40 | |
| Group C | 40 Positive cases | 23 | 27 | 36 | 39 |
Kruskal-Wallis’ H test.
Fig. 1.
Graphical Representation of Day wise Effect of Treatment in three Study groups considering RT-PCR results.
Overall improvement in the quality of life of the patients, on the basis of their mental and physical states, was observed after their treatment regimen which was more pronounced in the patients of Group A and B because of early recovery and significant decrease in mental stress.
3.5. Complications and their management
One of the patients in Group C was shifted to Level III hospital due to severe difficulty in breathing on 5th day of his admission. Two patients in Group A complained of loose stools with mild weakness after two days of consumption of decoction. The decoction was discontinued and they were managed by administering Kutaja ghan vati in a dose of 2 tablets 500 mg each, twice daily for two days. Three patients in group B complained of mild burning sensation in abdomen which was easily managed by advising them Dhanyaka siddha jala (Coriander water) and Shatpushpa (∼Fennel seeds) 3 g for consumption before meals.
4. Statistical analysis
Null Hypothesis was the distribution of days at which two consecutive test negative is the same across categories of the group. However, Kruskal–Wallis H test showed that there was a statistically significant difference in the number of days at which two consecutive real time PCR test comes negative and between the three groups (χ2 (2) = 23.35, p < 0.001). Pairwise comparison post hoc test was carried out on each pair of groups. The pairwise comparisons show the results of the test on each couple of groups and found very strong evidence (p < 0.001) of a difference between the groups A and C as well as groups B and C.
5. Discussion
The basic concept of disease in Ayurveda has been said to be an imbalance of the Biohumours (∼doshas) and derangement of digestive fire (∼Mandagni) which eventually leads to a decreased level of immunity, subsequently making body susceptible to any infectious agent. The basic line of management involves the correction of Agni to an optimum level and thus improving immunity.
The manifestation of disease can be compared to Sannipatika Jwara especially of Kaphaja [19]predominance wherein symptoms like Running nose, Cough, Drowsiness, Loss of Appetite, indigestion, headache are present or Vataja [10] predominance wherein symptoms like dyspnea, dry cough, running nose, pain in thoracic region, dryness of Mouth and abnormal sensation of taste and smell are present. On aggravation, symptoms of Ojakshaya can be seen which may result in death of the patient. The description of a disease involving a major area of land has been stated as Janapadodhwamsa by Charaka and Nidana vipreeta chikitsa (∼management contrasting to the causative factors) has been advised in such conditions [20]. The choice of drugs adopted here was for management of impaired digestion (Mandagni and Ama pachana), protection of respiratory system hence improvising the bodies’ defense mechanism (Table 6).
Table 6.
Properties of drugs used.
| Name of the drug | Pharmacological properties and medicinal use |
|---|---|
| Kantakari (Solanum xanthocarpum Schrad. And Wendl.) | Anti-asthmatic Anti-cough Anti-inflammatory Bronchodilatory |
| Guduchi (Tinospora cordifolia Thunb.) | Anti-inflammatory Anti-allergic Anti hepatotoxic Very low toxicity Rasayana |
| Shunthi (Zingiber officinale Roscoe.) | Appetizer, Carminative Anti-inflammatory effects |
| Pippali (Piper longum L.) | Anti-oxidant Anti-inflammatory Hepatoprotective Immunomodulatory action Anti-microbial Bioavailability enhancer [38] |
| Rasona (Alium sativum L.) | Immunomodulatory effects Anti-viral property |
As per the classics, there are six stages in a disease progression (∼Shad Kriya kala [21]). If the progress of the disease is not identified at one stage it progresses to the next more severe stage with more complications. Therefore, to manage disease progression, early identification and management is required or it would be subsequently be more difficult to manage and ultimately the patients’ probability of survival narrows out. Especially in a disease like COVID-19, which has a rapid spreading rate and if symptoms worsen it has proven to be fatal worldwide, so there is this need of earlier management for better prognosis.
According to the protocol, patients were to be discharged after their recovery and at least two consecutive reports of negative RTPCRs. This was the time in consideration for viral clearance [18]. It was noted that patients in Kashaya group recovered earlier than those on Shunthi churna and Rasona kalka (group B) followed by the control group. 40 patients reported negative in RT PCR on day 7 in group A.
In group B, 35 patients reported negative on day 5 and 39 on day 7. All 40 patients recovered on day 10. In the control group, 23 reported negative on day 5, 27 on day 7, 36 on day 10 and 39 on day 14.
The rationale for selection of this intervention was comparison of Ayurvedic drugs with the control group. Two Ayurvedic study groups were included to compare the effect of drugs acting only on Agni with the drugs acting on Agni as well as Pranavaha srotasa.
Patients in Kashaya group (A) and Churna group (B) presented with improved appetite, feeling of wellness and lightness with better bowel evacuation. The Kashaya group patients also reported better throat clearance on completion of regimen of ten days.
Vyaghryadi kashaya is indicated for Sannipatik jwara by Vagbhatta [17]. Owing to the presence of Kantakari, Guduchi and shunthi in equal quantities along with pippali churna it possesses collective properties of digestive, appetizer, anti-inflammatory, mucolytic and antipyretic actions. Kantakari is beneficial for patients with sore throat, causing reduction in local inflammation in throat and respiratory tract. Patients also reported better mucociliary clearance. As per another study on Solanum, an improvement in PEFR and the reduction in other symptom scores indicated bronchodilator effect and reduced edema in the airway lumen [22].
Patients reported better appetite owing to the presence of Shunthi and Pippali. Dietary inclusion of dry ginger has shown to improve growth, apparent nutrient digestibility, gut morphology, serum chemistry, and stimulation of balanced intestinal microflora [23]. Its supplementation has shown alleviation of inflammation by reducing levels of TNF-α and hs-CRP without elevation in IL-6 levels [24].
Piperine in Pippali has been shown to enhance the bioavailability of structurally and therapeutically diverse drugs, possibly by modulating membrane dynamics due to its easy partitioning and increasing permeability. Pippali was added in fine powdered form in a dose of 250 mg as its higher doses and long term usage are not recommended [25]. Owing to grishma ritu (∼Summer) it was used in a lesser dose.
Guduchi has been considered as a Rasayana known to promote longevity and delay ageing process [26]. In such acute infections its property to increase the number of WBCs would also help to neutralize the toxicity produced by any infectious pathogen. T. cordifolia aqueous extracts have been reported to influence the cytokine production, mutagenicity, stimulation and activation of immune effector cells. In-vitro evidences shown that it up-regulates the IL-6 cytokines facilitating the acute response to injuries, inflammation, activation of cytotoxic T cells and also B cell diffraction [[27], [28]].
S. vati whose prime constituent is Guduchi is a known anti-pyretic drug with potent Anti-inflammatory effects [29]. It was used here in a dosage of 500 mg twice daily.
The combined effects of Vyaghryadi Kashaya and S. vati were antipyretic, amapachana, appetizers, immunomodulatory, alleviating Kapha and Vata doshas. Kashaya also showed anti-inflammatory effects specifically on the respiratory channels which are chiefly affected in the novel Corona virus infection.
Drugs in Group B i.e. Shunthi and Rasona are capable of ama pachana (digestion of metabolic wastes) and provide immunomodulatory action (∼Rasayana). Better digestion and improved appetite was reported by most of the patients of this group.
Rasona has been mentioned as a potent alleviator of vata [30], a Rasayana in the patients of shosha (decreased physical strength) [31]. It is chiefly indicated in cough, asthma, hoarseness of voice and hiccups. It enables liquefaction and expectoration of Kapha (∼mucous) by its volatile oils. In a dose less than 1.5 mg/ml garlic extract has also shown antiviral properties [32].
Kashaya and S. vati had an overall better effect than Shunthi and R. kalka. This might be due to the enhanced anti-inflammatory effects of Kantakari, added immunomodulatory effects of Guduchi present in kashaya as well as S. vati and presence of Pippali as an enhancer for action of drugs.
Control group patients were administered Vitamin C in a dose of 500 mg twice daily. Vitamin C also known as Ascorbic acid is one of the long known super nutrients said to boost immunity. It maintains the immune system comprehensively through antioxidant ability, collagen synthesis or directly strengthening immune cells fight against infection. Placebo controlled trials have shown quite consistently that the duration and severity of common cold episodes are reduced in the vitamin C groups [33]. Controlled trials with human subjects reported a significantly lower incidence of Pneumonia in Vitamin C supplemented groups [34]. In control group also the disease progression to severe stages was not present. It could be possibly due to the combined effect of vitamin C, warm water intake, saline gargling and use of Onion.
Onion (Alium cepa L.) which was used by all patients has been said to alleviate vayu and improve appetite [35]. It has rich therapeutic properties of providing substantial relief in diseases such as common cold, fever, cough and respiratory ailments due to presence of Sulphur rich compounds and Quercetin in traces [36].
Warm water was advised to all the patients as it facilitates digestion and proper bowel evacuation along with alleviation of kapha and vayu [37] which may speed the disease recovery and have a smoothening effect on throat and upper respiratory tract by its mucolytic action.
Patients were generally advised to gargle with saline water using rock salt to clear the throat and nasal cavity to prevent any extra mucous formation. Besides it also reduces local inflammation and relieves pain.
Patients admitted were advised to follow a healthy routine along with meditation in the form of Pranayama to improve the functioning of lungs by increasing their vital capacities and ensure better clarity of nasopharyngeal region. It also maintains a good oxygen supply to brain and heart to de-escalate stress produced due to disease. It ensures upholding all the physical, mental, psychological and spiritual alignments to attain positivity, calmness and good mental health.
The results obtained in groups A and B are important because majority of the patients tested negative on 5th day and remaining on 7th day which is useful in limiting the probability of spread of virus in community. It may be stated that response to the infection was better addressed by combined effects of systemic and local action (on respiratory system) by drug intervention of group A and B as compared to only systemic effect produced by agni enhancement in group B.
Statistically significant results were noticed in symptoms like fever, sore throat, cough and irritability in group A. In group B, symptoms progression to more severe stage was checked but it was statistically non-significant.
The advantage of it lies in controlling the spread among society and prevention of disease progression to a more severe stage.
5.1. Limitations of study
Since this was a pilot study only asymptomatic cases or mild cases were registered. Owing to limited financial resources, the immunological biochemical markers like Immunoglobulins, Interleukins and TNFα were not conducted in the patients enrolled. Studies including such biochemical markers may ascertain the action of the Ayurvedic drugs in a more effectual manner.
6. Conclusion
The most significant finding in this study was that faster viral clearance and better symptomatic improvement was noticed in Groups A and B as compared to the control group. A restrain was observed in the disease progression to rigorous stages. It can be concluded that Ayurveda drugs having properties to act on agni and pranavaha srotasa can be used effectively against COVID-19 infection. Due to encouraging outcomes of this trial, these drugs can be used in clinical practice, too. This paves way to an integrated approach wherein limiting the community spread and progression to severe stages is the key for improving recovery rate and reducing mortality.
Source(s) of Funding
This pilot project was funded by State Program management unit of AYUSH, National Heath Mission, Uttar Pradesh in Lucknow. This unit was the single source of funds provided for this study.
Author CRediT statement
Adil Rais: Conceptualization, Methodology, Investigation, Writing-Original draft.
Devendra S Negi: Conceptualization, Methodology, Validation, Supervision.
Amita Yadav: Resources, Project administration.
Absar Ahmad: Data curation, Formal analysis, Writing-Original draft.
R Galib: Conceptualization, writing-review and editing.
Himanshu Arya: Funding Acquisition, Resources.
Ramji Verma: Funding acquisition, Project administration.
Mahendra Yadav: Investigation.
P.N. Ahirwar: Resources.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Statement on the Second meeting of the International Health Regulations(2005) Emergency Committee regarding the outbreak of Novel Corona virus(2019-nCoV) 30 January 2020. [Google Scholar]
- 2.COVID-19 Dashboard by center for systems Science and Engineering (CSSE) at John Hopkins University, Retrieved 21st October 2020.
- 3.How COVID 19 spreads Centers for Disease control and Prevention (CDC) April 2020, Archived from the Original on 14 May. 2020. [Google Scholar]
- 4.Q&A on Corona viruses (COVID-19) World Health Organization; 17 April 2020. Archived from original on 14 May 2020. [Google Scholar]
- 5.Hopkins C. 2020. Loss of Sense of Smell as marker of COVID-19 infection, Ear, nose and throat surgery body of United Kingdom. [Google Scholar]
- 6.Symptoms of Novel Corona virus (2019-nCoV) Centers for disease control and Prevention (CDC); April 4 2020. [Google Scholar]
- 7.The Great Lockdown . 2020. Worst Economic downturn since The Great Depression IMF Blog. [Google Scholar]
- 8.Yuen KF, et al. The Psychological causes of panic buying following a health crisis. Inter J Environ Res Pub Health 17(10):3513. 10.3390/ijerph17103513. [DOI] [PMC free article] [PubMed]
- 9.https://www.who.int/docs/default-source/coronaviruse/covidstrategy-update-14 April 2020.pdf?sfvrsn=29da3ba0_19
- 10.Acharya Y.T., editor. Commentary Ayurveda Dipika of Chakrapanidatta on Charak Samhita of Agnivesha, Chikitsa Sthana. Ch. 3, Jwarchikitsa Adhyaya Verse 100-101. 1st ed. Chaukhambha Prakashan; Varanasi: 2011. p. 406. [Google Scholar]
- 11.10, Nidana Sthana. Ch. 6/14. p. 222.
- 12.10, Chikitsa Sthana; Trimarmiya Chikitsa Adhyaya: Chapter 26/109. p. 604.
- 13.Paradkar H.S., editor. Commentary Sarvangasundara and Ayurved rasayana of Hemadri on Ashtanga Hridaya of Vagbhatta, Nidana sthana, Udara nidanadhyaya Chapter 12 Verse 1. 1st ed. Chaukhambha Surbharti Prakashan; Varanasi: 2014. p. 358. [Google Scholar]
- 14.Clinical management protocol: COVID-19, Government of India, MOHFW, Directorate general of health Services. https://www.mohfw.gov Version 3, June 13, 2020.
- 15.Acharya YT. Siddha yoga samgraha, Jwara rogadhikara, edtion-2013, Shri Baidyanath Ayurved bhavan, Naini, Allahabad, p. 4.
- 16.10, Sutra Sthana. Ch. 4/7. p. 31.
- 17.13, Chikitsa sthana, Chapter 1/61, p. 395.
- 18.Guidelines for Ayurveda Practitioners for COVID-19, CCRAS, Ministry of AYUSH, New Delhi, p. 36 [Accessed June 11, 2020].
- 19.10, Chikitsa Sthana. Ch. 3/97, p. 406.
- 20.10, Vimana Sthana. Ch. 3/6, p. 241.
- 21.Ambikadutt Shastri Kaviraj., editor. Ayurved Tatva Sandipika on Sushruta samhita of Sushruta, Sutra sthana chapter 21, Vrana prashamanam Adhyaya, Verse 37. 1st ed. Chaukhambha Orientalia; Varanasi: 2014. p. 64. [Google Scholar]
- 22.Govindan S., Viswanathan S., Vijayasekaran V., Alagappan R. Further studies on the clinical efficacy of Solanum xanthocarpum and Solanum trilobatum in bronchial asthma. Phytother Res. 2004;18(10):805–809. doi: 10.1002/ptr.1555. [DOI] [PubMed] [Google Scholar]
- 23.Oso A.O., Awe A.W., Awosoga F.G., et al. Effect of ginger (Zingiber officinale Roscoe) on growth performance, nutrient digestibility, serum metabolites, gut morphology, and microflora of growing Guinea fowl. Trop Anim Health Prod. 2013;45:1763–1769. doi: 10.1007/s11250-013-0430-3. [DOI] [PubMed] [Google Scholar]
- 24.Mahluji Sepide, et al. Anti-inflammatory effects of Zingiber officinale in type 2 Diabetic patients. Adv Pharmaceut Bull. 2013 Dec;3(2):273–276. doi: 10.5681/apb.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.10, Vimana Sthana. Ch. 1/16, p. 677.
- 26.10, Sutra Sthana. Ch. 4/50. 1st ed. Chaukhambha Prakashan; Varanasi: 2011. p. 98. [Google Scholar]
- 27.More P., Pai K. In vitro NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of macrophages after Tinospora cordifolia (guduchi) treatment. Immunopharmacol Immunotoxicol. 2012;34:368–372. doi: 10.3109/08923973.2011.606324. [DOI] [PubMed] [Google Scholar]
- 28.Sudhakaran D.S., Srirekha P., Devasree L.D., Premsingh S., Michael R.D. Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J Exp Biol. 2006;44(9):726–732. [PubMed] [Google Scholar]
- 29.Patgiri B., et al. Anti inflammatory activity of guduchi Ghana, (aqueous extract of Tinospora cordifolia Mers). Ayu J. 2014;35(1):108–110. doi: 10.4103/0974-8520.141958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acharya Y.T., editor. Commentary Ayurveda Dipika of Chakrapanidatta on Charak Samhita of Agnivesha, Sutra Sthana. Ch. 27, Annapana vidhi Adhyaya Ver. 176. 1st ed. Chaukhambha Prakashan; Varanasi: 2011. p. 546. [Google Scholar]
- 31.13, Uttarsthana 39/112, p. 821.
- 32.Tsai Y., Cole L.L., et al. Antiviral properties of Garlic: in vitro effects on Influenza B, Herpes simplex and Coxsackie virus. Planta Med. 1985;51(5):460e1. doi: 10.1055/s-2007-969553. [DOI] [PubMed] [Google Scholar]
- 33.Hemila H, Douglas RM, Vitamin C and Acute respiratory infections. Int J Tubercul Lung Dis July 5, 2020;3:756–61. [PubMed]
- 34.Hemila H. Vitamin C intake and susceptibility to pneumonia. Pediatr Infect Dis J. 1997;16:836–837. doi: 10.1097/00006454-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 35.10, Sutra Sthana. Ch.27/175. p. 545.
- 36.Sampath Kumar K.P., et al. Alliumcepa: a traditional medicinal herb and its health benefits. J Chem Pharm Res. 2010;2(1):283–291. [Google Scholar]
- 37.10, Vimana Sthana. Ch. 3/40. p. 702.
- 38.Satyapal S., Tripathi J.S., Rai N.P. An appraisal of the bioavailability enhancers in the light of recent pharmacological advances. Ayu. 2016 Jan-March;37(1):3–10. doi: 10.4103/ayu.AYU_11_15. [DOI] [PMC free article] [PubMed] [Google Scholar]