Abstract
MDSC are a heterogeneous population of immature myeloid cells that are released by biological stress such as tissue damage and inflammation. Conventionally, MDSC are known for their detrimental role in chronic inflammation and neoplastic conditions. However, their intrinsic functions in immunoregulation, wound healing, and angiogenesis are intended to protect from over-reactive immune responses, maintenance of immunotolerance, tissue repair, and homeostasis. Paradoxically, under certain conditions, MDSC can impair protective immune responses and exacerbate the disease. The transition from protective to harmful MDSC is most likely driven by environmental and epigenetic mechanisms induced by prolonged exposure to unresolved inflammatory triggers. Here, we review several examples of the dual impact of MDSC in conditions such as maternal-fetal tolerance, self-antigens immunotolerance, obesity-associated cancer, sepsis and trauma. Moreover, we also highlighted the evidence indicating that MDSC have a role in COVID-19 pathophysiology. Finally, we have summarized the evidence indicating epigenetic mechanisms associated with MDSC function.
Keywords: MDSC, immunosuppression, obesity, cancer, immune tolerance, wound healing, COVID-19, homeostasis, chronic inflammation, epigenetic regulation
Introduction
Inflammation is a complex physiological “first response” to any condition that may cause cellular and tissue damage [1]. While immune responses are necessary for the clearance of pathogens, nascent malignant cells, and virus-infected cells, dysregulation of the immune system and uncontrolled inflammatory responses can also contribute to disease pathology [2]. Therefore, regulatory mechanisms are required to maintain a fine balance between destroying infected or malignant cells while protecting against excessive collateral tissue damage and promoting immune tolerance. Myeloid-derived suppressor cells (MDSC) are immunoregulatory cells that are early responders to tissue insult whose primary function is aimed at promoting tissue repair and wound healing, which also leads to preventing uncontrolled inflammation and maintaining homeostasis within the immune response [3–5]. These activities are critical in situations requiring tolerance to self-antigen during limited tissue repair (as in surgery or small trauma) [5] or during maternal-fetal tolerance [6–9]. However, under prolonged tissue damage caused by chronic inflammation and cancer [10–12], extensive tissue damage from severe or extensive trauma [13–15] or chronic viral infections by hepatitis C (HCV), hepatitis B (HBV), human immunodeficiency virus (HIV), influenza, and severe acute respiratory syndrome (SARS)-associated coronavirus [16–18], MDSC are hijacked by the pathological process resulting in their prolonged expansion and an enhanced immunosuppressive function.
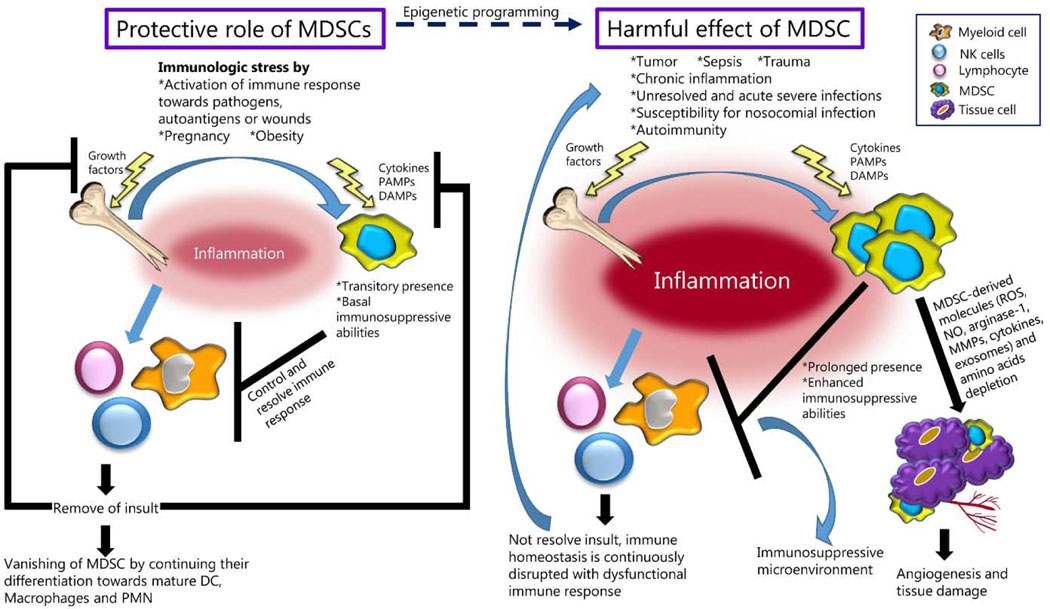
MDSC have two primary subtypes, granulocytic (G-MDSC) and monocytic (M-MDSC) that are found in different inflammatory conditions. Both subtypes of MDSC have pro- and anti-inflammatory properties mediated through the production of substances such as reactive oxygen species (ROS), matrix metalloproteinases (MMPs), arginase-1 (Arg-1), and the release of cytokines such as IL-6, IL-1β, and VEGF. The prolonged activation of MDSC can lead to tissue damage, promote angiogenesis, induce T cell dysfunction, all of which result in the development of an immunosuppressive microenvironment, which perpetuates chronic inflammation and in the case of cancer, promotes tumor growth and metastasis (Figure 1).
Figure 1. Comparison of the conditions where MDSC may have protective effects or cause abnormal pathological outcomes.
While clearance of initial insult results in the disappearance of MDSC and restoration of homeostasis, the unresolved inflammatory stimulus perpetuates the expansion and function of MDSC enhancing the inflammatory response, creating an immunosuppressive microenvironment, and facilitating angiogenesis and tissue damage. PAMPs stand for Pathogen-associated molecular patterns; DAMPs, Damage-associated molecular patterns; ROS, Reactive oxygen species; NO, Nitric oxide; MDSC, myeloid-derived suppressor cells; DC, dendritic cells, PMN, polymorphonuclear leukocytes.
In this review we examine the dual protective and pathogenic roles of MDSC in different types of biological processes and diseases, including the potential contribution of MDSC in the exacerbation of coronavirus disease 2019 (COVID-19). We will also discuss epigenetic changes which may in part be the key mechanisms responsible for the maladaptive response of MDSC.
1. Induction and activation of MDSC
Under normal conditions of myelopoiesis, hematopoietic stem cells (HSCs) differentiate into common myeloid progenitors (CMPs) and then into immature myeloid cells (IMCs). IMCs are released from bone marrow into the circulation where they complete their maturation into dendritic cells, macrophages, and granulocytes (neutrophils, basophils, or eosinophils). However, conditions of biologic stress or tissue insult, such as pregnancy [6–8, 19–21], infections [16, 17], wound healing [4, 5, 13, 22], or even psychological stress [23], stimulate emergency hematopoiesis resulting in the expansion and mobilization of IMCs from the bone marrow into the circulation at a much higher rate. The release of damage associated products (DAMPS), chemokines and other chemo-attractants cause IMCs to traffic to the sites of inflammation where they are stimulated by niche-specific environmental signals that sustain their immature state while display an initial discrete, and perhaps afterward enhanced, immunosuppressive function characteristic of MDSC [3].
Soluble mediators that trigger the mobilization of IMCs from bone marrow and activate MDSC vary widely across different inflammatory conditions. These factors include cytokines and growth factors such as granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage-CSF (GM-CSF), macrophage-CSF (M-CSF), stem cell factor (SCF), FMS-like tyrosine kinase 3 ligand (FLT3L), vascular endothelial growth factor (VEGF), interleukin (IL)-6, IL-13, and tumor necrosis factor-alpha (TNFα) among others [24]. Additional factors that induce the expression of proteins that prolong their survival or potentiate their immunosuppressive functions include pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) [25], damage-associated molecular patterns (DAMPs) such as calcium-binding proteins S100A8/A9 [26], and high-mobility group box 1 (HMGB1) [27]. Another critical mechanism in the activation of immunosuppressive functions in MDSC is the uptake of lipids that are rapidly incorporated into the mitochondria and undergo fatty acid β-oxidation (FAO) [28–31].
Activated MDSC produce cytokines and other soluble factors that have immunoregulatory effects on lymphocytes, NK cells, and other myeloid cells such as macrophages and dendritic cells [32]. Cytokines such as IL-10, and transforming growth factor-beta (TGFβ), ROS and RNS secreted by MDSC, can all inhibit T and NK cell proliferation, cytotoxicity and IFN production [32]. In addition, MDSC-derived mediators can promote the generation of immunosuppressive M2-type macrophages, tolerogenic dendritic cells (DC), and Th17 and regulatory T cells (Tregs) [10, 12, 32–34]. Furthermore, MDSC have a unique ability to modulate the availability of certain amino acids that are essential for protective T cell functions. Specifically, MDSC can uptake and deplete cysteine from the microenvironment, which inhibits cytotoxic and helper T cells. Similarly, MDSC can efficiently metabolize L-arginine through Arg-1, effectively depleting this amino acid that is essential to support T cell functions. Cell-to-cell interactions are also an important mechanism by which MDSC regulate T cells function. MDSC express programmed death-ligand 1 (PD-L1) and FasL that induce T cell anergy and apoptosis [32]. Also, in the sites of inflammation MDSC may actively phagocytize dead cells, invading microorganisms or dead cells that are eliminated in phagosomes containing ROS, reactive nitrogen species (RNS) and proteolytic and microbicidal enzymes including Arg-1. Arg-1 produced by MDSC metabolizes arginine to ornithine which stimulates the proliferation of fibroblasts, the production of collagen and effective wound healing. Together, these MDSC functions are intended to maintain immune homeostasis, restrict hyperactivation of pro-inflammatory cells, and prevent or reduce tissue damage [3]. These events are particularly crucial in situations such as maternal-fetal tolerance, prevention of immune responses against autologous antigens, and during the repair of damaged tissue. After clearance of the inflammatory source, the signals that induce MDSC to dissipate, which in turn promotes their clearance from the circulation or allows for their differentiation into mature myeloid cells with minimal impact on the systemic immune response [3].
Extensive or continued tissue damage as in cancer, chronic infections, sepsis, extensive trauma, or autoimmune disorders, results in prolonged emergency hematopoiesis with continued expansion of IMCs, and accumulation of MDSC with increased immunosuppressive functions. In these cases, MDSC become chronic inflammatory cells that impair a protective immune response which can contribute to cancer initiation and progression, a failure to clear infections, increasing tissue damage from autoimmune responses, and an inability to restore tissue homeostasis and regulate tissue repair. In addition to their immunosuppressive functions, the persistent production of cytokines, ROS, and MMPs by MDSC can lead to further tissue damage. MDSC can also contribute to angiogenesis via VEGF and MMP9 [35], and ROS, which also causes oxidative stress and regulates several kinases and transcription factors such as MAP kinases, NF-κB, AP-1, and HIF-1, which activate pro-inflammatory genes [36]. ROS also facilitates the uptake of exosomes through the inhibition of caveolin-1 leading to the metabolic reprogramming of surrounding cells [37]. Therefore, because of their potential for a detrimental role in certain diseases, it is critical to understand the biological and molecular mechanisms that redirect IMC into becoming MDSC and regulate their immunosuppressive functions.
2. MDSC in Clinical conditions and Pathological process
2.1. Obesity and cancer
Obesity is accompanied by multiple biological alterations including chronic low-grade inflammation and metabolic dysfunction. Mouse models of diet-induced obesity (DIO) have shown that MDSC accumulate in peripheral blood, liver, and fat tissue, contributing to controlling inflammation and re-establishing metabolic homeostasis [38]. However, their role is somewhat counterintuitive, i.e. they are not simple mediators of chronic inflammation and may have a beneficial role. In fact, the depletion of MDSC in murine DIO models increases the concentration of inflammatory markers and worsens insulin resistance. In contrast, adoptive transfer of MDSC reduces obesity-associated inflammation and improves insulin sensitivity [38]. Although MDSC were shown to induce adiposity, MDSC can also decrease the levels of circulating leptin which in turn could promote leptin sensitivity [39]. In humans, M-MDSC were shown to be increased in peripheral blood of overweight/obese males (BMI > 25 kg/m2) without diabetes or other complicating metabolic issues, compared to lean subjects (BMI < 25 kg/m2) [40]. However, the impact of MDSC in obesity and its related comorbidities is incompletely understood. Mechanistically, pro-inflammatory mediators responsible for adipocyte hyperplasia and hypertrophy such as TNFα, prostaglandin E2 (PGE2), IL-1β and IL-6 [41, 42] may also induce the expansion of MDSC [43–45] in an attempt to counter obesity-associated inflammation. However, other plausible explanations may be that dyslipidemia, frequently found in obesity, may also lead to the accumulation and enhance the immunosuppressive capacity of MDSC. In fact, exogenous lipid uptake by MDSC was shown to increase their immunosuppressive function [28].
Obesity is considered a major risk factor for the development of at least 13 types of cancer [46]. Therefore, it is reasonable to hypothesize that obesity-induced MDSC may help inhibit important anti-tumor mechanisms such as tumor surveillance and anti-tumor T cell responses, thus providing a biological link between obesity and the increased risk for developing cancer. Murine models have indeed confirmed that the presence of MDSC can accelerate the growth of the primary tumors and enhance metastatic tumors [39, 47, 48]. Specifically, DIO mice bearing renal cell carcinoma showed a robust accumulation of MDSC in tumors compared to lean mice and increased growth in tumors [48]. Similarly, the depletion of MDSC in DIO mice bearing breast tumors reduced tumor growth and restored protective antigen-driven T cell responses. In the same study, obesity-induced MDSC increased the development of spontaneous metastatic spread of tumors [39]. In these models, the increased immunosuppressive function of DIO-induced MDSC was attributed to increased PD-L1 expression [39]. Thus, together these studies suggest that the increased number of MDSC seen in obesity may be a potential explanation for the increased risk of developing cancer in obese individuals.
2.2. Wound healing and trauma
Extensive tissue damage caused by trauma or surgery leads to the release of bone marrow-derived cells (BMDC), among them MDSC, that contribute to tissue repair and the healing process. In trauma, MDSC may have a protective role in the early phase (within minutes to days) to curtail systemic hyper-inflammatory response, and in the phase of resolution and homeostasis (typically within days to weeks) [13] as a result of their important role in wound healing.
Conversely, MDSC may also contribute to the development of a persistent inflammatory-immunosuppressive and catabolic syndrome (PICS), a period of chronic critical illness seen within weeks to months in some injured patients. Patients with PICS have an increased risk of multi-organ dysfunction and secondary infections with subsequent morbidity and late mortality. MDSC found during PICS were shown to increase both pro-inflammatory and anti-inflammatory responses [49]. MDSC induced by extensive trauma also express high level of Arg-1, leading to a state of arginine deficiency and posttraumatic immune suppression characterized by T cell dysfunction [14]. Defective T cells after trauma lead to a higher susceptibility to posttraumatic infection [50]. In an animal model of surgical trauma, MDSC rapidly accumulated in the spleens of mice and were associated with reduced T cell number, decreased expression of TCR/CD3ζ-chain expression, and diminished IL-2 production. This effect was abrogated with an Arg-1 antagonist suggesting a potential role of MDSC producing Arg-1 [14]. Another animal study of polytrauma (laparotomy with cecectomy plus medial thigh dissection and femur fracture) induced the accumulation of MDSC accompanied by systemic inflammatory response syndrome in response to endogenously released DAMPs [15].
2.3. MDSC in Pregnancy and Neonates
The mechanisms allowing a successful gestation without triggering an immune response against the semi-allogeneic fetus are complex and involve multiple mechanisms including MDSC. Several studies showed that MDSC facilitate successful pregnancy by promoting maternal-fetal tolerance [6–8, 19–21]. In humans, circulating G-MDSC are increased in healthy pregnant women compared to non-pregnant controls and decline rapidly after childbirth [6]. These G-MDSC also have increased expression of Arg-1 and inducible nitric oxide synthase (iNOS) and suppress T cell proliferation. In addition, maintenance of maternal-fetal tolerance was associated with induction of Tregs by MDSC [7]. Furthermore, a decreased number of MDSC was associated with early miscarriage or unexplained recurrent pregnancy loss [21, 51]. The protective role of MDSC is further supported by animal studies where their accumulation in the circulation and uterus have been shown in pregnant mice. The depletion of MDSC with anti-Gr1 antibody caused implantation failure and infiltration of activated T cells in the uterus. In contrast, restoring MDSC levels by G-CSF injection, inhibited T cell responses and resulted in successful pregnancy [8]. In addition, the accumulation of MDSC induced by the injection of estradiol favors embryo implantation during in vitro fertilization (IVF) treatments. The mechanism appears to be related to the ability of estradiol to activate STAT3 and CCL2 chemokine which increases the expression of Indoleamine 2, 3-dioxygenase 1 (IDO1), Arg-1, and Cyclooxygenase 2 (COX2) in MDSC [52, 53] In addition, estradiol induces the expression of VEGF by MDSC which promote the development of maternal uterine spiral arteries and placental development during pregnancy, a fundamental step for the uterus to be receptive to implantation [54]. Although MDSC happen to be key players in a successful pregnancy, they could also contribute to the progression of pregnancy-associated lymphomas, which are relatively rare. However, accelerated tumor growth was found in pregnant mice inoculated with A20 murine lymphoma B cells compared to non-pregnant animals. Pregnant mice exhibited a greater infiltration of G-MDSC within the tumor compared with the control mice, suggesting that MDSC have a potential role in pregnancy-associated lymphoma progression [55].
Inflammation-mediated tissue damage is responsible for several severe neonatal diseases that occur during the first few weeks after birth including bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and sepsis. The expansion of MDSC observed in healthy neonates suggests that these cells may help control inflammation in general [56, 57] and regulate the immune responses in the gastrointestinal tract specifically [58]. Interestingly, full-term infants have higher numbers of MDSC and a lower risk for developing NEC compared to preterm infants [59]. These observations have further been corroborated by murine studies where newborn mice that developed NEC had reduced numbers of MDSC compared to mice that did not develop the disease [57]. A proposed mechanism is the ability of MDSC to downregulate TLR4 expression which results in reduced sensitivity to PAMPs derived from gut flora [58]. In fact, the expression of toll-like receptor 4 (TLR4) in the intestinal mucosa is increased during NEC, while TLR4 deletion in mice showed protection [60]. Interestingly, in addition to their conventional immunosuppressive function [61–63], G-MDSC in neonates have elevated microbicidal capacity and increased expression of genes associated with antimicrobial activity such as Cathepsin G (CTSG), Myeloperoxidase (MPO), Lysozyme (LYZ), Neutrophil Cytosolic Factor 1 (NCF1), lipocalin 2 (LCN2), neutrophil elastase (ELANE), and S100 Calcium Binding Protein A8/A9 (S100A8/9) [57]. This suggests that MDSC in addition to controlling the inflammatory response during microbial colonization of gut and lungs in neonates directly contributes to the eradication of pathogenic microorganisms. Besides this protective role, MDSC may also negatively influence the postnatal immune development which in turn will increase the neonate’s susceptibility to infections. This is proposed due to MDSC modulate adaptive immunity by inhibiting T helper type 1 (Th1) responses and inducing Th2 responses and Tregs [63], which weakens neonatal host immune defense. It is, therefore, the proper immune responses to microorganisms in the gastrointestinal tract depend on a balanced immune response in the neonatal period that appears to be closely linked to a balanced interaction between the neonatal microbiome and innate immunity, including MDSC.
2.4. Maintaining tolerance to autoantigens
Disturbance of the mechanisms that control the complete resolution of inflammation and complete the healing of tissues and homeostasis, can result in excessive and/or chronic immune activation, leading to chronic tissue damage that can promote the onset of autoimmune disease. MDSC have been shown to inhibit autoreactive T cells while inducing auto-antigen specific Tregs [64] and tolerogenic DC [32]. These mechanisms mediated by MDSC are essential to restore long-standing tolerance and prevent autoimmunity [9]. However, the role of MDSC in autoimmunity remains controversial in part due to different outcomes in various studies. This might be explained by the different models for autoimmunity used in the various studies, and the strategy to phenotype and identify MDSC. Nonetheless, the contradictory findings could be also an indication of the dual contribution of MDSC in protecting from or worsening illness depending on the severity and stage of the disease.
An example of how MDSC may limit tissue damage and protect from the development of T cell-mediated autoimmunity has been suggested in rheumatoid arthritis (RA), experimental autoimmune encephalomyelitis (EAE; [65]), and Type 1 diabetes (T1D, [66]).
Direct evidence for the beneficial role of MDSC was provided in a study where the adoptive transfer of MDSC in mice with RA reduced the severity of the condition and was accompanied by reduced numbers of CD4+ T cells and Th17 cells [67]. G-MDSC were also found in the synovial fluid of RA patients and possessed a protective effect by suppressing autologous autoreactive T cells [68]. MDSC also accumulated in synovia and suppressed DC maturation and proliferation of autoreactive T cells in a mouse model of RA [69]. G-MDSC were recently shown to contribute to the induction of immune tolerance during peptide immunotherapy in an experimental model of EAE [65]. On the other hand, expansion of MDSC were also correlated with EAE disease progression by driving a pathogenic Th17 response [70].
Several studies in mice also support the idea of a protective role for MDSC in T1D. In the nonobese diabetic (NOD) mouse model, MDSC contributed to the establishment of immune tolerance to pancreatic islets by directly inhibiting diabetogenic T cells and inducing Tregs [71]. Furthermore, the adoptive transfer of NOD-derived MDSC prevented the onset of diabetes in nondiabetic NOD/SCID injected with inflammatory T cells, which remained diabetes-free during the study period [66]. The protective effect of MDSC in T1D, determined by the co-transplantation of allograft islets with MDSC, has been attributed to the ability to decrease CD8+ T cell while increasing Tregs through the expression of PD-L1 [64]. In humans, MDSC were found to be increased in the circulation of diabetic patients; however, they showed minimal basal immunosuppressive function when freshly isolated, but this function was enhanced by ex-vivo activation with cytokines [72]. These findings suggest that activation of immune responses towards self-antigens could be the result of the compromised immunoregulatory capability of MDSC.
Alternatively, it was also suggested that MDSC may drive the pathogenesis of autoimmune diseases by increasing inflammation. For example, in systemic lupus erythematosus (SLE) patients, the percentage of circulating MDSC positively correlates with the exacerbation of the SLE disease activity index (SLEDAI) [73]. The accumulation of pro-inflammatory MDSC was described in experimental models of RA and EAE [10]. One common mechanism by which MDSC may increase the severity of autoimmune disorders is the ability to induce the differentiation of T cells towards Th17 cells via IL-17A, IL-1α or Arg-1 [34, 70, 73]. Specifically, L-arginine depletion by Arg-1 promotes the differentiation of Th17 cells by activating GCN2 and mTOR [73]. This again reveals the dual nature of MDSC in autoimmune conditions depending on disease context.
2.5. SARS-CoV-2 infection and sepsis
MDSC have also been described in viral infections such as influenza, hepatitis C virus (HCV), hepatitis B virus (HBV), human immunodeficiency virus (HIV), and cytomegalovirus (CMV). In general, the majority of studies report that MDSC are significantly elevated in these infections, suppress CD4+ T cell and NK cell function, increase Treg numbers, facilitating a persistent viral infection in detriment of the host [16, 17]. MDSC also inhibit the function of CD8+ T cells [74], likely through increased release of IL-10, expression of PD-L1, and production of Arg-1 [75, 76] which correlate with increased viral load and persistence of the infection [75]. In the current pandemic by the infection with SARS-CoV-2, there are multiple reports of dysregulation of several components of the immune system [77]. These include a reduction in T cell frequency and a massive increase in granulocytic cells [78]. Importantly, high granulocyte to lymphocyte ratios are associated with disease severity and reduced survival [79]. A recent study showed a significant increase in circulating G-MDSC in COVID-19 patients, which was associated with severity and poor outcomes [18]. It is unknown whether the increase of these cells results as a mechanism to control the severe inflammatory response induced by the SARS-CoV-2 infection; or contrarily, whether disease-derived factors induce the expansion and activation of MDSC to exacerbate inflammation.
One of the primary features identified in COVID-19 patients is the cytokine storm that contributes to the severity and leads to significant systemic tissue damage and potential mortality [80]. Increased cytokine levels and high numbers of circulating MDSC are also important features of sepsis [80], a life-threatening condition caused by the body’s response to an infection. In several studies, increased numbers of MDSC are associated with clinical worsening and increased occurrence of nosocomial infections and mortality in patients with sepsis [24]. One study found that increases in MDSC above 36% of all WBC in peripheral blood, was associated with higher rates of infections compared with ICU matched or normal controls [81]. This observation was supported by a separate study that found increases in nosocomial infections in patients with G-MDSC levels >30% WBC, compared with patients with lower levels of G-MDSC below 30% WBC [82]. Importantly, this same study found that a high number of MDSC at the time of admission correlated with earlier mortality, while decreased MDSC number correlated with shorter hospital stays.
Other reports, however, describe significant changes in phenotype and function of MDSC as sepsis progresses from the early pro-inflammatory phase to the late anti-inflammatory stage. Adoptive transfer of MDSC from mice with early sepsis (day 3) lead to increased pro-inflammatory cytokine production and earlier mortality of naïve mice compared to adoptively transferred MDSC from late phase. Interestingly, the MDSC from mice in early sepsis produce nitric oxide and pro-inflammatory cytokines, whereas MDSC from late sepsis express Arg-1, anti-inflammatory cytokines and had a more immature phenotype [83]. These findings coincide with the concept that MDSC become more suppressive in the presence of prolonged stimuli. Therefore, it may be important to carefully evaluate MDSC during COVID-19 given the experience in septic patients and models of sepsis. Because sepsis shares several characteristics with COVID-19, the knowledge of the role of MDSC in sepsis could potentially lead to understanding their impact on the severity of COVID-19 and potentially to identify new strategies to improve patient outcomes. To accomplish this, however, their roles and molecular mechanisms involved in their induction and activation need to be clearly delineated in the specific settings of SARS-CoV-2 infection.
3. Epigenetic mechanisms as a driver of MDSC expansion and function
The balance between tolerogenic, homeostatic, pro-inflammatory, and immunosuppressive activities of MDSC appears to be modulated by the microenvironmental signals that also dictate their expansion, survival, migration, phenotypic markers, and functional intensity. Conditions or pathologies that alter the environmental equilibrium, force cells to adapt to the particular milieu, and result in distinct polarization states that determine cellular function, which influence the disease outcome. Plasticity, known as the cell capability to reversibly assume more than one phenotype when exposed to different stimuli, is well exhibited by macrophages, which have the ability to adapt to the microenvironment to fill several roles such as pro-, anti-inflammatory, and regulatory with healing functions [84, 85]. Although less well-defined, phenotypic plasticity in MDSC is also displayed by M-MDSC which can differentiate towards G-MDSC [86], as well as the ability of MDSC to maintain their immature phenotype with different potency of immunosuppressive functions or continue their differentiation toward mature myeloid cells. The plasticity of myeloid cells may explain the exceptional phenotypic and functional characteristics and biochemical traits, that govern their different roles under different pathological conditions.
Despite the clinical relevance of MDSC in different pathological settings, the molecular pathways guiding the expansion and functional plasticity of these myeloid cells remain poorly understood. Compelling data support the concept that chronic inflammatory environments promote the outgrowth and transition into pathogenic long-lasting MDSC, which lead to the exacerbation of the inflammatory process and perpetuate disease pathology. Still, the molecular mechanisms are incompletely known. Epigenetic regulation is the process that allows genetically identical cells to differ in gene expression profiles and cellular phenotype. Epigenetics is then a crucial mechanism in the plasticity of myeloid cells, which are required to modify their functions depending on a multitude of different tissue environments.
Epigenetic processes include histone modifications by methylation or acetylation, DNA methylation at CpG islands in promoters, and differential expression of microRNAs (miRNAs) and long non-coding RNAs (lncRNAs). While DNA methylation and covalent modification of histones activate or silence gene transcription at the chromatin level, miRNAs and IncRNAs are critical to regulating protein expression at the post-transcriptional level. Several proteins are involved in epigenetic processes including histone acetyl transferases (HATs), histone deacetylases (HDACs), histone methyl transferases (HMTs), histone demethylases (HDMs), DNA methyltransferases (DNMTs), Ten-eleven translocation (TET) enzymes, and protein complexes, such as Mi-2/ Nucleosome Remodeling Deacetylase (NuRD) [87]. Although the epigenetic landscape that governs the expansion and function of MDSC are incompletely known, emerging evidence has revealed several epigenetic modifiers that are key players in the complex regulatory network controlling development, expansion, and functional outcomes of MDSC (Table 1). Pathways associated with STAT3, IRF8, PI-3K, NF-κB, C/EBPβ, and CHOP, can regulate the proliferation and activation of MDSC [88]. Additional regulatory mechanisms also include conditions leading to an increased endoplasmic reticulum (ER) stress, and activation of FAO [28, 89]. Epigenetic changes likely play an important role in regulating these pathways and therefore shaping the phenotype and function of MDSC and further, the outcome of different conditions. For example, several miRNAs have shown regulatory roles on MDSC in inflammation, infection, autoimmune disease, and cancer [90], such as miR-21, miR-181b, and miR-375 in sepsis, miR-124 in HCV, miR-223 in EAE and miR-10a, miR-30a and miR-6991–3p among others in cancer. Interestingly, in our ongoing study, we have found that miR-29A, as well as several lncRNAs, are up-regulated in PMN-MDSC isolated from peripheral blood from patients with morbid obesity (Body Mass Index [BMI] > 40 Kg/m2) compared to counterparts from healthy controls with a BMI < 25 (unpublished data).
Table 1.
Effect of epigenetic mediators on MDSC expansion and activation
| Mediator | Target/ mechanism | Effect | Ref | |
|---|---|---|---|---|
| miRNAs | miR-9 | ↓ RUNX1, a transcription factor that controls the expression of several genes that regulate myeloid differentiation including CSF2 (GM-CSF), MPO, IL3, CSF1R (M-CSFR) | Inhibits differentiation of MDSC into a mature myeloid cell and promote immunosuppressive function | [90] |
| miR-9 and miR-181a | Interfere with SOCS3 and PIAS3, respectively | Promote the development of early-stage MDSC | [99] | |
| miR-10a | Targets the RORα, thus activate NF-κB signaling. miR-10a also activates AMPK pathway | Promote expansion and immunosuppression | [90, 100] | |
| miR-15 family (miR-15a, miR-15b, miR-16, miR-195, miR-503 and miR-424) | Undetermined; however, miR424 increases the suppressive function, whereas the rest of miR-15 family members have an opposite effect by blocking PD-L1/PD-1 signaling | Inhibit immunosuppressive activity on T cells | [90] | |
| miR-17 family members (miR-17–5p, miR-20a, miR-106a) | ↓ STAT3 expression and STAT3-regulated ROS production. Also repress AML1, which result in the down-regulation of M-CSFR, thus blocking M-MDSC differentiation | Reduce suppressive function | [90, 101] | |
| miR-21 | Downregulates PTEN and thus activates PI-3K/Akt and MAPK signaling and increases the expression of PD-L1. miR-21 also targets STAT3 | Enhances the suppressive function | [90, 100] | |
| miR-21 and miR-181b | ↑ NFIA, and therefore transactivation of NF-κB | Enhance immunosuppression | [102] | |
| miR-21 and miR-155 | Synergistic effect of both miRNAs on MDSC induction via targeting SHIP-1 and PTEN, respectively, leading to STAT3 activation | Accelerate accumulation of functional MDSC | [103, 104] | |
| miR-27b, miR-126–3p, miR-320, and miR-342–3p | Undetermined | Enhance the expression of suppressive molecules | [105] | |
| miR-29a and miR-92a | Target HBP1 and protein kinase Prkar1a, respectively | Accelerate cycle progression and thus expansion and potentiate suppression function | [90, 100] | |
| miR-30a | Down-regulates SOCS3 mRNA, thus activates STAT3 signaling and up-regulates Arg-1 expression | Promote differentiation and activity of MDSC | [90] | |
| miR-34a | Inhibits apoptosis by suppressing the expression of N-myc or p2rx7/Tia1 | Promotes expansion of MDSC and enhances immunosuppression | [90, 106] | |
| miR-93/106b miRNA cluster | ↓ STAT3 expression and STAT3-regulated PD-L1 expression | [90] | ||
| miR-100 family (miR-99b/−100) and miR-125 family (miR-125a/−125) | Increase the expression of cytokines such as IL-6, CCL2, and TNFα to further activate the JAK/STAT pathway | Promote accumulation and suppression function | ||
| miR136 | Targets and degrade the transcription factor NFIA. Also, induce the expression of CD11b, CD14 and C/EBPε, several cytokines and ROS | Promotes maturation towards macrophages | ||
| miR-142–3p | Inhibits C/EBPβ/STAT3 pathway | Reduces the immunosuppressive activity | ||
| miR-143–3p | Modulates STAT3 and C/EBPβ signaling pathways | Prevents MDSC differentiation | ||
| miR-146a | ↓ TRAF6 and IRAK1 and negatively regulates myeloproliferation by controlling the expression of CSF1R gene | Interferes with myeloid cell maturation which possibly leads to expansion of MDSC | [107, 108] | |
| miR-155 | Down-regulate expression of SOCS1, thus activate STAT1 and up-regulate the expression of IDO. Also targets HIF-1α, thus down-regulating CXCL1, CXCL3 and CXCL8 expression in MDSCs | Induces MDSC and enhances their suppressive function via STAT1, although reduces accumulation in tumors via HIF-1α. | [109, 110] | |
| miR-185–5p | ↓ Chop | Inhibits MDSC generation and function | [92] | |
| miR-200c | ↓ PTEN, thus activates FOG2 which led to STAT3 and PI-3K/Akt signaling activation | Promotes suppressive function | [90] | |
| miR-210 | Increases Arg-1 activity, nitric oxide, IL-6, and CXCL12 production | Strengthens the suppression function | ||
| miR-223 and Let-7e | ↓ MEF2C and inhibit STAT3 activation | Suppress differentiation of IMCs into MDSC and downregulate the suppressive function | ||
| miR-375 | ↓ JAK/STAT3 | Reduces the number of MDSC | ||
| miR-424 | ↓ NFIA and therefore the expression of M-CSFR | Promotes differentiation of MDSC into mature cells | [111] | |
| miR-494 | ↓ PTEN, thus activates the PI-3K/Akt pathway and subsequent activation of survival pathways, upregulation of MMPs and enhances CXCR4-mediated MDSC chemotaxis | Leads to expansion and infiltration of MDSC | [90] | |
| miR-494–3p and miR-1260a | Increase intracellular calcium fluxes | Expansion of M-MDSC | [91] | |
| miR-690 | Attenuates C/EBPα expression | Maintenance of an immature, and therefore, functional population of MDSC | [90] | |
| miR-6991–3p | Suppress Galectin 9-mediated activation of JAK/STAT3 | Promotes apoptosis of MDSC | ||
| DNMT | DNMT3a and DNMT3b | Inhibit the expression or activity of STAT3 and therefore of Arg-1 and S100A8. Also, up-regulation of DNM3a induces hypermethylation in S1PR4, RUNX1, IL1RN, and CCR2 | Arrest the suppression activity. Function-specific DNA methylation pattern during MDSC generation | [93, 94] |
| HMT | SETD1B | Enrich H3K4me3 at the nos2 promoter, upregulating the expression of iNOS | Promotes suppressive function | [94] |
| HAT | CBP/EP300-BRD | regulator of H3K27 acetylation in MDSCs to further upregulate of STAT pathway-related genes, such as Ly6C2, Ccr2, Mmp9, and NOS2, and the expression of Arg-1 and iNOS | Inhibit transition from suppressive phenotype to an inflammatory phenotype through | [94] |
| HDAC | HDAC2 | Inhibition of rb1 gene expression, releasing the functional E2F transcription factor and activation of C/EBPβ and Inhibitor of ID-2. Abrogate expression of Rb-dependent genes | Promotes the phenotype switch from M-MDSC to G-MDSC | [94] |
| HDAC6 | Required for STAT3 phosphorylation and recruitment to the nucleus | Increase the suppressive activity of M-MDSC, but does not affect PMN-MDSC | [112] | |
| HDAC11 | Undetermined; however, it is possible that impairs STAT3 activation, and thus IL-10 and S100 proteins secretion | Negative regulator of expansion and function | [94] | |
| Dynamic between acetylation by HAT and deacetylation by HDACs | ↑ pathways related to Wnt, IL-6, MAPK, SNARE, JNK, and HIF-1 | Activate chemotaxis, cell migration, and anti-apoptosis | [113] | |
| Mi-2/ NuRD/ HDAC complex | p66a | Directly interacts with STAT3 suppressing its phosphorylation (Y705) and ubiquitination (K63), thus neutralize its function | Blocks the differentiation of cells into MDSC | [114] |
| lncRNA | Hotairm1 | ↑ expression of HOXA1, which ↓ Arg-1 levels and ROS production in MDSC. Binds S100A9 and leads its transport from cytosol to the nucleus, thus transforming S100A9 from a secreted mediator into a repressor protein | Reduces MDSC suppressor phenotype | [97] |
| RNCR3 (known as LINC00599 in human) | Sponges miR-185–5p to release its target gene Chop promoting Chop expression | Promotes MDSC differentiation and function | [92] | |
| lnc-C/EBPβ | Bind LIP isoform to stop the activity of C/EBPβ and also to WDR5 | Downregulate IL4il and promote PMN-MDSC but impede the differentiation of M-MDSC | [115] | |
| lnc-CHOP | Binds to the LIP isoform of C/EBPβ and CHOP and contributes to LAP isoform activation. lnc-CHOP induces accumulation of the epigenetic marker H3K4me3 in the promoter of Arg-1, NOX2, iNOS, and COX2 inducing their transcription | Promotes the immunosuppressive activity and generation of MDSC | [116] | |
| Olfr29-ps1 | N6-methyladenosine modification in Olfr29-ps1 sponges miR-214–3p and promotes MyD88 expression | Promotes the accumulation and immunosuppressive activity of MDSC | [117] | |
| lncRNA Pvt1 | ↑ c-myc | Promotes the immunosuppressive activity of G-MDSC by up-regulating the expression of Arg-1 and ROS production | [95] | |
| MALAT1 | Undetermined | Negatively regulates MDSC generation | [98] | |
| RUNXOR | ↓ RUNX1 expression | Promotes the generation and suppressive activity | [96] | |
| HOTAIR | ↑ CCL expression | Promotes recruitment and accumulation of MDSC | [118] | |
RUNX1 indicates runt-related transcription factor 1; CSF2, Colony Stimulating Factor 2; GM-CSF, Granulocyte-macrophage colony-stimulating factor; MPO, Myeloperoxidase; CSF1R, Colony Stimulating Factor 1 Receptor; M-CSFR, Macrophage Colony Stimulating Factor I Receptor; SOCS1/3, suppressor of cytokine signaling 1/3; Arg-1, arginase-1; PIAS3, protein inhibitor of activated STAT3; RORα, RAR-related orphan receptor alpha; NF-κB, nuclear factor kappa B; AMPK, AMP-activated protein kinase; PD-L1-PD1, programmed cell death 1 receptor/ programmed cell death ligand 1; STAT1/3, signal transducer and activator of transcription1/3; ROS, reactive oxygen species; AML1, acute myeloid leukemia 1; PTEN, phosphatase and tensin homolog; PI-3K/Akt, phosphatidylinositol-3-kinase/Akt; NFIA, nuclear factor 1 A-type; SHIP-1, 5’ polyphosphatase 1; HBP1, high-mobility group box transcription factor 1; Prkar1a, cAMP-dependent type I regulatory subunit alpha; CCL2, C-C motif chemokine ligand 2; TNFα, tumor necrosis factor alpha; JAK; Janus kinase; C/EBPα/β/ε, CCAAT enhancer binding protein alpha/beta/epsilon; IDO, indoleamine 2,3-dioxygenase; HIF-1α, hypoxia inducible factor 1 subunit alpha; CXCL1/3/8/12, chemokine (C-X-C motif) ligand 1/3/8/12; Chop, C/EBPβ homologous protein; FOG2, friend of Gata 2; MEF2C, Myocyte enhancer factor-2; CXCR4, C-X-C Motif Chemokine Receptor 4; DNMT; DNA methyltransferase; HMT; histone methyl transferase; HAT, histone acetyl transferase; HDACs, histone deacetylase; Mi-2/NuRD, Mi-2/Nucleosome Remodeling Deacetylase; S1PR4, Sphingosine-1-phosphate receptor 4; IL1RN, Interleukin 1 Receptor Antagonist; CCR2, C-C Motif Chemokine Receptor 2; H3K4me3, Trimethylation of Histone H3 at Lysine 4; iNOS, inducible NO synthase; CBP/EP300-BRD, Bromodomain (BRD) of the CREB (cyclic-AMP response element binding protein)-binding protein (CBP) and E1A-binding protein of 300 kDa (EP300); rb1, retinoblastoma 1; E2F, E2 factor; ID-2, DNA Binding 2; SNARE; SNAP receptor; HOXA1, Homeobox A1; S100A9, S100 calcium-binding protein A9; WDR5, WD Repeat Domain 5; NOX2, NADPH oxidase 2; COX2, cyclooxygenase-2; MyD88, Myeloid differentiation primary response 88.
Some examples of how different mechanisms can cause epigenetic changes that alter MDSC is shown by STAT3. STAT3 is a critical transcription factor in promoting the accumulation and activation of MDSC by regulating the expression of several immunosuppressive mediators such as Arg-1, IL-10, ROS, PD-L1, and S100 proteins. STAT3 is also an upstream regulator of C/EBPβ, which is indispensable for myelopoiesis and MDSC expansion [88]. Epigenetic modifiers, including several miRNAs (Table 1), are direct or indirect regulators of STAT3 expression, phosphorylation and signaling, as was recently described by Su and collaborators [90]. Other miRNAs, such as miR-494 and miR-1260a, which alter the PI-3K/Akt pathway or intracellular calcium fluxes [90, 91], induce the expansion of MDSC, while miR-210 enhances the immune suppressive function of MDSC by increasing Arg-1, NO, and IL-6, or miR-21, which downregulates PTEN, increases the expression of PD-L1 [90]. In contrast, miR-185-5p inhibit MDSC suppressive capacity by reducing CHOP expression [92]. Furthermore, DNA modification by DNMTs can also regulate the expression of STAT3. Two of these, DNMT3a and DNMT3b, were shown to inhibit the suppressive activity of MDSC by increasing methylation at the promoter region of STAT3 gene, and therefore reduce the expression of STAT3 target genes such as Arg-1 and S100A9 [93].
Histone modifications have the potential to significantly alter the chromatin landscape and induce changes in the expression of many genes simultaneously, which can modify the cellular phenotype. In MDSC, HDAC11 deficiency was shown to lead to more suppressive MDSC and increased tumor growth in an EL4 tumor model [94]. A different member of the HDAC family, HDAC2, has very intriguing effects on MDSC phenotype. In a preclinical cancer study, HDAC2 was shown to induce the differentiation of M-MDSC into G-MDSC by decreasing the expression of rb1 gene [94]. These DMNTs and HDACs are clinically significant because there are small molecule inhibitors that may be effective in inhibiting the actions of MDSC to improve cancer outcomes.
On the other hand, the role of lncRNAs on MDSC is less well understood. Some reports have shown that lncRNAs such as RNCR3 can enhance the suppressive capacity of MDSC by increasing the expression of CHOP. Similarly, lncRNA Pvt1 enhances the expression of Arg-1 and the production of ROS. RUNXOR, which can modulate the expression of Arg-1, was found to be increased in lung cancer patients [92, 95, 96]. Conversely, some lncRNAs inhibit MDSC.
Hotairm1 decreases Arg-1 and ROS levels, and MALAT1, which is decreased in the circulation of lung cancer patients, negatively regulates the differentiation of MDSC [97, 98]. While non-coding RNAs have few current therapeutic options, knowledge of the network of these RNAs in MDSC will lead to a better understanding of the biology of MDSC and could identify potential druggable protein targets that are altered in response to miRNAs and lncRNAs.
4. Conclusions remarks
The great plasticity and the lack of unique markers have made it difficult to specifically characterize and assign discrete and precise functions to MDSC under different conditions. Despite this, there is a growing body of evidence showing their importance in tissue repair, wound healing and maintaining a homeostatic balance between and protective immune response and a maladaptive damaging uncontrolled inflammation. The continued study of the basic mechanisms that regulate MDSC under normal conditions will allow us to better understand their behavior and role during disease. This will continue to be a complex process given the fact that in different pathologies or clinical conditions MDSC can be beneficial, while in others they clearly inhibit protective immune responses while intensifying the tissue damage. Innovative omics technologies (i.e., genomics, transcriptomics, proteomics, and metabolomics) will indeed allow us to better place their precise phenotype and their functions under different conditions. This will also be essential in the development of therapeutic approaches to either inhibit their induction/activity or induce their expansion and function for the benefit of patients.
Highlights:
MDSC are induced by biological stress, such as tissue damage and inflammation
MDSC suppress over-reactive immune response, maintain immunotolerance and homeostasis
Chronic exposure to inflammation bases the transition from protective to harmful MDSC
In chronic inflammation and neoplastic conditions, MDSC worsen disease
Understanding the underlying process in the transition may help for therapeutics
Acknowledgments
This work was supported by NIH funds 5P30GM114732-02, P20CA233374 and the Obesity / Cancer Pilot and Feasibility Grant (Grant # DK072476 Nutrition Obesity Research Center (NORC) from Pennington Biomedical Research center and Louisiana Cancer Research Center (LCRC-Louisiana State University) to MDSP; NIH funds (5P30GM114732-02, P20CA233374) to MD and AO. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Medzhitov R, Origin and physiological roles of inflammation, Nature 454 (2008) 428–435. [DOI] [PubMed] [Google Scholar]
- [2].Feehan KT, Gilroy DW, Is Resolution the End of Inflammation?, Trends in molecular medicine 25 (2019) 198–214. [DOI] [PubMed] [Google Scholar]
- [3].Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system, Nat Rev Immunol 9 (2009) 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mahdipour E, Charnock JC, Mace KA, Hoxa3 promotes the differentiation of hematopoietic progenitor cells into proangiogenic Gr-1+CD11b+ myeloid cells, Blood 117 (2011) 815–826. [DOI] [PubMed] [Google Scholar]
- [5].Ou L, Shi Y, Dong W, Liu C, Schmidt TJ, Nagarkatti P, Nagarkatti M, Fan D, Ai W, Kruppel-like factor KLF4 facilitates cutaneous wound healing by promoting fibrocyte generation from myeloid-derived suppressor cells, The Journal of investigative dermatology 135 (2015) 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kostlin N, Kugel H, Spring B, Leiber A, Marme A, Henes M, Rieber N, Hartl D, Poets CF, Gille C, Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses, European journal of immunology 44 (2014) 2582–2591. [DOI] [PubMed] [Google Scholar]
- [7].Kang X, Zhang X, Liu Z, Xu H, Wang T, He L, Zhao A, Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF-beta/beta-catenin pathway, Molecular human reproduction 22 (2016) 499–511. [DOI] [PubMed] [Google Scholar]
- [8].Ostrand-Rosenberg S, Sinha P, Figley C, Long R, Park D, Carter D, Clements VK, Frontline Science: Myeloid-derived suppressor cells (MDSCs) facilitate maternal-fetal tolerance in mice, Journal of leukocyte biology 101 (2017) 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Amodio G, Cichy J, Conde P, Matteoli G, Moreau A, Ochando J, Oral BH, Pekarova M, Ryan EJ, Roth J, Sohrabi Y, Cuturi MC, Gregori S, Role of myeloid regulatory cells (MRCs) in maintaining tissue homeostasis and promoting tolerance in autoimmunity, inflammatory disease and transplantation, Cancer immunology, immunotherapy : CII 68 (2019) 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Veglia F, Perego M, Gabrilovich D, Myeloid-derived suppressor cells coming of age, Nature immunology 19 (2018) 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ostrand-Rosenberg S, Myeloid derived-suppressor cells: their role in cancer and obesity, Curr Opin Immunol 51 (2018) 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gabrilovich DI, Myeloid-Derived Suppressor Cells, Cancer immunology research 5 (2017) 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA, Moldawer LL, A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma, Molecular medicine 17 (2011) 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB, CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress, J Immunol 176 (2006) 2085–2094. [DOI] [PubMed] [Google Scholar]
- [15].Cheng L, Xu J, Chai Y, Wang C, Han P, Dynamic changes in trauma-induced myeloid-derived suppressor cells after polytrauma are associated with an increased susceptibility to infection, Int J Clin Exp Pathol 10 (2017) 11063–11068. [PMC free article] [PubMed] [Google Scholar]
- [16].Dorhoi A, Du Plessis N, Monocytic Myeloid-Derived Suppressor Cells in Chronic Infections, Front Immunol 8 (2017) 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].O’Connor MA, Rastad JL, Green WR, The Role of Myeloid-Derived Suppressor Cells in Viral Infection, Viral immunology 30 (2017) 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Agrati C, Sacchi A, Bordoni V, Cimini E, Notari S, Grassi G, Casetti R, Tartaglia E, Lalle E, D’Abramo A, Castilletti C, Marchioni L, Shi Y, Mariano A, Song JW, Zhang JY, Wang FS, Zhang C, Fimia GM, Capobianchi MR, Piacentini M, Antinori A, Nicastri E, Maeurer M, Zumla A, Ippolito G, Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19), Cell death and differentiation 27 (2020) 3196–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kostlin N, Hofstadter K, Ostermeir AL, Spring B, Leiber A, Haen S, Abele H, Bauer P, Pollheimer J, Hartl D, Poets CF, Gille C, Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype, J Immunol 196 (2016) 1132–1145. [DOI] [PubMed] [Google Scholar]
- [20].Kostlin N, Ostermeir AL, Spring B, Schwarz J, Marme A, Walter CB, Poets CF, Gille C, HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4, European journal of immunology 47 (2017) 374–384. [DOI] [PubMed] [Google Scholar]
- [21].Nair RR, Sinha P, Khanna A, Singh K, Reduced Myeloid-derived Suppressor Cells in the Blood and Endometrium is Associated with Early Miscarriage, American journal of reproductive immunology 73 (2015) 479–486. [DOI] [PubMed] [Google Scholar]
- [22].Zhang X, Sarkar K, Rey S, Sebastian R, Andrikopoulou E, Marti GP, Fox-Talbot K, Semenza GL, Harmon JW, Aging impairs the mobilization and homing of bone marrow-derived angiogenic cells to burn wounds, Journal of molecular medicine 89 (2011) 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McKim DB, Yin W, Wang Y, Cole SW, Godbout JP, Sheridan JF, Social Stress Mobilizes Hematopoietic Stem Cells to Establish Persistent Splenic Myelopoiesis, Cell reports 25 (2018) 2552–2562 e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schrijver IT, Theroude C, Roger T, Myeloid-Derived Suppressor Cells in Sepsis, Front Immunol 10 (2019) 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang Y, Sun D, Zhou J, Tan C, Zhang H, Chen Z, Hao C, Zhang J, LPS expands MDSCs by inhibiting apoptosis through the regulation of the GATA2/let-7e axis, Immunol Cell Biol 97 (2019) 142–151. [DOI] [PubMed] [Google Scholar]
- [26].Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G, Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells, J Immunol 181 (2008) 4666–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, Tracey KJ, Ostrand-Rosenberg S, HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells, Cancer research 74 (2014) 5723–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC, Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells, Oncoimmunology 6 (2017) e1344804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, Ricciotti E, DiRusso C, Murphy ME, Vonderheide RH, Lieberman PM, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Lin C, Nefedova Y, Black P, Kagan VE, Gabrilovich DI, Fatty acid transport protein 2 reprograms neutrophils in cancer, Nature 569 (2019) 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, Partlova S, Garfall A, Vogl DT, Xu X, Knight SC, Malietzis G, Lee GH, Eruslanov E, Albelda SM, Wang X, Mehta JL, Bewtra M, Rustgi A, Hockstein N, Witt R, Masters G, Nam B, Smirnov D, Sepulveda MA, Gabrilovich DI, Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients, Sci Immunol 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, Valle LD, Trillo-Tinoco J, Maj T, Zou W, Rodriguez PC, Ochoa AC, Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies, Cancer immunology research 3 (2015) 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V, Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression, British journal of cancer 120 (2019) 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK, Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression, Seminars in cancer biology 22 (2012) 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang H, Wang S, Huang Y, Wang H, Zhao J, Gaskin F, Yang N, Fu SM, Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation, Clinical immunology 157 (2015) 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rivera LB, Bergers G, Intertwined regulation of angiogenesis and immunity by myeloid cells, Trends in immunology 36 (2015) 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ranneh Y, Akim AM, Hamid HA, Khazaai H, Fadel A, Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: a review, Applied Biological Chemistry 60 (2017) 327–338. [Google Scholar]
- [37].Weinberg F, Ramnath N, Nagrath D, Reactive Oxygen Species in the Tumor Microenvironment: An Overview, Cancers 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L, Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity, The Journal of biological chemistry 286 (2011) 23591–23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Clements VK, Long T, Long R, Figley C, Smith DMC, Ostrand-Rosenberg S, Frontline Science: High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells, Journal of leukocyte biology 103 (2018) 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bao Y, Mo J, Ruan L, Li G, Increased monocytic CD14(+)HLADRlow/- myeloid-derived suppressor cells in obesity, Mol Med Rep 11 (2015) 2322–2328. [DOI] [PubMed] [Google Scholar]
- [41].Chan PC, Liao MT, Hsieh PS, The Dualistic Effect of COX-2-Mediated Signaling in Obesity and Insulin Resistance, Int J Mol Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grivennikov SI, Greten FR, Karin M, Immunity, inflammation, and cancer, Cell 140 (2010) 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S, Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression, Cancer research 67 (2007) 10019–10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sade-Feldman M, Kanterman J, Ish-Shalom E, Elnekave M, Horwitz E, Baniyash M, Tumor necrosis factor-alpha blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation, Immunity 38 (2013) 541–554. [DOI] [PubMed] [Google Scholar]
- [45].Tomic S, Joksimovic B, Bekic M, Vasiljevic M, Milanovic M, Colic M, Vucevic D, Prostaglanin-E2 Potentiates the Suppressive Functions of Human Mononuclear Myeloid-Derived Suppressor Cells and Increases Their Capacity to Expand IL-10- Producing Regulatory T Cell Subsets, Front Immunol 10 (2019) 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Body fatness and weight gain and the risk of cancer. https://www.wcrf.org/dietandcancer/exposures/body-fatness (accessed 28 January 2020).
- [47].Condamine T, Ramachandran I, Youn JI, Gabrilovich DI, Regulation of tumor metastasis by myeloid-derived suppressor cells, Annu Rev Med 66 (2015) 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hale M, Itani F, Buchta CM, Wald G, Bing M, Norian LA, Obesity triggers enhanced MDSC accumulation in murine renal tumors via elevated local production of CCL2, PloS one 10 (2015) e0118784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Thompson KB, Krispinsky LT, Stark RJ, Late immune consequences of combat trauma: a review of trauma-related immune dysfunction and potential therapies, Military Medical Research 6 (2019) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].De AK, Kodys K, Puyana JC, Fudem G, Pellegrini J, Miller-Graziano CL, Only a subset of trauma patients with depressed mitogen responses have true T cell dysfunctions, Clinical immunology and immunopathology 82 (1997) 73–82. [DOI] [PubMed] [Google Scholar]
- [51].Li C, Zhang X, Kang X, Chen C, Guo F, Wang Q, Zhao A, Upregulated TRAIL and Reduced DcR2 Mediate Apoptosis of Decidual PMN-MDSC in Unexplained Recurrent Pregnancy Loss, Front Immunol 11 (2020) 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pan T, Zhong L, Wu S, Cao Y, Yang Q, Cai Z, Cai X, Zhao W, Ma N, Zhang W, Zhang H, Zhou J, 17beta-Oestradiol enhances the expansion and activation of myeloid-derived suppressor cells via signal transducer and activator of transcription (STAT)-3 signalling in human pregnancy, Clinical and experimental immunology 185 (2016) 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang Y, Qu D, Sun J, Zhao L, Wang Q, Shao Q, Kong B, Zhang Y, Qu X, Human trophoblast cells induced MDSCs from peripheral blood CD14(+) myelomonocytic cells via elevated levels of CCL2, Cellular & molecular immunology 13 (2016) 615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rabbani ML, Rogers PA, Role of vascular endothelial growth factor in endometrial vascular events before implantation in rats, Reproduction 122 (2001) 85–90. [DOI] [PubMed] [Google Scholar]
- [55].Horowitz NA, Bettman N, El Wahed AA, Kartz T, Myeloid-Derived Suppressor Cells As Potential Contributors to Pregnancy-Associated Lymphoma Progression, Blood 134 (2019) 3979. [Google Scholar]
- [56].Schwarz J, Scheckenbach V, Kugel H, Spring B, Pagel J, Hartel C, Pauluschke-Frohlich J, Peter A, Poets CF, Gille C, Kostlin N, Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period, Clin Exp Immunol 191 (2018) 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].He YM, Li X, Perego M, Nefedova Y, Kossenkov AV, Jensen EA, Kagan V, Liu YF, Fu SY, Ye QJ, Zhou YH, Wei L, Gabrilovich DI, Zhou J, Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation, Nature medicine 24 (2018) 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kostlin N, Schoetensack C, Schwarz J, Spring B, Marme A, Goelz R, Brodbeck G, Poets CF, Gille C, Granulocytic Myeloid-Derived Suppressor Cells (GR-MDSC) in Breast Milk (BM); GR-MDSC Accumulate in Human BM and Modulate T-Cell and Monocyte Function, Front Immunol 9 (2018) 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu Y, Perego M, Xiao Q, He Y, Fu S, He J, Liu W, Li X, Tang Y, Li X, Yuan W, Zhou W, Wu F, Jia C, Cui Q, Worthen GS, Jensen EA, Gabrilovich DI, Zhou J, Lactoferrin-induced myeloid-derived suppressor cell therapy attenuates pathologic inflammatory conditions in newborn mice, J Clin Invest 129 (2019) 4261–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ, A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair, J Immunol 179 (2007) 4808–4820. [DOI] [PubMed] [Google Scholar]
- [61].Gervassi A, Lejarcegui N, Dross S, Jacobson A, Itaya G, Kidzeru E, Gantt S, Jaspan H, Horton H, Myeloid derived suppressor cells are present at high frequency in neonates and suppress in vitro T cell responses, PloS one 9 (2014) e107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rieber N, Gille C, Kostlin N, Schafer I, Spring B, Ost M, Spieles H, Kugel HA, Pfeiffer M, Heininger V, Alkhaled M, Hector A, Mays L, Kormann M, Zundel S, Fuchs J, Handgretinger R, Poets CF, Hartl D, Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses, Clin Exp Immunol 174 (2013) 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kostlin N, Vogelmann M, Spring B, Schwarz J, Feucht J, Hartel C, Orlikowsky TW, Poets CF, Gille C, Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype, Immunology 152 (2017) 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chou HS, Hsieh CC, Charles R, Wang L, Wagner T, Fung JJ, Qian S, Lu LL, Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells, Transplantation 93 (2012) 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wegner A, Verhagen J, Wraith DC, Myeloid-derived suppressor cells mediate tolerance induction in autoimmune disease, Immunology 151 (2017) 26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY, Myeloid-derived suppressor cells prevent type 1 diabetes in murine models, J Immunol 185 (2010) 5828–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fujii W, Ashihara E, Hirai H, Nagahara H, Kajitani N, Fujioka K, Murakami K, Seno T, Yamamoto A, Ishino H, Kohno M, Maekawa T, Kawahito Y, Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis, J Immunol 191 (2013) 1073–1081. [DOI] [PubMed] [Google Scholar]
- [68].Kurko J, Vida A, Glant TT, Scanzello CR, Katz RS, Nair A, Szekanecz Z, Mikecz K, Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: a pilot study, BMC musculoskeletal disorders 15 (2014) 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Egelston C, Kurko J, Besenyei T, Tryniszewska B, Rauch TA, Glant TT, Mikecz K, Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis, Arthritis Rheum 64 (2012) 3179–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yi H, Guo C, Yu X, Zuo D, Wang XY, Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis, J Immunol 189 (2012) 4295–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hu C, Du W, Zhang X, Wong FS, Wen L, The role of Gr1+ cells after anti-CD20 treatment in type 1 diabetes in nonobese diabetic mice, J Immunol 188 (2012) 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Whitfield-Larry F, Felton J, Buse J, Su MA, Myeloid-derived suppressor cells are increased in frequency but not maximally suppressive in peripheral blood of Type 1 Diabetes Mellitus patients, Clinical immunology 153 (2014) 156–164. [DOI] [PubMed] [Google Scholar]
- [73].Wu H, Zhen Y, Ma Z, Li H, Yu J, Xu ZG, Wang XY, Yi H, Yang YG, Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus, Science translational medicine 8 (2016) 331ra340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zeng QL, Yang B, Sun HQ, Feng GH, Jin L, Zou ZS, Zhang Z, Zhang JY, Wang FS, Myeloid-derived suppressor cells are associated with viral persistence and downregulation of TCR zeta chain expression on CD8(+) T cells in chronic hepatitis C patients, Molecules and cells 37 (2014) 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, Singh KP, Thomas N, Das A, Chen A, Fusai G, Bertoletti A, Cantrell DA, Kennedy PT, Davies NA, Haniffa M, Maini MK, Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells, Nature medicine 21 (2015) 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Huang A, Zhang B, Yan W, Wang B, Wei H, Zhang F, Wu L, Fan K, Guo Y, Myeloid-derived suppressor cells regulate immune response in patients with chronic hepatitis B virus infection through PD-1-induced IL-10, J Immunol 193 (2014) 5461–5469. [DOI] [PubMed] [Google Scholar]
- [77].Mann ER, Menon M, Knight SB, Konkel JE, Jagger C, Shaw TN, Krishnan S, Rattray M, Ustianowski A, Bakerly ND, Dark P, Lord G, Simpson A, Felton T, Ho LP, Feldmann M, Grainger JR, Hussell T, Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19, Sci Immunol 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Miao H, Correction: Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study, Signal Transduct Target Ther 5 (2020) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, Feng J, Jia Q, Song Q, Zhu B, Wang J, Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19, Front Mol Biosci 7 (2020) 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chousterman BG, Swirski FK, Weber GF, Cytokine storm and sepsis disease pathogenesis, Seminars in immunopathology 39 (2017) 517–528. [DOI] [PubMed] [Google Scholar]
- [81].Uhel F, Azzaoui I, Gregoire M, Pangault C, Dulong J, Tadie JM, Gacouin A, Camus C, Cynober L, Fest T, Le Tulzo Y, Roussel M, Tarte K, Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis, American journal of respiratory and critical care medicine 196 (2017) 315–327. [DOI] [PubMed] [Google Scholar]
- [82].Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, Efron PA, the Sepsis CIRCI, Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock, Annals of surgery 265 (2017) 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Brudecki L, Ferguson DA, McCall CE, El Gazzar M, Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response, Infection and immunity 80 (2012) 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Malyshev I, Malyshev Y, Current Concept and Update of the Macrophage Plasticity Concept: Intracellular Mechanisms of Reprogramming and M3 Macrophage “Switch” Phenotype, BioMed research international 2015 (2015) 341308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat Rev Immunol 8 (2008) 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, Sarnaik A, Horna P, Sotomayor E, Gabrilovich DI, Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer, Nature immunology 14 (2013) 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhang C, Wang S, Liu Y, Yang C, Epigenetics in myeloid derived suppressor cells: a sheathed sword towards cancer, Oncotarget 7 (2016) 57452–57463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Condamine T, Mastio J, Gabrilovich DI, Transcriptional regulation of myeloid-derived suppressor cells, Journal of leukocyte biology 98 (2015) 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mohamed E, Cao Y, Rodriguez PC, Endoplasmic reticulum stress regulates tumor growth and anti-tumor immunity: a promising opportunity for cancer immunotherapy, Cancer immunology, immunotherapy : CII 66 (2017) 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Su Y, Qiu Y, Qiu Z, Qu P, MicroRNA networks regulate the differentiation, expansion and suppression function of myeloid-derived suppressor cells in tumor microenvironment, Journal of Cancer 10 (2019) 4350–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Basso D, Gnatta E, Padoan A, Fogar P, Furlanello S, Aita A, Bozzato D, Zambon CF, Arrigoni G, Frasson C, Franchin C, Moz S, Brefort T, Laufer T, Navaglia F, Pedrazzoli S, Basso G, Plebani M, PDAC-derived exosomes enrich the microenvironment in MDSCs in a SMAD4-dependent manner through a new calcium related axis, Oncotarget 8 (2017) 84928–84944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Shang W, Tang Z, Gao Y, Qi H, Su X, Zhang Y, Yang R, LncRNA RNCR3 promotes Chop expression by sponging miR-185–5p during MDSC differentiation, Oncotarget 8 (2017) 111754–111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sido JM, Yang X, Nagarkatti PS, Nagarkatti M, Delta9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8, Journal of leukocyte biology 97 (2015) 677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pan X, Zheng L, Epigenetics in modulating immune functions of stromal and immune cells in the tumor microenvironment, Cellular & molecular immunology 17 (2020) 940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zheng Y, Tian X, Wang T, Xia X, Cao F, Tian J, Xu P, Ma J, Xu H, Wang S, Long noncoding RNA Pvt1 regulates the immunosuppression activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice, Molecular cancer 18 (2019) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tian X, Ma J, Wang T, Tian J, Zheng Y, Peng R, Wang Y, Zhang Y, Mao L, Xu H, Wang S, Long non-coding RNA RUNXOR accelerates MDSC-mediated immunosuppression in lung cancer, BMC cancer 18 (2018) 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tian X, Ma J, Wang T, Tian J, Zhang Y, Mao L, Xu H, Wang S, Long Non-Coding RNA HOXA Transcript Antisense RNA Myeloid-Specific 1-HOXA1 Axis Downregulates the Immunosuppressive Activity of Myeloid-Derived Suppressor Cells in Lung Cancer, Front Immunol 9 (2018) 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Luo Y, Yang J, Yu J, Liu X, Yu C, Hu J, Shi H, Ma X, Long Non-coding RNAs: Emerging Roles in the Immunosuppressive Tumor Microenvironment, Frontiers in oncology 10 (2020) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, Guo X, Yu J, Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer, Oncogene 39 (2020) 4681–4694. [DOI] [PubMed] [Google Scholar]
- [100].Tian X, Shen H, Li Z, Wang T, Wang S, Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor microenvironment, Journal of hematology & oncology 12 (2019) 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang M, Liu Q, Mi S, Liang X, Zhang Z, Su X, Liu J, Chen Y, Wang M, Zhang Y, Guo F, Zhang Z, Yang R, Both miR-17–5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression, J Immunol 186 (2011) 4716–4724. [DOI] [PubMed] [Google Scholar]
- [102].Dai J, Kumbhare A, Youssef D, Yao ZQ, McCall CE, El Gazzar M, Expression of C/EBPbeta in myeloid progenitors during sepsis promotes immunosuppression, Molecular immunology 91 (2017) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chen S, Wang L, Fan J, Ye C, Dominguez D, Zhang Y, Curiel TJ, Fang D, Kuzel TM, Zhang B, Host miR155 promotes tumor growth through a myeloid-derived suppressor cell-dependent mechanism, Cancer research 75 (2015) 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY, Zen K, MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells, J Immunol 192 (2014) 1034–1043. [DOI] [PubMed] [Google Scholar]
- [105].Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sultmann H, Altevogt P, Umansky V, Momma S, Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment, Oncoimmunology 4 (2015) e1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chen S, Huang A, Chen H, Yang Y, Xia F, Jin L, Zhang J, miR-34a inhibits the apoptosis of MDSCs by suppressing the expression of N-myc, Immunology and cell biology 94 (2016) 563–572. [DOI] [PubMed] [Google Scholar]
- [107].Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D, miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice, The Journal of experimental medicine 208 (2011) 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhao JL, Rao DS, Boldin MP, Taganov KD, O’Connell RM, Baltimore D, NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies, Proceedings of the National Academy of Sciences of the United States of America 108 (2011) 9184–9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bruns H, Bottcher M, Qorraj M, Fabri M, Jitschin S, Dindorf J, Busch L, Jitschin R, Mackensen A, Mougiakakos D, CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D, Leukemia 31 (2017) 985–988. [DOI] [PubMed] [Google Scholar]
- [110].Wang J, Yu F, Jia X, Iwanowycz S, Wang Y, Huang S, Ai W, Fan D, MicroRNA-155 deficiency enhances the recruitment and functions of myeloid-derived suppressor cells in tumor microenvironment and promotes solid tumor growth, International journal of cancer 136 (2015) E602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rosa A, Ballarino M, Sorrentino A, Sthandier O, De Angelis FG, Marchioni M, Masella B, Guarini A, Fatica A, Peschle C, Bozzoni I, The interplay between the master transcription factor PU.1 and miR-424 regulates human monocyte/macrophage differentiation, Proceedings of the National Academy of Sciences of the United States of America 104 (2007) 19849–19854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cheng F, Lienlaf M, Wang HW, Perez-Villarroel P, Lee C, Woan K, Rock-Klotz J, Sahakian E, Woods D, Pinilla-Ibarz J, Kalin J, Tao J, Hancock W, Kozikowski A, Seto E, Villagra A, Sotomayor EM, A novel role for histone deacetylase 6 in the regulation of the tolerogenic STAT3/IL-10 pathway in APCs, J Immunol 193 (2014) 2850–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Sasidharan Nair V, Saleh R, Toor SM, Taha RZ, Ahmed AA, Kurer MA, Murshed K, Alajez NM, Abu Nada M, Elkord E, Transcriptomic profiling disclosed the role of DNA methylation and histone modifications in tumor-infiltrating myeloid-derived suppressor cell subsets in colorectal cancer, Clinical epigenetics 12 (2020) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Xin J, Zhang Z, Su X, Wang L, Zhang Y, Yang R, Epigenetic Component p66a Modulates Myeloid-Derived Suppressor Cells by Modifying STAT3, J Immunol 198 (2017) 2712–2720. [DOI] [PubMed] [Google Scholar]
- [115].Gao Y, Shang W, Zhang D, Zhang S, Zhang X, Zhang Y, Yang R, Lnc-C/EBPbeta Modulates Differentiation of MDSCs Through Downregulating IL4i1 With C/EBPbeta LIP and WDR5, Front Immunol 10 (2019) 1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gao Y, Wang T, Li Y, Zhang Y, Yang R, Lnc-chop Promotes Immunosuppressive Function of Myeloid-Derived Suppressor Cells in Tumor and Inflammatory Environments, J Immunol 200 (2018) 2603–2614. [DOI] [PubMed] [Google Scholar]
- [117].Shang W, Gao Y, Tang Z, Zhang Y, Yang R, The Pseudogene Olfr29-ps1 Promotes the Suppressive Function and Differentiation of Monocytic MDSCs, Cancer immunology research 7 (2019) 813–827. [DOI] [PubMed] [Google Scholar]
- [118].Fujisaka Y, Iwata T, Tamai K, Nakamura M, Mochizuki M, Shibuya R, Yamaguchi K, Shimosegawa T, Satoh K, Long non-coding RNA HOTAIR up-regulates chemokine (C-C motif) ligand 2 and promotes proliferation of macrophages and myeloid-derived suppressor cells in hepatocellular carcinoma cell lines, Oncology letters 15 (2018) 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]