Abstract
Background:
Prevention of Clostridioides difficile infection (CDI) is a national priority and may be facilitated by deployment of the Targeted Assessment for Prevention (TAP) Strategy, a quality improvement framework providing a focused approach to infection prevention. This article describes the process and outcomes of TAP Strategy implementation for CDI prevention in a healthcare system.
Methods:
Hospital A was identified based on CDI surveillance data indicating an excess burden of infections above the national goal; hospitals B and C participated as part of systemwide deployment. TAP facility assessments were administered to staff to identify infection control gaps and inform CDI prevention interventions. Retrospective analysis was performed using negative-binomial, interrupted time series (ITS) regression to assess overall effect of targeted CDI prevention efforts. Analysis included hospital-onset, laboratory-identified C. difficile event data for 18 months before and after implementation of the TAP facility assessments.
Results:
The systemwide monthly CDI rate significantly decreased at the intervention (β2, −44%; P = .017), and the postintervention CDI rate trend showed a sustained decrease (β1 + β3; −12% per month; P = .008). At an individual hospital level, the CDI rate trend significantly decreased in the postintervention period at hospital A only (β1 + β3, −26% per month; P = .003).
Conclusions:
This project demonstrates TAP Strategy implementation in a healthcare system, yielding significant decrease in the laboratory-identified C. difficile rate trend in the postintervention period at the system level and in hospital A. This project highlights the potential benefit of directing prevention efforts to facilities with the highest burden of excess infections to more efficiently reduce CDI rates.
Keywords: Targeted Assessment for Prevention Strategy, TAP, healthcare-associated infections, hospital infection prevention and control, quality improvement, cumulative attributable difference, Clostridioides difficile Infection, National Healthcare Safety Network, surveillance data for action, TAP Report, negative-binomial interrupted time series regression, standardized infection ratio, infection prevention interventions, HAI, reduction
Clostridioides difficile infection (CDI) is a prevalent healthcare-associated infection (HAI), with an estimated 450,000 cases associated with 29,000 deaths in the United States in 2011, and it remained one of the most common HAIs as of 2015.1,2 Prevention of CDI is a national priority, and the United States Department of Health and Human Services has established a 2020 reduction goal of 30% for hospital-onset CDI from the 2015 national baseline.3 To facilitate HAI prevention efforts among public health partners across the nation, the Centers for Disease Control and Prevention (CDC) developed the Targeted Assessment for Prevention (TAP) Strategy, a quality improvement framework that provides a focused approach to healthcare infection prevention.4 In addition to CDI, the TAP Strategy, accompanying tools, and the CDC’s technical assistance are also available for the prevention of central line–associated blood-stream infections (CLABSIs) and catheter-associated urinary tract infections (CAUTIs).
The TAP Strategy consists of 3 primary components: (1) targeting healthcare facilities and/or specific units with an excess burden of HAIs, (2) assessing targeted locations to identify gaps in infection prevention policies and practices using standardized assessment tools, and (3) preventing infections by implementing interventions to address identified gaps. Using this methodology, partners in prevention may maximize their resources to reach their HAI reduction goals more efficiently by targeting their efforts to the locations and gaps most in need of improvement.
The Centers for Medicare and Medicaid Services (CMS) Quality Innovation Networks–Quality Improvement Organizations (QIN-QIOs) and the CDC collaborated to pilot test the TAP Strategy in 2015 and 2016. During this piloting process, the CDC developed tools and provided direct technical assistance to the participating QIN-QIOs as they implemented the TAP Strategy among their participating hospitals. The Health Services Advisory Group (HSAG), the QIN-QIO for Arizona, California, Florida, Ohio, and the US Virgin Islands, worked with a 3-hospital system in Florida to prevent hospital-onset CDI.5 This article describes the process and outcomes of CDI TAP Strategy implementation in this healthcare system.
Methods
TAP implementation process
Using data from the National Healthcare Safety Network (NHSN, https://www.cdc.gov/nhsn/index.html), the most widely used HAI surveillance system in the United States, HSAG generated TAP reports to identify hospitals in Florida for participation in deploying the CDI TAP Strategy. The TAP report data reviewed were limited to hospitals that had previously conferred NHSN data rights to HSAG. Previously described by Soe et al,6 NHSN TAP reports utilize the cumulative attributable difference (CAD) metric to calculate the number of infections that must be prevented to reach an HAI reduction goal. TAP reports rank facilities by their CADs, allowing public health partners to prioritize HAI prevention efforts in areas in which the greatest impact may be achieved. HSAG identified hospital A among their Florida target facilities based on their CDI TAP report data.
Hospital A is a 528-bed hospital that combines with hospital B (311 beds) and hospital C (106 beds) to form a 3-hospital healthcare system in northeastern Florida area, which participated in this CDI TAP Strategy implementation project.7 All 3 hospitals are graduate-school affiliated, with at least 1 infection control practitioner; all have intensive care units (ICUs); and none have transplant services (heart, kidney, bone marrow) or burn units. Hospitals A and B have oncology units and provide chemotherapy, and hospital A has a cardiac ICU. The healthcare system reported that implementation of infection prevention policies and antimicrobial stewardship programs occur at the system level.
HSAG administered the CDI TAP facility assessments within this healthcare system. Created by CDC as a standardized method for assessing hospitals for gaps related to their CDI prevention policies and practices, the CDI TAP facility assessments capture awareness and perceptions among frontline, mid-level, and leadership personnel across the hospital.4 Available on the CDC’s TAP website (https://www.cdc.gov/hai/prevent/tap.html), the assessment consists of 5 domains: I. General Infrastructure, Capacity, and Processes; II. Antibiotic Stewardship; III. Early Detection and Isolation, Appropriate Testing; IV. Contact Precautions/Hand Hygiene; V. Environmental Cleaning. The assessment facilitates the targeting of prevention efforts to areas of greatest need.
Healthcare personnel from the 3 hospitals completed the assessments in July and August 2015. As part of technical assistance and partner support, completed assessments were sent to the CDC for data entry and summarization, and results were returned to HSAG in October 2015 to share with the participating hospitals. HSAG and the healthcare system worked together to prioritize their opportunities for improvement based on the CDI TAP facility assessment results. The healthcare system then implemented CDI prevention interventions specific to the priority areas identified.
Outcomes of TAP implementation
Data source and analysis
The analysis performed by CDC was based on laboratory-identified C. difficile (CDI LabID) event surveillance data reported from participating facilities in NHSN in accordance with the CMS reporting mandate (https://www.cdc.gov/nhsn/cms/index.html). Antibiotic use data were provided by the pharmacy system used by the 3 hospitals.
A retrospective analysis was performed to assess overall effect of targeted CDI prevention efforts by examining standardized infection ratios (SIRs) and CADs of hospital-onset CDI LabID events at the healthcare system and hospital levels for 18 months before and after the intervention. The intervention was defined as the implementation of the TAP facility assessments because completion of the assessments serves as the first engagement action among staff and may itself be an educational intervention that results in improved infection prevention practices. In addition, the quality improvement nature of this project and the retrospective time frame of the analysis limited the ability to define more optimal pre- and postintervention periods. Hospital C completed the assessments in July 2015, and hospitals A and B completed the assessments in August 2015.
To evaluate the trends of CDI LabID in both the pre- and postintervention periods, monthly incidence rates of hospital-onset CDI LabID events were analyzed using negative-binomial, interrupted time series (ITS) regression models. ITS models were developed separately at the system and individual hospital levels. The ITS approach is more informative and rigorous than a before-and-after design because it allows for comparison and quantification of pre- and postintervention trends (as opposed to a comparison of simple aggregated rates in the before-and-after model).8,9 To assess the true impact of the intervention and to ensure that increases or decreases in rate trend that preceded the intervention were properly accounted for in the analysis, parameter estimates generated by the ITS model provide the following 4 key pieces of information: (1) the preintervention rate trend (β1), (2) the rate change immediately after the intervention start (β2), (3) the difference between preintervention and postintervention rate trends (change in slope direction) (β3), and (4) the rate trend in the postintervention period (β1 + β3). Incidence rate ratio and percent change were also generated for each of these effects. When regression was modeled at the system level, interactions were tested between each hospital and β1, β2, and β3 to examine the validity of pooling data across multiple facilities.
Due to the nature of longitudinal data, possible interactions between covariates and time were tested for significance and adjusted for confounders as necessary. The following covariates were considered potential confounders in the ITS models: (1) defined daily dose (DDD) of ‘total’ antibiotics per 1,000 patient days by quarter (ie, ‘total’ DDD comprised the combined dose of quinolones, lincosamide, and third and fourth generation cephalosporins, assessed due to presence of interaction between time and total DDD, as some antibiotic stewardship efforts were reported prior to project period), (2) CDI test type (PCR-NAAT, EIA, others), and (3) monthly community-onset C. difficile prevalence rate (ie, community-onset C. difficile laboratory-identified event divided by the number of admissions to the hospital, as a percentage). Variables were retained in the models based on significance. Descriptions of analytical variables, except DDD, are available in the CDC NHSN protocol.10
Model diagnostics
Model fit statistics and residual graphs were examined for any influential points (high leverage and/or outlier). Because the analysis involved longitudinal data for multiple facilities, potential clustering was considered in 2 ways: (1) within-hospital correlation of errors over time and (2) specification of a random intercept (ie, to assess variation between facilities in baseline CDI LabID rates). Covariance tests were conducted to obtain statistical inferences for covariance parameters,11 and no evidence of clustering was found in either method. Statistical significance was defined as P ≤ .05. Data were analyzed and plotted using SAS version 9.4 software (SAS Institute, Cary, NC). This project was exempt from institutional review board review due to the quality improvement framework and the use of aggregate surveillance data previously reported by the hospitals.
Results
TAP implementation process
Among the Florida hospitals working with HSAG, hospital A was identified for participation with the second highest CAD value on the NHSN TAP report generated for CDI data reported from August 2014 through June 2015 (source: NHSN data). Although hospitals B and C were not identified for targeted outreach based on their CADs, they were offered participation in this project as a programmatic decision to deploy the TAP Strategy at a healthcare-system level.
The CDI TAP facility assessments were completed by 580 staff across the 3 participating hospitals. Most respondents were nurses or nurse assistants (n = 392, 68%), followed by patient care technicians, associate care providers, or other technicians (n = 107, 18%), and physicians, physician assistants, or nurse practitioners (n = 42, 7%). Select questions and corresponding frequencies of responses are presented in Table 1. Based on these data and contextual factors within the healthcare system, top priority areas for improvement were early detection and isolation of CDI patients, C. difficile testing practices, and antibiotic stewardship.
Table 1.
Clostridioides difficile Infection (CDI) Targeted Assessment for Prevention (TAP) Facility Assessment Frequency of Responses From Select Questionsa, Healthcare System TAP Strategy Implementation for CDI Prevention
| Question | % Never | % Rarely | % Sometimes | % Often | % Always | % Unknown |
|---|---|---|---|---|---|---|
| Early Detection and Isolation, Appropriate Testing | ||||||
| Are patients with diarrhea of other known causes tested for CDI? | 1 | 5 | 24 | 27 | 19 | 24 |
| Are patients without diarrhea tested for CDI? | 18 | 33 | 20 | 3 | 2 | 25 |
| Are patients tested for CDI cure? | 10 | 8 | 12 | 9 | 7 | 54 |
| Does your facility allow nurses to order C. difficile testing on patients with suspected CDI without a physician order (eg, through a nurse-driven protocol or standing order)? | 21 | 4 | 4 | 11 | 20 | 41 |
| Antibiotic Stewardship | ||||||
| Do ordering providers document in the medical record or during order entry a dose, duration, and indication for all antimicrobials at your facility? | 0 | 2 | 9 | 24 | 31 | 35 |
| In your facility, is it routine practice for specified antimicrobial agents to be approved by a physician or pharmacist at or soon after prescription (eg, preauthorization)? | 1 | 2 | 3 | 17 | 36 | 42 |
| Does your facility have a formal procedure for all ordering providers to review the appropriateness of all antibiotics at or after 48 h from the initial orders (eg, antibiotic time-out, postprescription review)? | 1 | 2 | 3 | 12 | 30 | 52 |
| Does your facility review current antibiotics for appropriateness in patients with new or recent CDI diagnosis? | 0 | 1 | 4 | 15 | 36 | 43 |
| Does your facility monitor antibiotic use (consumption) at the unit and/or facility level? | 0 | 1 | 4 | 14 | 37 | 43 |
Select questions identified based on the following response frequency thresholds: >33% ‘Unknown’ or > 50% unfavorable responses (ie, sum of ‘Never,’ ‘Rarely,’ ‘Sometimes,’ and ‘Unknown’ or sum of ‘Sometimes,’ ‘Often,’ ‘Always,’ and ‘Unknown’ based on question directionality).
As part of their ongoing CDI prevention efforts and in response to the CDI TAP facility assessment results, the healthcare system reported implementing a variety of interventions from August 2015 to August 2016 to target these priority areas (Fig. 1). For example, to address early detection and isolation of CDI patients and C. difficile testing practices, the healthcare system provided education to personnel, updated their CDI testing policy, implemented a CDI testing algorithm for nurses, and established a C. difficile testing audit tool for laboratory personnel. In addition, this healthcare system reported updating their electronic medical records to include a criteria-for-use C. difficile order form, requiring providers to confirm appropriate criteria were met prior to the test order. To improve antibiotic stewardship, interventions included physician education and implementation of an electronic criteria-for-use order form for fluoroquinolones, which required prescribers to select an appropriate indication upon order.
Fig. 1.
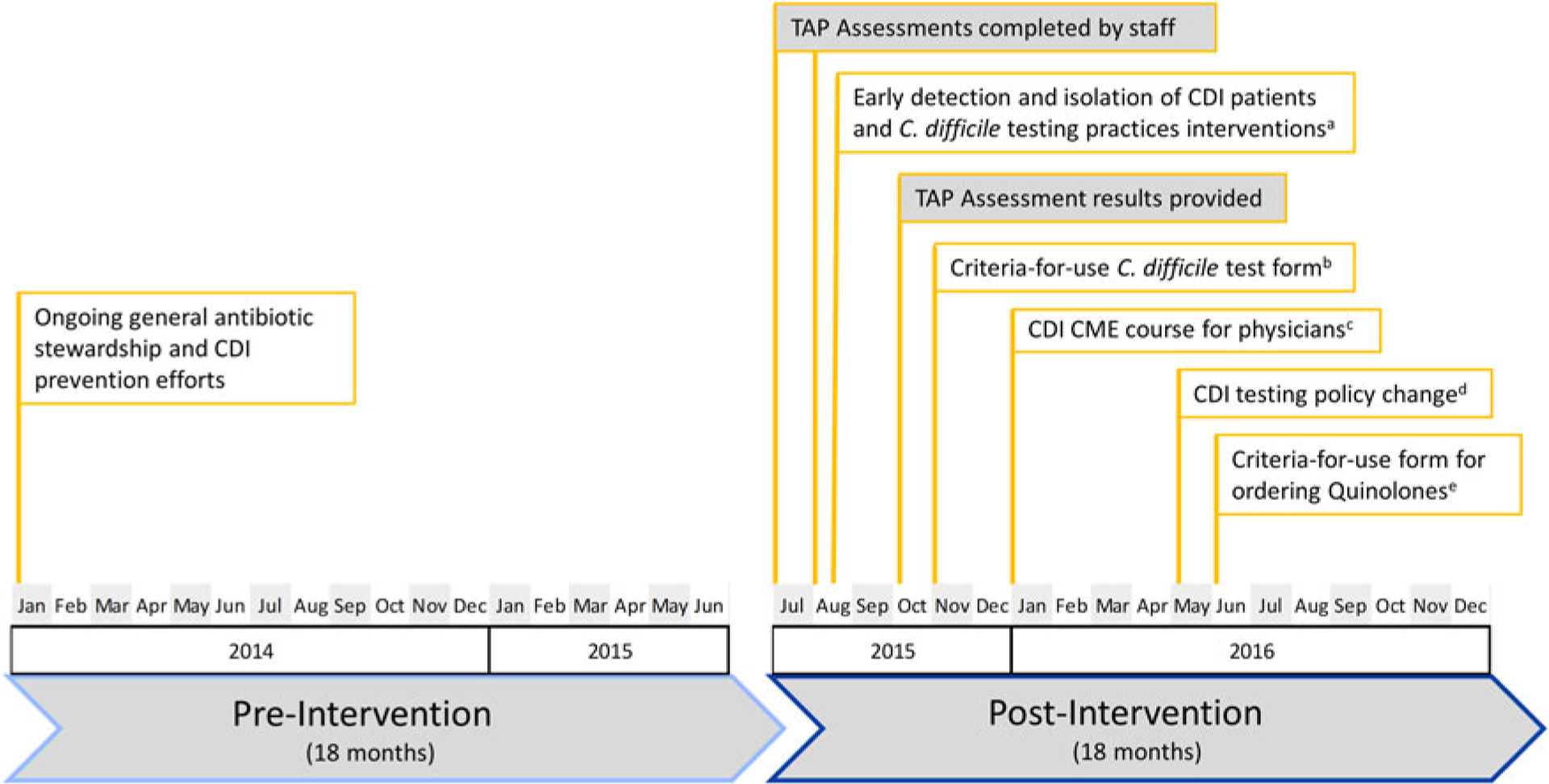
Timeline of Clostridioides difficile infection (CDI) Targeted Assessment for Prevention (TAP) Strategy implementation and related prevention activities in participating facilities, healthcare system TAP Strategy implementation for CDI prevention.
aTo address early detection and isolation of CDI patients and C. difficile testing practices, the healthcare system provided education to nurses and physicians regarding appropriate testing practices, implemented a CDI testing algorithm for nurses to guide appropriate specimen collection and implementation of contact precautions, and established a C. difficile testing audit tool for laboratory personnel to confirm specimen was appropriate for testing or rejection.
bThe healthcare system reported updating their electronic medical records to include a criteria-for-use C. difficile order form, requiring ordering providers to confirm appropriate criteria were met prior to the test order.
cThe healthcare system provided an in-person, infectious disease physician–led continuing medical education (CME) course for physicians focusing on CDI prevention, including appropriate testing practices and antibiotic stewardship.
dThe healthcare system reported updating their CDI testing policy to include an order cancellation for specimens not collected within 24 hours.
eThe healthcare system reported implementing an electronic criteria-for-use order form for fluoroquinolones. This order form required prescribers to select an appropriate reason for ordering the respective antibiotics, and initiated an auditing process if an order was placed in the absence of an appropriate selection.
Outcomes of TAP implementation
Participating facilities reported all 36 months of data to NHSN during the project period.
Unadjusted rates of continuous variables
Systemwide pooled mean hospital-onset CDI LabID rates decreased by 53.8% (95% CI, 43.5%–62.2%) from 1.14 per 1,000 patient days in the preintervention period to 0.53 in the postintervention period. Monthly CDI LabID rates were generally higher prior to the intervention, and they appeared to decrease over time in the intervention period, particularly at the system level and at hospital A, which had originally been identified with the highest CAD among the 3 healthcare system hospitals (Fig. 2). The pooled mean community-onset C. difficile prevalence at the system level decreased from 0.92% during preintervention period (range of monthly prevalence, 0%–3.04%) to 0.40% during postintervention period (range of monthly prevalence, 0.08%–1.76%). Systemwide DDD of ‘total’ antibiotics per 1,000 patient days was 1,247.7 in the first quarter of the preintervention period (2014, Q1) and declined to 543.4 in the last quarter of postintervention period (2016, Q4) with a brief period of uptick in the last quarter of 2015. This finding resulted in treating the inverse of DDD as a predictor in the regression models to linearize the association with CDI LabID rate.
Fig. 2.
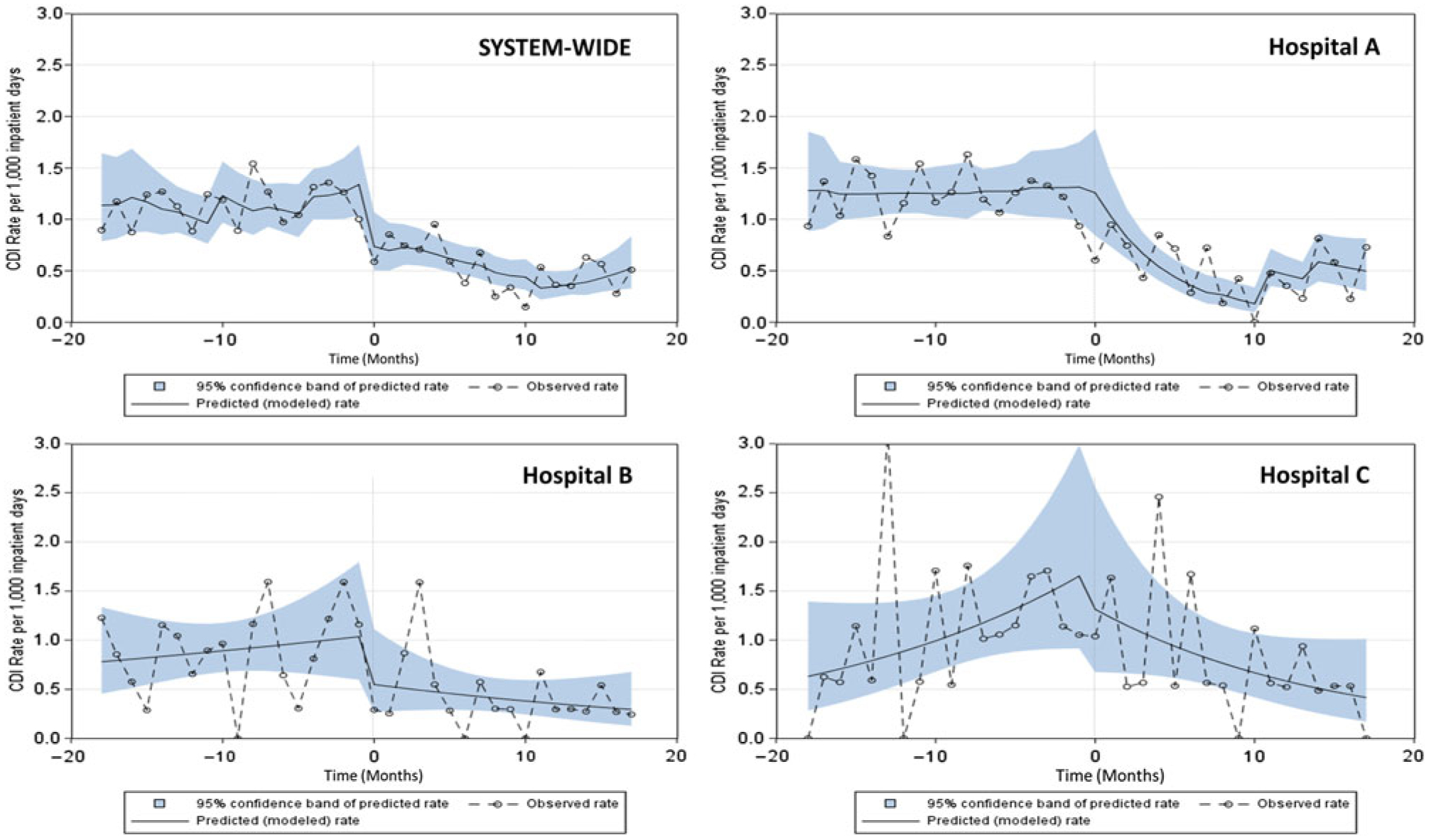
Healthcare system TAP Strategy implementation for CDI prevention. Observed and predicted (modeled) incidence rates of hospital-onset C. difficile laboratory-identified event (CDI) before and after intervention at the system level and by hospital. Predicted rates were estimated from final predictive models. Time=0 indicates the beginning of the intervention period (ie, completion of Targeted Assessment for Prevention [TAP] facility assessments). The pre- and postintervention periods each lasted for 18 months.
SIR and CAD during pre- and postintervention periods
Although not statistically significant, the SIR and CAD aggregated for the preintervention period were higher than that of postintervention period at the system level (ie, SIR decreased from 1.0 to 0.87; CAD decreased from 74 to 41) and in hospital A (ie, SIR decreased from 1.03 to 0.84; CAD decreased from 58 to 23) (Table 2). The remaining 2 facilities showed similar SIR and CAD values between the pre- and postintervention periods (Table 2).
Table 2.
Targeted Assessment for Prevention (TAP) Report Hospital-Onset C. difficile Laboratory-Identified Event (CDI) Data at Healthcare System Level and by Hospital, Healthcare System TAP Strategy Implementation for Clostridioides difficile Infection (CDI) Prevention
| Setting | Intervention Period (18 mo each) | Hospital- Onset CDI | Predicted No. | No. of Patient Days | Cumulative Attributable Difference ‘CAD’ (SIR goal = 0.7) | Standardized Infection Ratio ‘SIR’ | P Valuea |
|---|---|---|---|---|---|---|---|
| Healthcare system (all 3 hospitals) | Pre | 249 | 249.962 | 267,827 | 74.03 | 1.00 | .15 |
| Post | 209 | 239.771 | 263,940 | 41.16 | 0.87 | ||
| Hospital A | Pre | 180 | 174.496 | 178,106 | 57.85 | 1.03 | .07 |
| Post | 138 | 164.069 | 165,296 | 23.15 | 0.84 | ||
| Hospital B | Pre | 37 | 45.937 | 57,925 | 4.84 | 0.81 | .91 |
| Post | 43 | 51.963 | 65,111 | 6.63 | 0.83 | ||
| Hospital C | Pre | 32 | 29.529 | 31,796 | 11.33 | 1.08 | .74 |
| Post | 28 | 23.739 | 33,533 | 11.38 | 1.18 |
Two-sided mid-P method was used for statistical comparison of standardized infection ratios (SIRs) between pre- and post-intervention period. (Reference: Statistical tool SAS macro for comparing 2 SIRs. Available at https://www.cdc.gov/nhsn/ps-analysis-resources/index.html.)
ITS model estimates
Incidence rate ratios and percent change of CDI LabID rates for the ITS models are shown in Table 3. Monthly observed and predicted (modeled) CDI LabID incidence rates are shown in Fig. 2. As the systemwide model showed no significant interaction between hospital and β1, β2, and β3, hospital data were pooled for further analysis. The monthly hospital-onset CDI LabID rate was increasing during the preintervention period (β1, +25% per month, P = .002) (Table 3; Fig. 2). There was an initial significant decrease in monthly CDI LabID rates (β2, −44%; P = .017) at the start of the intervention, and the postintervention CDI LabID rate trend also showed a sustained decrease (β1 + β3, −12% per month; P = .008) (Table 3 and Fig. 2).
Table 3.
Summary of Coefficients, Incidence Rate Ratios, and Percent Changes in Monthly Incidence Rate of Hospital-Onset C. difficile Laboratory-Identified Event (CDI), Healthcare System Targeted Assessment for Prevention (TAP) Strategy Implementation for CDI Prevention Note. IRR, incidence rate ratio; NAAT, PCR-based nucleic acid amplification test; DDD, defined daily dose of ‘total’ select antibiotics (ie, combined dose of quinolones, lincosamide, third- and fourth-generation cephalosporins) per 1,000 patient days.
| Setting & Final Modela | Effect | Coefficient | IRR (95% CI) | % Change (95% CI)b | P Value |
|---|---|---|---|---|---|
| Healthcare System (All 3 Hospitals) Model-1c | Preintervention trend (β1) | 0.2267 | 1.25 (1.09–1.44) | 25 (9 to 44) | .0017 |
| Immediate effect of intervention (β2) | −0.5848 | 0.56 (0.35–0.90) | −44 (−66 to −10) | .0173 | |
| Change in pre- to postintervention slope (change in slope direction) (β3) | −0.3585 | 0.70 (0.57–0.85) | −30 (−43 to −15) | .0005 | |
| Postintervention trend (β1+ β3) | −0.1318 | 0.88 (0.80–0.96) | −12 (−20 to −4) | .0076 | |
| Hospital A Model-2d | Preintervention trend (β1) | 0.0005 | 1.00 (0.97–1.03) | 0 (−3 to 3) | .9757 |
| Immediate effect of intervention (β2) | 0.1611 | 1.17 (0.67–2.06) | 17 (−33 to 106) | .5618 | |
| Change in pre- to postintervention slope (change in slope direction) (β3) | −0.2991 | 0.74 (0.64–0.86) | −26 (−36 to −14) | .0002 | |
| Postintervention trend (β1 + β3) | −0.2987 | 0.74 (0.64–0.86) | −26 (−36 to −14) | .0003 | |
| Hospital B Model-3e | Preintervention trend (β1) | 0.0167 | 1.02 (0.96–1.07) | 2 (−4 to 7) | .5435 |
| Immediate effect of intervention (β2) | −0.5981 | 0.55 (0.21–1.42) | −45 (−79 to 42) | .2074 | |
| Change in pre- to postintervention slope (change in slope direction) (β3) | −0.0530 | 0.95 (0.86–1.04) | −5 (−14 to 4) | .2617 | |
| Postintervention trend (β1 + β3) | −0.0364 | 0.96 (0.89–1.04) | −4 (−11 to 4) | .3414 | |
| Hospital C Model-4e | Preintervention trend (β1) | 0.0567 | 1.06 (0.99–1.14) | 6 (−1 to 14) | .1085 |
| Immediate effect of intervention (β2) | −0.1621 | 0.85 (0.33–2.18) | −15 (−67 to 118) | .7277 | |
| Change in pre- to postintervention slope (change in slope direction) (β3) | −0.1244 | 0.88 (0.80–0.98) | −12 (−20 to −2) | .0217 | |
| Postintervention trend (β1 + β3) | −0.0677 | 0.93 (0.86–1.01) | −7 (−14 to 1) | .0878 |
Variables were retained in the models based on significance.
Percent change = (RR − 1) × 100.
Final predictive model 1: ln(λ) = β0 + β1(month) + β2(intervention) + β3 (month since intervention) + CDI test type (NAAT vs others) + (1/DDD) + (month*1/DDD) + (intervention month*1/DDD);
Model 2: ln(λ) = β0 + β1(month) + β2(intervention) + β3 (month since intervention) + (1/DDD) + (intervention month*1/DDD);
Model 3 and model 4: ln(λ) = β0 + β1(month) + β2(intervention) + β3 (month since intervention); n=36 months; offset = ln(no. inpatient days); λ = no. of CDIs.
For hospital A, there was no change in CDI LabID rate during the preintervention period and at the start of the intervention (Table 3 and Fig. 2); however, there was significant decrease in the CDI LabID rate trend during the postintervention period (β1 + β3, −26% per month, P = .003) (Table 3; Fig. 2). For hospital B, there was no significant change in CDI LabID rate over the entire project period (Table 3; Fig. 2). For hospital C, there was no significant change in CDI LabID rate during the preintervention and postintervention periods (Table 3; Fig. 2). However, as indicated by significant change (P = .02) in the direction of CDI LabID rate trend (β3), the increase in the monthly CDI LabID rate trend for hospital C was offset by the decreasing rate trend in the postintervention period (Table 3; Fig. 2).
Discussion
Through a collaborative partnership with CDC, HSAG, and this healthcare system, implementation of the TAP Strategy as a quality improvement framework was demonstrated in 3 participating hospitals. This project included the use of data for action to target facilities for participation and to direct prevention efforts to where they may have the greatest impact, systematic assessment of CDI prevention policies and practices, and the implementation of focused interventions to address specific gaps identified. At the system level, the CDI LabID rate decreased immediately after deployment of the CDI TAP facility assessments, possibly because of a combination of staff engagement, staff education about CDI prevention through assessment completion, heightened awareness of CDI-specific prevention efforts, and ongoing prevention activities. This decrease in trend was sustained in the postintervention period at the system level, which may be attributed to the immediate effect of TAP facility assessment deployment, implementation of the targeted interventions to address identified gaps, and ongoing prevention activities within the facilities. Notably, the healthcare system has reported continued success in maintaining the prevention activities initiated and decreased infection rates.
Although no significant change in the CDI LabID rate occurred in hospitals B and C, there was a significant decrease in the CDI LabID rate trend in the postintervention period in hospital A, the hospital originally identified in the CDI TAP report. This finding highlights the potential benefit of directing prevention efforts to facilities with the highest burden of excess infections; preventing those infections will more efficiently reduce the CDI rates at the system, group, state, and national levels. These findings align with the TAP Strategy methodology described by Soe et al6 and the use of the CAD metric to systematically prioritize prevention efforts to facilities that may have a greater impact on reaching overall HAI reduction goals.
There are several limitations regarding evaluation of TAP Strategy implementation. First, the TAP Strategy was designed as a quality improvement framework and was implemented among these hospitals with the goal of infection prevention, not with the purpose of studying the impact of this strategy. This was a retrospective review of the implementation process and analysis of associated data, which limited the ability to control for factors that may have influenced the results. These factors include potential varying degrees of engagement and intervention execution across the facilities, heterogeneity of additional and ongoing prevention activities across the facilities, lack of a control group, inability to establish more optimal pre- and postintervention project periods, and lack of patient-level information to confirm clinical diagnosis for each CDI LabID event. As outlined in Fig. 1, the healthcare system implemented some interventions prior to and immediately after deployment of the TAP facility assessments, but before they received the summary results of these assessments. This suggests that some prevention activities were either ongoing or in the planning stages prior to TAP Strategy deployment. As such, observed impact may be attributed to a cumulative effect of the TAP Strategy, additional CDI prevention activities, as well as ongoing and general infection prevention efforts at the system or hospital level that may influence CDI rates. Additional limitations specific to the statistical analysis include wide variations in monthly CDI LabID rates, use of different time intervals of measurement (monthly data of CDI rate and community-onset CDI prevalence, quarterly data of DDD and CDI test type), and availability of only aggregate hospital-level CDI LabID event data. These NHSN data are used as the standard national surveillance measurement for CDI; however, these data limit the ability to determine whether changes in the measure over time reflect reduced CDI incidence or C. difficile transmission versus changes in testing practices and other factors that might help minimize inappropriate testing.
This pilot project has demonstrated that a reduction in CDI LabID events is possible with implementation of the TAP Strategy and can serve as a model of coordinated and targeted prevention efforts. This model may be applied to other HAIs, as well as implemented by other partners across the continuum of prevention. Implementation may range from a single unit within a facility to areas of state and national deployment. As implementation processes may vary, the TAP Strategy is modifiable and scalable, allowing partners to adapt this quality improvement framework to align with their prevention priorities and goals. Facilities and healthcare systems should consider implementing the TAP Strategy, in addition to their ongoing prevention efforts, to improve processes and outcomes as they work toward the national goal of HAI reduction and elimination.
Acknowledgments.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support.
No financial support was provided relevant to this article.
Footnotes
Conflicts of interest. All authors report no conflicts of interest relevant to this article.
References
- 1.Lessa F, Mu Y, Bamberg W, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill S, O’Leary E, Janelle S, et al. Changes in prevalence of healthcare-associated infections in US hospitals. N Engl J Med 2018;379:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Targets and Metrics. Office of Disease Prevention and Health Promotion website. https://health.gov/hcq/prevent-hai-measures.asp. Published 2016. Accessed March 5, 2019.
- 4.The Targeted Assessment for Prevention (TAP) Strategy. Centers for Disease Control and Prevention website. https://www.cdc.gov/hai/prevent/tap.html. Published 2015. Accessed March 8, 2019.
- 5.What We Do. Health Services Advisory Group (HSAG) website. https://www.hsag.com/en/about/what-we-do-services/. Published 2014. Accessed March 5, 2019.
- 6.Soe M, Gould C, Pollock D, Edwards J. Targeted assessment for prevention of healthcare-associated infections: a new prioritization metric. Infect Control Hosp Epidemiol 2015;36:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St. Vincent’s Healthcare. Ascension website. https://www.jaxhealth.com/. Published 2007. Accessed March 5, 2019.
- 8.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 9.Gebski V, Ellingson K, Edwards J, Jernigan J, Kleinbaum D. Modelling interrupted time series to evaluate prevention and control of infection in healthcare. Epidemiol Infect 2012;140:2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Multidrug-resistant organism and Clostridioides difficile infection (MDRO/CDI) module. Centers for Disease Control and Prevention website. http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Published 2019. Accessed March 17, 2019.
- 11.SAS/STAT 9.2 User’s Guide, second edition. COVTEST Statement. SAS website. https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_glimmix_sect001.htm. Published 2009. Accessed March 17, 2019. [Google Scholar]


