Abstract
Background:
Carfilzomib, a next generation proteasome inhibitor, improves outcomes in patients with multiple myeloma (MM); however, a proportion of those treated develop renal failure due to adverse event, comorbidity, or myeloma progression. The rate of renal failure and associated risk factors remains unknown in real-world populations.
Patients and Methods:
Adults with relapsed/refractory MM who received carfilzomib between the years 2013-2016 were identified in the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked databases. Renal failure was defined using the corresponding International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) diagnostic codes and procedure codes for dialysis. Patients with a pre-existing diagnosis of renal failure were excluded to distinguish an adverse event from co-morbidity. Multivariate cox regression analysis was performed to identify the variables independently associated with the development of renal failure among MM patients utilizing carfilzomib.
Results:
1950 patients were included in the analysis. Renal failure developed in 22% of patients during the study period. The median time to development of renal failure from first carfilzomib administration was 1.6 months (range <0.1-23.3). Increasing age (adjusted hazard ratio [aHR] 1.01 per year, p=0.018), pre-existing heart failure (aHR 1.50, p=0.005), and pre-existing chronic kidney disease (aHR 2.00, p<0.001) were associated with a higher risk of developing renal failure.
Conclusion:
Renal failure occurred in up to 22% of patients on carfilzomib therapy. The exact cause and mechanism of renal failure cannot be determined from our study and may be multifactorial. Future studies are needed to further understand the cause of renal failure among patients on carfilzomib and devise strategies to mitigate the risk.
Keywords: renal failure, aged, hematological malignancy, multiple myeloma
Introduction
Multiple myeloma is an incurable hematological malignancy, which has seen tremendous progress in therapeutic agents over the past decade. Among them, carfilzomib, a next generation proteasome inhibitor, is now widely used among patients with relapsed/refractory multiple myeloma (MM) based upon pivotal landmark trials which have shown to improve outcomes for patients with MM including overall survival.[1,2]
While the increased efficacy of carfilzomib has led to important gains for patient with MM, carfilzomib is also associated with a unique toxicity profile, including cardiac, pulmonary and renal adverse events. Rates of cardiac and pulmonary toxicity have been characterized in randomized controlled trials [3-5] as well as among real-world patients.[6] However, there is a paucity of data regarding renal adverse events with carfilzomib use. Early case reports of renal toxicity with carfilzomib hypothesized many potential mechanisms including tumor lysis, cardiorenal syndrome and endothelial damage including thrombotic microangiopathy.[7-11] Renal failure could also be multifactorial due MM itself or other comorbidities, making the etiology of these events unclear. Ball et al. performed a systematic review and meta-analysis of randomized controlled trials of carfilzomib containing regimens and demonstrated that the cumulative rate of grade 3-5 renal adverse events was 8.3% with a pooled incidence ratio of 1.66 in the carfilzomib arm compared to the control arm.[12] In this analysis acute kidney injury was the most common adverse event.
Given that as many as 70% of real-world patients would not have met the stringent eligibility criteria for hallmark randomized controlled trials containing carfilzomib-based regimens, [13] there is a need understand the frequency of adverse events including renal failure among real-world patients using carfilzomib. Additionally, understanding risk factors associated with the development of renal failure is important in order to identify groups at highest risk of this adverse event and subsequently evaluate future strategies/interventions aimed at minimizing this risk.
To fulfill the above knowledge gaps, we used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked administrative database to estimate the frequency of renal failure among MM patients on carfilzomib therapy. We also aimed to investigate factors associated with an increased risk of developing renal failure while on carfilzomib therapy.
Methods
Data Source
The data source for this study was the SEER-Medicare linked database which provides cancer registry data from 18 geographic areas covering approximately 28% of the US population and is linked to billing claims for Medicare beneficiaries.[14] This linked database contains information regarding patient demographics, tumour characteristics and survival for those with a cancer diagnosis who reside in the coverage area. This data source broadly represents the health care experience of patients in the United States diagnosed with cancer and who are insured through traditional fee-for-service Medicare plans. This study was performed under a protocol approved by the Washington University School of Medicine Human Subject Committee.
Participants
Using the SEER-Medicare linked database, we identified adults (age >18) with MM (International Classification of Disease for Oncology [third edition] code 9732) receiving carfilzomib for relapsed or refractory myeloma from 2013 to 2016. Carfilzomib become commercially available in the United States in early 2013 and, at time of analysis, the SEER-Medicare database included claims through 2016. The Healthcare Commons Procedure Coding Systems (HCPCS) codes for carfilzomib (codes C9295 and J9047) were used to identify patients who had received carfilzomib at any time during their disease course in the study period. Carfilzomib that was provided via a clinical trial, and not billed to Medicare, was not available in the dataset. Patients who began carfilzomib within 6 months of MM diagnosis were excluded in attempt to create a more homogenous cohort of patients with relapsed or refractory MM, the population carfilzomib was indicated for upon its initial approval. Patients may have been receiving carfilzomib as a single-agent or in combination with dexamethasone, immunomodulatory drugs, or other treatments for MM. Interruption in carfilzomib-based treatment of >60 days was considered to be a change in treatment strategy and only data from the first carfilzomib regimen was included in the analysis.
The International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) was used to identify all the codes for renal failure and dialysis that have been previously reported in peer-reviewed literature with acceptable negative and positive predictive value[15] (Supplementary Table 1). Renal failure codes present within the 90 days prior to the initiation of carfilzomib were considered to be pre-existing conditions and those patients were excluded. Renal failure was defined from the first carfilzomib dose through 28 days after the last dose. Treatment details including year of carfilzomib administration and time from carfilzomib initiation to renal failure were recorded. Baseline co-morbidities present within 90 days prior to carfilzomib were also captured for hypertension, diabetes, congestive heart failure and chronic kidney disease and were defined using ICD-9/10 codes.
Statistical Analysis
Patient baseline demographic factors were summarized using measures of central tendency and proportions. The frequency of renal failure was reported using descriptive statistics. A multivariate cox regression, with participants censored 28-days after discontinuation of carfilzomib, was conducted to assess the association of baseline factors with the development of renal failure. P-values less than 0.05 were considered significant. All analyses were performed using SAS Enterprise Guide 5.1.
Results
A total of 2369 patients treated with carfilzomib for relapsed or refractory MM were identified between the years 2013-2016. There were 419 patients who had a history of renal failure prior to carfilzomib administration and were excluded, leaving 1950 evaluable patients. Baseline characteristics of the included patients are outlined in Table 1. The median age of the evaluable patients was 70 (range 30-94) and 55% were males. The majority of patients were white; 20% of patients were African American or belonged to another race. Among the evaluated co-morbidities, hypertension was most common, present in 55% of the cohort, and heart failure was the least common, in 10% of the cohort. The median time from MM diagnosis to the initiation of carfilzomib was 40 months (range 6-170). The median duration of treatment with carfilzomib was 3 months (range <1-40). The estimated median overall survival from initiation of carfilzomib was 17 months (95% CI 16-18).
Table 1:
Baseline characteristics of selected 1950 adults with multiple myeloma who received carfilzomib
| Demographics | (N=1950) |
|---|---|
| Age, years (median, range) | 70 (30-94) |
| Gender (N, %) | |
| Female | 881 (45) |
| Male | 1069 (55) |
| Ethnicity (N, %) | |
| Caucasian | 1567 (80) |
| Black | 275 (14) |
| Other | 108 (6) |
| Comorbidity/Performance Status Indicators | |
| Pre-existing co-morbidities (N, %) | |
| Hypertension | 1078 (55) |
| Diabetes | 421 (22) |
| Heart Failure | 202 (10) |
| Chronic Renal Failure | 425 (22) |
| Treatment characteristics | |
| Time from diagnosis to carfilzomib, months (median, range) | 40 (6-170) |
| Year of Carfilzomib usage (N, %) | |
| 2013 | 278 (15) |
| 2014 | 512 (26) |
| 2015 | 641 (33) |
| 2016 | 519 (27) |
| Duration of carfilzomib usage, months (median, range) | 3 ( <1- 40) |
| Overall Survival, months (median, 95% confidence interval)* | 17 (95% CI 16-18) |
Calculated from the time of initiation of carfilzomib
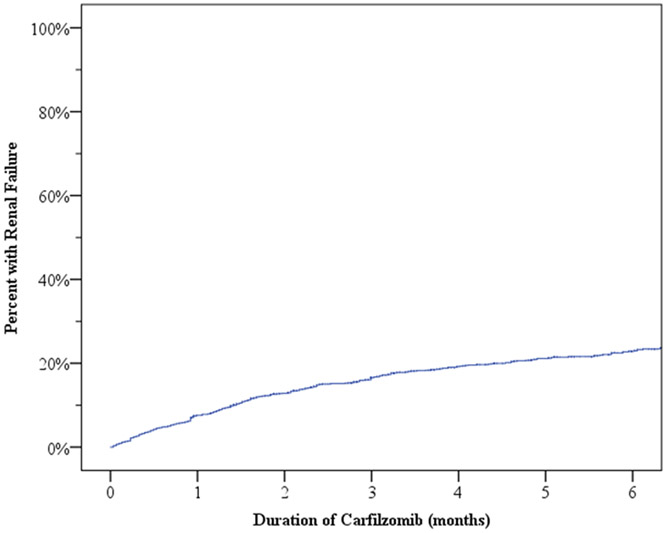
In total, approximately 22% (425/1950) of patients treated with carfilzomib during the study period had ICD-9/10 codes indicative of a new renal failure. The median time to development of renal failure from the time of carfilzomib initiation was 1.6 months (range <0.1-23.3). Cumulative incidence of renal failure during the first six months is shown in Figure 1. The incidence of renal failure was highest during the initial three months and then stabilized. Factors significantly associated with increased risk of renal failure are summarized in Table 2. Increasing age (adjusted hazard ratio [aHR] 1.01 per year, 95% Confidence Interval [CI] 1.00-1.03, p=0.018), pre-existing heart failure (aHR 1.50, 95% CI 1.13-1.98, p=0.005), and pre-existing chronic kidney disease (aHR 2.00, 95% CI 1.62-2.47, p<0.001) were all associated with a higher risk of developing renal failure among patients on carfilzomib. Gender, race, time from diagnosis to carfilzomib use, carfilzomib use year or a pre-existing history of hypertension and diabetes were not significantly associated with renal failure during carfilzomib use.
Fig 1:
Incidence of renal failure from the time of carfilzomib initiation
Table 2:
Multivariate cox regression of variables associated with renal failure among adults with relapsed/refractory MM utilizing carfilzomib
| Demographics | aHR | 95% Confidence Interval | P-value |
|---|---|---|---|
| Age (per year) | 1.01 | 1.00-1.03 | 0.018 |
| Gender | |||
| Male | REF | ||
| Female | 1.08 | 0.89-1.31 | 0.429 |
| Race | |||
| White | REF | ||
| Black | 1.09 | 0.83-1.43 | 0.532 |
| Other | 1.43 | 0.99-2.06 | 0.060 |
| Time from diagnosis to Carfilzomib (per month) | 1.00 | 1.00-1.00 | 0.671 |
| Carfilzomib utilization year (per year) | 0.93 | 0.83-1.01 | 0.069 |
| Pre-existing Hypertension | 1.07 | 0.86-132 | 0.528 |
| Pre-existing Diabetes | 1.12 | 0.90-1.41 | 0.315 |
| Pre-existing Heart Failure | 1.50 | 1.13-1.98 | 0.005 |
| Pre-existing Chronic Kidney Disease | 2.00 | 1.62-2.47 | <0.001 |
aHR adjusted hazard ratio
DISCUSSION
In our cohort of 1950 older adults with relapsed or refractory MM utilizing carfilzomib, renal failure occurred in up to 22% of patients. Increasing age, pre-existing heart failure and chronic kidney disease were associated with a higher risk of developing renal failure.
There are several differences in our selected cohort as compared to previously reported carfilzomib clinical trials ASPIRE and ENDEAVOR [3,4] including a higher median age of the patients in our study. Additionally, while we could not determine the functional status of patients from our registry cohort, in both ASPIRE and ENDEAVOR approximately 90% had an ECOG 0-1 representing a highly selected population, which is likely not reflective of real world patients.[16] Twenty percent of our cohort belonged to minority groups, but unfortunately, many clinical trials continue to under enroll[17] or not report the racial makeup of their cohorts, making it challenging to compare cohorts. The median duration on treatment and overall survival was also significantly shorter in our cohort than that reported in clinical trials. [3,4] The exact cause of this is unclear and whether it is on the basis of patient characteristics, toxicity/drug tolerance, or poorer response is unknown.
The cumulative incidence of renal failure among patients utilizing carfilzomib in our study was higher than that reported in clinical trials. Ball et al. recently summarized renal adverse event rates among four pivotal trials with carfilzomib-based regimens (ASPIRE, ENDEAVOR, FOCUS and CLARION) which included 2954 patients.[12] In their study, the cumulative rate of renal adverse events was 21.3% for all grades and 8.3% for grades 3-5 with acute kidney injury being the predominant reported event. Interestingly, renal adverse events were highest in the CLARION trial, [18] which included older transplant ineligible patients, likely reflecting a population of trial patients that more closely resembles our real-world patient cohort. While the above analysis done by Ball et al. had a much wider definition of renal toxicities including nephropathy, nephrotic syndrome, thrombotic microangiopathy, proteinuria, our study only defined renal adverse events as those coded as renal failure in order to minimize the effect of any confounding variables which would be increasingly difficult to distinguish in an administrative registry cohort.
With regards to the timing of renal failure, previous retrospective studies have shown that the median time to the development of renal failure among patient on carfilzomib is between 1-2 months, which is similar to the observation in our study.[19,7] The mechanism of renal failure in these studies is reported to be wide range including tumor lysis, albuminuria, and endothelial damage including thrombotic microangiopathy and cardiorenal syndrome.[11,9,10,20,21] In our study, we cannot determine the cause of renal failure and it may be multifactorial from the MM disease as well as other non-MM related causes present in the general population. Additionally, as our study relies on ICD codes for clinical diagnosis, we cannot ascertain the duration of renal adverse event and its subsequent management.
Risk factors for the development of renal adverse events with carfilzomib usage vary among studies. Dimopolous et al. conducted an observational study which suggested an increased risk of eGFR reduction with increasing doses of carfilzomib; however, did not find an effect of any baseline variables such as age, underlying diabetes or medications.[7] Conversely, Ball et al. did not find any relationship between carfilzomib dosing, duration, or setting (newly-diagnosed vs relapsed disease) in any of the pivotal carfilzomib trials.[12] In our study, age was associated with a marginal increase in the risk of renal failure. While no specific literature exists with regards to renal adverse events in older adults with MM, older adults are known to be at higher risk of both treatment related toxicity as well as treatment discontinuations and subsequent poor outcomes. [22,23]Additional age-associated vulnerabilities such as polypharmacy and accumulation of co-morbidities may also make older adults more susceptible to renal adverse events.[24] Pre-existing heart failure and pre-existing chronic kidney disease was associated with an increased risk of renal failure in our study. These patients developing renal failure may be natural progression of their disease, unrelated to carfilzomib, but it is possible that carfilzomib treatment exacerbated the underlying dysfunction. Regardless of etiology, identifying these risk factors may help identify patients that need to be monitored more carefully for developing renal failure during treatment.
To our knowledge, the current study is the first population-based study done among patients with MM utilizing carfilzomib which specifically tries to estimate both the incidence as well the risk factors for the development of renal failure during treatment. Our study has several limitations which must be acknowledged. First, the etiology of renal failure in patients with MM can be unclear as MM itself and common comorbidities such as HTN and diabetes can lead to renal failure. Moreover, pre-existing co-morbidities as well as renal adverse events were defined using ICD codes which has unavoidable limitations including under reporting, over reporting or miscoding. Additionally, ICD codes do not differentiate between the severity, reversibility, duration, clinical significance of the pre-existing co-morbidities or renal failure captured in our dataset. Carfilzomib administration was captured using HCPCS codes; however, we did not have additional important details regarding the dose and schedule of the drug which could have affected or been changed due to the development of adverse events. We also did not capture other drugs, including anti-MM agents, administered concurrently with carfilzomib. Many anti-MM treatments are oral formulations, and thus, their utilization would only be available on the subset of patients who had enrolled in the optional Medicare Part D (prescription benefit). Lastly, the SEER-Medicare database is largely representative of the older adult patients in the US enrolled in traditional fee-for-service Medicare and the results may not generalize to other populations. Based on these limitations, while our study does show that a high proportion of patients with relapsed or refractory MM develop renal failure, due to the study design, we cannot comment on causation. Additionally, this study should not be viewed as implying harm from carfilzomib as it is important to note that carfilzomib has been shown to improve outcomes in clinical trials even among patients with underlying renal impairment [25,26] emphasizing the need to individualize risk: benefit ratio for patients.
In conclusion, our study demonstrates that renal failure occurred among 22% patients during carfilzomib utilization. Although the etiology of renal failure among these patients is unclear, patients on carfilzomib should be monitored carefully for this adverse event. Future studies are needed to better define the risk for and cause of renal failure in older patients treated with carfilzomib in real-world clinical practice and to determine strategies and intervention to mitigate this risk especially among patient groups felt to be at highest risk.
Supplementary Material
Acknowledgments
Funding sources/Disclaimer: The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services Inc; and the SEER program tumor registries in the creation of the SEER-MHOS database.
Footnotes
Conflicts of Interest
Author HM has received honoraria/consultancy fees from Celgene, Takeda, Sanofi, Amgen, Janssen. Author MF declares that he has no conflict of interest. Author LS declares that she has no conflict of interest. Author RV has received research support from BMS/Takeda/Sanofi and Honoraria from BMS/Takeda/Sanofi/GSK/Karyopharm/Janssen/Oncopeptides/Securabio. Author TW has received research funding from Janssen and consulting fees from Carevive Systems, Seattle Genetics.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Availability of data and material: Not applicable
Code availability: Not applicable
References
- 1.Orlowski RZ, Moreau P, Niesvizky R, Ludwig H, Oriol A, Chng WJ, Goldschmidt H, Yang Z, Kimball AS, Dimopoulos M (2019) Carfilzomib-Dexamethasone Versus Bortezomib-Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Overall Survival, Safety, and Subgroups. Clin Lymphoma Myeloma Leuk 19 (8):522–530.e521. doi: 10.1016/j.clml.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 2.Siegel DS, Dimopoulos MA, Ludwig H, Facon T, Goldschmidt H, Jakubowiak A, San-Miguel J, Obreja M, Blaedel J, Stewart AK (2018) Improvement in Overall Survival With Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J Clin Oncol 36 (8):728–734. doi: 10.1200/jco.2017.76.5032 [DOI] [PubMed] [Google Scholar]
- 3.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos M-V, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P, Palumbo A (2014) Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. New England Journal of Medicine 372 (2):142–152. doi: 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]
- 4.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, Rosiñol L, Straub J, Suvorov A, Araujo C, Rimashevskaya E, Pika T, Gaidano G, Weisel K, Goranova-Marinova V, Schwarer A, Minuk L, Masszi T, Karamanesht I, Offidani M, Hungria V, Spencer A, Orlowski RZ, Gillenwater HH, Mohamed N, Feng S, Chng WJ (2016) Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 17 (1):27–38. doi: 10.1016/s1470-2045(15)00464-7 [DOI] [PubMed] [Google Scholar]
- 5.Shah C, Bishnoi R, Jain A, Bejjanki H, Xiong S, Wang Y, Zou F, Moreb JS (2018) Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma 59 (11):2557–2569. doi: 10.1080/10428194.2018.1437269 [DOI] [PubMed] [Google Scholar]
- 6.Fakhri B, Fiala MA, Shah N, Vij R, Wildes TM (2020) Measuring cardiopulmonary complications of carfilzomib treatment and associated risk factors using the SEER-Medicare database. Cancer 126 (4):808–813. doi: 10.1002/cncr.32601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Ziogas D, Eleutherakis-Papaiakovou E, Fotiou D, Migkou M, Kanellias N, Panagiotidis I, Ntalianis A, Papadopoulou E, Stamatelopoulos K, Manios E, Pamboukas C, Kontogiannis S, Terpos E, Kastritis E (2017) Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv 1 (7):449–454. doi: 10.1182/bloodadvances.2016003269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhaveri KD, Chidella S, Varghese J, Mailloux L, Devoe C (2013) Carfilzomib-related acute kidney injury. Clin Adv Hematol Oncol 11 (9):604–605 [PubMed] [Google Scholar]
- 9.Rosenthal A, Luthi J, Belohlavek M, Kortum KM, Mookadam F, Mayo A, Fonseca R, Bergsagel PL, Reeder CB, Mikhael JR, Stewart AK (2016) Carfilzomib and the cardiorenal system in myeloma: an endothelial effect? Blood Cancer J 6:e384. doi: 10.1038/bcj.2015.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shely RN, Ratliff PD (2014) Carfilzomib-associated tumor lysis syndrome. Pharmacotherapy 34 (5):e34–37. doi: 10.1002/phar.1397 [DOI] [PubMed] [Google Scholar]
- 11.Wanchoo R, Abudayyeh A, Doshi M, Edeani A, Glezerman IG, Monga D, Rosner M, Jhaveri KD (2017) Renal Toxicities of Novel Agents Used for Treatment of Multiple Myeloma. Clin J Am Soc Nephrol 12 (1):176–189. doi: 10.2215/CJN.06100616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball S, Behera TR, Anwer F, Chakraborty R (2020) Risk of kidney toxicity with carfilzomib in multiple myeloma: a meta-analysis of randomized controlled trials. Ann Hematol 99 (6):1265–1271. doi: 10.1007/s00277-020-04062-x [DOI] [PubMed] [Google Scholar]
- 13.Chari A, Romanus D, Palumbo A, Blazer M, Farrelly E, Raju A, Huang H, Richardson P (2020) Randomized Clinical Trial Representativeness and Outcomes in Real-World Patients: Comparison of 6 Hallmark Randomized Clinical Trials of Relapsed/Refractory Multiple Myeloma. Clin Lymphoma Myeloma Leuk 20 (1):8–17 e16. doi: 10.1016/j.clml.2019.09.625 [DOI] [PubMed] [Google Scholar]
- 14.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF (2002) Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40 (8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03 [DOI] [PubMed] [Google Scholar]
- 15.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL (2006) Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol 17 (6):1688–1694. doi: 10.1681/ASN.2006010073 [DOI] [PubMed] [Google Scholar]
- 16.Shah JJ, Abonour R, Gasparetto C, Hardin JW, Toomey K, Narang M, Srinivasan S, Kitali A, Zafar F, Flick ED, Rifkin RM (2017) Analysis of Common Eligibility Criteria of Randomized Controlled Trials in Newly Diagnosed Multiple Myeloma Patients and Extrapolating Outcomes. Clin Lymphoma Myeloma Leuk 17 (9):575–583 e572. doi: 10.1016/j.clml.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 17.Bhatnagar V, Gormley N, Kazandjian D, Goldberg K, McKee AE, Blumenthal G, Farrell AT, Pazdur R (2017) FDA Analysis of Racial Demographics in Multiple Myeloma Trials. Blood 130 (Supplement 1):4352–4352. doi: 10.1182/blood.V130.Suppl_1.4352.4352 [DOI] [Google Scholar]
- 18.Facon T, Lee JH, Moreau P, Niesvizky R, Dimopoulos M, Hajek R, Pour L, Jurczyszyn A, Qiu L, Klippel Z, Zahlten-Kumeli A, Osman M, Paiva B, San-Miguel J (2019) Carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood 133 (18):1953–1963. doi: 10.1182/blood-2018-09-874396 [DOI] [PubMed] [Google Scholar]
- 19.Kastritis E, Roussou M, Gakiopoulou C, Psimenou E, Gavriatopoulou M, Migkou M, Kanellias N, Dialoupi I, Ziogas DC, Eleutherakis-Papaiakovou E, Fotiou D, Papanota A-M, Giannouli S, Pouli A, Kartasis Z, Delavinia C, Efstathiou K, Tatouli I, Michas F, Kontogiannis S, Terpos E, Dimopoulos MA (2018) Carfilzomib-Associated Renal Toxicity Is Common and Unpredictable: An Analysis of 114 Patients. Blood 132 (Supplement 1):1966–1966. doi: 10.1182/blood-2018-99-112851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, O’Quinn R, Cohen AD, Stadtmauer EA, Ky B, Weiss BM (2018) Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol 4 (3):e174519. doi: 10.1001/jamaoncol.2017.4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, Carver JR, Cohen AD, Engelhardt BG, Garfall AL, Goodman SA, Harrell SL, Kassim AA, Jadhav T, Jagasia M, Moslehi J, O’Quinn R, Savona MR, Slosky D, Smith A, Stadtmauer EA, Vogl DT, Waxman A, Lenihan D (2019) Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J Clin Oncol 37 (22):1946–1955. doi: 10.1200/JCO.19.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wildes TM, Rosko A, Tuchman SA (2014) Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol 32 (24):2531–2540. doi: 10.1200/JCO.2014.55.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A, Rossi D, Cavalli M, Wijermans P, Ria R, Offidani M, Lahuerta JJ, Liberati AM, Mina R, Callea V, Schaafsma M, Cerrato C, Marasca R, Franceschini L, Evangelista A, Teruel AI, van der Holt B, Montefusco V, Ciccone G, Boccadoro M, San Miguel J, Sonneveld P, Palumbo A (2013) Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica 98 (6):980–987. doi: 10.3324/haematol.2012.075051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mina R, Bringhen S, Wildes TM, Zweegman S, Rosko AE (2019) Approach to the Older Adult With Multiple Myeloma. Am Soc Clin Oncol Educ Book 39:500–518. doi: 10.1200/EDBK_239067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimopoulos M, Siegel D, White DJ, Boccia R, Iskander KS, Yang Z, Kimball AS, Mezzi K, Ludwig H, Niesvizky R (2019) Carfilzomib vs bortezomib in patients with multiple myeloma and renal failure: a subgroup analysis of ENDEAVOR. Blood 133 (2):147–155. doi: 10.1182/blood-2018-06-860015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badros AZ, Vij R, Martin T, Zonder JA, Kunkel L, Wang Z, Lee S, Wong AF, Niesvizky R (2013) Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia 27 (8):1707–1714. doi: 10.1038/leu.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.