Abstract
Purpose
To compare the 12-month efficacy of intravitreous aflibercept (IVA) injection between eyes with pachychoroid neovasculopathy and neovascular age-related macular degeneration (AMD).
Methods
Retrospective, comparative case series analysis. Twenty-seven eyes with pachychoroid neovasculopathy and sixty-three eyes with neovascular AMD. All patients received three initial monthly, followed by bimonthly, IVA injections.
Results
Twelve months after initial treatment, the mean best-corrected visual acuity (BCVA) had improved both in pachychoroid neovasculopathy (from 0.28 to 0.14 logMAR; P = 0.001) and neovascular AMD (from 0.40 to 0.29 logMAR; P < 0.001). Twelve months after initial treatment, eyes with pachychoroid neovasculopathy exhibited decreased mean central retinal thickness (CRT) and subfoveal choroidal thickness (both, P < 0.001) and presence of polyps (P = 0.039) and improved integrity of external limiting membrane (ELM) (P = 0.008) and ellipsoid zone band (P = 0.001). At the 12-month follow-up, 77% and 68% of eyes with pachychoroid neovasculopathy and neovascular AMD, respectively, exhibited dry macula (P = 0.30). Baseline CRT was correlated with 12-month BCVA in eyes with pachychoroid neovasculopathy (P = 0.02). In eyes with neovascular AMD, CRT (P = 0.005) and presence of intact ELM (P = 0.007) were significant predictors of 12-month BCVA.
Conclusion
Periodic IVA injection leads to anatomical and functional improvement in eyes with pachychoroid neovasculopathy and in eyes with neovascular AMD.
Keywords: age-related macular degeneration, aflibercept, pachychoroid neovasculopathy, choroidal thickness
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness in developed countries. Early signs of AMD are soft drusen and extracellular deposits located between the retinal pigment epithelium (RPE) and Bruch’s membrane. Late AMD is characterized by two types of lesions: neovascular and dry AMD. Neovascular AMD is characterized by the formation of choroidal neovascularization (CNV), which causes hemorrhage or exudative changes resulting in deterioration of vision. Formation of large drusen and the subsequent hyperpigmentation have been reported to be closely associated with the evolution of CNV in AMD.1 Intravitreous anti-vascular endothelial growth factors (VEGFs) revolutionized the treatment of neovascular AMD and have been widely used for the 1st option.2–5
Recently, several researchers have reported a new clinical entity of CNV termed pachychoroid neovasculopathy.6–10 “Pachychoroid” indicates choroidal manifestations, including thickened choroid and dilation of outer choroidal vessels.6–13 It has been proposed that pachychorid pigment epitheliopathy (PPE), central serous chorioretinopathy (CSC), and pachychoroid neovasculopathy may be involved in the same pathophysiological process, including choroidal congestion and choroidal vascular hyperpermeability.7,8,14,15 Recently, Miyake et al showed that pachychoroid neovasculopathy differs from neovascular AMD not only phenotypically but also genetically9 suggesting that the mechanism of CNV development might differ between the two conditions. The results also raised a possibility that treatment effect and visual prognosis might differ between these two conditions. However, to date, there have been few reports on the effect of anti-VEGF therapy for pachychoroid neovasculopathy.16,17 The purpose of this study was to comparatively investigate the 1-year outcomes of intravitreous aflibercept (IVA) injection in eyes with pachychoroid neovasculopathy and neovascular AMD.
Methods
The study was approved by the institutional review board of Kyoto University Graduate School of Medicine, and all study protocols adhered to the tenets of the Declaration of Helsinki. All patients provided written informed consent to use clinical data.
Participants
We reviewed medical records of consecutive patients who initially visited the Macular Service, Department of Ophthalmology, Kyoto University Hospital between December 2012 and February 2016. The inclusion criteria were age >50 years, axial length <26.5 mm, presence of neovascular AMD or pachychoroid neovasculopathy. The exclusion criteria were as follows: history of CNV treatment and presence of other retinal diseases such as angioid streaks, uveitis, trauma, other secondary CNV, vitelliform macular dystrophy, diabetic retinopathy, and retinal vein or artery occlusion. Participants who dropped out within 1 year were excluded from analysis. If both eyes were eligible, only the right eye was used for analysis.
Definition of Pachychoroid Neovasculopathy and Neovascular AMD
In this study, pachychoroid neovasculopathy was diagnosed according to the following criteria:10,18,19 (1) CNV in either eye; (2) clinical features of the pachychoroid phenotype,11 such as reduced fundus tessellation on fundus photographs, choroidal vascular hyperpermeability on indocyanine green angiography (IA) images, and dilated choroidal vessels on optical coherence tomography (OCT) and IA images. Dilated choroidal vessels were defined as dilated outer choroidal vessels with attenuation and thinning of the choriocapillaris on OCT.11 On IA, dilated choroidal vessels extend from 1 or more vortex veins.20 (3) no drusen or only non-extensive hard drusen in both eyes (Age-Related Eye Disease Study [AREDS] level 1, no AMD21). Neovascular AMD was diagnosed in the presence of CNV and other findings corresponding to AREDS level 2 or higher21 (ie, extensive hard drusen, soft drusen, pseudodrusen, hyperpigmentation, or geographic atrophy). Diagnosis was made by two ophthalmologists; in case of discrepancy, a senior retinal specialist determined the final diagnosis.
Intervention and Multimodal Imaging
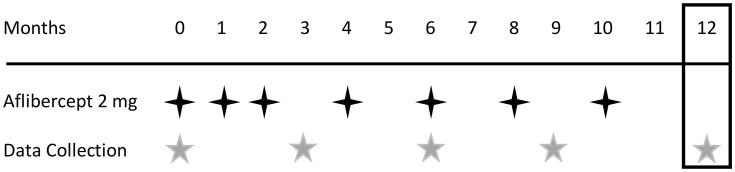
All participants received three courses of monthly and subsequent bimonthly aflibercept injection (2.0 mg). The number of injections in the 12-month study period was 3 + 4 (Figure 1). When there was a contraindication such as cerebral infarction or when patients did not consent to the treatment, the aflibercept course was skipped and the patients were excluded from analysis.
Figure 1.
Treatment protocol. Intervention schedule for patients with pachychoroid neovasculopathy and neovascular age-related macular degeneration.  Aflibercept injection (2 mg);
Aflibercept injection (2 mg);  clinical data collection;
clinical data collection;  efficacy endpoint.
efficacy endpoint.
All patients underwent ophthalmologic examination, including measurement of best-corrected visual acuity (BCVA) and intraocular pressure, indirect ophthalmoscopy, slit-lamp biomicroscopy, axial length measurement (IOL Master 500, Carl Zeiss Meditec, Dublin, CA), color fundus photography, spectral-domain OCT (SD-OCT), fundus autofluorescence, infrared reflectance, fluorescein angiography (FA), and IA at baseline and 12 months after initial treatment. BCVA measurement, fundus photography, and SD-OCT were performed at 3, 6, and 9 months after initial treatment.
Digital color fundus photographs (field, 40°) were obtained using a retinal camera (TRC NW6S, Topcon, Tokyo, Japan). Infrared reflectance, fundus autofluorescence, FA and IA images were acquired using a confocal scanning laser ophthalmoscope (HRA2; Heidelberg Engineering, Heidelberg, Germany) (field, 30°). For SD-OCT (Spectralis; Heidelberg Engineering), horizontal and vertical line scans were acquired through the fovea at a 30° angle. Inverted OCT images were acquired at the same position using the enhanced-depth imaging (EDI) technique.22 Fifty B-scans were automatically averaged to reduce speckle noise. Horizontal raster images with 240-µm spacing, covering 10- × 30-degree, were also acquired.
Image Analysis
Soft drusen was graded on the basis of fundus photography findings, in accordance with the AREDS severity scale for AMD, and confirmed by SD-OCT. In SD-OCT images, central retinal thickness (CRT) was defined as the mean distance between the vitreoretinal surface and RPE within a 1-mm circle from the center of the fovea, which was calculated automatically. Segmentation lines were manually corrected if there was a serious error. Subfoveal choroidal thickness (SFCT)—defined as the distance between Bruch’s membrane and the chorioscleral interface at the fovea—was manually measured in EDI-OCT images using the built-in calipers of the software. Both horizontal and vertical scans including the center of the fovea were used, and the average thickness values were calculated. Integrity of the external limiting membrane (ELM) and ellipsoid zone (EZ) bands and presence of vitreoretinal adhesion were evaluated in vertical and horizontal scans acquired through the fovea. Dry macula was defined by the absence of intra- or subretinal fluid throughout the raster scans. On FA images, we manually demarcated the outlines of lesions suggestive of CNV and measured the greatest linear dimension (GLD) and lesion size using the built-in calipers. Presence of polypoidal lesions was evaluated in IA images.
All assessments and measurements were independently performed by two masked examiners. Discrepancies in assessment were settled by open adjudication with the senior author. The mean values of measurements recorded by the two observers were used for analysis. If the differences between measurements recorded by the two examiners exceeded 15% of the mean of both values, a third examiner was invited to perform the measurement, and the value closest to that determined by the third examiner was selected.
Statistical Analysis
Data are presented as mean ± standard deviation, where applicable. For statistical analysis, visual acuity measured using the Landolt chart was converted to the logarithm of the minimum angle of resolution (logMAR). The chi-square test and McNemar’s test were used for the comparison of ratios between the study groups. The paired t-test was used for comparison of mean values of the same eyes acquired at different time points. The unpaired t-test was used for comparison of mean values between patients with neovascular AMD and pachychoroid neovasculopathy. Statistical significance was defined as P < 0.05. Statistical analysis was performed with the IBM SPSS software version 20.0 (IBM-SPSS Inc., Chicago, IL).
Results
The study sample consisted of 90 eyes (90 patients; 59 men (66%); mean age, 76 ± 7.5 years). While 27 eyes (30%) were diagnosed with pachychoroid neovasculopathy, 63 (70%) were diagnosed with neovascular AMD. In eyes with neovascular AMD, the frequency of type 1 and 2 CNV was 78% (49 eyes) and 22% (14 eyes), respectively. The baseline and 12-month characteristics of the participants are shown in Tables 1 and 2. Patients with pachychoroid neovasculopathy were significantly younger (mean age, 74 years vs 79 years, P = 0.023) and included a higher proportion of men (82% vs 59%; P = 0.037) than those with neovascular AMD. At baseline, the mean SFCT in the pachychoroid neovasculopathy group was significantly greater than that in the neovascular AMD group (338.2 μm vs 223.0 μm; P < 0.001). There was no significant difference in BCVA, GLD, lesion size, CRT, integrity of the ELM or EZ, or presence of vitreoretinal adhesion or polyps between the two groups at baseline.
Table 1.
Patient Characteristics at Baseline and 12 Months After Intravitreous Injection of Aflibercept
| Pachychoroid Neovasculopathy | Neovascular AMD | |||||
|---|---|---|---|---|---|---|
| Baseline | 12 Months | P value | Baseline | 12 Months | P value | |
| Age, years | 74.4 ± 8.1 | 78.5 ± 7.3 | ||||
| Sex (male/female) | 22/5 | 37/26 | ||||
| Number of eyes | 27 | 63 | ||||
| BCVA, logMAR (range) | 0.28 (0.13–0.44) | 0.14 (0.11–0.27) | 0.001* | 0.40 (0.31–0.50) | 0.29 (0.19–0.39) | < 0.001* |
| GLD, µm (range) | 3294 ± 1649 (2642–3947) | 3033 ± 1729 (2350–3717) | 0.090* | 3885 ± 1739 (3447–4323) | 3356 ± 1569 (2961–3751) | 0.001* |
| Lesion size, mm2 (range) | 5.17 ± 3.92 (3.62–6.72) | 5.01 ± 4.76 (3.13–6.89) | 0.811* | 9.95 ± 13.18 (6.63–13.27) | 6.08 ± 5.46 (4.70–7.45) | 0.013* |
| CRT, µm (range) | 346 ± 132 (294–399) | 197 ± 56 (174–219) | < 0.001* | 380 ± 180 (335–426) | 206 ± 119 (176–236) | < 0.001* |
| SFCT, µm (range) | 338 ± 66 (312–364) | 303 ± 81 (271–335) | < 0.001* | 223 ± 90 (200–246) | 192 ± 88 (170–215) | < 0.001* |
| Intact foveal ELM (±) | 13/14 | 21/6 | 0.008** | 18/45 | 30/33 | 0.004** |
| Intact foveal EZ (±) | 1/26 | 12/15 | 0.001** | 5/58 | 13/50 | 0.021** |
| Vitreoretinal adhesion (±) | 5/22 | 3/24 | 0.500** | 16/47 | 12/51 | 0.289** |
| Presence of polyps (±) | 13/14 | 5/22 | 0.039** | 32/31 | 14/49 | < 0.001** |
Notes: Data are presented as mean ± standard deviation or number of patients. *Paired t-test, **McNemar’s test.
Abbreviations: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; logMAR, logarithm of minimal angle of resolution; GLD, greatest lesion diameter; CRT, central retinal thickness; SFCT, subfoveal choroidal thickness; ELM, external limiting membrane; EZ, ellipsoid zone.
Table 2.
Comparison of Baseline Characteristics Between Patients with Pachychoroid Neovasculopathy and Neovascular AMD
| Pachychoroid Neovasculopathy | Neovascular AMD | P value | |
|---|---|---|---|
| Age, years | 74.4 ± 8.1 | 78.5 ± 7.3 | 0.023* |
| Sex (male/female) | 22/5 | 37/26 | 0.037** |
| BCVA logMAR | 0.28 ± 0.39 | 0.40 ± 0.38 | 0.709† |
| GLD, µm | 3294 ± 1649 | 3885 ± 1739 | 0.123† |
| Lesion size, mm2 | 5.17 ± 3.92 | 9.95 ± 13.18 | 0.245† |
| CRT, µm | 346 ± 132 | 380 ± 180 | 0.839† |
| SFCT, µm | 338 ± 66 | 223 ± 90 | <0.001† |
| Intact foveal ELM (±) | 13/14 | 18/45 | 0.073** |
| Intact foveal EZ (±) | 1/26 | 5/58 | 0.461** |
| Vitreoretinal adhesion (±) | 5/22 | 16/47 | 0.480** |
| Presence of Polyps (±) | 13/14 | 32/31 | 0.818** |
Notes: Data are presented as mean ± standard deviation or number of patients. *Unpaired t-test, **Chi-square test, †ANCOVA (adjusted for age and sex).
Abbreviations: AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; logMAR, logarithm of minimal angle of resolution; GLD, greatest lesion diameter; CRT, central retinal thickness; SFCT, subfoveal choroidal thickness; ELM, external limiting membrane; EZ, ellipsoid zone.
At 12 months after initial treatment, the mean BCVA had improved both in the pachychoroid neovasculopathy (from 0.28 to 0.14 logMAR; P = 0.001; Snellen equivalents: from 20/38 to 20/27; Figures 2 and 3) and neovascular AMD (from 0.40 to 0.29 logMAR; P < 0.001; Snellen equivalents: from 20/50 to 20/38) groups. There was no significant difference in change in BCVA between the two groups (P = 0.35). In eyes with pachychoroid neovasculopathy, the mean CRT and SFCT (P < 0.001) and presence of polyps (P = 0.039) had decreased, while the integrity of the ELM and EZ had improved (P = 0.008 and 0.001, respectively) at 12 months after initial treatment. In eyes with neovascular AMD, the mean CRT (P < 0.001), SFCT (P < 0.001), GLD (P = 0.001), and lesion size (P = 0.013) as well as the presence of polyps (P < 0.001) had decreased, while the integrity of the ELM and EZ had improved (P = 0.004 and 0.021, respectively) at 12 months after initial treatment.
Figure 2.
A case with pachychoroid neovasculopathy (before treatment). Images of the left eye of a 70-year-old man with pachychoroid neovasculopathy without any treatment history. The best-corrected visual acuity was 20/50. (A) Color fundus photograph shows subretinal hemorrhage and serous retinal detachment. Note that drusen are absent. (B) Optical coherence tomography (OCT) angiography image shows choroidal neovascularization (CNV). (C) Late-phase fluorescein angiography image shows leakage suggesting occult CNV. (D) Late-phase indocyanine green angiography image. (E) Enhanced-depth imaging OCT image shows serous retinal detachment and elevation of the retinal pigment epithelium, suggesting CNV. The choroid is thick throughout the macula, and choroidal vessels are dilated. Subfoveal choroidal thickness = 387 μm, central retinal thickness = 317 μm.
Figure 3.
A case with pachychoroid neovasculopathy (after treatment). Images of the same patient as in Figure 2, acquired 12 months after initial treatment. The best-corrected visual acuity had improved to 20/16. (A) Color fundus photograph shows resolution of subretinal hemorrhage and serous retinal detachment. (B) Optical coherence tomography (OCT) angiography image shows choroidal neovascularization. (C) Late-phase fluorescein angiography image shows reduction of leakage. (D) Late-phase indocyanine green angiography image. (E) Enhanced-depth imaging OCT image shows resolution of serous retinal detachment. Subfoveal choroidal thickness = 348 μm, central retinal thickness = 206 μm.
Tables 3 and 4 and Supplementary Figures 1–3 show the changes in BCVA, CRT, and SFCT between baseline and 3, 6, 9, and 12 months from initial treatment in both groups. There was a significant improvement in BCVA in both groups (both, P < 0.001) at 3 months after initial treatment; both groups also exhibited further improvement at later time points. There was a significant reduction in CRT in both groups (both, P < 0.001) at 3 months after initial treatment; the CRT remained almost stable over the subsequent period. There was a similar tendency in SFCT, which had decreased significantly at 3 months after initial treatment in both groups (both, P < 0.001) and remained almost stable afterwards.
Table 3.
Changes in Visual Acuity, Subfoveal Choroidal Thickness, and Central Retinal Thickness During Intravitreal Aflibercept Injection Treatment for Pachychoroid Neovasculopathy
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | Difference Between Baseline and 12 Months (95% CI) | P value* | |
|---|---|---|---|---|---|---|---|
| BCVA, logMAR | |||||||
| Mean ± SD | 0.28 ± 0.39 | 0.17 ± 0.32 | 0.15 ± 0.31 | 0.14 ± 0.35 | 0.14 ± 0.33 | 0.14 (0.06–0.21) | <0.001 |
| Median | 0.15 | 0.05 | 0.05 | 0.10 | 0.10 | ||
| Subfoveal choroidal thickness, µm | |||||||
| Mean ± SD | 338 ± 66 | 301 ± 85 | 307 ± 76 | 299 ± 81 | 303 ± 81 | 35 (16–51) | <0.001 |
| Median | 341 | 315 | 328 | 313 | 315 | ||
| Decrease from baseline (%) | 10.9 | 9.3 | 11.5 | 10.3 | |||
| Central retinal thickness, µm | |||||||
| Mean ± SD | 346 ± 132 | 217 ± 127 | 207 ± 65 | 193 ± 59 | 197 ± 56 | 149 (91–208) | <0.001 |
| Median | 317 | 183 | 203 | 184 | 191 | ||
| Decrease from baseline (%) | 37.2 | 40.4 | 44.2 | 43.2 |
Note: *Repeated measures ANOVA.
Abbreviations: CI, confidence interval; BCVA, best-corrected visual acuity; logMAR, logarithm of minimal angle of resolution; SD, standard deviation.
Table 4.
Changes in Visual Acuity, Subfoveal Choroidal Thickness, and Central Retinal Thickness During Intravitreal Aflibercept Injection Treatment for Neovascular Age-Related Macular Degeneration
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | Difference Between Baseline and 12 Months (95% CI) | P value* | |
|---|---|---|---|---|---|---|---|
| BCVA, logMAR | |||||||
| Mean ± SD | 0.40 ± 0.38 | 0.33 ± 0.41 | 0.32 ± 0.41 | 0.31 ± 0.42 | 0.29 ± 0.41 | 0.12 (0.05–0.17) | <0.001 |
| Median | 0.30 | 0.30 | 0.22 | 0.22 | 0.15 | ||
| Subfoveal choroidal thickness (µm) | |||||||
| Mean ± SD | 223 ± 90 | 193 ± 88 | 193 ± 88 | 189 ± 88 | 192 ± 88 | 30 (23–37) | <0.001 |
| Median | 210.5 | 167.5 | 166.5 | 171 | 177 | ||
| Decrease from baseline (%) | 13.4 | 13.7 | 15.1 | 13.7 | |||
| Central retinal thickness (µm) | |||||||
| Mean ± SD | 380 ± 180 | 202 ± 105 | 190 ± 85 | 190 ± 84 | 206 ± 119 | 174 (130–218) | <0.001 |
| Median | 338 | 174 | 164 | 170 | 172 | ||
| Decrease from baseline (%) | 46.9 | 49.9 | 50.1 | 45.8 |
Note: *Repeated measures ANOVA.
Abbreviations: CI, confidence interval; BCVA, best-corrected visual acuity; logMAR, logarithm of minimal angle of resolution; SD, standard deviation.
We classified patients with pachychoroid neovasculopathy into 2 groups; eyes with polyps and without polyps (Table 5). Eyes without polyps had larger lesion and worse BCVA than eyes with polyps. Final BCVA was improved in eyes without polyps, however, the difference was not significant (P=0.18).
Table 5.
Patients Classified with Pachychoroid Neovasculopathy
| Polypoidal Lesion (+) Group (n=11) | Polypoidal Lesion (–) Group (n=16) | P value | |
|---|---|---|---|
| Gender (men/women) | 10/1 | 12/4 | 0.618** |
| Age (years), mean ± SD | 69.9 ± 8.6 | 73.3 ± 7.2 | 0.417* |
| Initial conditions | |||
| BCVA (logMAR), mean ± SD | 0.22 ± 0.26 | 0.35 ± 0.44 | 0.480* |
| Lesion area (mm2), mean ± SD | 2.99 ± 2.77 | 5.18 ± 4.13 | 0.030* |
| GLD (µm), mean ± SD | 2602 ± 1398 | 3686 ± 1611 | 0.094* |
| CRT (µm), mean ± SD | 349.7 ± 99.3 | 343.7 ±147.1 | 0.911* |
| SFCT (µm), mean ± SD | 338.1 ± 48.7 | 338.3 ± 74.2 | 0.993* |
| Final conditions | |||
| BCVA (logMAR), mean ± SD | −0.01 ± 0.16 | 0.25 ± 0.36 | 0.042* |
| CRT (µm), mean ± SD | 174.2 ± 26.1 | 212.1 ± 63.8 | 0.085* |
Notes: *Unpaired t-test, **Chi-square test.
Abbreviations: SD, standard deviation; BCVA, best-corrected visual acuity; GLD, greatest linear dimension; CRT, central retinal thickness; SFCT, subfoveal choroidal thickness.
Table 6 shows the prevalence of dry macula at 3, 6, 9, and 12 months after initial treatment. At 3 months, 66% of eyes in both groups exhibited dry macula (P = 1.000). At 12 months, 77% and 68% of eyes with pachychoroid neovasculopathy and neovascular AMD, respectively, exhibited dry macula (P = 0.30). Complete regression of polyps was observed in 8 of 13 eyes with pachychoroid neovasculopathy and 18 of 32 eyes with neovascular AMD (P = 0.70).
Table 6.
Prevalence of Dry Macula in Patients with Pachychoroid Neovasculopathy and AMD Treated with Aflibercept
| 3 Months | 6 Months | 9 Months | 12 Months | |
|---|---|---|---|---|
| Overall (95% CI) | 0.66 (0.56–0.76) | 0.71 (0.57–0.77) | 0.75 (0.66–0.85) | 0.70 (0.60–0.79) |
| Pachychoroid Neovasculopathy | 0.66 (0.48–0.86) | 0.74 (0.56–0.92) | 0.81 (0.66–0.97) | 0.77 (0.61–0.95) |
| Neovascular AMD | 0.66 (0.55–0.79) | 0.69 (0.58–0.81) | 0.73 (0.62–0.84) | 0.68 (0.56–0.80) |
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval.
The results of linear regression analysis revealed that CRT at baseline was correlated with BCVA at 12 months in eyes with pachychoroid neovasculopathy (P = 0.02; R = 0.463); the association between presence of ELM at baseline and 12-month BCVA was marginal (P = 0.07; R = −0.353). In eyes with neovascular AMD, the results of linear regression analysis revealed that CRT (P = 0.001; R = 0.405), presence of intact ELM (P = 0.001; R = −0.395), lesion size (P = 0.003; R = −0.368), and presence of polypoidal lesions (P = 0.002; R = −0.390) at baseline were associated with 12-month BCVA. The results of multiple regression analysis revealed CRT (P = 0.005) and presence of intact ELM (P = 0.007) to be significant predictors of BCVA at 12 months. Data of each patient are presented in Supplemental File.
Discussion
Pachychoroid neovasculopathy is a recently recognized clinical entity of CNV, and little is known about the effect of anti-VEGF therapy for this condition. In this study, we comparatively investigated the 1-year outcomes of IVA injection in eyes with pachychoroid neovasculopathy and neovascular AMD. Our results revealed that three monthly and subsequent bimonthly IVA injections led to a significant improvement in BCVA and reduction in CRT and SFCT within a year from initial treatment, both in pachychoroid neovasculopathy and neovascular AMD.
In the present study, the prevalence of pachychorid neovasculopathy was about 30%, which was comparable with a previous study of Japanese patients.9 The mean BCVA was 0.28, which was similar to the study of Tagawa et al.10 Patients with pachychoroid neovasculopathy were significantly younger than those with neovascular AMD, as was also observed in the study by Miyake et al.9 The pachychoroid-driven mechanism operates independently of drusen, and the age of high susceptibility to CSC or PPE is the middle age.7,23 Therefore, in younger patients, pachychoroid-related diseases are likely to occur more frequently than AMD. In addition, among men included in the present study, pachychoroid neovasculopathy was more common than neovascular AMD; this trend might be explained by the relatively high prevalence of CSC among men reported in several studies.23–25 The mean SFCT in the present pachychoroid neovasculopathy group (338.2 µm) is comparable with the values previously reported by Pang and Freund (244–336 µm)8 and Miyake et al (310 µm).9 The mean SFCT in the present neovascular AMD group (223.0 µm) is also consistent with the values reported in previous studies (248.1–264.5 µm).26,27
The effects of IVA injection for neovascular AMD have been demonstrated in many studies.26–30 The VIEW studies showed that a course of three monthly injections of aflibercept followed by bimonthly injections was non-inferior to a course of monthly injections of ranibizumab in eyes with neovascular AMD.4,31 Consistent with these results, the present findings showed that, in eyes with neovascular AMD, the BCVA had improved significantly after three monthly and subsequent bimonthly IVA injections; eyes with pachychoroid neovasculopathy exhibited a similar response to IVA injection. Both groups of eyes exhibited a significant improvement in BCVA at 3 months after initial treatment, and further improvements were observed subsequently. There was no significant difference in change in BCVA between the pachychoroid neovasculopathy and neovascular AMD groups. In addition, both groups exhibited a significant reduction in CRT at 3 months after initial treatment; the CRT remained almost stable afterwards in both groups. At 12 months after initial treatment, dry macula and complete resolution of polyps were observed in approximately 70% and 60% of eyes, respectively, in both groups. Together, these results suggest that fixed-regimen IVA injection is effective for functional and anatomical improvement both in pachychoroid neovasculopathy and neovascular AMD, with no apparent difference in treatment efficacy between the two diseases.
In eyes with neovascular AMD, it has been reported that SFCT decreases after IVA injection.27,30,32,33 In a study by Koizumi et al, the mean SFCT in eyes with neovascular AMD decreased from 268 μm at baseline to 233 μm at 3 months from initial treatment and remained unchanged at 232 μm at 12 months from initial treatment (reduction, 36 μm [13.3%]).27 Consistent with these findings, the present results revealed that the SFCT had decreased by 13.6% from that at baseline in eyes with neovascular AMD, with most of the reduction occurring within the first 3 months. Eyes with pachychoroid neovasculopathy, too, exhibited similar changes in SFCT, where the mean SFCT decreased significantly within 3 months after initial treatment and remained almost stable afterwards; in these eyes, the SFCT had decreased by 36 μm (10.3%) within 12 months after initial treatment, which is consistent with the result reported by Padrón-Pérez et al where the SFCT decreased by 44 μm (10.3%).16 In asymptomatic individuals, the SFCT decreases by approximately 4 µm per year.34,35 Thus, the decrease in SFCT observed in the present study was more a result of aging than of IVA treatment. These results suggest that IVA injection has an effect on the choroid both in eyes with pachychoroid neovasculopathy and neovascular AMD. The mechanism of reduction of choroidal thickness is unclear; however, several hypotheses have been proposed. Inhibition of choroidal vascular hyperpermeability or direct or secondary vasoconstriction induced by a decrease in nitric oxide production might cause a decrease in choroidal thickness.36–38 Pachychoroid implies choroidal manifestations, including increased choroidal thickness and dilation of outer choroidal vessels, and appears to be involved in the evolution of CNV. Thus, it is possible that reduction in choroidal thickness after IVA treatment might lead to favorable outcomes in pachychoroid neovasculopathy. Further research is necessary to investigate the recurrence rate and long-term outcomes of IVA treatment for pachychoroid neovasculopathy.
In eyes with AMD, various anatomical factors have been reported to be associated with visual prognosis.39,40 Central retinal thickness has been used both as a quantitative outcome variable and as a measure for gauging the treatment effect in most clinical trials.41 Mathew et al demonstrated that the integrity of outer retinal layers at baseline is crucial for determining the final visual acuity at 12 months in eyes receiving ranibizumab treatment.42 Oishi et al reported that the presence of ELM is the most significant factor for visual acuity outcome after IVA in eyes with neovascular AMD.26 Consistent with these results, in patients with neovascular AMD in the present study, CRT and the presence of intact ELM at baseline were associated with BCVA at 12 months. In addition, the present results revealed that CRT at baseline was correlated with BCVA at 12 months in eyes with pachychoroid neovasculopathy, which indicates that CRT is the most significant factor for visual prognosis both in pachychoroid neovasculopathy and neovascular AMD. These results suggest that baseline CRT, which can be easily measured by OCT, is a useful predictor of visual function in pachychoroid neovasculopathy and neovascular AMD. Therefore, CRT measurement should be included in the OCT protocol before treatment. In our patients, lesion size was not affected by treatment outcomes maybe due to the small sample size.
There are several limitations to this study. First, the number of patients with pachychoroid neovasculopathy was relatively small, which is attributable to the rarity of this disease. Second, baseline demographics were different between pachychoroid neovasculopathy and neovascular AMD patients. In addition, all patients were Japanese. Further studies including more patients and other ethnic groups are required to evaluate the efficacy of IVA treatment for pachychoroid neovasculopathy. Despite these limitations, the present results demonstrated that periodic injection of aflibercept leads to anatomical and functional improvement in pachychoroid neovasculopathy and in eyes with neovascular AMD. Reduction in choroidal thickness due to IVA injection might affect the pathology of pachychoroid neovasculopathy. Therefore, further studies are necessary to determine the effect of reduction of choroidal thickness on treatment outcomes in pachychoroid neovasculopathy.
Funding Statement
This study was supported in part by the Japan Society for the Promotion of Science (JSPS), Tokyo, Japan (Grant-in-Aid for Scientific Research, no. 16K11321). The founders had no roles in the design and conduct of the study, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
Disclosure
None of the authors has a proprietary interest in any of the products described herein. Dr Manabu Miyata reports personal fees from Santen Pharmaceutical, personal fees from HOYA, grants from Alcon Japan, grants from Novartis Pharma, outside the submitted work. Dr Hiroshi Tamura reports personal fees from Novartis, grants from Findex, and personal fees from Suntory, outside the submitted work. Dr Akio Oishi reports personal fees from Bayer and non-financial support from Tokai Optical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Klein ML, Ferris FL, Armstrong J, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026–1031. doi: 10.1016/j.ophtha.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 4.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR Study. Ophthalmology. 2009;116(1):57–65.e5. doi: 10.1016/j.ophtha.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 6.Fung AT, Yannuzzi LA, Freund K. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina. 2012;32(9):1829–1837. doi: 10.1097/IAE.0b013e3182680a66 [DOI] [PubMed] [Google Scholar]
- 7.Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina. 2013;33(8):1659–1672. doi: 10.1097/IAE.0b013e3182953df4 [DOI] [PubMed] [Google Scholar]
- 8.Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina. 2015;35(1):1–9. doi: 10.1097/IAE.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 9.Miyake M, Ooto S, Yamashiro K, et al. Pachychoroid neovasculopathy and age-related macular degeneration. Sci Rep. 2015;5(1):16204. doi: 10.1038/srep16204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagawa M, Ooto S, Yamashiro K, et al. Characteristics of pachychoroid neovasculopathy. Sci Rep. 2020;10(1):16248. doi: 10.1038/s41598-020-73303-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina. 2016;36(3):499–516. doi: 10.1097/IAE.0000000000000742 [DOI] [PubMed] [Google Scholar]
- 12.Balaratnasingam C, Lee W-K, Koizumi H, et al. Polypoidal choroidal vasculopathy. Retina. 2016;36(1):1–8. doi: 10.1097/IAE.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 13.Lehmann M, Bousquet E, Beydoun T, Behar-Cohen F. Pachychoroid. Retina. 2015;35(1):10–16. doi: 10.1097/IAE.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 14.Gallego-Pinazo R, Dolz-Marco R, Gómez-Ulla F, et al. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol. 2014;3:111–115. [PMC free article] [PubMed] [Google Scholar]
- 15.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83 [DOI] [PubMed] [Google Scholar]
- 16.Padrón-Pérez N, Arias L, Rubio M, et al. Changes in choroidal thickness after intravitreal injection of anti-vascular endothelial growth factor in pachychoroid neovasculopathy. Invest Ophthalmol Vis Sci. 2018;59(2):1119–1124. doi: 10.1167/iovs.17-22144 [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto H, Hiroe T, Morimoto M, et al. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and type 1 neovascular age-related macular degeneration. Jpn J Ophthalmol. 2018;62(2):144–150. doi: 10.1007/s10384-018-0562-0 [DOI] [PubMed] [Google Scholar]
- 18.Hata M, Yamashiro K, Ooto S, et al. Intraocular vascular endothelial growth factor levels in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(1):292–298. doi: 10.1167/iovs.16-20967 [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A, Ooto S, Yamashiro K, et al. Pachychoroid geographic atrophy: clinical and genetic characteristics. Ophthalmol Retina. 2018;2(4):295–305. doi: 10.1016/j.oret.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 20.Pang CE, Shah VP, Sarraf D, Freund KB. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol. 2014;158(2):362–371.e2. doi: 10.1016/j.ajo.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report number 6. Am J Ophthalmol. 2001;132(5):668–681. doi: 10.1016/S0002-9394(01)01218-1 [DOI] [PubMed] [Google Scholar]
- 22.Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032 [DOI] [PubMed] [Google Scholar]
- 23.Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc. 1986;84:799–845. [PMC free article] [PubMed] [Google Scholar]
- 24.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63–68. doi: 10.1016/S0002-9394(99)00075-6 [DOI] [PubMed] [Google Scholar]
- 25.Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a Case–Control Study. Ophthalmology. 2004;111(2):244–249. doi: 10.1016/j.ophtha.2003.09.024 [DOI] [PubMed] [Google Scholar]
- 26.Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159(5):853–860.e1. doi: 10.1016/j.ajo.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 27.Koizumi H, Kano M, Yamamoto A, et al. Subfoveal choroidal thickness during aflibercept therapy for neovascular age-related macular degeneration. Ophthalmology. 2016;123(3):617–624. doi: 10.1016/j.ophtha.2015.10.039 [DOI] [PubMed] [Google Scholar]
- 28.Coscas F, Coscas G, Lupidi M, et al. Restoration of outer retinal layers after aflibercept therapy in exudative AMD: prognostic value. Invest Ophthalmol Vis Sci. 2015;56(6):4129–4134. doi: 10.1167/iovs.15-16735 [DOI] [PubMed] [Google Scholar]
- 29.Eleftheriadou M, Vazquez-Alfageme C, Citu CM, et al. Long-term outcomes of aflibercept treatment for neovascular age-related macular degeneration in a clinical setting. Am J Ophthalmol. 2017;174:160–168. doi: 10.1016/j.ajo.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 30.Hata M, Oishi A, Tsujikawa A, et al. Efficacy of intravitreal injection of aflibercept in neovascular age-related macular degeneration with or without choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci. 2014;55(12):7874–7880. doi: 10.1167/iovs.14-14610 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration. Ophthalmology. 2014;121(1):193–201. doi: 10.1016/j.ophtha.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 32.Koizumi H, Kano M, Yamamoto A, et al. Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(4):627–633.e1. doi: 10.1016/j.ajo.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 33.Mazaraki K, Fassnacht-Riederle H, Blum R, et al. Change in choroidal thickness after intravitreal aflibercept in pretreated and treatment-naive eyes for neovascular age-related macular degeneration. Br J Ophthalmol. 2015;99(10):1341–1344. doi: 10.1136/bjophthalmol-2015-306636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis R, Spaide RF. A Pilot Study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Wb W, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120(1):175–180. doi: 10.1016/j.ophtha.2012.07.048 [DOI] [PubMed] [Google Scholar]
- 36.Lowe J, Araujo J, Yang J, et al. Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res. 2007;85(4):425–430. doi: 10.1016/j.exer.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi: 10.1097/01.iae.0000242842.14624.e7 [DOI] [PubMed] [Google Scholar]
- 38.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274(3):H1054–8. doi: 10.1152/ajpheart.1998.274.3.H1054 [DOI] [PubMed] [Google Scholar]
- 39.Sayanagi K, Sharma S, Kaiser PK. Photoreceptor status after antivascular endothelial growth factor therapy in exudative age-related macular degeneration. Br J Ophthalmol. 2009;93(5):622–626. doi: 10.1136/bjo.2008.151977 [DOI] [PubMed] [Google Scholar]
- 40.Shin HJ, Chung H, Kim HC. Association between foveal microstructure and visual outcome in age-related macular degeneration. Retina. 2011;31(8):1627–1636. doi: 10.1097/IAE.0b013e31820d3d01 [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1–24. doi: 10.1016/j.preteyeres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 42.Mathew R, Richardson M, Sivaprasad S. Predictive value of spectral-domain optical coherence tomography features in assessment of visual prognosis in eyes with neovascular age-related macular degeneration treated with ranibizumab. Am J Ophthalmol. 2013;155(4):720–726.e1. doi: 10.1016/j.ajo.2012.11.003 [DOI] [PubMed] [Google Scholar]