ABSTRACT
Introduction
As the global severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic continues to spread, several variants have emerged. Variants B.1.1.7 and B.1.351 have attracted significant attention owing to their widespread transmission and possible immune evasion. A total of 19 SARS-CoV-2 vaccines based on original strains have entered clinical studies, including nine vaccines that have obtained emergency use or conditional marketing authorizations. However, newly emerging variants may affect their protective efficacy. Decreased efficacy of the Novartis, Johnson & Johnson, and AstraZeneca vaccines against B.1.351 has been reported. The spread of variants creates a tremendous challenge for the prevention and control of the SARS-CoV-2 pandemic via vaccination. Several response strategies, including accelerating massive rollouts of current vaccines, increasing vaccine immunogenicity by increasing vaccination doses, and accelerating next-generation vaccines against variants, have been suggested.
Areas covered
SARS-CoV-2 vaccine efficacy against variants and response strategies for emerging variants.
Expert opinion
Current SARS-CoV-2 vaccines authorized for emergency use or under clinical trials have shown certain advantages in providing adequate protection against new variants. We analyzed the effects of reported variants on neutralizing antibodies and the protective efficacy of different vaccines and propose strategies for applying current vaccines against variants and developing next-generation vaccines.
KEYWORDS: SARS-cov-2, variant, vaccine, protective efficacy, neutralization
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused substantial morbidity and mortality worldwide [1–3]. As the global SARS-CoV-2 pandemic continues, more than 4,000 variants have been reported. The D614G variant first appeared in the United States and began to sweep the world in mid-2020 [4,5]. Since the second half of 2020, more SARS-CoV-2 variants have emerged, including B.1.1.7 (501Y.V1 variant of concern [VOC] 202,012/01) first reported in the UK, B.1.351 first reported in South Africa (501Y.V2), and P.1 (501Y.V3) first reported in Brazil, causing widespread concern worldwide [6–8]. To date, 82 countries have reported cases caused by the B.1.1.7 variant [9]. The B.1.1.7 variant has a substantial transmission advantage, with the estimated difference in reproduction numbers between B.1.1.7 and non-B.1.1.7 variants ranging from 0.4 to 0.7, and the ratio of reproduction numbers varying between 1.4 and 1.8 [10]. B.1.351 has been reported in more than 40 countries [11], whereas P.1 has been detected in 20 countries [12]; however, the transmission characteristics and mortality of these two variants remain unclear. Since December 2020, B.1.1.7 and B.1.351 variants have been reported in Shanghai and Guangdong, China [13,14]. With an increasing number of cases reported, variants with mutation sites occurring in the receptor-binding domain (RBD) region of the viral spike protein have attracted extensive attention worldwide. As the RBD is the main target of neutralizing antibodies elicited by SARS-CoV-2 infection [15–17], these mutations could interact directly with the human angiotensin-converting enzyme 2 (hACE2) receptor and form part of the epitopes for hACE2-blocking neutralizing antibodies [18], which might lead to the ineffectiveness of immune protection provided by previous infections and vaccines [19]. In vitro studies have shown that the B.1.351 variant is refractory to neutralization by a number of monoclonal antibodies directed to the top of the RBD, including several that have received emergency use authorization [20,21]. In the present study, we reviewed the major variants and the effects of existing variants on neutralizing antibodies and vaccine protection and propose new ideas for applying current vaccines against variants and developing next-generation vaccines.
2. Major SARS-CoV-2 variants
2.1. D614G variant
In January 2020, all the original SARS-CoV-2 virus isolates were reported to have aspartic acid at amino acid position 614 of the spike protein (D614), which was gradually replaced by glycine (G614) in the epidemic strains reported later. Before March 2020, the D614G substitution frequency was less than 10% in global sequencing reports of SARS-CoV-2; however, it increased to more than 75% after June 2020 [5]. Studies on human respiratory cells and animal models have found that the D614G variant has higher infectivity and transmissibility than the original virus strain [22–24]. Clinical data indicated that disease severity was not related to D614G substitution. However, the effects of D614G in enhancing virus replication have been demonstrated in both in vitro studies based on human cells and respiratory ex vivo organ cultures of cattle and sheep, and in vivo studies using hamsters [25,26]. A higher viral load was also detected in the nasopharynx of patients infected with the G614 virus than in those infected with the D614 virus [27], which suggests that the D614G substitution might increase the adaptability of the virus to humans without causing a more serious disease response. In terms of vaccine protection, when the G614 pseudovirus was used to test the D614 or G614 virus-immune animal/human sera, the neutralization titer was 1.7 – to 2.0-fold higher than that of the D614 pseudovirus [28,29], indicating that the D614G mutation did not increase disease severity or affect the neutralizing activity of vaccine antibodies, which suggests that this variant would not present a threat to vaccine efficacy.
2.2. N439K variant
The N439K mutant was first discovered in Scotland in March 2020 and is the second most common mutation in the RBD region. By the end of 2020, N439K strains were detected in 34 countries [30]. Although the binding affinity to the hACE2 receptor of the spike protein was enhanced, the N439K viruses had similar in vitro replication fitness and caused infections with similar clinical outcomes compared to those by the wild-type. The N439K mutation confers resistance against several neutralizing monoclonal antibodies, including one authorized for emergency use by the US Food and Drug Administration, and reduces the activity of polyclonal sera from individuals recovered from infection [30]. Immune evasion mutations that maintain virulence and fitness, such as N439K, can emerge within the SARS-CoV-2 spike; however, the effect on the immune protection of the vaccines remains unknown.
2.3. B.1.1.7 variant
In December 2020, the UK reported a SARS-CoV-2 VOC, lineage B.1.1.7, also referred to as VOC 202,012/01 or 20I/501Y.V1 [6,31]. The emergence of B.1.1.7 is concerning given its increased transmissibility. As of 15 February 2021, B.1.1.7 comprises approximately 95% of new SARS-CoV-2 infections in England and has now been identified in at least 82 countries [32]. B.1.1.7 contains eight spike mutations and D614G (Figure 1), including two deletions (69–70del and 144del) in the N-terminal domain (NTD), one mutation (N501Y) in the RBD, and one mutation (P681H) near the furin cleavage site [33]. This mutation can increase the binding affinity of SARS-CoV-2 to ACE2 and enhance its ability to enter human cells, thereby accelerating the spread of the virus [10,34]. Early investigations from the UK suggested increased transmissibility of up to 71% over and above the previous circulating strains of SARS-CoV-2, which might contribute 0.39–0.93 to the R0 value estimates of the virus [35]. However, to date, there is no evidence that the B.1.1.7 variant demonstrates increased clinical severity of illness or immune escape capability [3,33,36–38].
Figure 1.
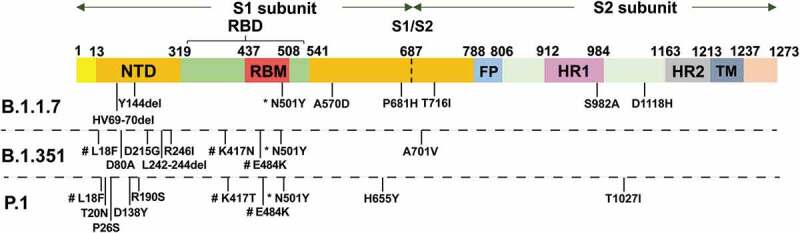
Schematic diagram of mutation sites for the B.1.1.7, B.1.351 and P.1 variants
* The mutation present in all three variants. # The mutation present in the B.1.351 and P.1 variants; notably, the mutation at site 417 is asparagine (N) in the B.1.351 variant and threonine (T) in the P.1 variant. NTD, N-terminal domain; RBD, receptor binding domain; RBM, receptor binding motif; FP, fusion peptide; HR, 7-peptide repeat; TM, transmembrane domain.
2.4. B.1.351 variant
In December 2020, South Africa announced the detection of a new B.1.351 variant of SARS-CoV-2. B.1.351 emerged in South Africa after the first epidemic wave in a severely affected metropolitan area, Nelson Mandela Bay [7]. B.1.351 contains nine spike mutations and D614G (Figure 1), including a cluster of mutations (e.g. 242–244del and R246I) in the NTD, three mutations (K417N, E484K, and N501Y) in the RBD, and one mutation (A701V) near the furin cleavage site [33]. There is a growing concern that the B.1.351 variant could impair the efficacy of current mAb therapies or vaccines because many of the mutations reside in the antigenic supersite in the NTD [16,39] or in the ACE2-binding site, which is a major target of potent virus-neutralizing antibodies [7,33]. For the three mutations (K417N, E484K, and N501Y) in the RBD, the N501Y mutation is also present in the UK B.1.1.7 variant [3], whereas the E484K mutation has been reported to be related to the escape of the B.1.351 variant from neutralizing antibodies [40]. In vitro studies have demonstrated that this mutation plays a crucial role in the loss of neutralizing activity of some monoclonal antibodies [41–44]. When the B.1.351 pseudovirus was used to detect the neutralization titers of convalescent sera and various vaccine-elicited immune sera, the results showed different degrees of reduction [33,45]. Furthermore, it has been found in clinical trials that the protective efficacy of the Novartis, Johnson & Johnson, and AstraZeneca-Oxford vaccines has decreased significantly in South Africa where this variant was prevalent [46–48]. Therefore, there is widespread concern regarding the protection provided by vaccines in the market and clinical trials against the B.1.351 variant.
2.5. P.1 variant
P.1 is a branch of the B.1.1.28 lineage, which was first discovered by the National Institute of Infectious Diseases in Japan in four travelers from Brazil. This variant was prevalent in northern Brazil in December 2020 [12]. P.1 contains a unique constellation of lineage-defining mutations, including several known biologically important mutations in the RBD, such as E484K, K417T, and N501Y (Figure 1) [8]. Similar to B.1.351, the P.1 variant was suspected to evade immunity in people who were vaccinated or previously infected [49]. To date, no study has shown that this variant affects vaccine protection.
2.6. Other variants
More variants continue to emerge worldwide, including the newly reported B.1.525 and A.23.1 [50,51]. For B.1.525, there are Q52R, E484K, Q677H, and F888L mutations in the spike, and a suite of deletions similar to B.1.1.7. The A.23.1 variant is the major virus lineage now observed in Kampala, Uganda. The mutations in the spike of the A.23.1 variant are F157L, V367F, Q613H, and P681R. Among them, Q613H is predicted to be functionally equivalent to the D614G mutation that was observed in the B.1 lineage found predominantly in Europe and the US [52]. Another lineage, B.1.429 (a.k.a. 20 C/CAL.20 C), containing the L452 R mutation, has risen to a high frequency in southern California [53]. The L452R mutation has been confirmed to escape LY-CoV555, a leading antibody that shows promise in humans and animal models [54]. As the effects of the B.1.525, A.23.1, and B.1.429 variants on the clinical severity and vaccine efficacy are not yet clear, it is essential that further studies are conducted on the new emerging variants.
3. Impact of major variants on vaccine efficacy
3.1. Neutralization of convalescent sera against variants
An important question for vaccine application is whether the authentic virus, under the selective pressure of the polyclonal immune response in convalescent or vaccinated people, can evolve to escape herd immunity and antibody treatment [55]. The convalescent serum of SARS-CoV-2 patients has been used in neutralization tests to investigate whether new variants exhibit immune evasion. The results not only indicate whether the variants cause reinfection, but also provide data on whether current vaccines remain effective. The studies listed in Table 1 illustrate that the neutralization titers against B.1.1.7 showed a slight decline, whereas the results for the B.1.531 variant were more worrisome. Notably, in two studies conducted by Gavin et al., the B.1.1.7 and B.1.531 variants used to test convalescent serum were both wild isolates, and the reduction ratios were 2.9 – and 13.3-fold, respectively [56,57]. Wibmer et al. assessed 44 plasma samples from individuals previously infected with SARS-CoV-2 using different pseudoviruses, including D614G (original), K417N, E484K, N501Y, and D614G (RBD only) or L18F, D80A, D215G, Δ242-244, K417N, E484K, N501Y, D614G, and A701V (501Y.V2). Nearly half (21 of 44, 48%) of the samples had no detectable neutralization activity, with only three samples (7%) retaining titers of inhibitory dilutions giving 50% neutralization (ID50) > 400 against the 501Y.V2 virus [45]. Wang et al. and Jangra et al. indicated that the E484K mutation (present in both B.1.351 and P.1 variants) significantly affected the neutralization activity [33,58], whereas Rathnasinghe et al. found that the N501Y substitution in the RBD spike did not mediate antibody evasion [59].
Table 1.
Results of convalescent plasma against SARS-CoV-2 variants
| Virus type | Variant | Control strains | No. of sample | Reduction fold (control/variant) |
Reference |
|---|---|---|---|---|---|
| pseudovirus | B.1.1.7 | D614G | 15 | 1.55 | Shen et al. [36] |
| pseudovirus | B.1.1.7 | WT | 20 | 2.7–3.8 | Wang et al. [33] |
| B.1.351 | 11.0–33.1 | ||||
| infectious cDNA clone | E484K mutation | WT | 30 | 2.4–4.2 | Jangra et al. [58] |
| live virus | B.1.1.7 | WT | 34 | 3.9 | Gavin R. et al. [56] |
| live virus | B.1.351 | WT | 34 | 13.3 | Gavin R. et al. [57] |
| mouse-adapted virus | N501Y MA-SARS-CoV-2 | WT | 30 | * | Rathnasinghe et al. [59] |
Annotation: WT = Wuhan reference strain; * No fold reduction in neutralization titers was mentioned in this study, although the results showed that N501Y did not mediate antibody escape.
3.2. Neutralization of vaccine-elicited sera against variants
3.2.1. Pfizer/BioNTech mRNA vaccine
Pseudoviruses and infectious cDNA clones have been employed more commonly to assess the neutralization ability of sera from vaccinated individuals against different variants (Table 2). Muik et al. tested serum samples from 40 participants who were vaccinated with the Pfizer/BioNTech mRNA vaccine (BNT162b2) in the previously reported German Phase 1/2 trial with pseudoviruses of B.1.1.7 and Wuhan reference strain (WT). Even though there was a slight reduction, the immune sera overall preserved neutralizing titers against B.1.1.7, which indicated that B.1.1.7 will not escape Pfizer/BioNTech vaccine-mediated protection [38]. Collier et al. also demonstrated that a pseudovirus with spike mutations in the B.1.1.7 variant (del69/70, del 144/145, N501Y, A570D, P681H, T716I, S982A, and D1118H) resulted in reduced sensitivity to sera from vaccines. Their results showed that 10 of the 23 sera from participants who received one dose of Pfizer/BioNTech vaccine showed reduced neutralization against the B.1.1.7 variant (fold change >3), and the highest fold change was approximately 6 with a median fold change of 3.85. However, when the sera were tested using pseudoviruses with N501Y, A570D, and 69/70 deletion mutations, no reduction in ability was observed [37]. Notably, as the sera were collected after 1 dose of a 2-dose regimen, the results only represent partial immunity.
Table 2.
Results of vaccine-elicited sera against SARS-CoV-2 variants
| Vaccine | Type of vaccine | Gene | Sample No. | Type of virus | Control strain | Variant | Reduction fold (control/variant) | Reference |
|---|---|---|---|---|---|---|---|---|
| Moderna vaccine | RNA vaccine | S | / | pseudovirus | D614G | K417N-E484K-N501Y-D614G | 2.7 | Wu et al. [62] |
| B.1.351 | 6.4 | |||||||
| Moderna vaccine | RNA vaccine | S | 12 | pseudovirus | WT | B.1.1.7 | 1.8 | Wang et al. [33] |
| B.1.351 | 8.6 | |||||||
| Pfizer/BioNTech vaccine | RNA vaccine | S | 10 | B.1.1.7 | 2 | |||
| B.1.351 | 6.5 | |||||||
| Moderna vaccine | RNA vaccine | S | 40 | pseudovirus | D614G | B.1.1.7 | 2.11 | Shen et al. [36] |
| NVX-CoV2373 | protein nanoparticle | S | 28 | 2.25 | ||||
| Pfizer/BioNTech vaccine | RNA vaccine | S | 40 | pseudovirus | WT | B.1.1.7 | 1.25 | Muik et al. [38] |
| Pfizer/BioNTech vaccine | RNA vaccine | S | 23 | pseudovirus | WT | B.1.1.7 | 3.85 | Collier et al. [37] |
| Pfizer/BioNTech vaccine | RNA vaccine | S | 20 | infectious cDNA clone | WT | Mutant N501Y | 0.68 | Xie et al. [60] |
| Mutant Δ69/70+ N501Y+D614G | 0.71 | |||||||
| Mutant E484K+N501Y+D614G | 1.23 | |||||||
| Pfizer/BioNTech vaccine | RNA vaccine | S | 5 | infectious cDNA clone | WT | E484K mutation | 3.4 | Jangra et al. [58] |
| Pfizer/BioNTech vaccine | RNA vaccine | S | 20 | infectious cDNA clone | N501 | Y501 | 1.46 | Shi et al. [61] |
| Pfizer/BioNTech vaccine | RNA vaccine | S | 25 | live virus | WT | B.1.1.7 | 3.3 | Gavin R. et al. [56] |
| AstraZeneca-Oxford vaccine | Adenovirus vector vaccine | S | 25 | live virus | WT | B.1.1.7 | 2.1 ~ 2.5 | |
| Pfizer/BioNTech vaccine | RNA vaccine | S | 25 | live virus | WT | B.1.351 | 7.6 | Gavin R. et al. [57] |
| AstraZeneca-Oxford vaccine | Adenovirus vector vaccine | S | 25 | live virus | WT | B.1.351 | 9 | |
| BBIBP vaccine | Inactive vaccine | full length |
12 | live virus | WT & D614G | B.1.351 | 1.6 | Gao et al. [63] |
| Zhifei vaccine | protein subunit vaccine | RBD | 12 | live virus | WT & D614G | B.1.351 | 1.6 | |
| Pfizer/BioNTech vaccine | RNA vaccine | S | 6 | mouse-adapted virus | WT | N501Y MA-SARS-CoV-2 | * | Rathnasinghe et al. [59] |
Annotation: S = spike; RBD = receptor binding domain; WT = Wuhan reference strain; * No fold reduction in geometric mean titers (GMT) was mentioned in this study, although the results showed that N501Y did not mediate antibody escape.
The neutralization ability of Pfizer/BioNTech vaccine immune sera against the B.1.351 variant declined significantly in comparison to that against the B.1.1.7 variant. Results from the study by Gavin R. et al. based on live viruses showed that there was a 7.6 fold reduction in the neutralization titers against the B.1.351 variant, whereas the fold decline was 3.3 against the B.1.1.7 strain [56,57]. In the study by Wang et al., 10 sera from clinical subjects who received the Pfizer/BioNTech vaccine at the clinical dose on days 0 and 21 were also assayed for neutralization against the major variants. The mean loss in neutralizing activity against the B.1.1.7 and B.1.351 variants was 2 – and 6.5-fold, respectively. The vaccine serum was also tested against each single-mutation pseudovirus. Similar to the convalescent plasma result, no single mutation could predictably account for the small loss in serum neutralizing activity against B.1.1.7, whereas there was loss in neutralizing activity against B.1.351 in the vaccine sera, which could be principally attributed to E484K [33].
Studies based on infectious cDNA clones have focused on the effect of the E484 mutation. Xie et al. engineered three SARS-CoV-2 viruses containing key spike mutations from B.1.1.7 and B.1.351 variants: N501Y from B.1.1.7 and B.1.351, 69/70-deletion+N501Y+D614G from B.1.1.7, and E484K+N501Y+D614G from B.1.351 using an infectious cDNA clone of SARS-CoV-2. A total of 20 BTN162b2 vaccine-elicited human sera were tested with the wild-type and mutant viruses, and the neutralizing antibody geometric mean titers (GMTs) of the sera against virus with E484K mutation declined to 1.23 fold, which was higher than that of other mutant viruses [60]. Jangra et al. reported a 3.4-fold reduction when the E484K rSARS CoV-2 virus was used in neutralization assays for sera samples from five individuals who received two doses of the Pfizer/BioNTech vaccine [58]. All these studies indicated that E484K, which existed not only in B.1.351 but also in the P.1 variant, is crucial for immunity.
Other studies have focused on the function of the N501Y mutation. In the study by Rathnasinghe et al., the sera from six individuals who had received two doses of the Pfizer/BioNTech vaccine had neutralizing antibody titers similar to the highest neutralization titers observed in convalescent sera against the mouse-adapted virus MA-SARS-CoV-2 with the N501Y mutation [59]. Shi et al. prepared an isogenic pair of SARS-CoV-2 containing the N501 or Y501 spike protein to detect serum neutralization in 20 participants immunized with two doses of Pfizer/BioNTech vaccine. The ratio of the 50% neutralization GMT against the Y501 virus to that against the N501 virus was 1.46 [61]. Both results suggested that the N501Y mutation did not compromise post-vaccination neutralization potential. Compared with the N501Y mutation common in the B.1.1.7, B.1.351, and P.1 variants, the strains with the E484K mutation would pose a serious threat to the protection efficacy of SARS-CoV-2 vaccines.
3.2.2. Moderna mRNA vaccine
The neutralization capacity of the Moderna SARS-Co-2 Moderna mRNA vaccine (mRNA-1273) vaccine against variants was similar to that of the Pfizer/BioNTech vaccine. In the study by Wang et al., sera obtained from 12 participants in a Phase 1 clinical trial of the Moderna vaccine were also assayed for neutralization against B.1.1.7, B.1.351, and WT pseudoviruses, and the results revealed no significant differences between the Moderna vaccine – and Pfizer/BioNTech vaccine-elicited sera against the B.1.1.7 and B.1.351 variants [33].
Shen et al. employed a lentivirus-based pseudovirus assay to show neutralization against the variant B.1.1.7 in 40 recipients of the Moderna vaccine and found that the B.1.1.7 variant was neutralized by the vaccine sera, although with modestly diminished susceptibility compared to the D614G variant. The median ID50 and ID80 titers of immune sera were, on average, 2.1-fold and 1.7-fold lower, respectively, against B.1.1.7 than that against D614G [36]. Wu et al. used two orthogonal vesicular stomatitis virus (VSV) and lentivirus pseudovirus neutralization (PsVN) assays that expressed spike variants of 20E (EU1), 20A.EU2, D614G-N439, mink cluster 5, B.1.1.7, and B.1.351 variants to assess the neutralizing capacity of sera from humans immune to the Moderna vaccine. No significant impact was found on the neutralization of Moderna vaccine-immune sera against the B.1.1.7 variant, whereas reduced neutralization was measured against the mutations present in B.1.351. VSV pseudoviruses with spikes containing K417N-E484K-N501Y-D614G and full B.1.351 mutations showed a 2.7 – and 6.4-fold reduction in GMT, respectively, compared to the D614G VSV pseudovirus [62].
3.2.3. AstraZeneca-Oxford vaccine
As one of the most widely used SARS-CoV-2 vaccines, the cross-neutralization ability of the AstraZeneca-Oxford vaccine (ChAdOx1) against these variants has attracted great attention. Gavin et al. reported that in participants who were vaccinated with the AstraZeneca-Oxford vaccine, the neutralization titers of sera at 14 and 28 days following the second dose showed a 2.5-fold (geometric mean, n = 15, p ≤ 0.0001) and 2.1-fold (geometric mean, n = 10, p < 0.002) reduction against B.1.1.7 strain, respectively [56]. They then measured neutralization of the B.1.351 variant using the AstraZeneca-Oxford vaccine-elicited serum obtained 14 or 28 days following the second vaccine dose, and found that GMTs against the B.1.351 variant were 9-fold lower than against the Victoria strain (p < 0.0001) [57]. The results indicated that the AstraZeneca-Oxford vaccine offers a more limited protection against the B.1.351 variant than to the B.1.1.7 variant.
3.2.4. Beijing Institute of Biological Products inactivated vaccine (BBIBP-CorV) and Zhifei Longcom recombinant protein vaccine (ZF2001)
When the wild B.1.351 variant was used by Gao et al. to test the immune sera of the BBIBP and Zhifei vaccines developed by Chinese companies, the results showed some differences from those of the mRNA vaccines based on pseudoviruses [63]. The wild B.1.351 variant was isolated in Guangdong, China, from a 55-year-old South African pilot who entered Guangzhou from Singapore [13]. A total of 24 samples were detected, and the result showed that the neutralization ability of both vaccines against the B.1.351 variant was reduced by 1.6-fold, the GMTs of immune serum elicited by the BBIBP vaccine decreased from 110.9 (95% CI: 76.7–160.2) to 70.9 (95% CI: 50.8–98.8), and those of the Zhifei vaccine decreased from 106.1 (95% CI: 75.0–150.1) to 66.6 (95% CI: 51.0–86.9) [63]. The authors considered that the protective effects of these two vaccines were not affected by the B.1.351 variant.
3.3. Effect of variants on vaccine protective efficacy
Recent results from the Phase 3 clinical trials of three vaccines from Novartis, Johnson & Johnson, and AstraZeneca-Oxford in South Africa showed that the locally circulating variants had affected the protective efficacy of the vaccines to varying degrees.
The UK Phase 3 clinical trials of NVX-CoV2373 developed by Novavax Inc. resulted in a point estimate of vaccine efficacy of 89.3% (95% CI: 75.2–95.4). Based on polymerase chain reaction performed on strains from 56 of the 62 cases, efficacy by strain was calculated to be 95.6% against the original SARS-CoV-2 strain and 85.6% against the UK variant. The South Africa Phase 2b clinical trial achieved its primary efficacy endpoint with an efficacy of 49.4% (95% CI: 6.172.8). Preliminary sequencing data were available for 27 of 44 SARS-CoV-2 events; of these, 92.6% (25 out of 27 cases) were the B.1.351 variant [48]. This result has raised concerns regarding the effectiveness of vaccines and the possibility of secondary infections in infected individuals.
During the Phase 3 ENSEMBLE clinical trial of Janssen’s SARS-CoV-2 vaccine candidate, for all participants from different geographies and those infected with an emerging viral variant, Janssen’s vaccine was 66% effective overall in preventing moderate to severe SARS-CoV-2. The level of protection against moderate to severe SARS-CoV-2 infection was 72% in the US, 66% in Latin America, and 57% in South Africa, and nearly all cases of SARS-CoV-2 (95%) in South Africa were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage [46].
Clinical trials of the AstraZeneca-Oxford vaccine in the UK indicated that the vaccine not only protected against the original pandemic virus, but also protected against the novel variant, B.1.1.7 [64]. However, the trial results in South Africa showed that the neutralization of the immune sera induced by the AstraZeneca-Oxford vaccine against the B.1.351 coronavirus variant was substantially reduced compared with that against the original strain, although it still had high efficacy against the original coronavirus non-B.1.351 variants. A two-dose regimen of the AstraZeneca-Oxford vaccine provided minimal protection against mild–moderate SARS-CoV-2 infection from the B.1.351 coronavirus variant [47,65].
4. Development and implementation strategies for vaccines targeting SARS-CoV-2 variants
The marked loss of neutralization ability and decline in the protective efficacy of current vaccines indicates the potential immune escape of B.1.351 variants. Nonetheless, in the study by Penny et al., most of the convalescent serum suffered less than a 4-fold reduction in total binding activity, suggesting that a considerable non-neutralizing antibody component is still able to bind the B.1.351 spike, which indicates the prospect of reinfection with antigenically distinct variants [45]. The immune escape SARS-CoV-2 variants could pose an unpredictable threat to the entire world, and global vaccine development and implementation strategies are urgently required. Recently, the US Food and Drug Administration stated that it is formulating rules for the accelerated review of updated vaccines against virus variants to fast-track the development and application of vaccines for SARS-CoV-2 mutants [66].
4.1. Rapid and massive vaccine rollouts using current SARS-CoV-2 vaccines
The clinical protection findings of the Johnson & Johnson and Novartis vaccines against the South African variant indicate that even though the protection efficacy of these vaccines is lower than those against previous epidemic strains, they are still higher than or close to the protection level criterion of at least 50% as defined by the World Health Organization (i.e. 57% and 49.4%, respectively) [46,48]. In other words, the vaccines retain a certain level of effectiveness against the variant. Hence, the urgent rollouts of current vaccines to immunize a large proportion of the population is currently the best option to manage the threat of emerging variants.
4.2. Improving the immunogenicity of current vaccines
Based on the protective efficacy of the current vaccines against the variants, raising the vaccine-induced immune response level would help prevent further transmission of variants. Different options should be considered, including increasing the number of doses and alternating vaccines to boost the immune response. For the one-shot adenovirus vaccine, two doses might further improve its protection against circulating strains and variants. Johnson & Johnson has launched a Phase 3 clinical trial to test the protective efficacy offered by two doses of the Ad26.COV2.spike vaccine. Moderna has considered adding a third Moderna vaccine dose after the first two doses to increase the neutralizing antibody titer, thus ensuring protective efficacy against SARS-CoV-2 variants, even if the titers declined [67].
Furthermore, the alternating vaccine immunization strategy may also be an effective measure to improve the immunogenicity of emergency vaccines. The Russian Direct Investment Fund announced plans to conduct a combined clinical trial in Ukraine, using the Sputnik V and AstraZeneca vaccines [68]. The University of Oxford has begun the first clinical trial to explore alternating between different SARS-COV-2 vaccines, in which over 800 volunteers will be recruited in England to evaluate four different combinations of prime and booster vaccinations of the Oxford-AstraZeneca and Pfizer vaccines [69]. Public Health England updated their guidance for SARS-CoV-2 vaccines, and announced that if the vaccine received as the first dose by an individual is not available, then an alternative vaccine may be used [70]. The US Food and Drug Administration is also taking notice of mixing and matching vaccines [71].
4.3. Developing next generation vaccines against variants
The next generation vaccines, such as multivalent vaccines, would be effective tools to control the spread of SARS-CoV-2 variants. Johnson & Johnson is now developing vaccines against variants and plans to add the original vaccine to form a bivalent vaccine. Moderna has developed a booster vaccine (mRNA-1273.351) against the newly emerging B.1.351 variant [72]. GlaxoSmithKline plc and CureVac N.V. jointly plan to develop next-generation mRNA multivalent vaccines [73]. The next generation of vaccines might usher in a new dawn in the prevention and control of SARS-CoV-2 worldwide.
5. Expert opinion
The SARS-CoV-2 pandemic is the most serious infectious disease that has afflicted humanity in the past 100 years and bringing it under control is a top priority. The main challenge against SARS-CoV-2 is the lack of understanding of the etiology and pathogenic mechanisms of the new emerging pathogen, the protection mechanism, immune endpoint, and immunized persistence of vaccines. A total of 19 SARS-CoV-2 vaccines have entered clinical trials within one year, including nine vaccines that have obtained authorizations for emergency use or conditional marketing. For the first time, the mRNA vaccine was used as a preventive vaccine and an adenovirus carrier vaccine was applied in a large-scale vaccination. Additionally, the success of other vaccines developed using different technologies also provides experience for the development and application of preventive vaccines in the future.
However, SARS-CoV-2 variants pose a tremendous challenge to the control of this epidemic. The B.1.351 and P.1 variants, which might lead to reinfections and immune escape, have raised questions regarding the effectiveness of vaccines that are currently being used in the massive vaccine rollouts. Most of the studies that reported a significant decline in neutralization titers against SARS-CoV-2 variants were based on pseudoviruses, whereas the study by Gao et al. that used the wild B.1.351 strain to detect the BBIBP and Zhifei vaccine-elicited sera showed a slight reduction. When using different methods/strains to detect the neutralizing activity of the variants, the comparability of the results is very important. In follow-up studies, the standardization of test methods is urgently needed, and it is extremely valuable to evaluate the neutralization ability of wild viruses or infectious cDNA clones, and to confirm the protective efficacy in clinical research. Additionally, sequencing and isolation of strains vary enormously from country to country, and the data currently available may not be truly representative of the existing global situation. Research on the monitoring of genetic variation should be strengthened, as well as basic research on pathogenic mechanisms and immune protection mechanisms.
This paper summarizes the major SARS-CoV-2 variants and their impact on the neutralization activity of convalescent serum and vaccine-elicited serum. Notably, given that certain vaccines continue to have protective effects against the B.1.351 variants, it is necessary to continue the rapid and massive vaccine rollouts against SARS-CoV-2 along with close monitoring of its genetic mutations. Other response strategies have been proposed, including improving the immunogenicity of current vaccines by increasing the number of doses and alternating vaccines and developing monovalent or multivalent vaccines against variants. These strategies provide a basis for the policy decisions of regulatory authorities, application agencies, and research and development manufacturers. It would be helpful to develop and apply vaccines against variants effectively.
The worldwide research and development and application of SARS-CoV-2 vaccines is an iconic event in the history of human vaccines. Further research on SARS-CoV-2 variants and next-generation vaccines would help to understand the law of virus mutation and develop an effective general vaccine against SARS-CoV-2. By responding to SARS-CoV-2 outbreaks, both the response abilities and strategies for new emerging infectious diseases, and the rapid and efficient vaccine research technology and application mode would be further improved.
Funding Statement
This work was supported by the National Key R&D Program of China (2020YFC0860500).
Article highlights
SARS-CoV-2 is prone to mutations. Close monitoring of the prevalence of different variants is a key aspect in preventing and controlling the SARS-CoV-2 epidemic.
The B.1.1.7 variant has become widespread worldwide. There is no evidence suggesting that it affects the neutralizing ability of convalescent sera and vaccine-elicited sera, as well as the protective efficacy of vaccines.
The results of studies based on pseudovirus and infectious cDNA clones with B.1.351-related mutations in the receptor-binding domain region showed that the neutralizing activity of both the convalescent sera and vaccine-elicited sera decreased, and the protective efficacy of some vaccines was also found to decline in clinical trials.
Current vaccines still have certain effects against the B.1.351 variant. Therefore, rapid and mass immunization with current SARS-CoV-2 vaccines would help control the spread of variants. Consideration should also be given to the balance of vaccination rates in different regions worldwide.
For current vaccines, increasing the number of doses to boost immune response or alternating vaccines could further enhance the immunogenicity and protective efficacy against variants.
The R&D of next generation vaccines against the B.1.351 variant should be conducted to improve vaccine protection.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Reviewers on this manuscript have received an honorarium for their review work. A reviewer on this manuscript has disclosed that they are an employee of University of Oxford working on ChAdOx1-nCoV19 vaccine studies. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Author Contributions
Conceptualization: Z.L., M.X.; Data curation: L.B., J.Z., Q.H.; Writing-original draft: L.B., Z.L., F.G.; Writing-review and editing: L.B., F.G., Q.H., Q.M.; Supervision: Z.L., M.X.; Funding acquisition: M.X.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang JW, Tambyah PA, Hui DS.. Emergence of a new SARS-CoV-2 variant in the UK. J Infect. 2020;S0163-4453(20):30786–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volz E, Hill V, Mccrone JT, et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184(1):64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations 2020 [cited 2021. February 15]. Available from: https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563
- 7.Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. Nature. 2021.DOI: 10.1038/s41586-021-03402-9. [DOI] [Google Scholar]
- 8.Faria NR, Claro IM, Candido D, et al. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings 2021. [cited 2021 February15]. Available from: https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586
- 9.PANGO lineages . B.1.1.7 report 2021. [cited 2021 February15]. Available from: https://cov-lineages.org/global_report_B.1.1.7.html
- 10.Volz E, Mishra S, Chand M, et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. medRxiv. 2021. DOI: 10.1101/2020.12.30.20249034. [DOI] [Google Scholar]
- 11.PANGO lineages . B.1.351 report 2021. [cited 2021 February15]. Available from: https://cov-lineages.org/global_report_B.1.351.html
- 12.PANGO lineages . P.1 report 2021. [cited 2021 February15]. Available from: https://cov-lineages.org/global_report_P.1.html
- 13.CGTN . Guangdong detects first COVID-19 case of variant found in S. Africa 2021. [cited 2021 February15]. Available from: https://news.cgtn.com/news/2021-01-06/Guangdong-detects-first-COVID-19-case-of-variant-found-in-S-Africa-WPW5sbHcju/index.html
- 14.Chen H, Huang X, Zhao X. et al. Notes from the field: the first case of new variant COVID-19 originating in the United Kingdom detected in a returning student — shanghai Municipality, China, December 14, 2020. China CDC Weekly. 2021;3(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccoli L, Park Y-J, Tortorici MA, et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–1042.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCallum M, Marco AD, Lempp F, et al. N-terminal domain antigenic mapping reveals a site of vulnerability for sars-cov-2. bioRxiv. 2021. DOI: 10.1101/2021.01.14.426475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerutti G, Guo Y, Zhou T, et al. Potent sars-cov-2 neutralizing antibodies directed against spike n-terminal domain target a single supersite. bioRxiv. 2021. DOI: 10.1101/2021.01.10.426120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes CO, Jette CA, Abernathy ME, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 variants from neutralization by convalescent plasma. Public Health. 2021. DOI: 10.1101/2021.01.26.21250224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. bioRxiv. 2021. DOI: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]; •• According to the results of this study, B.1.351 is more worrisome for not only being refractory to neutralization by most NTD mAbs but also to multiple individual mAbs to the receptor-binding motif on RBD, largely owing to an E484K mutation.
- 21.Garcia-Beltran WF, Lam EC, Denis KS, et al. Circulating SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. medRxiv. 2021. DOI: 10.1101/2021.02.14.21251704 [DOI] [Google Scholar]
- 22.Hou YJ, Chiba S, Halfmann P, et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2020;370(6523):1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniloski Z, Jordan TX, Ilmain JK, et al. The Spike D614G mutation increases SARS-CoV-2 infection of multiple human cell types. eLife. 2021;10. DOI: 10.7554/eLife.65365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozono S, Zhang Y, Ode H, et al. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun. 2021;12(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plante JA, Liu Y, Liu J, et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2020. DOI: 10.1038/s41586-020-2895-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Teodoro G, Valleriani F, Puglia I, et al. SARS-CoV-2 replicates in respiratory ex vivo organ cultures of domestic ruminant species. Vet Microbiol. 2021;252:108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenzo-Redondo R, Nam HH, Roberts SC, et al. A unique clade of SARS-CoV-2 viruses is associated with lower viral loads in patient upper airways. medRxiv. 2020. DOI: 10.1101/2020.05.19.20107144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muruato AE, Fontes-Garfias CR, Ren P, et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun. 2020;11(1):4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissman D, Alameh M-G, De Silva T, et al. D614G spike mutation increases SARS CoV-2 susceptibility to neutralization. Cell Host Microbe. 2021;29(1):23–31.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomson EC, Rosen LE, Shepherd JG, et al. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184(5):1171–1187.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The study shows that the N439K mutation confers resistance against several neutralizing monoclonal antibodies, including one authorized for emergency use by the US Food and Drug Administration.
- 31.Public Health England . Investigation of novel SARS-CoV-2 variant: variant of concern 202012/01. 2020. [cited 2021 Feb 15]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959360/Variant_of_Concern_VOC_202012_01_Technical_Briefing_3.pdf
- 32.O’Toole A, Hill V, Pybus OG, et al. Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 2021. [cited 2021 March6]. Available from: https://virological.org/t/tracking-the-international-spread-of-sars-cov-2-lineages-b-1-1-7-and-b-1-351-501y-v2/592 [DOI] [PMC free article] [PubMed]
- 33.Wang P, Nair MS, Liu L, et al. Increased resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7 to antibody neutralization. bioRxiv. 2021. DOI: 10.1101/2021.01.25.428137 [DOI] [Google Scholar]
- 34.Bal A, Destras G, Gaymard A, et al. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69–V70, France, August to December 2020. Eurosurveillance. 2021;26(3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Group NaERVTA . Meeting on SARS-CoV-2 variant under investigation VUI-202012/01. 2020. [cited 2021 February15]. Available from: https://khub.net/documents/135939561/338928724/SARS-CoV-2±variant±under±investigation%2C±meeting±minutes.pdf/962e866b-161f-2fd5-1030-32b6ab467896?t=1608470511452
- 36.Shen X, Tang H, Mcdanal C, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Social Science Electronic Publishing; 2021. Available from:https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3777473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier DA, Marco AD, Ferreira IATM, et al. Impact of SARS-CoV-2 B.1.1.7 spike variant on neutralisation potency of sera from individuals vaccinated with Pfizer vaccine BNT162b2. medRxiv. 2021. DOI: 10.1101/2021.01.19.21249840 [DOI] [Google Scholar]
- 38.Muik A, Wallisch A-K, Sänger B, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371(6534):eabg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerutti G, Guo Y, Zhou T, et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Virology. 2021. DOI: 10.1101/2021.01.10.426120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2020;29(1):44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baum A, Fulton BO, Wloga E, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369(6506):1014–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44–57.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Zheng H, Lin H, et al. Identification of common deletions in the spike protein of severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94(17):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9. DOI: 10.7554/eLife.61312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wibmer CK, Ayres F, Hermanus T, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021. DOI: 10.1101/2021.01.18.427166 [DOI] [PubMed] [Google Scholar]; •• The study confirmed that the B.1.351 variant exhibited complete escape from three classes of therapeutically relevant monoclonal antibodies.
- 46.Johnson & Johnson . Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its Phase 3 ENSEMBLE trial 2021. [cited 2021 February15]. Available from: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial
- 47.Witwatersrand U Oxford Covid-19 vaccine trial results 2021. [cited 2021 February15]. Available from: https://www.wits.ac.za/covid19/covid19-news/latest/oxford-covid-19-vaccine-trial-results.html
- 48.Novavax . Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK Phase 3 trial 2021. [cited 2021 February15]. Available from: https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3
- 49.Vaccine KK 2.0: moderna and other companies plan tweaks that would protect against new coronavirus mutations 2021. [cited 2021 February15]. Available from: https://www.sciencemag.org/news/2021/01/vaccine-20-moderna-and-other-companies-plan-tweaks-would-protect-against-new
- 50.PANGO lineages . A.23.1 report 2021. [cited 2021 February15]. Available from: https://cov-lineages.org/global_report_A.23.1.html
- 51.PANGO lineages . B.1.525 report 2021. [cited 2021 February15]. Available from: https://cov-lineages.org/global_report_B.1.525.html
- 52.Lule BD, Phan MVT, Ssewanyana I, et al. A SARS-CoV-2 lineage A variant (A.23.1) with altered spike has emerged and is dominating the current Uganda epidemic. medRxiv. 2021. DOI: 10.1101/2021.02.08.21251393 [DOI] [Google Scholar]
- 53.Zhang W, Davis BD, Chen SS, et al. Emergence of a novel SARS-CoV-2 variant in southern California. JAMA. 2021. DOI: 10.1101/2021.01.18.21249786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones BE, Brown-Augsburger PL, Corbett KS, et al. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. bioRxiv. 2020. DOI: 10.1101/2020.09.30.318972 [DOI] [Google Scholar]
- 55.Andreano E, Piccini G, Licastro D, et al. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. bioRxiv. 2020. DOI: 10.1101/2020.12.28.424451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Supasa P, Zhou D, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021. DOI: 10.1016/j.cell.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou D, Dejnirattisai W, Supasa P, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine induced sera. Cell. 2021. DOI: 10.1016/j.cell.2021.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jangra S, Ye C, Rathnasinghe R, et al. The E484K mutation in the SARS-CoV-2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post-vaccination sera. medRxiv. 2021. DOI: 10.1101/2021.01.26.21250543 [DOI] [Google Scholar]
- 59.Rathnasinghe R, Jangra S, Cupic A, et al. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post-vaccination human sera. medRxiv. 2021. DOI: 10.1101/2021.01.19.21249592 [DOI] [Google Scholar]
- 60.Xie X, Liu Y, Liu J, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021. DOI: 10.1038/s41591-021-01270-4 [DOI] [PubMed] [Google Scholar]
- 61.Xie X, Zou J, Fontes-Garfias CR, et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv. 2021. DOI: 10.1101/2021.01.07.425740 [DOI] [PubMed] [Google Scholar]
- 62.Wu K, Werner AP, Moliva JI, et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021. DOI: 10.1101/2021.01.25.427948 [DOI] [Google Scholar]
- 63.Huang B, Dai L, Wang H, et al. Neutralization of SARS-CoV-2 VOC 501Y.V2 by human antisera elicited by both inactivated BBIBP-CorV and recombinant dimeric RBD ZF2001 vaccines. bioRxiv. 2021. DOI: 10.1101/2021.02.01.429069. [DOI] [Google Scholar]; • This is the first study on the neutralization ability of inactivated SARS-CoV-2 vaccine against the B.1.351 variant.
- 64.Oxford University . Oxford vaccine effective against major B.1.1.7 ‘Kent’ coronavirus strain circulating in the UK 2021. [cited 2021 February15]. Available from: https://www.research.ox.ac.uk/Article/2021-02-05-oxford-vaccine-effective-against-major-b-1-1-7-kent-coronavirus-strain-circulating-in-the-uk
- 65.Oxford University . ChAdOx1 nCov-19 provides minimal protection against mild-moderate COVID-19 infection from B.1.351 coronavirus variant in young South African adults 2021. [cited 2021 February15]. Available from: https://www.research.ox.ac.uk/Article/2021-02-07-chadox1-ncov-19-minimal-protection-against-mild-covid-19-from-b-1-351-variant-in-young-sa-adults
- 66.Janet Woodcock MD Coronavirus (COVID-19) Update: FDA continues important work to support medical product development to address new virus variants 2021. [cited 2021 February15]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-continues-important-work-support-medical-product-development-address
- 67.CNN . Moderna expects vaccine will be protective against variants, but will test boosters to improve immunity. 2021. [cited 2021 February15]. Available from: https://www.wktv.com/content/news/573657962.html
- 68.Sputnik . The Russian direct investment fund is willing to start combined clinical trials of the Sputnik V and AstraZeneca vaccine in Ukraine 2021. [cited 2021 February15]. Available from: http://sputniknews.cn/covid-2019/202101031032825830/
- 69.Oxford University . Oxford leads first trial investigating dosing with alternating vaccines 2021. [cited 2021 February15]. Available from: https://www.research.ox.ac.uk/Article/2021-02-04-oxford-leads-first-trial-investigating-dosing-with-alternating-vaccines
- 70.Public Health England . COVID-19 vaccination programme Information for healthcare practitioners 2021. [cited 2021 February15]; Version 3.3 [Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/960977/COVID-19_vaccination_programme_guidance_for_healthcare_workers_11_February_2021_v3.3.pdf
- 71.Stephen M, Hahn MD. FDA Statement on Following the Authorized Dosing Schedules for COVID-19 Vaccines 2021. [cited 2021 February15]. Available from: https://www.fda.gov/news-events/press-announcements/fda-statement-following-authorized-dosing-schedules-covid-19-vaccines
- 72.Businesswire . Moderna COVID-19 Vaccine Retains Neutralizing Activity Against Emerging Variants First Identified in the U.K. and the Republic of South Africa 2021. [cited 2021 February15]. Available from: https://www.businesswire.com/news/home/20210125005439/en
- 73.GlaxoSmithKline. GSK and CureVac to develop next generation mRNA COVID-19 vaccines 2021. [cited 2021 February15]. Available from: https://www.gsk.com/en-gb/media/press-releases/gsk-and-curevac-to-develop-next-generation-mrna-covid-19-vaccines/


