Abstract
Colchicine is widely investigated for cardioprotection of COVID-19 patients since it can prevent the phenomenon of ‘cytokine storm’ and may reduce the complications arising from COVID-19. Despite the potentially beneficial effects of colchicine, there is no consensus on the appropriate dosage regimen and numerous schemes are currently used.
In this study, simulations were performed to identify the ability of dosage regimens to attain plasma levels in CVOID-19 patients, known to be generally safe and efficacious. Since renal and hepatic impairment, as well as, drug-drug interactions have been identified to be the most significant factors increasing colchicine toxicity, the impact of these interactions was assessed in the simulations.
Some dosage regimens lead to high colchicine concentrations, while others result in sub-therapeutic levels. Additional dosage schemes were proposed in this study aiming to be applied in patients with clearance insufficiency. Colchicine administration of 0.5 mg twice daily, can be considered safe and effective. In cases of clearance impairment, doses as low as 0.25 mg thrice or twice daily should be applied.
Colchicine is a narrow therapeutic index drug and dosage regimens tailored to patients’ needs should be designed.
Keywords: Colchicine, COVID-19, simulations, dosage regimens, cardioprotection, pharmacokinetics
Introduction
Colchicine is an alkaloid present in the plants Colchicium autumnale and Gloriosa superba, that has been used in therapeutics for its anti-inflammatory properties for ages (Putterman et al. 1991, Bhat et al. 2009, Terkeltaub 2009). Despite the anti-inflammatory actions of colchicine expressed through the different mechanisms, its role in pharmacotherapy remains controversial, due to the serious adverse effects that even low doses of the drug can induce (Solak et al. 2015a, 2015b, Slobodnick et al. 2018). Colchicine is currently categorised as a narrow therapeutic index drug (European Medicines Agency 2019).
Nowadays, colchicine is indicated for the treatment and prophylaxis of acute gout, in familial Mediterranean fever, for the management of Behçet disease, and cardiovascular diseases like pericarditis, stroke prevention, and IgA vasculitis (Saleh and Arayssi 2014, Schwier 2015, Audemard-Verger et al. 2017, Masson et al. 2020, Colchicine 500mcg tablets 2021, Colcrys Label 2012, Gloperba Label 2021). In addition, clinical studies support its cardioprotective effects and its beneficial effects in atherosclerosis, myocardial infarction, and cardioprotection (Gasparyan et al. 2015. Spartalis et al. 2018, Tardif et al. 2019, ClinicalTrials.gov Identifier: NCT04181996 2021, ClinicalTrials.gov Identifier: NCT02551094 2021) or versus atorvastatin (ClinicalTrials.gov Identifier: NCT04056039 2021).
In view of the recent COVID-19 pandemic caused by SARS-CoV-2, colchicine has been proposed as a therapeutic option for the management of the cardiac complications that the disease can induce. It has been observed that COVID-19 in many cases induced myocarditis and inflammation due to cardiorespiratory distress, that colchicine can alleviate primarily through inhibition of NLRP3 inflammasome (Deftereos et al. 2020a, Deftereos et al. 2020b). In this context, several clinical trials were conducted to investigate the anti-inflammatory and cardioprotective properties of colchicine in COVID-19 patients (ClinicalTrials.gov Identifier: NCT04322565 2021, ClinicalTrials.gov Identifier: NCT04322682 2021, Clinical trial: The ECLA PHRI COLCOVID Trial 2021, ClinicalTrials.gov Identifier: NCT04326790 2021). The COLCORONA trial showed that colchicine may reduce the composite rate of death or hospitalization by preventing the phenomenon of “cytokine storm” and subsequently reducing the complications arising from COVID-19 (COLCORONA trial 2021). Quite recently, the PRINCIPLE trial widened the age limits for participation and currently COVID-19 outpatients between 18 and 64 with shortness of breath or certain underlying health conditions, which put them at risk of severe illness, can join the trial (PRINCIPLE trial 2021). Thus, colchicine is under extensive investigation for its cardioprotective potential in COVID-19 patients.
Colchicine is a lipophilic drug exhibiting a highly variable bioavailability with a mean of 45% (Bhat et al. 2009, Davis et al. 2013, Tong et al. 2016). In plasma, 30–50% of the drug binds to albumin, while it quickly enters the peripheral leucocytes where it accumulates reaching concentrations exceeding those in plasma (Levy et al. 1991, Sapra et al. 2013, Tong et al. 2016). It has been proposed that leucocytes constitute the deep colchicine microcompartment, where the anti-inflammatory effects take place (Sapra et al. 2013). The distribution half-life of colchicine is 1–2.7 h. Colchicine is metabolised by the liver and the intestine where it undergoes oxidative demethylation by CYP3A4 (Bhat et al. 2009, Davis et al. 2013, Tong et al. 2016). Colchicine is primarily eliminated by hepatobiliary excretion through the stool, while only 10% of the drug undergoes renal excretion.
A relationship between colchicine plasma levels and pharmacological effects has not been clearly defined, since colchicine effects are related to the concentrations in leukocytes, rather than the ones in plasma (Rochdi et al. 1994, Niel and Scherrmann 2006). However, it has been proposed that the effective steady-state range of colchicine plasma concentrations is 0.5–3.0 μg/l (Molad 2002). The time-course of the pharmacodynamic effects of colchicine is closely correlated to that of the drug concentrations in leukocytes. The expression of colchicine toxicity results as an extension of its mechanism of action, implying that colchicine toxicity is dose-related. In observational studies, doses of 0.5–2.0 mg daily are considered relatively safe without major gastrointestinal adverse effects (Ehrenfeld et al. 1982), while doses over 2.0 mg are recommended only for very short periods (Ben-Chetrit and Levy 1998). Plasma levels above 3.0 μg/l have been proposed to be responsible for the induction of mild toxic effects in some healthy volunteers (Wallace et al. 1970, Wallace and Ertel 1973, Molad 2002). Even though setting up a dosage regimen is of paramount importance, consensus regarding the appropriate dosing scheme for each indication has not yet been reached and doses/duration of treatment applied in clinical practice or investigated in clinical trials vary significantly. This situation can be ascribed to the fact that many times, dosing selection is made empirically, and sometimes dose adjustment is needed according to the patient’s response (Gasparyan et al. 2015, Ozen et al. 2016, Masson et al. 2020). Table 1 provides a summarised list of the dosage schemes proposed for the treatment of Beçhet’s disease, familial Mediterranean fever, acute gout, pericarditis, atrial fibrillation, stroke prevention, myocardial infarction, coronary artery disease, and cardioprotection (Table 1). Due to the COVID-19 pandemic, a large number of clinical trials are currently ongoing for the investigation of colchicine use in COVID-19 patients (Table 2). Another approach is to apply modelling and simulation approaches in order to explore and propose the appropriate dosing regimen. These techniques have been successfully applied in the case of other medicines tested in COVID-19 patients, like chloroquine, hydroxychloroquine, etc. (Arnold and Buckner 2020, Karalis et al. 2020, Perinel et al. 2020, Yao et al. 2020, Karatza et al. 2021).
Table 1.
Representative dosing schemes of colchicine for various indications currently applied in clinical practice or investigated in clinical trials.
| Indication | Dosage scheme | References |
|---|---|---|
| Beçhet’s disease | 0.6 mg oral twice daily in a period of 5 weeks. | Raynor and Askari 1980 |
| 1–2 mg daily for 3-months | Woo et al.2012 | |
| Colchicine 1 mg/day | Saleh and Arayssi 2014 | |
| Familial Mediterranean fever | Adults and Children older than 12 years: 1.2–2.4 mg Children 6 to 12 years: 0.9–1.8 mg Children 4 to 6 years: 0.3–1.8 mg |
Colcrys Label 2012 |
| Adults: 1.0 mg per day | Cerquaglia et al.2005 | |
| 1.0–2.0 mg daily for 3 years | Ehrenfeld et al.1982 | |
| Adults: <3 mg Children: <2 mg |
Gül 2014 | |
| Children <5 years: a starting dose of ≤0.5 mg/day (≤0.6 mg/day in case of 0.6 mg tablets) Children 5–10 years: 0.5–1.0 mg/day (1.2 mg/day in case 0.6 mg tablets Adults and Children >10 years: 1.0–1.5 mg/day (1.8 mg/day in case of 0.6 mg tablets) |
Ozen et al.2016 Colchicine 500mcg tablets 2021 |
|
| Patients older than 16 years: 0.5 or 0.6 mg twice daily | Peters et al.1983 | |
| Acute gout | 0.6 mg once daily or twice daily (maximum dose 1.2 mg/day) | Colcrys Label 2012 |
| 1 mg (2 tablets of 0.5 mg) to be taken initially, followed by 0.5 mg 1 h later, and then followed, as needed, after 12 h, by continued colchicine (up to 0.5 mg twice daily) until the acute attack resolves Prophylaxis: 0.5 mg twice daily |
Colchicine 500mcg tablets 2021 |
|
| Low-dose: 1.2 mg followed by 0.6 mg in 1 h (1.8 mg total) High-dose: 1.2 mg followed by 0.6 mg every hour for 6 h (4.8 mg total) Single-dose: 0.6 mg |
Terkeltaub et al.2010 | |
| 0.5 mg twice daily for 4 days | Roddy et al.2020 | |
| Pericarditis | If weight >70 kg: 0.5 mg twice daily for 3 months If weight ≤70 kg: 0.5 mg daily for 3 months |
Tong et al.2016 |
| 1–2 mg on day 1, then 0.5–1 mg daily for 6 months | Tong et al.2016 | |
| Loading dose of 1–2 mg followed by maintenance dose of 0.5–1 mg/day | Schwier 2015 | |
| Loading dose of 1.2–1.8 mg followed by maintenance dose of 0.3–1.2 mg daily (once or twice daily) | Schwier 2015 | |
| Atrial fibrillation | 0.5 mg twice daily | Lennerz et al.2017 Deftereos et al.2012 |
| Stroke prevention | 0.5–1 mg once daily | Masson et al.2020 |
| Vascular inflammation prevention | 0.5 mg once daily for 60 months | ClinicalTrials.gov Identifier: NCT02898610 2021 (CONVINCE trial) |
| Myocardial infraction | 0.5 mg once daily | Tardif et al.2019 Bouabdallaoui et al.2020 (COLCOT trial) |
| Acute coronary syndrome | 0.5 mg twice daily for first month, then 0.5 mg daily for eleven months | Tong et al.2020 (COPS trial) |
| Coronary artery disease | 0.5 mg twice daily for 1 month | Nidorf and Thompson 2007 |
| 0.5 mg once daily for a median of 3 years | Nidorf et al.2013 (LoDoCo trial) Nidorf et al. 2020 (LoDoCo2 trial) |
|
| 2 mg loading dose followed by 0.5 mg twice daily for 5 days | Deftereos et al.2015 | |
| 0.5 mg twice daily for 6 months | Deftereos et al.2013 | |
| Cardio protection | 0.6 mg capsule once daily for 6 months | ClinicalTrials.gov Identifier: NCT04181996 2021 |
| 0.5 mg once daily | ClinicalTrials.gov Identifier: NCT02551094 2021 | |
| 0.5 mg twice daily for 1 year | ClinicalTrials.gov Identifier: NCT03704181 2021 | |
| Initial dose of 0.25 mg twice daily, with titration in the first 3 days according to tolerance up to a maximum dose of 0.5 mg twice daily for 4 weeks in decrease of troponin I of high sensitivity | ClinicalTrials.gov Identifier: NCT04056039 2021 |
Table 2.
Colchicine dosage schemes for cardioprotection in patients with COVID-19 pneumonia.
| Description | Study |
|---|---|
| Inpatient: 1 mg (or 0.5 mg in chronic kidney disease) once daily | ClinicalTrials.gov Identifier: NCT04322565 2021 (Italy, ColCOVID) |
| Early inpatient: 1 mg (reduced to 0.5 mg if severe diarrhoea) once daily | Scarsi et al.2020 |
| Early outpatient: 1 mg once daily (or 0.5 mg twice daily) for the first 3 days and then 0.5 mg once daily for 27 days | ClinicalTrials.gov Identifier: NCT04322682 2021 (Canada, COLCORONA) |
| Outpatient: 1 mg twice daily for the first day, then 1 mg daily until afebrile for 3 days | Della-Torre et al.2020 |
| Early outpatient >70 years: 0.5 mg twice daily for 3 days, then 0.5 mg once daily for 18 days |
ClinicalTrials.gov Identifier: NCT04416334 2021 (Spain, COLCHI-COVID) |
| Moderate to severe inpatient: 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days | Lopes et al.2020 |
| Inpatient: 0.6 mg twice daily for 30 days | ClinicalTrials.gov Identifier: NCT04355143 2021 (USA, COLHEART-19) |
| 0.5 mg twice daily for 3 days, then 0.5 mg once or twice daily for 7 days | ClinicalTrials.gov Identifier NCT04403243 2021 (Russia, COLORIT) |
| Early outpatient: 0.6 mg twice daily for 3 days, then 0.6 mg daily for 25 days. Early inpatient: 1.2 mg loading dose, then 0.6 mg 2 h later, then 0.6 mg twice daily for 28 days |
ClinicalTrials.gov Identifier: NCT04324463 2021 (Canada, ACTCOVID19) |
| Moderate to severe hospitalised patients before ARDS: 1.2 mg loading dose, then 0.6 mg 2 h later, then 0.6 mg twice daily for 14 days or until discharge | ClinicalTrials.gov Identifier: NCT04363437 2021 (USA, COMBATCOVID19) |
| Early inpatient: 0.5 mg twice daily for 30 days 1 mg twice daily if weight >100 kg | ClinicalTrials.gov Identifier: NCT04375202 2021 (Italy, COLVID-19) |
| Inpatient not receiving L/R: 1.5 mg followed by 0.5 mg after two h (day 1). The next day 0.5 mg twice daily for 14 days or until discharge. Inpatient receiving L/R: Loading dose of 0.5 mg (day 1). After 72 h from the loading dose, 0.5 mg every 72 h for 14 days or until discharge Inpatient under Colchicine treatment that are starting with L/R: 0.5 mg 72 h after starting L/R. then 0.5 mg every 72 h for 14 days or until discharge |
Clinical trial: The ECLA PHRI COLCOVID Trial 2021 (Argentina, ECLA PHRI COLCOVID) |
| Inpatient: Loading dose of 1 mg, then 0.5 mg 2 h after, followed by 0.5 mg twice daily during the next 7 days and 0.5 mg once daily until the completion of 14 days of total treatment. In patients receiving R/L or with eGFR < 50 ml/min/1.37 m2, weight <70 kg or age >75 years old, the dose is adjusted to the half |
ClinicalTrials.gov Identifier: NCT04667780 2021 (Pakistan) ClinicalTrials.gov Identifier: NCT04603690 2021 (Pakistan) |
| Early inpatient: Loading dose of 1 mg, then 0.5 mg 2 h later, then 0.5 mg twice daily for 7 days, then 0.5 mg daily for 28 days in total | ClinicalTrials.gov Identifier: NCT04350320 2021 (Spain, COL-COVID) |
| Early inpatient: Loading dose of 1.5 mg, then 0.5 mg twice daily until discharge | ClinicalTrials.gov Identifier: NCT04360980 2021 (Iran) |
| Inpatient: Loading dose of 1.5 mg, then 0.5 mg twice daily for 10 days | ClinicalTrials.gov Identifier NCT04367168 2021 (Mexico, ColchiVID) |
| Early inpatient: Loading dose of 1.5 mg, then 0.5 mg after 1 h, then 0.5 mg twice daily for as long as 3 weeks | ClinicalTrials.gov Identifier: NCT04326790 2021 (Greece, GRECCO-19) Deftereos et al.2020a, 2020b |
| Early inpatient: 0.5 mg twice daily or 1 mg once daily | ClinicalTrials.gov Identifier: NCT04326790 2021(Greece, GRECCO-19) |
| Early inpatient: 1.2 mg twice daily for the first day, then 0.6 mg daily for 13 days | ClinicalTrials.gov Identifier: NCT04527562 2021 (Bangladesh, COLCOVIDBD) |
| Early inpatient: Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg twice daily for a further 9 days or until hospital discharge. Maintenance dose 0.5 mg once daily when administered with CYP3A4 inhibitors (e.g. diltiazem, imatinib, letermovir), or renal impairment (eGFR < 30 mL/min/1.73 m2) either chronic or acute, or BW <70 kg |
ClinicalTrials.gov Identifier: NCT04381936 2021 (UK, RECOVERY) |
L/R: Lopinavir/Ritonavir; eGFR: estimated glomerular filtration rate.
The purpose of the present study was to identify the ability of colchicine regimens to attain plasma levels known to be generally safe and efficacious. Simulated plasma colchicine concentrations versus time profiles were generated for the majority of dosage schemes presented in Table 2, which included loading doses and administrations from one to three times daily. The predicted colchicine concentrations were assessed in terms of the desired levels to achieve efficacy and avoid toxicity. Special focus was made on certain conditions like renal/hepatic impairment of the patients, as well as colchicine interaction with other COVID-19 treatments, like lopinavir/ritonavir or azithromycin. Finally, new colchicine dosage regimens were explored through simulations intended to be used in patients with several levels of clearance impairment. Finally, specific dosage regimens were proposed for cardioprotection in COVID-19 patients.
Materials and methods
Model and doses selection for the simulations
A total of four different compartmental pharmacokinetic models of colchicine were identified in the literature (Thomas et al. 1989, Rochdi et al. 1994, Berkun et al. 2012, Ferron et al. 1996). In the study of Thomas et al., plasma samples were collected from nine healthy volunteers, pre-dose and at 0.25, 0.50, 1.00, 1.50, 2.00, 3.00, 4.00, 5.00, 6.00, 7.00, 9.00, 24.00, 32.00, and 48.00 h after oral administration of 0.5, 1.0, or 1.5 mg colchicine. The model that best described their data was a two-compartment model with zero-order absorption and lag time. The parameters estimated were found to be dose-dependent, while inter-individual variability was also estimated. In the study of Ferron et al., plasma samples were collected from 6 healthy volunteers, pre-dose and 0.08, 0.17, 0.25, 0.33, 0.42, 0.50, 0.58, 0.67, 0.75, 0.83, 0.92, 1.00, 1.17, 1.33, 1.50, 2.00, 3.00, 4.00, 5.00, 6.00, 7.00, 8.00, 9.00, 24.00, 32.00, 48.00 h after oral administration of 1.0 mg colchicine (Ferron et al. 1996). The model that best described their data was a three-compartments model with two successive zero-order absorption phases and lag time. They also reported inter-individual variability of the parameter estimates. In the study of Rochdi et al., plasma samples were collected from six healthy volunteers, pre-dose and at 5, 10, 15, 20, 30, 35, 40, 45, 50, 55, 60, 70, 80, and 90 min and then at 2.00, 3.00, 4.00, 5.00, 6.00, 7.00, 8.00, 9.00, 24.00, 32.00, and 48.00 h after intravenous administration of 0.5 mg colchicine. They developed a model based on intravenous data which included three-compartments, while they also estimated inter-individual variability. They also collected data after oral administration of 1.0 mg colchicine that was used for estimation of the absorption lag-time; however, the absorption process was not fully characterised. In the study of Berkun et al., for the population pharmacokinetic analysis, plasma samples were collected at steady-state from twelve healthy adults during multiple oral doses (0.6 mg twice daily) study, pre-dose and 0.25–0.50, 1.50–2.50, 3.00–5.00, 7.00–9.00, and 24.00 h after oral administration. The model that best described their data was a three-compartment model with zero-order absorption, while inter-individual variability was not reported. Age or weight was not identified as a significant covariate in any study.
After evaluation of the abovementioned population pharmacokinetic analyses, the model developed by Thomas et al. was selected in this study in order to perform the simulations of the colchicine dosage regimens (Figure 1). This choice was made since the Thomas et al. study included an adequate number of subjects (compared to the other three studies), the sampling scheme was appropriate for the characterization of colchicine pharmacokinetics, inter-individual variability was estimated, and the goodness of fit plots and criteria showed its nice descriptive ability (Thomas et al. 1989). The Thomas et al. study included three doses allowing thus the estimation of the dose-dependent pharmacokinetic parameters.
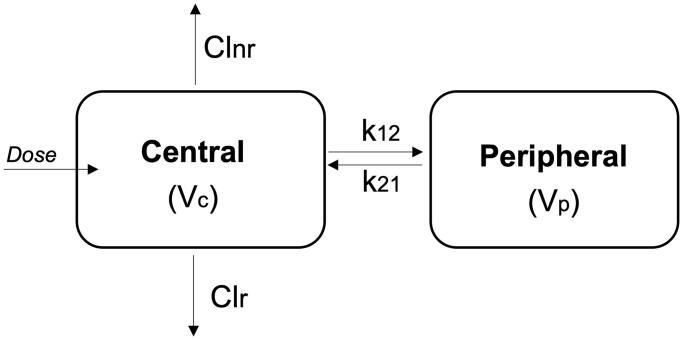
Figure 1.
A schematic representation of the pharmacokinetic model used for the simulations. This model was obtained from the study of Thomas et al. (1989). Colchicine is distributed in two compartments, the central and the peripheral compartment. Key: Vc, volume of distribution of the central compartment; Clr, first-order renal clearance from the central compartment; Clnr, first-order non-renal clearance from the central compartment; k12, first-order inter-compartmental transfer constant from the central to the peripheral compartment; k21, first-order inter-compartmental transfer constant from the peripheral to the central compartment.
In addition, given the strong effect of renal and/or hepatic impairment, as well as, the effect of drug–drug interactions on colchicine clearance, several additional scenarios of impaired clearance were also tested. Based on the study of Wason et al., the % difference in renal clearance compared to healthy volunteers is 13, 45, and 54% for mild (60–89 ml/min/1.73 m2), moderate (30–59 ml/min/1.73 m2), and severe (15–29 ml/min/1.73 m2) renal impairment, respectively (Wason et al. 2014). Given that renal clearance accounts for 10% of total colchicine clearance (Niel and Scherrmann 2006), the estimated population parameter of clearance for patients (with mild, moderate, and severe clearance) was used in our simulations.
Similarly, in the study of Leighton et al. it was shown that in cirrhotic patients, colchicine total clearance is reduced by almost 60% (Leighton et al. 1991). Another study reported that colchicine total clearance is reduced by 30% when co-administered with azithromycin, by 75% when co-administered with clarithromycin, by 40% when coadministered with diltiazem, by 52% when co-administered with verapamil and by 70% when co-administered with ketoconazole or ritonavir, due to CYP3A4 or P-gp mediated pharmacokinetic interactions (Terkeltaub et al. 2011).
Simulations
A pharmacokinetic model obtained from the literature was utilised for the simulations (Thomas et al. 1989). The latter consists of a two-compartment model with zero-order absorption and lag time. The average parameter values along with their variabilities are presented in Table 3. Due to the dose-dependency of the parameters, the parameter values are quoted for three dose levels (0.5, 1.0, and 1.5 mg). Inter-individual variability was also taken into account and a population of 500 patients was simulated. Three scenarios of impaired total colchicine clearance (30, 50, and 70%), which may account for renal and/or hepatic impairment and for pharmacokinetic drug interactions were explored in this study.
Table 3.
Pharmacokinetic parameter values used in the simulations of colchicine.
| Parameters | Parameter estimates | Inter-individual variabilitya |
|---|---|---|
| Dose: 0.5 mg | ||
| tlag (h) | 0.12 | 0.606 |
| Duration (h) | 0.99 | 0.551 |
| Vc/F (l) | 202 | 0.355 |
| Cl/F (l/h) normal renal function | 31.7 | 0.242 |
| Cl/F (l/h) 30% impaired | 22.19 | |
| Cl/F (l/h) 50% impaired | 15.85 | |
| Cl/F (l/h) 70% impaired | 9.51 | |
| Dose: 1.0 mg | ||
| tlag (h) | 0.16 | 0.418 |
| Duration (h) | 0.89 | 0.496 |
| Vc/F (l) | 229 | 0.452 |
| Cl/F (l/h) normal renal function | 33.7 | 0.248 |
| Cl/F (l/h) 30% impaired | 23.59 | |
| Cl/F (l/h) 50% impaired | 16.85 | |
| Cl/F (l/h) 70% impaired | 10.11 | |
| Dose: 1.5 mg | ||
| tlag (h) | 0.22 | 0.224 |
| Duration (h) | 0.79 | 0.321 |
| Vc/F (l) | 197 | 0.279 |
| Cl/F (l/h) 30% impaired | 23.59 | |
| Cl/F (l/h) 50% impaired | 16.85 | |
| Cl/F (l/h) 70% impaired | 10.11 | |
| For all doses: | ||
| k12 (h−1) | 0.22 | 0.268 |
| k21 (h−1) | 0.073 | 0.501 |
These values were obtained from the literature (Thomas et al. 1989). The structural model refers to two-compartment model with zero-order absorption and lag time. Due to dose-dependency of the parameters, the parameter values are quoted for three dose levels (0.5 mg, 1.0 mg, and 1.5 mg).
Inter-individual variability refers to the standard deviation of the parameter reported in Thomas et al. (1989).
: tlag, absorption lag time; Duration, duration of absorption calculated as the difference between end of absorption and tlag; Vc/F, apparent volume of distribution of the central compartment; Cl/F, apparent first-order clearance from the central compartment; k12, first-order inter-compartmental transfer constant from the central to the peripheral compartment; k21, first-order inter-compartmental transfer constant from the peripheral to the central compartment.
The inter-individual variability values, considered for all pharmacokinetic parameters, were those reported in the study of Thomas et al. (1989). For the simulations exploring the impact of clearance impairment on colchicine levels, only the average performance was simulated, in order to allow a clearer observation of the plots. The dosing schemes tested explored are summarised in Table 4. When co-administration with other medications was simulated, the pharmacokinetic parameter values were adjusted appropriately to be in accordance with the literature suggestions.
Table 4.
Colchicine dosage schemes used in the simulations.
| Name | Dosage scheme | Indication | References |
|---|---|---|---|
| A | 1 mg once daily |
|
Woo et al.2012 Saleh and Arayssi 2014 Ehrenfeld et al.1982 ClinicalTrials.gov Identifier: NCT04322565 2021 (Italy) |
| B | 0.5 mg once daily |
|
Ozen et al.2016 Colchicine 500mcg tablets SmPC 2021 Tardif et al.2019 Nidorf et al.2013 ClinicalTrials.gov Identifier: NCT02551094 2021 (Canada) ClinicalTrials.gov Identifier: NCT04322565 2021 (Italy) |
| C | 0.5 mg twice daily |
|
Colchicine 500mcg tablets SmPC 2021 Peters et al. 1983 Nidorf and Thompson 2007 Tong et al.2016 Lennerz et al.2017 ClinicalTrials.gov Identifier: NCT03704181 2021 (Brazil) Clinical Trials.gov Identifier: NCT04326790, 2020 (Greece, GRECCO-19). |
| D | Loading dose of 1.5 mg, followed by 0.5 mg after 2 h and then 0.5 twice daily for 14 days |
|
Schwier 2015 Clinical trial: The ECLA PHRI COLCOVID Trial 2021 (Argentina) |
| E | 0.5 mg twice daily for the first 3 days and then 0.5 once daily for 27 days |
|
ClinicalTrials.gov Identifier: NCT04322682 2021 (Canada, COLCORONA) |
| F | Loading dose of 1.5 mg, followed by 0.5 mg after 1 h and then 0.5 twice daily |
|
Schwier 2015 Deftereos et al.2015 Deftereos et al.2020b |
| G | Loading dose of 0.5 mg (day 1) – After 72 h from the loading dose, 0.5 mg every 72 h for 14 days or until discharge (co-administration with ritonavir, thus colchicine clearance would be 70% impaired clearance) |
|
Clinical trial: The ECLA PHRI COLCOVID Trial 2021 (Argentina) |
| H | Loading dose of 1 mg, followed then 0.5 mg twice daily (co-administration with azithromycin thus colchicine clearance would be 30% impaired clearance) |
|
Schwier 2015 Deftereos et al. 2020c |
| I | Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg twice daily for a further of nine days (10 days in total) or until hospital discharge Co-administration with CYP3A4 inhibitors (e.g. diltiazem, imatinib, letermovir): Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg once daily. |
|
ClinicalTrials.gov Identifier: NCT04381936 2021 (UK, Recovery trial) |
| J | Colchicine regimen was 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days. Patients with chronic kidney disease, with glomerular filtration rate under 30 mL/min/1.73 m2, colchicine dose is reduced to 0.25 mg thrice daily for 5 days, then 0.25 mg twice daily for 5 days. |
|
Lopes et al.2020 |
The indication and the bibliographic references are quoted.
Simulations were performed in Simulx® (Monolix 2019R2™, Simulation Plus). The R package ‘mlxR 4.2′ was used in order to perform the simulations (mlxR: Simulation of Longitudinal Data 2021).
Results
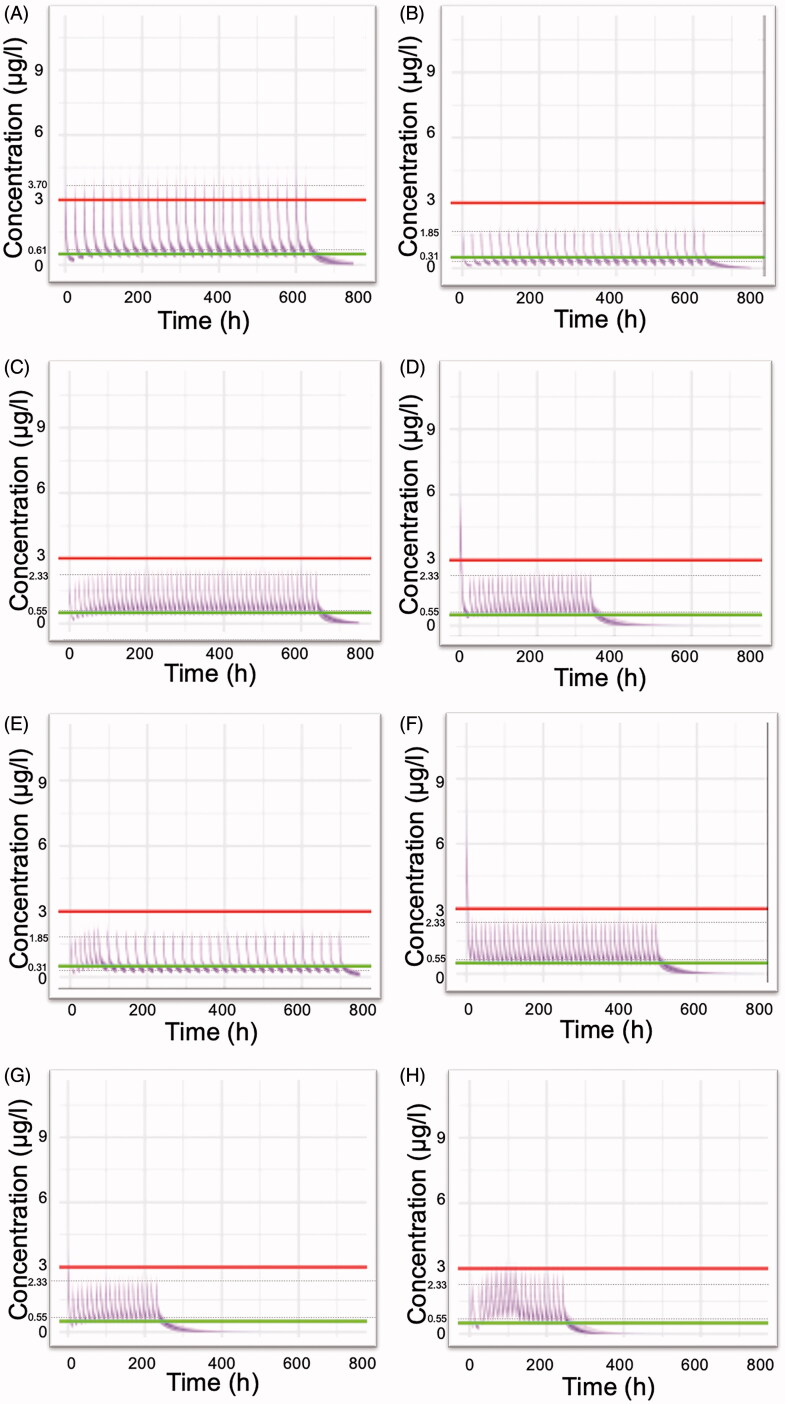
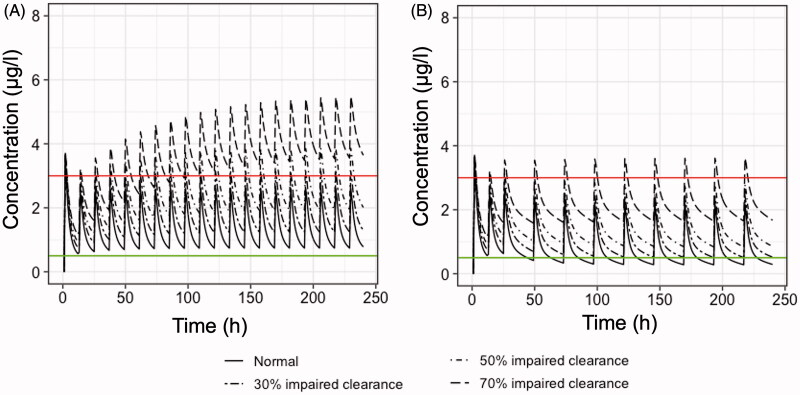
A previously developed pharmacokinetic model was used in order to perform simulations and to explore various dosing schemes of colchicine applied in clinical practice. Figure 2 reveals the anticipated concentration-time colchicine levels after administration of the dosing regimens listed in Table 4. Visual inspection of Figure 2 reveals that several regimens lead to increased plasma levels (e.g. Figure 2(A)), while some others (Figure 2(B,E)) result in levels that are lower than the minimum effective concentrations. When colchicine levels exceed the value of 3.0 μg/l, then there is a higher chance for the induction of toxic effects. Loading doses as high as 1.5 mg have increased chances to cause gastrointestinal discomfort that may lead to poor adherence to therapy (Figure 2(D,F)). A scheme implying 0.5 mg twice daily seemed to maintain more effective therapeutic levels, with a low probability to reach toxic plasma concentrations (Figure 2(C)). The dosing regimen utilised in the RECOVERY trial results in colchicine plasma levels that are in the desired region (Figure 2(G)). Also, satisfactory results are obtained from a scheme including thrice administration of 0.5 mg for the first five days (Figure 2(H)).
Figure 2.
Simulated plasma colchicine concentrations (μg/l) versus time (h) according to dosing schemes (Table 4) currently used in clinical practice or in clinical trials. (A) 1 mg once daily, (B) 0.5 mg once daily, (C) 0.5 mg twice daily, (D) Loading dose of 1.5 mg, followed by 0.5 mg after 2 h and then 0.5 twice daily for 14 days, (E) COLCORONA trial: 0.5 mg twice daily for the first 3 days and then 0.5 once daily for 27 days, (F) GRECCO-19 trial: Loading dose 1.5 mg, followed by 0.5 mg after 1 h and then 0.5 twice daily. (G) RECOVERY trial: Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg twice daily for a further of nine days (10 days in total) or until hospital discharge, (H) 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration. The inter-individual variability values, considered for all pharmacokinetic parameters, were those reported in the study of Thomas et al. (1989). Dashed lines indicate the average peak and trough concentration values at steady state.
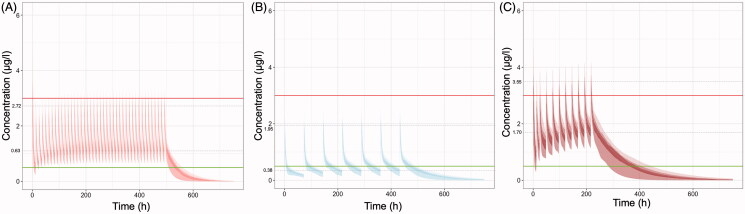
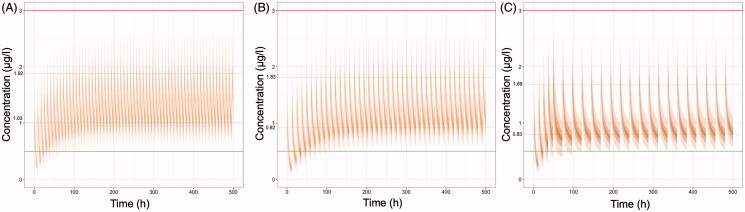
Simulated C-t profiles in case of co-administration of colchicine with other popular treatments in COVID-19, like lopinavir/ritonavir, azithromycin, or with CYP3A4 inhibitors (like diltiazem, imatinib, letermovir), are depicted in Figure 3. The dose adjustment proposed in the literature accounting for co-administration of azithromycin that causes a 30% reduction of total colchicine clearance (Terkeltaub et al. 2011) results in toxic plasma concentrations and therefore further dose reductions should be considered (Figure 3(A)). On the contrary, the dose adjustment proposed in the literature, accounting for co-administration of ritonavir that causes a 70% reduction of total colchicine clearance (Terkeltaub et al. 2011), results in sub-therapeutic trough concentrations (Figure 3(B)). The modified regimen of the RECOVERY trial suggested to be used when colchicine is co-administered with CYP3A4 inhibitors, still leads to high plasma levels (Figure 3(C)) and thus, increased risk for adverse effects.
Figure 3.
Simulated plasma colchicine concentrations (μg/l) versus time (h) in case of its co-administration with other medicines. (A) Co-administration with azithromycin (thus colchicine clearance would be reduced by 30%): Loading dose of 1 mg, followed then 0.5 twice daily, (B) Co-administration with lopinavir/ritonavir (thus colchicine clearance would be reduced by 70%): Loading dose of 0.5 mg and then after 72 h 0.5 mg every 72 h for 14 days or until discharge, (C) Co-administration with CYP3A4 inhibitors (e.g. diltiazem, imatinib, letermovir) [RECOVERY trial]: Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg once daily. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration. The inter-individual variability values, considered for all pharmacokinetic parameters, were those reported in the study of Thomas et al. (1989). Dashed lines indicate the average peak and trough concentration values at steady state.
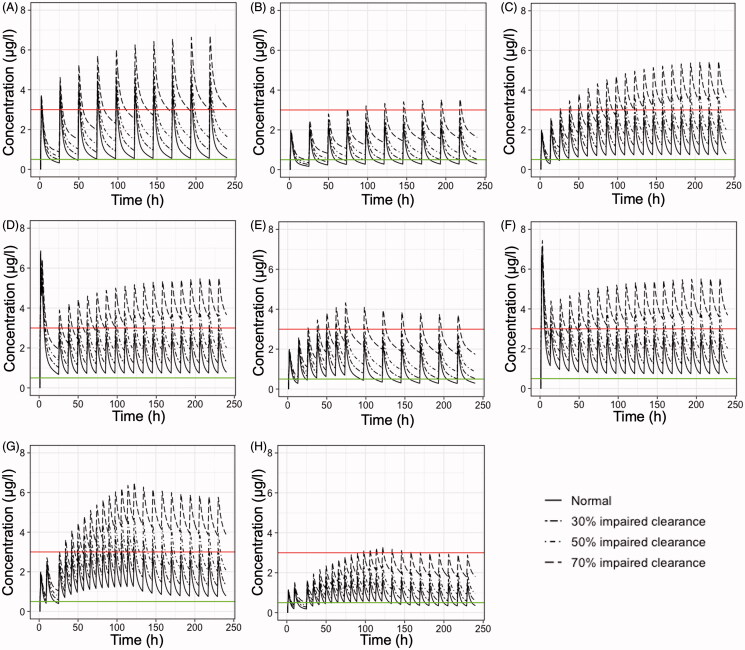
In order to investigate the impact of reduced clearance on colchicine plasma levels, simulations were performed utilizing several dosage regimens from the literature (Table 4). Figure 4 presents the results of the simulated plasma colchicine concentrations versus the time of patients with normal clearance, and three levels of impaired total clearance (i.e. 30, 50, and 70%). The latter can be attributed to pharmacokinetic interactions and/or co-existence of liver and hepatic diseases. The findings of Figure 4 are mainly in agreement with those from Figure 1; it is shown that for the majority of regimens, the concentration levels of colchicine fail to stay within the adequate range of plasma levels and that most of them lead to high concentrations with increased toxicity risk. The only exception is the scheme where colchicine is administered 0.25 mg thrice daily (Figure 4(H)) which can almost adequately account for 30, 50, or 70% reductions in colchicine clearance.
Figure 4.
Simulated mean plasma colchicine concentrations (μg/l) versus time (h) of patients with normal clearance, 30% impaired total clearance, 50% impaired total clearance, and 70% impaired total clearance. Eight different dosage regimens (Table 4) are simulated as following: (A) 1 mg once daily, (B) 0.5 mg once daily, (C) 0.5 mg twice daily, (D) Loading dose of 1.5 mg, followed by 0.5 mg after 2 h and then 0.5 twice daily for 14 days, (E) 0.5 mg twice daily for the first 3 days and then 0.5 once daily for 27 days, (F) Loading dose of 1.5 mg, followed by 0.5 mg after 1 h and then 0.5 mg twice daily, (G) 0.5 mg thrice daily for 5 days, then 0.5 mg twice daily for 5 days, (H) 0.25 mg thrice daily for 5 days, then 0.25 mg twice daily for 5 days. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration.
In the simulations performed in this study, special focus was placed on the dosing regimens proposed by the RECOVERY trial (CTI: NCT04381936). In this trial, it was generally proposed that a loading dose of 1 mg is administered, followed by 0.5 mg 12 h later, and then a maintenance dose 0.5 mg twice daily for a further 9 days or until hospital discharge. This is the typical scheme that is simulated in the case of normal clearance patients and at three levels of reduced clearance (30, 50, and 70%) (Figure 5(A)). Visual observation of Figure 5(A) clearly reveals that this scheme is appropriate only in cases of normal clearance and for clearance impairment up to 30%. When the elimination ability of the patients is reduced, then this dosage regimen would lead to increased colchicine levels. Nevertheless, it is stated in the RECOVERY trial that when colchicine is co-administered with CYP3A4 inhibitors or when creatinine clearance is significantly reduced, an alternative dosage should be applied. In particular, when the estimated glomerular filtration rate is less than 30 ml/min, then again, an initial loading dose of 1 mg is administered, followed by 0.5 mg 12 h later, and then a maintenance dose of 0.5 mg once daily. This dosage scheme is simulated in Figure 5(B), where it can be observed that an adequate range of plasma levels is achieved, for reduced clearances of 30% and 50%. However, when the clearance impairment is higher (i.e. 70%), then even this scheme leads to increased colchicine plasma levels.
Figure 5.
Simulated mean plasma colchicine concentrations (μg/l) versus time (h) of patients with normal clearance, 30% impaired total clearance, 50% impaired total clearance, and 70% impaired total clearance. The two dosing regimens of the RECOVERY trial are simulated (Table 4). (A) Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg twice daily for a further of 9 days (10 days in total) or until hospital discharge, (B) Loading dose of 1 mg, followed by 0.5 mg 12 h later, then maintenance dose 0.5 mg once daily. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration.
In order to account for all levels of clearance impairment, additional dosing regimens are simulated and proposed in this study (Figure 6). For patients with clearance reduction up to 30%, a dosage scheme using 0.25 mg thrice daily seems to be appropriate, since it leads to colchicine levels that are above the minimum effective concentration, and at the same time the achieved concentrations do not exceed the limit of 3.0 μg/l (Figure 6(A)). For clearance impairment between 30% and 50%, the 0.25 mg dose should be administered twice a day (Figure 6(B)). Finally, when clearance impairment is even more severe (e.g. 70% or more), then an appropriate dosage scheme could be 0.25 mg twice daily for the first two days followed by 0.25 mg per day (Figure 6(C)).
Figure 6.
Simulated plasma colchicine concentrations (μg/l) versus time (h) for three levels (30%, 50%, and 70%) of clearance impairment. For each level of impairment, a newly proposed dosage regimen is utilised. (A) 30% impairment: 0.25 mg thrice daily, (B) 50% impairment: 0.25 mg twice daily, (C) 70% impairment: 0.25 mg twice daily for the first two days and then 0.25 mg once daily. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration. The inter-individual variability values, considered for all pharmacokinetic parameters, were those reported in the study of Thomas et al. (1989). Dashed lines indicate the average peak and trough concentration values at steady state.
Discussion
In this study, simulations were performed to identify the ability of regimens to attain plasma levels known to be generally safe and efficacious. The dosage schemes, explored in this analysis, were taken from the literature since they were reported to be used for cardioprotection in COVID-19 patients. Colchicine mechanism of action resides on its ability to interfere with various biochemical pathways mediating the inflammatory process, like inhibition of neutrophil chemotaxis, impedance neutrophil adhesion, prevention of neutrophil recruitment, inhibition of superoxide production from neutrophils, and promotion of maturation of dendritic cells (Martinon et al. 2006, Niel and Scherrmann 2006, Nuki 2008, Leung et al. 2015, Tong et al. 2016).
The simulations resulted in plasma concentrations that are in line with those retrieved from the literature pharmacokinetic studies, providing evidence of the adequacy of the model selected (Achtert et al. 1989, Girre et al. 1989, Davis and Wason 2014, Wason et al. 2014). It was shown that high loading doses should be administered with caution, due to increased chance of toxicity, which is in accordance with previously published clinical studies showing that tolerability of the drug improves significantly when loading doses are not applied (Roubille et al. 2013). Several of the currently utilised dosage regimens lead either to increased or suppressed colchicine plasma values even in the case of patients with normal clearance ability. Among all dosage regimens, the scheme with colchicine administration of 0.5 mg twice daily led to the best results in terms of the colchicine plasma levels (Figure 2(C,G)). The latter maintained the drug in safe and effective plasma concentrations.
It should be underlined that avoiding toxicity is of paramount importance in the case of colchicine treatment. The clinical course of its toxicity entails three sequential overlapping phases. Initially, gastrointestinal mucosal damage with nausea, vomiting, diarrhoea, abdominal pain, hypovolemia, and leukocytosis manifest. Then, multi-organ failure, hemodynamic collapse, cardiac arrhythmias, or infectious or haemorrhagic complications become obvious. Patients that survive the second phase, enter a recovery phase in which there is a recovery of bone-marrow depression with rebound leukocytosis and resolution of organ failure (Putterman et al. 1991, Finkelstein et al. 2010). Gastrointestinal symptoms, related to increased peristaltic activity, are the most common complication quoted in both short- and long-term therapy with colchicine (Roubille et al. 2013, Schwier 2015).
No dose adjustments are necessary for patients with an impaired renal clearance of colchicine, as, in fact, it is only 10% of total clearance, while the predominant clearance is extra-renal (Girre et al. 1989). Wason et al., also in their study showed that patients with mild and moderate renal impairment and under haemodialysis do not need any dose adjustment (Wason et al. 2014). However, according to their study, patients with severe renal impairment resulted in higher exposure to the drug. In the study of Wallace et al., patients with the severe renal disease showed similar clearance to patients without, but clearance of patients with liver disease was significantly impaired (Wallace et al. 1970). In the same vein, Leighton et al., showed that in cirrhotic patients, colchicine means clearance was 4.22 ml/min/kg, when in normal subjects it was 10.65 ml/min/kg, indicating a 60% reduction (Leighton et al. 1991).
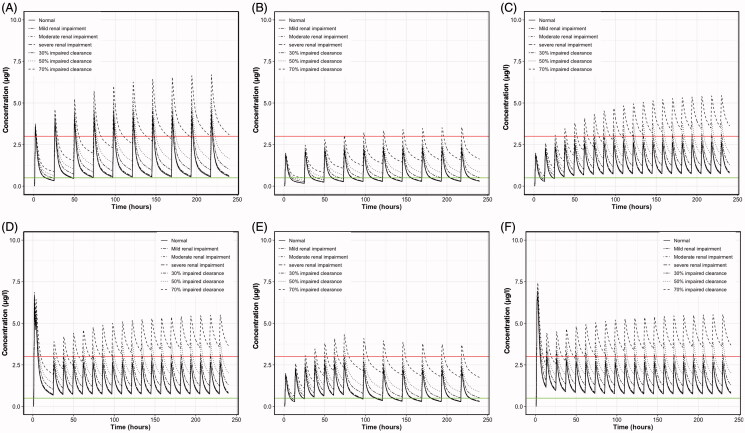
This study also showed the strong impact of a 30, 50, and 70% reduction in clearance, leading to accumulation of the drug. Most of the dosage regimens fail to reach adequate plasma levels of colchicine and lead to increased concentrations and potentially greater toxicity risk. However, a dosage regimen with colchicine administration of 0.25 mg thrice daily can account for 30, 50, or even 70% reductions in colchicine clearance (Figure 4(H)). The distinct impact of renal- and total-impairment on the colchicine levels is presented in the Appendix (Figure A1). These findings are in accordance with the literature since it is known that colchicine clearance is significantly reduced in patients with renal or hepatic failure. It is reported that colchicine half-life is prolonged twice in renal failure and ten times in the presence of cirrhosis and renal failure (Leighton et al. 1991, Bhat et al. 2009).
The dosages used in the RECOVERY trial were satisfactory for normal clearance and for impaired clearance patients with reductions up to 50% (Figure 5). However, when the colchicine clearance impairment is high (e.g. around 70% or more), then even this scheme can lead to increased colchicine plasma levels. In this context, new dosage regimens were proposed for patients with impaired clearance and the so-derived colchicine plasma levels were simulated (Figure 6). It was shown that appropriate doses, for maintaining colchicine plasma concentration within a safe and concomitantly therapeutic range, are 0.25 mg three times a day for patients with 30% impaired total colchicine clearance, 0.25 mg twice daily for patients with 50% impaired total colchicine clearance, and 0.25 mg twice daily for the first two days followed by 0.25 mg per day for patients with 70% impaired total colchicine clearance.
The use of loading doses or high initial doses of colchicine remains under discussion and requires special attention. A recent study (study with letter J in Table 4) stated that the use of relatively high doses during the first days of treatment (0.5 mg thrice daily for the first 5 days) might explain the reduction in CRP values of the patients and justify their better evolution at day 5 (Lopes et al. 2020). Nevertheless, simulations in this study showed that this dosage regimen can lead to high colchicine plasma levels. To the same conclusion come studies stating that the tolerability of colchicine improves significantly when loading doses are not applied (Roubille et al. 2013). In accordance with the recent suggestions of Lopes et al. (2020), a reduced dosage regimen with 0.25 mg thrice or twice daily, in patients with impaired clearance, leads to the desired concentration–time profile (Figure 4(H) and Figure 6).
In addition, this study also explored the situation of colchicine interactions with other medicines. Colchicine may be administered in patients for the management of pericarditis, cardiovascular diseases, or coronary artery disease, concomitantly with statins, digoxin, or verapamil which are CYP3A4 inhibitors. Similarly, for the management of autoimmune diseases, colchicine can be given together with cyclosporine, which is a P-gp inhibitor. In the treatment of COVID-19 patients, colchicine is often co-administered with azithromycin or other antibiotics that may also inhibit its clearance (Terkeltaub et al. 2011). It should be underlined that concomitant treatment with colchicine and drugs being CYP3A4 and/or P-gp inhibitors may result in fatal interactions if no dose adjustments are made. In Figure 3, the impact of three dosage regimens on colchicine plasma levels is explored. It becomes obvious that in some cases, sub-therapeutic levels are obtained, while when colchicine is co-administered with CYP3A4 inhibitors, increased plasma levels are observed with greater risk for adverse effects.
It should be mentioned that the pharmacokinetic model used for the simulations in this study, was initially developed for application within a dose range of 0.5–1.5 mg (Thomas et al. 1989). However, in this study, it was further used for simulating the plasma concentrations after doses of 0.25 mg. Despite the fact that an assumption was made that the parameters utilised would be as those in the case of 0.5 mg, this could also be supported by the fact that at a low dose no difference in pharmacokinetics is expected. The discrepancy in colchicine disposition identified in higher doses is due to the saturation phenomena, which are not expected to occur at lower doses (Thomas et al. 1989, Niel and Scherrmann 2006). It is worth stating that the selected pharmacokinetic model from the literature, was initially developed using data from nine subjects. Even though this number of subjects might seem low, however, this number was comparable (and in some cases higher) to the sample sizes utilized for the development of the other literature models. In addition, the selected model offered several advantages, such as coming from an adequate sampling scheme, offered estimates for inter-individual variability, and reported the values of the dose-dependent pharmacokinetic parameters.
Regarding the convenience of administrating the proposed 0.25 mg colchicine doses, it should be quoted that colchicine is available in the form of 0.5 mg tablets (Colchimex® 500 mcg tablet SmPC), which can be cut in half. Also, an oral solution is marketed in the US containing 0.6 mg/5 ml, of which 2 ml can be administered for safer use of the drug in these patients (Gloperba® label).
Conclusion
Colchicine appears as a promising drug for use in COVID-19 patients, since recent studies showed that it may reduce the rate of death and lung complications related to COVID-19. Numerous dosing regimens are currently utilised in clinical practice and clinical trials without any consensus. The purpose of the present study was to identify the ability of colchicine regimens to attain plasma levels known to be generally safe and efficacious. Additional dosage schemes were proposed in this study. Changes in pharmacokinetics, due to colchicine interaction with other popular treatments in COVID-19, like lopinavir/ritonavir or azithromycin, were also investigated. Some dosage regimens lead to relatively high colchicine levels, while some other administration schemes result in sub-therapeutic levels. It is concluded that 0.5 mg twice daily can be considered a safe and effective dosing scheme. Finally, in cases of colchicine clearance impairment, doses as low as 0.25 mg thrice or twice daily can be applied.
Appendix A.
Figure A1.
Mean simulated plasma colchicine concentrations (μg/l) versus time (h) of patients with normal clearance, mild renal impairment, moderate renal impairment, severe renal impairment, 30% impaired total clearance, 50% impaired total clearance, 70% impaired total clearance receiving dosing schemes A: 1 mg once daily, B: 0.5 mg once daily, C: 0.5 mg twice daily, D: Loading dose of 1.5 mg, followed by 0.5 mg after 2 h and then 0.5 twice daily for 14 days, E: 0.5 mg twice daily for the first 3 days and then 0.5 once daily for 27 days, F : Loading dose of 1.5 mg, followed by 0.5 mg after 1 h and then 0.5 twice daily. Upper line: 3 μg/l, plasma concentration that elicits toxic effects. Lower line: 0.5 μg/l, minimum effective plasma concentration.
Disclosure statement
The authors report no declarations of interest.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- Achtert, G., Scherrmann, J.M., and Christen, M.O., 1989. Pharmacokinetics/bioavailability of colchicine in healthy male volunteers. European journal of drug metabolism and pharmacokinetics, 14 (4), 317–322. [DOI] [PubMed] [Google Scholar]
- Arnold, S. and Buckner, F., 2020. Hydroxychloroquine for treatment of SARS-CoV-2 infection? Improving our confidence in a model-based approach to dose selection. Clinical and translational science, 13 (4), 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audemard-Verger, A., et al. , 2017. Characteristics and management of IgA vasculitis (Henoch-Schönlein) in adults: data from 260 patients included in a French multicenter retrospective survey. Arthritis & rheumatology, 69 (9), 1862–1870. [DOI] [PubMed] [Google Scholar]
- Ben-Chetrit, E. and Levy, M., 1998. Colchicine: 1998 update. Seminars in arthritis and rheumatism, 28 (1), 48–59. [DOI] [PubMed] [Google Scholar]
- Berkun, Y., et al. , 2012. Pharmacokinetics of colchicine in pediatric and adult patients with familial Mediterranean fever. International journal of immunopathology and pharmacology, 25 (4), 1121–1130. [DOI] [PubMed] [Google Scholar]
- Bhat, A., et al. , 2009. Colchicine revisited. Annals of the New York Academy of Sciences, 1173, 766–773. [DOI] [PubMed] [Google Scholar]
- Bouabdallaoui, N., et al. , 2020. Time-to-treatment initiation of colchicine and cardiovascular outcomes aftermyocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). European heart journal, 41 (42), 4092–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerquaglia, C., et al. , 2005. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: an update. Current drug targets inflammation and allergy, 4 (1), 117–124. [DOI] [PubMed] [Google Scholar]
- Clinical trial: Greece ., 2021. The Greek Study in the Effects of Colchicine in Covid-19 Complications Prevention (GRECCO-19). Available from: https://clinicaltrials.gov/ct2/show/NCT04326790 [Accessed on 24 January 2021].
- Clinical trial: The ECLA PHRI COLCOVID Trial ., 2021. Effects of colchicine on moderate/high-risk hospitalized COVID-19 patients (COLCOVID). Available from: https://clinicaltrials.gov/ct2/show/NCT04328480 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier NCT04367168 ., 2021. Colchivid. Available from: https://clinicaltrials.gov/ct2/show/NCT04367168 [accessed on 24 January 2021].
- ClinicalTrials.gov Identifier NCT04403243 ., 2021. Colchicine Versus Ruxolitinib and Secukinumab In Open Prospective Randomized Trial (COLORIT). Available from: https://clinicaltrials.gov/ct2/show/NCT04403243 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT02551094 ., 2021. Colchicine Cardiovascular Outcomes Trial (COLCOT). Available from: https://clinicaltrials.gov/ct2/show/NCT02551094 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT02898610 ., 2021. Colchicine for Prevention of Vascular Inflammation in Non-cardio Embolic Stroke (CONVINCE). Available from: https://clinicaltrials.gov/ct2/show/NCT02898610 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT03704181 ., 2021. Colchicine for Patients With Chagas’ Disease (B1 Stage) (COACH). Available from: https://clinicaltrials.gov/ct2/show/NCT03704181 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04056039 ., 2021. Atorvastatin vs Colchicine in Decrease of Troponin I of High Sensitivity in Patients with Rheumatoid Arthritis. (ACAR1). Available from: https://clinicaltrials.gov/ct2/show/NCT04056039 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04181996 ., 2021. Canadian Study of Arterial Inflammation in Patients With Diabetes and Vascular Events: EvaluatioN of Colchicine (CADENCE). Available from: https://clinicaltrials.gov/ct2/show/NCT04181996 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04322565 ., 2021. Colchicine Counteracting Inflammation in COVID-19 Pneumonia (ColCOVID-19). Available from: https://clinicaltrials.gov/ct2/show/NCT04322565 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04322682 ., 2021. Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA). Available from: https://clinicaltrials.gov/ct2/show/NCT04322682 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04324463 ., 2021. Anti-Coronavirus Therapies to Prevent Progression of Coronavirus Disease 2019 (COVID-19) Trial (ACTCOVID19). Available from: https://clinicaltrials.gov/ct2/show/NCT04324463 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04326790 ., 2021. Colchicine Twice Daily During 10 Days as an Option for the Treatment of Symptoms Induced by Inflammation in Patients With Mild and Severe Coronavirus Disease (ColchiVID). Available from: https://clinicaltrials.gov/ct2/show/NCT04326790 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04350320 ., 2021. Trial to Study the Benefit of Colchicine in Patients With COVID-19 (COL-COVID). Available from: https://clinicaltrials.gov/ct2/show/NCT04350320 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04355143 ., 2021. Colchicine to Reduce Cardiac Injury in COVID-19 (COLHEART-19). Available from: https://clinicaltrials.gov/ct2/show/NCT04355143 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04360980 ., 2021. The effects of standard protocol with or without colchicine in Covid-19 infection. Available from: https://clinicaltrials.gov/ct2/show/NCT04360980 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04363437 ., 2021. Colchicine in Moderate-severe Hospitalized Patients Before ARDS to Treat COVID-19 (COMBATCOVID19). Available from: https://clinicaltrials.gov/ct2/show/NCT04363437 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04375202 ., 2021. Colchicine in COVID-19: a Pilot Study (COLVID-19). Available from: https://clinicaltrials.gov/ct2/show/NCT04375202 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04381936 ., 2021. Randomised Evaluation of COVID-19 Therapy (RECOVERY). Available from: https://clinicaltrials.gov/ct2/show/NCT04381936 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04416334 ., 2021. Preemptive therapy with colchicine in patients older than 60 years with high risk of severe pneumoniae due to coronavirus (COLCHI-COVID). Available from: https://clinicaltrials.gov/ct2/show/NCT04416334 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04527562 ., 2021. Colchicine in Moderate Symptomatic COVID-19 Patients (COLCOVIDBD). Available from: https://clinicaltrials.gov/ct2/show/NCT04527562 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04603690 ., 2021. Study to Investigate the Benefits of Colchicine in Patients With COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04603690 [Accessed on 24 January 2021].
- ClinicalTrials.gov Identifier: NCT04667780 ., 2021. Study to investigate the treatment effect of colchicine in patients with COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04667780 [Accessed on 24 January 2021].
- Colchicine 500mcg tablets ., 2021. Summary of product characteristics (SmPC). Available from: https://www.medicines.org.uk/emc/product/6415/smpc#gref [Accessed on 24 January 2021].
- COLCORONA trial ., 2021. Colchicine reduces the risk of COVID-19-related complications. Available from: https://www.icm-mhi.org/en/pressroom/news/colchicine-reduces-risk-covid-19-related-complications [Accessed on 24 January 2021].
- Colcrys Label ., 2012. Highlights of prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022352s017lbl.pdf [Accessed on 24 January 2021].
- Davis, M.W. and Wason, S., 2014. Effect of steady-state atorvastatin on the pharmacokinetics of a single dose of colchicine in healthy adults under fasted conditions. Clinical drug investigation, 34 (4), 259–267. [DOI] [PubMed] [Google Scholar]
- Davis, M.W., Wason, S., and Digiacinto, J.L., 2013. Colchicine-antimicrobial drug interactions: what pharmacists need to know in treating gout. The consultant pharmacist, 28 (3), 176–183. [DOI] [PubMed] [Google Scholar]
- Deftereos, S., et al. , 2015. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation, 132 (15), 1395–1403. [DOI] [PubMed] [Google Scholar]
- Deftereos, S., et al. , 2012. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. Journal of the American College of Cardiology, 60 (18), 1790–1796. [DOI] [PubMed] [Google Scholar]
- Deftereos, S., et al. , 2013. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. Journal of the American College of Cardiology, 61 (16), 1679–1685. [DOI] [PubMed] [Google Scholar]
- Deftereos, S., et al. , 2020a. The Greek study in the effects of colchicine in Covid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic journal of cardiology, 61 (1), 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftereos, S., et al. , 2020b. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Network Open, 3 (6), e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftereos, S., et al. , 2020c. Colchicine as a potent anti-inflammatory treatment in COVID-19: can we teach an old dog new tricks? European heart journal. Cardiovascular pharmacotherapy, 6 (4), 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Torre, E., et al. , 2020. Treating COVID-19 with colchicine in community healthcare setting. Clinical immunology, 217, 108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld, M., et al. , 1982. Gastrointestinal effects of long-term colchicine therapy in patients with recurrent polyserositis (familial mediterranean fever). Digestive diseases and sciences, 27 (8), 723–727. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency ., 2019. EMA/CHMP/35552/2019. Available from: https://www.ema.europa.eu/en/ documents/scientific-guideline/colchicine-tablet-05-mg-1-mg-product-specific-bioequivalence-guidance_en.pdf [Accessed on 23 March 2021].
- Ferron, G.M., et al. , 1996. Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses. Journal of clinical pharmacology, 36 (10), 874–883. [DOI] [PubMed] [Google Scholar]
- Finkelstein, Y., et al. , 2010. Colchicine poisoning: the dark side of an ancient drug. Clinical toxicology, 48 (5), 407–414. [DOI] [PubMed] [Google Scholar]
- Gasparyan, A.Y., et al. , 2015. Colchicine as an anti-inflammatory and cardioprotective agent. Expert opinion on drug metabolism & toxicology, 11 (11), 1781–1794. [DOI] [PubMed] [Google Scholar]
- Girre, C., et al. , 1989. Model-independent pharmacokinetics of colchicine after oral administration to healthy volunteers. Fundamental & clinical pharmacology, 3 (5), 537–543. [DOI] [PubMed] [Google Scholar]
- Gloperba Label ., 2021. Highlights of prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210942s000lbl.pdf [Accessed on 24 January 2021].
- Gül, A., 2014. Treatment of familial Mediterranean fever: colchicine and beyond. The Israel Medical Association journal, 16 (5), 281–284. [PubMed] [Google Scholar]
- Karalis, V., Ismailos, G., and Karatza, E., 2020. Chloroquine dosage regimens in patients with COVID-19: safety risks and optimization using simulations. Safety science, 129, 104842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatza, E., et al. , 2021. Optimization of hydroxychloroquine dosing scheme based on COVID-19 patients' characteristics: a review of the literature and simulations. Xenobiotica; the fate of foreign compounds in biological systems, 51 (2), 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton, J.A., et al. , 1991. Colchicine clearance is impaired in alcoholic cirrhosis. Hepatology, 14 (6), 1013–1015. [PubMed] [Google Scholar]
- Lennerz, C., et al. , 2017. Colchicine for primary prevention of atrial fibrillation after open-heart surgery: systematic review and meta-analysis. International journal of cardiology, 249, 127–137. [DOI] [PubMed] [Google Scholar]
- Leung, Y.Y., Yao Hui, L.L., and Kraus, V.B., 2015. Colchicine-Update on mechanisms of action and therapeutic uses. Seminars in arthritis and rheumatism, 45 (3), 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, M., Spino, M., and Read, S.E., 1991. Colchicine: a state-of-the-art review. Pharmacotherapy, 11 (3), 196–211. [PubMed] [Google Scholar]
- Lopes, M., et al. , 2020. Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial. Available from: https://www.medrxiv.org/content/10.1101/2020.08.06.20169573v2 [Accessed on 24 January 2021]. [DOI] [PMC free article] [PubMed]
- Martinon, F., et al. , 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 440 (7081), 237–241. [DOI] [PubMed] [Google Scholar]
- Masson, W., et al. , 2020. Role of colchicine in stroke prevention: an updated meta-analysis. Journal of stroke and cerebrovascular diseases, 29 (5), 104756. [DOI] [PubMed] [Google Scholar]
- mlxR: Simulation of Longitudinal Data ., 2021. R package. Available from: https://cran.r-project.org/web/packages/mlxR/index.html [Accessed on 23 March 2021].
- Molad, Y., 2002. Update on colchicine and its mechanism of action. Current rheumatology reports, 4 (3), 252–256. [DOI] [PubMed] [Google Scholar]
- Nidorf, S.M., et al. , 2013. Low-dose colchicine for secondary prevention of cardiovascular disease. Journal of the American College of Cardiology, 61 (4), 404–410. [DOI] [PubMed] [Google Scholar]
- Nidorf, S.M., et al. , 2020. Colchicine in patients with chronic coronary disease. New England journal of medicine, 383 (19), 1838–1847. [DOI] [PubMed] [Google Scholar]
- Nidorf, M. and Thompson, P.L., 2007. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. The American journal of cardiology, 99 (6), 805–807. [DOI] [PubMed] [Google Scholar]
- Niel, E. and Scherrmann, J.M., 2006. Colchicine today. Joint bone spine, 73 (6), 672–678. [DOI] [PubMed] [Google Scholar]
- Nuki, G., 2008. Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Current rheumatology reports, 10 (3), 218–227. [DOI] [PubMed] [Google Scholar]
- Ozen, S., et al. , 2016. EULAR recommendations for the management of familial Mediterranean fever. Annals of the rheumatic diseases, 75 (4), 644–651. [DOI] [PubMed] [Google Scholar]
- Perinel, S., et al. , 2020. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clinical infectious diseases, 71 (16), 2227–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, R.S., Lehman, T.J., and Schwabe, A.D., 1983. Colchicine use for familial Mediterranean fever. Observations associated with long-term treatment. The western journal of medicine, 138 (1), 43–46. [PMC free article] [PubMed] [Google Scholar]
- PRINCIPLE trial ., 2021. PRINCIPLE COVID-19 treatments trial widens to under 50s and adds colchicine. https://www.principletrial.org/news/principle-covid-19-treatments-trial-widens-to-under-50s-adds-colchicine [Accessed on 23 March 2021].
- Putterman, C., et al. , 1991. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Seminars in arthritis and rheumatism, 21 (3), 143–155. [DOI] [PubMed] [Google Scholar]
- Raynor, A. and Askari, A.D., 1980. Behçet’s disease and treatment with colchicine. Journal of the American Academy of Dermatology, 2 (5), 396–400. [DOI] [PubMed] [Google Scholar]
- Rochdi, M., et al. , 1994. Pharmacokinetics and absolute bioavailability of colchicine after i.v. and oral administration in healthy human volunteers and elderly subjects. European journal of clinical pharmacology, 46 (4), 351–354. [DOI] [PubMed] [Google Scholar]
- Roddy, E., et al. , 2020. Open-label randomised pragmatic trial (CONTACT) comparing naproxen and low-dose colchicine for the treatment of gout flares in primary care. Annals of the rheumatic diseases, 79 (2), 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubille, F., et al. , 2013. Colchicine: an old wine in a new bottle? Anti-inflammatory & anti-allergy agents in medicinal chemistry, 12 (1), 14–23. [DOI] [PubMed] [Google Scholar]
- Saleh, Z. and Arayssi, T., 2014. Update on the therapy of Behçet disease. Therapeutic advances in chronic disease, 5 (3), 112–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapra, S., et al. , 2013. Colchicine and its various physicochemical and biological aspects. Medicinal chemistry research, 22, 531–547. [Google Scholar]
- Scarsi, M., et al. , 2020. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Annals of the rheumatic diseases, 79 (10), 1286–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwier, N.C., 2015. Pharmacotherapeutic considerations for using colchicine to treat idiopathic pericarditis in the USA. American journal of cardiovascular drugs, 15 (5), 295–306. [DOI] [PubMed] [Google Scholar]
- Slobodnick, A., et al. , 2018. Update on colchicine, 2017. Rheumatology, 57 (1), i4–i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solak, Y., Acikgoz, S.B., and Yildirim, M., 2015a. Colchicine toxicity: an exaggerated reality? The American journal of medicine, 128 (8), e11. [DOI] [PubMed] [Google Scholar]
- Solak, Y., Acikgoz, S.B., and Yildirim, M., 2015b. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clinical drug investigation, 35 (2), 149. [DOI] [PubMed] [Google Scholar]
- Spartalis, M., et al. , 2018. The beneficial therapy with colchicine for atherosclerosis via anti-inflammation and decrease in hypertriglyceridemia. Cardiovascular & hematological agents in medicinal chemistry, 16 (2), 74–80. [DOI] [PubMed] [Google Scholar]
- Tardif, J.C., et al. , 2019. Efficacy and safety of low-dose colchicine after myocardial infarction. The New England journal of medicine, 381 (26), 2497–2505. [DOI] [PubMed] [Google Scholar]
- Terkeltaub, R.A., 2009. Colchicine update: 2008. Seminars in arthritis and rheumatism, 38 (6), 411–419. [DOI] [PubMed] [Google Scholar]
- Terkeltaub, R.A., et al. , 2010. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis and rheumatism, 62 (4), 1060–1068. [DOI] [PubMed] [Google Scholar]
- Terkeltaub, R.A., et al. , 2011. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis and rheumatism, 63 (8), 2226–2237. [DOI] [PubMed] [Google Scholar]
- Thomas, G., et al. , 1989. Zero-order absorption and linear disposition of oral colchicine in healthy volunteers. European journal of clinical pharmacology, 37 (1), 79–84. [DOI] [PubMed] [Google Scholar]
- Tong, D.C., et al. , 2020. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation, 142 (20), 1890–1900. [DOI] [PubMed] [Google Scholar]
- Tong, D.C., Wilson, A.M., and Layland, J., 2016. Colchicine in cardiovascular disease: an ancient drug with modern tricks. Heart, 102 (13), 995–1002. [DOI] [PubMed] [Google Scholar]
- Wallace, S.L. and Ertel, N.H., 1973. Plasma levels of colchicine after oral administration of a single dose. Metabolism: clinical and experimental, 22 (5), 749–753. [DOI] [PubMed] [Google Scholar]
- Wallace, S.L., Omokoku, B., and Ertel, N.H., 1970. Colchicine plasma levels. Implications as to pharmacology and mechanism of action. The American journal of medicine, 48 (4), 443–448. [DOI] [PubMed] [Google Scholar]
- Wason, S., Mount, D., and Faulkner, R., 2014. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clinical drug investigation, 34 (12), 845–855. [DOI] [PubMed] [Google Scholar]
- Woo, M.Y., et al. , 2012. Differential effects of colchicine in blood mononuclear cells of patients with Behçet disease in relation to colchicine responsiveness. The British journal of dermatology, 167 (4), 914–921. [DOI] [PubMed] [Google Scholar]
- Yao, X., et al. , 2020. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical infectious diseases, 71 (15), 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.