Abstract
Traumatic brain injury (TBI) is the leading cause of injury-related death and disability in patients under the age of 46 years. Survivors of the initial injury often endure systemic complications such as pulmonary infection, and Pseudomonas aeruginosa is one of the most common causes of nosocomial pneumonia in intensive care units. Female patients are less likely to develop secondary pneumonia after TBI, and pre-clinical studies have revealed a salutary role for estrogen after trauma. Therefore, we hypothesized that female mice would experience less mortality after post-TBI pneumonia with P. aeruginosa. We employed a mouse model of TBI followed by P. aeruginosa pneumonia. Male mice had greater mortality and impaired lung bacterial clearance after post-TBI pneumonia compared with female mice. This was confirmed as a difference in sex hormones, as oophorectomized wild-type mice had mortality and lung bacterial clearance similar to male mice. There were differences in tumor necrosis factor-α secretion in male and female alveolar macrophages after P. aeruginosa infection. Finally, injection of male or oophorectomized wild-type female mice with estrogen restored lung bacterial clearance and prevented mortality. Our model of TBI followed by P. aeruginosa pneumonia is among the first to reveal sex dimorphism in secondary, long-term TBI complications.
Keywords: macrophage, phagocytosis, Pseudomonas aeruginosa, sex dimorphism, tumor necrosis factor-α
Introduction
Traumatic brain injury (TBI) is the leading cause of injury-related death and disability in patients under the age of 46 years.1 Survivors of the initial injury often endure significant systemic complications such as autonomic dysfunction, cardiac dysrhythmia, and pulmonary infection. For example, post-TBI pulmonary infection rates may be as high as 50–60% and may result in an infection-related mortality rate of ∼30%.2–5 Post-TBI complications are a major determinant of long-term patient outcome6–10 during their most productive years of life. Reasons for the increased incidence of post-TBI pneumonia are multifactorial, and include the neurological consequences of TBI, such as impairment of airway reflexes, decreased level of consciousness, dysphagia, and the need for mechanical ventilation. However, a recent review summarizing the incidence of post-TBI pulmonary complications revealed that decreased immune function and bacterial pneumonia subsequent to TBI occurs within the first 2 weeks after injury.11 Interestingly, neurological injury and mechanical ventilation may last much longer, but the incidence of bacterial pneumonia decreases. Therefore, this time discrepancy indicates that non-brain mechanisms may be responsible for increased susceptibility to bacterial pneumonia after severe TBI.
Nosocomial pneumonia is an underappreciated cause of mortality and end-organ dysfunction in critically ill and immunosuppressed patients.12 Pseudomonas aeruginosa is one of the most common causes of nosocomial pneumonia in intensive care units (ICUs).13–16 Further, P. aeruginosa is a bacterium that is not only virulent, but also exhibits antimicrobial resistance14,16,17 and places a significant economic burden on the healthcare system.18,19 P. aeruginosa is also a common source of sepsis and acute respiratory distress syndrome (ARDS) because of its ability to breach lung barrier functions, resulting in inadequate alveolar fluid clearance, inadequate gas exchange, and development of protein-rich edema.20–29 Finally, P. aeruginosa has a variety of mechanisms to evade host immunity including, but not limited to, formation of biofilms, regulatory changes in infection strategy, and quorum sensing.30,31 Overall, this indicates that P. aeruginosa is a relevant pathogen with significant ability to infect patient lungs after TBI.
Prior studies in humans and mice have demonstrated sex dimorphism with respect to survival after blunt and/or penetrating trauma, with females having better clinical outcomes.32 Specifically, female patients are less likely to develop secondary pneumonia after blunt and/or penetrating trauma or TBI.33,34 Pre-clinical studies have revealed a salutary role for estrogen after trauma.35–37 Interestingly, whereas earlier animal studies used both male and female mice,38,39 more recent studies have focused primarily on studying male mice and/or cells and adding estrogen(s) or other female hormones to study sex differences.40 To our knowledge, there are no studies that look specifically at sex-dependent effects of TBI and subsequent bacterial pneumonia.
Herein, we investigated whether mortality secondary to post-TBI P. aeruginosa would be increased and lung bacterial clearance would be decreased. The results demonstrated that mortality was increased and lung bacterial clearance was decreased in a murine model of TBI and subsequent P. aeruginosa pneumonia. Additionally, we examined whether there was a sex dimorphism at both the mouse and cellular level. The results demonstrate that sexual differences in cytokine production exist at the cellular level, and that mortality and lung bacterial clearance can be alleviated by administration of estrogen.
Methods
Reagents
All reagents were obtained from Fisher-Scientific (Waltham, MA, USA) unless otherwise specified. Estradiol was from Sigma-Aldrich (Saint Louis, MO, USA).
Mice
C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) for all experiments. Mice were housed in University of Alabama at Birmingham Animal Care Facilities under 12 h light/dark cycles and received Lab diet NIH-31 (PMI Nutritional International, Saint Louis, MO, USA) ad libitum. Mice were acclimated for at least 5 days prior to initiation of experiments. All animal experiments were conducted according to protocols approved by University of Alabama at Birmingham Animal Care and Use Committees and according to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
TBI model
Mice were anesthetized with ketamine (90–200 mg/kg, intramuscularly [IM]) and xylazine (10 mg/kg, IM) and placed in a stereotaxic frame (Benchmark™ Stereotaxic Instruments, Leica Biosystems, Buffalo Grove, IL, USA) with head holder (Kopf®, David Kopf Instruments, Tujunga, CA, USA) and ear bars. A 1 cm incision was made at the top of the skull to expose the bone, and a 5 mm left lateral craniotomy was performed using an electrical drill (Kopf®, David Kopf Instruments, Tujunga, CA, USA) attached on the stereotaxic arm as previously described.41 A controlled cortical impact (CCI) injury was produced with an electromagnetic impact device (Benchmark™ Stereotaxic Impactor, Leica Biosystems, Buffalo Grove, IL, USA) centered at 3.0 mm anterior from lambda and 2.7 mm left from midline with a depth of 2.5 mm, a velocity of 5.0 m/sec, and a dwell time of 100 ms. After impact, the skin incision was sutured closed and the mice were placed on a warming pad and returned to their home cages when they were fully ambulatory, typically 60–120 min after injury. For some experiments, mice underwent only the craniotomy, but not the CCI, as surgical controls. For some experiments, estradiol or vehicle (ethanol) was injected intravenously 1 h after TBI.
P. aeruginosa pneumonia model
Instillation of pneumonia was performed as previously described.42 Mice were instilled with pneumonia 24 h after TBI or craniotomy. Mice were anesthetized with ketamine (90–200mg/kg, IM) and xylazine (10 mg/kg, IM), and 25 μL of phosphate buffered saline (PBS) containing 5 x 107 colony-forming units of P. aeruginosa K-strain, a wild type strain (hereafter referred to as “PAK”) was instilled into both lungs via the trachea. Mice were allowed to recover for 15 min prior to being returned to their cage.
Survival
After instillation of pneumonia, mice were checked every 6 h after the instillation of PAK until death or survival at 36 h. Survival time was defined as the time from bacterial instillation to death.
Bacterial clearance
Lung bacterial clearance was measured as previously described.43 Six hours after instillation of PAK, lungs were collected in a sterile fashion. The lungs were homogenized in sterile containers and the homogenates were serially diluted and plated in triplicate on sheep-blood agar plates.
Isolation of peritoneal macrophages
Peritoneal macrophages were isolated as previously described.44 Briefly, peritoneal macrophages were collected 3 days after intraperitoneal injection of 4% Brewer thioglycollate and seeded on plates in Roswell Park Memorial Institute (RPMI) 1640. After 2 h, the plates were washed with serum-free RPMI 1640 to remove non-adherent cells. Macrophages were further cultured in RPMI 1640 (fetal bovine serum [FBS] 5%) and used for experiments the following day.
Cell culture
MH-S and AMJ2-C11 cells were acquired from ATCC® (CRL-2019 and CRL-2456, respectively, Manassas, VA, USA). MH-S cells were grown in RPMI supplemented with 10% FBS and 1% penicillin/streptomycin, and AMJ2-C11 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 5% FBS and 1% penicillin/streptomycin. All cells were cultured in a humidified incubator at 37°C and 5% CO2.
Phagocytosis
Phagocytosis assays were performed as previously described.45 Briefly, 1 x 106 cells were exposed to 107 colony forming units (CFU)/mL of PAK for 45 min at 37°C. Gentamicin (150 μg/mL) was added and cells were incubated for 1 h at 37°C. The medium was removed and cells were washed twice with sterile PBS, then lysed by adding 200 μL of hypotonic buffer (pH 7.2), and incubated on ice for 10 min. Sterile water (800 μL) was added to the cell suspension, which was serially diluted onto agar plates. The plates were incubated for 24 h at 37°C and the resulting colonies were counted. Comparisons were made between treatment groups at 1 h to determine the level of phagocytosis. For some experiments, cells were pre-treated with 10 nM estradiol or vehicle.
Cytokine measurement
Confluent macrophages were exposed to 107 CFU/mL of PAK for the times indicated in the respective figure legends. Supernatants were collected and centrifuged to remove cellular debris, and supernatants were stored at −80°C until they were assayed. Tumor necrosis factor (TNF)-α was from R & D Systems (Minneapolis, MN, USA). TNF-α was measured in the supernatants according to manufacturer instructions. Lysates from the same wells were collected and measured for protein content to normalize cytokine measurements. For some experiments, cells were pre-treated with 10 nM estradiol or vehicle.
Statistical analysis
For continuous results, normal distribution was verified using the Kolmogorov–Smirnov test. For normally distributed data, a Student t test was used to compare two experimental groups. Bonferroni correction, controlling for false positive error rate, was used to adjust for multiple comparisons. For survival results, Kaplan–Meier analysis followed by a log rank (Mantel–Cox) test was used to compare the survival between the two experimental groups of mice at 36 h. A p value of <0.05 was considered statistically significant. All statistical comparison of means was bilateral (two-tailed tests).
Results
Murine model of TBI and subsequent bacterial pneumonia
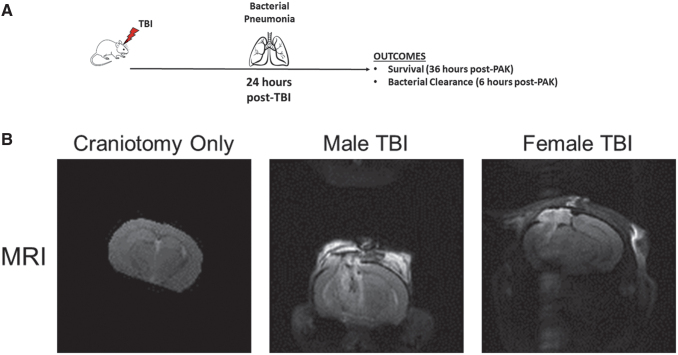
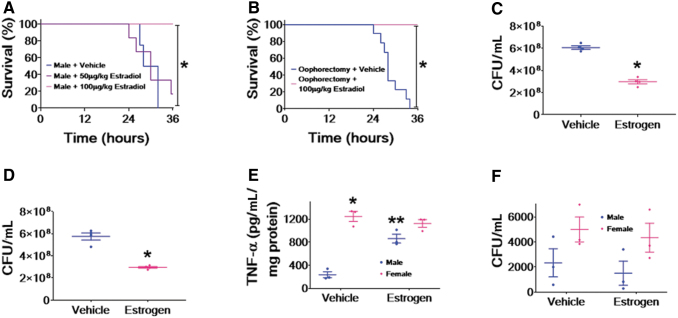
Bacterial pneumonia occurs in patients at high rates after sustaining a severe TBI.46–48 One of most common bacteria known to infect these patients is P. aeruginosa.16,49,50 Although altered mental status and mechanical ventilation are contributory to this high incidence of pneumonia, current evidence suggests that immune suppression secondary to severe TBI is also contributory.11 To better study the mechanism(s) of immune suppression, we created a model of severe TBI followed by instillation of bacterial pneumonia (Fig. 1A). Our mouse model began with a craniotomy followed by severe TBI using CCI. This allowed us to generate a reproducible brain injury. After 24 h, mice were instilled intratracheally with PAK. At designated times thereafter, we measured survival or lung bacterial clearance.
FIG. 1.
Murine model of traumatic brain injury and subsequent bacterial pneumonia. (A) Model of traumatic brain injury (TBI) and subsequent bacterial pneumonia. Mice are exposed to craniotomy and TBI as described in the Methods. Twenty-four hours later, mice are intratracheally instilled with Pseudomonas aeruginosa. Outcomes, in this case mortality and lung bacterial clearance, are measured at 36 and 6 h, respectively. (B) Magnetic resonance imaging (MRI) of mice brains 24 h after respective injuries. MRI was performed on mice after craniotomy alone, a male mouse that had undergone TBI and a female mouse that had undergone TBI. Mice that underwent craniotomy (n = 3) had little if any damage from the procedure, whereas male (n = 3) and female (n = 3) mice both had severe injury 24 h after TBI. A representative image from each group is shown.
To determine that our CCI model of TBI produced reproducible injury and craniotomy alone produced minimal or no injury, we performed magnetic resonance imaging (MRI) (Fig. 1B) of mouse brain slices 24 h after the surgical procedure. It is of note that this is the same time that PAK is instilled to produce pneumonia. Craniotomy alone did not lead to significant injury 24 h after surgery on MRI. However, in both male and female mice, TBI produced significant cortical and axonal lesions associated with the development of cerebral edema on MRI that were equal in severity at 24 h after the injury. These data indicate that craniotomy alone produces minimal, if any, damage to mice brains, but that CCI produces significant, reproducible, and equivalent injuries in male and female mice brains at 24 h.
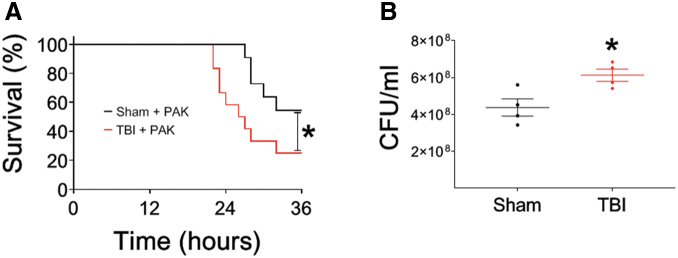
P. aeruginosa pneumonia after TBI leads to greater mortality and decreased bacterial clearance
Patients that sustain severe TBI have higher rates of subsequent bacterial pneumonia as a result of immune suppression secondary to the TBI.11 Therefore, we hypothesized that mice exposed to secondary bacterial pneumonia would have higher rates of mortality after severe TBI. To test this hypothesis, male mice were intratracheally instilled with PAK 24 h after craniotomy alone or severe TBI, and survival was measured (Fig. 2A). Mice that underwent craniotomy alone followed by PAK had ∼50% survival at 36 h. However, mice that received severe TBI followed by PAK had significantly greater mortality at the same time point. To better understand one potential mechanism of decreased survival after TBI and subsequent bacterial pneumonia, we measured lung bacterial clearance 6 h after intratracheal instillation of PAK after craniotomy alone or TBI (Fig. 2B). Mice that underwent TBI followed by PAK had significantly higher colony-forming units (CFU) of PAK isolated from the lung compared with the craniotomy-alone group. These data indicate that decreased bacterial clearance by mice that receive TBI is one method by which greater mortality may ensue.
FIG. 2.
Pseudomonas aeruginosa pneumonia after traumatic brain injury (TBI) leads to greater mortality and decreased bacterial clearance. For all experiments, mice received craniotomy or craniotomy with TBI according to protocol. Twenty-four hours later, P. aeruginosa K-strain (PAK) was instilled intratracheally and either mortality or bacterial clearance was measured. (A) P. aeruginosa-pneumonia leads to increased mortality after TBI (red line, n = 12) compared with craniotomy (black line, n = 11) in male mice, p < 0.05; *represents a significant difference compared with craniotomy. (B) Male mouse lungs were isolated 6 h after PAK instillation, and colony-forming units/mL were measured. Male mice that received TBI (red line) had decreased bacterial clearance compared with those that received craniotomy alone (black line); n = 4 for each group, p < 0.05; *represents a significant difference compared with craniotomy.
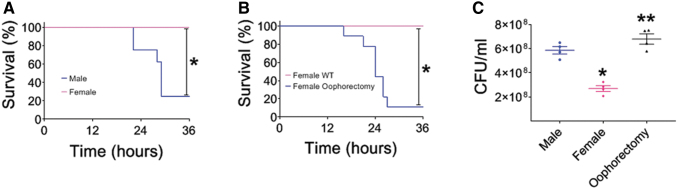
Increased mortality and decreased bacterial clearance after post-TBI bacterial pneumonia is sex dependent
Clinical studies reveal that women have better clinical outcomes after trauma than their male counterparts.32 More specifically, they have lower rates of secondary bacterial pneumonia after trauma.33,34 Therefore, we tested whether female mice have lower mortality after post-TBI bacterial pneumonia than male mice. To this end, we instilled PAK intratracheally into male and female mice 24 h after TBI and measured survival at 36 h (Fig. 3A). Our data demonstrate that female mice with post-TBI bacterial pneumonia have significantly less mortality than male mice. To determine whether this effect was secondary to female hormones, we performed a similar experiment using wild-type female mice and female mice that had been oophorectomized (Fig. 3B). Interestingly, oophorectomized female mice that underwent TBI followed by intratracheal instillation with PAK not only had significantly increased mortality compared with wild-type female mice, but also the mortality curve was similar to that of male mice. To determine whether mortality correlated with differences in lung bacterial clearance, we measured lung CFUs 6 h after intratracheal instillation of PAK after TBI in male wild-type female and oophorectomized female mice (Fig. 3C). Female mice that underwent TBI followed by PAK had significantly lower CFUs isolated from the lung than male mice that underwent TBI followed by PAK. Additionally, oophorectomized female mice had increased CFUs compared with wild-type female mice, which were also very similar to CFU levels isolated from male mice. These data indicate that female mice have increased survival and bacterial clearance compared with male mice and oophorectomized female mice that receive a TBI followed by instillation of bacterial pneumonia, indicating that these outcomes are not only sex dependent, but are also modified by female sex hormones.
FIG. 3.
Mortality and decreased bacterial clearance after post-traumatic brain injury (TBI) bacterial pneumonia are sex dependent. For all experiments, mice received TBI according to protocol. Twenty-four hours later, Pseudomonas aeruginosa K-strain (PAK) was instilled intratracheally, and either mortality or bacterial clearance was measured. (A) P. aeruginosa-pneumonia leads to increased mortality after TBI in male mice (blue line, n = 8) compared with female mice (pink line, n = 7), p < 0.05; *represents a significant difference compared with males. (B) P. aeruginosa pneumonia leads to increased mortality after TBI in oophorectomized female mice (blue line, n = 9) compared with wild-type female mice (pink line, n = 4), p < 0.05; *represents a significant difference compared with wild-type females. (C) Wild-type male, wild-type female, and oophorectomized female mice lungs were isolated 6 h after PAK instillation and colony-forming units/mL were measured. Wild-type females (pink diamonds) had improved bacterial clearance compared with wild-type males (blue circles); oophorectomized female mice (black triangles) had decreased bacterial clearance compared with wild-type females (pink diamonds); n = 4 for each group, p < 0.05; *represents a significant difference compared with wild-type males, **represents a significant difference compared with wild-type females.
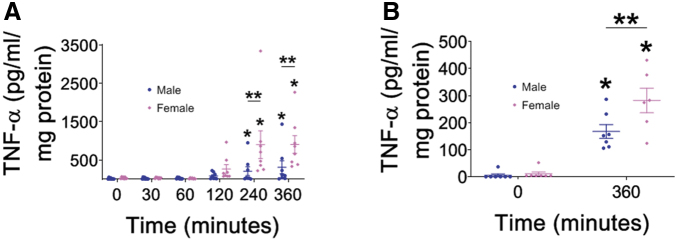
Differential TNF-α activation in male and female macrophages after exposure to P. aeruginosa
Alveolar macrophages are among the first immune cells to interact with invading pathogens of the lung. When alveolar macrophages interact with bacterial pathogens, they produce an inflammatory response that includes cytokines and chemokines, to activate their own phagocytic functions and recruit other immune cells to the area for host defense; one of the primary cytokines released by alveolar macrophages after bacterial activation is TNF-α.51 We hypothesized that one reason females may have fewer infections after TBI is increased cytokine activation upon infection with P. aeruginosa. Therefore, we exposed male and female alveolar macrophages to PAK and measured TNF-α over time (Fig. 4A). In both male and female alveolar macrophages, TNF-α secretion increased over time. Further, there was significantly increased TNF-α production at 240 and 360 min after PAK infection compared with unexposed controls. Interestingly, alveolar macrophage production in response to PAK infection was higher in female cells at both 240 and 360 min compared with their male counterparts. One potential reason for this difference could be that the male and female alveolar macrophages are independent cell lines. To test this hypothesis, we isolated primary macrophages from mice and exposed them to PAK infection (Fig. 4B). Both male and female primary macrophages had significantly increased TNF-α production at 6 h compared with unexposed control cells. Further, primary female macrophages exposed to PAK had greater TNF-α production at 6 h compared with their PAK-exposed primary male macrophage counterparts. These data indicate that one difference between male and female macrophages is greater TNF-α secretion from female macrophages compared with male macrophages.
FIG. 4.
Differential tumor necrosis factor (TNF)-α activation in male and female macrophages after exposure to Pseudomonas aeruginosa. Macrophages were exposed to P. aeruginosa for the times indicated and TNF-α was measured. (A) Male (MH-S) and female (AMJ2-C11) alveolar macrophages were exposed to PAK, and TNF-α was assayed at indicated time points. Both male and female macrophages produce greater quantities of TNF-α over time. At later time points, female alveolar macrophages (pink diamonds) make more TNF-α than male alveolar macrophages (blue circles), n = 8 for all time points assayed, TNF-α was measured in duplicate, p < 0.05; *represents a significant difference compared with control, **represents a significant difference comparing male and female alveolar macrophages at the same time point. (B) Primary male and female macrophages were exposed to PAK, and TNF-α was assayed at indicated time points. Both male and female primary macrophages produce greater quantities of TNF-α over time. At 6 h, female alveolar macrophages (pink diamonds) make more TNF-α than male alveolar macrophages (blue circles), n = 7 for all time points assayed, TNF-α was measured in duplicate, p < 0.05; *represents a significant difference compared with control, **represents a significant difference comparing male and female alveolar macrophages at the same time point.
P. aeruginosa phagocytosis in male and female macrophages is not sex dependent
We next sought to determine whether sex differences in P. aeruginosa-induced TNF-α secretion would lead to similar differences in phagocytosis. To this end, we infected male and female alveolar macrophages with PAK and measured phagocytosis (Fig. 5A). Both male and female alveolar macrophages were capable of P. aeruginosa phagocytosis. Although mean phagocytosis was increased in female alveolar macrophages compared with their male counterparts, this difference was not statistically significant. We also tested P. aeruginosa phagocytosis in male and female primary macrophages (Fig. 5B). Similar to male and female alveolar macrophages, male and female primary macrophages were capable of P. aeruginosa phagocytosis, but no differences between male and female primary macrophages were present.
FIG. 5.
Pseudomonas aeruginosa phagocytosis in male and female macrophages is not sex dependent. Macrophages were exposed to P. aeruginosa and phagocytosis was measured. (A) Male (blue circles) and female (pink diamonds) alveolar macrophages were exposed to Pseudomonas aeruginosa K-strain (PAK) and phagocytosis was assayed. Both male and female alveolar macrophages both phagocytose bacteria; n = 5. (B) Primary male (blue circles) and female (pink diamonds) alveolar macrophages were exposed to PAK and phagocytosis was assayed. Both male and female primary macrophages phagocytose bacteria; n = 6.
Estrogen alleviates increased mortality and decreased bacterial clearance in male and oophorectomized female mice after post-TBI bacterial pneumonia
Our data have indicated a sex-dependent difference in mortality and lung bacterial clearance in our murine model of P. aeruginosa pneumonia after TBI. However, there are multiple differences between male and female physiology including, but not limited to, genetics and sex hormone expression. One difference between males and reproductive-age females is the presence of estradiol. We tested whether estrogen could alleviate mortality in males and oophorectomized wild-type female mice that underwent TBI followed by P. aeruginosa pneumonia (Fig. 6A and B, respectively). Male mice that were injected with 100 μg/kg of estradiol after TBI and before instillation of PAK had improved survival compared with vehicle controls (Fig. 6A). Additionally, male mice that received 50 μg/kg of estradiol had a slight improvement in survival, but were not significantly different from male mice injected with vehicle. Wild-type oophorectomized female mice that were injected with 100 μg/kg of estradiol had significantly improved survival compared with oophorectomized wild-type female mice injected with vehicle (Fig. 6B).
FIG. 6.
Estrogen alleviates mortality and bacterial clearance in male and oophorectomized female mice after post-traumatic brain injury (TBI) bacterial pneumonia. For all in vivo experiments, mice received TBI according to protocol. Twenty-four hours later, Pseudomonas aeruginosa K-strain (PAK) was instilled intratracheally and either mortality or bacterial clearance was measured. Estrogen or vehicle was administered at the indicated doses. (A) P. aeruginosa pneumonia leads to decreased mortality after TBI in male mice that received 100 μg/kg estrogen (pink line, n = 10) compared with male mice that received 50 μg/kg estrogen (purple line, n = 6) or vehicle (blue line, n = 4), p < 0.05; *represents a significant difference compared with 50 μg/kg estrogen or vehicle. (B) P. aeruginosa pneumonia leads to decreased mortality after TBI in oophorectomized female mice that received 100 μg/kg estrogen (pink line, n = 10) compared with oophorectomized female mice that received vehicle (blue line, n = 9), p < 0.05; *represents a significant difference compared with vehicle. (C, D) Lungs from wild-type male mice that received 100 μg/kg estrogen or vehicle or oophorectomized female mice that received 100 μg/kg estrogen or vehicle were isolated 6 h after PAK instillation, and colony-forming units/mL were measured. For (C), wild-type male mice that received 100 μg/kg estrogen (pink diamonds) had improved bacterial clearance compared with those that received vehicle (blue circles); n = 4 for each group, p < 0.05; *represents a significant difference compared with vehicle. For (D), oophorectomized female mice that received 100 μg/kg estrogen (pink diamonds) had improved bacterial clearance compared with those that received vehicle (blue circles); n = 4 for each group, p < 0.05; *represents a significant difference compared with vehicle. (E) Male (blue circles) and female (pink diamonds) alveolar macrophages were pre-treated with 10 nM estradiol (or vehicle) for 30 min before exposure to PAK. Tumor nephrosis factor (TNF)-α was 6 h after exposure to PAK, n = 3 for all conditions assayed, TNF-α was measured in duplicate, p < 0.05; both * and ** represent a significant difference compared with male cells treated with vehicle and PAK. (F) Male (blue circles) and female (pink diamonds) alveolar macrophages were pre-treated with 10 nM estradiol (or vehicle) for 30 min before exposure to PAK and measurement of phagocytosis. All groups examined phagocytose bacteria; n = 3 per group.
We next determined whether reduced mortality from P. aeruginosa-mediated pneumonia after TBI was secondary to improved lung bacterial clearance. Male mice injected with 100 μg/kg of estradiol before instillation of PAK had improved lung bacterial clearance compared with male mice injected with vehicle (Fig. 6C). Further, wild-type oophorectomized female mice injected with 100 μg/kg of estradiol before PAK instillation had significantly improved lung bacterial clearance compared with oophorectomized wild-type female mice injected with vehicle (Fig. 6D). To determine whether these effects would also occur at the cellular level, we exposed male and female alveolar macrophages to estradiol before PAK exposure and measured TNF-α production and phagocytosis. Similar to what is shown in Figure 4, female alveolar macrophages make greater quantities of TNF-α than male alveolar macrophages when pre-treated with vehicle (Fig. 6E). However, when male alveolar macrophages are pre-treated with estrogen, they have a significant increase in PAK-induced TNF-α. Female alveolar macrophages do not respond to estradiol with a further increase in TNF-α. As seen in Figure 5, although there is a trend to greater levels of phagocytosis in female alveolar macrophages compared with male alveolar macrophages, there are no significant differences in the rate of phagocytosis between the cells (Fig. 6F), and pre-treatment with estradiol does not increase the rate of phagocytosis in either male or female alveolar macrophages.
Taken together, these data indicate that estradiol is one mechanism by which wild-type female mice have improved survival and lung bacterial clearance after TBI and subsequent P. aeruginosa pneumonia compared with their male counterparts.
Discussion
TBI is the leading cause of injury-related death and disability in patients under the age of 46 years.1 Numerous complications arise secondary to the initial injury and cause significant long-term morbidity. One of the most frequent complications secondary to TBI is bacterial pneumonia.2–5 The mechanisms of post-TBI bacterial pneumonia are multifactorial and include, but are not limited to, neurological consequences of TBI such as impairment of airway reflexes, decreased level of consciousness, dysphagia, and the need for mechanical ventilation. However, we have hypothesized that the increased incidence of post-TBI bacterial pneumonia is also caused by decreased immune function within the first 2 weeks after injury.11 In our model of TBI and subsequent bacterial pneumonia, we revealed increased mortality in mice that received TBI compared with those who received sham craniotomy. Importantly, male mice had higher mortality than their female counterparts. One mechanism by which this may occur is decreased bacterial clearance after TBI in male mice compared with female mice. Further, we demonstrated the essential role of female sex hormones in the sex dimorphism, as oophorectomized wild-type female mice had increased mortality and decreased bacterial clearance similar to male mice. Interestingly, we found a difference in the levels of TNF-α secreted after P. aeruginosa infection between male and female alveolar macrophages; however, there was no difference in phagocytosis. Finally, we demonstrated that estradiol is one female sex hormone that can alleviate post-TBI P. aeruginosa-mediated mortality, as administration of estradiol to male or oophorectomized wild-type female mice reduces mortality and improves lung bacterial clearance. These findings support an important role for estrogen(s) and sex dimorphism in post-TBI P. aeruginosa pneumonia, which may be mediated by differences in alveolar macrophage function.
In our study, this new model of TBI followed by P. aeruginosa pneumonia is used to study mortality and other indices of pulmonary function. There are numerous animal models of TBI that can accurately mimic focal or diffuse injury using direct impact or inertia-based injuries.52–54 For our purposes, we chose CCI. This method of injury was chosen because it creates a reproducible injury and a significant trauma that allows us to study extracranial pathology. CCI therefore allows us to create a consistent “trigger” injury and test the effects of subsequent pneumonia thereafter. Further, this model of post-TBI bacterial pneumonia allows us to test the effects of sex and drug therapy on a variety of pulmonary outcomes in current and future studies. Additionally, use of transgenic mice can be used within this model. Together, the benefits of this model will allow us to more precisely understand how TBI makes patients more susceptible to secondary bacterial pneumonia and long-term morbidity. Other groups have tested the effects of TBI on secondary bacterial pneumonia. In one model, Staphylococcus aureus was instilled after CCI, and the effect of tranexamic acid on pulmonary immune status was tested, but mortality was never measured.55 Further, another group studied the incidence of spontaneous bacterial pneumonia in mice after TBI with lateral fluid percussion injury.56 They found no increased bacterial load in the lung after 7 days. However, they did use a method of injury that creates a diffuse rather than a focal injury, which may lead to variations in immune suppression. Additionally, recent articles suggest that pneumonia is dependent on the lung microbiome, and mice have high variability in this regard dependent on many variables including cage, shipment, and vendor.57 Finally, another group has demonstrated that TBI before instillation of P. aeruginosa pneumonia improves survival.58–60 Interestingly (1) this group uses a model of diffuse axonal injury that produces mild, rather than severe TBI; (2) they instill the P. aeruginosa pneumonia 48 h after TBI in contrast to 24 h; and (3) they use entirely female mice of a different species. These data support our findings that post-TBI bacterial pneumonia with P. aeruginosa is sex dependent.
We found differences in mortality between male and female mice after post-TBI intratracheal instillation of P. aeruginosa pneumonia. We also revealed that increased mortality was associated with decreased bacterial lung clearance. Further, we demonstrated that these sex differences were related to the expression of female sex hormones, as oophorectomized female mice had increased mortality and decreased bacterial clearance similar to that of male mice. Clinical studies examining post-TBI, extracranial outcomes indicate that male patients are more likely to acquire secondary bacterial pneumonia than their female counterparts.33,34 It is also known in clinical trauma studies that females recover better than males from a number of trauma-related end-organ injuries.32 Pre-clinical rodent studies also indicate that female mice have better outcomes than male rodents in trauma-hemorrhage experiments.38,39 After these initial breakout studies, numerous other studies indicated that estrogen(s) and progesterone could alleviate end-organ injury post-trauma hemorrhage.40 However, limited studies were performed examining the exact difference between the sexes of mice; most studies focused on adding estrogen(s) to male mice just before or after the insult.
Another interesting finding in our study was the difference in secretion of TNF-α by male and female alveolar macrophages after P. aeruginosa infection, with females having significantly increased secretion compared with their male counterparts. Additionally, pre-treatment of male alveolar macrophages stimulated greater TNF-α secretion when exposed to PAK. Because these alveolar macrophages are both immortalized cell lines, we isolated primary macrophages from both male and female mice and again found that there was a difference. TNF-α is one of the key pro-inflammatory cytokines that is initially released when macrophages encounter an invading pathogen.51,61,62 Not only is TNF-α secretion necessary to aid recruitment of neutrophils and other immune cells to the site of the infection, it is also a key cytokine necessary for phagocytosis of P. aeruginosa by macrophages.61,63 Although we hypothesized that increased macrophage phagocytosis could be one mechanism that explained increased bacterial clearance and decreased mortality in female mice, our data do not support this hypothesis. Phagocytosis by male and female macrophages (and primary macrophages) is not significantly different in our studies, including after exposure to estradiol. However, none of these isolated macrophages was exposed to the immunosuppressive effects of TBI; therefore, exposing the cells to immunosuppressive mediators may lead to sex differences that explain the sex dimorphism in our murine model of post-TBI bacterial pneumonia.
In addition to revealing that mortality and lung bacterial clearance after post-TBI P. aeruginosa pneumonia is sex dependent, we reveal that administration of estradiol both alleviates increased mortality and improves lung bacterial clearance in male and oophorectomized female mice. As previously mentioned, estrogen has a salutary effect on male mice that have undergone trauma-hemorrhage.35–37 Pre-clinical studies in stroke and TBI also indicate that therapy with estrogen(s) and/or progesterone may have clinical benefits for patients.64–68 However, clinical trials exploring progesterone as a therapy for acute TBI have found no benefit to date.69,70 In our study, how estrogen improves lung bacterial clearance and improves mortality is not known. Estradiol can activate one of three receptors: estrogen receptor (ER) α, ERβ, and G protein-coupled ER (GPER).36,37,71–73 Typically, activation of ERα and ERβ occurs in a long-term mechanism involving activation/repression of transcription;74 however, GPER activation involves rapid signaling including the mobilization of Ca++ and phosphorylation of extracellular signal-regulated kinase (ERK) 1/2.75–78 Further studies will be required using receptor-specific agonists and/or antagonists or receptor knock-out mice and/or cells to determine the specific ER(s) involved in these processes and specific mechanisms. These question(s) are the source of intense investigation.
Our study has four specific limitations that are opportunities for further growth and utility of our murine model of TBI with subsequent P. aeruginosa pneumonia. First, although our model does exhibit a sex-dependent difference in mortality and lung bacterial clearance, we used a dose of P. aeruginosa that caused mortality in a minority of females that underwent TBI. In future studies, we will test higher doses of P. aeruginosa that induce greater levels of mortality, and examine pulmonary physiology indices at these doses to determine mechanism(s) of pneumonia-related death. Second, we measured lung bacterial clearance as our primary mechanistic end-point for pneumonia-related mortality after TBI. Although this is a useful measurement, there are multiple reasons that lung bacterial clearance may be altered, including, but not limited to: phagocytosis by immune cells, recruitment of immune cells, cytokine release, and permeability of the alveolar-capillary barrier. Future studies will further elucidate sex-dependent mechanisms of TBI and post-TBI, pneumonia-related alterations of pulmonary function and mortality. Third, although we did show differences in sex-dependent TNF-α secretion, we did not find a difference in phagocytosis. However, we have previously revealed that multiple mechanisms of systemic immune suppression inhibit immunity leading to bacterial pneumonia after TBI.11 The cells in our assay were not exposed to these conditions; therefore, they may not reflect the exact physiology that is present inside the murine lung after TBI. Future studies will elucidate these mechanisms via genetic or pharmacological inhibition of macrophages, mimicking what happens in vivo, to better understand these pathophysiological mechanisms. Fourth, although we have provided clear data for the effects of estrogen in this process, we have not studied testosterone. It is well described that having low testosterone levels after TBI leads to a worse prognosis and problems with rehabilitation79,80 and that testosterone can reduce neurodegeneration in mice after TBI.81 Although we could not find any data describing the effects of testosterone on post-TBI bacterial pneumonia, other studies do suggest that males with low testosterone are more susceptible to viral pneumonia.82,83 Therefore, future studies will focus on both clinical and pre-clinical models that examine the role of testosterone in post-TBI bacterial pneumonia.
Conclusion
In summary, we present a reproducible model of TBI using CCI followed by intratracheal instillation of P. aeruginosa. To our knowledge, this is the first study to examine sex differences in mortality and other pulmonary indices in post-TBI bacterial pneumonia with P. aeruginosa. We have demonstrated that decreased lung bacterial clearance is one mechanism by which increased mortality in males occurs. We have demonstrated that this is a sex- and estrogen-dependent effect, as oophorectomized wild-type female mice have greater mortality and decreased lung bacterial clearance, similar to male mice, which is alleviated by estradiol. Further, we have revealed that sex-dependent differences exist at the level of the macrophage. This is a model that has the power to elucidate differences in sex-related pulmonary physiology after TBI. Not only can this model be used to examine bacterial pneumonia after TBI, it can also be employed to understand sex differences in lung physiology after TBI alone. Additionally, the knowledge that isolated male and female macrophages have physiological differences in response to infection is powerful. Although this difference has only been demonstrated using TNF-α secretion, it could be the case for numerous other outcomes. If true, researchers would be able to genetically modify these specific populations to better understand sex differences at a molecular level. Our study reveals new findings in sex dimorphism after trauma, and new tools that can be used to better understand these physiological mechanisms and work toward sex-dependent therapies that can inform precision medicine for both sexes.
Funding Information
This work was supported by a Mentored Research Training Grant from the Foundation for Anesthesia and Education Research to B.M.W. and by grant R01 GM127584 from the National Institutes of Health to B.M.W. and J-F.P.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Shackford, S.R., Mackersie, R.C., Holbrook, T.L., Davis, J.W., Hollingsworth-Fridlund, P., Hoyt, D.B., and Wolf, P.L. (1993). The epidemiology of traumatic death. A population-based analysis. Arch. Surg. 128, 571–575 [DOI] [PubMed] [Google Scholar]
- 2. Rondina, C., Videtta, W., Petroni, G., Lujan, S., Schoon, P., Mori, L.B., Matkovich, J., Carney, N., and Chesnut, R. (2005). Mortality and morbidity from moderate to severe traumatic brain injury in Argentina. J. Head Trauma Rehabil. 20, 368–376 [DOI] [PubMed] [Google Scholar]
- 3. Hui, X., Haider, A.H., Hashmi, Z.G., Rushing, A.P., Dhiman, N., Scott, V.K., Selvarajah, S., Haut, E.R., Efron, D.T., and Schneider, E.B. (2013). Increased risk of pneumonia among ventilated patients with traumatic brain injury: every day counts! J. Surg. Res. 184, 438–443 [DOI] [PubMed] [Google Scholar]
- 4. Plurad, D.S., Kim, D., Bricker, S., Lemesurier, L., Neville, A., Bongard, F. and Putnam, B. (2013). Ventilator-associated pneumonia in severe traumatic brain injury: the clinical significance of admission chest computed tomography findings. J. Surg. Res. 183, 371–376 [DOI] [PubMed] [Google Scholar]
- 5. Rincon-Ferrari, M.D., Flores-Cordero, J.M., Leal-Noval, S.R., Murillo-Cabezas, F., Cayuelas, A., Munoz-Sanchez, M.A., and Sanchez-Olmedo, J.I. (2004). Impact of ventilator-associated pneumonia in patients with severe head injury. J. Trauma 57, 1234–1240 [DOI] [PubMed] [Google Scholar]
- 6. Cifu, D.X., Kreutzer, J.S., Marwitz, J.H., Rosenthal, M., Englander, J., and High, W. (1996). Functional outcomes of older adults with traumatic brain injury: a prospective, multicenter analysis. Arch. Phys. Med. Rehabil. 77, 883–888 [DOI] [PubMed] [Google Scholar]
- 7. Andelic, N., Bautz-Holter, E., Ronning, P., Olafsen, K., Sigurdardottir, S., Schanke, A.K., Sveen, U., Tornas, S., Sandhaug, M., and Roe, C. (2012). Does an early onset and continuous chain of rehabilitation improve the long-term functional outcome of patients with severe traumatic brain injury? J. Neurotrauma 29, 66–74 [DOI] [PubMed] [Google Scholar]
- 8. Shafi, S., Marquez de la Plata, C., Diaz-Arrastia, R., Shipman, K., Carlile, M., Frankel, H., Parks, J., and Gentilello, L.M. (2007). Racial disparities in long-term functional outcome after traumatic brain injury. J. Trauma 63, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 9. Ruet, A., Jourdan, C., Bayen, E., Darnoux, E., Sahridj, D., Ghout, I., Azerad, S., Pradat Diehl, P., Aegerter, P., Charanton, J., Vallat Azouvi, C., and Azouvi, P. (2018). Employment outcome four years after a severe traumatic brain injury: results of the Paris severe traumatic brain injury study. Disabil. Rehabil. 40, 2200–2207 [DOI] [PubMed] [Google Scholar]
- 10. Gause, L.R., Finn, J.A., Lamberty, G.J., Tang, X., Stevens, L.F., Eapen, B.C., and Nakase-Richardson, R. (2017). Predictors of satisfaction with life in veterans after traumatic brain injury: a VA TBI model systems study. J. Head Trauma Rehabil. 32, 255–263 [DOI] [PubMed] [Google Scholar]
- 11. Hu, P.J., Pittet, J.F., Kerby, J.D., Bosarge, P.L., and Wagener, B.M. (2017). Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L1–L15 [DOI] [PubMed] [Google Scholar]
- 12. Dela Cruz, C.S., Wunderink, R.G., Christiani, D.C., Cormier, S.A., Crothers, K., Doerschuk, C.M., Evans, S.E., Goldstein, D.R., Khatri, P., Kobzik, L., Kolls, J.K., Levy, B.D., Metersky, M.L., Niederman, M.S., Nusrat, R., Orihuela, C.J., Peyrani, P., Prince, A.S., Ramirez, J.A., Ridge, K.M., Sethi, S., Suratt, B.T., Sznajder, J.I., Tsalik, E.L., Walkey, A.J., Yende, S., Aggarwal, N.R., Caler, E.V., and Mizgerd, J.P. (2018). Future research directions in pneumonia. NHLBI Working Group Report. Am. J. Respir. Crit. Care Med. 198, 256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boucher, H.W., Talbot, G.H., Bradley, J.S., Edwards, J.E., Gilbert, D., Rice, L.B., Scheld, M., Spellberg, B., and Bartlett, J. (2009). Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48, 1–12 [DOI] [PubMed] [Google Scholar]
- 14. Giske, C.G., Monnet, D.L., Cars, O., Carmeli, Y. and ReAct-Action on Antibiotic Resistance (2008). Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 52, 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park, D.R. (2005). The microbiology of ventilator-associated pneumonia. Respir. Care 50, 742–763 [PubMed] [Google Scholar]
- 16. Pendleton, J.N., Gorman, S.P., and Gilmore, B.F. (2013). Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 11, 297–308 [DOI] [PubMed] [Google Scholar]
- 17. Spellberg, B., Guidos, R., Gilbert, D., Bradley, J., Boucher, H.W., Scheld, W.M., Bartlett, J.G., Edwards, J.Jr. and Infectious Diseases Society of America (2008). The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46, 155–164 [DOI] [PubMed] [Google Scholar]
- 18. Kaier, K., Heister, T., Gotting, T., Wolkewitz, M., and Mutters, N.T. (2019). Measuring the in-hospital costs of Pseudomonas aeruginosa pneumonia: methodology and results from a German teaching hospital. BMC Infect. Dis. 19, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Youssef, D., Bailey, B., El-Abbassi, A., Vannoy, M., Manning, T., Moorman, J.P., and Peiris, A.N. (2012). Healthcare costs of methicillin resistant Staphylococcus aureus and Pseudomonas aeruginosa infections in veterans: role of vitamin D deficiency. Eur. J. Clin. Microbiol. Infect. Dis. 31, 281–286 [DOI] [PubMed] [Google Scholar]
- 20. Ware, L.B., and Matthay, M.A. (2000). The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 [DOI] [PubMed] [Google Scholar]
- 21. Sawa, T., Shimizu, M., Moriyama, K., and Wiener-Kronish, J.P. (2014). Association between Pseudomonas aeruginosa type III secretion, antibiotic resistance, and clinical outcome: a review. Crit. Care 18, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiener-Kronish, J.P., Sakuma, T., Kudoh, I., Pittet, J.F., Frank, D., Dobbs, L., Vasil, M.L., and Matthay, M.A. (1993). Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J. Appl. Physiol. (1985) 75, 1661–1669 [DOI] [PubMed] [Google Scholar]
- 23. Sayner, S.L., Frank, D.W., King, J., Chen, H., VandeWaa, J., and Stevens, T. (2004). Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ. Res. 95, 196–203 [DOI] [PubMed] [Google Scholar]
- 24. Liu, A., Park, J.H., Zhang, X., Sugita, S., Naito, Y., Lee, J.H., Kato, H., Hao, Q., Matthay, M.A., and Lee, J.W. (2019). Therapeutic effects of hyaluronic acid in bacterial pneumonia in ex vivo perfused human lungs. Am. J. Respir. Crit. Care Med. 200, 1234–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganter, M.T., Roux, J., Su, G., Lynch, S.V., Deutschman, C.S., Weiss, Y.G., Christiaans, S.C., Myazawa, B., Kipnis, E., Wiener-Kronish, J.P., Howard, M., and Pittet, J.F. (2009). Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am. J. Respir. Cell. Mol. Biol. 40, 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carles, M., Lafargue, M., Goolaerts, A., Roux, J., Song, Y., Howard, M., Weston, D., Swindle, J.T., Hedgpeth, J., Burel-Vandenbos, F., and Pittet, J.F. (2010). Critical role of the small GTPase RhoA in the development of pulmonary edema induced by Pseudomonas aeruginosa in mice. Anesthesiology 113, 1134–1143 [DOI] [PubMed] [Google Scholar]
- 27. Kloth, C., Schirmer, B., Munder, A., Stelzer, T., Rothschuh, J., and Seifert, R. (2018). The role of Pseudomonas aeruginosa ExoY in an acute mouse lung infection model. Toxins (Basel) 10, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balczon, R., Prasain, N., Ochoa, C., Prater, J., Zhu, B., Alexeyev, M., Sayner, S., Frank, D.W., and Stevens, T. (2013). Pseudomonas aeruginosa exotoxin Y-mediated tau hyperphosphorylation impairs microtubule assembly in pulmonary microvascular endothelial cells. PLoS One 8, e74343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ochoa, C.D., Alexeyev, M., Pastukh, V., Balczon, R., and Stevens, T. (2012). Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J. Biol. Chem. 287, 25,407–25,418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faure, E., Kwong, K., and Nguyen, D. (2018). Pseudomonas aeruginosa in chronic lung infections: how to adapt within the host? Front. Immunol. 9, 2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riquelme, S.A., Ahn, D., and Prince, A. (2018). Pseudomonas aeruginosa and Klebsiella pneumoniae adaptation to innate immune clearance mechanisms in the lung. J. Innate Immun. 10, 442–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frink, M., Pape, H.C., van Griensven, M., Krettek, C., Chaudry, I.H., and Hildebrand, F. (2007). Influence of sex and age on mods and cytokines after multiple injuries. Shock 27, 151–156 [DOI] [PubMed] [Google Scholar]
- 33. Andermahr, J., Greb, A., Hensler, T., Helling, H.J., Bouillon, B., Sauerland, S., Rehm, K.E., and Neugebauer, E. (2002). Pneumonia in multiple injured patients: a prospective controlled trial on early prediction using clinical and immunological parameters. Inflamm. Res. 51, 265–272 [DOI] [PubMed] [Google Scholar]
- 34. Sharpe, J.P., Magnotti, L.J., Weinberg, J.A., Brocker, J.A., Schroeppel, T.J., Zarzaur, B.L., Fabian, T.C., and Croce, M.A. (2014). Gender disparity in ventilator-associated pneumonia following trauma: identifying risk factors for mortality. J. Trauma Acute Care Surg. 77, 161–165 [DOI] [PubMed] [Google Scholar]
- 35. Weniger, M., Angele, M.K. and Chaudry, I.H. (2016). The role and use of estrogens following trauma. Shock 46, 4–11 [DOI] [PubMed] [Google Scholar]
- 36. Kawasaki, T., and Chaudry, I.H. (2012). The effects of estrogen on various organs: therapeutic approach for sepsis, trauma, and reperfusion injury. Part 2: liver, intestine, spleen, and kidney. J. Anesth. 26, 892–899 [DOI] [PubMed] [Google Scholar]
- 37. Kawasaki, T., and Chaudry, I.H. (2012). The effects of estrogen on various organs: therapeutic approach for sepsis, trauma, and reperfusion injury. Part 1: central nervous system, lung, and heart. J. Anesth. 26, 883–891 [DOI] [PubMed] [Google Scholar]
- 38. Choudhry, M.A., Schwacha, M.G., Hubbard, W.J., Kerby, J.D., Rue, L.W., Bland, K.I., and Chaudry, I.H. (2005). Gender differences in acute response to trauma-hemorrhage. Shock 24 Suppl. 1, 101–106 [DOI] [PubMed] [Google Scholar]
- 39. Wichmann, M.W., Zellweger, R., DeMaso, C.M., Ayala, A., and Chaudry, I.H. (1996). Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock and resuscitation. Cytokine 8, 853–863 [DOI] [PubMed] [Google Scholar]
- 40. Bosch, F., Angele, M.K., and Chaudry, I.H. (2018). Gender differences in trauma, shock and sepsis. Mil. Med. Res. 5, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brody, D.L., Mac Donald, C., Kessens, C.C., Yuede, C., Parsadanian, M., Spinner, M., Kim, E., Schwetye, K.E., Holtzman, D.M., and Bayly, P.V. (2007). Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma 24, 657–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Che, P., Wagener, B.M., Zhao, X., Brandon, A.P., Evans, C.A., Cai, G.Q., Zhao, R., Xu, Z.X., Han, X., Pittet, J.F., and Ding, Q. (2020). Neuronal Wiskott-Aldrich syndrome protein regulates Pseudomonas aeruginosa-induced lung vascular permeability through the modulation of actin cytoskeletal dynamics. FASEB J. 34, 3305–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wagener, B.M., Anjum, N., Evans, C., Brandon, A., Honavar, J., Creighton, J., Traber, M.G., Stuart, R.L., Stevens, T., and Pittet, J.F. (2020). Alpha-tocopherol attenuates the severity of Pseudomonas aeruginosa-induced pneumonia. Am. J. Respir. Cell Mol. Biol. 63, 234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang, S., Park, D.W., Stigler, W.S., Creighton, J., Ravi, S., Darley-Usmar, V., and Zmijewski, J.W. (2013). Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J. Biol. Chem. 288, 26,013–26,026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wagener, B.M., Hu, P.J., Oh, J.Y., Evans, C.A., Richter, J.R., Honavar, J., Brandon, A.P., Creighton, J., Stephens, S.W., Morgan, C., Dull, R.O., Marques, M.B., Kerby, J.D., Pittet, J.F., and Patel, R.P. (2018). Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: a preclinical experimental study. PLoS Med 15, e1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li, Y., Liu, C., Xiao, W., Song, T., and Wang, S. (2020). Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit. Care 32, 272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharma, R., Shultz, S.R., Robinson, M.J., Belli, A., Hibbs, M.L., O'Brien, T.J., and Semple, B.D. (2019). Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain Behav. Immun. 79, 63–74 [DOI] [PubMed] [Google Scholar]
- 48. Gamberini, L., Giugni, A., Ranieri, S., Meconi, T., Coniglio, C., Gordini, G., and Bardi, T. (2019). Early-onset ventilator-associated pneumonia in severe traumatic brain injury: is there a relationship with prehospital airway management? J. Emerg. Med. 56, 657–665 [DOI] [PubMed] [Google Scholar]
- 49. Bucci, M. (2018). No more ESKAPE. Nat. Chem. Biol. 14, 989. [DOI] [PubMed] [Google Scholar]
- 50. Mulani, M.S., Kamble, E.E., Kumkar, S.N., Tawre, M.S., and Pardesi, K.R. (2019). Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol 10, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raoust, E., Balloy, V., Garcia-Verdugo, I., Touqui, L., Ramphal, R., and Chignard, M. (2009). Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4, e7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiong, Y., Mahmood, A., and Chopp, M. (2013). Animal models of traumatic brain injury. Nat. Rev. Neurosci. 14, 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O'Connor, W.T., Smyth, A., and Gilchrist, M.D. (2011). Animal models of traumatic brain injury: a critical evaluation. Pharmacol. Ther. 130, 106–113 [DOI] [PubMed] [Google Scholar]
- 54. Mayer, A.R., Dodd, A.B., Vermillion, M.S., Stephenson, D.D., Chaudry, I.H., Bragin, D.E., Gigliotti, A.P., Dodd, R.J., Wasserott, B.C., Shukla, P., Kinsler, R., and Alonzo, S.M. (2019). A systematic review of large animal models of combined traumatic brain injury and hemorrhagic shock. Neurosci. Biobehav. Rev. 104, 160–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Draxler, D.F., Awad, M.M., Hanafi, G., Daglas, M., Ho, H., Keragala, C., Galle, A., Roquilly, A., Lyras, D., Sashindranath, M., and Medcalf, R.L. (2019). Tranexamic acid influences the immune response, but not bacterial clearance in a model of post-traumatic brain injury pneumonia. J. Neurotrauma 36, 3297–3308 [DOI] [PubMed] [Google Scholar]
- 56. Sun, M., Brady, R.D., Wanrooy, B., Mychasiuk, R., Yamakawa, G.R., Casillas-Espinosa, P.M., Wong, C.H.Y., Shultz, S.R., and McDonald, S.J. (2020). Experimental traumatic brain injury does not lead to lung infection. J. Neuroimmunol. 343, 577239. [DOI] [PubMed] [Google Scholar]
- 57. Dickson, R.P., Erb-Downward, J.R., Falkowski, N.R., Hunter, E.M., Ashley, S.L., and Huffnagle, G.B. (2018). The lung microbiota of healthy mice are highly variable, cluster by environment, and reflect variation in baseline lung innate immunity. Am. J. Respir. Crit. Care Med. 198, 497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vaickus, M., Hsieh, T., Kintsurashvili, E., Kim, J., Kirsch, D., Kasotakis, G., and Remick, D.G. (2019). Mild traumatic brain injury in mice beneficially alters lung NK1R and structural protein expression to enhance survival after Pseudomonas aeruginosa infection. Am. J. Pathol. 189, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang, S., Stepien, D., Hanseman, D., Robinson, B., Goodman, M.D., Pritts, T.A., Caldwell, C.C., Remick, D.G., and Lentsch, A.B. (2014). Substance P mediates reduced pneumonia rates after traumatic brain injury. Crit. Care Med. 42, 2092–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hsieh, T., Vaickus, M.H., Stein, T.D., Lussier, B.L., Kim, J., Stepien, D.M., Duffy, E.R., Chiswick, E.L., and Remick, D.G. (2016). The role of substance P in pulmonary clearance of bacteria in comparative injury models. Am. J. Pathol. 186, 3236–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tzepi, I.M., Giamarellos-Bourboulis, E.J., Carrer, D.P., Tsaganos, T., Claus, R.A., Vaki, I., Pelekanou, A., Kotsaki, A., Tziortzioti, V., Topouzis, S., Bauer, M., and Papapetropoulos, A. (2012). Angiopoietin-2 enhances survival in experimental sepsis induced by multidrug-resistant Pseudomonas aeruginosa. J. Pharmacol. Exp. Ther. 343, 278–287 [DOI] [PubMed] [Google Scholar]
- 62. Jabir, M.S., Hopkins, L., Ritchie, N.D., Ullah, I., Bayes, H.K., Li, D., Tourlomousis, P., Lupton, A., Puleston, D., Simon, A.K., Bryant, C., and Evans, T.J. (2015). Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy. Autophagy 11, 166–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kowalski, M.P., and Pier, G.B. (2004). Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J. Immunol. 172, 418–425 [DOI] [PubMed] [Google Scholar]
- 64. Golz, C., Kirchhoff, F.P., Westerhorstmann, J., Schmidt, M., Hirnet, T., Rune, G.M., Bender, R.A., and Schafer, M.K.E. (2019). Sex hormones modulate pathogenic processes in experimental traumatic brain injury. J. Neurochem. 150, 173–187 [DOI] [PubMed] [Google Scholar]
- 65. Selvaraj, U.M., Zuurbier, K.R., Whoolery, C.W., Plautz, E.J., Chambliss, K.L., Kong, X., Zhang, S., Kim, S.H., Katzenellenbogen, B.S., Katzenellenbogen, J.A., Mineo, C., Shaul, P.W., and Stowe, A.M. (2018). Selective nonnuclear estrogen receptor activation decreases stroke severity and promotes functional recovery in female mice. Endocrinology 159, 3848–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang, H., Lin, S., Chen, X., Gu, L., Zhu, X., Zhang, Y., Reyes, K., Wang, B., and Jin, K. (2019). The effect of age, sex and strains on the performance and outcome in animal models of stroke. Neurochem. Int. 127, 2–11 [DOI] [PubMed] [Google Scholar]
- 67. Brotfain, E., Gruenbaum, S.E., Boyko, M., Kutz, R., Zlotnik, A., and Klein, M. (2016). Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr. Neuropharmacol. 14, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu, X., Frechou, M., Schumacher, M., and Guennoun, R. (2019). Cerebroprotection by progesterone following ischemic stroke: multiple effects and role of the neural progesterone receptors. J. Steroid Biochem. Molec. Biol. 185, 90–102 [DOI] [PubMed] [Google Scholar]
- 69. Skolnick, B.E., Maas, A.I., Narayan, R.K., van der Hoop, R.G., MacAllister, T., Ward, J.D., Nelson, N.R., Stocchetti, N. and SYNAPSE Trial Investigators (2014). A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476 [DOI] [PubMed] [Google Scholar]
- 70. Wright, D.W., Yeatts, S.D., Silbergleit, R., Palesch, Y.Y., Hertzberg, V.S., Frankel, M., Goldstein, F.C., Caveney, A.F., Howlett-Smith, H., Bengelink, E.M., Manley, G.T., Merck, L.H., Janis, L.S., Barsan, W.G., and NETT Investigators (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Prossnitz, E.R., and Barton, M. (2014). Estrogen biology: new insights into GPER function and clinical opportunities. Mol. Cell Endocrinol. 389, 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barton, M., Filardo, E.J., Lolait, S.J., Thomas, P., Maggiolini, M., and Prossnitz, E.R. (2018). Twenty years of the G protein-coupled estrogen receptor GPER: historical and personal perspectives. J. Steroid Biochem. Molec. Biol. 176, 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Prossnitz, E.R., and Barton, M. (2011). The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 7, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kovats, S. (2015). Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 294, 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gonzalez de Valdivia, E., Broselid, S., Kahn, R., Olde, B., and Leeb-Lundberg, L.M.F. (2017). G protein-coupled estrogen receptor 1 (GPER1)/GPR30 increases ERK1/2 activity through PDZ motif-dependent and -independent mechanisms. J. Biol. Chem. 292, 9932–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kabir, M.E., Singh, H., Lu, R., Olde, B., Leeb-Lundberg, L.M., and Bopassa, J.C. (2015). G protein-coupled estrogen receptor 1 mediates acute estrogen-induced cardioprotection via MEK/ERK/GSK-3beta pathway after ischemia/reperfusion. PLoS One 10, e0135988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Meyer, M.R., Field, A.S., Kanagy, N.L., Barton, M., and Prossnitz, E.R. (2012). GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life Sci. 91, 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Revankar, C.M., Cimino, D.F., Sklar, L.A., Arterburn, J.B., and Prossnitz, E.R. (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 79. Hohl, A., Zanela, F.A., Ghisi, G., Ronsoni, M.F., Diaz, A.P., Schwarzbold, M.L., Dafre, A.L., Reddi, B., Lin, K., Pizzol, F.D., and Walz, R. (2018). Luteinizing hormone and testosterone levels during acute phase of severe traumatic brain injury: prognostic implications for adult male patients. Front. Endocrinol. (Lausanne) 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Young, T.P., Hoaglin, H.M., and Burke, D.T. (2007). The role of serum testosterone and TBI in the in-patient rehabilitation setting. Brain Inj. 21, 645–649 [DOI] [PubMed] [Google Scholar]
- 81. Carteri, R.B., Kopczynski, A., Rodolphi, M.S., Strogulski, N.R., Sartor, M., Feldmann, M., De Bastiani, M.A., Duval Wannmacher, C.M., de Franceschi, I.D., Hansel, G., Smith, D.H., and Portela, L.V. (2019). Testosterone administration after traumatic brain injury reduces mitochondrial dysfunction and neurodegeneration. J. Neurotrauma 36, 2246–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vom Steeg, L.G., Vermillion, M.S., Hall, O.J., Alam, O., McFarland, R., Chen, H., Zirkin, B., and Klein, S.L. (2016). Age and testosterone mediate influenza pathogenesis in male mice. Am. J. Physiol. Lung Cell Mol. Physiol. 311, L1234–L1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rastrelli, G., Di Stasi, V., Inglese, F., Beccaria, M., Garuti, M., Di Costanzo, D., Spreafico, F., Greco, G.F., Cervi, G., Pecoriello, A., Magini, A., Todisco, T., Cipriani, S., Maseroli, E., Corona, G., Salonia, A., Lenzi, A., Maggi, M., De Donno, G., and Vignozzi, L. (2020). Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]