Abstract
The mortality of traumatic brain injury (TBI) has been largely static despite advances in monitoring and imaging techniques. Substantial variance exists in outcome, not fully accounted for by baseline characteristics or injury severity, and genetic factors likely play a role in this variance. The aims of this systematic review were to examine the evidence for a link between the apolipoprotein E4 (APOE4) polymorphism and TBI outcomes and where possible, to quantify the effect size via meta-analysis. We searched EMBASE, MEDLINE, CINAHL, and gray literature in December 2017. We included studies of APOE genotype in relation to functional adult TBI outcomes. Methodological quality was assessed using the Quality in Prognostic Studies Risk of Bias Assessment Instrument and the prognostic studies adaptation of the Grading of Recommendations Assessment, Development and Evaluation tool. In addition, we contacted investigators and included an additional 160 patients whose data had not been made available for previous analyses, giving a total sample size of 2593 patients. Meta-analysis demonstrated higher odds of a favorable outcome following TBI in those not possessing an ApoE ɛ4 allele compared with ɛ4 carriers and homozygotes (odds ratio 1.39, 95% confidence interval 1.05 to 1.84; p = 0.02). The influence of APOE4 on neuropsychological functioning following TBI remained uncertain, with multiple conflicting studies. We conclude that the ApoE ɛ4 allele confers a small risk of poor outcome following TBI, with analysis by TBI severity not possible based on the currently available published data. Further research into the long-term neuropsychological impact and risk of dementia is warranted.
Keywords: genetics, living systematic reviews, outcome, prognosis, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a substantial health problem, which shows substantial variance in outcome—only about a third of which can be accounted for by known covariates.1 There is an increasing interest in exploring whether some of the unexplained variance may arise from genetic differences in processes involved in cognitive reserve, injury mechanisms, repair mechanisms, or neurodegenerative processes. Several genes have been explored in this context, and the polymorphisms studied include those coding for brain-derived neurotrophic factor, cytokine, neurotransmitter and mitochondrial gene families, and other individual candidate genes. However, the most common focus of study in this context has been variations in the APOE gene, which codes for apolipoprotein E. Since the original report2 of worse outcomes in TBI patients possessing the ɛ4 allele of apolipoprotein E (APOE), a large number of studies have tested the influence of sequence variants in specific genes on mortality, functional, and neuropsychological outcomes. The mechanisms by which APOE polymorphisms might modulate these outcomes are detailed in Supplementary Appendix 1: Background to APOE. This manuscript is one of a pair of systematic reviews addressing the effect of genetic variation in TBI and will concentrate on the effect of the ɛ4 allele of APOE. A companion systematic review, which addresses non-APOE genes, has recently been published.3
APOE is undoubtedly the most extensively studied gene in the field of TBI. It codes for a 34kDa protein, which has a central role in central nervous system lipid transport, including movement of cholesterol into cells to aid repair processes in damaged neurones. Three common alleles have been characterized (ɛ2, 3, and 4), which code for protein isoforms E2, E3, and E4. The literature to date supports an association between possession of the ɛ4 allele and a variety of negative neuropsychiatric outcomes, including a dose-dependent increase in the risk of late onset Alzheimer's disease as well as intracerebral haemorrhage.4 There is some evidence that APOE2 may exert a neuroprotective effect opposite to that of E4, but its relatively low population incidence is a limiting factor in research.
The neurochemical mechanisms for toxic effects of APOE4 have been reviewed extensively.5 In brief, it is thought that the E4 isoform (which uniquely contains an arginine replacing a cysteine at residue 112) exhibits a property known as domain interaction, whereby an exposed arginine at residue 61 interacts with the C-terminal domain. This change in the tertiary structure of the peptide results in aberrant cleavage within the endoplasmic reticulum, releasing neurotoxic fragments into the cytosol, where they impair mitochondrial and cytoskeletal function, potentially leading to cellular apoptosis. There is evidence that APOE4 inhibits neurite outgrowth (unlike E2/E3, which encourage it) and that release of pro-inflammatory mediators (interleukin [IL] 6, nitric oxide) from stimulated microglia is greater in the presence of E4. Traumatic brain injury involves a mechanical insult triggering a complex pathogenic process, with inflammation and neurotoxicity featuring prominently in the development of secondary brain injury.6 As the E4 isoform has been shown to exhibit a number of pathological functions with respect to these processes, it has been hypothesized that TBI patients who are homo- or heterozygous for the ɛ4 allele may experience a more severe TBI for a given cause of injury, with greater secondary injury and impaired capacity for recovery.
With regard to the previously published data on this topic, there have been many reviews that have tried to collate the available data at different times. For brevity, we have concentrated on the most recent of these, which examine the effect of APOE genotype on global functional outcome from TBI, both of which reported an increased incidence of poor outcome in carriers of the risk of the ɛ4 allele.7,8 A separate meta-analysis examining purely cognitive outcome measures found that no firm association between APOE alleles and post-TBI function could be demonstrated.9 A narrative systematic review of APOE and TBI outcomes reported a deleterious effect of APOE4 on functional outcomes in severe TBI but no consistent association in milder injury.10
The aim of this meta-analysis and systematic review is to provide a comprehensive report of the association between APOE variants (focusing on the effect of possession of an ɛ4 allele) and outcome in adults suffering TBI. We have divided outcomes of interest into “global” measures such as the Glasgow Outcome Score, which represent overall levels of disability after injury, and more detailed neuropsychiatric and cognitive assessments such as measures of verbal reasoning and executive functioning. We report a meta-analysis of global outcomes, and narratively summarize the evidence regarding neuropsychiatric and cognitive recovery. It is important to point out that our meta-analysis includes substantial unpublished data not available to those authors of the recent meta-analyses discussed above; concordances and discordances with these reviews are covered in detail in the Discussion section.
Methods
This review was conducted and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.11 A protocol was registered on June 10, 2014, with the University of York's International Prospective Register of Systematic Reviews (PROSPERO) database (registration number CRD42014013623; available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013623).
This review is being prepared as a “living systematic review” as part of the Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) project (www.center-tbi.eu).12 A living systematic review is a high quality up-to-date online summary of health research that is updated as new research becomes available.13 In practice, this means that the searches will be re-run and any new studies incorporated into the review. We will seek to publish regular updates.
Search methods for identification of studies
In May 2014, EMBASE, MEDLINE, and CINAHL (all via National Institute for Health and Care Excellence Healthcare Databases) and Google Scholar were searched for published studies, and conference abstracts published in peer reviewed journals indexed in the above databases. Developed with search experts at Monash University's National Trauma Research Institute, the search strategies used a combination of keywords and MeSH terms (Supplementary Appendix 2: Search Strategies). Study reference lists were manually reviewed to identify relevant publications not identified by the search strategy. Conference abstracts prompted further PubMed searches to discover whether the data had subsequently been published in full. Searches were re-run in August 2015, November 2016 and December 2017 using the identical protocol.
Selection criteria
Citations were downloaded into Endnote (Thomson Reuters), duplicates removed, and were then screened by one author (CAM) on title/abstract using the following selection criteria:
-
1.
Adult TBI patients (aged over 16 years).
-
2.
A functional outcome measure of any type, reported by patient genotype—this included the Glasgow Outcome Scale (GOS), Glasgow Outcome Scale-Extended (GOS-E), modified Rankin Scale (mRS), Disability Rating Scale (DRS), Neurobehavioral Rating Scale (NRS), as well as neuropsychological measures.
-
3.
English language.
Studies were excluded if they dealt with in vitro/animal work, or included non-TBI/pediatric patients and did not report separate outcome data for the adult TBI cohort. Studies reporting nonfunctional outcome measures, such as histological findings at post mortem, were also excluded.
After screening, the remaining citations were reviewed in full text independently by two authors (CAM and VFJN/FAZ) to assess them for eligibility. Disagreements regarding eligibility were resolved by consensus, and referral to a third reviewer (DKM) was not required.
Quality assessment
Risk of bias was assessed using the Quality In Prognostic Studies (QuIPS) criteria, a validated domain-based tool for quality assessment of prognostic studies.14 Two authors (CAM and VFJN/FAZ) independently completed the QuIPS for each study, and then reached a final judgement on each of the six domains by consensus. In line with the guidance given by the team who developed QuIPS, no overall rating of quality is assigned to each study.
Data extraction
Citations and full text files were uploaded to Covidence (www.covidence.org). Two authors (CAM, and either VFJN or FAZ) worked independently, resolving disagreements through consensus. The following characteristics were extracted:
-
1.
Inclusion/exclusion criteria.
-
2.
Baseline characteristics, where possible for each genotype within the cohort:
a. Cohort gender composition.
b. Age (mean ± standard deviation [SD] if available).
c. TBI severity according to the Glasgow Coma Scale (GCS) which was quantified, wherever possible, as mean GCS ± SD, or GCS grouped according to existing guidelines for classification.15
-
3.
Outcome data (see below).
-
4.
Funding source(s).
In the case of studies covering global functional outcomes (e.g., GOS/GOS-E, mRS, NRS, DRS, mortality), scores were extracted at all available time-points for each genotype. The total numbers of patients assigned a given score at each time-point were extracted and used to calculate the number of patients with a “favorable” outcome. Categorical scales were dichotomized in line with previously recognized methods for defining “favorable” outcomes (i.e., GOS 4 to 5, GOS-E 5 to 8.)16 When authors reported self-defined favorable or unfavorable outcomes without a breakdown of the underlying raw categorical data, this was extracted instead. If ordinal data were not available, the mean scores and standard deviations (or standard errors/95% confidence intervals) were extracted. In studies dealing with neuropsychological scales or other outcomes (e.g., measures of fatigue), reports of statistically significantly differences between genotype results (at the alpha level selected by the study's authors) were extracted, with a narrative note made of nonsignificant results. In the case of no significant results being reported, a narrative note of negative findings was made.
Statistical analysis
Studies were subdivided for analysis by outcome measures. Where studies were sufficiently homogenous (in terms of gene studied, patient characteristics, and outcome measured), they were pooled statistically using RevMan 5.3 (Nordic Cochrane Centre 2014).17 Only studies reporting global functional outcome scores in relation to APOE genotype were entered into meta-analysis.
The quality of evidence contributing to each pooled outcome was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, modified for prognostic studies.18 This examines eight factors; six that can downgrade the evidence (phase of investigation, study limitations, inconsistency, indirectness, imprecision, publication bias) and two that can increase it (moderate or large effect size, exposure–response gradient).
For the meta-analysis, outcome was extracted for dichotomized genotypes (APOE4 carriers vs. non-carriers), with outcome scores dichotomized as GOS 4-5/GOS-E 5-8 representing a “favorable outcome.” The primary meta-analysis was of outcome data at 6 months; one study (Ponsford and colleagues)19 did not report 6-month data, and so the next available time-point (12 months) was used instead. Throughout the review, where not otherwise specified, “unfavorable outcome” or “poor outcome” is in reference to a GOS score of 1–3, or a GOS-E of 1–4, with “good” or “favorable” outcome referring to GOS 4–5 or GOS-E 5–8.
We employed the random effects model implemented in RevMan (Nordic Cochrane Centre 2014).17 Between-study heterogeneity was explored with the chi-squared test, and quantified using the I2 statistic. Significant heterogeneity was defined as I2 > 50%. A pooled effect estimate for the total study population was calculated as Mantel-Haenzel odds ratios and 95% confidence intervals (see http://handbook.cochrane.org/chapter_9/9_4_4_3_random_effects_method.htm for details). We defined the clinical importance of the observed associations as small (odds ratio [OR] <2.5), moderate (2.5–4.25), or large (> 4.25), in line with the definition proposed in a recent Cochrane prognostic review.18
Sensitivity analysis
During the data extraction process, it became clear that there was variation in the manner in which outcome data were reported and interpreted by authors. Willmott and colleagues20 for example use a GOS-E score of 7 to 8 (living independently/return to work) as a marker of “good recovery” for their analyses. We recognize that identical odds ratios might be assumed if the GOS-E is considered as an ordinal scale, as is done with proportional odds regression analysis.21 In addition, some studies reported outcome data over significantly longer time scales, ranging from 36 months to 25 years. The time-point chosen for meta-analysis and outcome dichotomization employed was not based on a priori scientific evidence, but reflected the most common practice of authors in the field. Post hoc sensitivity analyses were therefore constructed as follows:
-
1.
Six-month outcome data (or next available time-point) with GOS-E dichotomized in line with Willmott and colleagues.
-
2.
Last available time-point, with GOS 4/5 or GOS-E 5–8 representing “favorable outcome.”
-
3.
Last available time-point, with the Wilmott dichotomization of GOS-E.
-
4.
Six-month outcome data (or next available time-point) but omitting studies rated as high risk of bias in one or more QuIPS domains.
Assessment of publication bias
For studies included in the meta-analysis, we examined funnel plot asymmetry (which may indicate the presence of publication bias), using RevMan 5.3 software (the Nordic Cochrane Centre). Where data were unable to be pooled, we assessed the likelihood of publication bias qualitatively, based on included study characteristics and the advice of experts within and beyond the author team about the possibility of relevant unpublished studies.
Results
Search results
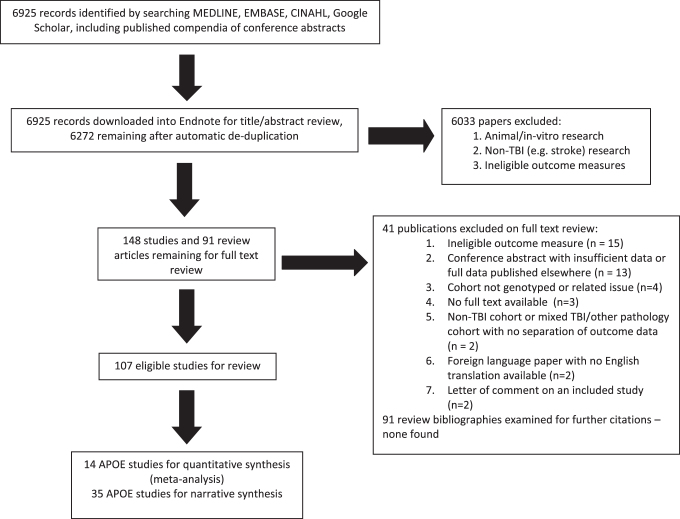
A total of 6925 citations were identified through database searches (Fig. 1). After removing duplicates, 6272 were screened on citation and abstract, with 6033 excluded. We obtained 239 citations in full text (including 91 review articles), of which 41 studies were excluded. The reasons for exclusion included non-TBI study populations and ineligible outcome measures (Supplementary Table S1).
FIG. 1.
Study selection flowchart.
Included studies
Forty-nine studies examining APOE2,19,22–68 were identified. Of these, 21 reported a global outcome such as GOS or GOSE,2,16,19–33,55,59–61 and of these studies, 142,16,19,22–28,31,32,55 provided sufficient detail for meta-analysis (see Table 1, which includes all 21 studies and specifies the studies that were included in the meta-analysis). Study designs consisted of 38 prospective cohort studies, 10 retrospective cohort studies, and one case-control study. Population sizes were heterogeneous; 21 studies included 100 or more patients and the remaining 28 less than 100.
Table 1.
Characteristics of Studies of Apolipoprotein E Gene (Measuring Glasgow Outcome Scale/Glasgow Outcome Scale–Extended)
| Study ID | Setting (country) | Design | Participants |
Outcome reported | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number (n = ) | Age (m, SD or range) | Gender (M, %) | Injury Severity |
|||||||
| GCS (m ± SD) | GCS 3–8 (n, %) | GCS 9–12 (n, %) | GCS 13–15 (n, %) | |||||||
| Alexander 200722 | ITU (USA) | Pros. cohort | APOE4+: 97 | 33.7 ± 14.6 | 72 (74.2) | 5.7 ± 1.4 | NR | NR | NR | APOE4+ had higher (better) GOS at 3, 6, 12 months and slightly lower at 24 months; none statistically significant once GCS and age controlled for. |
| APOE4-: 26 | 31 ± 14.5 | 21 (80.8) | 5.4 ± 1.4 | NR | NR | NR | ||||
| Ariza 200623 | Hosp. (Spain) | Pros. cohort | APOE4+: 67 | 28.7 ± 11.47 | 53 (79.7) | 7.82 ± 2.24 | NR | NR | NR | No statistically significant association between genotype and GOS-E at mean 215 (SD 23) days post- injury. |
| APOE4-: 10 | 37.7 ± 18.31 | 7 (70) | 7.1 ± 2.81 | NR | NR | NR | ||||
| Chamelian 200424 | Clinic (Canada) | Pros. cohort | APOE4-: 71 | 34.1 ± 12.3 | 44 (62) | NR | NR | (91) | (9) | No statistically significant association between genotype and GOS at 6 months. |
| APOE4+: 19 | 31.2 ± 13.3 | 10 (52.6) | NR | NR | (88.2) | (11.8) | ||||
| Chiang 200325 | Hosp. (Taiwan) | Pros. cohort | APOE4+:81 | 42.6 (15 - 86) | 61 (76.3) | NR | 30 (37) | 20 (24.7) | 31 (38.3) | Significantly more APOE4+ had unfavorable 6-month GOS |
| APOE4-: 19 | 46.4 (22 - 79) | 16 (84.2) | NR | 10 (52.6) | 4 (21.1) | 5 (26.3) | ||||
| Diaz-Arrastia 2003246 | Hosp. (USA) | Pros. cohort | APOE4-: 77 | 38.8 ± 19.7 | 52 (67.5) | 11 ± 4.5 | 25 (32.5 ) | 9 (14.8) | 43 (56.3) | No statistically significant association between genotype and GOS-E at 6 months. |
| APOE4+: 29 | 40 ± 19 | 18 (62.1) | 10.8 ± 4.7 | 10 (33.3) | 4 (14.8) | 15 (51.8) | ||||
| Friedman 199927 | Clinic (Israel) | Pros. cohort | APOE4-: 42 | 38.2 ± 14.6 | NR | NR | (55.6) | NR | NR | Statistically significant worse outcome on proprietary outcome scale at 6 months for APOE4+. |
| APOE4+: 27 | 31.8 ± 14.1 | NR | NR | (80) | NR | NR | ||||
| Hiekkanen 200928 | Hosp. (Finland) | Pros. cohort | APOE4-: 26 | 41.4 ± 17.4 | 19 (73.1%) | NR | 9 (34.6) | 12 (46.2%) | 5 (19.2%) | No statistically significant association between genotype and GOS-E at 12 months. |
| APOE4+: 11 | 46.3 ± 14.6 | 8 (72.3%) | NR | 2 (18.2) | 6 (54.5%) | 3 (27.3%) | ||||
| Millar 200329 | Clinic (UK) | Retr. cohort | APOE4-: 279 | 24.9 ± 15.6 | 210 (75.3) | NR | 184 (82.9) | 17 (7.7) | 21 (9.5) | No statistically significant association between genotype and GOS at 6 months or GOS-E at 15–25 years post-injury. |
| APOE4+: 116 | 21.5 ± 14.2 | 81 (69.2) | NR | 88 (89.8) | 4 (4.1) | 6 (6.1) | ||||
| Nathoo 200330 | Hosp. (S Africa) | Pros. cohort | APOE4-: 65 | 24.8 ± 9.1 | NR | 12.3 ± 2.8 | NR | NR | NR | No statistically significant association between genotype and GOS at 6 months. |
| APOE4+: 45 | 28.4 ± 13.7 | NR | 12.8 ± 2.1 | NR | NR | NR | ||||
| Nielson 201763 | Hosp. (USA) | Pros. cohort | Total: 586 | 43.3 ± 18.5 | 419 (71.5%) | 42 (7.6) | 28 (5.1) | 480 (87.3) | No statistically significant association between genotype and GOS-E at 3 or 6 months. | |
| Olivecrona 201031 | ITU (Sweden) | Pros. cohort | APOE4-: 28 | 33 ± 13.2 | NR | 5.3 ± 1.59 | NR | NR | NR | Statistically significant worse dichotomized GOS in APOE4+ at 3 months but not at 12 or 24 months |
| APOE4+: 18 | 38.7 ± 17 | NR | 5.2 ± 1.7 | NR | NR | NR | ||||
| Olivecrona 201232 | Hosp. (Sweden) | Pros. cohort | APOE4-: NR | 33.0 ± 2.5 (SEM) | NR | 5 (3 – 8) | NR | NR | NR | No statistically significant association between genotype and GOS-E at 3 months. |
| APOE4+: NR | 38.7 ± 4.0 (SEM) | NR | 6 (3 – 8) | NR | NR | NR | ||||
| Olivecrona 201764 | ITU (Sweden) | Retr. cohort | APOE4- DC: 8 | 35.2 ± 4.4 | 5 (62.5) | 4 (3–7) | 46 (100) | Statistically significant higher incidence of decompressive craniectomy in APOE4+ group but no reported association between genotype and GOS-E at 6 months. | ||
| APOE4+ DC: 11 | 40.1 ± 5.1 | 6 (54.5) | 5 (3–8) | |||||||
| Non-DC: 27 | 33.2 ± 2.8 | 20 (74.1) | 6 (3–8) | |||||||
| Ost 200833 | ITU (USA) | Pros. cohort | APOE4-: 70 | NR | NR | NR | NR | NR | NR | Statistically significantly higher mortality in APOE4+ at 12 months; no significant association between genotype and overall GOS-E at 12 months reported. |
| APOE4+: 26 | NR | NR | NR | NR | NR | NR | ||||
| Ponsford 201119 | Rehab. (Aust.) | Pros. cohort | APOE4-: 329 | NR | NR | NR | 253 (56.4) | 67 (14.9) | 129 (28.7) | Statistically significant higher levels of severe disability in APOE4+ and overall significant association between APOE4+ and worse GOS-E at 1–2 years. |
| APOE4+: 124 | NR | NR | NR | 86 (55.8) | 27 (17.5) | 41 (26.6) | ||||
| Pruthi 201034 | Hosp. (India) | Pros. cohort | APOE4-: 61 | 42.5 ± 14.4 | 48 (78.7) | NR | NR | NR | NR | No statistically significant association between genotype and GOS at 6 months. |
| APOE4+: 12 | 35.7 ± 11.1 | 10 (83.3) | NR | NR | NR | NR | ||||
| Røe 201662 | Hosp. (Norway) | Pros. Cohort | APOE4-: 107 | 40 ± 18 | 105 (81) | 129 (100) | No statistically significant association between genotype and GOS-E at 12 months. | |||
| APOE4+: 22 | ||||||||||
| Teasdale 200535 | Hosp. (UK) | Pros. cohort | APOE4-: 630 | 35 ± 21.6 | 536 (81) | NR | 191 (30) | 121 (19) | 332 (52) | No statistically significant association between APOE4+ and dichotomized GOS at 6 months; significant interaction between APOE4+ genotype and worse outcome once initial motor score, CT findings and pupil reaction controlled for. |
| APOE4+: 303 | 35 ± 21.8 | 261 (81) | NR | 76 (24) | 57 (18) | 181 (58) | ||||
| Teasdale 19972 | Hosp. (UK) | Pros. cohort | APOE4-: 63 | 41.9 | NR | NR | 11 (18.3) | 11 (18.3) | 38 (63.3) | Statistically significant association between APOE4+ and worse outcome (dichotomized GOS) at 6 months. |
| APOE4+: 29 | 34.3 | NR | NR | 11 (37.9) | 8 (27.6) | 10 (34.5) | ||||
| Willemse-van Son 200836 | Hosp. (Neth.) | Pros. cohort | APOE4-: 59 | 33.6 ± 12.9 | 45 (73) | 6.9 ± 3.0 | NR | NR | NR | Statistically significant association between APOE4+ genotype and improved outcome (GOS) at 18 and 36 but not 3/6/12/24 months. |
| APOE4+: 17 | 33.2 ± 11.4 | 12 (71) | 6.8 ± 2.7 | NR | NR | NR | ||||
| Yousuf 201558 | Hosp. (India) | Pros. cohort | APOE4-: 117 | 41 ± 15.7 | 96 (82.1%) | 24 (20.5%) | 42 (35.9%) | 51 (43.89%) | No statistically significant association between genotype and GOS at 6 months. | |
| APOE4+: 33 | 38 ± 14.5 | 27 (81.8%) | 9 (18.18%) | 12 (36.36%) | 12 (36.36%) | |||||
Italicized studies could not be entered into meta-analysis due to paucity of data.
Aust., Australia; DC, decompressive craniectomy; GCS Glasgow Coma Scale; GOS, Glasgow Outcome Score; GOS-E, Glasgow Outcome Scale–Extended; Hosp., hospital; ICP, intracranial pressure; ITU, Intensive therapy unit; m, mean; M, male; mod., moderate; Neth., Netherlands; NRS – R, Neurobehavioural Rating Scale – Revised; NR, not reported; Pros., prospective; rehab, rehabilitation; retr., retrospective; S, South; SD, standard deviation; SEM, standard error of the mean; sev., severe.
Our meta-analysis included data on 2593 subjects, of which 160 were additional individual patient data from two previous publications,22,28 which were kindly provided by the study authors as the published manuscript did not contain enough data to be included in previous meta-analyses. We also contacted the corresponding authors of three other papers23,24,33 to source raw data, but did not receive responses, preventing us from including these studies in our meta-analysis. We nonetheless include more patients than two recent meta-analyses.7,8
Risk of bias
The studies all constitute observational research, and in most there were areas of methodological weakness, even in otherwise well conducted studies. In 25 studies, one or more domains were judged to be at high risk of bias. For 13 studies, this was due to concern regarding significant attrition of the study cohort, especially if demographic homogeneity between patients who did and did not drop out was not demonstrated by the authors. The included studies' risk of bias ratings for each of the six QuIPs domains can be seen in Supplementary Table S2.
Apolipoprotein E genotype and meta-analysis of functional outcomes
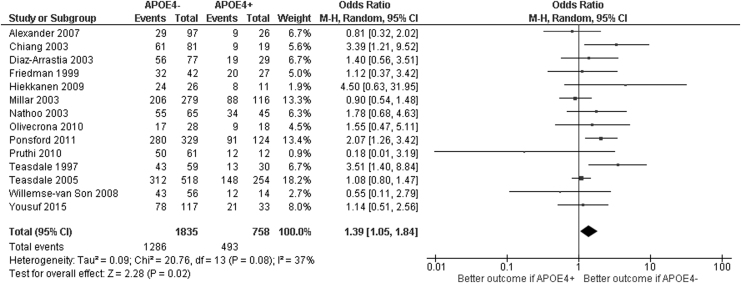
Twenty-one studies investigated the relationship between APOE genotype and functional outcome in adult patients. Fourteen of these studies reported data in sufficient detail (or provided it on request) to allow entry into the meta-analysis.
The absence of the APOE4 genotype was associated with a significant increase in the odds of a favorable outcome in patients with TBI (odds ratio [OR] 1.39, 95% confidence interval [CI] 1.05 to 1.84; p = 0.02; Fig. 2). The low quality of the evidence meant that the confidence in this effect size was low. Due to the prevalence of mixed severity patient populations, subgroup analysis by TBI severity was deemed inappropriate—only two papers (Alexander and colleages22 and Olivecrona31) studied purely severe TBI cohorts. The results of the four sensitivity analyses provided effect sizes which were consistent with the primary meta-analysis (Supplementary Figs. S1–S4). Moderate heterogeneity was noted within the studies, with an I2 statistic of 37%. The sensitivity analysis including only high-quality studies (Supplementary Fig. 4) demonstrated a slightly stronger effect estimate (OR 1.58, 95% CI 1.11 – 2.24; p = 0.01) but with slightly higher overall heterogeneity (I2 45%).
FIG. 2.
Meta-analysis of the effect of apolipoprotein E4 (APOE4) on traumatic brain injury outcome.
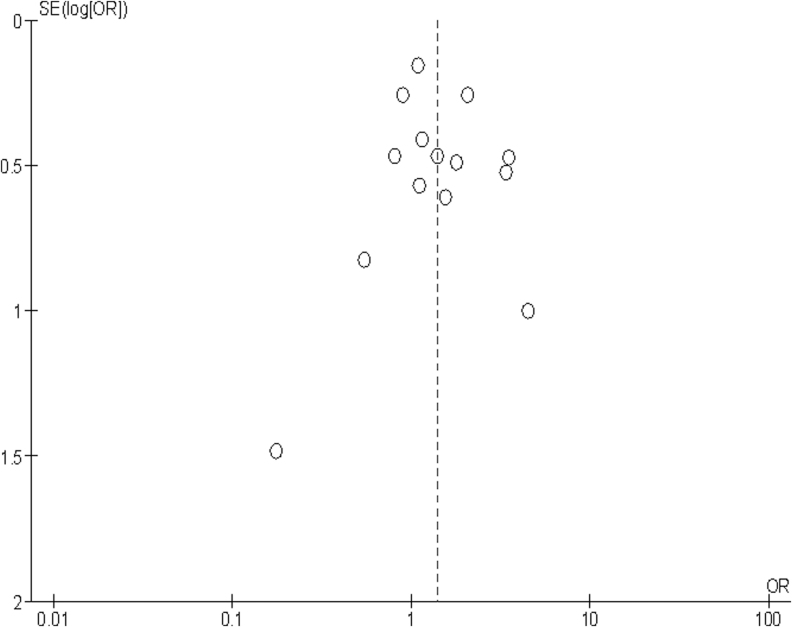
The quality of the evidence for the association between APOE and functional outcome was rated as low due to serious study limitations: most studies were rated as moderate risk of bias. According to the GRADE framework for prognostic studies, this means that our confidence in the effect estimate is limited and that the true effect may be substantially different from the estimate of the effect.18 Details of the GRADE framework assessment can be found in Supplementary Table S3. A funnel plot showed no evidence of publication bias in the reviewed literature (Fig. 3).
FIG. 3.
Funnel plot for primary meta-analysis: global outcome (6-month data or closest, all studies).
Studies not included in meta-analysis
Studies that provided global outcome measures, but insufficient detail for meta-analysis, and those that reported on end-points other than global outcomes are summarized in Table 2. For studies that were not included in the meta-analysis, this table provides readers with a summary of the reported direction of effect (or absence of effect) of ApoE genotype on the end-points of interest.
Table 2.
Summary of Non–Meta-Analysis Papers' Results
| Type of outcome | Effect of APOE e4 allele on outcome (total study cohort size in parentheses) |
||
|---|---|---|---|
| Positive impact | No impact/uncertain | Negative impact | |
| Global scales (GOS, GOS-E, DRS, FIM) and Clinical (seizure incidence, need for DC, mortality) | Ariza 2006 (77)* | Lichtman 2000 (31) | |
| Chamelian 2004 (90)* | Olivecrona 2017 (46)* | ||
| Mejia 2016 (170) | Öst 2008 (96)* | ||
| Miller 2010 (322) | Jiang 2006 (110) | ||
| Nielson 2017 (586)* | Total n = 283 | ||
| Olivecrona 2012 (48)* | |||
| Røe 2016 (129)* | |||
| Total n = 1422 | |||
| Neuropsychological | Han 2007 (78) | Eramudugolla 2014 (6333) | Anderson 2009 (51) |
| Han 2009 (46) | Ariza 2006 (77) | ||
| Kristman 2008 (318) | Banks 2016 (120) | ||
| Lee 2017 (189) | Crawford 2002 (110) | ||
| Liberman 2002 (78) | Merritt 2016 (42) | ||
| Padgett 2016 (142) | Müller 2009 (59) | ||
| Shadli 2011(19) | Noé 2010 (67) | ||
| Hodgkinson (100) | Sundström 2004 (34) | ||
| Total n = 7225 | Teasdale 2000 (39) | ||
| Yue 2017 (114) | |||
| Total n = 713 | |||
| Dementia incidence | Rapoport 2008 (49) | Isoniemi 2006 (61) | |
| Sundström 2007a (31) | |||
| Sundström 2007b (71) | |||
| Total n = 163 | |||
| Total number of subjects studied | 78 | 8696 | 1159 |
APOE studies classified by type of outcome and authors' interpretation of results. Studies included in meta-analysis not included. TBI cohort sizes for each paper given in brackets. Papers reporting multiple outcomes listed in “Negative impact” if any outcome measure statistically significant for negative outcome and remainder of outcomes showed no effect. Studies with an asterisk (*) are also featured in Table 1.
GOS, Glasgow outcome scale; GOS-E, Glasgow outcome scale-extended; DRS, disability rating scale; FIM, functional independence measure; DC, decompressive craniectomy.
Among studies that collected but did not fully publish global outcome measures, conflicting results were found. In studies of severe TBI patients, Lichtman and colleagues47 reported worse outcomes for APOE4+ patients on the Functional Independence Measure (driven by poor motor recovery), and Mejia and colleagues67 describe an association between APOE3+ genotypes and improved DRS scores at 6 months (including APOE 3/4 heterozygote patients). Chamelian and colleagues 24 found no difference in mean GOS at 6 months among mild-moderate TBI patients while Ost and colleagues33 found an increase in mortality among APOE4+ men. Nielson and colleagues could not demonstrate an association between poor outcome and APOE genotype in their topological data analysis of the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) cohort.63 Miller and colleagues48 found no effect of APOE genotype on early or delayed post-traumatic seizure occurrence in a severe TBI cohort. Jiang and colleagues43 suggest that APOE4 carriage increases the odds of early clinical deterioration within the first 7 days after injury, with Olivecrona and Koskinen64 observing higher maximum intracranial pressures and an increased risk of requiring decompressive craniectomy within 36 h of injury. Jiang and colleagues extend their findings to the A-419T polymorphism in the APOE gene promoter region in a separate paper42 and Lendon and colleagues,45 studying the G-219T promoter SNP, found the TT genotype experienced significantly worse outcomes at 6 months on the GOS scale.
Apolipoprotein E and neuropsychological outcomes
Studies investigating the effects of APOE4 carriage on neuropsychological outcomes also reported conflicting results. A broad overview of how the published evidence has been reported can be found in Supplementary Tables S4 and S5. With regard to measures of working memory and verbal recall, Han and colleagues39 found that APOE4 carriers actually outperform APOE4– patients at 5 weeks post-injury, whereas Anderson and colleagues37 and Crawford and colleagues38 demonstrated the opposite effect at 6 months. Shadli and colleagues,52 Padgett and colleagues,61 and Millar and colleagues29 all found no difference in performance on multiple tests of executive functioning, working memory, and verbal recall, despite in the case of Millar and colleagues having demonstrated a significant difference in GOS between genotypes. Han and colleagues40 also found that change in job status (to a less demanding workload) following TBI was predicted by number of post-concussive symptoms and pre-morbid IQ among APOE4- patients, but by degree of memory impairment alone in APOE4+ patients, perhaps suggestive that a higher pre-morbid IQ does not protect against post-TBI cognitive impairment to the same degree among APOE4+ patients.
Hodgkinson analyzed 100 patients with severe TBI undergoing rehabilitation, and could find no cognitive differences between APOE genotypes at 6 months.68 Liberman and colleagues46 showed that APOE4+ predicts worse performance on tests of mental arithmetic (Paced Auditory Serial Addition Test 2.8 serial addition) 3 weeks after injury, but that patients have recovered to similar levels regardless of genotype by 6 weeks. A large Australian cohort of prior TBI patients analyzed by Eramudugolla and colleagues demonstrated significantly worse episodic memory performance in young APOE carriers (ages 20–24 at recruitment), and slower reaction times in middle aged APOE4+ TBI patients. The effect size in the younger cohort was driven chiefly by those with moderate or severe injuries, and no APOE4 × TBI interaction could be detected in the oldest (60–64 years old) cohort.57 Merritt and Arnett,60 studying college athletes who had suffered a mild TBI in the last 3 months, showed an increase in post-concussive symptom burden among APOE4 carriers, and Banks and Bernick59 found worse verbal memory performance among APOE4+ combat sports participants. This latter finding was nonetheless not associated with a decrease in hippocampal or thalamic volume, leaving the physiological underpinnings of APOE4 mediated cognitive impairment unclear.
In the longer term, although the quality of evidence is variable, several publications suggest that APOE4 carriage predisposes to some degree of accelerated cognitive decline in the context of TBI, but no complete agreement between studies. Rapoport and colleagues51 found no increased incidence of dementia in the 2 years following TBI, regardless of genotype, whereas Isoniemi and colleagues41 followed patients for an average of 30 years from injury and found that APOE4+ subjects display greater deterioration on a composite neuropsychological battery, performing on average 7.4 SD below the norm for age. This difference, however, was driven entirely by the development of dementia in six of the 19 APOE4+ subjects, with no cognitive decline in the other 13. No APOE4– patients had developed dementia at follow-up, consistent with the well documented dementia risk associated with the ɛ4 allele. Sundström and colleagues have reported on head injury patients from the Betula longitudinal study being conducted in Sweden, which has recruited 4000 subjects from the general population since 1988 and carries out five yearly tests relating to aging, cognition, and health. They report a significant deterioration in task performance among APOE4+ patients in three of nine cognitive domains tested,53 with increased measures of fatigue54 and an increased risk of developing dementia during the study period.55 They found an odds ratio for developing dementia in the years following TBI in APOE4+ subjects (compared to APOE4- non-TBI controls) of 5.2 (95% CI 2–14), compared with 3.0 (95% CI 1.9–4.7) for APOE4+ non-TBI controls, and 0.9 (95% CI 0.4–1.8) for APOE4– TBI patients. This suggests that the dementia risk in APOE4 carriers may be amplified following TBI.
Synthesis of the main results
We found 14 studies eligible for meta-analysis. Our main finding in this regard is that the overall effect of the APOE4 allele on early functional outcomes from TBI is a negative impact, which is quantified as small, based on criteria in a recent Cochrane prognostic review.15 Over longer time scales such as those addressed by Isoniemi and colleagues41 and Sundström and colleagues,53–55 the evidence suggests that TBI may provide an additive factor in the already elevated background risk of dementia among APOE4+ patients. This increased incidence of dementia may account for worse performance on neurocognitive testing during long term follow-up.
It is worth noting that the drivers of poor outcome in some of the studies relating APOE4 allele carriage to cognitive outcome were not uniform across the study populations, and may have been heavily influenced by performance on a subset of cognitive tests, or the development of late dementia. While the implications for clinical practice remain to be defined, taken together, it seems reasonable to conclude that the APOE4 allele may have an adverse effect on neurocognitive recovery from TBI and dementia incidence over the long-term, with a small impact on the acute clinical course and early outcome.
Discussion
An extensive research effort has been expended on uncovering associations between candidate genetic variants and TBI outcomes. It is likely that genetic variation constitutes a contributing influence rather than being a dominant driver of outcome in TBI, and the integration of this emerging information into clinical practice is still a work in progress. Genetic profiling might provide an additional prognostic factor that could be used to refine current prognostic schemes, with the more accurate prognostication allowing better risk adjustment in research and audit. It might also aid therapy stratification, either by targeting treatment based on risk of poor outcome, or based on mechanistic differences in patient subgroups. The first of these options would be realized by any improvement in overall prognostication. The second, while possible in principle,69 will only be possible in practice if we identify mechanistic correlates that drive the differences in outcome impact from genetic variation. Because the clinical course of TBI is indeed a complex trait, successfully identifying genetic variants with an effect on outcome will require large sample sizes of homogenously characterized individuals. With regard to prognosis, there are currently no genetic variants that have been incorporated into existing models, although APOE represents an obvious candidate in this context.
On the basis of our meta-analysis, we conclude that APOE ɛ4 has a small impact on shorter-term outcomes (over a time scale of months to 2 years), as measured by functional assessments such as GOS or GOS-E. The conclusion of earlier publications—that the effect of APOE4 is more pronounced among severe TBI patients—could not be tested with sufficient statistical rigor based on the available evidence.
Assessment of data and analysis quality
We are confident that we identified all relevant published studies in the field of TBI genetics using a comprehensive search strategy, in line with PRISMA guidance. Unpublished data, which have been provided to us, were unavailable to previous systematic meta-analyses,7,8 which added an additional 160 patients, resulting in a total of 2593 patients.
There are nonetheless limitations to this review. The summarized studies are underpowered, and the likelihood is high that negative results exist but have never been published. Only one reviewer carried out the initial screening of studies (although full text review was carried out by two separate authors). It is likely we have missed studies published in the non-English literature. With the exception of four papers entered into meta-analysis, the remaining studies cover largely Caucasian populations, and in almost all studies the majority of patients were male. This former observation reflects the ethnic composition of the countries that are involved in leading TBI research and the latter the demographics of real-world TBI populations. We are confident that our main finding, that APOE4 has a small effect on initial recovery, is an accurate assessment of the true effect size, and is generalizable to adult non-penetrating TBI populations.
As can be seen from the Risk of Bias judgements (Supplementary Table 2), methodological quality was variable, and most publications were rated as having medium or high Risk of Bias in one or more domains. There are three major methodological limitations that were common across the studies analyzed. The majority of studies contained mixed severity cohorts with generally small cohort sizes; the few larger cohorts contained both pediatric and adult patients across all severities and attempt to control for this later through regression analysis. Current large multi-center international research collaborations that form part of the International Traumatic Brain Injury Research Initiative (InTBIR; https://intbir.nih.gov) aim to address these issues by recruiting large, well-characterized TBI cohorts.
The effect size that we demonstrate are similar to those demonstrated in genetic association studies in other acute neurological diseases such as stroke,70 and if confirmed, could be important to contribute to a multivariable prognostic profile. Further, given the substantial interest in developing amyloid modifying therapies in Alzheimer's disease, these potentially present a target for therapy and therapy stratification. However, while our analysis is in keeping with an effect of APOE e4 carriage on outcome, our confidence in the magnitude of this effect size is limited by the quality and heterogeneity of the contributing studies. We note that publication bias for positive candidate gene association studies has been demonstrated in other clinical contexts.71,72 Our funnel plot (Fig. 3) suggested no such bias in our analysis.
The other major issues relate to the research and statistical approaches used by many groups. The traditional alpha for statistical significance (0.05) is frequently thought to be inadequately rigorous when considering multiple gene assays, necessitating large and well-designed trial populations. Hirschhorn and colleagues73 and Nakaoka and Inoue74 highlight further potential methodological pitfalls in their reviews of commonly reported genetic associations, such as linkage disequilibrium and ethnic admixture as potential causes of false positive results. Both Hirschhorn and colleagues73 and Lohmueller and colleagues75 emphasize the need for very large cohorts in order to suitably power studies, and suggest that data should always be published in sufficient detail to enable meta-analysis. This latter criterion is notably not met in the current TBI genetic literature.
Agreements and disagreements with other reviews
Two other meta-analyses of the effects of the APOE4 allele on functional TBI outcomes have been published. Zhou and colleagues7 used seven studies, all contained in our analysis, but excluded one29 when sensitivity analysis showed that its inclusion was responsible for the significant between-study heterogeneity. On the basis of the remaining six studies, they concluded that APOE4 carriage was associated with a higher risk of poor outcomes 6 months post-injury (risk ratio 1.49, 95% CI, 1.11–2.00). A second meta-analysis by Zeng and colleagues8 contained data from 13 studies. Of these, nine are included in our meta-analysis. The remaining four comprise a study of pediatric TBI,76 a study in which the outcome measured was worsening of CT scan findings,77 a study in a journal not listed on the databases we searched,78 and results taken from a letter of reply79 to a paper we deemed ineligible.80 Zeng and colleagues did not report on six papers (comprising 915 patients) that are included in our review, including Ponsford and colleagues,19 which is the second largest cohort that we included. The authors did not include a list of their excluded studies, so it is not possible to ascertain why studies we chose to include did not form part of their analysis. Zeng and colleagues concluded that the APOE4 allele was associated with lower odds of a good prognosis (OR = 0.68, 95% CI: 0.48–0.96; p = 0.027), and performed subgroup analyses, which revealed a slightly larger effect size if only severe TBI is included.
Our overall result is similar to these two preceding meta-analyses—we find that there is an odds ratio indicative of favorable outcomes among APOE4 non-carriers of 1.39 (95% CI 1.05 to 1.84; p = 0.02). As our study selection process identified only two papers with purely severe TBI patients, we did not deem it appropriate to perform a subgroup analysis of severe TBI. Sensitivity analysis revealed similar effect sizes regardless of time-point post-injury or definition of “favorable” versus “unfavorable” outcomes.
A narrative systematic review of the APOE TBI literature by Lawrence and colleagues10 containing 69 studies reported an overall negative influence of APOE4 on outcome and incidence of dementia following TBI, with a more marked effect in severe TBI. The same authors conclude that APOE4 may impair neuropsychological functioning following severe TBI. The review contains multiple studies excluded from our review, as they included pediatric TBI research, and papers with non-functional outcomes such as measures of cerebral blood flow and coagulation. The authors also stratify papers (including many discussed in this review) by TBI severity, even if the studies contain heterogeneous cohorts with a simple or absolute majority of one degree of TBI severity and do not report separate data for each injury strata. We considered employing a subgroup analysis of this nature for our meta-analysis (severe vs. mild/moderate injury), but following advice from our statisticians and methodologists, decided it was not appropriate.
Padgett and colleagues performed a meta-analysis of studies analyzing APOE genotype in relation to cognitive function in the first 12 months post-TBI.9 They report that no significant differences could be demonstrated for either general cognitive function or test subdomains analyzing verbal, visual and working memory. Meta-analysis of cognitive subdomains involved pooling results of different tests (e.g., California Verbal Learning Test and Rey Auditory Verbal Learning Test [RAVLT]) and sections of tests analyzing differing aspects of the same subdomain (e.g., RAVLT immediate and delayed recall). We decided not to perform a pooled meta-analysis of such studies, as the cohorts and cognitive batteries involved in the published literature are heterogeneous. The studies covered by Padgett and colleagues are all discussed individually in our review. Necessarily due to the design of meta-analyses, many studies discussed in our manuscript are omitted from the Padgett publication (for example Eradmudugolla and colleagues' study of a >6000 patient cohort) although the authors note these in their Discussion section. Padgett and colleagues raise the need for future TBI genomic research to use large cohorts and investigate gene × gene and allele dose interactions, a recommendation with which we wholeheartedly agree.
Mechanisms by which APOE4 might contribute to worse outcome
The mechanisms by which APOE4 carriage might drive worse outcomes in TBI has been the subject of much investigation and speculation, and has included direct neurotoxicity, modulation of tau biology, abnormal cerebrovascular function, effects on the blood–brain barrier, inflammation, and oxidant injury. In addition, pre-injury level of education is one of the strongest predictors of outcome from mild traumatic brain injury (alongside age and pre-existing psychiatric disorder).2 It is therefore plausible that any pre-morbid genetic disposition to reduced cognitive function may indirectly impair recover. An analysis of the relevant literature is beyond the scope of this review, but is briefly summarized in Supplementary Appendix 2.
Conclusion
The APOE4 allele has a small impact on the acute clinical course following TBI, but the evidence for its effect on neuropsychological recovery remains incomplete. Genetic association studies of complex traits have repeatedly demonstrated that with sufficient sample sizes, genetic influences can be identified. There is no reason to suspect that outcome from TBI should be any different. What have been missing are large cohorts of TBI patients, who have been both richly and homogeneously characterized. Now that such cohorts are being ascertained, there is every reason to be optimistic that the genetic influences on TBI outcome will emerge. These discoveries will be the first step toward making genetics useful in TBI care.
Supplementary Material
Author Disclosure Statement
The authors received funding from the European Union FP 7th Framework program under grant agreement No 602150 (CENTER-TBI). No competing financial interests exist.
DKM is supported by funding from the National Institute for Health Research (NIHR; UK) through a Senior Investigator Award and funding to the Cambridge NIHR Biomedical Research Centre.
FAZ has received salary support for dedicated research time, during which this project was completed. Such salary support came from: the Cambridge Commonwealth Trust Scholarship, the University of Manitoba Clinician Investigator Program, the Royal College of Surgeons of Canada—Harry S. Morton Travelling Fellowship in Surgery, R. Samuel McLaughlin Research and Education Award, the Manitoba Medical Service Foundation, the University of Manitoba-Faculty of Medicine Dean's Fellowship Fund, and the Royal College of Surgeons of Canada-Harry S. Morton Travelling Fellowship.
VFJN is supported by an Academy of Medical Sciences/Health Foundation Clinician Scientist Fellowship.
Supplementary Material
Supplementary Appendix 1: Background to APOE
Supplementary Appendix 2: Search Strategies
References
- 1. Lingsma, H.F., Yue, J.K., Maas, A.I., Steyerberg, E.W., and Manley, G.T. (2015). Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK-TBI pilot study. J. Neurotrauma 32, 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teasdale, G.M., Nicoll, J.A.R., Murray, G., and Fiddes, M. (1997). Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 350, 1069–1071 [DOI] [PubMed] [Google Scholar]
- 3. Zeiler, F.A., McFadyen, C., Newcombe, V., Synnot, A., Donoghue, E.L., Ripatti, S., Steyerberg, E.W., Gruen, R.L., McAllister, T., Rosand, J., Palotie, A., Maas, A., and Menon, D. (2018). Genetic influences on patient oriented outcomes in TBI: a living systematic review of non-APOE single nucleotide polymorphisms. J. Neurotrauma. 2018 Aug 10; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verghese, P.B., Castellano, J.M., and Holtzman, D.M. (2011). Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 10, 241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahley, R.W. and Huang, Y. (2012). Apolipoprotein E sets the stage: response to injury triggers neuropathology. Neuron 76, 871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corps, K.N., Roth, T.L., and McGavern, D.B. (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou, W., Xu, D., Peng, X., Zhang, Q., Jia, J., and Crutcher, K.A. (2008). Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J. Neurotrauma 25, 279–290 [DOI] [PubMed] [Google Scholar]
- 8. Zeng, S., Jiang, J.X., Xu, M.H., Xu, L.S., Shen, G.J., Zhang, A.Q., and Wang, X.H. (2014). Prognostic value of apolipoprotein E epsilon4 allele in patients with traumatic brain injury: a meta-analysis and meta-regression. Genet. Test Mol. Biomarkers 18, 202–210 [DOI] [PubMed] [Google Scholar]
- 9. Padgett, C.R., Summers, M.J., and Skilbeck, C.E. (2016). Is APOE ɛ4 associated with poorer cognitive outcome following traumatic brain injury? A meta-analysis. Neuropsychology 30, 775–790 [DOI] [PubMed] [Google Scholar]
- 10. Lawrence, D.W., Comper, P., and Hutchison, M.G. (2014). The role of apolipoprotein E (APOE) in outcome following traumatic brain injury (TBI): a systematic review. Brain Inj. 28, 841. [DOI] [PubMed] [Google Scholar]
- 11. Moher, D., Liberati, A., Tetzlaff, J., and Altman, D.G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maas, A.I., Menon, D.K., Steyerberg, E.W., Citerio, G., Lecky, F., Manley, G.T., Hill, S., Legrand, V., and Sorgner, A. (2015). Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80 [DOI] [PubMed] [Google Scholar]
- 13. Elliott, J.H., Turner, T., Clavisi, O., Thomas, J., Higgins, J.P., Mavergames, C., and Gruen, R.L. (2014). Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 11, e1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayden, J.A., van der Windt, D.A., Cartwright, J.L., Cote, P., and Bombardier, C. (2013). Assessing bias in studies of prognostic factors. Ann. Intern. Med. 158, 280–286 [DOI] [PubMed] [Google Scholar]
- 15. Teasdale, G., Maas, A., Lecky, F., Manley, G., Stocchetti, N., and Murray, G. (2014). The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 13, 844–854 [DOI] [PubMed] [Google Scholar]
- 16. McMillan, T., Wilson, L., Ponsford, J., Levin, H., Teasdale, G., and Bond, M. (2016). The Glasgow Outcome Scale—40 years of application and refinement. Nat. Rev. Neurol. 12, 477–485 [DOI] [PubMed] [Google Scholar]
- 17. Utada, Y., Haga, S., Kajiwara, T., Kasumi, F., Sakamoto, G., Nakamura, Y., and Emi, M. (2000). Allelic loss at the 8p22 region as a prognostic factor in large and estrogen receptor negative breast carcinomas. Cancer 88, 1410–1416 [PubMed] [Google Scholar]
- 18. Huguet, A., Hayden, J.A., Stinson, J., McGrath, P.J., Chambers, C.T., Tougas, M.E., and Wozney, L. (2013). Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst. Rev. 2, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ponsford, J., McLaren, A., Schönberger, M., Burke, R., Rudzki, D., Olver, J., and Ponsford, M. (2011). The association between apolipoprotein E and traumatic brain injury severity and functional outcome in a rehabilitation sample. J. Neurotrauma 28, 1683–1692 [DOI] [PubMed] [Google Scholar]
- 20. Willmott, C., Ponsford, J., McAllister, W., and Burke, R. (2013). Effect of COMT Val158Met genotype on attention and response to methylphenidate following traumatic brain injury. Brain Inj. 27, 1281–1287 [DOI] [PubMed] [Google Scholar]
- 21. McHugh, G.S., Butcher, I., Steyerberg, E.W., Lu, J., Mushkudiani, N., Marmarou, A., Maas, A.I., and Murray, G.D. (2007). Statistical approaches to the univariate prognostic analysis of the IMPACT database on traumatic brain injury. J. Neurotrauma 24, 251–258 [DOI] [PubMed] [Google Scholar]
- 22. Alexander, S., Kerr, M.E., Kim, Y., Kamboh, M.I., Beers, S.R., and Conley, Y.P. (2007). Apolipoprotein E4 allele presence and functional outcome after severe traumatic brain injury. J. Neurotrauma 24, 790–797 [DOI] [PubMed] [Google Scholar]
- 23. Ariza, M., Pueyo, R., Matarín, M.M., Junqué, C., Mataró, M., Clemente, I., Moral, P., Poca, M.A., Garnacho, A., and Sahuquillo, J. (2006). Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 77, 1191–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chamelian, L., Reis, M., and Feinstein, A. (2004). Six-month recovery from mild to moderate traumatic brain injury: the role of APOE-ɛ4 allele. Brain 127, 2621–2628 [DOI] [PubMed] [Google Scholar]
- 25. Chiang, M.F., Chang, J.G., Hu, C.J., Dunn, L., and Nicoll, J.A.R. (2003). Association between apolipoprotein E genotype and outcome of traumatic brain injury. Acta Neurochir. 145, 649–654 [DOI] [PubMed] [Google Scholar]
- 26. Diaz-Arrastia, R., Gong, Y., Fair, S., Scott, K.D., Garcia, M.C., Carlile, M.C., Agostini, M.A., and Van, P.C.P. (2003). Increased risk of late posttraumatic seizures associated with inheritance of APOE epsilon4 allele. Arch. Neurol. 60, 818–822 [DOI] [PubMed] [Google Scholar]
- 27. Friedman, G., Froom, P., Sazbon, L., Grinblatt, I., Shochina, M., Tsenter, J., Babaey, S., Yehuda, B., and Groswasser, Z. (1999). Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology 52, 244–248 [DOI] [PubMed] [Google Scholar]
- 28. Hiekkanen, H., Kurki, T., Brandstack, N., Kairisto, V., and Tenovuo, O. (2009). Association of injury severity, MRI-results and ApoE genotype with 1-year outcome in mainly mild TBI: a preliminary study. Brain Inj. 23, 396–402 [DOI] [PubMed] [Google Scholar]
- 29. Millar, K., Nicoll, J.A.R., Thornhill, S., Murray, G.D., and Teasdale, G.M. (2003). Long term neuropsychological outcome after head injury: relation to APOE genotype. J. Neurol. Neurosurg. Psychiatry 74, 1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nathoo, N., Chetty, R., van Dellen, J.R., Connolly, C., and Naidoo, R. (2003). Apolipoprotein E polymorphism and outcome after closed traumatic brain injury: influence of ethnic and regional differences. J. Neurosurg. 98, 302–306 [DOI] [PubMed] [Google Scholar]
- 31. Olivecrona, M., Wildemyr, Z., and Koskinen, L.O.D. (2010). The apolipoprotein E 4 allele and outcome in severe traumatic brain injury treated by an intracranial pressure-targeted therapy. J. Neurosurg. 112, 1113–1119 [DOI] [PubMed] [Google Scholar]
- 32. Olivecrona, Z. and Koskinen, L.O.D. (2012). The release of S-100B and NSE in severe traumatic head injury is associated with APOE ɛ4. Acta Neurochir. 154, 675. [DOI] [PubMed] [Google Scholar]
- 33. Ost, M., Nylén, K., Csajbok, L., Blennow, K., Rosengren, L., and Nellgård, B. (2008). Apolipoprotein E polymorphism and gender difference in outcome after severe traumatic brain injury. Acta Anaesthesiol. Scand. 52, 1364–1369 [DOI] [PubMed] [Google Scholar]
- 34. Pruthi, N., Chandramouli, B.A., Kuttappa, T.B., Rao, S.L., Subbakrishna, D.K., Abraham, M.P., Mahadevan, A., and Shankar, S.K. (2010). Apolipoprotein e polymorphism and outcome after mild to moderate traumatic brain injury: a study of patient population in India. Neurol. India 58, 264–269 [DOI] [PubMed] [Google Scholar]
- 35. Teasdale, G.M., Murray, G.D., and Nicoll, J.A.R. (2005). The association between APOE ɛ4, age and outcome after head injury: a prospective cohort study. Brain 128, 2556–2561 [DOI] [PubMed] [Google Scholar]
- 36. Willemse-van Son, A.H.P., Ribbers, G.M., Hop, W.C.J., van Duijn, C.M., and Stam, H.J. (2008). Association between apolipoprotein-ɛ4 and long-term outcome after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 79, 426–430 [DOI] [PubMed] [Google Scholar]
- 37. Anderson, G.D., Temkin, N.R., Dikmen, S.S., Diaz-Arrastia, R., Machamer, J.E., Farhrenbruch, C., Miller, J.W., and Sadrzadeh, S.M.H. (2009). Haptoglobin phenotype and apolipoprotein E polymorphism: Relationship to posttraumatic seizures and neuropsychological functioning after traumatic brain injury. Epilepsy Behav. 16, 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crawford, F.C., Vanderploeg, R.D., Freeman, M.J., Singh, S., Waisman, M., Michaels, L., Abdullah, L., Warden, D., Lipsky, R., Salazar, A., and Mullan, M.J. (2002). APOE genotype influences acquisition and recall following traumatic brain injury. Neurology 58, 1115–1118 [DOI] [PubMed] [Google Scholar]
- 39. Han, S.D., Drake, A.I., Cessante, L.M., Jak, A.J., Houston, W.S., Delis, D.C., Filoteo, J.V., and Bondi, M.W. (2007). Apolipoprotein E and traumatic brain injury in a military population: evidence of a neuropsychological compensatory mechanism? J. Neurol. Neurosurg. Psychiatry 78, 1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han, S.D., Suzuki, H., Drake, A.I., Jak, A.J., Houston, W.S., and Bondi, M.W. (2009). Clinical, cognitive, and genetic predictors of change in job status following traumatic brain injury in a military population. J. Head Trauma Rehabil. 24, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isoniemi, H., Tenovuo, O., Portin, R., Himanen, L., and Kairisto, V. (2006). Outcome of traumatic brain injury after three decades—relationship to ApoE genotype. J. Neurotrauma 23, 1600–1608 [DOI] [PubMed] [Google Scholar]
- 42. Jiang, Y., Sun, X., Gui, L., Xia, Y., Tang, W., Cao, Y., and Gu, Y. (2007). Correlation between APOE -491AA promoter in epsilon4 carriers and clinical deterioration in early stage of traumatic brain injury. J. Neurotrauma 24, 1802–1810 [DOI] [PubMed] [Google Scholar]
- 43. Jiang, Y., Sun, X., Xia, Y., Tang, W., Cao, Y., and Gu, Y. (2006). Effect of APOE polymorphisms on early responses to traumatic brain injury. Neurosci. Lett. 408, 155–158 [DOI] [PubMed] [Google Scholar]
- 44. Kristman, V.L., Tator, C.H., Kreiger, N., Richards, D., Mainwaring, L., Jaglal, S., Tomlinson, G., and Comper, P. (2008). Does the apolipoprotein epsilon 4 allele predispose varsity athletes to concussion? A prospective cohort study. Clin. J.Sport Med. 18, 322–328 [DOI] [PubMed] [Google Scholar]
- 45. Lendon, C.L., Harris, J.M., Pritchard, A.L., Nicoll, J.A.R., Teasdale, G.M., and Murray, G. (2003). Genetic variation of the APOE promoter and outcome after head injury. Neurology 61, 683–685 [DOI] [PubMed] [Google Scholar]
- 46. Liberman, J.N., Stewart, W.F., Wesnes, K., and Troncoso, J. (2002). Apolipoprotein E ɛ4 and short-term recovery from predominantly mild brain injury. Neurology 58, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 47. Lichtman, S.W., Seliger, G., Tycko, B., and Marder, K. (2000). Apolipoprotein E and functional recovery from brain injury following postacute rehabilitation. Neurology 55, 1536–1539 [DOI] [PubMed] [Google Scholar]
- 48. Miller, M.A., Conley, Y., Scanlon, J.M., Ren, D., Kamboh, M.I., Niyonkuru, C., and Wagner, A.K. (2010). APOE genetic associations with seizure development after severe traumatic brain injury. Brain Inj. 24, 1468–1477 [DOI] [PubMed] [Google Scholar]
- 49. Müller, K., Ingebrigtsen, T., Wilsgaard, T., Wikran, G., Fagerheim, T., Romner, B., and Waterloo, K. (2009). Prediction of time trends in recovery of cognitive function after mild head injury. Neurosurgery 64, 698. [DOI] [PubMed] [Google Scholar]
- 50. Noé, E., Ferri, J., Colomer, C., Moliner, B., and Chirivella, J. (2010). APOE genotype and verbal memory recovery during and after emergence from post-traumatic amnesia. Brain Inj. 24, 886–893 [DOI] [PubMed] [Google Scholar]
- 51. Rapoport, M., Wolf, U., Herrmann, N., Kiss, A., Shammi, P., Reis, M., Phillips, A., and Feinstein, A. (2008). Traumatic brain injury, apolipoprotein E-4, and cognition in older adults: A two-year longitudinal study. J. Neuropsychiatry Clin. Neurosci. 20, 68–73 [DOI] [PubMed] [Google Scholar]
- 52. Shadli, R.M., Pieter, M.S., Yaacob, M.J., and Rashid, F.A. (2011). APOE genotype and neuropsychological outcome in mild-to-moderate traumatic brain injury: a pilot study. Brain Inj. 25, 596–603 [DOI] [PubMed] [Google Scholar]
- 53. Sundstrom, A., Marklund, P., Nilsson, L.G., Cruts, M., Adolfsson, R., Van, C., and Nyberg, L. (2004). APOE influences on neuropsychological function after mild head injury: within-person comparisons. Neurology 62, 1963–1966 [DOI] [PubMed] [Google Scholar]
- 54. Sundström, A., Nilsson, L., Cruts, M., Adolfsson, R., Van, C., and Nyberg, L. (2007). Fatigue before and after mild traumatic brain injury: pre-post-injury comparisons in relation to Apolipoprotein E. Brain Inj. 21, 1049–1055 [DOI] [PubMed] [Google Scholar]
- 55. Sundstrom, A., Nilsson, L.G., Cruts, M., Adolfsson, R., Van, C., and Nyberg, L. (2007). Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int. Psychogeriatr. 19, 159–165 [DOI] [PubMed] [Google Scholar]
- 56. Teasdale, T.W., Jorgensen, O.S., Ripa, C., Nielsen, A.S., and Christensen, A.L. (2000). Apolipoprotein E and subjective symptomatology following brain injury rehabilitation. Neuropsychol. Rehabil. 10, 151–166 [Google Scholar]
- 57. Eramudugolla, R., Bielak, A.M., Bunce, D., Easteal, S., Cherbuin, N., and Anstey, J. (2014). Long-term cognitive correlates of traumatic brain injury across adulthood and interactions with APOE genotype, sex, and age cohorts. J. Int. Neuropsychol. Soc. 20, 444. [DOI] [PubMed] [Google Scholar]
- 58. Yousuf, A., Khursheed, N., Rasool, I., Kundal, V., Jeelani, H., and Afroze, D. (2015). Genetic variation of ApoE gene in ethnic Kashmiri population and its association with outcome after traumatic brain injury. J. Mol. Neurosci. 56, 597–601 [DOI] [PubMed] [Google Scholar]
- 59. Banks, S. and Bernick, C. (2015). ApoE status related to memory scores but not hippocampal or thalamic volume in combat sports. Neurology 8425982050 [Google Scholar]
- 60. Merritt, V.C. and Arnett, P.A. (2016). Apolipoprotein E (APOE) e4 allele is associated with increased symptom reporting following sports concussion. J. Int. Neuropsychol. Soc. 22, 89–94 [DOI] [PubMed] [Google Scholar]
- 61. Padgett, C.R., Summers, M.J., Vickers, J.C., McCormack, G.H., and Skilbeck, C.E. (2016). Exploring the effect of the apolipoprotein E (APOE) gene on executive function, working memory, and processing speed during the early recovery period following traumatic brain injury. J. Clin. Exp. Neuropsychol. 38, 551–560 [DOI] [PubMed] [Google Scholar]
- 62. Røe, C., Andelic, N., Anke, A., Skandsen, T., Wehling, E., and Eiklid, K. (2016). 0213 Outcome after severe TBI—the influence of ApoE in an Norwegian cohort. Brain Inj. 30, 481–81727196965 [Google Scholar]
- 63. Nielson, J.L., Cooper, S.R., Yue, J.K., Sorani, M.D., Inoue, T., Yuh, E.L., Mukherjee, P., Petrossian, T.C., Paquette, J., Lum, P.Y., Carlsson, G.E., Vassar, M.J., Lingsma, H.F., Gordon, W.A., Valadka, A.B., Okonkwo, D.O., Manley, G.T., and Ferguson, A.R.; TRACK-TBI Investigators. (2017). Uncovering precision phenotype-biomarker associations in traumatic brain injury using topological data analysis. PloS One 12, e0169490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Olivecrona, Z. and Koskinen, L.O.D. (2017). APOE epsilon4 positive patients suffering severe traumatic head injury are more prone to undergo decompressive hemicraniectomy. J. Clin. Neurosci. 42, 139–142 [DOI] [PubMed] [Google Scholar]
- 65. Yue, J.K., Robinson, C.K., Burke, J.F., Winkler, E.A., Deng, H., Cnossen, M.C., Lingsma, H.F., Ferguson, A.R., McAllister, T.W., Rosand, J., Burchard, E.G., Sorani, M.D., Sharma, S., Nielson, J.L., Satris, G.G., Talbott, J.F., Tarapore, P.E., Korley, F.K., Wang, K.K.W., Yuh, E.L., Mukherjee, P., Diaz-Arrastia, R., Valadka, A.B., Okonkwo, D.O., and Manley, G.T. (2017). Apolipoprotein E epsilon 4 (APOE-epsilon4) genotype is associated with decreased 6-month verbal memory performance after mild traumatic brain injury. Brain Behav. 7, e00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee, H.H., Yeh, C.T., Ou, J.C., Ma, H.P., Chen, K.Y., Chang, C.F., Lai, J.H., Liao, K.H., Lin, C.M., Lin, S.Y., Wu, D., Huang, Y.H., Hu, C.J., and Hong, C.T. (2017). The association of apolipoprotein E allele 4 polymorphism with the recovery of sleep disturbance after mild traumatic brain injury. Acta Neurol. Taiwan 26, 13–19 [PubMed] [Google Scholar]
- 67. Mejia, L.P., Navarro, J.C., Jerry, G., and Robertson, C. (2016). ApoE e3 allele and DRS outcome in patients with severe traumatic brain injury. Neurology 86, Supplement. S46.007. [Google Scholar]
- 68. Hodkingson, A., Gillett, L., and Simpson, G.K. (2009). Does apolipoprotein E play a role in outcome after severe traumatic brain injury? Brain Impairment 10, 162–168 [Google Scholar]
- 69. Hingorani, A.D., Windt, D.A., Riley, R.D., Abrams, K., Moons, K.G., Steyerberg, E.W., Schroter, S., Sauerbrei, W., Altman, D.G., and Hemingway, H. (2013). Prognosis research strategy (PROGRESS) 4: stratified medicine research. BMJ 346, e5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Falcone, G.J., Malik, R., Dichgans, M., and Rosand, J. (2014). Current concepts and clinical applications of stroke genetics. Lancet Neurol. 13, 405–418 [DOI] [PubMed] [Google Scholar]
- 71. Bosker, F.J., Hartman, C.A., Nolte, I.M., Prins, B.P., Terpstra, P., Posthuma, D., van Veen, T., Willemsen, G., DeRijk, R.H., de Geus, E.J., Hoogendijk, W.J., Sullivan, P.F., Penninx, B.W., Boomsma, D.I., Snieder, H., and Nolen, W.A. (2011). Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol. Psychiatry 16, 516–532 [DOI] [PubMed] [Google Scholar]
- 72. Duncan, L.E. and Keller, M.C. (2011). A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am. J. Psychiatry 168, 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hirschhorn, J.N., Lohmueller, K., Byrne, E., and Hirschhorn, K. (2002). A comprehensive review of genetic association studies. Genet. Med. 4, 45–61 [DOI] [PubMed] [Google Scholar]
- 74. Nakaoka, H. and Inoue, I. (2009). Meta-analysis of genetic association studies: methodologies, between-study heterogeneity and winner's curse. J. Hum. Genet. 54, 615–623 [DOI] [PubMed] [Google Scholar]
- 75. Lohmueller, K.E., Pearce, C.L., Pike, M., Lander, E.S., and Hirschhorn, J.N. (2003). Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat. Genet. 33, 177–182 [DOI] [PubMed] [Google Scholar]
- 76. Lo, T.Y., Jones, P.A., Chambers, I.R., Beattie, T.F., Forsyth, R., Mendelow, A.D., and Minns, R.A. (2009). Modulating effect of apolipoprotein E polymorphisms on secondary brain insult and outcome after childhood brain trauma. Childs Nerv. Syst. 25, 47–54 [DOI] [PubMed] [Google Scholar]
- 77. Jiang, Y., Sun, X.C., Gui, L., Tang, W.Y., Zhen, L.P., Gu, Y.J., and Wu, H.T. (2008). Lack of association between apolipoprotein E promoters in epsilon4 carriers and worsening on computed tomography in early stage of traumatic brain injury. Acta Neurochir. Suppl. 105, 233–236 [DOI] [PubMed] [Google Scholar]
- 78. Gu, Y., Gao, X.J., and Xu, T. (2007). Association between the apolipoprotein E gene polymorphism and traumatic brain injury. Chin. J. Nervous Mental Dis. 33, 385–388 [Google Scholar]
- 79. Sorbi, S., Nacmias, B., Piacentini, S., Repice, A., Latorraca, S., Forleo, P., and Amaducci, L. (1995). ApoE as a prognostic factor for post-traumatic coma. Nat. Med. 1, 852. [DOI] [PubMed] [Google Scholar]
- 80. Nicoll, J.A., Roberts, G.W., and Graham, D.I. (1995). Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat. Med. 1, 135–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.