Dear editor,
Follow-up studies in COVID-19 survivors have found persistent symptoms(fatigue, dyspnea, muscle pain et, al.), impaired pulmonary function, abnormal chest CT images in COVID-19 survivors even after 110 days and 6 month of follow-up. 1 , 2 Several studies also have reported that cardiac involvement, including myocardial edema, fibrosis, and cardiac dysfunction, detected by using multi-parameter cardiac magnetic resonance (CMR) techniques were identified in recovered COVID-19 patients during early convalescence.[3], [4], [5], [6], [7], [8] However, whether COVID-19 has a continuous influence on the cardiovascular system in late convalescence is unknown. Therefore, we used traditional CMR sequences to evaluate cardiac abnormalities in late convalescence comprehensively, including cardiac function, myocardial deformation, and myocardial tissue characteristics, and explore its related risk factors.
34 recovered COVID-19 patients at Chengdu Public Health Clinical Medical Centre were prospectively enrolled and followed-up from Jan 1 to Oct 20, 2020. Diagnosis and discharging of COVID-19 patients were based on guidelines of the Chinese Center for Disease Control and Prevention.9 Six months after discharging from hospital, gadolinium enhanced CMR scan (1.5T, Signa HDxt; GE Medical systems, USA) was performed and 20 healthy controls were enrolled too. All the patients and healthy controls signed informed consent and the institutional ethics board of our institutes approved this study (No. 2020.43). Electrocardiography, echocardiography, laboratory test, and clinical characters at admission were collected. Cardiac abnormalities were defined as a combination of elevated myocardial enzyme and injury marker, abnormal echocardiographic and electrocardiographic results. Patients was divided into two subgroups, subgroup with/without cardiac abnormalities at admission.
Biventricular function, myocardial deformation, myocardial edema and fibrosis were evaluated with postprocessing software Cvi42 (Circle Cardiovascular Imaging, Calgary, Canada). Cardiac dysfunction was a combination of left ventricular ejection fraction (LVEF) less than 50%, right ventricular ejection fraction (RVEF) less than 45%, and LV deformation dysfunction. CMR abnormalities was a combination of myocardial edema, fibrosis, and cardiac dysfunction.
At admission, 23 (67.65%) patients had cardiac abnormalities, 7 (20.59%) patients had elevated myocardial enzyme, 2 (5.88%) patients had elevated myocardial injury maker, 3 (8.82%) and 20 (58.82%) patients reported abnormal echocardiographic and electrocardiographic results. None of these 34 patients reported cardiovascular-related symptoms or signs during follow-up.
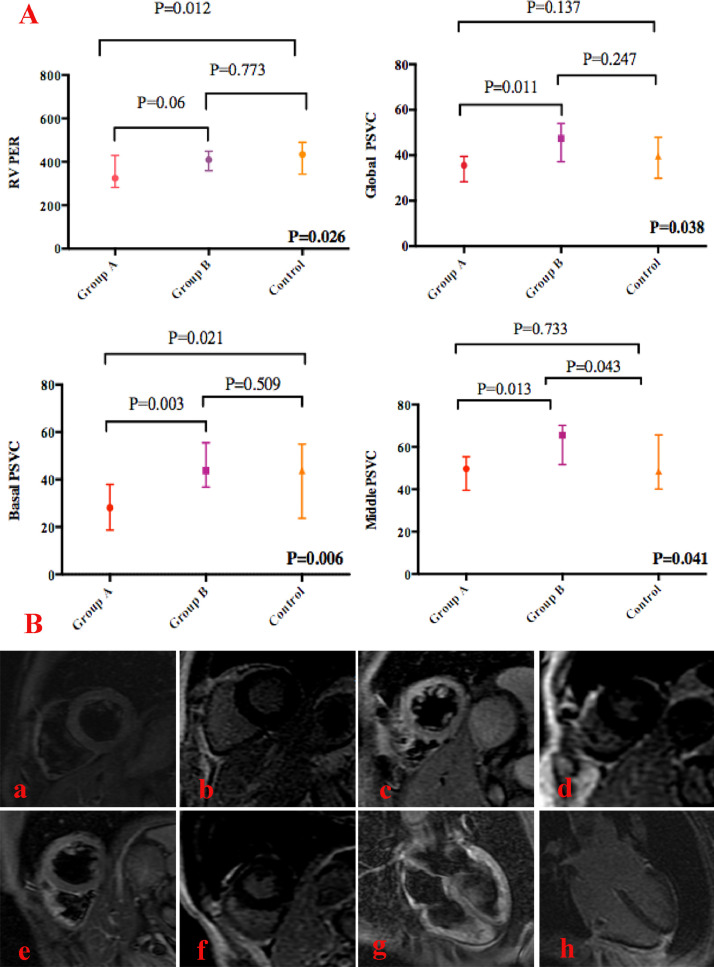
Cardiac function and LV myocardial deformation indexes were compared between the subgroups of patients and controls. All of the 34 recovered COVID-19 patients had normal LVEF (>50%). Nevertheless, right ventricular (RV) systolic dysfunction (RVEF<45%) was found in 5 (14.71%) patients. Meanwhile, right ventricular peak ejection rate (RVPER) in recovered COVID-19 patients with cardiac abnormalities at admission was significantly reduced compared to the controls (Fig. 1 A). The other biventricular function indexes were not significantly different among subgroups of patients with/without cardiac abnormalities at admission and controls (all p > 0.05). Although similar to healthy controls, the global peak systolic velocity circumferential (PSVC) in patients with cardiac abnormalities at admission was lower than patients without cardiac abnormalities (p = 0.011) (Fig. 1A). A similar phenomenon was found in the basal (p = 0.003) and middle PSVC (p = 0.013) (Fig. 1A). Additionally, basal PSCV in patients with cardiac abnormalities at admission was significantly reduced compared to healthy controls (p = 0.021) (Fig. 1A). ROC analysis showed that basal PSVC could differentiate subclinical left ventricular dysfunction with good sensitivity and high specificity (p = 0.004). The area under the curve was 0.822, and the cut-off was 34.455 for basal PSVC (sensitivity: 0.909 and specificity: 0.652). According to the cut-off value, 16 (47.06%) patients had reduced basal myocardial deformation.
Fig. 1.
A. Comparison of RVPER and PSVC between subgroups of recovered COVID-19 patients and controls; Group A represents patients with cardiac abnormalities at admission; Group B represents patients without cardiac abnormalities. Right ventricular peak filling rate(RVPFR); Peak systolic velocity circumferential(PSVC). B. Myocardial edema and fibrosis of recovered COVID-19 patients in late convalescence; a,b. A common type COVID-19 without cardiac abnormalities. No edema or LGE was identified. c,d. A mild type COVID-19 patient with mid mitral valvular regurgitation at admission on echocardiography, but turned to normal on the day of CMR. Edema was found in the anterior, anterolateral, and anteroseptal walls, and there was no evidence of LGE. e,f. A severe type COVID-19 patient with ICU admission and elevated LDH at admission and the day of CMR, other tests were normal. Edema was identified in anterior, anterolateral wall of basal and middle segment, and intra-wall LGE was identified. g,h. A mild type COVID-19 patient with mild tricuspid valvular regurgitation at admission and the day of CMR on echocardiography. CMR showed myocardial edema and sub-epicardial LGE at the lateral wall.
Except for dysfunction of segmental LV deformation and RV systolic function, myocardial abnormality was also identified. Edema was found in 10 (29.41%) and fibrosis in 2 (5.80%) patients. 70% of edema and all the fibrosis was in subgroup of patients with cardiac abnormalities at admission (Fig. 1B).
Finally, 19 (55.88%) patients developed cardiac dysfunction and 22 (64.71%) patients had abnormal CMR findings in our study. Further comparisons were performed to evaluate the characteristics of patients with abnormal CMR finding (Table 1 ). Patients with LV deformation dysfunction were older and had a higher percentage of elevated lactic dehydrogenase (LDH), elevated creatine kinase, echocardiographic abnormalities, and cardiac abnormalities at admission (all p < 0.05). For patients with right ventricle dysfunction/cardiac dysfunction/CMR abnormalities, a higher percentage of elevated LDH was identified (all p < 0.05). Severe or critically severe COVID-19 was more prevalent in patients with myocardial edema (40.00% vs 8.00%, p = 0.048).
Table 1.
Comparison of clinical characters at admission in recovered COVID-19 patients with different CMR models.
|
Dysfunction of LV deformation |
Right ventricle systolic dysfunction |
|||||
|---|---|---|---|---|---|---|
| with(n = 16) | Without (n = 18) | P | with(n = 5) | without(n = 29) | P | |
| Age | 60(49.5,64.5) | 41(34,49) | 0.021 | 34(33.5,43) | 49(38,62) | 0.079 |
| Disease course | 23(16,44.3) | 19(15,28) | 0.226 | 27(19,35) | 19(15,28.5) | 0.273 |
| Severe or critically severe type | 2(12.5%) | 4(22.22%) | 0.672 | 2(40.00%) | 4(13.79%) | 0.205 |
| CT score | 5(3,5) | 3(1,5) | 0.091 | 5(1,9) | 4(2,5) | 0.710 |
| Elevated hs-CRP | 13(81.25%) | 9(50.00%) | 0.152 | 5(100%) | 17(58.62%) | 0.137 |
| Elevated LDH | 6(37.5%) | 1(5.56%) | 0.035 | 3(60.00%) | 4(13.79%) | 0.048 |
| Elevated CK | 4(25.00%) | 0 | 0.039 | 1(20.00%) | 3(10.34%) | 0.488 |
| Elevated K-MB | 1(6.25%) | 0 | 0.471 | 0 | 1(3.45%) | 1.000 |
| Elevated NT-HSST | 1(6.25%) | 0 | 0.471 | 0 | 1(3.45%) | 1.000 |
| Elevated Myoglobin | 1(6.25%) | 0 | 0.471 | 0 | 1(3.45%) | 1.000 |
| Echocardiographic abnormalities | 13(81.25%) | 7(38.89%) | 0.017 | 1(20.00%) | 19(65.52%) | 0.135 |
| Electrocardiographic abnormalities | 2(12.50%) | 1(5.56%) | 0.591 | 0 | 3(10.34%) | 1.000 |
| Cardiac abnormalities | 15(93.75%) | 8(44.44%) | 0.003 | 3(60.00%) | 20(68.97%) | 1.000 |
| Cardiac dysfunction | Myocardial edema | |||||
| with(n = 19) | without(n = 15) | p | with(n = 10) | without(n = 24) | P | |
| Age | 55(34,63.0) | 45(34,49) | 0.159 | 40.5(33,60) | 49(35.5,62) | 0.710 |
| Disease course | 23(16,42) | 18(15,23) | 0.187 | 21.5(15.8,31.5) | 19(15,30) | 0.953 |
| Severe or critically severe type | 3(15.79%) | 3(20.00%) | 1.000 | 4(40.00%) | 2(8.33%) | 0.048 |
| CT score | 5(3,5) | 3(1,5) | 0.223 | 3.5(1.0,8.5) | 4.5(2,5) | 0.647 |
| Elevated hs-CRP | 16(84.21%) | 6(40.00 %) | 0.012 | 7(70.00%) | 15(62.50%) | 1.000 |
| Elevated LDH | 7(36.84%) | 0 | 0.011 | 3(30.00%) | 4(16.67%) | 0.394 |
| Elevated CK | 4(21.05%) | 0 | 0.113 | 2(20.00%) | 2(8.33%) | 0.564 |
| Elevated CK-MB | 1(5.26%) | 0 | 1.000 | 0 | 1(4.17%) | 1.000 |
| Elevated NT-HSST | 1(5.26%) | 0 | 1.000 | 0 | 1(4.17%) | 1.000 |
| Elevated myoglobin | 1(5.26%) | 0 | 1.000 | 0 | 1(4.17%) | 1.000 |
| Echocardiographic abnormalities | 13(68.42%) | 7(46.67%) | 0.296 | 6(60.00%) | 14(58.33%) | 0.462 |
| Electrocardiographic abnormalities | 2(10.53%) | 1(6.25%) | 1.000 | 0 | 3(12.50%) | 0.549 |
| Cardiac abnormalities | 16(84.21%) | 7(46.67%) | 0.030 | 7(70.00%) | 16(66.67%) | 1.000 |
| CMR abnormality | Abbreviations: as shown in Table 1 | |||||
| with(n = 22) | without(n = 12) | P | ||||
| Age | 52.5(34,62.3) | 48(35.5,57.3) | 0.588 | |||
| Disease course | 21.5(15,31.5) | 19(15.3,29) | 0.665 | |||
| Severe or critically severe type | 4(18.18%) | 2(16.67%) | 1.000 | |||
| CT score | 4.5(2.8,5.0) | 3(1.3,5) | 0.398 | |||
| Elevated hs-CRP | 16(72.73%) | 6(50.00%) | 0.265 | |||
| Elevated LDH | 7(31.82%) | 0 | 0.036 | |||
| Elevated CK | 4(18.18%) | 0 | 0.273 | |||
| Elevated CK-MB | 1(4.55%) | 0 | 1.000 | |||
| Elevated TNT-HSST | 1(4.55%) | 0 | 1.000 | |||
| Elevated Myoglobin | 1(4.55%) | 0 | 1.000 | |||
| Echocardiographic abnormalities | 15(68.18%) | 5(46.15%) | 0.163 | |||
| Electrocardiographic abnormalities | 2(9.09%) | 1(8.33%) | 1.000 | |||
| Cardiac abnormalities | 17(80.95%) | 6(41.67%) | 0.060 | |||
To evaluate risk factors for CMR abnormalities, variables with p < 0.1 in Table 1 were included in univariable logistic regression. Referring to dysfunction of LV deformation, elevated LDH (OR:10.20, 95%CI: 1.07 to 97.41, p = 0.044), presence of echocardiographic abnormalities (OR:6.81, 95%CI: 1.41 to 32.83, p = 0.017), and presence of cardiac abnormalities at admission (OR:18.75, 95%CI: 2.02 to 173.9, p = 0.010) were risk factors. Patients with elevated LDH at admission also had a higher risk for RV systolic dysfunction (OR: 9.38, 95% CI: 1.17 to 74.84, p = 0.035). Severe or critically severe COVID-19 was a risk factor for myocardial edema. We also found that the presence of cardiac abnormalities at admission (OR: 6.30, 95% CI: 1.30 to 30.53, p = 0.022) was a risk factor for the presence of CMR abnormalities and cardiac dysfunction (OR: 6.10, 95% CI: 1.24 to 30.09, p = 0.027) at six months of follow-up.
In this recovered COVID-19 cohort, we screened for cardiac sequela in the late convalescence using CMR. We found that cardiac involvement, including RV systolic dysfunction, segmental LV deformation decrease, myocardial edema and fibrosis were not uncommon even after six months of recovery. Findings in our study was consistent with and a supplement to previous researches in early convalescence.[3], [4], [5], [6], [7], [8] Abnormal findings in COVID-19 survivors after 110 days and 6 months imply continuous inflammation, which may be the reason for the lasting cardiac involvement in our patients.1 , 2 What's more, our study also identified that elevated LDH, the presence of echocardiographic abnormalities, the presence of cardiac abnormalities at admission, and the severity of COVID-19 were risk factors for cardiac sequelae in COVID-19 survivors in the late convalescent stage. This was consistent with previous study that focused on risk stratification of cardiac involvement in COVID-19 patients during hospitalization.10 This implies that physicians should pay more attention to these high risk patients during follow-up, and CMR could be performed to screen out cardiovascular involvement in late convalescence.
Declaration of Competing Interest
The authors report no conflicts of interest.
Acknowledgments
Acknowledgments
We thank the reviewers for their professional opinion.
Funding
This work was supported by 2020 Novel coronavirus pneumonia prevention and control technology project of Chengdu (NO. 2020-YF05- 00007-SN); Clinical research finding of Chinese society of cardiovascular disease(CSC) of 2019 (HFCSC2019B01); National Natural Science Foundation of China (82071874, 81971586, 81901712, 81771887, and 81771897); Sichuan Science and Technology Program (2020YFS0050, 2020YJ0029, 2017TD0005); and Fundamental Research Funds for the Central Universities (SCU2020D4132).
References
- 1.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knight D.S., Kotecha T., Razvi Y., Chacko L., Brown J.T., Jeetley P.S. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajpal S., Tong M.S., Borchers J., Zareba K.M., Obarski T.P., Simonetti O.P. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Li R., Zhou Z., Jiang H., Yan Z., Tao X. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021;23:14. doi: 10.1186/s12968-021-00710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark D.E., Parikh A., Dendy J.M., Diamond A.B., George-Durrett K., Fish F.A. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR) Circulation. 2021;143:609–612. doi: 10.1161/CIRCULATIONAHA.120.052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of the People's Republic of China. Diagnosis and Treatment Protocol of Novel Coronavirus (trial version 7th). National Health Commission of the Peo- ple's Republic of China Website. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 10.Fan Q., Zhu H., Zhao J., Zhuang L., Zhang H., Xie H. Risk factors for myocardial injury in patients with coronavirus disease 2019 in China. ESC Heart Fail. 2020;7(6):4018–4117. doi: 10.1002/ehf2.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]