Abstract
Background:
Understanding metabolic mechanisms associated with cognitive changes preceding an Alzheimer’s disease (AD) diagnosis could advance our understanding of AD progression and inform preventive methods.
Objective:
We investigated the metabolomics of the early changes in executive function and delayed recall, the earliest aspects of cognitive function to change in the course of AD development, in order to better understand mechanisms that could contribute to early stages and progression of this disease.
Methods:
This investigation used longitudinal plasma samples from the Wisconsin Registry for Alzheimer’s Prevention (WRAP), a cohort of participants who were dementia free at enrollment and enriched with a parental history of AD. Metabolomic profiles were quantified for 2,324 fasting plasma samples among 1,200 participants, each with up to three study visits, which occurred every two years. Metabolites were individually tested for association with executive function and delayed recall trajectories across age.
Results:
Of 1,097 metabolites tested, levels of seven were associated with executive function trajectories, including an amino acid cysteine S-sulfate and three fatty acids, including erucate (22 : 1n9), while none were associated with delayed recall trajectories. Replication was attempted for four of these metabolites that were present in the Vietnam Era Twin Study of Aging (VETSA). Although none reached statistical significance, three of these associations showed consistent effect directions.
Conclusion:
Our results suggest potential metabolomic mechanisms that could contribute to the earliest signs of cognitive decline. In particular, fatty acids may be associated with cognition in a manner that is more complex than previously suspected.
Keywords: Alzheimer’s disease, amino acids, cognition, executive function, fatty acids, longitudinal analysis, metabolomics
INTRODUCTION
Recent technological advances have made metabolomic studies increasingly feasible for Alzheimer’s disease (AD) researchers [1–3]; however, most of these studies have been limited to cross-sectional approaches comparing patients with either AD or mild cognitive impairment (MCI) to controls. In these early stages of AD metabolomics research, few metabolites have been found to be associated with AD in more than one study [1, 4]. Because neuropathological changes that lead to the development of AD occur decades before its clinical presentation [5–8], longitudinal investigations preceding its diagnosis could add to our current knowledge. In particular, understanding how metabolites correlate with subtle changes in cognition prior to AD diagnosis could help identify causal mechanisms contributing to its onset. This is supported by several studies that have identified metabolites associated with cognitive decline, suggesting that metabolomics could provide key insights into the prevention of cognitive decline and AD [9–13].
Executive function and memory deficits occur in the very early stages of AD, prior to deficits of language and visuospatial functions [14–16]. Studies have shown that these deficits are associated with AD pathology (such as amyloid and tau accumulation) in patients beginning in their 60s [17–19] and are associated with subsequent global cognitive decline [20]. Metabolite levels associated with early cognitive changes in executive function and memory could be particularly indicative of underlying biological mechanisms and pathways contributing to the pathology of AD and could ultimately inform stronger predictive models for this disease.
Using participants from the Wisconsin Registry for Alzheimer’s Prevention (WRAP), we investigated whether longitudinally measured plasma metabolite levels predicted age-related cognitive changes (i.e., trajectories) for executive function and memory (delayed recall), which were also measured longitudinally. WRAP participants are enriched for a parental history of AD, and as such, we anticipate cognitive changes to occur earlier in this population, as they could reflect early changes related to AD pathology. Results from each of these association analyses were further explored using Mendelian randomization (MR) and a metabolite pathway analysis. Replication analyses were performed using an independent sample of participants from the Vietnam Era Twin Study of Aging (VETSA).
MATERIALS AND METHODS
Participants
Study participants were from WRAP, an ongoing longitudinal study of initially dementia free middle-aged adults that allows for the enrollment of siblings and is enriched for a parental history of AD. Further details of the study design and methods used have been previously described [21, 22]. Analyses did not include the baseline WRAP visit due to subsequent protocol changes regarding sample collection procedures and tests included in the neuropsychological battery. Analyses included up to three study visits for each participant, which occurred every two years (spanning a time period of up to four years), with plasma samples and cognitive measures collected concurrently within the same study visit. This study was conducted with the approval of the University of Wisconsin, University of California, and Boston University Institutional Review Boards in accordance with the Helsinki Declaration, and all participants provided signed informed consent before participation.
Biological samples
Plasma collection and sample handling
Fasting blood samples for this study were drawn the morning of each study visit, which was also the day cognitive testing was completed. Blood was collected in 10mL ethylenediaminetetraacetic acid (EDTA) vacutainer tubes. They were immediately placed on ice, and then centrifuged at 3,000 revolutions per minute for 15 min at room temperature. Plasma was pipetted off within 1 h of collection. Plasma samples were aliquoted into 1.0 mL polypropylene cryovials and placed in –80°C freezers within 30min of separation. Samples were never thawed before being shipped overnight on dry ice to Metabolon, Inc. (Durham, NC), where they were again stored in –80°C freezers and thawed once before testing.
Metabolomic profiling and quality control
An untargeted plasma metabolomics analysis was performed by Metabolon, Inc. using Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Quantification was performed as previously described [23]; details regarding sample processing, MS analyses, metabolite identification and quantification (resulting in metabolite “levels” described here) are outlined in the Supplementary Material. Metabolites within nine super pathways were identified: amino acids, carbohydrates, cofactors and vitamins, energy, lipids, nucleotides, partially characterized molecules, peptides, and xenobiotics.
Up to three longitudinal plasma samples were available for each participant. Metabolites with an interquartile range of zero (i.e., those with very low or no variability due to individuals having almost identical levels of the given metabolite) were excluded from analyses (n = 178 metabolites). After removing these metabolites, samples were missing a median of 11.7% metabolites, while metabolites were missing in a median of 1.2% of samples. Missing metabolite values were imputed to the lowest level of detection for each metabolite. Metabolite values were median-scaled and log-transformed to normalize metabolite distributions [24]. If a participant reported that they did not fast or withhold medications and caffeine for at least eight hours, the sample was excluded from analyses (n = 159 samples). A total of 1,097 metabolites among 2,324 samples remained for analyses.
DNA collection and genomics quality control
DNA extraction, sample preparation, genotyping, quality control, the calculation of principal components of ancestry, and imputation are described in detail in Darst et al., 2019 [25]. Briefly, 1,340 samples were genotyped using the Illumina Multi-Ethnic Genotyping Array at the University of Wisconsin Biotechnology Center. Samples missing > 5% of genotypes, with inconsistent self-reported and genetic sex, or whose genetic ancestry was not of European descent were excluded. After excluding variants that were missing in > 5% of individuals, monomorphic, or not in Hardy-Weinberg equilibrium, and using the HRC Imputation Checking Tool [26] for additional quality control, 898,220 bi-allelic autosomal variants among 1,198 WRAP participants remained for imputation, which was performed with the Michigan Imputation Server v1.0.3 [27], using the Haplotype Reference Consortium (HRC) v.r1.1 2016 [28] as the reference panel and Eagle2 v2.3 [29] for phasing. Post-imputation quality control (i.e., excluding imputed variants with an imputation quality score R2 < 0.80, MAF < 0.001, or that were out of HWE), led to a total of 10,499,994 imputed and genotyped variants available for analyses.
Cognitive phenotypes
Composite scores were calculated for executive function and delayed recall based on a previous analysis [19]. Each composite score was calculated from three neuropsychological tests, which were each converted to z-scores using baseline means and standard deviations. The executive function composite score included the Trails Making Test Part B (TMTB) [30] total time to completion, Stroop Neuropsychological Screen Test [31] color-word interference total items completed in 120 s, and Wechsler Abbreviated Intelligence Scale-Revised (WAIS-R) digit symbol coding subtest total items completed in 90 s [32]. The delayed recall composite score included the Rey Auditory Verbal Learning Test (RAVLT) [33] long-delay free recall, Wechsler Memory Scale-Revised Logical Memory (WMS-R LM) [34] delayed recall, and Brief Visuospatial Memory Test (BVMT-R) [35] delayed recall. The TMTB was multiplied by negative one prior to being converted to z-scores, so that higher z-scores indicated better performance. These z-scores were then averaged to derive executive function and delayed recall composite scores at each visit for each individual. We previously found that in the WRAP cohort, these two composite scores had significantly lower intraindividual variability (suggesting lower measurement error) and greater sensitivity to age-related decline than corresponding factor scores [36].
Statistical analyses
Metabolome-wide association studies
All associations were tested using linear mixed effects regression models implemented in the SAS MIXED procedure. The use of mixed models enables the inclusion of longitudinally measured metabolites and longitudinally measured cognitive function. To assess whether metabolite levels were associated with age-related cognitive trajectories, an interaction term between metabolite level and age at study visit was used to predict cognitive composite scores (i.e., executive function and delayed recall). The 1,097 metabolites were each tested in separate models, as were the two cognitive outcomes (1,097*2 = 2,194 models). Models included fixed effects for metabolite level, age at study visit (centered on mean baseline age), and potential confounders or variables that could add noise to the model: sex, self-reported ancestry, cholesterol-lowering medication use (the most commonly used class of medications in our sample), sample storage time (which could impact metabolite levels), education, a genetic risk score for APOE (described previously, and found to be associated with cognitive function [37]), and practice effects (using visit number, as practice effects can influence cognitive scores). Random intercepts were included for within-subject correlations due to repeated measures (i.e., longitudinal measures) and within-family correlations due to the enrollment of siblings. All 1,200 participants had complete covariate data and one or both cognitive measures; however, some were missing either executive function (complete observations for at least one visit: 1,176, at least two visits: 890, and three visits: 205) or delayed recall (complete observations for at least one visit: 1,199, at least two visits: 908, and three visits: 212), and these participants were excluded from respective analyses. The two sets of p-values resulting from testing executive function and delayed recall trajectories were separately corrected for multiple testing using the Benjamini-Hochberg [38] adjustment with an alpha of 0.05.
Mendelian randomization
MR [39] was used to assess whether levels of any individual metabolite identified in our association analyses (i.e., metabolites associated with either executive function or delayed recall trajectories) could causally influence cognition. Metabolic quantitative trait loci (mQTLs) were identified as genomic variants influencing metabolite levels with a p < 0.001 using genome-wide association study (GWAS) summary statistics provided by the authors of a recent publication by Long et al., 2017 [40]. A polygenic score (PGS) was created for each metabolite identified in our association analyses that also had GWAS summary statistics available. PGSs were defined as the sum of an individual’s metabolite-increasing alleles weighted by the effect sizes from GWAS summary statistics. PGSs were created using the additive allelic scoring function in PLINK 1.9 [41] after LD pruning variants within each PGS (R2 > 0.50). The strength of the PGSs as instrumental variables was determined by assessing the relationship between each PGS and the corresponding measured metabolite levels using Pearson r and the F-statistic; otherwise, measured metabolite levels were not included in MR analyses. To be consistent with our discovery models, interactions between each PGS and age were tested for association with cognition using linear mixed effects regression models. Models included fixed effects for mean centered age, sex, education, practice effects, the PGS, and the first four principal components of ancestry to account for potential population stratification. They also included random intercepts for repeated measures and sibling relationships. Due to our limited sample of non-European ancestry individuals and the strong potential for confounding due to population stratification in genetic association studies, MR analyses were limited to European ancestry individuals who had genetic data (N = 1,111 with 2,191 samples total).
Metabolite pathway analysis
Results from association analyses were further investigated using a metabolite pathway analysis. Metabolites included in this analysis were those associated with either executive function or delayed recall trajectories with an unadjusted p < 0.05 and that had a Kyoto Encyclopedia of Genes and Genomics (KEGG) compound ID [42]. Metabolites on our panel with KEGG compound IDs were used as the reference panel for this analysis. The pathway analysis was conducted using MetaboAnalyst 4.0 and included both an overrepresentation analysis, which was assessed using a hypergeometric test, and a pathway topology analysis, which was assessed using relative-betweenness centrality [43]. The overrepresentation analysis tests whether a user-defined list of metabolites represents a particular pathway of metabolites more than expected by chance. The pathway topology analysis considers the structure of a pathway by assessing how connected metabolites are within a pathway. If a pathway contains metabolites that connect dense clusters of other metabolites, the pathway would have a high impact score, as changes to its metabolites would likely have a strong impact on other metabolites within the pathway.
Replication
Participants
Findings were replicated using VETSA, a prospective longitudinal study of middle-aged adults and protective influences on cognitive aging in a community-dwelling sample comprised of individual male twins drawn from the larger Vietnam Era Twin Registry [44]. The Registry is defined on the basis of military service sometime between 1965 and 1975. VETSA participants are reasonably representative of American men of the same age in terms of lifestyle and health characteristics based on Center for Disease Control data [45]. Nearly 80% reported no combat exposure. Participants were not selected or excluded on the basis of any diagnostic characteristics. The only criteria were that participants had to be 51–59 years old at recruitment and both twins in a pair were willing to participate. Participants were administered identical protocols at the University of California, San Diego or Boston University. In this analysis, both twins from a pair were examined at the same site. The complete protocol has been described previously [44].
Metabolomic profiling and quality control
Plasma samples were collected at baseline for 60 VETSA participants. Similar to WRAP, untargeted plasma metabolomics in the VETSA cohort were measured by Metabolon, Inc. using UPLC-MS/MS. Also consistent with WRAP, fasting blood samples (8 + h) for this study were drawn in the morning on the same day cognitive testing was completed. Plasma samples were collected in 10 mL EDTA tubes, aliquoted, and stored at –80°C. Metabolomic data preprocessing procedures were identical to those used in WRAP.
Cognitive phenotypes
At each visit, VETSA participants undergo an extensive neurocognitive test battery comprising 13 neuropsychological tests (23 scores) covering seven cognitive domains [45, 46] which were designed to avoid ceiling effects in middle-aged adults. Seven neuropsychological tasks were used to calculate a common executive function measurement, including Stroop, AX-Continuous Performance Test, Delis-Kaplan Executive Function System (D-KEFS) Trail Making Test, D-KEFS category switching, WMS-III letter numbering, reading span, and WMS-III digit span, as previously detailed [47]. As metabolomic samples were measured at baseline, baseline cognitive measures were used for replication analyses.
Statistical analyses
Replication analyses were performed for putative metabolites identified in WRAP using linear mixed models, with cognitive function as the dependent variable and an interaction term between metabolite level and age as the primary predictor of interest. Models included fixed effects for age, metabolite level, zygotic status, and education and a random effect for twin pair. p-values of < 0.05 were considered significant.
Since VETSA was limited to baseline samples, significant WRAP findings were also re-analyzed in WRAP using only the first sample available (i.e., excluding subsequent data points and reducing the analysis to cross-sectional data). The purpose of this was to improve the comparability between results in the two studies. These cross-sectional models were identical to the initial mixed models used in WRAP with the exception of excluding the random intercept that accounted for within-subject correlations as no repeated measurements were included in cross-sectional models.
RESULTS
Participants
A total of 1,200 WRAP participants with 2,324 longitudinal plasma samples (1.9 visits on average per participant) were available for analyses. At baseline for the current study, 69.2% of participants were female, 93.8% were non-Hispanic White, and participants were 60.8 years old with a bachelor’s degree, on average (Table 1). Executive function and delayed recall cognitive measurements for each participant and each study visit are shown in Supplementary Figure1. Participant seach had 1,097 plasma metabolites available for analyses, 347 (31.6%) of which were of unknown chemical structure. Properties of each metabolite, such as biochemical name, super pathway, and sub pathway are described in Supplementary Table 1.
Table 1.
WRAP participant characteristics at each study visit (2,324 samples total)
| Characteristic | Visit 1 | Visit 2 | Visit 3 |
|---|---|---|---|
| (N = 1,200) | (N = 912) | (N = 212) | |
| Age, mean y (SD, range) | 60.8 (6.7, 40–78) | 63.1 (6.7, 43–81) | 63.8 (7.0, 45–77) |
| Female, N (%) | 830 (69.2) | 628 (68.9) | 141 (66.5) |
| Years of education, mean (SD) | 16.3 (2.9) | 16.3 (2.9) | 16.6 (3.0) |
| Non-Hispanic White, N (%) | 1,125 (93.8) | 873 (95.7) | 211 (99.5) |
| APOE ε4 carrier, N (%) | 457 (38.1) | 354 (38.8) | 84 (39.6) |
| Cholesterol lowering medication, N (%) | 381 (31.8) | 298 (32.7) | 69 (32.6) |
| Sample storage, mean days, (SD) | 1,518.8 (403.9) | 718.5 (294.3) | 219.4 (147.6) |
| Executive Function Composite Score, mean (SD)* | −0.10 (0.85) | −0.09 (0.83) | 0.04 (0.84) |
| Delayed Recall Composite Score, mean (SD)* | 0.02 (0.81) | 0.16 (0.77) | 0.31 (0.70) |
Study visits indicate when the first, second, and third metabolomic samples were available, which do not correspond with the first, second, and third WRAP study visits (analyses did not include the baseline WRAP visit due to subsequent protocol changes regarding sample collection procedures and tests included in the neuropsychological battery).
An increase in cognitive scores is observed over time within individuals likely due to practice effects and self-selection bias (i.e., non-random return patterns for longitudinal visits). See Supplementary Figure 1 for details regarding cognitive scores across age within and across individuals.
Metabolome-wide association studies
Executive function
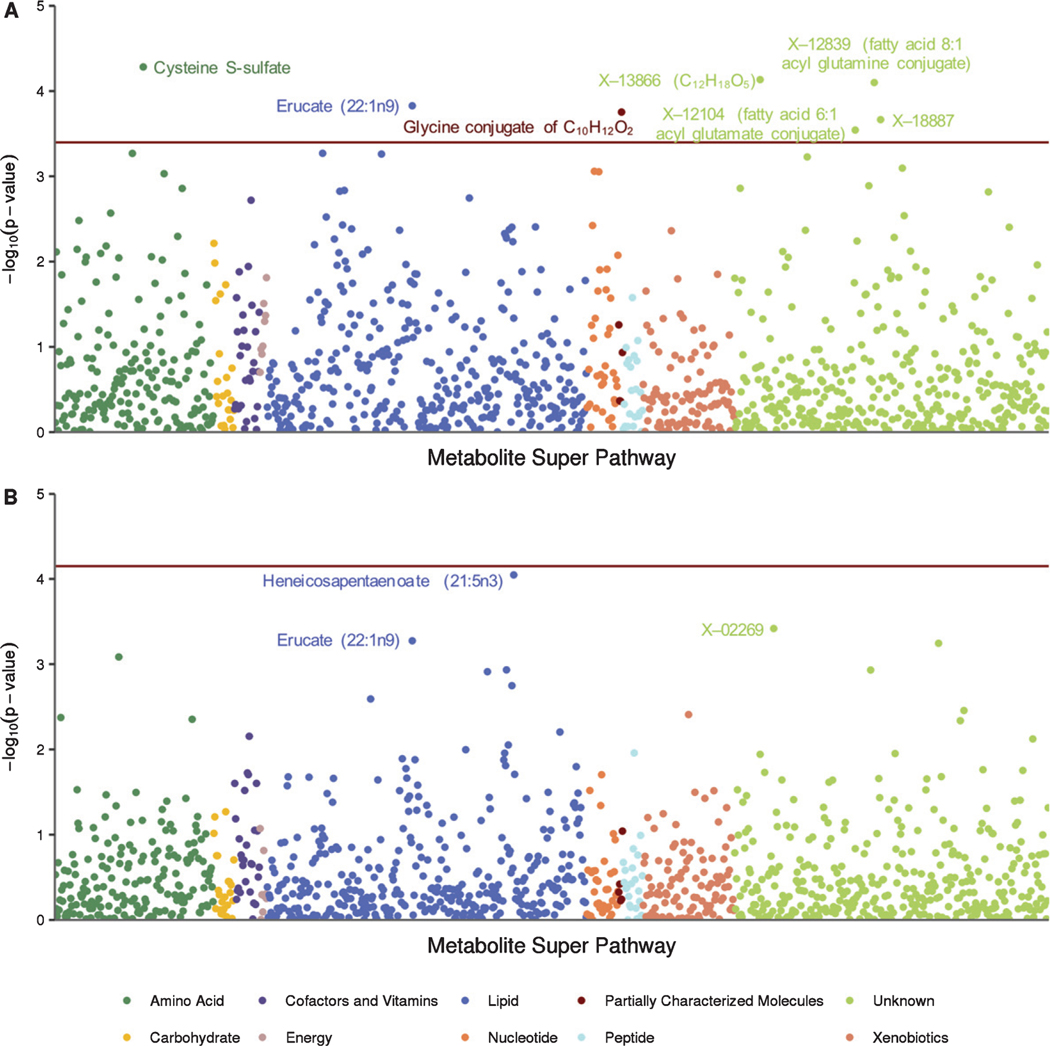
All metabolome-wide association results are detailed in Supplementary Table 1. Seven metabolite-by-age interactions were significantly associated with executive function (Figs. 1A, 2, and Table 2). Levels of cysteine S-sulfate, an amino acid, had the strongest association (unadjusted p = 5.2e–05), with lower levels of cysteine S-sulfate associated with poorer executive function with increasing age (Fig. 2A). The six other significant metabolites showed the opposite relationship with age and executive function, such that higher metabolite levels were associated with poorer executive function with increasing age (Fig. 2B–G). These metabolites included erucate (22 : 1n9) (a monosaturated omega-9 fatty acid), four partially characterized molecules (glycine conjugate of C10H12O2, fatty acid 8 : 1 acyl glutamine conjugate, fatty acid 6 : 1 acyl glutamine conjugate, and C12H18O5), and one unknown metabolite (X-18887). A composite score of these seven metabolites was calculated by averaging the seven metabolites within each sample (after negating cysteine S-sulfate due to the opposite effect observed). The association between the interaction of this metabolite composite score and age was highly associated with executive function (p = 3.4e–14; Supplementary Figure 2).
Fig. 1.
Manhattan plot of metabolome-wide association results for cognitive composite scores. A) Seven metabolite*age interactions were significantly associated with executive function. B) No metabolite*age interactions were significantly associated with delayed recall. Both sets of results used a Benjamini-Hochberg adjusted p-value threshold (red horizontal line).
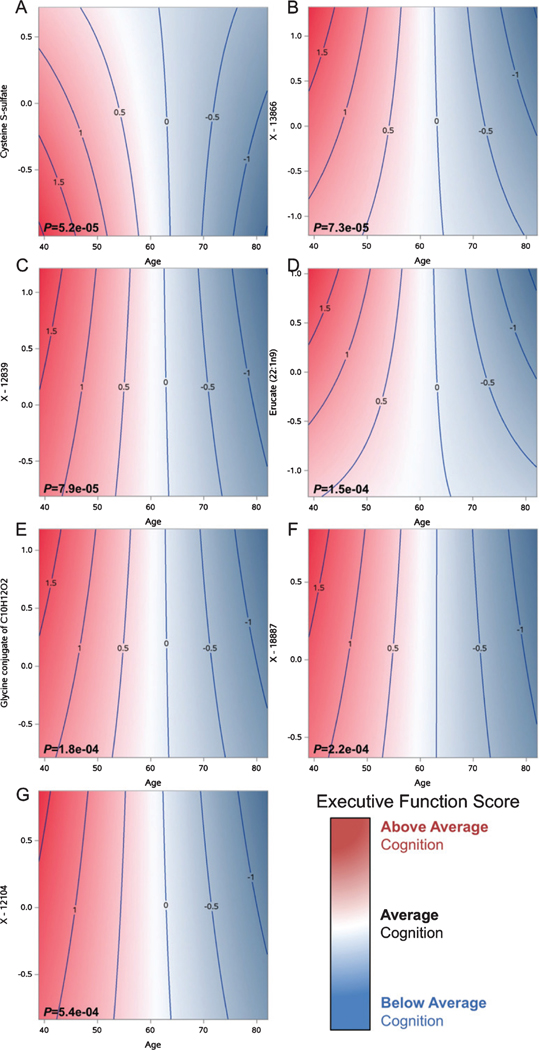
Fig. 2.
Contour plots showing executive function trajectories by seven metabolite levels. The x-axis represents age, y-axis represents standardized metabolite levels, and z-axis represents the standardized executive function composite score. In younger ages, higher levels of most metabolites are associated with above average cognition (indicated by the darker red regions), whereas in older ages, higher levels are associated with below average cognition (indicated by the darker blue regions), with the exception of cysteine s-sulfate, where there opposite is true. Unadjusted p-values are indicated for each test.
Table 2.
Top ten metabolite*age interactions on executive function
| Metabolite | Super pathway | Sub pathway | p |
|---|---|---|---|
| Cysteine S-sulfate | Amino acid | Methionine, cysteine, SAM and taurine metabolism | 5.2e-05 |
| X – 13866 (C12H18O5) | Partially characterized molecules | Partially characterized molecules | 7.3e-05 |
| X – 12839 (fatty acid 8 : 1 acyl glutamine conjugate) | Partially characterized molecules | Partially characterized molecules | 7.9e-05 |
| Erucate (22 : 1n9) | Lipid | Long chain fatty acid | 1.5e-04 |
| Glycine conjugate of C10H12O2 |
Partially characterized molecules | Partially characterized molecules | 1.8e-04 |
| X – 18887 | Unknown | Unknown | 2.2e-04 |
| X – 12104 (fatty acid 6 : 1 acyl glutamine conjugate) | Partially characterized molecules | Partially characterized molecules | 5.4e-04 |
| Dihomo-linolenoyl-choline | Lipid | Fatty acid metabolism (Acyl choline) | 5.4e-04 |
| N6-acetyllysine | Amino acid | Lysine metabolism | 5.5e-04 |
| Heptenedioate (C7 : 1-DC) | Lipid | Fatty acid, Dicarboxylate | 5.9e-04 |
p-values are unadjusted. Bold p-values are statistically significant using a Benjamini-Hochberg adjustment for multiple comparisons.
Delayed recall
No metabolite-by-age interactions were associated with delayed recall after adjusting for multiple comparisons. The three strongest interactions included heneicosapentaenoate (21: 5n3) (a polysaturated fatty acid, unadjusted p = 9.0e–05), X – 02269 (an unknown metabolite, unadjusted p = 3.8e–04), and erucate (22 : 1n9) (unadjusted p = 5.3e–04) (Fig. 1B). Four of the seven metabolites associated with executive function showed a similar relationship with delayed recall, although none were statistically significant (erucate (22 : 1n9), X – 13866, X – 12104, and cysteine S-sulfate, all unadjusted p-values <0.20) (Supplementary Fig. 3).
Mendelian randomization
GWAS summary statistics were available for three of the seven metabolites associated with executive function (cysteine S-sulfate, erucate (22 : 1n9), and X-13866, an unknown metabolite) and used to create a PGS for each metabolite. The three PGSs were fairly weak instruments, with correlations with corresponding metabolites ranging from r = –0.03 to 0.004 and the largest F-statistic = 1.71, well below the commonly used F-statistic threshold of 10 [48] (Supplementary Table 2). Not surprisingly, associations between executive function and the PGSs-by-age were not significant (each p ≥ 0.54). Thus, MR analyses were insufficient to draw conclusions about the nature of the relationship between the metabolites and executive function.
Metabolite pathway analysis
Of the 1,097 metabolites tested, only 291 had KEGG compound IDs that were recognized by MetaboAnalyst and could be used as the reference panel for the pathway analysis. A total of 254 metabolites met the inclusion threshold of an unadjusted p < 0.05 for the cognitive metabolite pathway analysis; however, only 82 of these were identified metabolites with KEGG compound IDs. These metabolites most strongly represented pathways involved in inositol phosphate, ether lipid, and amino sugar and nucleotide metabolism, although none of the pathways identified were statistically significant (Supplementary Figure 4 and Supplementary Table 3).
Replication
Sixty non-Hispanic White men from the VETSA cohort were available for replication analyses and were 57 years of age on average at baseline (SD = 2.3) with an associate degree, on average (Supplementary Table 4). This included 15 pairs of monozygotic twins and 15 pairs of dizygotic twins, for 30 twin pairs total. All of these 30 pairs had no cognitive impairment (NCI) at Wave 1 (year 2003 to 2008) but were discordant for the development of mild cognitive impairment (MCI) in VETSA at Wave 2 (five years later), meaning that within a twin pair, one twin developed MCI and the other twin remained NCI.
Of the seven metabolites associated with executive function in WRAP, four were present in VETSA (cysteine S-sulfate, erucate (22 : 1n9), X-13866, and X-18887) and the interaction between these metabolites and age was tested for association with executive function. Three of these metabolites showed effect trajectories that were strikingly consistent with WRAP findings (Supplementary Figure 5), although they fell short of statistical significance (cysteine S-sulfate, p = 0.35; erucate, p = 0.11; and X-13866, p = 0.09), while one metabolite was not at all associated (X-18887: p = 0.93) (Supplementary Table 1). A composite score of these four metabolites was calculated by averaging the four metabolites within each sample (after negating cysteine S-sulfate due to the opposite effect observed), and the interaction between this metabolite composite score and age was tested for association with executive function. The trajectory was consistent with results from WRAP, although the association fell short of statistical significance (p = 0.08; Supplementary Fig. 5).
To improve the comparability of VETSA and WRAP findings, we also tested the association between the seven significant metabolites identified in WRAP and executive function using cross-sectional models that were limited to the first sample available. Although these analyses had greatly reduced power compared to the full initial analyses (as it excluded 1,124/2,324 or 48.4% of samples), the interaction between age and each of the seven metabolites were associated with executive function with p ≤ 0.16, with three age*metabolite interactions having a p < 0.05 (unknown metabolites X – 13866, X – 12839, and X – 12104; Supplementary Figure 6). All interaction effect directions were consistent with the initial findings based on the full sample.
DISCUSSION
In our longitudinal metabolomics investigation of cognitive trajectories, we identified seven metabolites that are associated with changes in executive function, one of the earliest aspects of cognition to decline in AD progression. Replication of four of these metabolites in an independent cohort showed that three of them, namely an amino acid cysteine S-sulfate, a fatty acid erucate (22 : 1n9), and an unknown metabolite, had strikingly consistent effect directions, although p-values did not reach statistical significance. We did not identify metabolites significantly associated with changes in delayed recall, another aspect of cognition that declines early in AD progression. Our study suggests that levels of these specific metabolites correspond with executive function trajectories in late middle-aged adults with increased risk for AD.
The associations we observed between metabolite levels and executive function trajectories could provide insights into mechanisms contributing to cognitive decline. In particular, lower levels of the amino acid cysteine S-sulfate were associated with poorer executive function with increasing age. The involvement of cysteine metabolism in AD has been implicated in a pathway analysis of previous AD metabolomics studies [1], and our results further suggest that this relationship could depend on age. Cysteine S-sulfate is a glutamate receptor agonist that can lead to calcium influx in nerve cells and neurotoxicity when present in high levels [49, 50]. In a previous investigation, we reported the heritability of plasma cysteine S-sulfate to be 46.8%, suggesting that it is strongly influenced by both genetic and environmental factors, and we reported that levels of cysteine S-sulfate increase with age [51]. Potential dietary sources of cysteine S-sulfate include hazelnut, star anise, agar, and sorghum (The Food Database, FooDB). Cysteine S-sulfate has been shown to drive excitotoxic neurodegeneration in individuals with molybdenum cofactor deficiency, an autosomal recessive inborn error of metabolism characterized by early childhood death [52]. This supports our finding that high levels of cysteine S-sulfate may be detrimental to cognitive function earlier in life. However, experiments using model organisms will be crucial to understand the mechanisms by which cysteine S-sulfate could have protective effects later in life.
The opposite pattern was seen for the six other metabolites associated with executive function, which included three fatty acids, where higher metabolite levels were associated with poorer executive function with increasing age. One possible explanation for this could be that these particular metabolites may be metabolized faster in younger years and slower in older years, and compensation is needed in younger years to account for the quick metabolism. We previously found that plasma levels of these six metabolites significantly increase with age [51], supporting this hypothesis. One of the three fatty acids was erucate (22 : 1n9), an omega-9 fatty acid that readily crosses the blood-brain barrier [53] and has been shown to enhance memory performance in mice [54]. Erucate is mainly found in Brassica family of plants, including kale, mustard, Brussel sprouts, and broccoli, and it makes up ∼40–50% of the oils in mustard seed, rapeseed, and wallflower seed (FooDB). Taken together with erucate’s low heritability of 15.6% [51], this suggests that plasma levels of erucate are predominantly influenced by dietary factors.
Fatty acids have long been suspected to influence cognitive performance, but studies have had mixed findings regarding their role, particularly of omega-3 fatty acids [55, 56]. Our results suggest that this role may be difficult to define because the implications of these metabolite levels change as individuals age. This is further supported by similar relationships we found for two partially characterized fatty acids: fatty acid 8 : 1 acyl glutamine conjugate and fatty acid 6 : 1 acyl glutamine conjugate, both of which also have low heritabilities of 28.4% and 13.3%, respectively [51]. More information about these two metabolites could prove useful in understanding the relationship between fatty acids and cognitive function. A recent study reported higher levels of docosapentaenoate (22 : 5 n-6), a long-chain polyunsaturated fatty acid, to be associated with less decline of information-processing speed in a sample of midlife African Americans [10], supporting the association between higher fatty acids levels and better cognition function in midlife. Beyond cognitive performance, omega fatty acids have also been shown to be dysregulated in certain brain regions of patients with AD pathology [50], further strengthening the potential relevance of fatty acids. Given the importance of dietary factors on circulating fatty acid levels, our findings could be followed up in model organism experiments controlling for dietary fatty acid intake using an age-dependent dosing scheme while monitoring cognitive performance across the lifespan.
This study has several limitations. The pathway analysis we performed was highly limited due to the large number of metabolites in our panel that did not have KEGG compound IDs with several of our significant findings being partially characterized or unknown metabolites. This is a notable challenge in the field of metabolomics and greatly underscores the importance of continued efforts to identify and characterize metabolites. The PGSs we developed for our MR assessment were weak instrumental variables and did not allow us to determine whether levels of the metabolites we identified are causally related to executive function. Although our replication in VETSA was not based on longitudinal data, the executive function score was not measured identically, and VETSA was limited to men with a narrower age range and less education on average than WRAP participants, the striking similarity of the effects of cysteine S-sulfate and erucate on executive function across age suggests that our findings are robust. However, the relatively small sample size of the replication cohort is likely why replication analyses did not reach statistical significance. It is also possible that dietary differences between WRAP and VETSA could have contributed to the lack of statistical significance in VETSA, given the potential importance of dietary factors to the identified metabolites. Replicating metabolomics findings is a current challenge in the field of metabolomics, as differences in sample handling, laboratory platforms, and quantification techniques can lead to variation in metabolite levels and different sets of metabolites identified [3]. Replication with a larger sample, ideally one with longitudinally measured metabolites and longitudinally measured cognitive function from a healthy aging population, will be necessary to further validate our observed associations. Further, future investigations using other analytic approaches to measure executive function could serve as a means to identify additional metabolites associated with cognitive trajectories.
The lack of findings for delayed recall does not rule out the possibility that metabolites could be involved in this aspect of cognition—we previously reported that the executive function composite score had notably lower intraindividual variability and greater sensitivity to age-related decline relative to the delayed recall composite score [36], which could explain why we only identified metabolites associated with executive function. It is also possible that the relevant metabolites may not have been captured in our investigation (e.g., the plasma metabolites measured may be relevant to vascular cognitive impairment, in which executive function is commonly impaired early on), that our sample was not large enough to identify weak associations, or that our cohort has not yet experienced sufficient cognitive decline to identify metabolite levels associated with delayed recall trajectories. While we observed decline in cognition with age, although to a lesser degree for delayed recall than executive function (Supplementary Fig. 1), practice effects and non-random return patterns (i.e., participants with better cognition being more likely to return for follow-up visits) often resulted in participants having improved cognitive measures in later study visits (as seen in Table 1 and Supplementary Fig. 1). This likely weakened our findings, as adjusting for practice effects does not account for non-random return patterns. Future investigations using other analytic approaches to measure both executive function and delayed recall could serve as a means to identify additional metabolites associated with cognitive trajectories. As metabolomics technologies continue to improve, the field will be able to quantify and identify metabolites more comprehensively and it will become more feasible to generate metabolomics data in large cohorts, which could contribute to an improved understanding of the biological mechanisms contributing to cognitive decline and AD progression.
Using a large panel of longitudinal metabolomics data, we found that levels of certain plasma metabolites, including amino acid cysteine S-sulfate and fatty acid erucate, were associated with age-related changes in executive function, one of the earliest aspects of cognitive function to change in the course of AD development. It will be important for future population-based and experimental studies to investigate whether these metabolites are causally associated with executive function, which could lead to the elucidation of mechanisms influencing early stages of AD and ultimately inform preventative measures.
Supplementary Material
ACKNOWLEDGMENTS
BFD was supported by an NLM training grant to the Computation and Informatics in Biology and Medicine Training Program [grant number NLM 5T15LM007359]. This research was also supported by the NIH [grant numbers R01AG054047, R01 AG27161, UL1TR000427, and P2C HD047873], Wisconsin Alumni Research Foundation UW2020, Helen Bader Foundation, Northwestern Mutual Foundation, Extendicare Foundation, and State of Wisconsin. The authors thank the University of Wisconsin Madison Biotechnology Center Gene Expression Center for providing Illumina Infinium genotyping services. GWAS summary statistics for several metabolites excluded from analyses in the Long et al., 2017 Nature Genetics publication were generously calculated and provided by the authors of this manuscript, particularly Elizabeth Cirulli, PhD. The authors also thank Joshua Coon, PhD, for providing expertise in early stages of this investigation. We especially thank the WRAP participants. The VETSA study was supported by NIA [grant numbers R01 AG050595, R01 AG022381, R01 AG062483 and R01 AG059329 and P01 AG059329]. It was also, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA/NIH or the VA. The U.S. Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance in the conduct of the VET Registry, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0176r3).
SUPPLEMENTARYMATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-200176.
Handling Associate Editor: Roger Dixon
REFERENCES
- [1].Enche Ady CNA, Lim SM, Teh LK, Salleh MZ, Chin AV, Tan MP, Poi PJH, Kamaruzzaman SB, Abdul Majeed AB, Ramasamy K (2017) Metabolomic-guided discovery of Alzheimer’s disease biomarkers from body fluid. J Neurosci Res 95, 2005–2024. [DOI] [PubMed] [Google Scholar]
- [2].Trushina E, Mielke MM (2014) Recent advances in the application of metabolomics to Alzheimer’s disease. Biochim Biophys Acta 1842, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gonzalez-Dominguez R, Sayago A, Fernandez-Recamales A (2017) Metabolomics in Alzheimer’s disease: The need of complementary analytical platforms for the identification of biomarkers to unravel the underlying pathology. J Chromatogr B Analyt Technol Biomed Life Sci 1071, 75–92. [DOI] [PubMed] [Google Scholar]
- [4].Jiang Y, Zhu Z, Shi J, An Y, Zhang K, Wang Y, Li S, Jin L, Ye W, Cui M, Chen X (2019) Metabolomics in the development and progression of dementia: A systematic review. Front Neurosci 13, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1, a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van der Lee SJ, Teunissen CE, Pool R, Shipley MJ, Teumer A, Chouraki V, Melo van Lent D, Tynkkynen J, Fischer K, Hernesniemi J, Haller T, Singh-Manoux A, Verhoeven A, Willemsen G, de Leeuw FA, Wagner H, van Dongen J, Hertel J, Budde K, Willemsvan Dijk K, Weinhold L, Ikram MA, Pietzner M, Perola M, Wagner M, Friedrich N, Slagboom PE, Scheltens P, Yang Q, Gertzen RE, Egert S, Li S, Hankemeier T, van Beijsterveldt CEM, Vasan RS, Maier W, Peeters CFW, Jorgen Grabe H, Ramirez A, Seshadri S, Metspalu A, Kivimaki M, Salomaa V, Demirkan A, Boomsma DI, van der Flier WM, Amin N, van Duijn CM (2018) Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement 14, 707–722. [DOI] [PubMed] [Google Scholar]
- [10].Bressler J, Yu B, Mosley TH, Knopman DS, Gottesman RF, Alonso A, Sharrett AR, Wruck LM, Boerwinkle E (2017) Metabolomics and cognition in African American adults in midlife: The atherosclerosis risk in communities study. Transl Psychiatry 7, e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li D, Misialek JR, Boerwinkle E, Gottesman RF, Sharrett AR, Mosley TH, Coresh J, Wruck LM, Knopman DS, Alonso A (2017) Prospective associations of plasma phospholipids and mild cognitive impairment/dementia among African Americans in the ARIC Neurocognitive Study. Alzheimers Dement (Amst) 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, Hall WJ, Fisher SG, Peterson DR, Haley JM, Nazar MD, Rich SA, Berlau DJ, Peltz CB, Tan MT, Kawas CH, Federoff HJ (2014) Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med 20, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Low DY, Lefevre-Arbogast S, Gonzalez-Dominguez R, Urpi-Sarda M, Micheau P, Petera M, Centeno D, Durand S, Pujos-Guillot E, Korosi A, Lucassen PJ, Aigner L, Proust-Lima C, Hejblum BP, Helmer C, Andres-Lacueva C, Thuret S, Samieri C, Manach C (2019) Diet-related metabolites associated with cognitive decline revealed by untargeted metabolomics in a prospective cohort. Mol Nutr Food Res 63, e1900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lafleche G, Albert MS (1995) Executive function deficits in mild Alzheimer’s disease. Neuropsychology 9, 313–320. [Google Scholar]
- [15].Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L (2006) Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol 21, 15–21. [DOI] [PubMed] [Google Scholar]
- [16].Albert MS (1996) Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A 93, 13547–13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, Bendlin BB, Asthana S, Johnson SC, Zetterberg H, Carlsson CM (2018) Age-accelerated cognitive decline in asymptomatic adults with CSF beta-amyloid. Neurology 90, e1306–e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Betthauser TJ, Koscik RL, Jonaitis EM, Allison SL, Cody KA, Erickson CM, Rowley HA, Stone CK, Mueller KD, Clark LR, Carlsson CM, Chin NA, Asthana S, Christian BT, Johnson SC (2020) Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 143, 320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clark LR, Racine AM, Koscik RL, Okonkwo OC, Engelman CD, Carlsson CM, Asthana S, Bendlin BB, Chappell R, Nicholas CR, Rowley HA, Oh JM, Hermann BP, Sager MA, Christian BT, Johnson SC (2016) Beta-amyloid and cognitive decline in late middle age: Findings from the Wisconsin Registry for Alzheimer’s Prevention study. Alzheimers Dement 12, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC, Bondi MW (2012) Specific measures of executive function predict cognitive decline in older adults. J Int Neuropsychol Soc 18, 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sager MA, Hermann B, La Rue A (2005) Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol 18, 245–249. [DOI] [PubMed] [Google Scholar]
- [22].Johnson SC, Koscik RL, Jonaitis EM, Clark LR, Mueller KD, Berman SE, Bendlin BB, Engelman CD, Okonkwo OC, Hogan KJ, Asthana S, Carlsson CM, Hermann BP, Sager MA (2018) The Wisconsin Registry for Alzheimer’s Prevention: A review of findings and current directions. Alzheimers Dement (Amst) 10, 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Evans AM, Bridgewater BR, Liu Q, Mitchell MW, Robinson RJ, Dai H, Stewart SJ, DeHaven CD, Miller LAD (2014) High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 4, 1–7. [Google Scholar]
- [24].van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ (2006) Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genomics 7, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Darst BF, Lu Q, Johnson SC, Engelman CD (2019) Integrated analysis of genomics, longitudinal metabolomics, and Alzheimer’s risk factors among 1,111 cohort participants. Genet Epidemiol 43, 657–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rayner NW, Robertson N, Mahajan A, McCarthy MI (2016) A suite of programs for pre- and post-imputation data checking. In The American Society of Human Genetics, Vancouver, Canada. [Google Scholar]
- [27].Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh PR, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C(2016)Next-generation genotype imputation service and methods. Nat Genet 48, 1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, Luo Y, Sidore C, Kwong A, Timpson N, Koskinen S, Vrieze S, Scott LJ, Zhang H, Mahajan A, Veldink J, Peters U, Pato C, van Duijn CM, Gillies CE, Gandin I, Mezzavilla M, Gilly A, Cocca M, Traglia M, Angius A, Barrett JC, Boomsma D, Branham K, Breen G, Brummett CM, Busonero F, Campbell H, Chan A, Chen S, Chew E, Collins FS, Corbin LJ, Smith GD, Dedoussis G, Dorr M, Farmaki AE, Ferrucci L, Forer L, Fraser RM, Gabriel S, Levy S, Groop L, Harrison T, Hattersley A, Holmen OL, Hveem K, Kretzler M, Lee JC, McGue M, Meitinger T, Melzer D, Min JL, Mohlke KL, Vincent JB, Nauck M, Nickerson D, Palotie A, Pato M, Pirastu N, McInnis M, Richards JB, Sala C, Salomaa V, Schlessinger D, Schoenherr S, Slagboom PE, Small K, Spector T, Stambolian D, Tuke M, Tuomilehto J, Van den Berg LH, Van Rheenen W, Volker U, Wijmenga C, Toniolo D, Zeggini E, Gasparini P, Sampson MG, Wilson JF, Frayling T, de Bakker PI, Swertz MA, McCarroll S, Kooperberg C, Dekker A, Altshuler D, Willer C, Iacono W, Ripatti S, Soranzo N, Walter K, Swaroop A, Cucca F, Anderson CA, Myers RM, Boehnke M, McCarthy MI, Durbin R, Haplotype Reference Consortium (2016) A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 48, 1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loh PR, Danecek P, Palamara PF, Fuchsberger C, Y AR, H KF, Schoenherr S, Forer L, McCarthy S, Abecasis GR, Durbin R, A LP (2016) Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet 48, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Reitan RM, Wolfson D (1985) The Halstead–Reitan Neuropsychological Test Battery: Therapy and clinical interpretation, Neuropsychological Press, Tucson, AZ. [Google Scholar]
- [31].Trenerry MR, Crosson B, DeBoe J, Leber WR (1989) Stroop Neuropsychological Screening Test Manual, Psychological Assessment Resources. [Google Scholar]
- [32].Wechsler D (1981) Wechsler Adult Intelligence Scale - Revised, The Psychological Corporation, San Antonio. [Google Scholar]
- [33].Schmidt M (1996) Rey Auditory Verbal Learning Test: A Handbook, Western Psychological Services, Los Angeles, California. [Google Scholar]
- [34].Wechsler D (1987) Wechsler Memory Scale-Revised, The Psychological Corporation, Harcourt Brace Jovanovich, inc for Psychological Corp, New York. [Google Scholar]
- [35].Benedict RH (1997) Brief Visuospatial Memory Test-Revised. Psychological Assessment Resources, Inc., Odessa, FL. [Google Scholar]
- [36].Jonaitis EM, Koscik RL, Clark LR, Ma Y, Betthauser TJ, Berman SE, Allison SL, Mueller KD, Hermann BP, Van Hulle CA, Christian BT, Bendlin BB, Blennow K, Zetterberg H, Carlsson CM, Asthana S, Johnson SC (2019) Measuring longitudinal cognition: Individual tests versus composites. Alzheimers Dement (Amst) 11, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Darst BF, Koscik RL, Racine AM, Oh JM, Krause RA, Carlsson CM, Zetterberg H, Blennow K, Christian BT, Bendlin BB, Okonkwo OC, Hogan KJ, Hermann BP, Sager MA, Asthana S, Johnson SC, Engelman CD (2017) Pathway-specific polygenic risk scores as predictors of amyloid-beta deposition and cognitive function in a sample at increased risk for Alzheimer’s disease. J Alzheimers Dis 55, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 57, 289–300. [Google Scholar]
- [39].Smith GD, Ebrahim S (2003) ‘Mendelian randomization’: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32,1–22. [DOI] [PubMed] [Google Scholar]
- [40].Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, Brewerton S, Turpaz Y, Perkins BA, Evans AM, Miller LA, Guo L, Caskey CT, Schork NJ, Garner C, Spector TD, Venter JC, Telenti A (2017) Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet 49, 568–578. [DOI] [PubMed] [Google Scholar]
- [41].Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xia J, Wishart DS (2016) Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics 55, 14.10.1–14.10.91. [DOI] [PubMed] [Google Scholar]
- [44].Kremen WS, Franz CE, Lyons MJ (2013) VETSA: The Vietnam Era Twin Study of Aging. Twin Res Hum Genet 16, 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schoenborn CA, Heyman KM (2009) Health characteristics of adults aged 55 years and over: United States, 2004–2007. Natl Health Stat Report, pp. 1–31. [PubMed] [Google Scholar]
- [46].Kremen WS, Beck A, Elman JA, Gustavson DE, Reynolds CA, Tu XM, Sanderson-Cimino ME, Panizzon MS, Vuoksimaa E, Toomey R, Fennema-Notestine C, Hagler DJ Jr., Fang B, Dale AM, Lyons MJ, Franz CE (2019) Influence of young adult cognitive ability and additional education on later-life cognition. Proc Natl Acad Sci U S A 116, 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gustavson DE, Panizzon MS, Franz CE, Friedman NP, Reynolds CA, Jacobson KC, Xian H, Lyons MJ, Kremen WS (2018) Genetic and environmental architecture of executive functions in midlife. Neuropsychology 32, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Stock JH, Wright JH, Yogo M (2002) A survey of weak instruments and weak identification in generalized method of moments. J Bus Econ Stat 20, 518–529. [Google Scholar]
- [49].Olney JW, Misra CH, de Gubareff T (1975) Cysteine-S-sulfate: Brain damaging metabolite in sulfite oxidase deficiency. J Neuropathol Exp Neurol 34, 167–177. [DOI] [PubMed] [Google Scholar]
- [50].Snowden SG, Ebshiana AA, Hye A, An Y, Pletnikova O, O’Brien R, Troncoso J, Legido-Quigley C, Thambisetty M (2017) Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med 14, e1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Darst BF, Koscik RL, Hogan KJ, Johnson SC, Engelman CD (2019) Longitudinal plasma metabolomics of aging and sex. Aging (Albany NY) 11, 1262–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kumar A, Dejanovic B, Hetsch F, Semtner M, Fusca D, Arjune S, Santamaria-Araujo JA, Winkelmann A, Ayton S, Bush AI, Kloppenburg P, Meier JC, Schwarz G, Belaidi AA (2017) S-sulfocysteine/NMDA receptor-dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. J Clin Invest 127, 4365–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Golovko MY, Murphy EJ (2006) Uptake and metabolism of plasma-derived erucic acid by rat brain. J Lipid Res 47, 1289–1297. [DOI] [PubMed] [Google Scholar]
- [54].Kim E, Ko HJ, Jeon SJ, Lee S, Lee HE, Kim HN, Woo ER, Ryu JH (2016) The memory-enhancing effect of erucic acid on scopolamine-induced cognitive impairment in mice. Pharmacol Biochem Behav 142, 85–90. [DOI] [PubMed] [Google Scholar]
- [55].Mazereeuw G, Lanctot KL, Chau SA, Swardfager W, Herrmann N (2012) Effects of omega-3 fatty acids on cognitive performance: A meta-analysis. Neurobiol Aging 33, 1482 e1417–1429. [DOI] [PubMed] [Google Scholar]
- [56].Cederholm T, Salem N Jr., Palmblad J (2013) omega-3 fatty acids in the prevention of cognitive decline in humans. Adv Nutr 4, 672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.