Supplemental Digital Content is Available in the Text.
Cage-lid hanging behavior is impaired by sustained pain in mice and can be used as an ethologically valid and translationally relevant pain outcome measure.
Keywords: Behavior, Mice, Preclinical, Assessment, Analgesia
Abstract
The development of new analgesic drugs has been hampered by the inability to translate preclinical findings to humans. This failure is due in part to the weak connection between commonly used pain outcome measures in rodents and the clinical symptoms of chronic pain. Most rodent studies rely on the use of experimenter-evoked measures of pain and assess behavior under ethologically unnatural conditions, which limits the translational potential of preclinical research. Here, we addressed this problem by conducting an unbiased, prospective study of behavioral changes in mice within a natural homecage environment using conventional preclinical pain assays. Unexpectedly, we observed that cage-lid hanging, a species-specific elective behavior, was the only homecage behavior reliably impacted by pain assays. Noxious stimuli reduced hanging behavior in an intensity-dependent manner, and the reduction in hanging could be restored by analgesics. Finally, we developed an automated approach to assess hanging behavior. Collectively, our results indicate that the depression of hanging behavior is a novel, ethologically valid, and translationally relevant pain outcome measure in mice that could facilitate the study of pain and analgesic development.
1. Introduction
Despite a growing need for new analgesics, there has been little progress in translating preclinical advances into safe and efficacious clinical interventions for chronic pain.48 A major contributor to this translational gap is the limited relevance of existing preclinical outcome measures to the human pain experience.29,33,37 Clinical manifestations of chronic pain include physical, emotional, and cognitive changes that are often difficult to measure in rodents.30 In addition, many behavioral outcome measures in rodents are assessed under ethologically “unnatural” and potentially stress-evoking testing conditions.15,21,35,41 Furthermore, preclinical pain studies often rely on outcome measures that are experimenter-evoked, such as nociceptive withdrawal thresholds to mechanical or thermal stimuli.28,29 Besides the fact that the hypersensitivity states (ie, allodynia and hyperalgesia) being modeled are rather rare symptoms of chronic pain in humans,2,39 this approach to pain assessment can introduce confounding influences such as bias and experimenter-induced stress.10,17,41 Although spontaneous behaviors are increasingly used as outcome measures of pain models, many have significant limitations.10,28,43 These behaviors are often far more robust under conditions of acute, but not chronic, pain.23,42,47 For example, grimace scales, which quantify facial expressions associated with pain, are progressively less suitable as the duration of pain increases.23,42
Nonessential or elective behaviors offer promising avenues for identifying novel behavioral measures of pain that can be objectively quantified under ethologically relevant conditions. These natural, spontaneous, and often complex homecage behaviors include playing, grooming, socializing, and nest building.19 Elective behaviors, sometimes called “luxury” behaviors, are voluntary and healthy rodents are motivated to perform them despite possibly limited survival benefits.19,38 Elective behaviors in rodents may be indicators of well-being and are often affected by poor health.1,5,19,20 Analogous elective behaviors in humans, such as social interactions and participation in extracurricular activities, are consistently impacted by chronic pain.8,11,13 Thus, elective behaviors might offer a window into the inner state and well-being of rodents and make these behaviors potential translationally relevant measures of pain.
To identify novel behavioral measures of pain in mice with translational relevance, we conducted an unbiased, prospective study of mouse behavioral changes in a natural homecage environment using various assays featuring sustained pain. Using automated video tracking, we found that cage-lid hanging was the only homecage behavior reliably impacted by pain over its duration. Here, we defined hanging behavior as a mouse climbing onto the metal lid of their homecage and suspending themselves, upside-down, off the floor. We found that hanging behavior was reduced in pain assays in a stimulus intensity-dependent manner that could be restored with analgesics. Next, we created an ethological profile of hanging across different sexes, ages, and strains of mice. Finally, we developed and validated new tools to facilitate observation and automate quantification of hanging, thus streamlining pain assessment in mice. Overall, our study has demonstrated that hanging is an elective behavior that can act as a robust and translationally relevant measure of pain in mice.
2. Methods
2.1. Animals
All experiments were approved by the Downtown Animal Care Committee at McGill University and the Animal Ethics and Compliance Program at the University of Toronto and conducted in accordance with Canadian Council on Animal Care (CCAC) guidelines. Male and female C57BL/6N and CD-1 mice were tested in 4 different facilities: McGill University (Mogil Lab), University of Toronto St. George campus (Bonin Lab), University of Toronto at Mississauga (UTM; Martin Lab), and The Centre for Phenogenomics (TCP) in Toronto. Mice tested at McGill University were all purchased from Charles River Laboratories, St. Constant, QC (CD-1, n = 16). Mice tested at the University of Toronto were all purchased from Charles River Laboratories (CD-1, n = 94; C57BL/6N, n = 94). C57BL/6N mice tested in the UTM campus were bred in-house (n = 76) or purchased from Charles River (n = 20). C57BL/6N mice tested at TCP (n = 35) were bred in-house. All mice purchased from Charles River were allowed a one-week habituation period after arrival at the facility. The mice were housed in same-sex cages containing 3-4 animals per cage. Mice bred at the UTM were housed with same-sex littermates in groups of 4 to 5 per cage at weaning. For most experiments, either two-month-old CD-1 male and female mice or C57BL/6N male mice were used (details for each experiment are provided in the Results section). In experiments exploring the effects of sex and age on hanging behavior, two-month-old C57BL/6N male and female mice were used. Mice used to assess osteoarthritis (OA) pain were 24- to 25-weeks old at the time of assessment. Experiments conducted at TCP to assess automated detection of cage lid interaction were assessed using four-month-old C57BL/6N male and female mice.
All facilities were temperature-controlled (20 ± 1°C) with 12 hours:12 hours light: dark cycle at UTM, McGill University, and TCP. Experiments in Toronto had a 14 hours:10 hours light: dark cycle (6 am-8 pm). When a reverse light cycle was used, animals were given one week to acclimatize to the new cycle before experiment commencement. The UTM and TCP conducted experiments during the light period only. In all facilities, environmental enrichment was provided by placing a cotton nesting square, cork bedding, and a red, transparent plastic mouse house inside each cage, except TCP, where enrichment was using corncob bedding and shredded paper with no red mouse house. Mice at the University of Toronto, UTM, and McGill were housed in ventilated Allentown Micro-Vent cages with automated water delivery. Mice at TCP were housed in Tecniplast Green Line cages with automatic ventilation and water delivery. For all homecage experiments, mice were tested in a clean cage of the same manufacturer and make as their original homecage at weaning. All mice had access to food and water ad libitum. For all experiments, animals were randomly assigned into treatment groups, and experimenters were blinded to treatment. A new cohort of mice was used for each experiment.
2.2. Pain assays
2.2.1. Spared nerve injury
Spared nerve injury (SNI) was performed as previously described40: Mice were first anesthetized with halothane anesthesia (1.75%-2.5%). After skin and muscle incision, 2 of the 3 terminal branches of the sciatic nerve (sural and tibial) were tightly ligated with a 9-0 silk suture (Ethicon, Somerville, NJ). Next, the ligated branches were transected distal to the ligature, and ∼2 mm of each distal nerve stump was removed. In all animals, overlying muscle and skin layers were closed separately by using 6-0 silk sutures (Ethicon) and 7.5-mm suture clips (Harvard Apparatus, Holliston, MA), respectively.40 A baseline recording of hanging behavior was taken before the surgery (day −1, preoperative) and compared with recordings taken after surgery (days 1, 4, 7, 14, and 28, postoperative). In all sessions, mice were filmed for 24 hours, starting immediately after lights off. Mice were returned to their homecage in between testing periods (Mogil and Martin labs).
2.2.2. Plantar complete Freund's adjuvant
A volume of 15 μL of complete Freund's adjuvant (CFA) (0.5 mg/mL heat-killed Mycobacterium) was injected subcutaneously into the plantar surface of the right hind paw. A baseline recording of hanging behavior was taken before injection (day −1, pre-operative). On test days (days 1, 4, 7, 11, and 14, postoperative), mice were filmed for 24 hours, starting immediately after lights off. Mice were returned to their homecage in between testing periods (Mogil lab). In some CFA experiments conducted at TCP (Toronto), cage lid interaction was assessed using an automated capacitance sensing device. In these experiments, 16-week-old C57BL/6N mice were tested at baseline and 3 hours after intraplantar CFA injection (20 μL) for mechanical sensitivity using the SUDO von Frey filament up-down method,7 heat sensitivity using the Hargreaves (radiant heat paw-withdrawal) test,16 and tested again at 1 day postinjection for cage lid interaction in the homecage (Flenniken lab).
2.2.3. Formalin
A volume of 10 μL of 1% formalin was injected subcutaneously into the plantar surface of the right hind paw. The mice were then individually placed in a second homecage for testing, which contained bedding, food, and water. Mice were filmed for 2 hours after the injection. The early phase of the formalin test was defined as the first 5 minutes after injection and the late phase as 15 to 60 minutes after injection. This experiment was conducted during the dark cycle, and animals were housed in a reverse light: dark cycle for one week before testing (Bonin lab).
2.2.4. Capsaicin
A volume of 5 μL of 0.5%, wt/vol CAP (dissolved in 80% saline, 10% Tween 80, and 10% ethanol) was injected subcutaneously into the plantar surface of the right hind paw. The mice were then individually placed in a second homecage for testing, which contained bedding, food, and water. These experiments were conducted during the light cycle, and mice were filmed for 2 hours after the injection (Bonin lab).
2.2.5. Anterior cruciate ligament transection
To model posttraumatic osteoarthritis in male C57BL/6N mice, anterior cruciate ligament (ACL) transection was performed as previously described.32 Sham surgery, where all surgical procedures were conducted except the ACL transection, was used as a control. Mice were housed with cagemates postsurgery, and hanging behavior was assessed between 12 and 13 weeks postsurgery, and then, percent hanging was normalized to age-matched mice that received sham surgery. Behavior was recorded overnight, and mice were filmed for 9 hours (Bonin lab).
2.2.6. Cyclophosphamide cystitis
Cyclophosphamide (CYP) was dissolved in physiological saline and was injected i.p. at 3 different doses (30, 100, or 300 mg/kg). Cyclophosphamide is converted to acrolein in the liver and collects in the bladder, causing painful cystitis and allodynia in the lower abdomen of mice.6 The mice were then placed individually in a second homecage for testing, which contained bedding, food, and water. Mice were filmed for 9 hours after injection. These experiments were conducted during the dark cycle, and mice were housed in a reverse light: dark cycle for one week before the experiment (Bonin lab).
2.2.7. Systemic lipopolysaccharide
Lipopolysaccharide (LPS) was dissolved in physiological saline and injected i.p. at 3 different doses (1.5, 15, and 150 µg/kg). Lipopolysaccharide is a proinflammatory mediator that causes sickness behavior in mice12 and causes generalized hyperalgesia.26 The mice were then individually placed in a second homecage for testing, which contained bedding, food, and water. Mice were filmed for 9 hours after injection. These experiments were conducted during the dark cycle, and mice were housed in a reverse light: dark cycle for one week before the experiment (Bonin lab).
2.3. Analgesic drugs
The following analgesic compounds were used in this study: ketoprofen (5 mg/kg), tramadol (30 mg/kg), gabapentin (10, 30, and 100 mg/kg) and (±)-trans-U-50,488H (10 and 25 mg/kg). Gabapentin was a gift from Yves De Koninck (Université Laval), and all other drugs were obtained from Millipore Sigma Chemical Co. (Toronto, Canada). Drugs were dissolved in physiological saline, and appropriate vehicle-treated groups were assessed simultaneously. All drugs were administered i.p. in a volume of 0.01 mL/g body weight.
2.4. Homecage hanging experiments
For homecage behavioural tests, mice were placed individually in a clean Allentown Micro-Vent cage (30 × 12 × 13 cm) with minimal bedding and were covered with a stainless steel cage lid that contained food and a water bottle. For all experiments, mice were tested individually. A camcorder (Sony CX405) was set up beside the cage allowing for an unobstructed side view. In addition, a background was placed behind the cage to create a contrast that enabled the optimal detection of the mice (CD-1: dark background, C57BL/6N: white background). The experimenter left the behavioral testing room during video recording. Videos were recorded in MPEG format and then converted to MP4 format (320 × 240) for analysis.
2.5. HangBox hanging experiments
A holding chamber, the “HangBox,” was created to encourage and allow an isolated analysis of the hanging behavior of mice. The HangBox was designed as a small, transparent Plexiglas chamber (15 × 15 × 12.5-cm height) enclosed with a modified stainless steel cage lid repurposed from the cage lids used on Allentown Micro-Vent cages. The steel lid was placed with the angle facing upwards to facilitate mouse interaction and the lid ends were fixed to the sides of the HangBox 7 cm above the floor, while the peak of the lid is suspended approximately 12 cm above the floor. Mice were placed in the box for one to 2 hours at a time without bedding, food, or other environmental enrichment. A camcorder (Sony CX405) was placed in front of the HangBox to record hanging. These experiments were conducted during the dark cycle and mice were housed in a reverse light: dark cycle for one week before the experiment (Bonin lab).
2.6. Video-based analysis of hanging and behavior
HomeCageScan (CleverSys Inc, Reston, VA) software program was used to quantify mouse behaviour in some experiments. The software program analyzes and quantifies 38 predetermined behaviors that were reclassified into 8 combined categories for statistical analysis in accordance with previous studies14 (Supplementary Table 1, available at http://links.lww.com/PAIN/B220). In other experiments, EthoVision XT 12 (Noldus Information Technology, Wageningen, the Netherlands) was used to quantify hanging behavior and distance traveled by each mouse throughout the experiment. Using EthoVision, hanging behavior was defined as when the center of a mouse's body was located in the designated “Hang Zone” approximately 3 cm from the stainless steel cage lid and no paws were in contact with the cage floor. This analysis approach was verified by comparing hanging behavior measured by automated scoring to that measured by manual scoring. Zone parameters were validated in pilot studies before proceeding with experiments. There was a significant correlation (r = 0.99, n = 101, P < 0.001) between hanging behavior scored from automated and manual methods. EthoVision was used for all experiments except the initial characterization of homecage behaviors after SNI and CFA using HomeCageScan (Mogil lab).
2.7. Automated detection of cage lid interaction
To automate the detection of mouse interaction with the cage lid, an Arduino-based device was developed to measure the capacitance of the cage lid. Mouse contact with the cage lid creates a spike in the measured capacitance with a sustained increase in the capacitance while mice are hanging. Cage lid capacitance was continually assessed at a rate of 4 kHz, digitized at 28-bit resolution, and output to a computer at a rate of 4 Hz. Up to 4 cages were recorded simultaneously from each device. For each mouse, all measured capacitance values were visualized as a histogram. The generated histograms typically had a bimodal distribution, with a predominant peak at lower values corresponding to baseline lid capacitance and a second peak corresponding to the combined capacitance of the lid and mouse. Time spent hanging was calculated as the area of the second peak over the total histogram area. The experiments using electronic assessment of CFA-impaired hanging were conducted using Tecniplast Green Line cages at TCP. The metal lids used in Green Line cages had a large net capacitance with high baseline variability, which prevented the detection of total hanging time using the histogram approach described above. For automated detection of cage lid interaction in Green Line cages, the capacitance data were high-pass filtered by subtracting a moving average of 500 samples to remove baseline variability. After filtering, a contact detection threshold was set at >3 root mean square (RMS) above baseline, and the frequency of contact events was quantified as a measure of cage lid interaction.
2.8. Statistical analyses
Statistical analyses were performed using GraphPad Prism v.8 or IBM SPSS Statistics for Windows v.20. In all figures, results are expressed as the mean ± SEM. In most experiments, results were analyzed statistically using one-way analysis of variance (ANOVA) to the saline/control group, followed by a post hoc Dunnett test. Analgesic reversal of formalin, CYP, and LPS depressed hanging behavior were analyzed statistically using one-way ANOVA to the noxious stimulus group, followed by a post hoc Dunnett test. Two-tailed t-tests or two-way ANOVA were used as appropriate. Group differences at the level of P < 0.05 were considered statistically significant.
3. Results
3.1. Hanging behavior is impaired in assays of pain
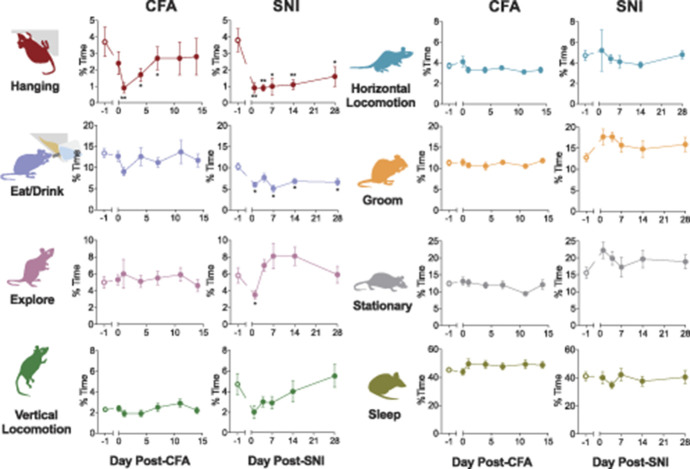
To identify novel behavioral correlates of pain in mice, we evaluated the effect of sustained pain on typical homecage behaviors. Naive, 8- to 12-week old, individually housed, male and female CD-1 mice were exposed to one of 2 commonly used pain assays: intraplantar injection of CFA and SNI. Intraplantar injection of CFA into the mouse hind paw was used to induce persistent inflammatory pain; in our hands and in this strain, mechanical allodynia resolves by approximately 14 days postinjection. SNI was used to induce chronic neuropathic pain in the mouse hind limb that in our hands does not resolve even by 3 months postsurgery. Mouse behavior was recorded for 24 hours in a homecage environment at baseline and several 24-hour periods after the injury. Automated video tracking and behavioral classification software program was used to determine the percent of time mice spent participating in 8 distinct behaviors: hanging, eating or drinking, exploring, vertical locomotion, horizontal locomotion, grooming, stationary or inactivity, and sleeping.
We found that CFA had a significant effect on hanging behavior, which was decreased by 73% from baseline one day after the injection (F(6,42) = 3.6, P = 0.005; Fig 1). Hanging behavior remained significantly reduced through day 7, with evidence of recovery by day 14. Complete Freund's adjuvant had no significant effect on the percent of time mice spent engaging in any other homecage behaviors on day one postinjection (sleep: F(6,49) = 1.4, P = 0.23; horizontal: F(6,49) = 2.0, P = 0.08; vertical: F(6,35) = 1.7, P = 0.15; stationary: F(6,49) = 1.0, P = 0.40; groom: F(6,49) = 0.80, P = 0.62; explore: F(6,42) = 1.1, P = 0.37; eating/drinking: F(6,49) = 1.9, P = 0.10). Although not significant, there was a trend towards decreased eating/drinking on day one postinjection.
Figure 1.
Hanging behavior is impaired in models of inflammatory and neuropathic pain. (A) The effect of injection of complete Freund's adjuvant (CFA) or spared nerve injury (SNI) on 8 distinct homecage behaviors of CD-1 mice was quantified using automated video scoring (CleverSys HomeCageScan). Data are shown as the percentage of time mice participated in each behavior over 24 hours (average ± SEM). Note that hanging behavior is reduced after both CFA and SNI, but only recovers to normal levels after CFA, not after SNI. *P < 0.05, **P < 0.01, ***P < 0.001 compared with baseline (−1).
An effect on hanging behavior was also found using the SNI assay, where hanging was decreased by 78% from baseline on day one after surgery (F(5,40) = 10.5, P < 0.001) and did not recover by day 28 (Fig. 1). Spared nerve injury also significantly reduced eating/drinking behavior by 42% (F(5,40) = 4.6, P =0.002; Fig. 1) and exploration by 40% (F(5,40) = 2.5, P =0.02; Fig. 1) one day postsurgery. Although exploration recovered back to baseline by day 28, eating/drinking remained significantly impaired. There was no significant difference in other homecage behaviors across the time course of the study (stationary: F(5,40) = 1.1, P = 0.39; grooming: F(5,40) = 1.1, P = 0.36; sleeping: F(5,40) = 0.60, P = 0.69; horizontal: F(5,40) = 0.30, P = 0.89, Fig. 1). Although not significant, there was a strong trend towards decreased vertical locomotion after SNI (vertical: F(5,40) = 2.4, P = 0.053). Collectively, these results suggest that hanging is the homecage behavior most reliably impacted by traditional persistent pain assays.
We next investigated whether effects on hanging behavior generalize to other preclinical pain assays, another hanging measurement technique, and another mouse strain. First, we tested whether assays of shorter-term pain in the hind paw (formalin and capsaicin) decrease hanging. In this experiment, we streamlined the analysis process by selectively measuring hanging behavior using Ethovision (Noldus) tracking software (see Methods for details). We found that injection of formalin (1%, 10 µL) or capsaicin (0.5%, 5 µL) reduced hanging behavior by 78% and 67%, respectively, compared with baseline hanging (formalin: t(11) = 15.7, P < 0.001, n = 12; capsaicin: t(7) = 4.97, P =0.002, n = 8; Fig. 2A). We additionally assessed the impact of osteoarthritis on hanging behavior using the ACL transection model. At 12 weeks postsurgery, we observed a 61% reduction in hanging compared with age-matched mice that received a sham surgery (t (7) = 4.43, P=0.003, n = 8; Fig. 2A). We further verified that SNI reduced hanging behavior in C57BL/6N mice compared with baseline activity (46% reduction; t(11) = 6.90, P < 0.001, n = 12, Fig. 2A), and confirmed that intraplantar CFA impaired hanging behavior in C57BL/6N mice (32% reduction; t(9) = 2.86, P=0.02, n = 10, Fig. 2A). These results indicated that hanging behavior is significantly decreased in pain assays targeting the hind limbs. Furthermore, the impairment in hanging observed after CFA or SNI is consistent across 2 mouse strains.
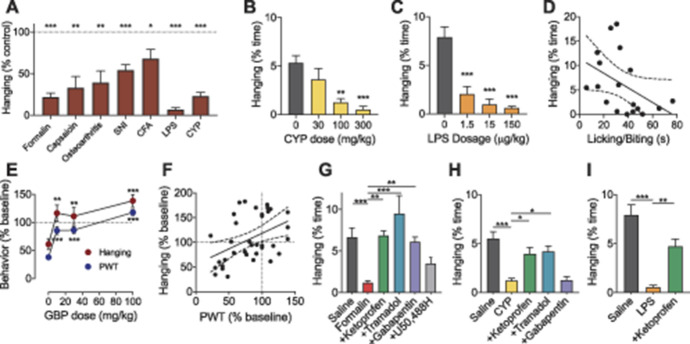
Figure 2.
Impairments in hanging behavior are noxious stimulus intensity-dependent and reversed by analgesics. (A) The effect of nociceptive assays targeting the plantar region or systemic circulation on hanging behavior of C57BL/6N mice. Values represent time hanging after different assays normalized to control (saline injection or sham surgery as appropriate); intraplantar formalin (1% in 10 µL); intraplantar capsaicin (0.5% in 5 µL); osteoarthritis induced by ACL transection; spared nerve injury (SNI); intraplantar CFA; lipopolysaccharide (LPS, 15 µg/kg); and cyclophosphamide (CYP, 100 mg/kg). Dose-dependent reductions in hanging behavior were observed after i.p. injection of (B) CYP (30, 100, and 300 mg/kg) and (C) LPS (1.5, 15, and 150 μg/kg). (D) Intraplantar injection of formalin (1%, 10 μL) induced nocifensive licking and biting behavior in C57BL/6N mice. The total amount of time spent licking or biting 45 minutes after injection was inversely correlated with the amount of time mice participated in hanging behavior (r = −0.44, P = 0.05). (E) Dose-dependent effect of gabapentin (10, 30, and 100 mg/kg, i.p., n = 9, crossover design) on hanging behavior or mechanical paw withdrawal threshold (PWT) assessed using the SUDO assay with von Frey filaments one week after SNI. Data are shown normalized to presurgery values. (F) The amount of time mice spent hanging and paw PWT relative to presurgical levels for all mice in graph E were correlated such that decreased time hanging was associated with lower PWT after SNI (n = 36, r = 0.39, P =0.02). (G–I) Analgesics reversed the reduction of hanging behavior seen in different pain models. (G) The effect of formalin (1%, 10 μL, intraplantar) on hanging was reversed with either ketoprofen (5 mg/kg, i.p.), tramadol (30 mg/kg, i.p.), or gabapentin (100 mg/kg), but not reversed with U50,488H (10 mg/kg, i.p.). (H) The effect of CYP (100 mg/kg, i.p.) on hanging was reversed by ketoprofen (5 mg/kg, i.p.) and tramadol (30 mg/kg, i.p.) but not gabapentin (100 mg/kg, i.p.). (I) The effect of LPS (15 µg/kg, i.p.) was reversed by ketoprofen (5 mg/kg, i.p.). Data represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with noxious stimulus group. CFA, complete Freund's adjuvant; ACL, anterior cruciate ligament.
Because targeting the hind limb may impede the physical ability of the mouse to hang, we next explored effects on hanging behavior using assays that do not target body parts involved in hanging. We began by investigating the effect of systemic inflammation on hanging behavior. To model systemic inflammation, we injected animals with LPS (15 µg/kg, i.p.). Lipopolysaccharide is a proinflammatory mediator that causes sickness behavior in mice12 and causes generalized hyperalgesia.26 We found that LPS significantly reduced the hanging behavior by 93% (t (11) = 38.5, P < 0.001, n = 12, Fig. 2A). Next, we used a bladder cystitis model, cyclophosphamide (CYP, 100 mg/kg, i.p.), to induce cystitis and allodynia in the lower abdomen of mice.6 Acute injection of CYP decreased hanging behavior by 77% (t(10) = 17.5, P < 0.001, n = 11, Fig. 2A). These data suggest that systemic models that do not target the hind paw also reduce hanging in mice.
3.2. Impairments in hanging behavior are dose-dependent and reversed by analgesics
Having demonstrated that hanging behavior is impaired in various pain assays, we next investigated whether the extent of impairment is correlated with the likely degree of pain experienced by mice, and whether it is reduced by analgesic treatments. We first examined whether pain intensity affected the degree to which hanging behavior was impaired. We administered CYP (30, 100, 300 mg/kg; i.p.) or LPS (1.5, 15, 150 µg/kg; i.p.) to mice and their behavior was recorded over 9 hours during their active period. For both CYP and LPS, hanging behavior decreased in a dose-dependent (and thus, presumably noxious stimulus intensity-dependent) manner (Figs. 2B and C). Although 30 mg/kg CYP had no effect on hanging behavior, 100 and 300 mg/kg CYP caused a 76% and 90% reduction in hanging behavior, respectively (F(3, 39) = 7.4, P < 0.001, CYP0: n = 21; CYP30: n = 7, P = 0.41; CYP100: n = 7, P < 0.006; CYP300: n = 8, P < 0.001, Fig. 2B). Even at a low dose, LPS (1.5 µg/kg) decreased hanging behavior by 73% (F(3, 33) = 16.4, P < 0.0001, LPS0: n = 15; LPS1.5: n = 8, P < 0.001, Fig. 2C). Higher doses of LPS, 15 µg/kg and 150 µg/kg, almost completely depressed hanging with decreases of 87% and 92%, respectively (LPS15: n = 6, P < 0.0001; LPS150: n = 8, P < 0.0001, Fig. 2C).
We further tested the correlation between impaired hanging and pain behavior using the formalin assay, measuring the time spent licking or biting the hind paw after injection of dilute formalin solution into a rodent's paw.44 After intraplantar formalin administration (1%, 10 µL), both hind paw licking/biting and hanging behaviors were recorded over one hour. We found a significant negative correlation between hanging behavior and licking/biting behavior (r = −0.44, n = 18, P = 0.05, Fig. 2D).
We next assessed whether the analgesic gabapentin dose-dependently restores hanging behavior after SNI in a manner correlated with hypersensitive withdrawal responses from von Frey tests in mice. This experiment used a crossover design, with a 72-hour washout period between injections. Spared nerve injury robustly decreased hanging behavior and mechanical paw withdrawal thresholds one week after surgery (Fig. 2E). The impairment of both hanging and withdrawal threshold were significantly reversed by gabapentin at doses from 10 to 100 mg/kg compared with saline treatment (hanging: F(3,24) = 9.2, P < 0.0001; paw withdrawal: F(3,24) = 36.8, P < 0.0001, Fig. 2E). This result also demonstrates strong parallels of both paw-withdrawal threshold and hanging in response to gabapentin dose. Notably, the time spent hanging correlated significantly with the withdrawal threshold, indicating that increased mechanical allodynia was associated with decreased hanging (r = 0.45, P=0.005, n = 36, Fig. 2F).
Next, we asked whether the effects of formalin on hanging behavior can be reversed by analgesics. We administered formalin (1%, 10 µL, intraplantar) to mice pretreated with either conventional analgesics such as ketoprofen (5 mg/kg, i.p.), tramadol (30 mg/kg, i.p.), gabapentin (100 mg/kg, i.p.), or the selective kappa opioid agonist U50,488H (10 mg/kg, i.p.). Centrally acting kappa opioid agonists generally induce analgesia in preclinical pain assays but have failed to show efficacy as analgesics in clinical trials.24,36 Previous studies showed that U50,488H dose-dependently produced analgesia in the mouse formalin assay,46 tail-withdrawal assay,27 and acetic acid writhing assay,3 but failed to reverse acid-induced conditioned place aversion in mice.3 We found that ketoprofen, tramadol, and gabapentin reversed the effect of formalin on hanging behavior. However, U50,488H did not significantly reverse formalin-induced depression of hanging behavior (F(5, 87) = 7.6, P < 0.001, formalin: n = 21; vehicle: n = 23, P < 0.001; +ketoprofen: n = 12, P = 0.001; +tramadol: n = 12, P < 0.001; +gabapentin: n = 13, P = 0.005; +U50,488H: n = 10, P = 0.50, Fig. 2G). Notably, administration of these analgesics alone had no significant effect on the time mice spent engaging in hanging behavior (F(4,61) = 0.7, P = 0.57) (Fig. S1A, available at http://links.lww.com/PAIN/B220), indicating the analgesics do not cause sedation or motor impairment at the doses used. We further confirmed that U50,488H at both 10 mg/kg and 25 mg/kg significantly reduced the total time mice spent licking the formalin-injected hind paw (1%, 10 µL) (Fig. S1B, available at http://links.lww.com/PAIN/B220), consistent with the analgesic effects of kappa-opioid agonists seen in reflexive measures of pain but not observed in more complex behavioural measures indicative of a pain “state.”33 This suggests that pain likely drives the formalin-induced impairment in hanging, and hanging behavior is a translationally relevant outcome measure of pain in mice.
We additionally tested whether analgesics could also reverse the effect of systemic inflammation on hanging behavior. Mice were coadministered CYP (100 mg/kg, i.p.) with ketoprofen (5 mg/kg), tramadol (30 mg/kg), or gabapentin (100 mg/kg). We found that ketoprofen and tramadol, but not gabapentin, reversed the effect of CYP on hanging behavior (F(4, 63) = 9.5, P < 0.001, CYP: n = 11; vehicle: n = 21, P < 0.001; +ketoprofen: n = 12, P = 0.03; +tramadol: n = 12, P = 0.01; +gabapentin: n = 12, P > 0.99, Fig. 2H). Our present findings are consistent with previous studies that show that gabapentin does not affect CYP-induced pain-related behaviors. Finally, we coadministered LPS (15 µg/kg; i.p.) with ketoprofen (5 mg/kg). We found that ketoprofen reverses the effect of LPS on hanging behavior (F(2, 36) = 21.2 P < 0.001, LPS: n = 12; vehicle: n = 15, P < 0.001; +ketoprofen: n = 12, P = 0.002, Fig. 2I). Specifically, our results with analgesics suggest that changes in hanging behavior are driven predominantly by pain in mice and thus, these impairments can act as a sensitive measure of pain.
3.3. Hanging behavior varies with age and strain
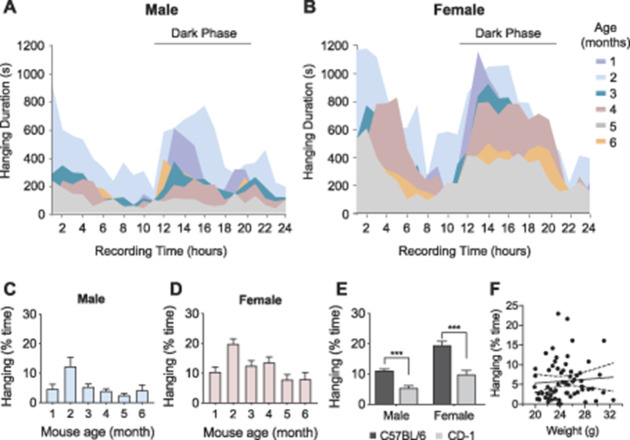
We next examined the ethological basis of hanging by evaluating the behavior for 5 months in pain-naive male and female C57BL/6N mice, starting when the mice were one month old. Mice were assessed for 24 hours once a month and returned to their homecages between testing sessions. In all groups of mice, hanging behavior was greatest during the dark phase and when mice were first introduced into the observation cage (Figs. 3A and B). Mice tend to be more active during the dark phase and have a natural drive to explore a novel cage environment, which is consistent with our results.4,18 To investigate the effects of age and sex on hanging behavior, we performed a two-way ANOVA on hanging behavior from mice aged 2, 4, and 6 months with age and sex as between-subject factors. We observed a main effect of age (F(5, 84) = 9.9, P < 0.0001) and sex (F(1, 84) = 42.6, P < 0.0001) but no interaction of age and sex (F(5, 84) = 0.7, P = 0.65, Figs. 3C and D). Overall, females showed significantly more hanging behavior than males, and mice exhibited the most hanging behavior at 2 months old.
Figure 3.
Hanging behavior is dependent on circadian and physiological factors of age, sex, and strain. (A) Hanging behavior in 1- to 6-month-old male (n = 8) and (B) female (n = 8) C57BL/6N mice over a 24-hour period. Peak hanging periods occurred upon introduction of mouse to the testing cage and during the dark phase. (C) Hanging behavior of male (n = 8) and (D) female (n = 8) C57BL/6N mice expressed as total percentage of time of a 24-hr period at different ages. Two-month-old mice show significantly higher hanging behavior than older and younger mice in both males and females. (E) The effect of sex and strain on hanging behavior. C57BL/6N mice (n = 26 male, 26 female) showed significantly more hanging behavior than CD-1 mice (n = 17 male, 17 female). Female mice showed more hanging than age-matched male mice regardless of strain. (F) Hanging behavior of 2-month-old naive male C57BL/6N mice (n = 64) did not correlate with the weight of mice. Data represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 as indicated.
Because previous studies have shown that strains of mice differ in behavior, including pain-related behavior,31 we next performed a series of experiments investigating the effects of strain on hanging. We performed a two-way ANOVA with strain and sex as between-subject factors. Because we had previously observed robust hanging behavior in two-month-old mice, we used this age group exclusively for the present experiment. We observed a main effect of strain (F(1, 82) = 33.9, P < 0.0001) and sex (F(1, 82) = 49.4, P < 0.0001) but no sex-by-strain interaction (F(1, 82) = 3.1, P = 0.08, Fig. 3E). As in our previous experiment, female mice showed significantly more hanging behavior than males (Fig. 3E). Furthermore, we observed that hanging behavior was greater in C57BL/6N mice than in CD-1 mice (Fig. 3E).
These results indicate that hanging behavior varies with age, sex, and strain. However, because these 3 variables are associated with weight differences, our findings might also be explained by differential weight. We investigated the effect of weight on hanging behavior by analyzing the combined data from all the naive 2-month-old male C57BL/6N mice across our experimental groups. We found no correlation between the weight of the mouse and hanging behavior in this group of animals (r = 0.07, P = 0.28, n = 65, Fig. 3F).
3.4. Development of automated approaches for assessing hanging behavior
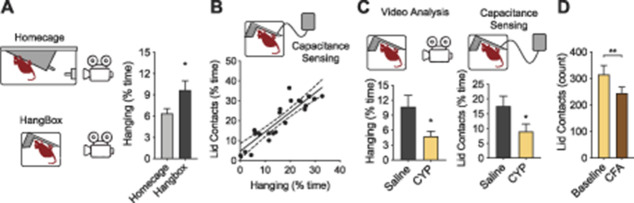
In our previous experiments, we measured hanging behavior in a standard homecage environment through automated video scoring (HomeCageScan and EthoVision). However, a homecage environment is a more complex setting where multiple behaviors may compete with hanging behavior. We reasoned that a specialized testing environment designed to encourage hanging might increase the frequency of behavior and facilitate observation of the behavior of interest. Accordingly, we developed a specialized testing apparatus, the HangBox, to measure hanging behavior. The HangBox, measuring 15 × 15 × 12.5-cm height, is considerably smaller than a typical mouse homecage. The HangBox contains a slanted grid for hanging and does not include any other objects or bedding, which could encourage other behaviors such as burrowing. Using our automated video scoring method, we compared hanging behavior in the HangBox to that in the homecage environment. As we predicted, hanging behavior was significantly more frequent when using the HangBox, increasing by 52% (homecage: n = 36; HangBox: n = 24, t (58) = 2.5, P = 0.02, Fig. 4A).
Figure 4.
Hanging behavior can by isolated as a measure of pain using a dedicated hanging environment and automated detection strategies. (A) Hanging behavior was assessed using a normal homecage and a dedicated hanging assay comprised of a small box with an angled metal grid (HangBox). Male C57BL/6N mice spent significantly more time engaged in hanging in the HangBox (n = 24) compared with the homecage (n = 36). (B) Lid contact in the HangBox was detected using a capacitance sensing device for automatic assessment of hanging behavior. Automated detection of the amount of time mice were in contact with the metal lid through capacitance detection strongly correlated with amount of time mice were engaged in hanging as assessed by video scoring (r = 0.90, n = 24). (C) The effect of cyclophosphamide (CYP) on hanging behavior of male C57BL/6N mice in the HangBox measured by both EthoVision and the capacitance sensing device. CYP (n = 12) decreased hanging in the HangBox assay compared with saline injected mice (n = 12) when hanging behavior was assessed by either video (EthoVision) or capacitance sensing. (D) Intraplantar CFA (n = 35) significantly reduced cage lid interaction frequency as measured by capacitance sensing one day after injection (compared with baseline). Data represented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with saline/baseline. CFA, complete Freund's adjuvant.
The current approach to quantifying hanging behavior requires video recordings and analysis of each cage, which can be labor-intensive and costly in terms of analysis time and data storage requirements. To increase the throughput of hanging behavior measurement, we next developed an electronic device that could automatically quantify hanging behavior within the mouse cage. Because hanging involves the mouse contacting the metal cage lid, our device can detect hanging by electronically measuring changes in the cage lid capacitance associated with mouse contact. Using the HangBox, we compared the duration of hanging assessed electronically with the duration of hanging assessed from video scoring in two-month-old male C57BL/6N mice. Mice received either systemic saline (n = 12) or CYP to model cystitis (n = 12). Notably, the lid contact time measured by capacitance includes both contact during rearing and sniffing as well as actual hanging, making the contact time by capacitance larger than the hanging time alone measured by video analysis. Nevertheless, we observed a significant correlation between cage lid interaction time measured electronically and by direct video analysis (r = 0.90, P < 0.0001, n = 24, Fig. 4B). We then used the capacitance sensing device to quantify hanging behavior over 2 hours (with the HangBox) and observed a robust decrease in hanging after CYP when assessed using both automated video scoring (t(22) = 2.4, P = 0.03, Fig. 4C) and electronic assessment (t(22) = 2.1, P = 0.047, Fig. 4C).
Finally, we used the capacitance sensing device to measure hanging behavior in a homecage environment one day after intraplantar administration of CFA (Fig. 4D). 16-week-old C57BL/6N mice were placed in a clean homecage for one hour after injection during which cage lid capacitance was continuously measured. We also assessed thermal and mechanical withdrawal thresholds before and after injection using the Hargreaves and von Frey tests, respectively (n = 35). Intraplantar CFA reduced cage lid interaction from baseline (t(34) = 3.0, P = 0.005), as well as reduced latency to withdraw from thermal stimuli (44% reduction, t(34) = 8.9, P < 0.0001) and reduced mechanical paw withdrawal threshold (43% reduction, t(34) = 10.8, P < 0.0001). Notably, the change in lid interaction after CFA significantly correlated with both the changes in the Hargreaves (r = 0.40, P = 0.02) and von Frey (r = 0.36, P = 0.02) tests.
4. Discussion
Our study aimed to identify changes in spontaneous behaviors associated with pain in mice that could be measured in natural homecage environments. Conducting an unbiased, prospective analysis of mouse behavior, we determined that cage-lid hanging is the only homecage behavior that is reliably impacted by multiple assays featuring sustained pain. Notably, the impairment in hanging was noxious stimulus intensity-dependent and could be reversed by analgesics, indicating that this behavior has broad suitability as an outcome measure in preclinical pain research and analgesic development. In our characterization of cage-lid hanging behavior in pain-free mice, we observed that cage-lid hanging varies with the age, sex, and strain of the mouse. To facilitate the automated measurement of hanging behavior, we developed the “HangBox,” a standardized arena that increases the incidence and facilitates observation of hanging behavior. We also created a capacitance-sensing device that can automatically quantify hanging without the use of video recording. Of course, one of the main advantages of hanging as a pain outcome measure is that the behavior can be assessed without the stress of removing rodents from their homecage for testing. Use of the HangBox obviates this advantage, in exchange for streamlining assessment of hanging. Thus, we expect that users will adopt the version that best suits their experimental purposes.
To see whether impairment of cage-lid hanging behavior in mice is generalizable across different types of pain, we measured the behavior in multiple assays. In 5 common assays that target the hind limb (formalin, capsaicin, ACL transection, CFA, and SNI), we found that hanging behavior was significantly impaired (by as much as 76%). We also found that cage-lid hanging behavior was reduced in assays that did not target the hind limb, including a systemic inflammation assay (LPS) and a model of bladder cystitis (CYP). Thus, our data suggest that it is pain per se, not a physical inability to hang, that causes the reduction in cage-lid hanging. This is further supported by evidence that the degree of hanging impairment was stimulus intensity-dependent and reversed by conventional analgesics. Of note, our data showing that gabapentin reversed the effect of formalin but not the effect of CYP on cage-lid hanging behavior are consistent with previous studies showing a lack of gabapentin analgesia in the CYP model.45 Moreover, we found that the kappa-opioid agonist U50,488H failed to reverse formalin-impaired hanging despite reducing formalin-induced hind paw licking. This is consistent with observations that kappa-opioid agonists are antinociceptive in reflexive or spontaneous pain measures but fail to restore pain-depressed unconditioned behaviours such as nesting that may have more clinical relevance.34 As a result of this analgesic profile, kappa agonists have been suggested to be tested as a negative control in the development of new translational pain assays, given the largely failed efforts to develop this class of drugs into clinically viable analgesics.33 These findings therefore indicate that the clinical effects of analgesics can be recapitulated in the cage-lid hanging assay, and further suggest this assay may be of use for analgesic drug screening. Collectively, our results indicate that an impairment in cage-lid hanging behavior can act as a translationally relevant and sensitive, if indirect, measure of ongoing pain in mice.
The perception of pain in humans is complex and varies with factors such as age and sex/gender. We found that younger mice exhibited increased cage-lid hanging behavior, with the peak of hanging behavior occurring at 2 months of age. The age-related decline in hanging may be a function of the decreased overall activity and exploratory drive associated with aging.22,25 In addition, we found that C57BL/6N mice hang significantly more than CD-1 mice. Strain-specific differences in cage-lid hanging are not surprising because robust strain differences in other complex behaviors, including those related to pain responsiveness, have been previously reported.31 It is advantageous that C57BL/6N mice exhibit high cage-lid hanging behavior because this strain is used as the genetic background of many extant transgenic mouse strains.
It is unclear what physiological or neurological processes regulate hanging behavior, and likewise, what processes are altered by pain to reduce hanging. Further research into the physiological factors driving hanging behavior is necessary to understand their relationship to nociceptive processing.
Our goal was to identify a novel, spontaneous, and translationally relevant pain outcome measure that could be measured in a natural homecage environment without direct experimenter involvement. Current preclinical pain research often uses experimenter-evoked pain outcome measures, and experiments are conducted under highly artificial and potentially stressful conditions.15,21,28,29,35,41 These factors, along with difficulty in measuring rodent behaviors relevant to the human pain experience,9,30 significantly limit the clinical relevance, and thus the ultimate translatability, of preclinical pain research. Cage-lid hanging behavior may represent a novel pain outcome measure that circumvents many of these problems. Cage-lid hanging can be assessed within a homecage environment, obviating the need to remove the mouse from their homecage and be subjected to handling and novelty stress. Furthermore, Cage-lid hanging is a spontaneous behavior in which mice engage voluntarily and can be assessed objectively and remotely. Hanging behavior can also be measured through wholly automated scoring, which minimizes subjectivity. Finally, hanging may be more aligned than other common outcome measures with the clinical reality of chronic pain, given that the behavior may reflect a mouse's internal emotional and/or motivational state.1,5,19,20 Overall, we believe cage-lid hanging is a translationally relevant pain-associated behavior that can be assessed with minimal stress and experimenter interaction and is ideal for cases where high-throughput testing is required.
Our observation that the depression of cage-lid hanging is a behavioral measure of pain was replicated across 4 different laboratories, demonstrating the robustness and consistency of this novel pain outcome measure. The tools we have developed, including the HangBox and the capacitance-sensing device that automates hanging quantification, can greatly facilitate the assessment of hanging behavior. It is possible that evaluating changes in cage-lid hanging could be useful in other rodent models of disease, such as sensory disorders, motor disorders (such as Huntington disease and ALS), cancer, and diabetes. Overall, we have identified and characterized a novel, ethologically valid pain outcome measure that may improve the translational potential of preclinical pain research and the development of new analgesic drugs.
Conflict of interest statement
The authors have no conflicts of interest to declare. R.P. Bonin, I. Lecker, A.J.D'Souza, D. Dubins, and J.S. Mogil have a patent US20180007862A1 pending related to this work.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B220.
Acknowledgments
The authors thank Graziella Molska for technical assistance, Josiane Mapplebeck for editorial assistance, and Maya Selitser for assistance with video analysis. This research was supported by funding from CIHR (Foundation Grant 154281 to JSM), the University of Toronto Connaught Fund (RPB), Natural Sciences and Engineering Research Council of Canada (RGPIN-2016-05538 to RPB, PDF to IL), the ACLAM Foundation (C.Cho, R.P. Bonin), the National Institutes of Health NIH 3UM1OD023221-07S1 (TCP), the University of Toronto Centre for the Study of Pain (M. Zain, R.P. Bonin), the Leslie Dan Faculty of Pharmacy (R.P. Bonin), and Blue Mountain Botanical Medicines Inc. (R.P. Bonin).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
H. Zhang, I. Lecker, J. Mogil, and R. Bonin contributed equally to this study.
Contributor Information
Hantao Zhang, Email: hantao.zhang@mail.utoronto.ca.
Irene Lecker, Email: irene.lecker@utoronto.ca.
Chereen Collymore, Email: chereen.collymore@uottawa.ca.
Anastassia Dokova, Email: afdokova@unc.edu.
Maian Christine Pham, Email: maian.pham@mail.utoronto.ca.
Sarah F. Rosen, Email: srosen625@gmail.com.
Hayley Crawhall-Duk, Email: hayley.crawhall-duk@umontreal.ca.
Maham Zain, Email: maham.zain@mail.utoronto.ca.
Megan Valencia, Email: megan.valencia@mail.utoronto.ca.
Helena Fetter Filippini, Email: helena.fetterfilippini@utoronto.ca.
Jerry Li, Email: jerbear.li@mail.utoronto.ca.
Chulmin Cho, Email: chulmin.cho@utoronto.ca.
Vassilia Michailidis, Email: vassilia.michailidis@mail.utoronto.ca.
Paul D. Whissell, Email: abigail.dsouza@mail.utoronto.ca.
Ingita Patel, Email: ingita.patel@mail.utoronto.ca.
Hendrik W. Steenland, Email: wsneurotek@gmail.com.
Wai-Jane Virginia Lee, Email: wlee2021@dents.uwo.ca.
Massieh Moayedi, Email: m.moayedi@utoronto.ca.
Toni-Lee Sterley, Email: tonilee.sterley@ucalgary.ca.
Jaideep S. Bains, Email: jsbains@ucalgary.ca.
Jo Anne Stratton, Email: jo.stratton@mcgill.ca.
John R. Matyas, Email: jmatyas@ucalgary.ca.
Jeff Biernaskie, Email: jeff.biernaskie@ucalgary.ca.
David Dubins, Email: d.dubins@UTORONTO.CA.
Igor Vukobradovic, Email: igor@lunenfeld.ca.
Alexandr Bezginov, Email: alex.bezginov@phenogenomics.ca.
Ann M. Flenniken, Email: flenniken@lunenfeld.ca.
Loren J. Martin, Email: lj.martin@utoronto.ca.
Jeffrey S. Mogil, Email: jeffrey.mogil@mcgill.ca.
References
- [1].Arras M, Rettich A, Cinelli P, Kasermann HP, Burki K. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 2007;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain 2004;5:491–7. [DOI] [PubMed] [Google Scholar]
- [3].Bagdas D, Muldoon PP, AlSharari S, Carroll FI, Negus SS, Damaj MI. Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology 2016;102:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bains RS, Wells S, Sillito RR, Armstrong JD, Cater HL, Banks G, Nolan PM. Assessing mouse behaviour throughout the light/dark cycle using automated in-cage analysis tools. J Neurosci Methods 2018;300:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, Keeling LJ, Winckler C, Forkman B, Dimitrov I, Langbein J, Bakken M, Veissier I, Aubert A. Assessment of positive emotions in animals to improve their welfare. Physiol Behav 2007;92:375–97. [DOI] [PubMed] [Google Scholar]
- [6].Bon K, Lichtensteiger CA, Wilson SG, Mogil J. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol 2003;170:1008–12. [DOI] [PubMed] [Google Scholar]
- [7].Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain 2014;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- [9].Crofford LJ. Chronic pain: where the body meets the brain. Trans Am Clin Climatol Assoc 2015;126:167–83. [PMC free article] [PubMed] [Google Scholar]
- [10].Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 2017;10:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Duenas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016;9:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fink MP. Animal models of sepsis. Virulence 2014;5:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fortier MA, Chou J, Maurer EL, Kain ZN. Acute to chronic postoperative pain in children: preliminary findings. J Pediatr Surg 2011;46:1700–5. [DOI] [PubMed] [Google Scholar]
- [14].Fuzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 2016;7:11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gonder JC, Laber K. A renewed look at laboratory rodent housing and management. ILAR J 2007;48:29–36. [DOI] [PubMed] [Google Scholar]
- [16].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. PAIN 1988;32:77–88. [DOI] [PubMed] [Google Scholar]
- [17].Hirst JA, Howick J, Aronson JK, Roberts N, Perera R, Koshiaris C, Heneghan C. The need for randomization in animal trials: an overview of systematic reviews. PLoS One 2014;9:e98856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hughes RN. Intrinsic exploration in animals: motives and measurement. Behav Process. 1997;41:213–26. [DOI] [PubMed] [Google Scholar]
- [19].Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 2014;234:139–46. [DOI] [PubMed] [Google Scholar]
- [20].Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci 2010;4:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klinck MP, Mogil JS, Moreau M, Lascelles BDX, Flecknell PA, Poitte T, Troncy E. Translational pain assessment: could natural animal models be the missing link? PAIN 2017;158:1633–46. [DOI] [PubMed] [Google Scholar]
- [22].Lalonde R, Badescu R. Exploratory drive, frontal lobe function and adipsia in aging. Gerontology 1995;41:134–44. [DOI] [PubMed] [Google Scholar]
- [23].Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010;7:447–9. [DOI] [PubMed] [Google Scholar]
- [24].Lazenka ML, Moerke MJ, Townsend EA, Freeman KB, Carroll FI, Negus SS. Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats. Psychopharmacology (Berl) 2018;235:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Macri S, Adriani W, Chiarotti F, Laviola G. Risk taking exploration of a plus-maze is greater in adolescent than in a juvenile or adult mice. Anim Behav 2002;64:541–6. [Google Scholar]
- [26].Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res 1993;623:321–4. [DOI] [PubMed] [Google Scholar]
- [27].McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology 2006;31:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci 2009;10:283–94. [DOI] [PubMed] [Google Scholar]
- [29].Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals?. PAIN 2004;112:12–15. [DOI] [PubMed] [Google Scholar]
- [30].Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. PAIN 2010;151:12–17. [DOI] [PubMed] [Google Scholar]
- [31].Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. PAIN 1999;80:67–82. [DOI] [PubMed] [Google Scholar]
- [32].Mousseau M, Burma NE, Lee KY, Leduc-Pessah H, Kwok CHT, Reid AR, O'Brien M, Sagalajev B, Stratton JA, Patrick N, Stemkowski PL, Biernaskie J, Zamponi GW, Salo P, McDougall JJ, Prescott SA, Matyas JR, Trang T. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv 2018;4:eaas9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Negus SS. Core outcome measures in preclinical assessment of candidate analgesics. Pharmacol Rev 2019;71:225–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. PAIN 2015;156:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Otis C, Gervais J, Guillot M, Gervais JA, Gauvin D, Pethel C, Authier S, Dansereau MA, Sarret P, Martel-Pelletier J, Pelletier JP, Beaudry F, Troncy E. Concurrent validity of different functional and neuroproteomic pain assessment methods in the rat osteoarthritis monosodium iodoacetate (MIA) model. Arthritis Res Ther 2016;18:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol 1996;19:92–7. [DOI] [PubMed] [Google Scholar]
- [37].Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Preclinical Pain C, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. PAIN 2008;139:243–7. [DOI] [PubMed] [Google Scholar]
- [38].Richardson CA. The power of automated behavioural homecage technologies in characterizing disease progression in laboratory mice: a review. Appl Anim Behav Sci 2015;163:19–27. [Google Scholar]
- [39].Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, Scoffings D, Phillips A, Guo J, Laing RJ, Abdi S, Decosterd I, Woolf CJ. A novel tool for the assessment of pain: validation in low back pain. PLoS Med 2009;6:e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shields SD, Eckert WA, III, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain 2003;4:465–70. [DOI] [PubMed] [Google Scholar]
- [41].Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods 2014;11:629–32. [DOI] [PubMed] [Google Scholar]
- [42].Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 2011;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents—challenges and opportunities. Eur J Neurosci 2014;39:1881–90. [DOI] [PubMed] [Google Scholar]
- [44].Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. PAIN 1992;51:5–17. [DOI] [PubMed] [Google Scholar]
- [45].Wantuch C, Piesla M, Leventhal L. Pharmacological validation of a model of cystitis pain in the mouse. Neurosci Lett 2007;421:250–2. [DOI] [PubMed] [Google Scholar]
- [46].Wettstein JG, Grouhel A. Opioid antagonist profile of SC nor-binaltorphimine in the formalin paw assay. Pharmacol Biochem Behav 1996;53:411–16. [DOI] [PubMed] [Google Scholar]
- [47].Whittaker AL, Howarth GS. Use of spontaneous behaviour measures to assess pain in laboratory rats and mice: how are we progressing? Appl Anim Behav Sci 2014;151:1–12. [Google Scholar]
- [48].Yezierski RP, Hansson P. Inflammatory and neuropathic pain from bench to bedside: what went wrong? J Pain 2018;19:571–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B220.