Supplemental Digital Content is Available in the Text.
Imagining pressure pain to be self-induced led to increased pressure pain thresholds. Such sensory attenuation of pain was also seen in actual self-induced pressure.
Keywords: Sensory attenuation, Self-inflicted pain, Imagery, Efference copy, Forward models, Pain thresholds
Abstract
During self-induced pain, a copy of the motor information from the body's own movement may help predict the painful sensation and cause downregulation of pain. This phenomenon, called sensory attenuation, enables the distinction between self-produced stimuli vs stimuli produced by others. Sensory attenuation has been shown to occur also during imagined self-produced movements, but this has not been investigated for painful sensations. In the current study, the pressure pain thresholds of 40 healthy participants aged 18 to 35 years were assessed when pain was induced by the experimenter (other), by themselves (self), or by the experimenter while imagining the pressure to be self-induced (imagery). The pressure pain was induced on the participants left lower thigh (quadriceps femoris) using a handheld algometer. Significant differences were found between all conditions: other and self (P < 0.001), other and imagery (P < 0.001), and self and imagery (P = 0.004). The mean pressure pain threshold for other was 521.49 kPa (SE = 38.48), for self 729.57 kPa (SE = 32.32), and for imagery 618.88 kPa (SE = 26.67). Thus, sensory attenuation did occur both in the self condition and the imagery condition. The results of this study may have clinical relevance for understanding the mechanisms involved in the elevated pain thresholds seen in patients with self-injury behavior and the low pain thresholds seen in patients with chronic pain conditions. Imagery of sensory attenuation might also be used to alleviate the pain experience for patients undergoing procedural pain.
1. Introduction
Self-generated touch is perceived as less intense compared with touch by someone else.20 This phenomenon, known as sensory attenuation, has been observed in response to both nonpainful1,6,19–22 and painful pressure8,39 to the body.
In terms of neural mechanisms, self-generated touch has been shown to activate the secondary somatosensory cortex and the cerebellum to a lesser extent than externally generated touch,5,19 and the functional connectivity between cerebellum and somatosensory cortex was shown to correlate with the participants' attenuation at the perceptual level.19 In terms of computational mechanisms, sensory attenuation is believed to occur when a copy of the motor command (ie, an efference copy) allows the brain to predict and attenuate the somatosensory consequences of the self-generated movement.19 The prediction and downregulation of the intensity of self-generated movements is considered to be one of the reasons as to why it is difficult to tickle oneself.4 Sensory attenuation enables humans to differentiate between sensations that result from self-generated movements and those that result from external events, which is an important ability for the detection of potentially threatening external stimuli.4
Humans have a unique ability to imagine a wide range of situations, emotions, and actions. Imagining a movement of the body can increase the physical arousal (eg, heart rate and respiration) with levels comparable to the arousal caused by actual movements,11 and motor areas in the human brain are activated by imaginary movements.13 A previous study has shown that humans can imagine that externally generated touch is generated by themselves if the imagined self-touch matches the external stimulus in space and time.18 The imagined self-touch reduced intensity of the tactile sensations with magnitudes comparable to those of actual self-generated touch.18 Hence, the mental act of imagining that external touch is self-produced can attenuate somatosensory signaling. So far, these effects have not been tested in the context of painful stimuli.
The aim of this study was to test whether externally induced pressure pain would be attenuated if the participant imagined it to be self-induced. Pressure pain thresholds were assessed in 3 different conditions: (1) pressure applied by the experimenter (other), (2) pressure applied by the participants themselves (self), and (3) pressure applied by the experimenter while the participants imagined, with their eyes closed, that they themselves were applying the pressure (imagery). Our prespecified hypotheses were: (H1) pain thresholds will be higher when participants are applying pressure to themselves compared to pressure applied by the experimenter (self > other); and (H2) pain thresholds will be higher when the experimenter is applying the pressure but participants are imagining that they are applying the pressure themselves, compared to the condition where the experimenter is applying the pressure without imagery (imagery > other).
2. Methods
The study was approved by the Regional Ethical Review Board in Stockholm (2018/1367-31/1 and 2019-03076). The analysis plan was preregistered on the Open Science Framework August 9, 2019 (osf.io/ra9ug) before the data set was opened.
2.1. Study participants
Participants were recruited through advertisements at universities in Stockholm, Sweden, and on recruitment websites for studies (www.studentkaninen.se and https://ki-behavioraltesting.sona-systems.com). Inclusion criteria required that participants were: (1) aged 18 to 35 years, and (2) in good health. The narrow age range was chosen to decrease the risk of an age effect because lower pressure pain thresholds have been shown in older adults.36 The power calculation was based on pilot testing of the self and other conditions where a medium effect size between conditions (Cohen's d = 0.66) was found. A sample size of 36 participants would render 90% power (α = 0.05) to detect a difference between self and other. To account for unforeseen data loss, the sample size was set to a minimum of 40 participants. The participants took part in another pain experiment after this experiment33 (total time approximately 60 minutes) and they received 2 cinema tickets for their participation. The participants were asked to withhold from using any drugs and/or as-needed medications that could affect their pain perception (eg, analgesics, sleep medication, and tranquilizers) 24 hours before the visit.
2.2. Equipment
A handheld pressure algometer (Somedic Algometer version II, Hörby, Sweden) was used to induce pain and measure pressure pain thresholds in kilopascal (kPa). A second identical algometer was used to simulate self-produced pressure. When an algometer is pressed against a surface, it records the pressure and stops recording as soon as it is lifted from the surface. A digital display shows the highest applied pressure during the event. The algometers had a 1-cm2 round rubber tip, and the pressure slope was set to indicate when an even pressure of 50 kPa/s was applied. The Somedic algometers have shown excellent interrater and intrarater reliability.31 The same algometer was used to apply and assess the pressure during other (Fig. 1A), self (Fig. 1B), and imagery (Fig. 1C) (see description of procedure further below). During imagery, the participants held the identical algometer, but this algometer did not record any pressures used for the assessments. The 2 algometers were firmly attached to each other with self-adhesive plastic velcro. A 5-cm vertical offset between the algometers was used to ensure only one of the algometers was in contact with the participant's leg. The probes of the algometers were placed 3.7 cm apart. The proximity of the 2 pressed-together algometers ensured that the imagined pressure and the executed pressure from the experimenter's algometer were close enough to enable attenuation.20
Figure 1.
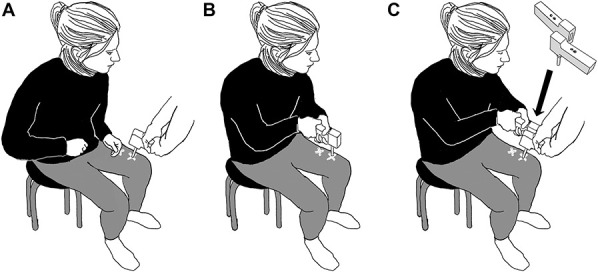
(A–C) The illustrations show a participant during the different conditions of the experiment. (A) The pressure is applied by the experimenter (other), (B) the pressure is applied by the participant (self), and (C) the pressure is applied by the experimenter while the participants imagine that they are applying the pressure (imagery).
2.3. Measures
The Pain Catastrophizing Scale (PCS) was used to assess whether catastrophic thinking was associated with low pressure pain thresholds during externally induced pain (other). This association has previously been found in a clinical pain population,30 and we were interested to assess whether it could be found also in our healthy participants. The PCS consists of 13 items rated on a 5-point scale between the endpoints (0) not at all and (4) all the time. The Swedish translation of the PCS has shown excellent internal consistency (α = 0.92).17 The participants' experience of agency of the applied pressure during imagery was assessed with the question “To what extent did you experience that you applied the pressure?” rated on a 7-point scale with the endpoints and midpoint defined as, Not at all (0), Partly (3), and Completely (6).
2.4. Study procedure
Before the training and testing, oral and written information about the study was given and written informed consent was collected.
2.5. Training procedure
The participants sat on a chair with their feet flat on the ground with an approximate 90-degree angle between thigh and calf. A cross of thin surgical tape was placed approximately 17 cm above the kneecap on the participants' left front lower thigh (quadriceps femoris), indicating the point where the pressure was to be applied during the training. The training and testing sites were separated to avoid sensitization of the testing site. The participants were familiarized to the handheld algometers and practiced how to apply pressure while maintaining an even pressing speed (50 kPa/s) and instructed to lift the algometer from their leg when the pressure pain threshold was reached (self). The participants also practiced indicating (by saying “now”) when the pressure pain threshold was reached when the experimenter applied the pressure (other and imagery). The pain threshold was defined as the instant when the pressure went from nonpainful (ie, 0 on a pain scale 0-10) to minimally painful (ie, 1 or more on a pain scale 0-10). The endpoint 10 was defined as the worst possible pain that the participants could imagine from the algometer. Instructions to the participants were: “When you feel pressure but no pain, that represents 0 on a pain scale 0 to 10. When you start to feel the slightest pain, ie, a 1 on a scale 1 to 10, I want you to indicate” (by either lifting the algometer or saying “now,” depending on the condition). The training procedure for the imagery condition took place after the self and other testing. During imagery testing, the participants were holding the identical algometer with closed eyes and practiced to imagine that they were motorically pressing it against their leg without applying any pressure. They first practiced without the experimenter applying any pressure with the other algometer, then while the experimenter applied pressure with the other algometer (algometers not attached together), and last while the experimenter applied pressure and the 2 algometers were attached to each other. In the first 2 rounds of practicing imagery, the participants' algometers were checked so that no pressure or only very low pressure was applied by the participant. If pressure ≥100 kPa was applied, the participant continued to practice until no pressure or only low pressure <100 kPa was applied.
2.6. Testing procedure
All conditions were tested 3 times each, in one of 2 test sequences:
(1) other, self, other, self, other, self, imagery, imagery, imagery
(2) self, other, self, other, self, other, imagery, imagery, imagery
Imagery was always tested last because we thought that it would be easier for the participants to imagine the self-induced pain if they had performed it a few times before. The sequences (1 and 2) were counterbalanced within the sample. The testing cross was placed approximately 10 cm above the kneecap, approximately 7 cm below the training cross. The same experimenter, who was trained to apply the pressure at the speed of 50 kPa/s, conducted all tests. The instruction to indicate when the pressure pain threshold was reached, as practiced in the training procedure, was shortly repeated before each trial. After each testing trial, the maximum pressure was registered from the algometer's display. The experimenter monitored whether there were any arm movements from the participants during imagery and did not observe any. The PCS was administered before testing and the question about experience of agency during imagery was administered immediately after testing.
2.7. Statistical analysis
Differences between the other, self, and imagery conditions were analyzed with a mixed-effects model. Condition (other, self, and imagery) and order of stimulus (1, 2, 3, 4, 5, 6, 7, 8, and 9) were prespecified as fixed effects in the model, and by-subject intercepts and by-subject slopes for the effect of condition and order were prespecified as random effects. Order of stimulus was included in the model to assess whether there were any significant effects of order (sensitization or habituation). Cohen's d effect sizes were calculated as the difference between the model implied means divided by the square root of the sums of all variance related to random effects in the model.9,40 Each participant's mean value of the 3 repetitions of other, self, and imagery, respectively, were calculated and used in correlation analyses. Spearman's correlation was used to assess the correlations between the experience of agency during imagery and imagery-induced attenuation and also between the PCS and the other condition (pressure pain threshold assessed by the experimenter). Imagery-induced attenuation was defined as the difference in pain thresholds between imagery and other. To assess responders to self-attenuation in the self and imagery conditions, the number of participants with a 10% increased pain threshold compared to the other condition was calculated for self and imagery, respectively. The statistical calculations were conducted in Stata16 IC34 and R.35
3. Results
3.1. Participant characteristics
A total of 42 participants completed all the tests. The mean age of the participants was 25.1 years (SD = 4.5). None of the participants reported having taken any pain medication or psychoactive substances within the last 24 hours before the study. Two participants were excluded from data analysis due to: (1) being older than 35 years, which was noticed first after data were collected, and (2) not being able to reach the pain threshold during the self condition (ie, participant was not able to press hard enough on the own leg). Therefore, 40 participants were included in the analyses of which 21 were women, 18 men, and 1 did not want to define as either man or woman. The mean self-appraised experience of agency during imagery was 2.75 (SD = 1.24), corresponding to partly experiencing agency (3). The mean pain catastrophizing assessed by the PCS was 17.68 (SD = 7.92).
3.2. Pain thresholds
There were 360 pain threshold measurements in total, 120 each for the 3 conditions, equally distributed across participants. The mean pain threshold across all participants and conditions was 622.46 kPa (SE = 14.86; 95% confidence interval 592.16-650.47).
3.3. Pain thresholds between conditions
Significant differences in pain thresholds were found between the other and self conditions (P < 0.001), between other and imagery (P < 0.001) and between self and imagery (P = 0.004). In the mixed model, the model implied mean for other was 521.49 kPa (SE = 38.48). For imagery, the mean was 618.88 kPa (SE = 26.67), and for self 729.57 kPa (SE = 32.32). The model-implied means and SEs are illustrated in Figure 2. Model estimates for the mixed-effects model are presented in the online supplementary eTable 1 (available as supplemental digital content at http://links.lww.com/PAIN/B229).
Figure 2.
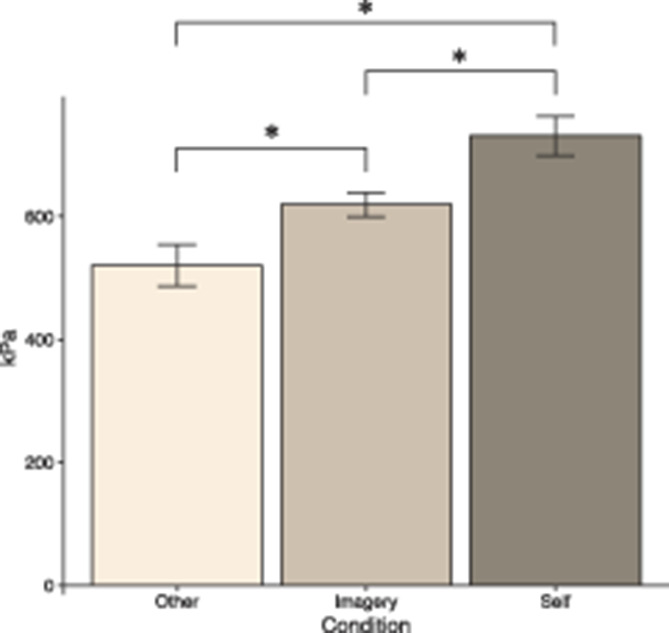
Model-implied mean pressure pain thresholds (kPa) for the conditions other, imagery, and self. The error bars represent 2 SEs. Statistically significant differences between conditions are indicated with *.
The effect size for imagery vs other was Cohen's d = 0.28, and for self vs other, the effect size was d = 0.61, and for self vs imagery, the effect size was d = 0.24. There was no effect of order (indicating no sensitization or habituation) in the model (P = 0.932). The observed means and SEs for the conditions in all trials are presented in Table 1.
Table 1.
Pressure pain thresholds.
| other 1 | other 2 | other 3 | self 1 | self 2 | self 3 | imag 1 | imag 2 | imag 3 | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 524.6 | 520.8 | 513.9 | 709.8 | 740.2 | 730.0 | 621.1 | 624.2 | 607.3 |
| SE | 35.6 | 34.2 | 32.7 | 55.9 | 53.6 | 51.0 | 41.3 | 37.2 | 36.6 |
Observed means and SEs for pressure pain thresholds in all experimental trials, represented by pressure (kPa) units (n = 40). The order of the other, self, and imagery trials was mixed.
imag, imagery.
3.4. Responder analysis
A majority of the participants 28/40 (70%) were responders in terms of pain attenuation in the imagery condition as they reported at least 10% higher pain thresholds compared to the other condition. Almost all participants, 36/40 (90%), were responders in one or both of the self or imagery conditions. A minority of participants had increased pain thresholds only in the self condition 8/40 (20%), or only in the imagery condition 4/40 (10%). Only a few participants 4/40 (10%) did not increase their pain threshold in any of the self or imagery conditions.
3.5. Questionnaire data correlations
There was no significant correlation between the experience of agency during imagery and imagery-induced pain attenuation (rs = 0.14, P = 0.398). Also, we found no correlation between self-reported pain catastrophizing measured by the PCS and pressure pain thresholds during other (rs = −0.09, P = 0.581).
4. Discussion
Previous research has shown that pain can be attenuated if the painful stimuli are self-induced, compared to externally induced pain.8,39 Our study showed that imagining an externally induced pain stimulus to be self-induced also led to attenuation of pain, indicated by increased pain thresholds. This suggests that the imagery condition included motor predictions (ie, efference copy) needed for self-attenuation of pain. This might be the first indication of a feed-forward mechanism of pain inhibition through imagining that pain is self-inflicted rather than externally induced. We observed that 70% of the participants experienced at least 10% higher pain thresholds during imagery compared with other, indicating robustness of the results (ie, results not only driven by a few participants with large effects). These results support our hypothesis (H2). Our data also confirm that self-attenuation of painful stimuli is possible, indicated by increased pain thresholds during self-generated pressure, compared to externally induced pressure. This has previously been shown8,39 and was in line with our hypothesis (H1).
It is well established that imagined motor actions may lead to similar physiological, psychological, and neural response as executed actions,11,13 which rendered it likely that attenuation of imagined self-induced pain would occur. In a study of force perception, sensory attenuation during imagined self-induced pressure was comparable to that of real self-induced pressure.18 Because self-attenuation is an adaptive response that helps promote survival (through increased attention to externally induced stimuli), there should be value from similar attenuation of painful sensations. However, in the current study, the attenuation in the self condition was significantly higher compared to the imagery condition. One possible explanation to this difference is that it may be easier to imagine a stimulus that is not painful because pain might not entail the same sensory acuity. This is supported by the average experience of agency that corresponded to partly experiencing agency during imagery. Another reason could be that pain is highly salient because it serves as a warning signal for potential threats to our tissues10 and might therefore be more resilient to attenuation through imagery compared to tactile stimuli. Furthermore, perceptual effects elicited by mental imagery are often weaker than those evoked by the corresponding veridical perception.2,3 Differences between imagery and perception may be important for the ability to distinguish between real and imagined sensory signals, for example, to discriminate actual experiences from hallucinations.3
Before an actual bodily movement is executed, a motor command is sent from the motoric cortices to the muscles. A copy of that motor command (ie, an efference copy) is sent to predictive computational units (ie, forward models) to predict the state of the moving body and the sensory consequences of the movement.32,41 The prediction of the sensory consequences of the self-generated movements—including painful consequences—enables its downregulation (ie, sensory attenuation).5,18,21,33 Here, we provide evidence for hypoalgesic responses to pressure pain when one is imagining that externally inflicted pressure is self-induced. We propose that in both the self and the imagery conditions, efference copies are used in forward models to predict the actual or imaginary movements, and that this prediction produces the sensory attenuation.
Sensory attenuation of self-induced pain may have clinical implications for patients with self-injury behavior. Nonsuicidal self-injury behavior is defined as intentional self-harm to the body without suicidal intent24 and is associated with psychiatric comorbidity, functional disability, and an increased risk for suicide.37 Pain thresholds and tolerance are elevated among individuals with self-harm,23,25 and this antinociceptive pain profile has been suggested to decrease the threshold for more serious self-harming behaviors and underlie the increased risk of suicide.27 Also, there is some evidence suggesting that pain thresholds and tolerance are normalized after the self-injury behaviors stop.29 More research is needed to further assess the pain's role in self-injury behavior and to increase the understanding of whether self-injury behavior in fact alters pain regulation.
Sensory attenuation of self-induced and imagery-induced pain may also have clinical implications for patients with longstanding pain. Patients with longstanding pain conditions often avoid movements that provoke pain and try to distract themselves from pain sensations, which can be seen as the opposite of self-induced or imagery-induced pain. Avoidance of movements that provoke pain, or avoidance of the pain sensations themselves, may be reinforced in the short term by pain relief, but in the long run, the avoidance may maintain the pain problems. The role of avoidance as maintaining factor of long-standing pain is described in the fear-avoidance model for chronic pain.38 In this model, the fear of pain and related catastrophic thoughts leads to behavioral avoidance, which in turn feeds into a vicious circle that maintains pain and functional disability. In exposure therapy for longstanding pain, the patient provokes pain by engaging in physically active exposure exercises. Mindfulness, in which the patient practices to pay close attention to the pain sensation, is often included. Exposure therapy has been shown to be an effective treatment for different pain conditions such as fibromyalgia,16 functional abdominal pain disorders,7,26,28 and chronic back pain.15 The sensory attenuation described in this study constitutes a possible mechanism of change in exposure-based treatments for longstanding pain. The patient's agency and focus on the pain experience may be particularly beneficial. Finally, the results in this study may also have clinical implications for procedural pain. Even if patients are not able to execute painful procedures themselves, a mental image of controlling the procedure may be induced, and thereby decrease the experienced pain.
The participants were trained to apply the pressure to their own leg at the same speed as the test leader (50 kPa/s). However, the speed of the participant's pressure was not monitored and corrected after the training sessions because it was thought to distract the participants' ability to determine their pain threshold. This is a limitation to the study because pressure speed may have had an effect on perceived pressure pain and affected the self condition. A strength, however, is that the same experimenter performed all testing, which is likely to have minimized experimenter-related variance in pressure application during the other and imagery conditions. Another limitation is the lack of a control for the self and imagery conditions. However, previous studies of coauthors of this study have demonstrated that sensory attenuation of self-generated and motor imagery-induced nonpainful pressure is only attained when the experienced pressure matches the performed20 or imagined18 movement of applying the pressure very well, both in terms of timing and location. The controls for timing and location show that alternative explanations, such as distraction, cognitive load, or general anticipation of touch, did not explain the sensory attenuation during self and imagery in nonpainful pressure.
We did not find a significant correlation between self-assessed agency during the imagery condition and the level of attenuation during imagery (compared with “other”). Hence, the self-reported sense of agency does not seem to explain the sensory attenuation. This could be explained by a general difficulty of self-assessing one's own experience, a phenomenon also seen in self-appraisal of one's own pain sensitivity.12 It is also possible that the self-assessment scale used in the study (“To what extent did you experience that you applied the pressure?”) did not match very well with the task. It is possible that participants would answer “low” to that question (because they know they are not physically applying the pressure) but at the same time have a very vivid imagined feeling of applying pressure. A question more directly designed to capture the imagined experience of applying pressure might have rendered a stronger correlation between the voluntary aspect of the motor imagery and attenuation.
The conclusion of this study is that sensory attenuation occurs during imagery as well as during genuine self-induced pressure pain. Imagining that externally generated pressures were self-induced produced higher pain thresholds. The results may have clinical implications for patients with self-injury behavior (with reported high pain thresholds), patients with longstanding pain (with reported low pain thresholds), and patients undergoing procedural pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B229.
Acknowledgements
This study was supported by the Lundblad Family Donation to K. Jensen. H.H. Ehrsson was supported by a Distinguished Professor Grant from the Swedish Research Council. K. Kilteni was supported by a VR Starting Grant (Registration number 2019-01909) from the Swedish Research Council. The authors thank Robin Fondberg for technical support.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Jens Fust, Email: jens.fust@ki.se.
Viktor Vadenmark-Lundqvist, Email: viktor.lundqvist@stud.ki.se.
H. Henrik Ehrsson, Email: henrik.ehrsson@ki.se.
Konstantina Kilteni, Email: konstantina.kilteni@ki.se.
Karin Birgitta Jensen, Email: karin.jensen@ki.se.
References
- [1].Bays PM, Wolpert DM, Flanagan JR. Perception of the consequences of self-action is temporally tuned and event driven. Curr Biol 2005;15:1125–8. [DOI] [PubMed] [Google Scholar]
- [2].Berger CC, Ehrsson HH. Mental imagery changes multisensory perception. Curr Biol 2013;23:1367–72. [DOI] [PubMed] [Google Scholar]
- [3].Berger CC, Ehrsson HH. The fusion of mental imagery and sensation in the temporal association cortex. J Neurosci 2014;34:13684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blakemore SJ, Wolpert D, Frith C. Why can't you tickle yourself?. Neuroreport 2000;11:R11–6. [DOI] [PubMed] [Google Scholar]
- [5].Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci 1998;1:635–40. [DOI] [PubMed] [Google Scholar]
- [6].Boehme R, Hauser S, Gerling GJ, Heilig M, Olausson H. Distinction of self-produced touch and social touch at cortical and spinal cord levels. Proc Natl Acad Sci U S A 2019;116:2290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bonnert M, Olén O, Lalouni M, Benninga MA, Bottai M, Engelbrektsson J, Hedman E, Lenhard F, Melin B, Simrén M, Vigerland S, Serlachius E, Ljótsson B. Internet-delivered cognitive behavior therapy for adolescents with irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol 2017;112:152–62. [DOI] [PubMed] [Google Scholar]
- [8].Braid L, Cahusac PMB. Decreased sensitivity to self-inflicted pain. PAIN 2006;124:134–9. [DOI] [PubMed] [Google Scholar]
- [9].Brysbaert M, Stevens M. Power analysis and effect size in mixed effects models: a tutorial. J Cogn 2018;1:1052–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci 2003;26:303–7. [DOI] [PubMed] [Google Scholar]
- [11].Decety J, Jeannerod M, Durozard D, Baverel G. Central activation of autonomic effectors during mental simulation of motor actions in man. J Physiol 1993;461:549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Edwards RR, Fillingim RB. Self-reported pain sensitivity: lack of correlation with pain threshold and tolerance. Eur J Pain 2007;11:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 2003;90:3304–16. [DOI] [PubMed] [Google Scholar]
- [14].Fust J, Lalouni M, Vadenmark Lundqvist V, Wärnberg E, Jensen KB. Offset analgesia and onset hyperalgesia with different stimulus ranges. medRxiv 2020:1–14. doi: 10.1101/2020.06.01.20113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Glombiewski JA, Holzapfel S, Riecke J, Vlaeyen JWS, de Jong J, Lemmer G, Rief W. Exposure and CBT for chronic back pain: an RCT on differential efficacy and optimal length of treatment. J Consult Clin Psychol 2018;86:533–45. [DOI] [PubMed] [Google Scholar]
- [16].Hedman-Lagerlöf M, Hedman-Lagerlof E, Axelsson E, Ljótsson B, Engelbrektsson J, Hultkrantz S, Lundbäck K, Björkander D, Wicksell RK, Flink I, Andersson E. Internet-delivered exposure therapy for fibromyalgia: a randomized controlled trial. Clin J Pain 2018;34:532–42. [DOI] [PubMed] [Google Scholar]
- [17].Kemani MK, Grimby-Ekman A, Lundgren J, Sullivan M, Lundberg M. Factor structure and internal consistency of a Swedish version of the Pain Catastrophizing Scale. Acta Anaesthesiol Scand 2019;63:259–66. [DOI] [PubMed] [Google Scholar]
- [18].Kilteni K, Andersson BJ, Houborg C, Ehrsson HH. Motor imagery involves predicting the sensory consequences of the imagined movement. Nat Commun 2018;9:1617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kilteni K, Ehrsson HH. Functional connectivity between the cerebellum and somatosensory areas implements the attenuation of self-generated touch. J Neurosci 2020;40:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kilteni K, Ehrsson HH. Sensorimotor predictions and tool use: hand-held tools attenuate self-touch. Cognition 2017;165:1–9. [DOI] [PubMed] [Google Scholar]
- [21].Kilteni K, Engeler P, Ehrsson HH. Efference copy is necessary for the attenuation of self-generated touch. iScience 2020;23:100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kilteni K, Houborg C, Ehrsson HH. Rapid learning and unlearning of predicted sensory delays in self-generated touch. Elife 2019;8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kirtley OJ, O'Carroll RE, O'Connor RC. Pain and self-harm: a systematic review. J Affect Disord 2016;203:347–63. [DOI] [PubMed] [Google Scholar]
- [24].Klonsky ED, Muehlenkamp JJ. Self-injury: a research review for the practitioner. J Clin Psychol 2007;63:1045–56. [DOI] [PubMed] [Google Scholar]
- [25].Koenig J, Thayer JF, Kaess M. A meta-analysis on pain sensitivity in self-injury. Psychol Med 2016;46:1597–612. [DOI] [PubMed] [Google Scholar]
- [26].Lalouni M, Ljótsson B, Bonnert M, Ssegonja R, Benninga M, Bjureberg J, Högström J, Sahlin H, Simrén M, Feldman I, Hedman-Lagerlof E, Serlachius E, Olén O. Clinical and cost effectiveness of online cognitive behavioral therapy in children with functional abdominal pain disorders. Clin Gastroenterol Hepatol 2019;17:2236–44.e11. [DOI] [PubMed] [Google Scholar]
- [27].Law KC, Khazem LR, Jin HM, Anestis MD. Non-suicidal self-injury and frequency of suicide attempts: the role of pain persistence. J Affect Disord 2017;209:254–61. [DOI] [PubMed] [Google Scholar]
- [28].Ljótsson B, Hesser H, Andersson E, Lackner JM, Alaoui El S, Falk L, Aspvall K, Fransson J, Hammarlund K, Löfström A, Nowinski S, Lindfors P, Hedman E. Provoking symptoms to relieve symptoms: a randomized controlled dismantling study of exposure therapy in irritable bowel syndrome. Behav Res Ther 2014;55:27–39. [DOI] [PubMed] [Google Scholar]
- [29].Ludäscher P, Greffrath W, Schmahl C, Kleindienst N, Kraus A, Baumgärtner U, Magerl W, Treede R-D, Bohus M. A cross-sectional investigation of discontinuation of self-injury and normalizing pain perception in patients with borderline personality disorder. Acta Psychiat Scand 2009;120:62–70. [DOI] [PubMed] [Google Scholar]
- [30].Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan S-T, Wasan AD, Kaptchuk TJ, McDonnell C, Carriere J, Rosen B, Gollub RL, Edwards RR. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. PAIN 2019;160:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pelfort López J, Torres Claramunt R, Sánchez Soler JF, Hinarejos Gómez PA, Leal Blanquet J, Valverde D, Monllau García JC. Pressure algometry is a useful tool to quantify pain in the medial part of the knee: an intra- and inter-reliability study in healthy subjects. Orthopaed Traumatol Surg Res 2015;101:559–63. [DOI] [PubMed] [Google Scholar]
- [32].Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 2008;185:359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: the neuroscience of force escalation. Science 2003;301:187. [DOI] [PubMed] [Google Scholar]
- [34].Stata S. StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. n.d, 2019. [Google Scholar]
- [35].Team RC. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2013, 2015. [Google Scholar]
- [36].Tumi El H, Johnson MI, Dantas PBF, Maynard MJ, Tashani OA. Age-related changes in pain sensitivity in healthy humans: a systematic review with meta-analysis. Eur J Pain 2017;21:955–64. [DOI] [PubMed] [Google Scholar]
- [37].Victor SE, Klonsky ED. Correlates of suicide attempts among self-injurers: a meta-analysis. Clin Psychol Rev 2014;34:282–97. [DOI] [PubMed] [Google Scholar]
- [38].Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. PAIN 2000;85:317–32. [DOI] [PubMed] [Google Scholar]
- [39].Wang Y, Wang JY, Luo F. Why self-induced pain feels less painful than externally generated pain: distinct brain activation patterns in self-and externally generated pain. PLoS One 2011;6:e23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Westfall J, Kenny DA, Judd CM. Statistical power and optimal design in experiments in which samples of participants respond to samples of stimuli. J Exp Psychol Gen 2014;143:2020–45. [DOI] [PubMed] [Google Scholar]
- [41].Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci 2000;3:1212–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B229.


