Abstract
Background
The global healthcare burden of COVID-19 continues to rise. There is currently limited information regarding the disease progression and the need for hospitalizations in patients who present to the Emergency Department (ED) with minimal or no symptoms.
Objectives
This study identifies bounceback rates and timeframes for patients who return to the ED due to COVID-19 after initial discharge on the date of testing.
Methods
Using the NorthShore University Health System's (NSUHS) Enterprise Data Warehouse (EDW), we conducted a retrospective cohort analysis of patients who were tested positive for COVID-19 and were discharged home on the date of testing. A one-month follow-up period was included to ensure the capture of disease progression.
Results
Of 1883 positive cases with initially mild symptoms, 14.6% returned to the ED for complaints related to COVID-19. 56.9% of the mildly symptomatic bounceback patients were discharged on the return visit while 39.5% were admitted to the floor and 3.6% to the ICU. Of the 1120 positive cases with no initial symptoms, only four returned to the ED (0.26%) and only one patient was admitted. Median initial testing occurred on day 3 (2–5.6) of illness, and median ED bounceback occurred on day 9 (6.3–12.7). Our statistical model was unable to identify risk factors for ED bouncebacks.
Conclusion
COVID-19 patients diagnosed with mild symptoms on initial presentation have a 14.6% rate of bounceback due to progression of illness.
Keywords: COVID-19, Coronavirus, Bounceback rates, Emergency department
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) emerged from China's Hubei province in December 2019 (1). The resulting infection, named “coronavirus disease 2019” (COVID-19) by the World Health Organization, has since become a global pandemic, with more than 120 million confirmed cases worldwide and resulting in over 2.6 million deaths (2,3) as of October 2020. The United States has become the epicenter of the pandemic with 7.7 million total cases and 214,000 deaths.
COVID-19 has become a robust area of research with more than 30,000 citations listed in PubMed through September 2020. The clinical manifestations and disease course has been studied by multiple groups both in the virus' country of origin and worldwide. The median incubation time is approximately 4–6 days, though may extend to 24 days in rare cases (4). Initial symptoms are usually mild and are flu-like in nature, but can advance quickly and precipitously in select patients. Median time to admission, dyspnea, development of acute respiratory distress syndrome (ARDS), and intensive care unit (ICU) admission are 7, 8, 9, 10.5 days, respectively (5).
Patients with relatively mild initial presentation of COVID-19 can progress to severe illness and lethal decline. This poses a clinical conundrum for healthcare personnel regarding disposition, and patient counseling with particular attention to anticipatory guidance and return precautions. With an uncertain disease course, an initial mild presentation may provide false reassurance to both patients and providers.
NorthShore University HealthSystem (NSUHS) is a five-hospital system located primarily in the northern suburbs of Chicago, Illinois. NSUHS became the first hospital system in Illinois to have on-site COVID-19 testing when it began testing patients with an institutionally developed PCR test on March 12, 2020. As one of the first non-public health laboratories to perform SARS-CoV-2 testing, we were able to compile comprehensive regional data regarding the spread of COVID-19 infection during the early stages of spread across the US. As the predominant healthcare provider locally, we have also been able to follow these patients over time as their disease course unfolds. The aim of this paper is to describe emergency department (ED) bounceback rates in asymptomatic and mildly symptomatic patients diagnosed with COVID-19 to better characterize disease progression.
2. Methods and materials
2.1. Specimen testing
In addition to the five main hospitals, NSUHS also established four Immediate Care Centers (ICCs) as testing locations at the onset of the coronavirus pandemic in March 2020. Specimens were collected at these nine locations. In addition to traditional in-person visits, drive-thru testing was available at the four ICCs via online appointments.
NSUHS utilized two testing modalities during the study period. The initial NorthShore SARS-Cov-2 assay is a real time (RT) PCR assay based on the Center for Disease Control (CDC) designed and published protocol (6). Primers and probes, as well as plasmid DNA constructs, containing all four described targets from Integrated DNA Technologies were ordered and the performance validated on a Roche LC480II instrument. Our studies supported the use of a single viral target, N1, which permitted higher throughput testing than the original design. Analytic sensitivity and specificity were established at approximately five viral genomes per RT-PCR reaction with no cross reactivity with other respiratory viral or bacterial pathogens. Nineteen patient specimens, eight positive and eleven negative, were also tested in parallel with the Illinois Department of Public Health (IDPH) for confirmation with 100% concordance. Later in the evaluation of the assay, an Abbott m2000 assay was used to further evaluate the NorthShore assay by testing 107 samples in parallel on both machines. Clinical sensitivity and specificity using contrived patient specimens across a range of viral loads were determined to be 100% and 98%, respectively. The test swabs collected for derivation of the test characteristics were obtained by nasopharyngeal swab. Our in-house assay was launched on March 12, 2020 with an initial capacity of approximately 120 tests per day. On March 21, 2020, testing was augmented to 1200–1500 tests per day with acquisition of the Abbott Molecular m2000 platform. Specimens were collected via nasopharyngeal or oropharyngeal swab and sent to our centralized lab for testing. The location of the swab for the cohort of study patients, either nasopharyngeal or oropharyngeal, was not included in the data analysis as this information was not available by chart review.
2.2. Study population and design
Patients were included if they tested positive within NSUHS between March 12, 2020 and April 16, 2020. Patients were either asymptomatic or mildly symptomatic at the time of presentation. “Asymptomatic” patients were defined as having no symptoms. These patients often presented because of work obligations or due to a close contact with COVID-19. “Mildly symptomatic” was defined as patients that were appropriate for discharge by the treating physician from initial encounter at the ICC or the ED. Although objective vital sign data was collected and reviewed by the treated physician, no single clinical criteria or algorithms were used to identify patients appropriate for discharge. Rather, the decision was made the by the treating physician. Patients who were admitted to the hospital or were already inpatient status at the time of testing were excluded. Patients were also excluded if they had not had NSUHS healthcare encounter in the past two years to help limit those lost to followup.
Since NSUHS was the first hospital system in Illinois to develop in-house testing, our institution accepted testing specimens from various outside networks, including IDPH and nearby hospitals, to assist in testing for the entire state. These patients were excluded as follow-up information could not reliably be obtained. Additionally, our fifth hospital, Swedish Covenant Hospital, acquired by NSUHS at the beginning of the year, was not included as our health records are not yet synced and such follow-up information could not be reviewed. Patients who tested positive at employee health were also excluded.
Testing protocols changed rapidly during the collection period. Initially, patients were tested only if they had traveled to or had contact with a patient from an endemic area and had presenting symptoms consistent with known COVID-19 presentations. As positive cases and NSUHS testing capacity increased, testing was expanded to include all mildly symptomatic patients regardless of contact or travel history. By the end of the study period, asymptomatic patients with possible exposure to a confirmed or suspected case were also able to be tested. The final decision regarding who to test always fell to the discretion of the treating physician.
Bounceback patients were defined as positive cases that had a return visit to the ED within the study period. Data collection on bounceback visits extended to May 20, 2020 to allow for a thirty-day day followup period for those patients that tested positive on or before April 16, 2020. Patients that tested positive after April 16, 2020 were excluded, as a 30 day followup could not be guaranteed.
2.3. Data sources and definitions
Data was collected via the electronic medical record (EMR) in conjunction with Northshore's Enterprise Data Warehouse (EDW). Data points obtained included COVID-19 testing date, demographic data, and encounter details for both initial presentation and follow-up visits, including chief complaint and disposition.
Missing or incomplete data and date of symptom onset was manually extracted by our research team and reviewed by an ED physician. Bounceback ED disposition was defined as either discharge, admit to the floor, or admit to the ICU. If the patient stated they were asymptomatic on the day of testing, we counted this as day zero of illness. For patients with multiple bouncebacks to the ED, the visit with the highest acuity disposition was included in analysis. During the return visit, only the first chief complaint listed by the patient was included.
2.4. Statistical analysis
Descriptive statistics were used to summarize the data. Results are reported as medians and interquartile ranges or counts and percentages, as appropriate.
To understand how different patient characteristics may have contributed to bounceback rates, we created a logistic regression model for classifying patients, and calculated the odds ratios of each individual predictor. Odds ratios were then compared to evaluate how incremental changes in each variable would influence the likelihood of a return visit to the ED that resulted in hospitalization. Clinical variables were derived either from a patient survey or by mapping finalized ICD codes according to the standard Clinical Classification Software (CCS) definitions. CCS categories were used to indicate whether patients had certain conditions in their past medical history. The number of days elapsed between key time points, such as the date of COVID-19 testing or the first ED visit, were also measured; we ultimately used the time difference between a patient's first ED visit and the earliest date of either their COVID-19 test or e-visit as a model variable. A complete list of variables used in our evaluation is provided in our Supplementary Information (Supplemental Tables 1 and 2).
This work was submitted to the NorthShore Institutional Review Boards and was approved with an exempt review (EH20–178) as a quality initiative project.
3. Results
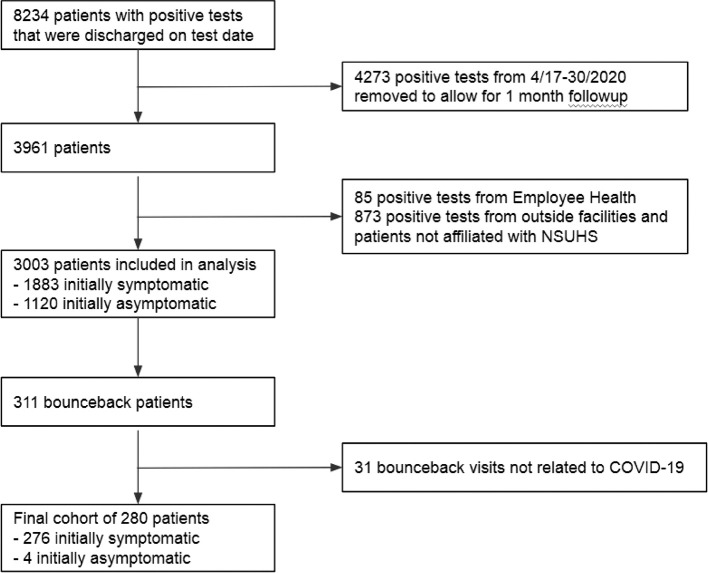
During the study period, NSUHS testing resulted in 8234 positive cases that were discharged on the date of testing. After applying the exclusions listed in Fig. 1 , 3003 patients that were diagnosed with COVID-19 and discharged on their initial visit were included in the final analysis. This cohort was further broken down into mildly symptomatic (1883, 62.7%) or asymptomatic patients (1120, 37.3%) based on their initial presentation. 280 patients (9.3%) returned to the ED during the study period with COVID-19-related chief complaints. Only four were initially asymptomatic. Table 2 describes the outcomes of the bounceback visits. 21 patients (0.7%) died from COVID-19 during the study period, all of whom initially presented mildly symptomatic. One patient returned to the ED seven times, while nine patients had three bounceback visits. 42 patients had two bounceback visits.
Fig. 1.
Flow diagram for study cohort.
Table 2.
Bounceback visit Information (N = 280).
| Day of illness | Symptomatic (N = 276) | Asymptomatic (N = 4) |
|---|---|---|
| At positive test | 3 [2–5.6] | 0 |
| At bounceback visit | 9 [6.3–12.7] | 10 [4–15] |
| Disposition at bounceback visit | ||
| Discharge | 157 (56.9% of symptomatic bounceback patients, 8.3% of all symptomatic patients) | 3 (75% of asymptomatic bounceback patients, 0.26% of all asymptomatic patients) |
| Admit floor | 110 (39.5%, 5.8%) | 1 (25%, 0.09%) - Hyponatremia |
| Admit ICU | 10 (3.6%, 0.5%) | |
| Admission Diagnosis for Bounceback Patients | ||
| COVID-19 (99.2% of 120 patients) Nausea, Vomiting, Diarrhea (0.8%) | Hyponatremia (100%) | |
| Chief Complaint at bounceback visit | ||
| Pulmonary | 154 (55.8% of symptomatic bounceback patients) - Shortness of breath (83.1% of symptomatic pulmonary bounceback patients) - Cough (16.8%) |
3 (75% of asymptomatic bounceback patients) - Shortness of breath (66.7% of asymptomatic pulmonary bounceback patients) - Cough (33.3%) |
| Cardiac | 36 (13.0%) - Chest pain (83.3%) - Palpitations (8.3%) - Syncope (8.3%) |
|
| Infectious | 36 (13.0%) - Fever/chills (75%) - Flu-like illness (25%) |
|
| GI | 20 (7.2%) - Nausea and vomiting (65%) - Diarrhea (25%) - Abdominal pain (10%) |
|
| Constitutional | 16 (5.8%) - Dehydration (25%) - Weakness/Fatigue (56.3%) - Musculoskeletal pain (18.8%) |
1 (25%) - Weakness |
| COVID Testing | 12 (4.3%) - Repeat testing (100%) |
|
| Psychiatric | 2 (0.7%) - Anxiety (100%) |
|
Patient characteristics for the 276 mildly symptomatic bounceback visits are summarized in Table 1 . The median age (IQR) of patients was 52 (42–61), with 51.1% female and 46.0% obese. The bounceback population consisted of 38.4% Caucasians, 11.6% African American, 20.3% Hispanic/Latino, and 10.5% Asian. The majority of patients had private insurance (61.6%). English (90.2%) was the most commonly spoken language. More than twice as many patients who returned to the ED had their initial testing performed at the ICC than the ED (64.9% and 30.0%, respectively).
Table 1.
Population characteristics of bounceback group (N = 280).
| Demographics | Symptomatic (N = 276) | Asymptomatic (N = 4) |
|---|---|---|
| Age | 52 [42–61] | 65 [60–71] |
| Female | 141 (51.1%) | 3 (75%) |
| BMI | 29 [26–33] | 25 [23–27] |
| BMI ≥ 30 | 127 (46.0%) | |
| Pregnant | 1 (0.4%) | |
| Patient has PCP | 250 (90.6%) | 4 (100%) |
| Race | ||
| African American | 32 (11.6%) | 1 (25%) |
| American Indian or Alaskan Native | 2 (0.7%) | |
| Asian | 29 (10.5%) | 1 (25%) |
| Pacific Islander or Hawaiian Native | 1 (0.4%) | |
| Caucasian | 106 (38.4%) | |
| Other | 106 (38.4%) | 2 (50%) |
| Ethnicity | ||
| Hispanic/Latino | 56 (20.3%) | |
| Non-Hispanic | 220 (79.7%) | 4 (100%) |
| Insurance | ||
| Medicaid | 39 (14.1%) | |
| Medicare | 54 (19.6%) | 1 (50%) |
| Private | 170 (61.6%) | 2 (50%) |
| Self-Pay/Unknown | 13 (4.7%) | 1 (25%) |
| Testing Location | ||
| ICC | 179 (64.9%) | 1 (25%) |
| Drive-Thru | 14 (5.1%) | |
| ED | 83 (30.0%) | 3 (75%) |
| Language | ||
| English | 249 (90.2%) | 3 (75%) |
| Spanish | 22 (8.0%) | |
| Russian | 2 (0.7% | |
| Gujarati | 1 (0.4%) | |
| Korean | 1 (0.4%) | |
| Arabic | 1 (0.4%) | |
| Tagalog | 1 (0.4%) | |
Mildly symptomatic patient bounceback details are listed in Table 2 . The median day of initial testing occurred on day 3 (2–5.6). The median day of illness for their bounceback presentation occurred on day 9 (6.3–12.7). The most common chief complaints on the return visit were pulmonary in nature, such as cough, shortness of breath, or hypoxia (55.8%), followed by cardiac and infectious complaints (13.0% each). Asymptomatic patient details can also be found in Table 1, Table 2.
In order to identify risk factors for bounceback visits, our combined data set of both mildly symptomatic and asymptomatic patients was separated randomly: 80% of the patients were chosen for model training, while the remaining 20% was used for model evaluation. We found that COVID patients who previously had a stroke had a substantially higher chance of being hospitalized after returning to the ED (OR = 4.4901, p = 0.0006). Additionally, female patients were more likely than their male counterparts to re-visit the ED and become hospitalized (OR = 1.6336, p = 0.0136). Patients who had a shorter elapsed time between their first ED visit and the date of their earliest COVID-19 test or e-visit had some protection from this outcome (OR = 0.9175, p = 0.0019). We also found in our sample that Hispanic patients had a decreased chance of a second ED visit (OR = 0.5216, p = 0.0140). Despite these findings, the overall model performed poorly as a predictive tool (AUC = 0.5628, AUC 95% CI = [0.5030, 0.6226]); the positive predictive value of this model was 0.2159, and the model sensitivity was 0.5758 when half the population was flagged. These metrics suggested that while some patient variables may be more influential than others, which patients will return to the ED is nearly arbitrary.
4. Discussion
ED bounceback rates are a commonly studied metric used to assess progression of disease or illness, quality of ED care, and patient compliance ([12], [13], [14], [15], [16]). Gabayan et al. examined over 5 million ED visits encompassing all presentations and found a 2.6% bounceback admission rate at 7 days (7). To our knowledge, our current study is the first to examine bounceback visits for patients recently diagnosed with COVID-19. The majority (85.3%) of COVID-19 positive patients in our study population who initially presented with a mildly symptomatic illness severity did not bounce back to the ED. For mildly symptomatic patients, we report a bounceback admission rate of 6.4%, suggesting that approximately 1 in every 16 patients who test positive with mild symptoms will later require admission. The majority of these patients return to the ED with pulmonary, cardiac, or infectious symptoms. CDC data from May 2020 show that 14% of all patients that contract COVID-19 will require admission to the hospital, which is significantly higher than we found with our cohort (8). There are many explanations for this discrepancy. First, we excluded patients from our study that were admitted to the hospital on the day of testing. Second, NSUHS implemented aggressive testing at the onset of the pandemic which allowed for all patients to be tested at a time when many locations across the United States reserved tests for those patients with moderate or severe illness. This may have resulted in a higher number of positive cases with mild or no symptoms due to testing capability and capacity. Third, there are numerous racial healthcare disparities that are well-documented in the United States healthcare system. Our analysis also shows that the majority of our cohort had private insurance (61.4%) and access to primary care physicians (90.7%), which may also contribute to improved outcomes. A recent study by Price-Haywood et al. showed that in a large cohort of patients, African-American patients had higher hospital admission rates than Caucasians (9). Hsu et al. drew similar conclusions with Hispanic patients (10). In our mildly symptomatic cohort at NSUHS, only 11.8% of patients were African-American and 20% Hispanic, which may have further contributed to our lower admission rates given these defined disparities.
Patients with return visits most commonly presented on day of illness 6 to 12, which is similar to prior research that examines the progression of illness for patients with COVID-19 (5). Based on this data, ED physicians should offer patients anticipatory guidance that they may experience worsening symptoms for up to two weeks after symptom onset. A large portion of our mildly symptomatic bounceback cohort experienced symptoms that were not associated with a respiratory illness (44.2%). In particular, patients should be advised to be aware of digestive symptoms, as recent evidence shows these patients have a worse prognosis than those with strictly respiratory symptoms (11).
Our study cohort included 1120 patients that were asymptomatic at the time of testing (37% of cohort). Only four of these patients returned to the ED in the study period, and only one required admission to the hospital. It is difficult to draw conclusions from this low rate of return in asymptomatic patients, but this data suggests that patients who are asymptomatic but still test positive via PCR can expect a relatively mild course of illness.
4.1. Limitations
Our study has several limitations. Early availability of testing and rapid augmentation of throughput in the collection period led to nearly 5000 positive tests from outside hospital systems or the IDPH. Unfortunately, follow up data on these patients could not be reliably obtained and were excluded from the cohort. Review of the population characteristics and disease progression in healthcare workers poses an area of interest for potential further review. In addition, NSUHS is a single healthcare system with a largely suburban population sample, which may limit generalizability for return visits and need for later admission. We were also unable to find risk factors to help identify patients that will return to the ED after their initial COVID-19 diagnosis. This may be due to heterogeneity of patient presenting symptoms, with some patients being asymptomatic. The testing assay used during the study period had a 100% sensitivity and 98% specificity. As such, there is a small number of false-positive patients that may increased the number of patients in the study cohorts.
Patient followup was performed using NSUHS's EMR as detailed above. Patients were not contacted directly at 30 days, and we cannot guarantee that patients did not go to another healthcare system for followup care. We believe we mitigated this potential for error in several ways. First, 90% of the mildly symptomatic cohort had a primary care physician within the NSUHS network, and we assumed that those patients would not seek additional care at an outside facility. Secondly, patients were included in the analysis only if they had been to a NSUHS location in the past 2 years, further reducing chance that patients would be lost to followup. Lastly, we are confident that all patient deaths during the study period were accurately captured, as the EMR used at NSUHS is linked to IDPH databases such that deaths are recorded regardless of their location within the state.
5. Conclusions
This study identified ED bounceback rates for patients with COVID-19 who were discharged on their initial ED visit. Of the cohort of discharged mildly symptomatic patients, 14.6% of patients returned to the ED, and 6.4% of the initial cohort was admitted to the hospital. Patients returned to the ED on median day of illness 9. Due to many asymptomatic patients that were present in the study, risk factors associated with return visits were unable to be identified. Future studies should focus on larger data sets of mildly symptomatic patients to help better identify risk factors associated with ED bounceback visits.
Grant
There were no grants or financial support for this paper. We received no external assistance for manuscript support.
Declaration of Competing Interest
There were no conflicts of interest for any authors on this paper. No one, other than the stated authors, had control over the data analysis, interpretation, wording or conclusions described in the paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajem.2021.04.050.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- 1.Guan W., Ni Z., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Naming the coronavirus disease (COVID-19) and the virus that causes it. February 11 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Accessed March 15, 2021.
- 3.John Hopkins University & Medicine COVID-19 Dashboard by the Center for Systems, Science and Engineering at Johns Hopkins University. https://coronavirus.jhu.edu/map.html Accessed March 15, 2021.
- 4.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;21 doi: 10.1001/jama.2020.2565. Published online February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 04 Feb 2020. Real-time RT-PCR panel for detection 2019-Novel Coronavirus. [Google Scholar]
- 7.Gabayan G.Z., Asch S.M., Hsia R.Y., et al. Factors associated with short-term bounce-back admissions after emergency department discharge. Ann Emerg Med. 2013;62(2):136–144. doi: 10.1016/j.annemergmed.2013.01.017. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu H.E., Ashe E.M., Silverstein M., et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an urban safety-net medical center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:864–869. doi: 10.15585/mmwr.mm6927a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan L., Mu M., Hg Ren, Yang P.C. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng J., Shroff A., Khan N., Jain S. Emergency department return visits resulting in admission: do they reflect quality of care? Am J Med Qual. 2016;31(6):541–551. doi: 10.1177/1062860615594879. [DOI] [PubMed] [Google Scholar]
- 13.Montoy J.C.C., Tamayo-Sarver J., Miller G.A., Baer A.E., Peabody C.R. Predicting emergency department “bouncebacks”: a retrospective cohort analysis. West J Emerg Med. 2019;20(6):865–874. doi: 10.5811/westjem.2019.8.43221. Published 2019 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellerin G., Gao K., Kaminsky L. Predicting 72-hour emergency department revisits. Am J Emerg Med. 2018;36(3):420–424. doi: 10.1016/j.ajem.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Hu K.W., Lu Y.H., Lin H.J., Guo H.R., Foo N.P. Unscheduled return visits with and without admission post emergency department discharge. J Emerg Med. 2012;43(6):1110–1118. doi: 10.1016/j.jemermed.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 16.Sklar D.P., Crandall C.S., Loeliger E., Edmunds K., Paul I., Helitzer D.L. Unanticipated death after discharge home from the emergency department. Ann Emerg Med. 2007;49(6):735–745. doi: 10.1016/j.annemergmed.2006.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2