Keywords: air pollution, COVID-19, gut, intestinal barrier, lung
Abstract
As countries continue to industrialize, major cities experience diminished air quality, whereas rural populations also experience poor air quality from sources such as agricultural operations. These exposures to environmental pollution from both rural and populated/industrialized sources have adverse effects on human health. Although respiratory diseases (e.g., asthma and chronic obstructive pulmonary disease) are the most commonly reported following long-term exposure to particulate matter and hazardous chemicals, gastrointestinal complications have also been associated with the increased risk of lung disease from inhalation of polluted air. The interconnectedness of these organ systems has offered valuable insights into the roles of the immune system and the micro/mycobiota as mediators of communication between the lung and the gut during disease states. A topical example of this relationship is provided by reports of multiple gastrointestinal symptoms in patients with coronavirus disease 2019 (COVID-19), whereas the rapid transmission and increased risk of COVID-19 has been linked to poor air quality and high levels of particulate matter. In this review, we focus on the mechanistic effects of environmental pollution on disease progression with special emphasis on the gut-lung axis.
INTRODUCTION
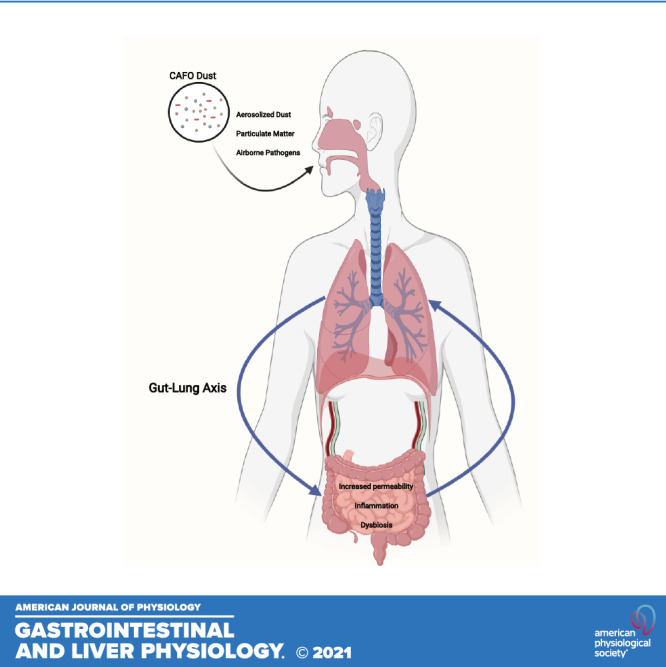
Ambient air pollution poses a major threat to human health with an estimated 4.2 million deaths per year due to the onset of cardiovascular disease and chronic respiratory illnesses linked to exposure to toxic air pollutants (1). Although there are regulatory policies to reduce air pollution, especially in urban areas where air quality levels exceed recommended restrictions for particulate matter (PM), communities continue to breathe air containing high concentrations of airborne toxins (1). Moreover, health disparities in the United States are seen among minorities and immigrants who account for the majority of seasonal farm workers frequently exposed to PM in agricultural dusts (2, 3). Strikingly, many farm workers live below the poverty level with limited access to healthcare services thus increasing their risk of developing respiratory disorders from exposure to airborne pollutants (4–7). Most air pollution is man-made and derived from fossil fuels including toxins from car exhaust and industrial waste (8–10). In addition, agricultural enterprises including concentrated animal feeding operations include a variety of dusts, vapors, and fumes that can promote and exacerbate respiratory diseases including chronic obstructive pulmonary disease (COPD), hypersensitivity pneumonitis (11, 12), and organic dust toxic syndrome (Fig. 1) (13, 14). More specifically, farmers who are in daily contact with livestock (e.g., pigs) are exposed to dust composed of microorganisms (Tables 1 and 2) originating from animal dander and fecal matter (31, 35). Although the inhalation of dust and other airborne pollutants are major factors in the development of cardiovascular and respiratory complications (48), recent studies have also shown that urban airborne particulate matter can have adverse effects on the gastrointestinal (GI) tract (e.g., barrier function and microbial composition) and immune system (Fig. 1) (18, 35, 56). In addition, the hazards of farming have been widely acknowledged with the primary environmental threat being exposure to pathogenic microbes and toxins generated during agricultural and swine farm field operations (57). Therefore, the purpose of this review is to examine the effects of airborne pollution, including agricultural- and concentrated animal feeding operation (CAFO)-associated particulate matter exposures, on the progression of gastrointestinal diseases through altered gut and lung bacterial and fungal communities. Moreover, we will emphasize the role of these microorganisms on immune responses through the gut-lung axis (GLA).
Figure 1.
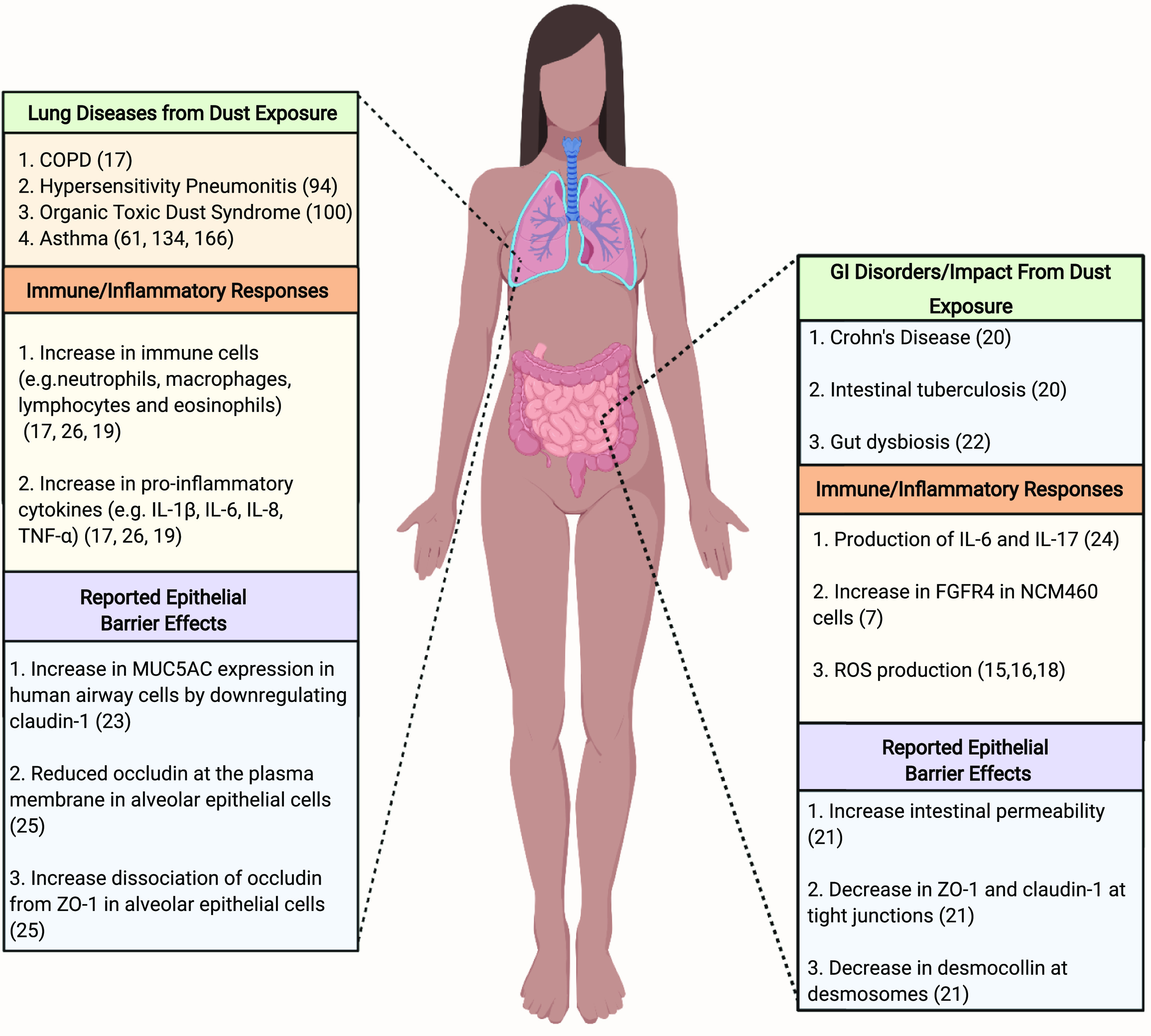
Gut and lung diseases and the reported immune responses associated with agricultural and concentrated animal feeding operation (CAFO) dust exposure.
Table 1.
Major bacterial composition of CAFO dust
| Bacterial Class | CAFO | Disease(s)/Impact | References |
|---|---|---|---|
| Staphylococcus spp. (S. aureus, S. simulans, S. epidermidis, S. chromogenes, S. pasteuri, S. hyicus, S. haemolyticus, S. equorum) | Swine farm | Pneumonia | (27–31) |
| Bacillus spp. (Bacillus cereus) | Poultry farm, swine farm | Respiratory infections, diarrhea, bacteremia | (27–32) |
| Mycobacterium tuberculosis | Swine farm | Pneumonia, Intestinal tuberculosis, Crohn’s disease | (33, 34) |
| Methanobrevibacter, Methanothermobacter, and Methanosphaera | Pre-filtered SFD | Linked to inflammatory bowel disease | (35–37) |
| Ruminococcus | Swine farm | Associated with respiratory allergies and Crohn’s disease | (31, 35, 15) |
| Lactobacillus | Swine farm | Pulmonary infections, Bacteremia, endocarditis | (31, 35, 38) |
| Eubacterium spp. | Swine farm | Associated upper respiratory tract infections and cystic fibrosis | (31, 35, 39, 40) |
| Clostridium spp. (C. perfringens) | Poultry farm, swine farm | Can assist the expansion of regulatory T-cellsPrevents growth of commensal bacteria found in the gut | (31,32, 35) |
| Betaproteobacteria | Swine farm | Low abundance associated with increase in serum IL-6 in acute respiratory disease syndrome patients | (32, 41, 42) |
| Actinobacteria (genus: Micrococcus) | Swine farm | Increase in gut associated with IBDPulmonary hypertension | (32, 43, 44) (27–30, 45) |
| Aerococcus (Aerococcus viridans) | Swine farm | Endocarditis | (27–31) |
| Enterococcus | Swine farm | Upper and lower airway infections | (27–30, 46) |
CAFO, concentrated animal feeding operation; IBD, inflammatory bowel disease; IL-6, interleukin-6; SFD, swine farm dust.
Table 2.
Major fungal/yeast composition of CAFO dust
| Fungi/Yeast | CAFO | Disease(s)/Impact | References |
|---|---|---|---|
| Aspergillus | Swine farm | Enterocolitis, appendicitis, colonic ulcers and GI bleeding | (16,17, 35, 47–50) |
| Acremonium | Swine farm | Colonize the lungs and GI tract | (35, 17, 48, 49, 50, 51) |
| Penicillium spp. | Swine farm | Associate with the onset of pneumonia | (35, 17, 48–50, 52) |
| Cladosporium | Swine farm | Respiratory infection; causes infection in immunocompromised individuals | (35, 17, 48–50, 53) |
| Filobasidium uniguttulatus (Yeast) | Swine farm | Meningitis | (31, 54) |
| Cryptococcus | Swine farm | Acute or chronic infection of the lungs | (31, 55) |
CAFO, concentrated animal feeding operation; GI, gastrointestinal.
Swine Farm Building Environment and Clinical Ramifications
Concentrated animal feeding operations.
Because of the increasing demand for animal products worldwide, small livestock farms have been replaced with large-scale farming productions known as concentrated animal feeding operations (58). These industrial agricultural facilities that raise animals for the consumption of meat, eggs, or milk release toxic gases (e.g., ammonia, hydrogen sulfide) and particles that contribute to the development of respiratory diseases in farm workers (59) and people living in close proximity to CAFOs (60). Moreover, endotoxins found in the dust collected from CAFOs act as the primary factor in the onset of asthma and other respiratory conditions (61–63). A considerable amount of research has shown that exposure to endotoxins is especially prevalent in swine farm workers (64–66). Raising swine is a lucrative agricultural enterprise for US farmers with sales generating 26.3 billion dollars in 2017 (67). The success of swine farms depends on the large-scale indoor confinement of pigs and the commitment of full-time employees (68). Consequently, swine farm workers are frequently exposed to particulate matter in dust including microbial components (e.g., endotoxins, bacteria, and mites) and plant- and animal-derived materials (e.g., pollen and ammonia, respectively). Interestingly, swine farm laborers are reported to have a higher prevalence of occupational respiratory symptoms in comparison with other agricultural workers (11, 69). A clinical study reported that exposure to swine farm air for 2–5 h resulted in the thickening of nasal mucosa, lung function decline, an increase in immune cell infiltrates [e.g., neutrophils, macrophages (MΦ), lymphocytes, and eosinophils], and proinflammatory cytokines [e.g., interleukin (IL)-1β, IL-6, tumor necrosis factor α (TNF-α), and IL-8] in the bronchoalveolar lavage fluid of healthy participants (11, 19, 70). These findings were also corroborated in animal studies by Charavaryamath et al. (71) which demonstrated that swine farm dust (SFD) exposure induced lung inflammation and the recruitment of neutrophils in Sprague–Dawley rats. A more recent study by Roque et al. assessed endotoxin levels in swine barn air. In comparison with pigs raised in barns with low-bacterial endotoxin levels, there was an increase of white blood cells in pigs exposed to high-endotoxin levels. In addition, peripheral blood mononuclear cells (PBMC) collected from high-exposure pigs had greater plasma immunoglobulin (Ig) G and IgE levels but lower IgA levels than that produced by PBMCs from low-exposure pigs (66). The inflammatory capabilities of SFD have also been demonstrated in various in vitro experiments using human bronchial epithelial cells and pulmonary carcinoma cell lines (19, 72). Similar studies have indicated that an increase in proinflammatory cytokines following SFD exposure promoted the adhesion of lymphocytes through the upregulation of intracellular adhesion molecule-1 (73, 74), which contains five Ig superfamily domains (75). Although respiratory immune responses of SFD have been characterized in animal studies, the effects of particulate matter in SFD on the intestinal immune system continue to be an underdeveloped area of investigation.
Respiratory and Systemic Responses to Particulate Matter
Particulate matter, found in ambient and urban air pollution, is a key pollutant linked to chronic airway inflammation, cardiovascular disease, and inflammatory bowel disease (9, 76–78). The components of particulate matter are defined by their aerodynamic equivalent diameter (AED), which determines the particles’ potential to cause disease (79). Depending on particle AED, different regions of the human respiratory tract are penetrated by varying amounts of particulate matter (79, 80). Particle penetration into respiratory regions can be identified as either “inhalable fractions” or “respirable fractions” (80). Inhalable fractions are defined as the mass fraction of total airborne particles inhaled through the nose and mouth whereas respirable fractions can penetrate the unciliated airways (80). These categories recognize the important role of particle deposition and AED in the induction of disease in different regions of the respiratory tract. Ultrafine particles, with an AED of 0.1 µm (PM0.1), pose a major health risk because of their size and their ability to absorb toxins. PM0.1 is formed by the coalescence of ions and gaseous molecules produced by combustion (e.g., vehicle and power plant emissions) (81). These particles can easily penetrate the lungs and translocate through alveolar epithelial cells. This allows for the subsequent transport of toxic cellular fragments via the surface of PM0.1 (82) that can enter the bloodstream and promote inflammation in distal organs (81, 83). Furthermore, the coalescence of ultrafine particulate matter can form larger particles such as PM2.5 (2.5 µm). PM2.5 is an indicator of “fine inhalable particles” which include combustion emissions, organic compounds, and metals (84, 85). “Inhalable particles,” such as pollen, have a diameter of 10 µm (PM10) or smaller and can typically be seen without the assistance of an electron microscope (86). Particles with an AED of 10 µm or larger directly impact the nasopharyngeal membranes (81) and can be swallowed following mucociliary clearance (87). Respiratory studies show that particles with a smaller AED may have the ability to infiltrate the terminal bronchioles and alveoli that are typically inaccessible to larger particles (78, 88). Thus, evidence suggests that the pathogenicity of particulate matter depends on their size (80, 89). PM2.5 and PM10 have distinctive detrimental effects on respiratory and gastrointestinal health (55, 78, 86, 90, 91). To understand the pulmonary mechanisms involved in PM pathogenicity, Chan et al. (92) exposed mice to traffic-related PM10 for 3 wk and found a significant increase in lymphocytes, macrophages, IL-1β expression, and the apoptosis marker, caspase 3, in bronchoalveolar lavage fluid. Because of its small diameter, PM2.5 can easily penetrate the lungs and activate inflammatory signaling cascades, triggering inflammatory cytokine expression and promoting systemic inflammation (93, 94). More specifically, when bronchial epithelial cells are exposed to extracts of swine dust representing PM2.5 and smaller fractions, there is an increase in IL-6 and IL-8 release which is further dependent on the activation of protein kinase-α (PKCα) and -ε (PKCε) isoforms (94, 95). Similarly, C57BL/6J mice chronically exposed to different concentrations of PM2.5 show distinct transcriptional profiles in the lungs associated with immune and cardiovascular disease pathways (96). Although fewer in number, there are studies that examined the mechanisms underlying the harmful effects of PM exposure in the gastrointestinal system. Kish et al. exposed wild-type 129/SvEv mice to PM10 for 7–14 days. This exposure altered immune gene expression, increased proinflammatory cytokine secretion, and barrier permeability in the small intestine (97). In addition, IL-10-deficient mice fed a chow diet supplemented with PM10 exhibited microbial dysbiosis and altered short chain fatty acid composition followed by elevated cytokine expression in the colon (97). In 2011, Mutlu et al. performed comparable in vitro studies in human epithelial colorectal adenocarcinoma cell (Caco-2) monolayers exposed to PM. PM exposure increased mitochondrially derived reactive oxygen species (ROS), intestinal permeability, the activation of nuclear factor-κB (NF-κB) and target gene IL-6, and Caco-2 cell death (98). They validated these findings in vivo by demonstrating that a single oral gavage treatment of PM (200 µg) decreased the colocalization and mRNA levels of zona occluden protein 1 (ZO-1), increased the levels of IL-6 mRNA, and induced apoptosis along the gastrointestinal tract of C57BL/6 mice (98). Overall, these data illustrate the disruptive effects of particulate matter on the respiratory and gastrointestinal systems, respectively. However, additional experimental research is needed to establish a well-defined gut-lung axis following exposure to airborne pollutants (99, 100).
Particulate matter and intestinal permeability.
The intestinal epithelium acts as a selective permeable barrier between luminal contents (e.g., intestinal microbiota) and the immune system (82, 101, 102). The barrier is supported by three distinct structural components: tight junctions that provide the first line of defense against toxins and enteric pathogens and selectively regulate paracellular permeability; the adherens junctions, which are protein complexes at cell-cell junctions that are linked to the actin cytoskeleton and combine with tight junctions to form the apical junctional complex; and desmosomes that form adhesive bonds between cells and provide mechanical strength to tissues (102, 103). Past studies have indicated that the gastrointestinal tract is exposed to high levels of pollutant particulate matter ostensibly from the inhalation of PM and the ingestion of contaminated food and water (9). As a result, studies have shown that particulate matter can obtain access to the gastrointestinal tract through several routes. After mucociliary clearance, particulate matter can pass via the esophagus, through the stomach, and enter the intestinal lumen where it can directly affect epithelial cells and be metabolized by commensal gut microbes causing the release of toxic metabolites (9, 82, 104, 105). Prior research also suggests that once deposited in the lungs, particulate matter behaves similar to gas molecules, moving through the alveoli via diffusion and translocating to the circulatory system (9, 106). Airborne microorganisms (bacteria, fungi, and viruses) are primary components of PM2.5 (107, 108). When microorganisms are inhaled and reach the alveolar space, they are subsequently phagocytosed by alveolar macrophages that further stimulate immune cell activity (9). However, there are microbes that utilize alveolar macrophages to circumvent host defenses. For example, the airborne pathogen Mycobacterium tuberculosis can reside in the phagosomes of macrophages where it can gain access to the cytosol, control host cell death (33, 34), and increase the risk of granulomatous disorders such as intestinal tuberculosis and Crohn’s disease (20). The bacteria and/or spores not destroyed by alveolar macrophages can move through the circulatory system to the intestines. In addition to inhalation, particles and microorganisms released by industrial waste and vehicle exhaust can contaminate food and water supplies, thereby suggesting another route of oral/gastrointestinal exposure (109, 110). Recent epidemiological studies have shown a direct link between particulate matter exposure and intestinal defects (111). Inflammation is a normative response to environmental stressors, even being important for wound healing (111). Commensal gut bacteria modulate the production of reactive oxygen species and various growth factors responsible for intestinal epithelial migration and proliferation (112). Therefore, dysbiosis of the gut microbiome induced by harmful pollutants (113) may severely impair critical processes necessary for intestinal epithelial wound healing (114–116). However, the protective or detrimental role of PM-induced inflammation on gut epithelial cells continues to be an active area of investigation (111). Chronic PM exposure for 12 mo in mice resulted in epithelial lesions and the confluence of inflammatory cells in murine colons (111). In addition, normal human colon epithelial cells (NCM460) exposed to PM for 48–72 h showed an increased expression of fibroblast growth factor receptor 4 (FGFR4) (111), which is associated with the development of colon cancer (117). In 2019, Li et al. (111) demonstrated that FGFR4 activates the phosphatidylinositol-3-kinase (PI3K)/Akt pathway and the removal of FGFR4 prevented PM-induced colorectal cancer in FGFR4−/− mice. Moreover, PM exposure has been suggested to increase the risk of inflammatory bowel diseases and colorectal cancer (118) by altering gut epithelial tight junctions via the production of reactive oxygen species (9, 27, 77) generated by Fenton’s reaction of transition metals commonly detected in PM (119). More recently, a study has shown that levels of zona occluden protein 1 (ZO-1), claudin-1, and the desmosome protein, desmocollin, were decreased in enteroids following exposure to PM2.5 culminating in increased intestinal permeability (21). Consequently, an increase in intestinal permeability allows for pathogenic bacteria, viruses, and fungi to migrate to the lamina propria and interact with intestinal immune cells and commensal microbes (120–122). Ultimately, these interactions increase immune cell activity evidenced by proinflammatory responses from macrophages and dendritic cells, which worsen intestinal permeability and furthers dysbiosis (Fig. 2) (9).
Figure 2.
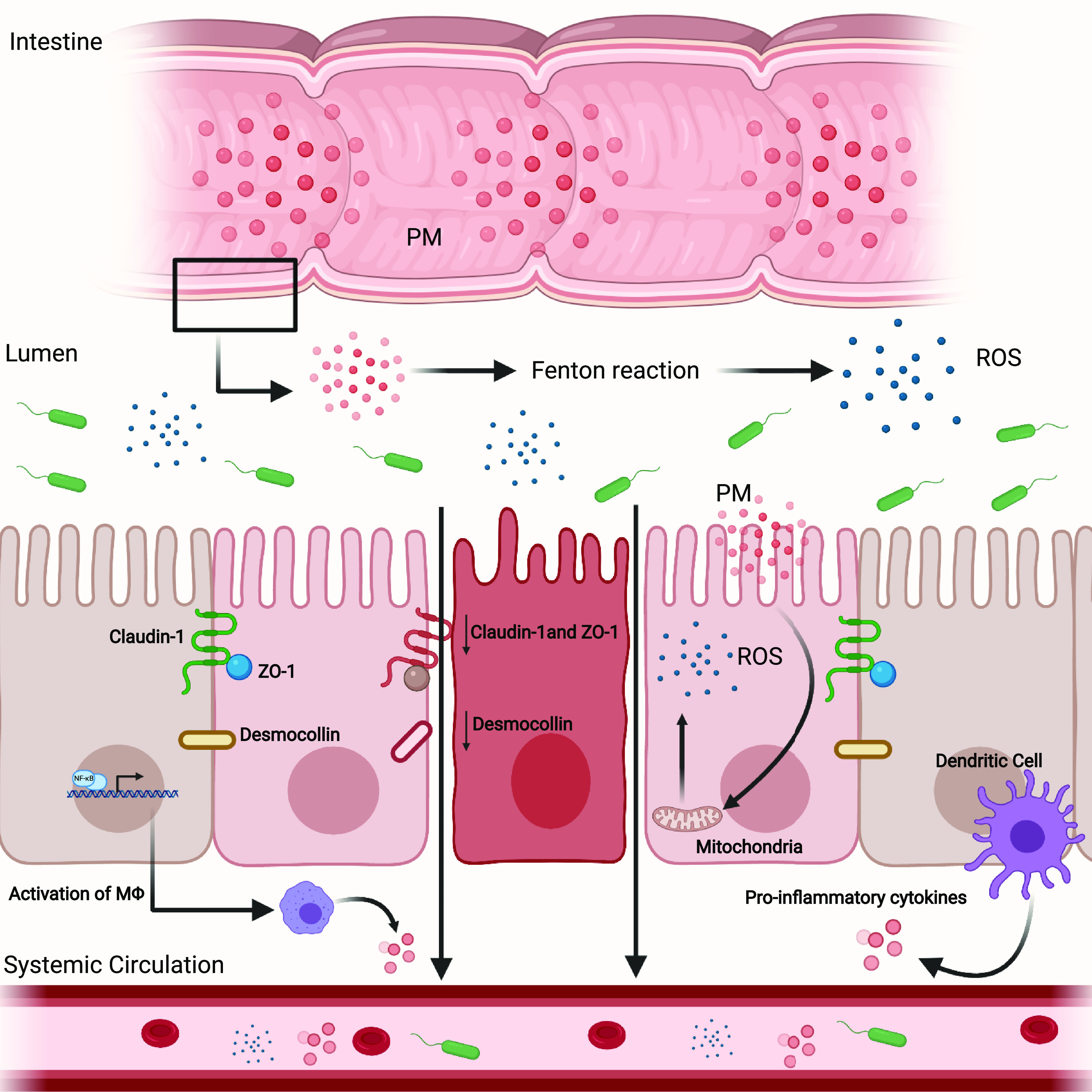
Particulate matter (PM) toxicity and reactive oxygen species (ROS). PM toxicity may come from the generation of hydroxyl radicals and ROS through Fenton’s reaction. Following inhalation, particulate matter can reach the gastrointestinal tract, undergo a catalytic process, and promote systemic inflammation through the production of ROS and the subsequent release of proinflammatory cytokines/chemokines from macrophages (MΦ) and dendritic cells (9, 123–125). In addition, PM exposure can promote mitochondrially derived ROS in intestinal epithelial cells (98), thereby altering the expression of tight-junction proteins, intestinal barrier function, and increasing permeability which allows for the translocation and systemic migration of bacteria/bacterial products and proinflammatory cytokines/chemokines (21).
Respiratory and Gastrointestinal Conditions Associated with Microbial Products in Agricultural Dust
Effects of microbial and fungal communities found in swine farm dust.
Agricultural workers are at extreme risk of developing chronic airway inflammation and severe respiratory illnesses when exposed to workplace dust (126, 127). These risks are further inflated by the absence of face respirators (57). Farmers who maintain crops may develop a type of hypersensitivity pneumonitis referred to as “farmer’s lung” (128, 129). This severe respiratory condition is triggered by the inhalation of mold spores (e.g., Micropolyspora faeni and Aspergillus fumigatus; ∼2–10 µm) that grow in hay and grain (129, 130). Mold spores have the capability of attaching to airborne dust particles (129, 130) and preventing normal lung function (131) such as gas exchange (132). Comparably, swine farm workers also increase their risk of lung function decline and chronic bronchitis from long-term exposure to swine farm dust (133, 134). “Feed particles” are the major component of agricultural dust and SFD (35); however, it is likely that microbes found in SFD provoke the proinflammatory responses linked to respiratory and gastrointestinal diseases. Metagenomic analyses on settled dust collected from two different swine confinement facilities have shown that “pre-filtered” SFD contains archaeal DNA (Methanobrevibacter, Methanothermobacter, and Methanosphaera) (35). However, swine farm dust is largely composed of Staphylococcus, Micrococcus, and Bacillus spp., all of which can metabolize harmful environmental particles and promote bacteremia and ROS production (27–30). In addition to bacteria, fungal species ubiquitous to the environment have also been identified in SFD (Acremonium, Aspergillus, Penicillium, and Cladosporium) (Table 2) although they are less abundant and understudied (17, 35, 48, 50, 135). Past reports indicate infections caused by Acremonium species, although rare, affect immunocompetent individuals by colonizing the lungs and gastrointestinal tract (51). Clinical case studies in immunosuppressed patients have reported the ability of Aspergillus infections to develop into serious gastrointestinal complications including enterocolitis, appendicitis, colonic ulcers, and gastrointestinal bleeding (47, 136). Although the microbial composition of SFD has been characterized, the pathological features and mechanisms of SFD that are responsible for intestinal barrier dysfunction during chronic exposure remain an understudied area of investigation.
Gut-Lung Axis and Microbial Interactions
The gut-lung axis (GLA) describes a two-way interchange between the microbiota and immune system of the gut and lungs (137). In a healthy individual, the microbiomes of the respiratory tract and gastrointestinal system are comprised of distinctive genomes (archaea, eukaryotes, viruses, bacteria, and fungi) that live symbiotically to maintain homeostasis in their respective organs (138). In 2019, Lee et al. (139) indicated that the lung microbiota, as assessed via sputum samples, is primarily composed of microbes from the phyla Firmicutes, Proteobacteria, Bacteroidetes, Actinobacteria, and Fusobacteria. Further examination also revealed similarities between healthy sputum and saliva samples. However, in comparison with saliva, sputum is more abundant in the genera Granulicatella (phyla: Firmicutes) (139). A recent study examining the pharyngeal microbiota of outdoor farmers’ market workers chronically exposed to smog with PM2.5 and PM10 reported an increase in Granulicatella (140) that is directly linked to the onset of lung cancer and endocarditis (141). Interestingly, the lungs, once considered a sterile environment (142), have low-colony density but high-microbial diversity (16). Comparably, murine lung microbiome studies show a similar composition (143). Although the lung microbial community differs from the intestinal microbiota, similar phyla are found (e.g., Bacteroidetes, Firmicutes, and Proteobacteria) and play an important role in establishing the immune system (56). Previous studies have shown that the lung and gut microbiota are essential to the interactions between both mucosal sites and the development of disease. A recent study indicated that lung microbial dysbiosis and dysfunction was associated with the development of inflammatory bowel disease in patients who reported no history of smoking. However, no definitive pathogenic mechanism has been identified (144). In addition, respiratory diseases induced by environmental pollution promote dysbiosis of the pulmonary and intestinal microbiota indicated by an outgrowth of Proteobacteria and Firmicutes (39). It is well documented that changes in the pulmonary flora are directly associated with the onset of respiratory infections including pneumonia and influenza (106). Pulmonary research suggests PM2.5 pathobionts can invade deeply into the lungs and release toxins that damage airway epithelial cells and selectively destroy pulmonary microbes (144, 145). Consequently, pathogenic bacteria and their toxins can translocate through endothelial cells and circulate in the blood to distal organs and tissues (93) including the intestines, culminating in altered gut flora, intestinal barrier dysfunction, and incitement of an immune response. Yet, alterations in the intestinal microbiota have been linked to changes in pulmonary immune responses, inflammation, and disease progression (Fig. 3) (137, 146). For example, the development of asthma, notably in children, has been associated with the reduced abundance of Bifidobacteria and an increase in Clostridia in the intestine (147). In addition, Arrieta et al. (148) have also shown that the over-representation of gut fungal species, specifically Pichia kudriavzevii, is associated with the development of atopic wheeze in children. Moreover, in human and murine models, modulation of the gut microbiota due to antibiotic intake increases the risk of lung diseases and allergic inflammation (149–152). Although the exact mechanisms continue to be examined, studies have shown that gut bacteria interact with the mucosal immune system via metabolites, including proinflammatory and regulatory signals (22, 137, 153, 154).
Figure 3.
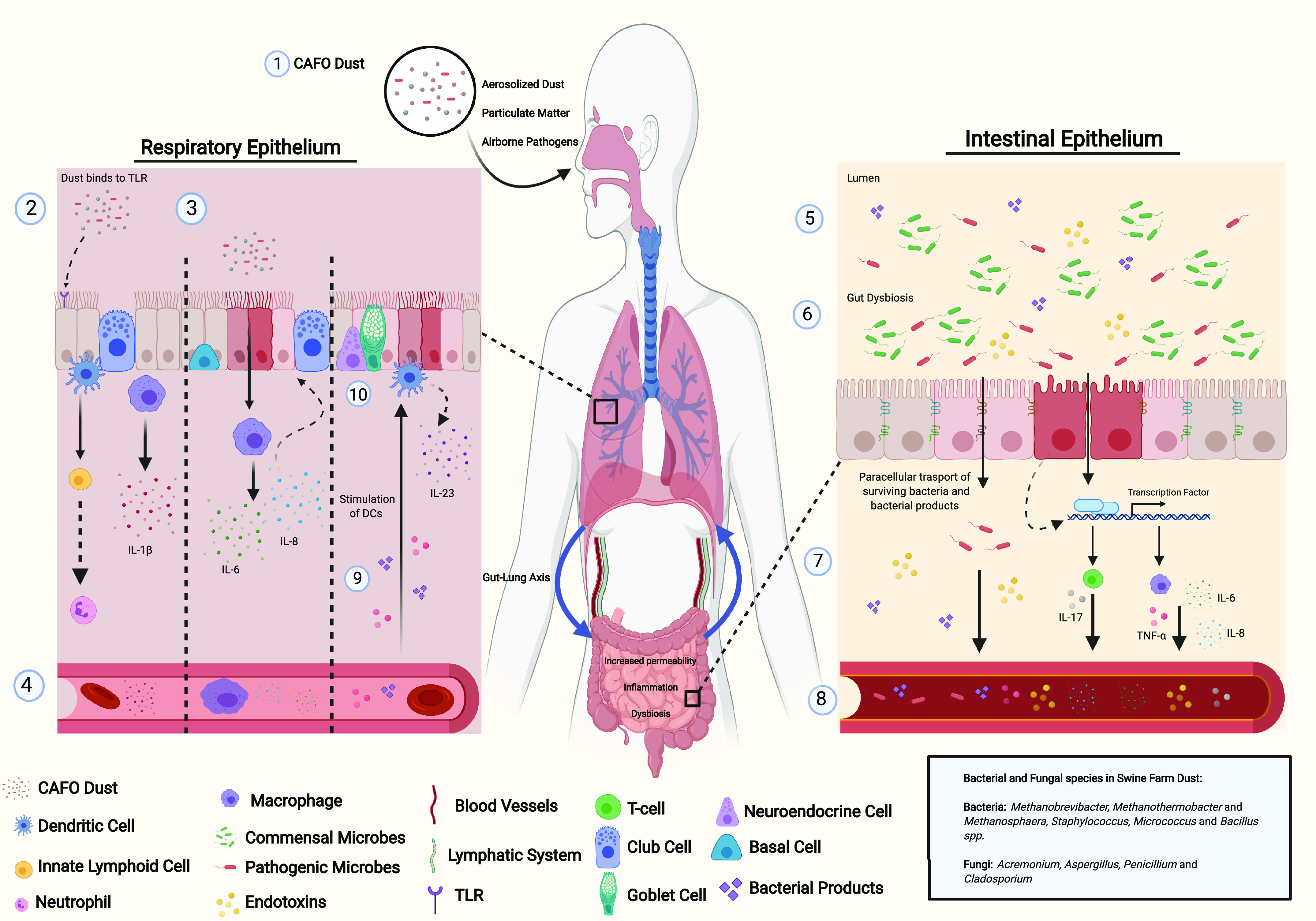
Particulate matter induction of inflammation along the gut-lung axis. (1) When inhaled or ingested dust can reach the alveolar space and promote immune responses. (2) Particulate matter in concentrated animal feeding operations (CAFO) dust, like swine farm dust (SFD), can bind to Toll-like receptors (TLR) on respiratory epithelial cells and activate innate lymphoid cells (ILC). ILCs recruit neutrophils, responsible for killing pathogens. In addition, ILCs such as ILC3 can differentiate into T-helper cells (i.e. Th17) and release interleukin (IL)-17A,F and IL-22. Furthermore, alveolar macrophages release IL-1B inducing pulmonary inflammation. (3) Particulate matter bypasses the respiratory epithelium and is phagocytosed by alveolar macrophages which release proinflammatory cytokines, IL-6, and IL-8. (4) The components of particulate matter (e.g., bacteria and fungi) and proinflammatory cytokines then travel through the circulatory and/or lymphatic system to the intestine. (5) Particulate matter can enter the gut via mucociliary clearance and/or circulation from the lungs. (6) Bacteria, endotoxins, and fungi from CAFO dust induces intestinal dysbiosis, increases permeability, the paracellular transport of pathogens, and promotes the activation of transcription factors such as nuclear factor-κB (NF-κB), which mediates the activation of macrophages in the intestine and releases IL-6, IL-8, and tumor necrosis factor α (TNF-α) (7). (8) Via the intestinal vasculature/mesenteric lymph nodes, proinflammatory cytokines, surviving bacteria, and bacterial products can reach the basolateral membrane of the respiratory epithelium (9) thus stimulating dendritic cells (DC) to produce IL-23 thus promoting further epithelial damage (10).
Immune cell contributions to the gut-lung axis.
It has been suggested that the respiratory microbiome acts as a “gatekeeper,” providing colonization resistance against environmental pathogens (119) and metabolizing pollutants (142). These preventative measures, along with the respiratory mucosa (e.g., mucus production) (119) and ciliary clearance (155), block pathogens and particulate matter from reaching the airway epithelium (70, 144, 156, 157). However, if bacteria or foreign materials invade this barrier, airway epithelial cells express pattern recognition receptors, secrete antimicrobial peptides, and incite an inflammatory response through the activation of mitogen-activated protein kinases (155), thereby serving as the primary line of defense (135). Together, interactions between the respiratory microbiota and the epithelial barrier influence pulmonary immune responses. Of note, prior studies have shown that long-term exposure to urban particulate matter can have severe effects on lung microbial composition and immunity (158, 159). In murine models, Li et al. (89) demonstrated T-helper (Th) 2 cell-mediated immune responses and acute inflammation are linked to toll-like receptor (TLR) 2 and TLR4 activation following exposure to PM2.5. More specifically, urban particulate matter-activated dendritic cells (UPM-DC) are responsible for Th1, Th2, and Th17 differentiation through major histocompatibility class II availability (93). Th1, Th2, and Th17 effector phenotypes are directly implicated in the exacerbation of asthma and chronic lung inflammation (158, 160, 161). In addition, particulate matter also activates dendritic cells to release the proinflammatory cytokine, interleukin-23 (123, 124). IL-23 incites T-cell immunity and stimulates innate lymphoid cell (ILC) (100, 162) production of cytokines responsible for the defense against extracellular bacteria and fungi (23, 163, 164). Moreover, Th17 cells play a significant role in host defense against extracellular pathogens by recruiting neutrophils and macrophages to infected tissues and provide compensatory support in response to epithelial barrier defects (165, 166). In addition, there is evidence that suggests respiratory infections not only alter the lung microbiome but are also responsible for directing signals of infection from the lungs to the gut causing alterations in gut microbiome dynamics (137, 167). This interaction is seen in Balb/c mice infected with aerosolized M. tuberculosis where they exhibit a rapid shift in gut microbial diversity which may indicate these alterations are as a result of M. tuberculosis infection and immune signaling from the lungs (167). During a respiratory infection, bacteria and immune cells can translocate across lung epithelial cells and reach the gastrointestinal tract via lymphatic or blood circulation to activate local intestinal immunity (65, 137, 168). Prior studies have reported that swine farm dust is primarily composed of Ruminococcus, Lactobacillus, Eubacterium, and Clostridium species (31, 35). Clostridium species have been reported to assist in the expansion of regulatory T-cells, which are linked to the suppression of immune responses in the colon of germ-free mice (169). Likewise, spore-forming Clostridium perfringens (C. perfringens) demonstrate rapid proliferation while secreting membrane and cytoskeleton disrupting, pore-forming, and tight-junction disintegrating toxins that prevent the growth of commensal bacteria found in the gut (31). Enterotoxin is linked to food poisoning and nonfoodborne diarrhea through the disruption of claudin tight-junction proteins in gut epithelial cells (31). The disruption of tight-junction proteins in response to environmental pathogens has a direct impact on commensal gut microbes and the pulmonary immune system (170). An increase in intestinal permeability allows for the systemic migration of gut bacteria and their metabolites (short-chain fatty acids; SCFA) to the lungs (168, 171, 172). More specifically, gut-derived metabolites can pass systemically to the bone marrow and promote hematopoiesis (137, 173). Under inflammatory conditions, hematopoietic stem cells can differentiate and give rise to dendritic cell precursors that disseminate to the lungs and mature into CD11b+ dendritic cells (21, 136, 172) that have been shown to be responsible for inducing allergic airway hypersensitivity (174). SCFAs can also affect lung immunity by enhancing CD8+ T-cell activity (22), promoting forkhead box P3 (FOXP3) expression (175) and regulating the production of proinflammatory cytokines (TNF-α, IL-2, IL-6, IL-10) through the activation of free fatty acid receptor 3 (122, 176). Together, these findings demonstrate the level of communication between the gut and lungs in response to alterations of the gut microbiota and intestinal permeability. The gut-lung axis is a two-way system that involves the interactions between their respective microbiota and immune cells. There has been increasing evidence of host-microbe and microbe-microbe interactions shaping immune responses in respiratory diseases and the development of subsequent effects in the gut (177–179). Nevertheless, further investigation is needed to explore the potential pathogenic effects of the gut-lung interaction following agriculture and CAFO exposure.
Air Pollution and COVID-19
An additional and highly topical example of a pathogenic influence on the lung also manifesting symptoms in the gastrointestinal tract is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The outbreak of coronavirus disease 2019 (COVID-19) in December 2019 has led to a global pandemic affecting over 90 million individuals and leading to more than 1.9 million deaths (as of January 14, 2021) (www.who.int) with most patients suffering from respiratory symptoms. It has been reported that SARS coronaviruses infect immune cells and the lung epithelium thereby enhancing proinflammatory cytokine and chemokine production and leading to severe acute respiratory syndrome (180). However, there is increasing awareness of the high prevalence of extrapulmonary symptoms, in particular, those arising from the gastrointestinal tract. Viral infections, such as influenza, can promote dysbiosis in the gut microbiota and increase gut permeability (181). Indeed, studies identified that 46% of patients had GI symptoms, diarrhea (29.3%) being the most frequent (114, 127, 182). More specifically, SARS-CoV-2 activates angiotensin-converting enzyme 2 (ACE2) and promotes enteritis and the risk of diarrhea (183, 184). SARS-CoV-2 uses transmembrane protease serine 2 receptors (TMRPSS2) and TMPRSS4 to gain entry into small intestinal epithelial cells and has been shown to promote enterocyte dysfunction and increase intestinal permeability (185–188). In a manuscript recently deposited in medRxiv, preliminary evidence, generated using multiomics screening approaches on serum samples, suggests that severe COVID-19 may be associated with increased intestinal permeability to microbial products (e.g., lipopolysaccharides/lipopolysaccharide binding protein, β-glucan), altered amino acid metabolism, and compromised gut enterocyte function (189). Moreover, hospitalized patients diagnosed with severe COVID-19 displayed higher levels of plasma zonulin. In the gut, elevated levels of zonulin increase intestinal permeability, microbial-mediated myeloid inflammation, and allow for the translocation of microbes and their products (e.g., lipopolysaccharides/lipopolysaccharide binding protein, β-glucan) from the gut into the systemic circulation (189). Although these data are not yet published, they do begin to identify a mechanism by which GI symptoms and effects of COVID-19 on gastrointestinal function contribute to more severe COVID-19 outcomes.
Risk factors for infection and more severe disease include age, with the elderly (>65 yr old) being most vulnerable, and individuals with underlying medical conditions including respiratory disease, cardiovascular disease, and chronic inflammatory conditions such as obesity and diabetes (190). Obesity, the most common metabolic disease and global epidemic characterized by chronic low-grade inflammation, is implicated in COVID-19 severity in patients with a body mass greater than 40–45 kg/m2 (191–193). Obesity can significantly influence the respiratory system by reducing lung volume and capacity via mechanical changes (e.g., patterns of fat distribution particularly along the chest wall, abdomen, and upper airway). Nevertheless, there is also risk of systemic inflammation through the production of inflammatory cytokines (e.g., TNF-α, IL-1B, IL-6) by excess adipose tissue. In addition, metabolic dysregulation in obese patients promotes intestinal barrier dysfunction (194–197). Reports also state that adipose tissue has higher expression of ACE2 in comparison with the lungs, therefore, serving as a reservoir for viral infections. Increased ACE2 mRNA expression in the ileum of patients with inflammatory bowel disease (IBD) and non-IBD subjects was associated with a higher body mass index (BMI), whereas obesity is also associated with a higher risk of infections that can underlie or exacerbate many conditions that have more severe outcomes in obese subjects (198). Moreover, ACE2 is upregulated in visceral and subcutaneous adipose tissue in obese patients, thereby increasing the risk of ACE2 shedding and the redistribution of the receptor to other tissues (199) via transcriptional upregulation and activation of a disintegrin and metalloproteinase domain 17 (ADAM17) (200, 201).
An additional vulnerability appears to be the association between increased deaths from COVID-19 in areas with high levels of air pollution, more specifically, elevated exposure to the toxic component nitrogen dioxide (NO2). Anthropogenic activity, such as fossil fuel combustion, releases NO2 into the atmosphere and exposure has been linked to metabolic disorders (96), COPD (202), and lung injury (203). Moreover, NO2 promotes the production of proinflammatory cytokines and lung epithelial cell death (204). In 2020, Ogen (205) assessed long-term exposure to NO2 in European countries and found a strong correlation between high levels of NO2 (>100 µmol/m2) and COVID-19 fatalities. Nitrogen dioxide can also react with other chemicals and produce secondary pollutants such as ozone and PM (206). A recent nationwide cross-sectional study revealed that an increase of just 1 µg/m3 of PM corresponded to an 8% increase in COVID-19 deaths (207). Although the mechanisms by which air pollution modifies severity of COVID-19 responses have yet to be determined, very little is known about the host factors that determine mild or even asymptomatic responses compared with life-threatening or fatal outcomes. As with many inflammatory diseases, it is the combination of specific host and environmental factors in certain individuals that provokes more severe disease outcomes. What does appear to be emerging from evidence generated thus far is that environmental air pollutants have a positive correlation with overall COVID-19 severity (207). Furthermore, members of racial and ethnic minority groups are at a greater risk of contracting COVID-19 because of social inequalities and health disparities (208). This increased vulnerability is also seen among seasonal agricultural workers in COVID-19 high-risk rural counties in the US (209) because of factors including confined group housing conditions as well as limited access to healthcare and personal protective equipment (44, 209, 210). Moreover, although the specific influences of aerosolized agricultural dust on COVID-19 are unknown, it is not unreasonable to suggest that given its causal role in many respiratory conditions, agricultural dust may represent a potential additional risk factor for COVID-19 infection, or more severe outcomes, in agricultural workers. This may be particularly relevant to the high levels of COVID-19 in Imperial County, California (149). This rural and agriculture-intense inland county has a number of disadvantages as it battles the pandemic including limited access to healthcare, high levels of poverty, obesity, and asthma hospitalizations (209, 211), as well as poor air quality with higher levels of particulate matter exposure than the state or national averages (24, 119, 212).
CONCLUSIONS
Farmers and farm workers are regularly exposed to agriculture dust (57). Adverse respiratory health effects including reduced lung function and shortness of breath (213, 214) are directly linked to the components of agriculture particulate matter such as chemicals, bacteria, fungi, and viruses (35, 76). Interestingly, chronic lung disorders and respiratory infections as a result of particulate matter exposure are often accompanied by gastrointestinal diseases (appendicitis, bowel infections, and irritable bowel disease) (47, 215, 216). The source of these GI disorders has been linked to lung microbial alterations followed by a robust immune response of the respiratory system. In parallel, gastrointestinal diseases have also been shown to be a comorbidity for respiratory disorders. Although the respiratory effects of air pollution and agriculture dust have been well investigated, there is a lack of studies that examine the consequences of inhaled particulate matter from agriculture and CAFO dust on intestinal barrier function and immunity. Further investigation is especially important for minority populations who are generally employed as seasonal farm workers with limited healthcare access. Thus, identifying the mechanistic properties of the interactions seen between both mucosal sites is an important area of exploration and imperative to understanding the physiological consequences of chronic exposure to harmful environmental pollutants while also promoting awareness of the health disparities in minority and immigrant populations.
GRANTS
This study was supported by National Institutes of Health Grants 2R01DK091281 (to D. F. McCole), 1R01AI153314 (to D. F. McCole), and R00ES025819 (T. M. Nordgren).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S.C. prepared figures; M.S.C. and D.F.M. drafted manuscript; M.S.C., T.M.N., and D.F.M. edited and revised manuscript; M.S.C., T.M.N., and D.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Figures were created with BioRender.com.
REFERENCES
- 1.WHO. Health, Environment and Climate Change: Report by the Director-General. EB142/12. 2018. WHO, 2016, p. 1–7. [http://apps.who.int/gb/ebwha/pdf_files/EB142/B142_12-en.pdf]. [Google Scholar]
- 2.Lighthall D. The poor health of farm workers. West J Med 175: 223–224, 2001. doi: 10.1136/ewjm.175.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenker MB, Christiani D, Cormier Y, Dimich-Ward H, Doekes G, Dosman J , et al. Respiratory health hazards in agriculture. Am J Respir Crit Care Med 158: S1–S76, 1998. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 4.Elliott L, von Essen S. COPD in farmers: what have we learnt? Eur Respir J 47: 16–18, 2016. doi: 10.1183/13993003.01768-2015. [DOI] [PubMed] [Google Scholar]
- 5.Guillien A, Puyraveau M, Soumagne T, Guillot S, Rannou F, Marquette D, Berger P, Jouneau S, Monnet E, Mauny F, Laplante JJ, Dalphin JC, Degano B. Prevalence and risk factors for COPD in farmers: a cross-sectional controlled study. Eur Respir J 47: 95–103, 2016. doi: 10.1183/13993003.00153-2015. [DOI] [PubMed] [Google Scholar]
- 6.Hu R, Shi L, Lee DC, Haile GP. Access to and disparities in care among migrant and seasonal farm workers (MSFWs) at U.S. health centers. J Health Care Poor Underserved 27: 1484–1502, 2016. doi: 10.1353/hpu.2016.0107. [DOI] [PubMed] [Google Scholar]
- 7.Linaker C, Smedley J. Respiratory illness in agricultural workers. Occup Med (Lond) 52: 451–459, 2002. doi: 10.1093/occmed/52.8.451. [DOI] [PubMed] [Google Scholar]
- 8.Environmental Protection Agency. US Environmental Protection Agency Greenhouse Gas Emissions. Greenhouse Gas Emissions. [https://www3.epa.gov/climatechange/ghgemissions/].
- 9.Salim SY, Jovel J, Wine E, Kaplan GG, Vincent R, Thiesen A, Barkema HW, Madsen KL. Exposure to ingested airborne pollutant particulate matter increases mucosal exposure to bacteria and induces early onset of inflammation in Neonatal IL-10-deficient mice. Inflamm Bowel Dis 20: 1129–1138, 2014. doi: 10.1097/MIB.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 10.Winkler SL, Anderson JE, Garza L, Ruona WC, Vogt R, Wallington TJ. Vehicle criteria pollutant (PM, NOx, CO, HCs) emissions: how low should we go? NPJ Clim Atmos Sci 1: 26, 2018. doi: 10.1038/s41612-018-0037-5. [DOI] [Google Scholar]
- 11.Charavaryamath C, Singh B. Pulmonary effects of exposure to pig barn air. J Occup Med Toxicol 1: 10–14, 2006. doi: 10.1186/1745-6673-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordgren TM, Bailey KL. Pulmonary health effects of agriculture. Curr Opin Pulm Med 22: 144–149, 2016. doi: 10.1097/MCP.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert SA, Von Essen S, Jacobitz K, Crouch R, Lintner CP. Organic dust toxic syndrome: a review. J Toxicol Clin Toxicol 41: 185–193, 2003. doi: 10.1081/clt-120019136. [DOI] [PubMed] [Google Scholar]
- 14.Von Essen SG, Auvermann BW. Health effects from breathing air near CAFOs for feeder cattle or hogs. J Agromed 10: 55–64, 2005. doi: 10.1300/J096v10n04_08. [DOI] [PubMed] [Google Scholar]
- 15.Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH, Ni YH. Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 154: 154–167, 2018. doi: 10.1053/j.gastro.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Costa AN, Costa FMD, Campos SV, Salles RK, Athanazio RA. The pulmonary microbiome: challenges of a new paradigm. J Bras Pneumol 44: 424–432, 2018. doi: 10.1590/S1806-37562017000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CW, Chung H, Huang CF, Su HJ. Exposure of workers to airborne microorganisms in open-air swine houses. Appl Environ Microbiol 67: 155–161, 2001. doi: 10.1128/AEM.67.1.155-161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142: 46–54.e42, 2012. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Manninen A, Malmberg P, Larsson K. Inhalation of swine-house dust increases the concentrations of interleukin-1 beta (IL-1 beta) and interleukin-1 receptor antagonist (IL-1ra) in peripheral blood. Respir Med 92: 1022–1027, 1998. doi: 10.1016/S0954-6111(98)90349-3. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee R, Balaji M, Sasikala M, Anuradha S, Rao G, Nageshwar Reddy D. Granulomas of intestinal tuberculosis and Crohn’s disease can be differentiated by CD73 cell surface marker expression: a pilot study. Dig Dis Sci 58: 2301–2307, 2013. doi: 10.1007/s10620-013-2667-0. [DOI] [PubMed] [Google Scholar]
- 21.Woodby B, Schiavone ML, Pambianchi E, Mastaloudis A, N Hester S, M Wood S, Pecorelli A, Valacchi G. Particulate matter decreases intestinal barrier-associated proteins levels in 3D human intestinal model. Int J Environ Res Public Health 17: 3234, 2020. doi: 10.3390/ijerph17093234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scales BS, Dickson RP, Huffnagle GB. A tale of two sites: how inflammation can reshape the microbiomes of the gut and lungs. J Leukoc Biol 100: 943–950, 2016. doi: 10.1189/jlb.3MR0316-106R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 17: 765–774, 2016. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Environmental Health Sciences. Imperial County Community Air Monitoring Project. 2020. [www.niehs.nih.gov/resaerch/supported/translational/community/imperial/index.cfm]
- 25.Caraballo JC, Yshii C, Westphal W, Moninger T, Comellas AP. Ambient particulate matter affects occludin distribution and increases alveolar transepithelial electrical conductance. Respirology 16: 340–349, 2011. doi: 10.1111/j.1440-1843.2010.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JR. Why emergency physicians should care about the Salton sea. West J Emerg Med 18: 1008–1009, 2017. doi: 10.5811/westjem.2017.8.36034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 3: 17001, 2017. doi: 10.1038/npjbiofilms.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crook B, Robertson JF, Glass SA, Botheroyd EM, Lacey J, Topping MD. Airborne dust, ammonia, microorganisms, and antigens in pig confinement houses and the respiratory health of exposed farm workers. Am Ind Hyg Assoc J 52: 271–279, 1991. doi: 10.1080/15298669191364721. [DOI] [PubMed] [Google Scholar]
- 29.Miltiadous G, Elisaf M. Native valve endocarditis due to Micrococcus luteus: a case report and review of the literature. J Med Case Rep 5: 251, 2011. doi: 10.1186/1752-1947-5-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer G, Campbell W, Jenks J, Beesley C, Katsivas T, Hoffmaster A, Mehta SR, Reed S. Persistent Bacillus cereus bacteremia in 3 persons who inject drugs, San Diego, California, USA. Emerg Infect Dis 22: 1621–1623, 2016. doi: 10.3201/eid2209.150647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White JK, Nielsen JL, Madsen AM. Microbial species and biodiversity in settling dust within and between pig farms. Environ Res 171: 558–567, 2019. doi: 10.1016/j.envres.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Luiken REC, Van Gompel L, Bossers A, Munk P, Joosten P, Hansen RB, Knudsen BE, García-Cobos S, Dewulf J, Aarestrup FM, Wagenaar JA, Smit LAM, Mevius DJ, Heederik DJJ, Schmitt H; EFFORT-group. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ Int 143: 105971, 2020. doi: 10.1016/j.envint.2020.105971. [DOI] [PubMed] [Google Scholar]
- 33.Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol 35: 563–583, 2013. doi: 10.1007/s00281-013-0388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Queval CJ, Brosch R, Simeone R. The macrophage: a disputed fortress in the battle against Mycobacterium tuberculosis. Front Microbiol 8: 2284, 2017. doi: 10.3389/fmicb.2017.02284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boissy RJ, Romberger DJ, Roughead WA, Weissenburger-Moser L, Poole JA, LeVan TD. Shotgun pyrosequencing metagenomic analyses of dusts from swine confinement and grain facilities. PLoS One 9: e95578, 2014. doi: 10.1371/journal.pone.0095578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghoshal U, Shukla R, Srivastava D, Ghoshal UC. Irritable bowel syndrome, particularly the constipation-predominant form, involves an increase in Methanobrevibacter smithii, which is associated with higher methane production. Gut Liver 10: 932–938, 2016. doi: 10.5009/gnl15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang C, Weidenbach K, Gutsmann T, Heine H, Schmitz RA. The intestinal archaea Methanosphaera stadtmanae and Methanobrevibacter smithii activate human dendritic cells. PLoS One 9: e99411, 2014. doi: 10.1371/journal.pone.0099411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherid M, Samo S, Sulaiman S, Husein H, Sifuentes H, Sridhar S. Liver abscess and bacteremia caused by lactobacillus: role of probiotics? Case report and review of the literature. BMC Gastroenterol 16: 4–9, 2016. doi: 10.1186/s12876-016-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsland BJ, Trompette A, Gollwitzer ES. The gut-lung axis in respiratory disease. Ann Am Thorac Soc 12: S150–S156, 2015. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 40.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol 17: 1–20, 2019. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyo M, Nishioka K, Nakaya T, Kida Y, Tanabe Y, Ohshimo S, Shime N. Unique patterns of lower respiratory tract microbiota are associated with inflammation and hospital mortality in acute respiratory distress syndrome. Respir Res 20: 246, 2019. doi: 10.1186/s12931-019-1203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vestergaard DV, Holst GJ, Basinas I, Elholm G, Schlünssen V, Linneberg A, Šantl-Temkiv T, Finster K, Sigsgaard T, Marshall IPG. Pig farmers' homes harbor more diverse airborne bacterial communities than pig stables or suburban homes. Front Microbiol 9: 870, 2018. doi: 10.3389/fmicb.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, Arasaradnam RP. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog 12: 1–8, 2020. doi: 10.1186/s13099-019-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuiran R, Roberts N. Farmworkers are among those at highest risk for COVID-19, studies show. Public Broadcasting Service, 21 July 2020. [www.pbs.org/wgbh/frontline/article/covid-19-farmworkers-among-highest-risk-studies-show/]
- 45.Oudiz RJ, Widlitz A, Beckmann XJ, Camanga D, Alfie J, Brundage BH, Barst RJ. Micrococcus-associated central venous catheter infection in patients with pulmonary arterial hypertension. Chest 126: 90–94, 2004. doi: 10.1378/chest.126.1.90. [DOI] [PubMed] [Google Scholar]
- 46.Savini V, Gherardi G, Astolfi D, Polilli E, Dicuonzo G, D'Amario C, Fazii P, D'Antonio D. Insights into airway infections by enterococci: a review. Recent Pat Antiinfect Drug Discov 7: 36–44, 2012. doi: 10.2174/157489112799829774. [DOI] [PubMed] [Google Scholar]
- 47.Kazan E, Maertens J, Herbrecht R, Weisser M, Gachot B, Vekhoff A, Caillot D, Raffoux E, Fagot T, Reman O, Isnard F, Thiebaut A, Bretagne S, Cordonnier C. A retrospective series of gut aspergillosis in haematology patients. Clin Microbiol Infect 17: 588–594, 2011. doi: 10.1111/j.1469-0691.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee SA, Adhikari A, Grinshpun SA, McKay R, Shukla R, Reponen T. Personal exposure to airborne dust and microorganisms in agricultural environments. J Occup Environ Hyg 3: 118–130, 2006. doi: 10.1080/15459620500524607. [DOI] [PubMed] [Google Scholar]
- 49.Létourneau V, Nehmé B, Mériaux A, Massé D, Duchaine C. Impact of production systems on swine confinement buildings bioaerosols. J Occup Environ Hyg 7: 94–102, 2010. doi: 10.1080/15459620903425642. [DOI] [PubMed] [Google Scholar]
- 50.Viegas C, Carolino E, Sabino R, Viegas S, Veríssimo C. Fungal contamination in swine: a potential occupational health threat. J Toxicol Environ Health A 76: 272–280, 2013. doi: 10.1080/15287394.2013.757205. [DOI] [PubMed] [Google Scholar]
- 51.Fincher RM, Fisher JF, Lovell RD, Newman CL, Espinel-Ingroff A, Shadomy HJ. Infection due to fungus Acremonium (cephalosporium). Medicine (Baltimore) 70: 398–409, 1991. doi: 10.1097/00005792-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Oshikata C, Tsurikisawa N, Saito A, Watanabe M, Kamata Y, Tanaka M, Tsuburai T, Mitomi H, Takatori K, Yasueda H, Akiyama K. Fatal pneumonia caused by Penicillium digitatum: a case report. BMC Pulm Med 13: 16, 2013. doi: 10.1186/1471-2466-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grava S, Lopes FA, Cavallazzi RS, Grassi MF, Svidzinski TI. A rare case of hemorrhagic pneumonia due to Cladosporium cladosporioides. J Bras Pneumol. 42: 392–394, 2016. doi: 10.1590/S1806-37562016000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan W, Liao W, Hagen F, Theelen B, Shi W, Meis JF, Boekhout T. Meningitis caused by Filobasidium uniguttulatum: case report and overview of the literature. Mycoses 55: 105–109, 2012. doi: 10.1111/j.1439-0507.2011.02054.x. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol Suppl 105: 2412–2419, 2010. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 20: e12966, 2018. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 57.Rumchev K, Gilbey S, Mead-Hunter R, Selvey L, Netto K, Mullins B. Agricultural dust exposures and health and safety practices among Western Australian wheatbelt farmers during harvest. Int J Envrion Res Public Health 16: 7–9, 2019. doi: 10.3390/ijerph16245009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hribar C. Understanding concentrated animal feeding operations and their impact on communities. Environment Health; 1–22, 2010. [http://www.cdc.gov/nceh/ehs/docs/understanding_cafos_nalboh.pdf] [2019 Nov 28]. [Google Scholar]
- 59.Heederik D, Sigsgaard T, Thorne PS, Kline JN, Avery R, Bønløkke JH, Chrischilles EA, Dosman JA, Duchaine C, Kirkhorn SR, Kulhankova K, Merchant JA. Health effects of airborne exposures from concentrated animal feeding operations. Environ Health Perspect 115: 298–302, 2007. doi: 10.1289/ehp.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz AA, Peppard P, Gangnon RE, Malecki KMC. Residential proximity to concentrated animal feeding operations and allergic and respiratory disease. Environ Int 130: 104911, 2019. doi: 10.1016/j.envint.2019.104911. [DOI] [PubMed] [Google Scholar]
- 61.Attwood P, Brouwer R, Ruigewaard P, Versloot P, de Wit R, Heederik D, Boleij JS. A study of the relationship between airborne contaminants and environmental factors in Dutch swine confinement buildings. Am Ind Hyg Assoc J 48: 745–751, 1987. doi: 10.1080/15298668791385507. [DOI] [PubMed] [Google Scholar]
- 62.Donham KJ, Zavala DC, Merchant JA. Respiratory symptoms and lung function among workers in swine confinement buildings: a cross-sectional epidemiological study. Arch Environ Health 39: 96–101, 1984. doi: 10.1080/00039896.1984.10545842. [DOI] [PubMed] [Google Scholar]
- 63.Rasmussen SG, Casey JA, Bandeen-Roche K, Schwartz BS. Proximity to industrial food animal production and asthma exacerbations in Pennsylvania, 2005–2012. Int J Envrion Res Public Health 14: 362, 2017. doi: 10.3390/ijerph14040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basinas I, Schlünssen V, Takai H, Heederik D, Omland Ø, Wouters IM, Sigsgaard T, Kromhout H. Exposure to inhalable dust and endotoxin among danish pig farmers affected by work tasks and stable characteristics. Ann Occup Hyg 57: 1005–1019, 2013. doi: 10.1093/annhyg/met029. [DOI] [PubMed] [Google Scholar]
- 65.Heederik D, Brouwer R, Biersteker K, Boleij JS. Relationship of airborne endotoxin and bacteria levels in pig farms with the lung function and respiratory symptoms of farmers. Int Arch Occup Environ Health 62: 595–560, 1991. doi: 10.1007/BF00381114. [DOI] [PubMed] [Google Scholar]
- 66.Roque K, Shin KM, Jo JH, Lim GD, Song ES, Shin SJ, Gautam R, Lee JH, Kim YG, Cho AR, Kim CY, Kim HJ, Lee MS, Oh HG, Lee BC, Kim JH, Kim KH, Jeong HK, Kim HA, Heo Y. Association between endotoxin levels in dust from indoor swine housing environments and the immune responses of pigs. J Vet Sci 19: 331–338, 2018. doi: 10.4142/jvs.2018.19.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.United States Department of Agriculture-National Agricultural Statistics Service. United States Summary and State Data. 2017 Census of Agriculture. Washington, DC: USDA NASS, 2019, 1. [AC-07-A-51]. [Google Scholar]
- 68.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 9: 185–196, 2003. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 69.Iversen M, Dahl R, Korsgaard J, Hallas T, Jensen EJ. Respiratory symptoms in Danish farmers: an epidemiological study of risk factors. Thorax 43: 872–877, 1988. doi: 10.1136/thx.43.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malmberg P, Larsson K. Acute exposure to swine dust causes bronchial hyperresponsiveness in healthy subjects. Eur Respir J 6: 400–404, 1993. [PubMed] [Google Scholar]
- 71.Charavaryamath C, Janardhan KS, Townsend HG, Willson P, Singh B. Multiple exposures to swine barn air induce lung inflammation and airway hyper-responsiveness. Respir Res 6: 50, 2005. doi: 10.1186/1465-9921-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmberg L, Larsson B-M, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 53: 260–264, 1998. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathisen T, Von Essen SG, Wyatt TA, Romberger DJ. Hog barn dust extract augments lymphocyte adhesion to human airway epithelial cells. J Appl Physiol (1985) 96: 1738–1744, 2004. doi: 10.1152/japplphysiol.00384.2003. [DOI] [PubMed] [Google Scholar]
- 74.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol (1985) 93: 289–296, 2002. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 75.Owens RM, Gu X, Shin M, Springer TA, Jin MM. Engineering of single Ig superfamily domain of intercellular adhesion molecule 1 (ICAM-1) for native fold and function. J Biol Chem 285: 15906–15915, 2010. doi: 10.1074/jbc.M110.104349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kundu S, Stone EA. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ Sci Process Impacts 16: 1360–1370, 2014. doi: 10.1039/c3em00719g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Opstelten JL, Beelen RMJ, Leenders M, Hoek G, Brunekreef B, van Schaik FDM, Siersema PD, Eriksen KT, Raaschou-Nielsen O, Tjønneland A, Overvad K, Boutron-Ruault M-C, Carbonnel F, de Hoogh K, Key TJ, Luben R, Chan SSM, Hart AR, Bueno-de-Mesquita HB, Oldenburg B. Exposure to ambient air pollution and the risk of inflammatory bowel disease: a european nested case–control study. Digest Dis Sci 61: 2963–2971, 2016. doi: 10.1007/s10620-016-4249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. J Thorac Dis 8: E69–E74, 2016. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan M, Lednicky JA, Wu C-Y. Collection, particle sizing and detection of airborne viruses. J Appl Microbiol 127: 1596–1611, 2019. doi: 10.1111/jam.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown JS, Gordon T, Price O, Asgharian B. Thoracic and respirable particle definitions for human health risk assessment. Part Fibre Toxicol 10: 12, 2013. doi: 10.1186/1743-8977-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med 52: 311–317, 2020. doi: 10.1038/s12276-020-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oberdorster G. Lung dosimetry: pulmonary clearance of inhaled particles. Aersol Sci Technol 18: 279–289, 1993. doi: 10.1080/02786829308959605. [DOI] [Google Scholar]
- 83.Cassee FR, Muijser H, Duistermaat E, Freijer JJ, Geerse KB, Marijnissen JCM, Arts JHE. Particle size-dependent total mass deposition in lungs determines inhalation toxicity of cadmium chloride aerosols in rats. Application of a multiple path dosimetry model. Arch Toxicol 76: 277–286, 2002. doi: 10.1007/s00204-002-0344-8. [DOI] [PubMed] [Google Scholar]
- 84.Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol 6: 24–19, 2009. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morakinyo OM, Mokgobu MI, Mukhola MS, Hunter RP. Health outcomes of exposure to biological and chemical components of inhalable and respirable particulate matter. Int J Environ Res Public Health 13: 592–522, 2016. doi: 10.3390/ijerph13060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.U.S. Environmental Protection Agency. Particulate Matter (PM) Basics. 2018 https://www.epa.gov/pm-pollution/particulate-matter-pm-basics#effects.
- 87.Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 9: a028241, 2017. doi: 10.1101/cshperspect.a028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glencross DA, Ho TR, Camiña N, Hawrylowicz CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med 151: 56–68, 2020. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- 89.Li R, Zhou R, Zhang J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett 15: 7506–7514, 2018. doi: 10.3892/ol.2018.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes 5: 215–219, 2014. doi: 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vallès Y, Francino MP. Air pollution, early life microbiome, and development. Curr Environ Health Rep 5: 512–521, 2018. doi: 10.1007/s40572-018-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan YL, Wang B, Chen H, Ho KF, Cao J, Hai G, Jalaludin B, Herbert C, Thomas PS, Saad S, Oliver BGG. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am J Physiol Lung Cell Mol Physiol 317: L424–L430, 2019. doi: 10.1152/ajplung.00232.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu T, Chen X, Xu Y, Wu W, Tang W, Chen Z, Ji G, Peng J, Jiang Q, Xiao J, Li X, Zeng W, Xu X, Hu J, Guo Y, Zou F, Du Q, Zhou H, He Y, Ma W. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: evidence from a population-based epidemiological study. Environ Int 130: 104882, 2019. doi: 10.1016/j.envint.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 94.Nordgren TM, Heires AJ, Wyatt TA, Poole JA, Levan TD, Cerutis DR, Romberger DJ. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res 14: 51, 2013. doi: 10.1186/1465-9921-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wyatt TA, Slager RE, Heires AJ, DeVasure JM, VonEssen SG, Poole JA, Romberger DJ. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am J Respir Cell Mol Biol 42: 706–715, 2010. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shin S, Bai L, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Goldberg MS, Copes R, Kopp A, Chen H. Association between road traffic noise and incidence of diabetes mellitus and hypertension in Toronto, Canada: a population-based cohort study. J Am Heart Assoc 9: e013021, 2020. doi: 10.1161/JAHA.119.013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, Madsen KL. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One 8: e62220, 2013. doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A, Jakate S, Keshavarzian A, Budinger GR, Mutlu GM. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol 8: 19, 2011. doi: 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, Macpherson A, Neurath MF, Ali RAR, Vavricka SR, Fiocchi C. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 15: 39–49, 2018. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 100.Feng J, Cavallero S, Hsiai T, Li R. Impact of air pollution on intestinal redox lipidome andmicrobiome. Free Radic Biol Med 151: 99–110, 2020. doi: 10.1016/j.freeradbiomed.2019.12.044. [DOI] [PubMed] [Google Scholar]
- 101.Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med 50: 103, 2018. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 124: 3–22, 2009. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Catalioto RM, Maggi CA, Giuliani S. Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem 18: 398–426, 2011. doi: 10.2174/092986711794839179. [DOI] [PubMed] [Google Scholar]
- 104.Kreyling WG. Interspecies comparison of lung clearance of “insoluble” particles. J Aerosol Med Pulm D 3: S-93–S-110, 1990. doi: 10.1089/jam.1990.3. [DOI] [Google Scholar]
- 105.Möller W, Häussinger K, Winkler-Heil R, Stahlhofen W, Meyer T, Hofmann W, Heyder J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol (1985) 97: 2200–2206, 2004. doi: 10.1152/japplphysiol.00970.2003. [DOI] [PubMed] [Google Scholar]
- 106.Kim KH, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int 74: 136–143, 2015. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Guo M, Liu G, Yu G, Wang P, Wang H, Chai T. Detection and analysis of fine particulate matter and microbial aerosol in chicken houses in Shandong Province, China. Poult Sci 97: 995–1005, 2018. doi: 10.3382/ps/pex388. [DOI] [PubMed] [Google Scholar]
- 108.Zhai Y, Li X, Wang T, Wang B, Li C, Zeng G. A review on airborne microorganisms in particulate matters: composition, characteristics and influence factors. Environ Int 113: 74–90, 2018. doi: 10.1016/j.envint.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 109.Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: an environmental factor contributing to intestinal disease. J Crohns Colitis 5: 279–286, 2011. doi: 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 110.Sun F, Yun D, Yu X. Air pollution, food production and food security: a review from the perspective of food system. J Int Agric 16: 2945–2962, 2017. doi: 10.1016/S2095-3119(17)61814-8. [DOI] [Google Scholar]
- 111.Li X, Cui J, Yang H, Sun H, Lu R, Gao N, Meng Q, Wu S, Wu J, Aschner M, Chen R. Colonic injuries induced by inhalational exposure to particulate-matter air pollution. Adv Sci (Weinh) 6: 1900180, 2019. doi: 10.1002/advs.201900180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers 6: 1539595, 2018. doi: 10.1080/21688370.2018.1539595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bailey MJ, Naik NN, Wild LE, Patterson WB, Alderete TL. Exposure to air pollutants and the gut microbiota: a potential link between exposure, obesity, and type 2 diabetes. Gut Microbes 11: 1188–1202, 2020. doi: 10.1080/19490976.2020.1749754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31: 677–689, 2009. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 115.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by t-bet deficiency in the innate immune system. Cell 131: 33–45, 2007. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pineton de Chambrun G, Body-Malapel M, Frey-Wagner I, Djouina M, Deknuydt F, Atrott K, Esquerre N, Altare F, Neut C, Arrieta MC, Kanneganti TD, Rogler G, Colombel JF, Cortot A, Desreumaux P, Vignal C. Aluminum enhances inflammation and decreases mucosal healing in experimental colitis in mice. Mucosal Immunol 7: 589–601, 2014. doi: 10.1038/mi.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho SH, Hong CS, Kim HN, Shin MH, Kim KR, Shim HJ, Hwang JE, Bae WK, Chung IJ. FGFR4 Arg388 is correlated with poor survival in resected colon cancer promoting epithelial to mesenchymal transition. Cancer Res Treat 49: 766–777, 2017. doi: 10.4143/crt.2016.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14: 9–21, 2017. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Man WH, De Steenhuijsen Piters WAA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15: 259–270, 2017. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 157: 121–114, 2014. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu H, Zhang X, Zhang H, Yao X, Zhou M, Wang J, He Z, Zhang H, Lou L, Mao W, Zheng P, Hu B. Effect of air pollution on the total bacteria and pathogenic bacteria in different sizes of particulate matter. Environ Pollut 233: 483–493, 2018. doi: 10.1016/j.envpol.2017.10.070. [DOI] [PubMed] [Google Scholar]
- 122.Rutting S, Xenaki D, Malouf M, Horvat JC, Wood LG, Hansbro PM, Oliver BG. Short-chain fatty acids increase tnfα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 mapk. Am J Physiol Lung Cell Mol Physiol 316: L157–L174, 2019. doi: 10.1152/ajplung.00306.2018. [DOI] [PubMed] [Google Scholar]
- 123.Blank F, Rothen-Rutishauser B, Gehr P. Dendritic cells and macrophages form a transepithelial network against foreign particulate antigens. Am J Respir Cell Mol Biol 36: 669–677, 2007. doi: 10.1165/rcmb.2006-0234OC. [DOI] [PubMed] [Google Scholar]
- 124.Castañeda AR, Pinkerton KE, Bein KJ, Magaña-Méndez A, Yang HT, Ashwood P, Vogel CFA. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol Lett 292: 85–96, 2018. doi: 10.1016/j.toxlet.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol 2011: 1–9, 2011. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.House JS, Wyss AB, Hoppin JA, Richards M, Long S, Umbach DM, Henneberger PK, Beane Freeman LE, Sandler DP, Long O'Connell E, Barker-Cummings C, London SJ. Early-life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J Allergy Clin Immunol 140: 249–256.e14, 2017.. doi: 10.1016/j.jaci.2016.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Von Essen S, Fryzek J, Nowakowski B, Wampler M. Respiratory symptoms and farming practices in farmers associated with an acute febrile illness after organic dust exposure. Chest 116: 1452–1458, 1999. doi: 10.1378/chest.116.5.1452. [DOI] [PubMed] [Google Scholar]
- 128.Cano-Jiménez E, Acuña A, Botana MI, Hermida T, González MG, Leiro V, Martín I, Paredes S, Sanjuán P. Farmer’s lung disease. A review. Arch Bronconeumol 52: 321–328, 2016. doi: 10.1016/j.arbres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Grisso R, Gay S, Hetzel G, Stone B. Farmer’ s Lung: Causes and Symptoms of Mold and Dust Induced Respiratory Illness. Blacksburg, VA: Virginia Polytechnic Institute and State University, 2009, 442-602(BSE-287P). [Google Scholar]
- 130.Segura-Medina P, Vargas MH, Aguilar-Romero JM, Arreola-Ramírez JL, Miguel-Reyes JL, Salas-Hernández J. Mold burden in house dust and its relationship with asthma control. Respir Med 150: 74–80, 2019. doi: 10.1016/j.rmed.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 131.Basu T, Seyedmousavi S, Sugui JA, Balenga N, Zhao M, Kwon Chung KJ, Biardel S, Laviolette M, Druey KM. Aspergillus fumigatus alkaline protease 1 (Alp1/Asp f13) in the airways correlates with asthma severity. J Allergy Clin Immunol 141: 423–425.e7, 2018.. doi: 10.1016/j.jaci.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwaiblmair M, Beinert T, Vogelmeier C, Fruhmann G. Cardiopulmonary exercise testing following hay exposure challenge in farmer’s lung. Eur Respir J 10: 2360–2365, 1997. doi: 10.1183/09031936.97.10102360. [DOI] [PubMed] [Google Scholar]
- 133.Akpinar-Elci M, Pasquale DK, Abrokwah M, Nguyen M, Elci OC. United airway disease among crop farmers. J Agromed 21: 217–223, 2016. doi: 10.1080/1059924X.2016.1179239. [DOI] [PubMed] [Google Scholar]
- 134.van Dijk CE, Smit LAM, Hooiveld M, Zock J-P, Wouters IM, Heederik DJJ, Yzermans CJ. Associations between proximity to livestock farms, primary health care visits and self-reported symptoms. BMC Fam Pract 17: 22, 2016. doi: 10.1186/s12875-016-0421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leiva-Juárez MM, Kolls JK, Evans SE. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 11: 21–34, 2018. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dias C, Duarte-Ribeiro F, Pipa S, Mota M. A rare and potentially catastrophic infection: primary intestinal aspergillosis-case report in an HIV patient. Case Rep Infect Dis 2018: 3269847, 2018. doi: 10.1155/2018/3269847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, Delhaes L. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10: 9, 2020. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol 9: 1835, 2018. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee SY, Aogáin MM, Fam KD, Chia KL, Ali NA, Tika BM, Yap MMC, Yap EPH, Chotirmall SH, Lim CL. Airway microbiome composition correlates with lung function and arterial stiffness in an age-dependent manner. PLoS One 14: e0225636, 2019. doi: 10.1371/journal.pone.0225636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu S, Qin P, Wang J. High-fat diet alters the intestinal microbiota in streptozotocin-induced type 2 diabetic mice. Microorganisms 7: 176, 2019. doi: 10.3390/microorganisms7060176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mur LA, Huws SA, Cameron SJ, Lewis PD, Lewis KE. Lung cancer: a new frontier for microbiome research and clinical translation. Ecancermedicalscience 12: 866, 2018. doi: 10.3332/ecancer.2018.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Adar SD, Huffnagle GB, Curtis JL. The respiratory microbiome: an underappreciated player in the human response to inhaled pollutants? Ann Epidemiol 26: 355–359, 2016. doi: 10.1016/j.annepidem.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barfod KK, Roggenbuck M, Hansen LH, Schjørring S, Larsen ST, Sørensen SJ, Krogfelt KA. The murine lung microbiome in relation to the intestinal and vaginal bacterial communities. BMC Microbiol 13: 303, 2013. doi: 10.1186/1471-2180-13-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mendez R, Banerjee S, Bhattacharya SK, Banerjee S. Lung inflammation and disease: a perspective on microbial homeostasis and metabolism. IUBMB Life 71: 152–165, 2019. doi: 10.1002/iub.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li N, He F, Liao B, Zhou Y, Li B, Ran P. Exposure to ambient particulate matter alters the microbial composition and induces immune changes in rat lung. Respir Res 18: 1–10, 2017. doi: 10.1186/s12931-017-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol 11: 1–14, 2020. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 107: 129–134, 2001. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 148.Arrieta MC, Arévalo A, Stiemsma L, Dimitriu P, Chico ME, Loor S, Vaca M, Boutin RCT, Morien E, Jin M, Turvey SE, Walter J, Parfrey LW, Cooper PJ, Finlay B. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J Allergy Clin Immunol 142: 424–434.e10, 2018. doi: 10.1016/j.jaci.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Imperial County Public Health Department. COVID-19. [http://www.icphd.org/health-information-and-resources/healthy-facts/covid-19/ %0D%0A%0D%0A]
- 150.Looft T, Allen HK. Collateral effects of antibiotics on mammalian gut microbiomes. Gut Microbes 3: 463–467, 2012. doi: 10.4161/gmic.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noverr MC, Falkowski NR, McDonald RA, McKenzie AN, Huffnagle GB. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect Immun 73: 30–38, 2005. doi: 10.1128/IAI.73.1.30-38.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 13: 440–447, 2012. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O'Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol 196: 4839–4847, 2016. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ohnmacht C. Microbiota, regulatory T cell subsets, and allergic disorders. Allergo J Int 25: 114–123, 2016. doi: 10.1007/s40629-016-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cooper DM, Loxham M. Particulate matter and the airway epithelium: the special case of the underground? Eur Respira Rev 28: 190066, 2019. doi: 10.1183/16000617.0066-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Invernizzi R, Lloyd CM, Molyneaux PL. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology, 160: 171–182, 2020. doi: 10.1111/imm.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mathieu E, Escribano-Vazquez U, Descamps D, Cherbuy C, Langella P, Riffault S, Remot A, Thomas M. Paradigms of lung microbiota functions in health and disease, particularly, in asthma. Front. Physiol 9: 1168, 2018. doi: 10.3389/fphys.2018.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, Lee TH. Urban particulate matter-activated human dendritic cells induce the expansion of potent inflammatory Th1, Th2, and Th17 effector cells. Am J Respir Cell Mol Biol 54: 250–262, 2016. doi: 10.1165/rcmb.2015-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao C, Liao J, Chu W, Wang S, Yang T, Tao Y, Wang G. Involvement of TLR2 and TLR4 and Th1/Th2 shift in inflammatory responses induced by fine ambient particulate matter in mice. Inhal Toxicol 24: 918–927, 2012. [Erratum in Inhal Toxicol 25: 178, 2013]. doi: 10.3109/08958378.2012.731093. [DOI] [PubMed] [Google Scholar]
- 160.Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest 39: 526–549, 2010. doi: 10.3109/08820131003615498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol 151: 297–307, 2010. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 162.Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol 24: 424–430, 2012. doi: 10.1016/j.coi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mortha A, Burrows K. Cytokine networks between innate lymphoid cells and myeloid cells. Front Immunol 9: 191, 2018. doi: 10.3389/fimmu.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343: 1249288, 2014. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Edelblum KL, Sharon G, Singh G, Odenwald MA, Sailer A, Cao S, Ravens S, Thomsen I, El Bissati K, McLeod R, Dong C, Gurbuxani S, Prinz I, Mazmanian SK, Turner JR. The microbiome activates CD4 T-cell-mediated immunity to compensate for increased intestinal permeability. Cell Mol Gastroenterol Hepatol 4: 285–297, 2017. doi: 10.1016/j.jcmgh.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Robinette ML, Colonna M. Immune modules shared by innate lymphoid cells and T cells. J Allergy Clin Immunol 138: 1243–1251, 2016. doi: 10.1016/j.jaci.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Winglee K, Eloe-Fadrosh E, Gupta S, Guo H, Fraser C, Bishai W. Aerosol mycobacterium tuberculosis infection causes rapid loss of diversity in gut microbiota. PLoS One 9: e97048, 2014. doi: 10.1371/journal.pone.0097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon J-Y, Bernalier-Donadille A, Vasson M-P, Filaire E. Desired turbulence? Gut-lung axis, immunity, and lung cancer. J Oncol 2017: 5035371, 2017. doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–334, 2011. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chiu L, Bazin T, Truchetet ME, Schaeverbeke T, Delhaes L, Pradeu T. Protective microbiota: from localized to long-reaching co-immunity. Front Immunol 8: 1678, 2017. doi: 10.3389/fimmu.2017.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 48: 39–49, 2018. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 48: 992–1005.e8, 2018.. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 173.Dang AT, Marsland BJ. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol 12: 843–850, 2019. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 174.Mesnil C, Sabatel CM, Marichal T, Toussaint M, Cataldo D, Drion PV, Lekeux P, Bureau F, Desmet CJ. Resident CD11b+Ly6C− lung dendritic cells are responsible for allergic airway sensitization to house dust mite in mice. PLoS One 7: e53242, 2012. doi: 10.1371/journal.pone.0053242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vieira R de S, Castoldi A, Basso PJ, Hiyane MI, Câmara NOS, Almeida RR. Butyrate attenuates lung inflammation by negatively modulating Th9 cells. Front Immunol 10: 67, 2019. doi: 10.3389/fimmu.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 3: 858–876, 2011. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bradley CP, Teng F, Felix KM, Sano T, Naskar D, Block KE, Huang H, Knox KS, Littman DR, Wu H-JJ. Inducing gut-lung axis Th17 cells expressing dual TCRs. Cell Host Microbe 22: 697–704.e4, 2017. doi: 10.1016/j.chom.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Núñez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE2. Cell Host Microbe 15: 95–102, 2014. doi: 10.1016/j.chom.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, Arditi M, Underhill DM, Iliev ID. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19: 865–873, 2016. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aktas B, Aslim B. Gut-lung axis and dysbiosis in COVID-19. Turk J Biol 44: 265–272, 2020. doi: 10.3906/biy-2005-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang J, Li F, Wei H, Lian Z-X, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiotamediated Th17 cell-dependent inflammation. J Exp Med 211: 2397–2410, 2014. [Erratum in J Exp Med 211: 2683, 2014]. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nordgren TM, Charavaryamath C. Agriculture occupational exposures and factors affecting health effects. Curr Allergy Asthma Rep 18: 65, 2018. doi: 10.1007/s11882-018-0820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 159: 765–767.e2, 2020. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao N, Zhou ZL, Wu L, Zhang XD, Han SB, Bao HJ, Shu Y, Shu XG. An update on the status of COVID-19: a comprehensive review. Eur Rev Med Pharmacol Sci 24: 4597–4606, 2020. doi: 10.26355/eurrev_202004_21046. [DOI] [PubMed] [Google Scholar]
- 185.Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open 3: e2011335, 2020. doi: 10.1001/jamanetworkopen.2020.113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res 226: 57–69, 2020. doi: 10.1016/j.trsl.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367: 1444–1448, 2020. [ 10.1126/science.abb2762] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SPJ, Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 5: eabc3582, 2020. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Giron LB, Dweep H, Yin X, Wang H, Damra M, Goldman AR, Gorman N, Palmer CS, Tang H, Shaikh MW, Forsyth CB, Balk RA, Zilberstein NF, Liu Q, Kossenkov A, Keshavarzian A, Landy A, Abdel-Mohsen M. . Severe COVID-19 is fueled by disrupted gut barrier integrity. medRxiv 2020. [2020.11.13.20231209] [Google Scholar]
- 190.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic—a focused review for clinicians. Clin Microbiol Infect 26: 842–847, 2020. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lockhart SM, O'Rahilly S. When two pandemics meet: why is obesity associated with increased COVID-19 mortality? Med (NY) 1: 33–42, 2020. doi: 10.1016/j.medj.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract 14: 295–300, 2020. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 193.Ritter A, Kreis NN, Louwen F, Yuan J. Obesity and COVID-19: molecular mechanisms linking both pandemics. Int J Mol Sci 21: 5793, 2020. doi: 10.3390/ijms21165793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Belančić A. Gut microbiome dysbiosis and endotoxemia—additional pathophysiological explanation for increased COVID-19 severity in obesity. Obes Med 20: 100302, 2020. doi: 10.1016/j.obmed.2020.100302.[Mismatch] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chakaroun RM, Massier L, Kovacs P. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: perpetrators or bystanders? Nutrients 12: 1082, 2020. doi: 10.3390/nu12041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol 18: 923–929, 2012. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Teixeira TF, Souza NC, Chiarello PG, Franceschini SC, Bressan J, Ferreira CL, Peluzio MC. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr 31: 735–740, 2012. doi: 10.1016/j.clnu.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 198.Potdar AA, Dube S, Naito T, Li K, Botwin G, Haritunians T, Li D, Casero D, Yang S, Bilsborough J, Perrigoue JG, Denson LA, Daly M, Targan SR, Fleshner P, Braun J, Kugathasan S, Stappenbeck TS, McGovern DPB. Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease. Gastroenterology 160: 809–822.e7, 2021. doi: 10.1053/j.gastro.2020.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kruglikov IL, Shah M, Scherer PE. Obesity and diabetes as comorbidities for COVID-19: underlying mechanisms and the role of viral–bacterial interactions. Elife 9: 1–21, 2020. doi: 10.7554/eLife.61330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201..Li R, Uttarwar L, Gao B, Charbonneau M, Shi Y, Chan JS, Dubois CM, Krepinsky JC. High glucose up-regulates ADAM17 through HIF-1a in mesangial cells. J Biol Chem 290: 21603–21614, 2015. doi: 10.1074/jbc.M115.651604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202..Abbey DE, Colome SD, Mills PK, Burchette R, Beeson WL, Tian Y. Chronic disease associated with long-term concentrations of nitrogen dioxide. J Expo Anal Environ 3: 181–202, 1993. [PubMed] [Google Scholar]
- 203..Bowatte G, Lodge CJ, Knibbs LD, Lowe AJ, Erbas B, Dennekamp M, Marks GB, Giles G, Morrison S, Thompson B, Thomas PS, Hui J, Perret JL, Abramson MJ, Walters H, Matheson MC, Dharmage SC. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol 139: 122–129.e1, 2017. doi: 10.1016/j.jaci.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 204..Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YMW. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biol 234: 71–80, 2002. doi: 10.1023/A:1015973530559. [DOI] [PubMed] [Google Scholar]
- 205..Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci Total Environ 726: 138605, 2020. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.U.S. Environmental Protection Agency. Nitrogen dioxide (NO2) pollution. 2019. [https://www.epa.gov/nov2-pollution]
- 207.Wu X, Nethery RC, Sabath BM, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv, 2020.doi: 10.1101/2020.04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). 2020. [https://www.cdc.gov/coronavirus/2019-ncov/index.html]
- 209.National Center for Farmworker Health. “MSAWs and COVID-19.” July 2020. [www.ncfh.org/msaws-and-covid-19.html]
- 210.Villarejo D. CIRS Research Report Increased Risks and Fewer Jobs: Evidence of California Farmworker Vulnerability During the COVID-19 Pandemic. California: California Institute for Rural Studies, 2020, p. 1–9. [Google Scholar]
- 211.Tulimiero M, Garcia M, Rodriguez M, Cheney AM. Overcoming barriers to health care access in rural Latino communities: an innovative model in the eastern Coachella Valley. J Rural Health, 2020, p. 1–10. doi: 10.1111/jrh.12483. [DOI] [PubMed] [Google Scholar]
- 212.Jones BA, Fleck J. Shrinking lakes, air pollution, and human health: evidence from California’s Salton Sea. Sci Total Environ 712: 136490, 2020. doi: 10.1016/j.scitotenv.2019.136490. [DOI] [PubMed] [Google Scholar]
- 213.Schenker MB. Inorganic agricultural dust exposure causes pneumoconiosis among farmworkers. Proc Am Thorac Soc 7: 107–110, 2010. doi: 10.1513/pats.200906-036RM. [DOI] [PubMed] [Google Scholar]
- 214.Schenker MB, Pinkerton KE, Mitchell D, Vallyathan V, Elvine-Kreis B, Green FHY. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ Health Perspect 117: 988–994, 2009. doi: 10.1289/ehp.0800144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lipsett M, Hurley S, Ostro B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ Health Perspect 105: 216–222, 1997. doi: 10.1289/ehp.97105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Orazzo F, Nespoli L, Ito K, Tassinari D, Giardina D, Funis M, Cecchi A, Trapani C, Forgeschi G, Vignini M, Nosetti L, Pigna S, Zanobetti A. Air pollution, aeroallergens, and emergency room visits for acute respiratory diseases and gastroenteric disorders among young children in six Italian cities. Environ Health Perspect 117: 1780–1785, 2009. doi: 10.1289/ehp.0900599. [DOI] [PMC free article] [PubMed] [Google Scholar]


