Abstract
Objective
During the coronavirus disease 2019 (COVID-19) pandemic, exploring insulin resistance and beta-cell activity is important for understanding COVID-19‒associated new-onset diabetes. We assessed insulin sensitivity and fasting insulin secretion in patients with COVID-19 without diabetes on admission and at 3 and 6 months after discharge.
Methods
This 6-month prospective study assessed data from the records of 64 patients without diabetes diagnosed with COVID-19 at Wenzhou Central Hospital, China. Each patient was followed up at 3 and 6 months after discharge. Repeated measures analysis of variance was used to investigate differences in multiple measurements of the same variable at different times. Linear regression analysis was performed to analyze the contributor for changes in the triglyceride-glucose (TyG) index.
Results
Fasting C-peptide levels in patients at baseline were lower than the normal range. Compared with the baseline results, patients had significantly elevated fasting C-peptide levels (0.35 ± 0.24 vs 2.36 ± 0.98 vs 2.52 ± 1.11 μg/L; P < .001), homeostasis model assessment for beta-cell function (0.42, interquartile range [IQR] 0.36-0.62 vs 2.54, IQR 1.95-3.42 vs 2.90, IQR 2.02-4.23; P < .001), and TyG indices (8.57 ± 0.47 vs 8.73 ± 0.60 vs 8.82 ± 0.62; P = .006) and decreased fasting glucose levels (5.84 ± 1.21 vs 4.95 ± 0.76 vs 5.40 ± 0.68 mmol/L; P = .003) at the 3- and 6-month follow-up. Male gender, age, interferon-alfa treatment during hospitalization, and changes in total cholesterol and high-density lipoprotein levels were significantly associated with changes in the TyG index.
Conclusion
Our study provided the first evidence that COVID-19 may increase the risk of insulin resistance in patients without diabetes.
Key words: coronavirus disease 2019, COVID-19, insulin resistance, insulin secretion, triglyceride-glucose index
Abbreviations: ACE2, angiotensin-converting enzyme 2; BMI, body mass index; COVID-19, coronavirus disease 2019; FBG, fasting blood glucose; HDL, high-density lipoprotein; HOMA, homeostatic model assessment; HOMA-CP, homeostasis model assessment for beta-cell function; HOMA-IR, homeostasis model assessment for insulin resistance; IFN, interferon; IL, interleukin; IQR, interquartile range; LDL, low-density lipoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TC, total cholesterol; TyG, triglyceride-glucose
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic. According to the World Health Organization (https://www.who.int/emergencies/diseases/novel-coronavirus-2019), by late March 2021, the disease had reached 223 countries, areas, or territories, with over 123 million infection cases and over 2 719 000 confirmed deaths. It is undeniable that COVID-19 has had a severe negative impact on human health.
Diabetes and the degree of hyperglycemia are related to an increased risk of COVID-19 severity and mortality.1, 2, 3 An early study showed that underlying diabetes was common among patients with COVID-19 admitted to intensive care units.4 Furthermore, the presence of typical diabetic complications, such as chronic kidney disease and cardiovascular disease, increases the risk of COVID-19 mortality.3 A potential reason for this increased risk may be that hyperglycemia supports viral proliferation. It was reported that elevated glucose levels directly increased SARS-CoV-2 replication in human monocytes.5 In addition, immune system dysregulation6 and an impaired inflammatory response7 may be contributing factors to the susceptibility to SARS-CoV-2 infection and severe conditions in patients with diabetes.
Meanwhile, a growing number of studies have observed that patients with COVID-19 can progress to new-onset diabetes or acute complications of pre-existing diabetes, including hyperosmolarity and diabetic ketoacidosis.8, 9, 10, 11 One study reported 29 patients without diabetes who developed hyperglycemia during treatment for SARS-CoV-2 infection, some of whom had normal glycated hemoglobin levels on admission.12 These studies indicate that there may be a bidirectional relationship between diabetes and COVID-19 and suggest a complex pathophysiology of COVID-19-associated diabetes. However, data regarding insulin sensitivity and pancreatic islet activity in COVID-19 patients are scarce, impeding our understanding of a potential diabetogenic effect of COVID-19.1
The purpose of this study was to explore the insulin sensitivity and beta-cell activity in COVID-19 patients without pre-existing diabetes on admission and at 3 and 6 months after discharge, thereby providing clinical evidence for the understanding of COVID-19‒associated hyperglycemia and new-onset diabetes.
Methods
Subjects
All data for this prospective study were obtained from patients with confirmed COVID-19 from Wenzhou, China, a city seriously affected by SARS-CoV-2 infections. The initial cohort comprised 149 patients with confirmed COVID-19 admitted to Wenzhou Central Hospital from January 17 to February 9, 2020. Among them, 85 patients were excluded, including patients with pre-existing diabetes (n = 6) or cancer (n = 1), children and adolescents (n = 5), and those who refused to participate in follow-up (n = 73). Finally, 64 patients were enrolled and analyzed in the study. None of the included patients had pre-existing diabetes, a history of insulin or oral hypoglycemic medication usage, cancer, cachexia, severe debilitating illness, hepatic failure, end-stage chronic kidney disease, hematologic system diseases, pancreatitis, or schizophrenia. The therapeutic principles included general support therapy, monitoring of organ functions, active management of high fever, antiviral treatment with lopinavir-ritonavir and interferon-alfa, and oxygen uptake, if necessary.13 Baseline anthropometric parameters of patients with COVID-19 were obtained on the day of admission. Because most patients were not fasting on the day of admission, baseline biochemical indices obtained from fasting blood samples the next morning after admission were analyzed.
Patients discharged from the hospital were quarantined for 2 weeks and did not continue pharmacologic treatment after discharge. Each patient was followed up at 3 and 6 months after discharge. The follow-up tests were conducted at Wenzhou Central Hospital on an outpatient basis. No participants were lost to follow-up. At the 3-month and 6-month postdischarge follow-up, biochemical indices were assessed using early morning fasting blood samples. All follow-up contact work was performed by physicians (X.H. and X.X.). All medical data were checked by 2 medical doctors (M.C. and B.Z.), and the lead authors (C.H., J.L., and S.Q.) adjudicated any different interpretations between the 2 medical doctors.
Ethical Statements
This study was approved by the Research Ethics Review Committees of Wenzhou Medical University (No. K2020-01-005(5)). All patients provided informed consent prior to participating in the study, which was conducted according to the revised (2013) Declaration of Helsinki.
Diagnostic and Classification Criteria
SARS-CoV-2 infection was diagnosed by reverse-transcription polymerase chain reaction assays of samples taken from upper nasopharyngeal swabs. Sample collection, reverse-transcription polymerase chain reaction, and interpretation of results were performed as previously described.14 The diagnosis, classification, and discharge criteria for COVID-19 patients were based on the guidelines issued by the National Health Commission of China.15 The severity was classified as follows: (1) mild: mild symptoms, no pneumonia in imaging diagnosis; (2) moderate: fever, respiratory tract symptoms, and pneumonia in imaging diagnosis; (3) severe: either respiratory rate ≥30 beats/min, or finger oxygen saturation ≤93% at rest, or arterial blood oxygen partial pressure/oxygen concentration ≤300 mm Hg; or (4) critical: respiratory failure requiring mechanical ventilation, organ failure requiring care in the intensive care unit, or shock.
Homeostatic Model Assessment and the Triglyceride-Glucose Index
Homeostatic model assessment (HOMA) is a measure of insulin sensitivity and beta-cell function. Because C-peptide is a marker of endogenous insulin secretion, the generally accepted assumption is the negligible extraction of C-peptide by the liver and constant metabolic clearance under physiologic conditions.16 Therefore, for HOMA for beta-cell function (HOMA-CP), C-peptide data are used to evaluate beta-cell function if both insulin and C-peptide data are available.17 HOMA-CP and HOMA for insulin resistance (HOMA-IR) were calculated using the formulas (glucose [mg/dL] × C-peptide [ng/mL] × 1.8395 / 135) and (fasting plasma insulin [mU/L] × fasting blood glucose [FBG; mmol/L] / 22.5), respectively.17, 18, 19 No hypoglycemic or malignant event was observed in our patients, which enhanced the validation of the HOMA model used in this study. The triglyceride-glucose (TyG) index, which is widely used as a reliable marker of insulin resistance, was determined.20 The TyG was calculated using the formula ln (fasting triglycerides [mg/dL] × FBG [mg/dL] / 2).11 , 20 Guerrero-Romero et al20 recommended the value of the TyG index as 4.68 for identification of insulin resistance.
Statistical Analyses
Data were double-entered, validated, and anonymized before analysis. The types of missing values were random. Therefore, we did not fill in the missing data by mean-value imputation. Outliers, which were defined as being >3 standard deviations above the mean, were removed. Repeated measures analysis of variance was performed to investigate differences in multiple measurements of the same variable taken from the same patient at different times. To study the within-time point effects, we performed post hoc tests, which are pairwise comparisons of either all possible combinations of means or selected means of interest.21 In post hoc analyses, the sphericity assumption was tested using the Mauchly test, and Greenhouse-Geisser adjusted P values were presented when sphericity was not assumed. The Pearson or Spearman correlation analysis was used to investigate the association between different values of the variable measured at 3 or 6 months after discharge and at baseline, respectively. Linear regression analysis was performed to analyze the relationship between the changes in the TyG index and the changes in other variables. The variables that demonstrated significant associations with changes in the TyG index in the bivariate correlation and univariate linear regression analyses, as well as those with r or β values ≥0.3, were adjusted for in the multivariate linear regression analysis. All statistical analyses were performed using SPSS 25.0 (IBM Corp) and Prism software (Prism 8.0, GraphPad). Results were considered statistically significant at a P value <.05.
Results
As shown in Table 1 , the average age of the patients (35/64, 54.7% male) was 44.33 years, and the average body mass index (BMI) was 23.87 kg/m2. The number of patients classified as having mild or moderate COVID-19 was 54 of 64 (84.4%), and the remaining 10 (15.6%) patients were severely or critically ill. Common comorbidities among these patients included hypertension (13/64, 20.3%), nonalcoholic fatty liver disease (3/64, 4.7%), coronary disease (1/64, 1.6%), and hepatitis B virus infection (4/64, 6.3%). During hospitalization, 60 of 64 (93.8%) patients were treated with interferon-alfa by aerosolization twice a day, 63 of 64 (98.4%) received lopinavir-ritonavir syrup twice a day, 52 of 64 (81.3%) received an umifenovir electuary 3 times a day, and 2 of 64 (3.1%) needed mechanical ventilation. None of the patients was treated with glucocorticoids.
Table 1.
Anthropometric Parameters of Coronavirus Disease 2019 Patients in This Study (N = 64)
| Characteristics | Baseline |
|---|---|
| Age (years) | 44.33 ± 13.51 |
| Male sex (n, %) | 35 (54.7%) |
| BMI (kg/m2) | 23.87 ± 3.91 |
| SBP (mm Hg) | 140.73 ± 17.50 |
| DBP (mm Hg) | 88.82 ± 14.13 |
| HR (bpm) | 89.5 (84.0, 103.0) |
| Body temperature (°C) | 38.0 (37.6, 38.5) |
| Classification of severity | |
| Mild/moderate | 54 (84.4%) |
| Severe/critical | 10 (15.6%) |
| Comorbidity (n, %) | |
| Diabetes | 0 (0%) |
| NAFLD | 3 (4.70%) |
| Hypertension | 13 (20.3%) |
| Coronary disease | 1 (1.60%) |
| Cancer | 0 (0%) |
| Mental disease | 0 (0%) |
| Immune system disease | 0 (0%) |
| Other infectious diseases | |
| HBV (+) | 4 (6.30%) |
| Tuberculosis | 0 (0%) |
| HIV (+) | 0 (0%) |
| Syphilis | 0 (0%) |
| Mechanical ventilation | 2 (3.1%) |
| Pharmacologic treatment during hospitalization | |
| Glucocorticoids | 0 (0%) |
| Interferon-alfa (500 U, twice a day) | 60 (93.8%) |
| Lopinavir-ritonavir (500 mg, twice a day) | 63 (98.4%) |
| Umifenovir (0.2 g, 3 times a day) | 52 (81.3%) |
| Antibiotics | 13 (20.3%) |
Abbreviations: BMI = body mass index; DBP = diastolic blood pressure; HBV = hepatitis B virus; HR = heart rate; NAFLD = nonalcoholic fatty liver disease; SBP = systolic blood pressure.
Continuous data are presented as means ± standard deviations or medians (interquartile range) based on the data distribution. Categorical variables are presented as number (%).
Fasting C-peptide levels in patients at baseline (0.35 ± 0.24 μg/L) were below the lower end of the normal range (1.1-4.4 μg/L). Compared with the baseline results, patients had significantly elevated fasting C-peptide levels (0.35 ± 0.24 vs 2.36 ± 0.98 vs 2.52 ± 1.11 μg/L; P < .001), HOMA-CP (0.42, interquartile range [IQR] 0.36-0.62 vs 2.54, IQR 1.95-3.42 vs 2.90, IQR 2.02-4.23; P < .001), and mean TyG indices (8.57 ± 0.47 vs 8.73 ± 0.60 vs 8.82 ± 0.62, P = .006) during the follow-up (Table 2 ). Conversely, patients had significantly decreased FBG levels 3 months (4.95 ± 0.76 mmol/L) and 6 months (5.40 ± 0.68 mmol/L) after discharge compared with admission levels (5.84 ± 1.21 mmol/L; P = .003). In addition, we found a significant tendency toward an increased lipid profile (triglycerides, total cholesterol [TC], high-density lipoprotein [HDL], and low-density lipoprotein [LDL]); alanine aminotransferase, alkaline phosphatase, creatine kinase, hemoglobin, and uric acid levels; and lymphocyte and thrombocyte counts after hospital discharge. We also observed significantly decreased lactate dehydrogenase and C-reactive protein levels during the follow-up. However, we did not find significant changes in the fasting plasma insulin levels and HOMA-IR after the hospital discharge of our patients (Table 2).
Table 2.
Changes in Characteristics Between the Baseline, 3-Month Follow-up, and 6-Month Follow-up in Coronavirus Disease 2019 Patients (N = 64)
| Characteristics (normal range) | Baseline | 3-month follow-up | 6-month follow-up | F value | P value |
|---|---|---|---|---|---|
| FBG (3.8-6.1 mmol/L) | 5.84 ± 1.21 | 4.95 ± 0.76 | 5.40 ± 0.68 | 8.260 | .003a |
| FPI (3-25 mU/L) | 15.82 ± 11.7 | 17.26 ± 13.69 | 20.0 ± 18.03 | 1.315 | .293 |
| Fasting C-peptide (1.1-4.4 μg/L) | 0.35 ± 0.24 | 2.36 ± 0.98 | 2.52 ± 1.11 | 27.402 | <.001a |
| HOMA-CP | 0.42 (0.36, 0.62) | 2.54 (1.95, 3.42) | 2.90 (2.02, 4.23) | 18.755 | <.001a |
| HOMA-IR | 4.07 ± 2.20 | 4.10 ± 3.46 | 4.96 ± 4.46 | 0.940 | .414 |
| TyG index | 8.57 ± 0.47 | 8.73 ± 0.60 | 8.82 ± 0.62 | 5.595 | .006a |
| TC (3.1-5.2 mmol/L) | 3.59 ± 0.75 | 4.83 ± 0.87 | 5.63 ± 0.88 | 57.482 | <.001a |
| TG (0.34-1.7 mmol/L) | 1.11 (0.90, 1.67) | 1.53 (0.99, 2.38) | 1.34 (1.09, 2.64) | 8.176 | .001a |
| HDL (1.04-2.49 mmol/L) | 1.19 (0.97, 1.36) | 1.69 (1.40, 1.99) | 1.56 (1.20, 1.76) | 23.294 | <.001a |
| LDL (1.63-3.12 mmol/L) | 1.97 ± 0.84 | 2.41 ± 0.80 | 3.25 ± 0.84 | 35.067 | <.001a |
| ALT (9-50 U/L) | 25.67 ± 14.20 | 34.21 ± 23.59 | 42.0 ± 33.07 | 5.112 | .010a |
| AST (15-40 U/L) | 25.0 (19.50, 33.50) | 27.0 (20.0, 32.0) | 28.0 (22.50, 35.0) | 0.977 | .384 |
| ALP (45-125 U/L) | 50.54 ± 13.28 | 73.00 ± 18.11 | 78.59 ± 24.16 | 38.998 | <.001a |
| GGT (10-60 U/L) | 34.00 (20.00, 50.00) | 27.00 (12.00, 51.00) | 30.00 (14.00, 43.00) | 2.528 | 0.108 |
| LDH (120-250 U/L) | 213.12 ± 57.62 | 179.58 ± 33.77 | 183.31 ± 37.82 | 7.221 | 0.007a |
| CK (50-310 U/L) | 69.40 (47.40, 92.55) | 82.60 (63.60, 117.80) | 99.80 (89.65, 151.40) | 12.359 | <.001a |
| Cr (57-111 μmol/L) | 69.92 ± 16.39 | 70.81 ± 12.83 | 69.50 ± 12.52 | 0.240 | .787 |
| WBC (3.5-9.5 × 109/L) | 4.72 ± 1.50 | 5.35 ± 1.54 | 5.35 ± 1.57 | 3.260 | .051 |
| Neutrophils (1.8-6.3 × 109/L) | 2.65 (2.10, 3.43) | 2.80 (2.10, 3.60) | 2.60 (2.10, 3.63) | 0.426 | .561 |
| Lymphocytes (1.1-3.2 × 109/L) | 1.10 (0.80, 1.50) | 1.90 (1.60, 2.40) | 2.10 (1.60, 2.40) | 35.651 | <.001a |
| Thrombocytes (125-350 × 109/L) | 178.12 ± 45.70 | 211.65 ± 54.40 | 217.81 ± 55.67 | 12.143 | .001a |
| Hb (male 120-160; female 110-150 g/L) | 135.38 ± 15.42 | 143.62 ± 13.78 | 144.11 ± 12.25 | 10.084 | .001a |
| CRP (0-6 mg/L) | 12.90 (3.05, 31.08) | 0.90 (0.50, 1.60) | 1.00 (0.58, 2.0) | 22.697 | <.001a |
| UA (208-428 μmol/L) | 231.58 ± 83.68 | 331.82 ± 97.11 | 312.48 ± 117.05 | 23.452 | <.001a |
Abbreviations: ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CK = creatine kinase; Cr = creatinine; CRP = C-reactive protein; FBG = fasting blood glucose; FPI = fasting plasma insulin; GGT = gamma-glutamyl transpeptidase; Hb = hemoglobin; HDL = high-density lipoprotein; HOMA-CP = homeostasis model assessment for beta-cell function; HOMA-IR = homeostasis model assessment for insulin resistance; LDH = lactate dehydrogenase; LDL = low-density lipoprotein; TC = cholesterol; TG = triglycerides; TyG = triglyceride-glucose index; UA = uric acid; WBC = white blood cell.
Continuous data are presented as means ± standard deviations or medians (interquartile range) based on the data distribution.
Data were analyzed by repeated measures analysis of variance.
P < .05 was considered statistically significant.
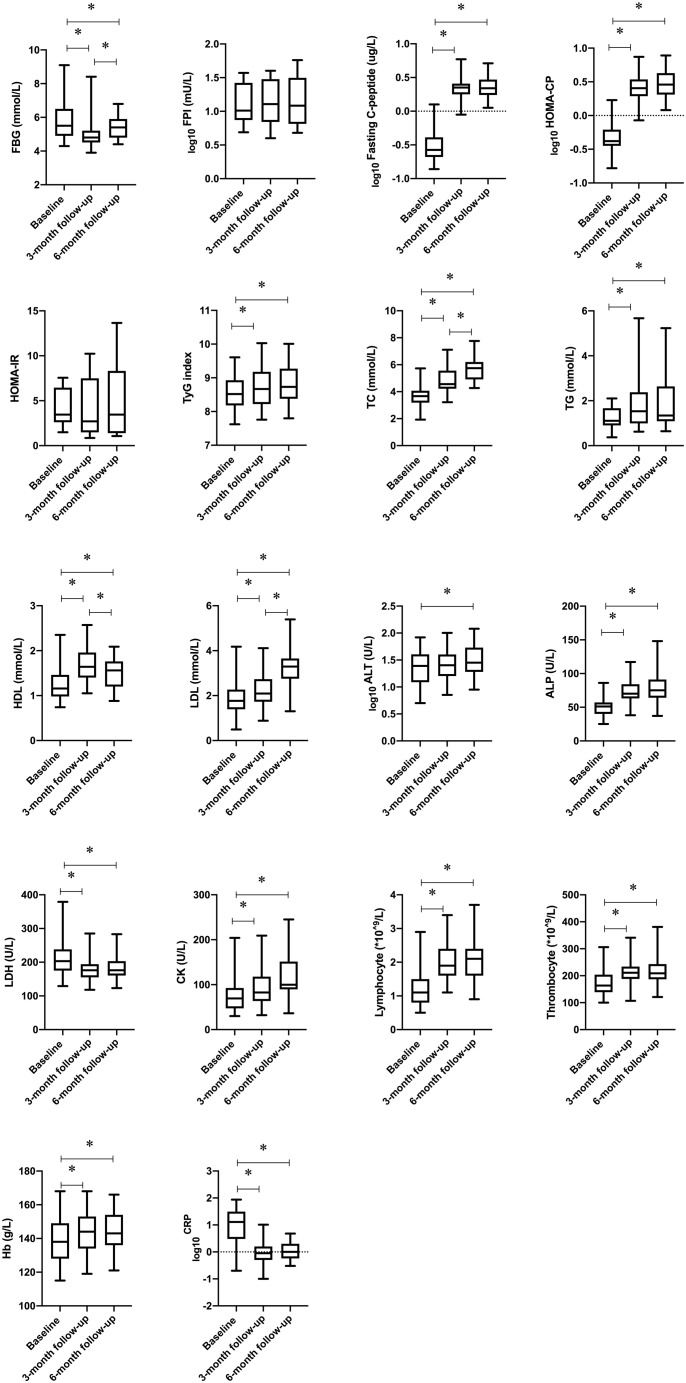
Within-time point contrasts of variables for the baseline, 3-month follow-up, and 6-month follow-up in the COVID-19 patients are presented in Supplementary Table 1 and the Figure . The FBG levels had significantly decreased at both the 3-month and 6-month follow-up. However, the FBG levels were significantly higher at the 6-month follow-up compared with the levels measured 3 months after discharge. The fasting C-peptide levels, HOMA-CP, and mean TyG index had significantly increased at the 3-month follow-up and remained unchanged at the 6-month follow-up. For the lipid profile, TC, triglycerides, HDL, and LDL levels were significantly higher at both the 3-month and 6-month follow-up than at admission in the COVID-19 patients. Relative to the measurements at the 3-month follow-up, the COVID-19 patients had significantly higher TC and LDL levels and lower HDL levels at 6 months after discharge. In addition, when compared with the baseline, levels of alkaline phosphatase, lactate dehydrogenase, C-reactive protein, uric acid, and hemoglobin as well as the lymphocyte and thrombocyte count were consistently and significantly changed both at the 3-month and 6-month follow-up. No significant difference was found in these parameters between the 3-month and 6-month follow-up.
Figure.
Within-time points contrasts in variables for the baseline, 3-month follow-up, and 6-month follow-up in coronavirus disease 2019 patients without diabetes. Box plots convey the level, spread, and symmetry of the distribution of data values. Sphericity-assumed modeling or Greenhouse-Geisser adjustments (sphericity not assumed) were applied to test within-time points effects. Logarithmic transformation was used for some variables due to the high discrete degree (log10). ALP = alkaline phosphatase; ALT = alanine aminotransferase; CK = creatine kinase; CRP = C-reactive protein; FBG = fasting blood glucose; FPI = fasting plasma insulin; Hb = hemoglobin; HDL = high-density lipoprotein; HOMA-CP = homeostasis model assessment for beta-cell function; HOMA-IR = homeostasis model assessment for insulin resistance; LDH = lactate dehydrogenase; LDL = low-density lipoprotein; TC = total cholesterol; TG = triglycerides; TyG = triglyceride-glucose.
The factors associated with changes in the TyG index after the COVID-19 patients were discharged are presented in Supplementary Table 2. Bivariate simple correlation analysis indicated that the changes in the TyG index were positively correlated with the changes in TC levels (r = 0.327, P = .014) and were negatively correlated with the changes in HDL levels (r = −0.328, P = .014) at the 3-month follow-up. Furthermore, a linear regression analysis performed at the 3-month follow-up demonstrated that age, gender, and changes in TC levels contributed to the changes in the TyG index (Supplementary Table 3). In addition, the multivariate linear regression analysis, which was performed at the 3-month follow-up, demonstrated that male gender (β [95% CI]: −0.312 [−0.590, −0.034]; P = .028), age (0.012 [0.001, 0.023], P = .031), interferon-alfa treatment during hospitalization (0.540 [0.029, 1.051]; P = .039), the increases in TC (0.217 [0.069, 0.366], P = .005), and the decreases in HDL (−0.477 [−0.881, −0.074], P = .021) levels were significantly associated with the increases in the TyG index (Table 3 ). However, we did not find a significant contributor to changes in the TyG index in the linear regression analysis performed at the 6-month follow-up (Supplementary Table 4 and Supplementary Table 5).
Table 3.
Multivariable-Adjusted Association Between Changes in the TyG Index and Anthropometric Parameters and Changes in Variables for Coronavirus Disease 2019 Patients at the 3-Month Follow-up
| Variables | ΔTyG-3 |
||
|---|---|---|---|
| β | 95% CI | P value | |
| Model 1 | |||
| Gender | −0.427 | −0.712, −0.142 | .004a |
| Age | 0.016 | 0.005, 0.027 | .004a |
| Model 2 | |||
| Gender | −0.422 | −0.708, −0.136 | .005a |
| Age | 0.018 | 0.007, 0.029 | .001a |
| BMI (kg/m2) | 0.005 | −0.032, 0.042 | .791 |
| Interferon-alfa | 0.609 | 0.064, 1.155 | .029a |
| Model 3 | |||
| Gender | −0.312 | −0.590, −0.034 | .028a |
| Age | 0.012 | 0.001, 0.023 | .031a |
| BMI (kg/m2) | 0.013 | −0.022, 0.048 | .445 |
| Interferon-alfa | 0.540 | 0.029, 1.051 | .039a |
| ΔTC-3 | 0.217 | 0.069, 0.366 | .005a |
| ΔHDL-3 | −0.477 | −0.881, −0.074 | .021a |
Abbreviations: β = regression coefficient; BMI = body mass index; HDL = high-density lipoprotein; TC = total cholesterol; TyG = triglyceride-glucose index.
ΔVariable-3 is the mean difference value of the variable between 3 months after discharge and at baseline. Linear regression analysis was performed to analyze the relationship between the changes in the TyG index and changes in other variables.
P < .05 was considered statistically significant.
Discussion
An increasing number of reports have revealed the detrimental consequences of SARS-CoV-2 infection to multiple organs.22, 23, 24, 25, 26, 27 However, studies are lacking that examine the metabolic parameters, such as insulin sensitivity and insulin secretion, in COVID-19 patients. To our knowledge, our study provides the first evidence that COVID-19 may increase the risk of insulin resistance in patients without pre-existing diabetes. In addition, COVID-19 may cause fasting insulin secretion to decrease during the early stages of SARS-CoV-2 infection, although this can be transient and reversible.
The TyG index values obtained in this study indicated that the COVID-19 patients had insulin resistance on admission. A previous study demonstrated that the TyG index could predict the risk of severe illness and mortality in patients with COVID-19.11 In addition, the TyG index was an independent predictor of the development of metabolic syndrome and type 2 diabetes.28 Unfortunately, data regarding insulin sensitivity were unavailable for our patients before they had COVID-19; thus, we could not rule out the possibility that insulin resistance was present in these patients prior to SARS-CoV-2 infection because the average BMI of patients with COVID-19 in this study was higher than the BMI recommended by the World Health Organization as being overweight for Asians. The changes in the TyG index values of the COVID-19 patients during their follow-up suggest that SARS-CoV-2 infections aggravate insulin resistance, which persisted at both the 3-month and 6-month follow-up. Furthermore, although fasting C-peptide secretion increased persistently, the FBG levels were even significantly higher at the 6-month follow-up than at the 3-month follow-up, highlighting the fact that reduced insulin sensitivity persists and still affects glucose metabolism 6 months after COVID-19 onset. This characteristic may be a sequela of COVID-19 that should be investigated further.
Studies have shown that acute viral respiratory infections are associated with the rapid development of transient insulin resistance in normal and overweight individuals.29 , 30 Virally induced inflammation and immune dysfunction increase insulin resistance by several mechanisms. The functions of the liver and skeletal muscle, which are major organs that are responsible for insulin-mediated glucose disposal, can be affected by the large burden of inflammatory cells.31 Eketunde et al32 reported that diffuse alveolar damage and inflammatory cell infiltration in the lungs, lymphocyte infiltration in the liver, myocardial inflammation, and focal pancreatitis were common postmortem findings in patients with fatal COVID-19. More recent studies demonstrated that patients with COVID-19 had high levels of circulating interferon (IFN) γ, interleukin (IL) 1ß, and IL6.33 , 34 Patients with COVID-19 also exhibit elevated levels of other inflammatory markers, such as ferritin and D-dimer.34 These findings indicate a proinflammatory milieu caused by SARS-CoV-2 infection.
It is also recognized that mechanisms linking COVID-19 and diabetes overlap with immunoregulatory pathways.35 Virus-induced IFN activity may increase insulin resistance in muscle, driving hyperinsulinemia to maintain euglycemia and boost antiviral CD8+ T-cell responses.29 In addition, both increased IFN-γ production and activated natural killer cells establish a detrimental effect on glucose disposal by exacerbating systemic inflammation in adipose tissues and muscle.36 It has been demonstrated that IFN-γ, IL6, monocytes, neutrophils, natural killer cells, and CD4+ T-cells significantly increased while CD8+ T-cells decreased in patients with COVID-19 after longer-term infection.37 It has also been indicated that inborn errors of type I IFN immunity correlated with beta-cell immunity underlie fatal COVID-19.38 Moreover, drug-induced insulin resistance should also be considered because in this study, some patients received interferon-alfa during the course of their treatment. Indeed, studies have indicated that the risks for type 1 diabetes and type 2 diabetes increased during and after interferon-alfa treatment.39 , 40 To sum up everything that has been stated so far, we speculated that persistent and aggravated insulin resistance is one of the possible pathophysiologic mechanisms of COVID-19‒associated new-onset diabetes or a worsened outcome in COVID-19 patients with pre-existing diabetes. Indeed, a recent study has highlighted that type 2 diabetes and coronavirus infections share pathways with therapeutic implications, suggesting that COVID-19‒related diabetes and type 2 diabetes may share the same disease pathophysiology.35 Unfortunately, at this stage, we do not have definitive data or an appropriate control group to distinguish whether or not the elevated risk of insulin resistance is associated with any type of acute viral infection rather than specifically related to SARS-CoV-2 infection.
We showed that COVID-19 patients without pre-existing diabetes had decreased pancreatic islet activity during the early stages of SARS-CoV-2 infection. This result corresponded with those from several recently published studies of impaired insulin secretion in COVID-19 patients. Hollstein et al8 reported a 19-year-old White male who, in the absence of autoantibodies, had insulin-dependent diabetes 3 weeks after an asymptomatic SARS-CoV-2 infection, with a serum C-peptide level of 0.62 μg/L. A retrospective study performed in China reported that 42 of 658 (6.4%) hospitalized COVID-19 patients presented with ketosis on admission, of whom 27 (64%) patients did not have pre-existing diabetes.10 In another study in Singapore, a case of diabetic ketoacidosis precipitated by COVID-19 in a 37-year-old male patient with newly diagnosed diabetes was reported.9 In addition, increased amylase and lipase levels and focal pancreatic enlargement or pancreatic duct dilatation in some COVID-19 patients were reported,41 suggesting that SARS-CoV-2 infection may have resulted in pancreatic beta-cell injury. This is of interest because pancreatic islet cells demonstrate a high expression of angiotensin-converting enzyme 2 (ACE2) receptors.41 ACE2 is the main receptor for SARS-CoV-2.42 Yang et al43 recently confirmed that human pancreatic beta-cells are permissive to SARS-CoV-2 infection, suggesting that SARS-CoV-2 might result in the alteration of the pancreatic beta-cell function. Of note, SARS coronavirus has been confirmed to induce pancreatic beta-cell damage by using ACE2.44 Chee et al9 hold the opinion that the downregulation of ACE2 due to SARS-CoV-2 infection leads to unopposed angiotensin II and impedes insulin secretion.45 Beta-cell dedifferentiation accompanied by a reduction in ACE2 was also found in high-fat diet mice.46 Previous animal studies have shown that ACE2 deficiency resulted in impaired beta-cell proliferation, increased beta-cell oxidative stress, and hyperglycemia.47 , 48 Whether the decreased insulin secretions that occur in COVID-19 patients persist or remit when the disease is resolved is still unclear.1 Our observations of increased fasting C-peptide levels and HOMA-CP at the 6-month follow-up implied a transient and reversible disturbance of beta-cell activity in COVID-19 patients. However, data regarding the pathologic changes of beta-cells and the long-term changes of beta-cell activity in COVID-19 patients remain scarce and need to be explored.
It is worth noting that the plasma insulin levels are determined by the balance between insulin secretion and clearance.16 Insulin clearance is conceptualized as the sum of hepatic clearance and extrahepatic clearance.16 Recent data on COVID-19 have shed light on the effect of the disease on the liver, skeletal muscle, kidney, and heart.23 , 49, 50, 51 Thus, we speculate that the insulin clearance rate decreases in individuals with SARS-CoV-2 infection. This may partially explain the inconsistency between the C-peptide and insulin levels, thereby reducing the value of HOMA-IR in estimating insulin resistance in this study.
We admit that the conclusions from the analysis are relatively preliminary because the beta-cell activity and peripheral insulin action measurements based on fasting hormones may not be stable. Insulin secretion capacity is better modeled by the glucose tolerance test. Unfortunately, we had no data on the glucose tolerance test in this study; otherwise, we could have further explored postprandial insulin secretion capacity. In addition, data regarding insulin sensitivity and pancreatic islet activity, biochemical indices, economic status, diet, and lifestyle were unavailable for our patients before SARS-CoV-2 infection, which limits the conclusions of this study. Furthermore, the research design could have caused a selection bias because all patients were of the same race and enrolled from 1 city, which weakens the generalizability of this study to other races. In the process of analysis, our sample size limited the analysis based on the classification of severity and caused a high discrete degree in some variables as well. Moreover, no significant contributor for changes in the TyG index in the linear regression analysis performed at the 6-month follow-up was found, which is mainly owing to the small sample size. Further prospective studies involving large patient cohorts are required to evaluate the prognoses of pancreatic islet functioning and insulin sensitivity in COVID-19 patients.
In conclusion, our results suggest that COVID-19 may increase the risk of insulin resistance in patients without pre-existing diabetes. At the same time, the results also indicate that there may be decreases in insulin secretion with SARS-CoV-2 infection, although these decreases could be transient and reversible. Thus, it is suggested that parameters regarding insulin sensitivity and pancreatic islet activity in patients with COVID-19 history are monitored.
Acknowledgment
We acknowledge Professor Fredric B. Kraemer (Division of Endocrinology, Gerontology and Metabolism, Stanford University School of Medicine, and VA Palo Alto Health Care System, California) for his kind support in editing and proofreading this manuscript. This work was supported by the National Key R&D Program of China (2018YFC1314101), the National Natural Science Foundation of China (81970677), the Projects of Artificial Intelligence Early Warning and Multi-point Trigger Monitoring system for Infectious Diseases integrated with Medical Prevention (WKJ-ZJ-2138), the Outstanding Clinical Discipline Project of Shanghai Pudong (PWYgy2018-10), the Key Scientific and Technological Innovation Projects of Wenzhou (ZY202004), and the Science and Technology Project of Zhejiang Province (2017C33215). The funders had no role in the design and conduct of the study, the completion of the analysis, the interpretation of the data, or the content and preparation of the manuscript.
Author Contributions
All authors have met the requirements for authorship. M.C. and B.Z. are co-first authors. C.H., J.L., and S.Q. had the idea for and designed the study. M.C., B.Z., D.C., X.H., and X.X. followed up with the patients and collected data. M.C., B.Z., and W.J.S. processed statistical data and drafted the manuscript. All authors reviewed and approved the final version of the manuscript.
Disclosure
The authors have no multiplicity of interest to disclose.
Supplementary Material
References
- 1.Rubino F., Amiel S.A., Zimmet P. New-onset diabetes in Covid-19. N Engl J Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L., She Z.-G., Cheng X. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman N., Knighton P., Kar P. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323(21):2195–2198. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Codo A.C., Davanzo G.G., Monteiro L.B. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32(3):437–446.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson K., Morris J., Bridson T., Govan B., Rush C., Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144(2):171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esposito K., Nappo F., Marfella R. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 8.Hollstein T., Schulte D.M., Schulz J. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat Metab. 2020;2(10):1021–1024. doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- 9.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren H., Yang Y., Wang F. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol. 2020;19(1):58. doi: 10.1186/s12933-020-01035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith S.M., Boppana A., Traupman J.A. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2021;93(1):409–415. doi: 10.1002/jmv.26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H., Wu J., Hong L. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnosis and treatment guidelines for 2019 novel coronavirus pneumonia (version 7). China NHaHCotPsRo. Accessed March 3, 2020. http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11815/202003/W020200305456621460977.pdf
- 16.Piccinini F., Bergman R.N. The measurement of insulin clearance. Diabetes Care. 2020;43(9):2296–2302. doi: 10.2337/dc20-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 18.LaBarre J.L., Peterson K.E., Kachman M.T. Mitochondrial nutrient utilization underlying the association between metabolites and insulin resistance in adolescents. J Clin Endocrinol Metab. 2020;105(7):2442–2455. doi: 10.1210/clinem/dgaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Zhou Z.-G., Qi H.-Y., Chen X.-Y., Huang G. Replacement of insulin by fasting C-peptide in modified homeostasis model assessment to evaluate insulin resistance and islet beta cell function. Article in Chinese. Zhong Nan Da Xue Bao Yi Xue Ban. 2004;29(4):419–423. [PubMed] [Google Scholar]
- 20.Guerrero-Romero F., Simental-Mendía L.E., González-Ortiz M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 21.Liu C., Cripe T.P., Kim M.-O. Statistical issues in longitudinal data analysis for treatment efficacy studies in the biomedical sciences. Mol Ther. 2010;18(9):1724–1730. doi: 10.1038/mt.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao R., Qiu Y., He J.-S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y.-T., Shao S.-C., Hsu C.-K., Wu I.-W., Hung M.-J., Chen Y.-C. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):346. doi: 10.1186/s13054-020-03009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sardu C., D’Onofrio N., Balestrieri M.L. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro-González D., Sánchez-Íñigo L., Pastrana-Delgado J., Fernández-Montero A., Martinez J.A. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the Vascular-Metabolic CUN cohort. Prev Med. 2016;86:99–105. doi: 10.1016/j.ypmed.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Šestan M., Marinović S., Kavazović I. Virus-induced interferon-γ causes insulin resistance in skeletal muscle and derails glycemic control in obesity. Immunity. 2018;49(1):164–177.e6. doi: 10.1016/j.immuni.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Narita R., Abe S., Kihara Y., Akiyama T., Tabaru A., Otsuki M. Insulin resistance and insulin secretion in chronic hepatitis C virus infection. J Hepatol. 2004;41(1):132–138. doi: 10.1016/j.jhep.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Groop L.C., Bonadonna R.C., DelPrato S. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84(1):205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eketunde A.O., Mellacheruvu S.P., Oreoluwa P. A review of postmortem findings in patients with COVID-19. Cureus. 2020;12(17):e9438. doi: 10.7759/cureus.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020;41(3):457–470. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wensveen F.M., Jelenčić V., Valentić S. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. 2015;16(4):376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 37.Lin L., Luo S., Qin R. Long-term infection of SARS-CoV-2 changed the body’s immune status. Clin Immunol. 2020;218:108524. doi: 10.1016/j.clim.2020.108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastard P., Rosen L.B., Zhang Q. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zornitzki T., Malnick S., Lysyy L., Knobler H. Interferon therapy in hepatitis C leading to chronic type 1 diabetes. World J Gastroenterol. 2015;21(1):233–239. doi: 10.3748/wjg.v21.i1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoungas S., Patel A., Chalmers J. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 41.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130.e2. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiersinga W.J., Rhodes A., Cheng A.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 43.Yang L., Han Y., Nilsson-Payant B.E. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125–136.e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlsson P.O., Berne C., Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41(2):127–133. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 46.Xuan X., Gao F., Ma X. Activation of ACE2/angiotensin (1-7) attenuates pancreatic β cell dedifferentiation in a high-fat-diet mouse model. Metabolism. 2018;81:83–96. doi: 10.1016/j.metabol.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Roca-Ho H., Palau V., Gimeno J., Pascual J., Soler M.J., Riera M. Angiotensin-converting enzyme 2 influences pancreatic and renal function in diabetic mice. Lab Invest. 2020;100(9):1169–1183. doi: 10.1038/s41374-020-0440-5. [DOI] [PubMed] [Google Scholar]
- 48.Shoemaker R., Yiannikouris F., Thatcher S., Cassis L. ACE2 deficiency reduces β-cell mass and impairs β-cell proliferation in obese C57BL/6 mice. Am J Physiol Endocrinol Metab. 2015;309(7):E621–E631. doi: 10.1152/ajpendo.00054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai Q., Huang D., Yu H. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta A., Madhavan M.V., Sehgal K. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 51.Most Z.M., Hendren N., Drazner M.H. Striking similarities of multisystem inflammatory syndrome in children and a myocarditis-like syndrome in adults: overlapping manifestations of COVID-19. Circulation. 2021;143(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.050166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.