Summary
Regulatory T cells (Treg cells) represent a CD4+ T-cell lineage that plays a critical role in restraining immune responses to self and foreign antigens and associated inflammation. Due to the suppressive function of Treg cells, inhibition or ablation of these cells can be used to boost the immunity against malignant cells. On the other hand, augmenting the activity of Treg cells can be employed for the treatment of inflammatory or autoimmune diseases and allogeneic conflicts associated with transplantation. Graft-versus-host disease (GvHD) is a leading cause of morbidity and mortality after haematopoietic stem cell transplantation (HSCT). In this review, we describe basic biological properties of Treg cells and their role in GvHD. We focus on the application of adoptive transfer of Treg cells and the therapeutic modulation of their activity for the prevention and treatment of GvHD in pre-clinical models and in clinical settings. We also discuss the main obstacles to applying Treg cell-based therapies for GvHD in clinical practice.
Keywords: Treg cells, GVHD, immunotherapy, IL-2, rapamycin
Biology of Treg cells and their therapeutic use for autoimmune diseases
Regulatory T cells (Treg cells) represent a distinct lineage of CD4+ T cells that restrain the immune response against self and foreign antigens (Table I) (Sakaguchi, et al 2011). These cells, which constitute 5–10% of CD4+ cells, exert their suppressive function both by direct cell-cell interactions as well as by secretion of inhibitory cytokines such as interleukin (IL)-10 or transforming growth factor β (TGF-β) (Josefowicz, et al 2012). The transcription factor FOXP3 is a lineage-specific marker of Treg cells which is critical for their function (Fontenot, et al 2003, Hori, et al 2003). In addition, Treg cells express high amounts of Il-2 receptor α-chain (IL-2Rα; also termed IL2RA, CD25), but do not produce IL-2 themselves. Treg cells can be broadly classified into two groups according to their developmental origin: thymic regulatory T cells (tTreg cells) and extrathymic or peripheral Treg cells (pTreg cells). tTreg cells are generated in the thymus from CD4+ single-positive thymocytes and express T-cell receptors (TCR) with an increased affinity for self-peptides (Josefowicz, et al 2012). On the other hand, pTreg cells develop from conventional CD4+ T cells in the periphery after encountering an antigen in the presence of high amounts of TGF-β (Kanamori, et al 2016). When the conversion of conventional CD4+ T cells to Treg cells occurs in vitro these cells are termed induced Treg cells (iTreg cells). Currently, tTreg cells and pTreg cells/iTreg cells cannot be reliably differentiated by specific markers. Recent studies indicate that Treg cells are much more heterogeneous than implied from this classification. First, Treg cells that reside in non-lymphoid organs (tissue Treg cells) exhibit gene expression profiles distinct from those of Treg cells found in secondary lymphoid organs. Furthermore, different tissue Treg cells were reported to differ in their transcriptomes (Li, et al 2018). Secondly, different populations of Treg cells can be delineated based on the expression of distinct transcription factors, which affect their function. For example, Treg cells which express the transcription factor T-bet specifically inhibit TH1 and CD8+ T cell activation (Levine, et al 2017).
Table I.
General properties and subpopulations of Treg cells
| • 5–10% of CD4+ T cells |
| • Play a role in suppressing an immune response |
| • Express the transcription factor FOXP3 and the interleukin-2 receptor α-chain (IL-2Rα; IL2RA, CD25) |
| • Surface stain markers - CD4+CD25hiCD127lo |
| • Secrete the inhibitory cytokines IL-10 and TGF-β |
| • Subpopulations of Treg cells: |
| ○ Thymic Treg cells - generated in the thymus. |
| ○ Peripheral Treg cells - develop from conventional CD4+ cells in the periphery. |
| ○ Induced Treg cells – induced in vitro from conventional CD4+ cells. |
| ○ Tissue Treg cells – Treg cells that reside in non-lymphoid organs. |
| ○ “naïve Treg cells” express CD44loCD62Lhi; “effector-like” Treg cells express CD44hiCD62Llo. CD62L is a homing molecule that enables migration of Treg cells into lymph nodes. |
| • Properties of Treg cells in different graft sources: Bone marrow Treg cells (compared to peripheral blood Treg cells) - high numbers, high expression of CD62L. Cord blood Treg cells - naïve phenotype, high expression of CD25. |
The initial discovery of Treg cells resulted from a search for a population of immune suppressive cells capable of preventing autoimmunity and inflammation in rodents subjected to neonatal thymectomy. This quest culminated in the discovery first of CD25 and later of FOXP3 as a definitive marker of Treg cells (Sakaguchi 2011). Treg cell deficiency in humans, resulting from inactivating mutations in the FOXP3 gene leads to the development of IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome (Bennett, et al 2001). Similarly, fatal aggressive systemic disease is observed in Treg cell-deficient mice (Brunkow, et al 2001). In many autoimmune diseases, including type I diabetes mellitus (DM), multiple sclerosis and rheumatoid arthritis, a defect in the Treg cell number or function has been described (Grant, et al 2015). Several attempts have been made to harness Treg cells for treating autoimmune diseases. For example, an IL-2 antibody that stabilizes IL-2 in a conformation that leads to its preferential binding to the IL-2 receptor and to STAT5 phosphorylation, was able to induce remission of type I DM in mice and protect mice against GvHD (Trotta, et al 2018). Although these strategies have not been reduced to clinical practice, they emphasize the potential of Treg cell-based therapies for autoimmune diseases (Bluestone and Tang 2018).
Treg cells in GvHD patients
During the last several decades it has been established that allogeneic haematopoietic cell transplantation (HSCT) can lead to the cure of several haematological malignancies, mainly acute leukaemia, as well as of genetic immune deficiencies (Appelbaum 2007). Despite the effectiveness of this treatment, HSCT is a challenging procedure with life-threatening complications. One of these common complications is GvHD, which is a leading cause of morbidity and mortality after HSCT.
Several studies have been conducted in human patients to elucidate the association between GvHD severity and relative abundance of Treg cells. One of the main limitations of early GvHD studies is that unambiguous quantification of Treg cells based on expression of cell surface markers, such as CD25, is not attainable because CD25 is also expressed by activated T cells. The detection of Treg cells was improved in more recent studies by gating on CD25high and CD127low cells or by detecting FOXP3 expression using intracellular staining or by quantifying its transcript level in tissues. The different methods for Treg cell detection might explain, at least partially, the inconsistency in studies that examined the correlation between peripheral blood (PB) Treg cells and GvHD severity. Most of the studies found an inverse correlation between the number of Treg cells and the development of acute GvHD (aGvHD) (Fujioka, et al 2013, Li, et al 2010, Magenau, et al 2010, Rieger, et al 2006, Ukena, et al 2011), but some studies did not observe this association (Noel, et al 2008, Sanchez, et al 2004). The results are more inconsistent in case of chronic GvHD (cGvHD), which was associated with a reduced relative Treg cell abundance in some (Li, et al 2010, Zorn, et al 2005), but not in other studies (Clark, et al 2004, Sanchez, et al 2004, Ukena, et al 2011). Several studies also assessed the association between the numbers or proportion of Treg cells in the graft and GvHD. Most of these studies found an inverse correlation between graft or donor Treg cells and GvHD, mainly aGVHD (Lu, et al 2011, McIver, et al 2013, Pabst, et al 2007, Rezvani, et al 2006), but not all of them (Arimoto, et al 2007, Stanzani, et al 2004). The Treg cell content can also vary in different graft sources. Compared to bone marrow (BM) grafts, the number of Treg cells in PB grafts is lower and these cells have lower expression of CD62L (also termed SELL) (Blache, et al 2010). On the other hand, cord blood (CB) Treg cells are mostly naïve and, compared to PB Treg cells, have higher expression of CD25, which enables easier isolation of these cells (Godfrey, et al 2005). However, it is not completely clear whether the suppressive activity of CB Tregs cells is comparable to that of expanded PB Treg cells (Fujimaki, et al 2008, Godfrey, et al 2005).
It is likely that the importance of Treg cells in GvHD could be assessed in a more direct and convincing manner through their analyses in GvHD target organs rather than in the PB. One study analysed the number of infiltrating Treg cells in intestinal biopsies using immunohistochemical detection of FOXP3 expression (Rieger, et al 2006). The FOXP3+/CD8+ T cell ratios in patients with acute and chronic GvHD was similar to healthy controls and lower than in patients with infectious inflammation or in allograft patients without GvHD, implying that GvHD is associated with an insufficient up-regulation in Treg cell numbers in intestinal GvHD lesions. This notion is supported by analysing FOXP3+ Treg cells in skin biopsies (Fondi, et al 2009). In contrast to these studies, another study did not find an inverse association between gastric Treg cells and gastric GvHD (Lord, et al 2011), which raises the possibility of differences between GvHD target organs.
Thus, despite the limitations mentioned above and some conflicting results, overall, these studies imply that GvHD (mainly aGvHD) is associated with a diminished proportion of Treg cells.
Therapeutic use of Treg cells for GvHD in preclinical models
Several pre-clinical studies have been conducted in experimental GvHD models to explore the therapeutic potential of Treg cells for GvHD prevention (the main findings of these studies are summarized in Table II). Although these studies have several limitations, they established the notion that Treg cells have a significant potential to ameliorate GvHD.
Table II.
Summary of main findings in pre-clinical studies of Treg cells for GvHD
| Major finding | Representative references |
|---|---|
| Depletion of Treg cells from the graft exacerbates GvHD, and supplementation of Treg cells ameliorates GvHD | Cohen et al (2002), Taylor et al (2002) |
| Treg cells inhibit the early expansion of allogeneic conventional T cells in lymphoid organs and GvHD target organs | Edinger et al (2003) |
| The best therapeutic effect of transplanted Treg cells for GvHD is achieved when Treg cells are infused in early phases of inflammation | Nguyen et al (2007) |
| The graft-versus-tumour effect is unimpeded upon infusion of Treg cells | Edinger et al (2003), Jones et al (2003), Martelli et al (2014), Nguyen et al (2007), Trenado et al (2003) |
| Treg cells can ameliorate GvHD in haploidentical mouse models (in addition to fully allogeneic models) | Wysocki et al (2005), Zhang et al (2013) |
| Treg cells are beneficial in chronic GvHD mouse models (in addition to acute GvHD models) | Anderson et al (2004), Zhao et al (2008) |
| Treg cells have a therapeutic role in ameliorating GvHD in xenogeneic models | Hippen et al (2008), Hippen et al (2011), Mutis et al (2006) |
| The expression of FOXP3 in induced Treg cells is unstable, which impairs their ability to protect against GvHD | Beres et al (2011), Koenecke et al (2009) |
| Rapamycin with or without low-dose IL-2 can improve the stability of FOXP3 in induced Treg cells | Shin et al (2011), Zhang et al (2013) |
GvHD: graft-versus-host disease; IL-2: interleukin 2; Treg: T regulatory.
In early studies, depletion of Treg cells from the graft using depleting CD25 antibody led to an earlier onset of GvHD, while supplementation of CD4+CD25+ Treg cells significantly delayed or prevented the development of GvHD (Cohen, et al 2002, Taylor, et al 2002). Importantly, the CD4+CD25+ Treg cell population was not able to induce GvHD on its own.
In one study, lethally irradiated BALB/c hosts were injected with C57BL/6-derived T cell-depleted BM cells with and without splenic CD4+CD25- and CD4+CD25+ T cells. At a 1:1 ratio of CD4+CD25+ Treg cells and effector CD4+CD25- T cells, the recipient mice were protected from aGvHD with 93% of animals surviving for 100 days. The protective effect of Treg cells was dependent, at least partially, on IL-10 production (Hoffmann, et al 2002). Another study by the same group demonstrated, using bioluminescence imaging, that CD4+CD25+ Treg cells inhibited the early expansion of allogeneic conventional T (Tconv) cells in lymphoid organs and GvHD target organs (Edinger, et al 2003).
In another study, Treg cell localization was tracked over time in vivo in a mouse GvHD model by using whole body bioluminescence imaging (BLI) (Nguyen, et al 2007). Within the first 24–48 h after transplantation, donor Treg cells localized to the peripheral lymph nodes and spleen. By day 4, Treg cells had migrated to the liver and the gut followed by skin infiltration between days 5 and 6. This study also found that the best therapeutic effect of transplanted Treg cells is achieved in early phases of inflammation, during which lower Treg cell numbers were also required to protect from GvHD. Moreover, the best therapeutic effect of infused Treg cells was achieved when Treg cells were administered prior to the Tconv cell transfer. In contrast, by 3 weeks after HSCT, the addition of Treg cells reduced the morbidity and mortality of GvHD at a significantly lower rate.
Although the results of these pioneering pre-clinical studies were encouraging, they raised several questions regarding the application of this treatment to human patients. One of the cardinal issues is the number of Treg cells, which are required to obtain a significant anti-GvHD effect. In these pre-clinical studies, Treg cells were usually administrated in similar proportions to donor T cells, whereas a lower ratio of Treg cells, similar to their physiological proportion, had no protective effect against GvHD (Hoffmann, et al 2002). This obstacle could be mitigated by in vitro expansion of Treg cells, which showed initial encouraging results. However, in vitro expansion of Treg cells might lead to preferential expansion of Tconv cells (Riley, et al 2009). Another strategy, which might be more efficient for treating human patients, is to generate in vitro induced Treg cells (iTreg cells) from conventional CD4+ T cells, usually upon stimulation with CD3 and CD28 antibodies in the presence of IL-2 and TGF-β. However, FOXP3 expression in iTreg cells is typically unstable and these cells do not convey significant protection against aGvHD (Beres, et al 2011, Koenecke, et al 2009). Several pharmacological manipulations have been attempted to improve the stability of FOXP3 expression, which include CpG methylation targeting agents, histone deacetylase inhibitors and the mechanistic target of rapamycin (mTOR) inhibitor, rapamycin (Lal, et al 2009, Polansky, et al 2008). Furthermore, rapamycin, which inhibits conventional T cell expansion while sparing Treg cells, can be combined with low-dose IL-2 administration to improve the stability of FOXP3 expression and augment the suppressive properties of iTreg cells, including FOXP3 expressing CD8+ T cells (see below) (Shin, et al 2011, Zhang, et al 2013). According to one of these studies (Zhang, et al 2013), in vivo administration of rapamycin led to migration and expansion of iTreg cells in the gastrointestinal tract and spleen and to a reduction in pro-inflammatory cytokine levels. A different strategy to overcome the low numbers of Treg cells is to enhance their suppressive activity. Priming of donor Treg cells in the presence of tumour necrosis factor-α (TNF-α) has been shown to increase FOXP3 expression, and to enhance Treg cell suppressive function in a GvHD model (Pierini, et al 2016). Alternatively, recipient Treg cells could be expanded in vitro before HSCT using a tumour necrosis factor receptor 2 (TNFR2) agonist (Chopra, et al 2016). In addition, Treg cells that express a constitutively active form of STAT5b (STAT5b-CA) have been shown to be more potent in suppressing GvHD compared to wild type Treg cells (Vogtenhuber, et al 2010). It has also been found that ex vivo fucosylation of human Treg cells reduced the number of Treg cells required to ameliorate GvHD in a mouse xenogeneic GvHD model (Parmar, et al 2015). Finally, it has been suggested that disruption of the vimentin network can enhance Treg suppressive activity in a GvHD mouse model (McDonald-Hyman, et al 2018).
In addition to the number of Treg cells required to achieve a therapeutic anti-GvHD effect, an important issue in considering their therapeutic application is whether the graft-versus-tumour (GvT) effect is unimpeded upon infusion of Treg cells. Several studies demonstrated a GvT effect in the presence of Treg cells (Edinger, et al 2003, Jones, et al 2003, Martelli, et al 2014, Nguyen, et al 2007, Trenado, et al 2003). However, a more recent study, where blast crisis chronic myeloid leukaemia (CML) cells were used as the tumour, showed that adoptive transfer of Treg cells impaired the graft-versus-leukaemia (GvL) effect (Zhang, et al 2013). Therefore, the effect of Treg cells on GvT might be dependent on several parameters, such as the effector to Treg cell ratio, the specific tumour type and the extent of tumour burden, which should be explored in-depth.
Another important issue is the transplant type and its relation to the efficiency of Treg cell treatment. Many pre-clinical studies that demonstrated a beneficial effect of Treg cell treatment were conducted in a fully allogeneic model, where usually a HSC graft of a C57BL/6 (H-2b) mouse origin was transplanted into BALBc (H-2d) recipients (Edinger, et al 2003, Hoffmann, et al 2002). However, some of the studies were performed in a haploidentical GvHD mouse model, which extends the findings to a setup that is more similar to the human disease (Cohen, et al 2002, Taylor, et al 2002, Wysocki, et al 2005, Zhang, et al 2013). Notably, in one of the studies, the therapeutic effect of Treg cells was less pronounced in a haploidentical GvHD model (Taylor, et al 2002). The therapeutic effect of Treg cells has been also demonstrated in the case of minor histocompatibility antigen disparity (Jones, et al 2003). More studies are warranted to explore the therapeutic efficacy of Treg cell transfer in antigenically different GvHD models.
While preclinical studies discussed above concentrated on aGvHD, several studies assessed the effect of Treg cells in mouse models of cGvHD. The most common cGvHD model is the B10.D2 (H-2d) graft into BALB/c (H-2d) donor, which, while being major histocompatibility complex (MHC)-compatible, features multiple minor histocompatibility antigen (miHA) mismatches. This mouse model shares many features with cGvHD observed in human HSCT patients. In one of the studies, it was observed that donor-type CD103+ Treg cells from cGVHD mice were more potent in ameliorating ongoing cGvHD when transferred to other mice in which cGvHD was induced compared to CD25hi Treg cells isolated from healthy mouse donors (Zhao, et al 2008). According to another study, host CD4+CD25+ T cells, which survive irradiation, play a role in ameliorating cGvHD, in addition to donor CD4+CD25+ cells (Anderson, et al 2004).
Finally, in these pre-clinical studies, Treg cells are usually administered together with the graft as a prophylactic treatment for GvHD (Cohen, et al 2002, Edinger, et al 2003, Taylor, et al 2002). However, in human patients, Treg cells might be required clinically for GvHD treatment and not only for prophylaxis, and this issue should also be better explored.
The therapeutic role of Treg cells in ameliorating GvHD has been also observed in a xenogeneic model where human PB mononuclear cells (PBMCs) were injected into immunodeficient mice. This model has its own limitations given that, in this case, the presentation of mouse antigens depends on human antigen presenting cells (APCs) that present these antigens in the context of class MHC and, therefore, mainly enables the evaluation of the role of CD4+ T cells. When human PBMCs were transferred into RAG2γc−/− mice, depletion of CD25+ Treg cells exacerbated the lethality of xenogeneic GvHD (Mutis, et al 2006). Conversely, co-administration of Treg cells with autologous PBMCs reduced the development of lethal GvHD. The protection from GvHD was associated with a significant increase in the plasma levels of IL-10 and γ-interferon. This therapeutic effect of Treg cells in xenogeneic GvHD models has been also observed when PB Treg cells were expanded in vitro (Hippen, et al 2011) or when purified CB Treg cells were used for adoptive transfer (Hippen, et al 2008) with allogeneic PBMCs at a 1:1 ratio.
CD8+FOXP3+ Treg cells represent another less-studied population of cells with a likely immunoregulatory function. These cells, which are induced during GvHD, have been suggested to exhibit suppressor function and to ameliorate GvHD in mouse models (Robb, et al 2012, Sawamukai, et al 2012). In addition, these cells are thought to exhibit cytotoxic activity against tumour cells, activity which could prevent tumour relapse during GvHD (Zheng, et al 2013). Although CD8+FOXP3+ T cells are present in limited numbers, the latter can be increased upon TCR-induced stimulation in the presence of rapamycin and IL-2. However, FOXP3 expression is less stable in CD8+FOXP3+ cells compared to their CD4+ counterparts. Recently, it has been suggested that the generation of CD8+ iTreg cells from JAK2-deficient T cells enables high FOXP3 expression in these cells (Iamsawat, et al 2018), which raises the possibility that JAK2 inhibitors could promote stability of CD8+ iTreg cells. Another recent study demonstrated that down-regulation of BIM (also termed BCL2L11) in CD8+ Treg cells enabled prolonged survival of these cells, leading to improvement in GvHD (Agle, et al 2018).
Apart from ameliorating GvHD, several studies in mice demonstrated that CD25+ Treg cells can prevent BM graft rejection (Hanash and Levy 2005, Joffre, et al 2004, Steiner, et al 2006, Taylor, et al 2004) and to improve immune reconstitution after transplantation in mouse models (Trenado, et al 2003).
Features of Treg cells capable of ameliorating GvHD in pre-clinical models
Several studies were conducted to define characteristics of Treg cells capable of ameliorating GvHD. Two studies demonstrated that the expression of CD62L, a homing molecule enabling migration into lymph nodes, is important for ameliorating GvHD as well as for BM engraftment (Ermann, et al 2005, Taylor, et al 2004). In these studies, donor Treg cells were separated into CD62Lhigh or CD62Llow expressing cells. While the infusion of CD62Llow Treg cells did not protect mice from lethal GvHD, CD62Lhigh Treg cells inhibited the expansion of effector T cells in the spleen and in secondary lymphoid organs. In another study, it has been shown that Treg cells lacking the chemokine receptor CCR5 were less effective in preventing lethal GvHD. The lack of CCR5 correlated with impaired accumulation of Treg cells in the liver, lung, spleen and mesenteric lymph nodes in the second week after transplantation, implying that this receptor is important for later recruitment of Treg cells to lymphoid tissues and GvHD target organs (Wysocki, et al 2005). Thus, Treg cells expressing specific chemokine receptors can be therapeutically important in the recruitment of Treg cells to specific GvHD target organs. Indeed, Treg cells transfected with the chemokine receptor CXCR3 were able to better migrate to the liver, lung and intestine when compared to control Treg cells, leading to enhanced control of GvHD in these organs (Hasegawa, et al 2008). Taken together, these studies, as well as others mentioned above (Edinger, et al 2003, Nguyen, et al 2007), emphasise that the therapeutic effect of Treg cells depends both on their presence in lymphoid organs and in GvHD target organs. However, this issue, as well as the exact mechanism of the suppressive effect of Treg cells in ameliorating GvHD, should be better explored in future studies.
Another important issue is the significance of using antigen-specific Treg cells to ameliorate GvHD compared to using polyclonal Treg cells. This strategy can potentially lead to more efficient Treg-mediated suppression with a lower requirement of infused cells. Although this notion has been much more established in autoimmune diseases (especially type I DM) (Bluestone, et al 2015, Veerapathran, et al 2011), some studies also demonstrated an advantage of antigen-specific Treg cells in ameliorating GvHD, in which the expanded Treg cells were cultured in the presence of cells expressing recipient-type alloantigens (Cohen, et al 2002, Trenado, et al 2003) or even exogenous antigen (nondonor, nonrecipient) (Martin, et al 2013). Recently, it has been shown that antigen-specific Treg cells can be produced by expressing chimeric antigen receptor (CAR) (MacDonald, et al 2016). This study demonstrated that Treg cells that express a HLA-A2-specific CAR were more efficient than Treg cells expressing an irrelevant CAR in preventing GvHD in a xenogeneic mouse model.
Finally, the importance of human leucocyte antigen (HLA) matching between Treg cells and Tconv cells has also been studied. GvHD protection can be achieved by donor type Treg cells, rather than by host type Treg cells (Hoffmann, et al 2002). Interestingly, Treg cells produced from third-party donors are also very efficient in protecting mice from GvHD, with comparable efficiency to donor-derived Treg cells in an MHC minor mismatch model (but with some reduced efficiency in a complete allogeneic model) (Pierini, et al 2015). This finding implies that third-party Treg cells are a useful source of Treg cells and that, mechanistically, Treg cells can be effective independently of Treg/Tconv HLA matching.
Expanding Treg cells for the treatment of GvHD patients
The encouraging pre-clinical results led to several clinical studies of Treg cell therapy for human GvHD patients. Clinical translation of this therapeutic approach raised several questions: What is the preferred source of cells for adoptive transfer (PB versus CB)? Should natural Treg cells be isolated or should Treg cells be generated in vitro? How should Treg cells be expanded in vitro? Should the Treg cells be polyclonal or be enriched for specific antigens? Should additional medications be administered to improve the Treg cell treatment efficiency? Figure 1 summarizes the prevalent sources of therapeutic Treg cells and the main methods for expanding them in vitro and in vivo.
Figure 1. A summary of the main available sources of Treg cells and of the methods for expading them in vitro and in vivo for GvHD treatment.
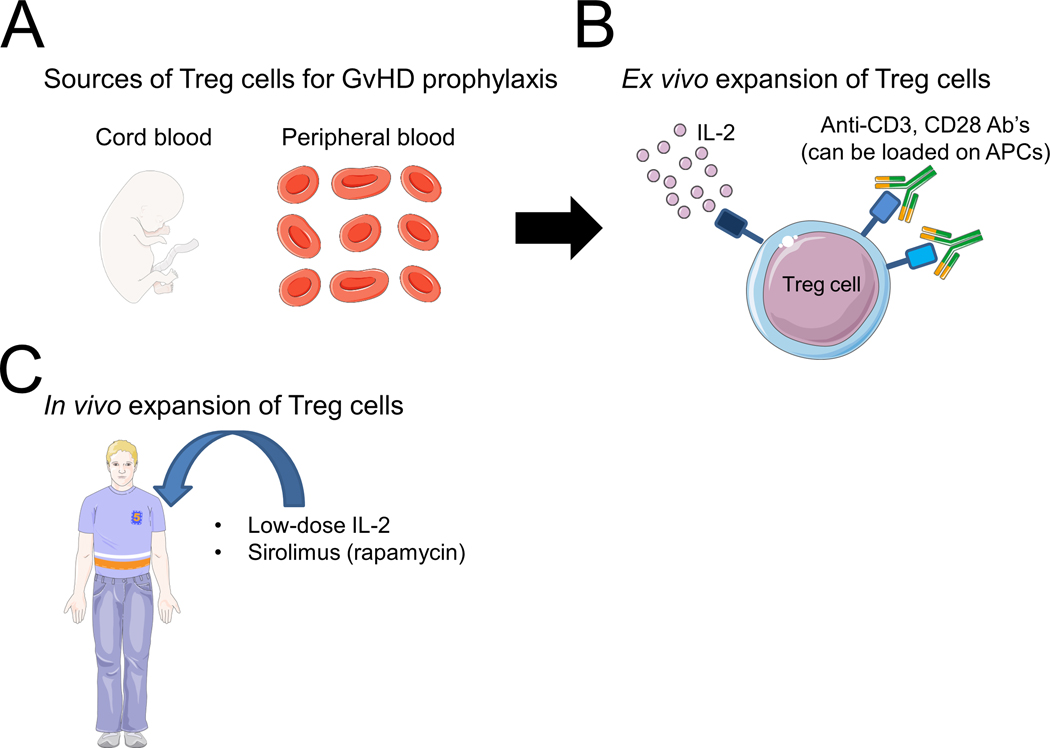
Ab’s: antibodies; APCs: antigen presenting cells; GvHD: graft-versus-host disease; IL-2: interleukin 2; Treg: T regulatory.
The figure was produced using Servier Medical Art (www.servier.com).
The isolation of human natural Treg cells poses the following difficulties. Firstly, it is a rare population of cells. Secondly, as mentioned above, Treg cells cannot be unambiguously defined by cell surface markers. In clinical studies of Treg cells for GvHD, Treg cells are usually isolated by positive selection with CD25 antibody, with or without a prior negative selection stage with anti-CD8 and anti-CD19 antibodies. Although this method leads to significant enrichment of CD4+CD25+FOXP3+ cells (Di Ianni, et al 2009), the purity of FOXP3+ cells is not high enough [50–80%, (Martelli, et al 2014, Peters, et al 2008)], but can reach 87% after expansion (Brunstein, et al 2016). The problem of human Treg cell purity has been largely solved by sorting CD4+CD25hiCD127lo cells introduced by Bluestone and colleagues, which markedly improved the purity of human Treg cell isolation (in this case in type I DM patients) (Putnam, et al 2009).
As for the expansion of Treg cells, several studies demonstrated the utility of ex vivo expansion of human natural Treg cells to preserve their functional activity. Stimulation of Treg cells isolated from the PB with CD3 and CD28 monoclonal antibodies and high amounts of IL-2, led to a marked expansion of the cells and to the enhancement of their suppressor function (Godfrey, et al 2004). In other studies, Treg cells were expanded in vitro using artificial antigen presenting cells (aAPC) loaded with CD3 antibody with remarkable efficiency, while preserving the expression of FOXP3 and the suppressive functions of the cells. These cells have been shown to ameliorate GvHD in a xenogeneic mouse model (Hippen, et al 2011). Another proposed strategy to expand functionally-potent human Treg cells is based on the use of a TNFR2 agonist (Okubo, et al 2013).
Umbilical cord blood (UCB) is another potential source of therapeutic Treg cells. The purity of Treg cells isolated from UCB is higher compared to cells isolated from adult PB, probably because of the presence of activated effector CD4+ T cells with variable levels of CD25 expression in the adult PB as the result of their antigenic exposure (Godfrey, et al 2004). However, the numbers of Treg cells present in UCB samples are limiting. UCB-derived Treg cells can be expanded successfully using cell-based aAPCs preloaded with CD3 and CD28 antibodies (Godfrey, et al 2005). UCB-derived Treg cell expansion can be further improved upon incorporation of OX40L or 4–1BBL into aAPCs. This approach enabled increased survival of Treg cells in a xenogeneic GvHD model (Hippen, et al 2008).
Finally, Treg cells can be generated in vitro in the presence of rapamycin, which enhances TGF-β-dependent FOXP3 expression and limits activation and expansion of effector T cells (Battaglia, et al 2005). In vivo administration of rapamycin to primates has also been shown to enhance the half-life and stability of adoptively transferred Treg cells (Singh, et al 2014). According to a recent study, Treg cells can be induced by stimulation of naïve CD4+ T cells in conditions of low tryptophan plus kynurenines. However, adoptive transfer of these Treg cells in a xenogeneic GvHD model did not prolong survival, despite transient clinical improvement (Hippen, et al 2017). Lastly, even freezing and thawing of murine and human Treg cells might impair their function by reducing the expression of CD62L, which, as mentioned above, contributes to the protective effect of Treg cells in vivo (Florek, et al 2015).
Clinical studies of adoptively transferred Treg cells for GvHD prophylaxis and treatment
Several clinical studies examined the efficiency of Treg cells for GvHD (summarised in Table III); however, not one was a randomized control study. The first clinical study of infused Treg cells for GvHD prophylaxis was performed in adults transplanted with UCB-derived cells (Brunstein, et al 2011). This phase I study evaluated the safety profile of UCB Treg cells in 23 patients with various haematological malignancies. Treg cells were isolated from a third-party CB product and then expanded ex vivo for ~18 days using IL-2 and CD3- and CD28-antibody-coated beads (median expansion of 211-fold). The Treg cell dose target was achieved in 74% of all cultures, and all Treg cell cultures were suppressive in vitro. The patients received a dose of 0.1–30 × 105 UCB Treg cells/kg one day after double UCB transplantation (a subset of patients received an additional dose at day +15). After infusion, UCB-derived Treg cells could be detected in the PB for 14 days. Compared to a historical control group, the incidence of grade 2–4 aGvHD was reduced (61% vs 43%). However, as expected from a phase I study, the evidence for the overall efficacy of this regimen was not unequivocal, especially because the incidence of GvHD has been reported to be lower in other studies (Komanduri and Champlin 2011). In addition, the ratio of Treg:Tconv cells in this study was 1:5, which is lower compared to the murine studies, and the use of a calcineurin inhibitor could have affected the function of the Treg cells. Subsequent analysis of the outcomes of this study revealed a significant cumulative incidence of opportunistic infections during the first 30 days compared with historical controls, which did not affect non-relapse mortality or progression-free survival (Brunstein, et al 2013). No evidence of faster immune reconstitution was observed in the Treg cell treatment group compared to historical controls, as evaluated by the fraction of immune cell subsets on day +180. In a subsequent study, better expansion of Treg cells was accomplished by stimulating them with K562 cells that were modified to express the Fc receptor CD64 and the co-stimulatory molecule CD86, enabling the administration of 3–100 × 106 UCB Treg cells/kg (Brunstein, et al 2016). The incidence of aGvHD in this study was 9% among the 11 patients treated with this regimen, compared to a rate of 45% in contemporaneous controls exposed to the same conditioning regimen. The incidence of cGvHD was also lower. There was no difference between the groups in haematopoietic cell recovery and chimerism, infections, non-relapse associated mortality, relapse and disease-free survival (Brunstein, et al 2016). Similar to the previous study, Treg cells were not detected in the PB beyond 14 days despite higher infusion doses.
Table III.
Main clinical studies with therapeutic use of Tregs cells for GvHD.
| Number of patients, diagnoses | Conditioning regimen | Donor source | Treg cell source | Treg cell expansion | Treg cell dose, Treg:Tconv ratio | Treg cell administered | Outcome | Possible adverse effects related to Treg cell infusion | References |
|---|---|---|---|---|---|---|---|---|---|
| 23, various haematological malignancies (48% with acute leukaemia) | Nonmyeloablative regimen (Cy, Flu, TBI 200 cGy) | UCB. | UCB (from a third unit). | Expanded in vitro for ~18 days with anti-CD3/anti-CD28 antibody-coated beads and IL-2. | 0.1–30 × 105 Treg/kg, Treg:Tconv 1:5. | Day +1. | Compared to historical controls, the incidence of aGvHD was reduced (61% vs. 43%). | Increased incidence of opportunistic infections during the first 30 days compared with historical control subjects (with no effect on non-relapse mortality or progression-free survival). | Brunstein et al (2011, 2013) |
| 11, various haematological malignancies (54% with acute leukaemia and 45% with high-risk disease). | Nonmyeloablative regimen (Cy, Flu, TBI 200 cGy). | UCB. | UCB (from a third unit). | Expanded in vitro for ~18 days with aAPCs expressing CD86 and loaded with anti-CD3 antibody and IL-2. | 3–100 × 106 Treg/kg (dose escalation with each successive patient). | Day +1. | The incidence of grade 2–4 aGvHD at 100 days was 9% vs 45% of contemporary controls. The incidence of cGVHD at one year was zero in the Treg-treated group and 14% in controls. | No difference in haematopoietic recovery and chimerism, infections, non-relapse mortality, relapse and disease-free survival between Treg recipients and controls. | Brunstein et al (2016) |
| 28, high risk acute leukaemia (with one high-grade non-Hodgkin lymphoma). | TBI-based conditioning | Haploidentical donor (purified CD34+ cells and Tconv at day 0). | Naturally occurring Treg cells of the donor. | No expansion. | 2–4 × 106 Treg/kg, Treg:Tconv 4:1–2:1. |
Day −4. | Only 2/26 developed aGVHD. No patient had developed cGVHD at a follow-up of 11 months. Only one patient relapsed. | No fatal infections occurred after the first 2 months post-transplantation. | Di Ianni et al (2011) |
| 43 (24 were included also in the previous study), high risk acute leukaemia. | TBI-based conditioning | Haploidentical donor (purified CD34+ cells and Tconv at day 0). | Naturally occurring Treg cells of the donor. | No expansion | 2.5 × 106 Treg/kg, Treg:Tcon 2:1 | Day −4. | 6/41 (15%) patients developed ≥ grade 2 aGVHD, similar to historical controls (11%). One patient developed cGVHD. Only 2/41 patients have relapsed (significantly lower than historical controls). | Specific T cells for opportunistic pathogens appeared significantly earlier compared with standard haploidentical transplantation. | Martelli et al (2014) |
aAPCs: artificial antigen presenting cells; aGvHD: acute graft-versus-host disease; cGvHD: chronic graft-versus-host disease; Cy: cyclophosphamide; Flu: fludarabine; GvHD: graft-versus-host disease; IL-2: interleukin 2; TBI: total body irradiation; Tconv: conventional T cells; Treg: T regulatory; UCB: umbilical cord blood.
Another clinical study examined the effect of early infusion of Treg cells in a setup of haploidentical HSCT (Di Ianni, et al 2011). This study included 28 patients with high risk haematological malignancies who underwent haploidentical HSCT. In this transplant protocol, after conditioning, the patients received an infusion of freshly-isolated Treg cells. Four days later, the patients received donor CD34+ stem cells as well as donor Tconv cells. The Tconv:Treg ratio in this study was 1:2 in most patients (and 1:4 in the first group of four patients). The study examined the utility of early infusion of Treg cells, followed by infusion of Tconv cells, on GvHD and immunological reconstitution in these patients compared to historical controls. Secondary endpoints included the incidence and severity of infections and transplant-related mortality, in addition to examination of post-transplant leukaemia relapse. The patients did not receive any post-transplant immunosuppression. In this study, unlike the abovementioned studies with UCB-derived Treg cells, naturally occurring Treg cells were infused. These cells prevented GvHD in the absence of any post-transplant immunosuppression as only two patients developed aGvHD and no patient developed cGvHD. In addition, the immune recovery of the patients was rapid, with a fast increase in PB T cell subsets and broadening of their T-cell receptor repertoire. T cells specific for pathogens inducing opportunistic infections rebounded earlier with fewer episodes of CMV reactivation. Finally, the graft-versus-leukaemia effect seemed to persist, because only one relapse has occurred at a median follow-up of 12 months in a population of high-risk patients. A subsequent study by the same group examined whether adoptive transfer of Treg and Tconv cells prevented post-transplant leukaemia relapse in 43 patients with high-risk acute leukaemia who underwent haploidentical transplantation after conditioning with total body irradiation (Martelli, et al 2014). The graft included CD34+ cells, Treg cells (mean 2.5 × 106 cells/kg) and Tconv cells (mean 1.1 × 106 cells/kg). The Treg cells were administrated at day −4 and the Tconv cells at day 0. The patients did not receive post-transplant immunosuppression and 15% of them developed ≥grade 2 aGvHD. At a median follow-up of 46 months, the relapse incidence was very low (0.05) and significantly better than historic controls. Thus, Treg cells can suppress GvHD without losing the effect on GvL. In this study, there was early immune reconstitution of CD4+ and CD8+ T cells specific for opportunistic pathogens compared with standard haploidentical transplantation.
It must be noted that the utility of Treg cell infusion for the treatment of GvHD rather than its prophylaxis has not been tested in large-scale studies. In one report, two patients with GvHD received a treatment with ex vivo expanded donor Treg cells (Trzonkowski, et al 2009). One of these patients responded, and the dose of corticosteroids could be tapered. Repeated stimulation of Treg cells led to a significant decrease in their numbers. In another study, five patients with steroid-refractory cGvHD received expanded PB Treg cells that had been isolated from their donors (Theil, et al 2015). Following Treg cell infusion, there was symptom improvement in two patients, and four patients could reduce their immunosuppressive therapy. Two patients developed skin tumours, yet it was unclear whether it was related to the Treg cell therapy.
7. Indirect measures to activate Treg cells in GvHD patients
In addition to harnessing immunosuppressive Treg cells directly as a cell therapeutic agent, other, indirect interventions have been employed to activate and expand these cells for the treatment of GvHD. Low-dose IL-2 is an example of such an established strategy. IL-2, originally named T cell growth factor or TCGF, is an essential cytokine required for the activation of T cells, particularly CD8+ T cells, and therefore can increase the GvL effect. However, low doses of IL-2 preferentially activate Treg cells due to their characteristically abundant expression of the high-affinity IL-2 receptor CD25. In a phase I study, patients with active cGvHD who were refractory to glucocorticoid treatment were treated daily with escalating doses of low-dose IL-2 (0.3×106, 1×106, or 3×106 iu per m2 of body-surface area) for 8 weeks (Koreth, et al 2011). The maximum tolerated dose of IL-2 was 1×106 iu/m2/day. GvHD was assessed at baseline, after eight weeks of IL-2 administration and four weeks after the discontinuation of IL-2. None of the patients had a relapse or progression of their cGvHD. Out of 23 patients, 12 patients developed partial response and 11 patients had a stable disease. The glucocorticoid dose was tapered by 60% and there were no significant adverse effects to the IL-2 treatment. Mechanistically, following low dose IL-2 treatment, the numbers of Treg cells increased along with a selective increase in the phosphorylation of the transcription factor STAT5, the main downstream target of IL-2 signalling, in Treg cells and a decrease in STAT5 phosphorylation in Tconv cells (Koreth, et al 2011, Matsuoka, et al 2013). In a separate phase II study, 35 patients with steroid-refractory cGVHD received daily doses of IL-2 (1×106 iu/m2/day) for 12 weeks (Koreth, et al 2016). Sixty-one percent of the patients had a clinical response at multiple cGvHD sites, including the liver, skin, gastrointestinal tract and lungs. Following treatment with IL-2, Treg and natural killer (NK) cell counts rose significantly without significant changes in the number of conventional CD4+ or CD8+ cells. During two years of extended IL-2 therapy, both the clinical response and Treg cell immune response persisted (Koreth, et al 2016). An additional prospective phase II study demonstrated that ultra-low doses of IL-2 administered three times per week for 12 weeks can be effective for GvHD prophylaxis (Matsuoka, et al 2013). In the group of patients who received prophylactic treatment with IL-2, there was no 2–4 grade aGHVD compared to 12% at the control group. In addition, the prophylactic treatment with IL-2 was not associated with any significant toxicities. As expected, due to this treatment, the fraction of Treg cells increased from 4.8% to 11.1%. Another study tested the prophylactic utility of IL-2 in combination with sirolimus and tacrolimus. Although the addition of IL-2 increased the fraction of Treg cells at day 30, by day 90, Treg cells decreased and there was no reduction in acute or chronic GvHD (Betts, et al 2017). Therefore, the prophylactic regimen of IL-2 should be further explored in future studies. Overall, the beneficial effect of low dose IL-2 emphasizes the potential therapeutic role for Treg cells in the prevention and treatment of GvHD.
Additional immunomodulatory therapies have also been shown to affect Treg cell numbers in GvHD besides IL-2. One study suggested that in vivo expansion of Treg cells may occur upon administration of a novel liposomal formulation of a synthetic derivative of alpha-galactosyl-ceramide, a surrogate ligand that binds to CD1d and activates NKT cells (Chen, et al 2017, Duramad, et al 2011). Patients received this drug on day 0 of allogeneic HSCT, and the incidence of GvHD was lower among patients who responded to this drug. It was suggested that this drug can synergize with sirolimus to promote Treg cell expansion. Another study of GvHD in non-human primates demonstrated the utility of the mTOR inhibitor sirolimus (rapamycin) and a blocking antibody against OX40L, which enabled better control of GvHD and maintained Treg numbers (Tkachev, et al 2017). In addition, antibody-mediated blockade of the IL-6 receptor has been shown to ameliorate GvHD and to increase the absolute number of Treg cells (Chen, et al 2009). Hypomethylating agents (HMAs), including decitabine and azacitidine, have been shown in mouse models to induce FOXP3 expression in CD4+CD25- T cells in vitro and in vivo, which enabled better control of GvHD (Choi, et al 2010, Ehx, et al 2017). Administration of HMAs to human patients after transplantation was shown to expand Treg cells and was associated with a low risk of GvHD (Goodyear, et al 2012). However, the exact role of HMAs in prevention of GvHD should be better explored in further studies.
Finally, one of the revolutionary conditioning regimens for haploidentical transplants is in vivo T-cell depletion by post-transplant cyclophosphamide. This treatment has been shown to deplete CD8+ T cells but spare Treg cells, with the prophylactic efficacy of this treatment dependent on donor FOXP3+ Treg cells (Ganguly, et al 2014). In two GvHD mouse models with matched MHC, post-transplant cyclophosphamide treatment was associated with a relative preservation of donor Treg cells, and Treg-depleted allografts abrogated the GvHD-prophylactic activity of post-transplant cyclophosphamide. The suggested mechanism of Treg cell resistance is their high expression level of aldehyde dehydrogenase (Kanakry, et al 2013).
Summary and future directions
Since their discovery over two decades ago as a subset of CD25+CD4+ T cells, numerous studies have demonstrated the potent immunosuppressive activity of Treg cells. The preclinical and clinical research efforts, inspired by basic studies of Treg cell biology, have established their potential to ameliorate GvHD. This notion has received additional support from observations that other effective approaches for GvHD management enhance Treg cell activity, foremost, low-dose IL-2 treatment. However, Treg cell therapy for GvHD has not yet entered clinical practice due to several remaining obstacles including the high dose of Treg cells required to prevent GvHD and the difficulty of expanding in vitro generated Treg cells. It is likely that genetic engineering of Treg cells with an enhanced immunosuppressive capacity and an improved ability to expand and maintain their identity will enable their use as a potent mainstream therapeutic modality for GvHD prevention and treatment.
Acknowledgments
We thank Jacob G. Verter for language editing. S.E. is a Cancer Research Institute Irvington Fellow supported by the Cancer Research Institute.
Footnotes
Disclosure of interest: The authors report no conflict of interest.
References
- Agle K, Vincent BG, Piper C, Belle L, Zhou V, Shlomchik W, Serody JS & Drobyski WR (2018) Bim regulates the survival and suppressive capability of CD8(+) FOXP3(+) regulatory T cells during murine GVHD. Blood, 132, 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD & Shlomchik MJ (2004) Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood, 104, 1565–1573. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR (2007) Hematopoietic-cell transplantation at 50. N Engl J Med, 357, 1472–1475. [DOI] [PubMed] [Google Scholar]
- Arimoto K, Kadowaki N, Ishikawa T, Ichinohe T & Uchiyama T. (2007) FOXP3 expression in peripheral blood rapidly recovers and lacks correlation with the occurrence of graft-versus-host disease after allogeneic stem cell transplantation. Int J Hematol, 85, 154–162. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A & Roncarolo MG (2005) Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood, 105, 4743–4748. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF & Ochs HD (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet, 27, 20–21. [DOI] [PubMed] [Google Scholar]
- Beres A, Komorowski R, Mihara M & Drobyski WR (2011) Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clin Cancer Res, 17, 3969–3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts BC, Pidala J, Kim J, Mishra A, Nishihori T, Perez L, Ochoa-Bayona JL, Khimani F, Walton K, Bookout R, Nieder M, Khaira DK, Davila M, Alsina M, Field T, Ayala E, Locke FL, Riches M, Kharfan-Dabaja M, Fernandez H & Anasetti C. (2017) IL-2 promotes early Treg reconstitution after allogeneic hematopoietic cell transplantation. Haematologica, 102, 948–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blache C, Chauvin JM, Marie-Cardine A, Contentin N, Pommier P, Dedreux I, Francois S, Jacquot S, Bastit D & Boyer O. (2010) Reduced frequency of regulatory T cells in peripheral blood stem cell compared to bone marrow transplantations. Biol Blood Marrow Transplant, 16, 430–434. [DOI] [PubMed] [Google Scholar]
- Bluestone JA & Tang Q. (2018) Treg cells-the next frontier of cell therapy. Science, 362, 154–155. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH & Tang Q. (2015) Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med, 7, 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF & Ramsdell F. (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet, 27, 68–73. [DOI] [PubMed] [Google Scholar]
- Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR & Wagner JE (2011) Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood, 117, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen KL, McKenna DH, Curtsinger J, McGlave PB & Wagner JE (2013) Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol Blood Marrow Transplant, 19, 1271–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR & Wagner JE (2016) Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood, 127, 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M & Drobyski WR (2009) Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood, 114, 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YB, Efebera YA, Johnston L, Ball ED, Avigan D, Lekakis LJ, Bachier CR, Martin P, Duramad O, Ishii Y, Han S, Jung YJ, Lee D, Kunkel L, Negrin RS & Bui JD (2017) Increased Foxp3(+)Helios(+) Regulatory T Cells and Decreased Acute Graft-versus-Host Disease after Allogeneic Bone Marrow Transplantation in Patients Receiving Sirolimus and RGI-2001, an Activator of Invariant Natural Killer T Cells. Biol Blood Marrow Transplant, 23, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, Piwnica-Worms DR & DiPersio JF (2010) In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood, 116, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Biehl M, Steinfatt T, Brandl A, Kums J, Amich J, Vaeth M, Kuen J, Holtappels R, Podlech J, Mottok A, Kraus S, Jordan-Garrote AL, Bauerlein CA, Brede C, Ribechini E, Fick A, Seher A, Polz J, Ottmuller KJ, Baker J, Nishikii H, Ritz M, Mattenheimer K, Schwinn S, Winter T, Schafer V, Krappmann S, Einsele H, Muller TD, Reddehase MJ, Lutz MB, Mannel DN, Berberich-Siebelt F, Wajant H & Beilhack A. (2016) Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion. J Exp Med, 213, 1881–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M, Begum G, Mahendra P, Craddock C, Moss P & Chakraverty R. (2004) Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood, 103, 2410–2416. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Trenado A, Vasey D, Klatzmann D & Salomon BL (2002) CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med, 196, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ianni M, Del Papa B, Cecchini D, Bonifacio E, Moretti L, Zei T, Ostini RI, Falzetti F, Fontana L, Tagliapietra G, Maldini C, Martelli MF & Tabilio A. (2009) Immunomagnetic isolation of CD4+CD25+FoxP3+ natural T regulatory lymphocytes for clinical applications. Clin Exp Immunol, 156, 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F & Martelli MF (2011) Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood, 117, 3921–3928. [DOI] [PubMed] [Google Scholar]
- Duramad O, Laysang A, Li J, Ishii Y & Namikawa R. (2011) Pharmacologic expansion of donor-derived, naturally occurring CD4(+)Foxp3(+) regulatory T cells reduces acute graft-versus-host disease lethality without abrogating the graft-versus-leukemia effect in murine models. Biol Blood Marrow Transplant, 17, 1154–1168. [DOI] [PubMed] [Google Scholar]
- Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S & Negrin RS (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med, 9, 1144–1150. [DOI] [PubMed] [Google Scholar]
- Ehx G, Fransolet G, de Leval L, D’Hondt S, Lucas S, Hannon M, Delens L, Dubois S, Drion P, Beguin Y, Humblet-Baron S & Baron F. (2017) Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology, 6, e1314425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG & Strober S. (2005) Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood, 105, 2220–2226. [DOI] [PubMed] [Google Scholar]
- Florek M, Schneidawind D, Pierini A, Baker J, Armstrong R, Pan Y, Leveson-Gower D, Negrin R & Meyer E. (2015) Freeze and Thaw of CD4+CD25+Foxp3+ Regulatory T Cells Results in Loss of CD62L Expression and a Reduced Capacity to Protect against Graft-versus-Host Disease. PLoS One, 10, e0145763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondi C, Nozzoli C, Benemei S, Baroni G, Saccardi R, Guidi S, Nicoletti P, Bartolozzi B, Pimpinelli N, Santucci M, Bosi A & Massi D. (2009) Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant, 15, 938–947. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA & Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol, 4, 330–336. [DOI] [PubMed] [Google Scholar]
- Fujimaki W, Takahashi N, Ohnuma K, Nagatsu M, Kurosawa H, Yoshida S, Dang NH, Uchiyama T & Morimoto C. (2008) Comparative study of regulatory T cell function of human CD25CD4 T cells from thymocytes, cord blood, and adult peripheral blood. Clin Dev Immunol, 2008, 305859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Tamaki H, Ikegame K, Yoshihara S, Taniguchi K, Kaida K, Kato R, Inoue T, Nakata J, Ishii S, Soma T, Okada M & Ogawa H. (2013) Frequency of CD4(+)FOXP3(+) regulatory T-cells at early stages after HLA-mismatched allogeneic hematopoietic SCT predicts the incidence of acute GVHD. Bone Marrow Transplant, 48, 859–864. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazar BR, Levy RB & Luznik L. (2014) Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood, 124, 2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR & Porter SB (2004) In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood, 104, 453–461. [DOI] [PubMed] [Google Scholar]
- Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR & Porter SB (2005) Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood, 105, 750–758. [DOI] [PubMed] [Google Scholar]
- Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, Nunnick J, Khanum R, Raghavan M, Cook M, Snowden JA, Griffiths M, Russell N, Yin J, Crawley C, Cook G, Vyas P, Moss P, Malladi R & Craddock CF (2012) Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood, 119, 3361–3369. [DOI] [PubMed] [Google Scholar]
- Grant CR, Liberal R, Mieli-Vergani G, Vergani D & Longhi MS (2015) Regulatory T-cells in autoimmune diseases: challenges, controversies and--yet--unanswered questions. Autoimmun Rev, 14, 105–116. [DOI] [PubMed] [Google Scholar]
- Hanash AM & Levy RB (2005) Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood, 105, 1828–1836. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Inoue A, Kohno M, Lei J, Miyazaki T, Yoshie O, Nose M & Yasukawa M. (2008) Therapeutic effect of CXCR3-expressing regulatory T cells on liver, lung and intestinal damages in a murine acute GVHD model. Gene Ther, 15, 171–182. [DOI] [PubMed] [Google Scholar]
- Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, Wagner JE, June CH, Riley JL & Blazar BR (2008) Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4–1BB expressed on artificial antigen-presenting cells. Blood, 112, 2847–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE & Blazar BR (2011) Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med, 3, 83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen KL, O’Connor RS, Lemire AM, Saha A, Hanse EA, Tennis NC, Merkel SC, Kelekar A, Riley JL, Levine BL, June CH, Turka LA, Kean LS, MacMillan ML, Miller JS, Wagner JE, Munn DH & Blazar BR (2017) In Vitro Induction of Human Regulatory T Cells Using Conditions of Low Tryptophan Plus Kynurenines. Am J Transplant, 17, 3098–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Ermann J, Edinger M, Fathman CG & Strober S. (2002) Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med, 196, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T & Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science, 299, 1057–1061. [DOI] [PubMed] [Google Scholar]
- Iamsawat S, Daenthanasanmak A, Voss JH, Nguyen H, Bastian D, Liu C & Yu XZ (2018) Stabilization of Foxp3 by Targeting JAK2 Enhances Efficacy of CD8 Induced Regulatory T Cells in the Prevention of Graft-versus-Host Disease. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O, Gorsse N, Romagnoli P, Hudrisier D & van Meerwijk JP (2004) Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood, 103, 4216–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SC, Murphy GF & Korngold R. (2003) Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant, 9, 243–256. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF & Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol, 30, 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, Fuchs EJ, Jones RJ, Hess AD & Luznik L. (2013) Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med, 5, 211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Nakatsukasa H, Okada M, Lu Q & Yoshimura A. (2016) Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol, 37, 803–811. [DOI] [PubMed] [Google Scholar]
- Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Forster R & Prinz I. (2009) Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol, 39, 3091–3096. [DOI] [PubMed] [Google Scholar]
- Komanduri KV & Champlin RE (2011) Can Treg therapy prevent GVHD? Blood, 117, 751–752. [DOI] [PubMed] [Google Scholar]
- Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J & Soiffer RJ (2011) Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med, 365, 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Kim HT, Jones KT, Lange PB, Reynolds CG, Chammas MJ, Dusenbury K, Whangbo J, Nikiforow S, Alyea EP 3rd, Armand P, Cutler CS, Ho VT, Chen YB, Avigan D, Blazar BR, Antin JH, Ritz J & Soiffer RJ (2016) Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood, 128, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE & Bromberg JS (2009) Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol, 182, 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, Hoyos BE, Putintseva EV, Chaudhry A, Dikiy S, Fujisawa S, Chudakov DM, Treuting PM & Rudensky AY (2017) Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature, 546, 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C & Mathis D. (2018) TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell, 174, 285–299 e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhai Z, Xu X, Shen Y, Zhang A, Sun Z, Liu H, Geng L & Wang Y. (2010) Decrease of CD4(+)CD25(+) regulatory T cells and TGF-beta at early immune reconstitution is associated to the onset and severity of graft-versus-host disease following allogeneic haematogenesis stem cell transplantation. Leuk Res, 34, 1158–1168. [DOI] [PubMed] [Google Scholar]
- Lord JD, Hackman RC, Gooley TA, Wood BL, Moklebust AC, Hockenbery DM, Steinbach G, Ziegler SF & McDonald GB (2011) Blood and gastric FOXP3+ T cells are not decreased in human gastric graft-versus-host disease. Biol Blood Marrow Transplant, 17, 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SY, Liu KY, Liu DH, Xu LP & Huang XJ (2011) High frequencies of CD62L(+) naive regulatory T cells in allografts are associated with a low risk of acute graft-versus-host disease following unmanipulated allogeneic haematopoietic stem cell transplantation. Clin Exp Immunol, 165, 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, Broady R & Levings MK (2016) Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest, 126, 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenau JM, Qin X, Tawara I, Rogers CE, Kitko C, Schlough M, Bickley D, Braun TM, Jang PS, Lowler KP, Jones DM, Choi SW, Reddy P, Mineishi S, Levine JE, Ferrara JL & Paczesny S. (2010) Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant, 16, 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E, Del Papa B, Zei T, Iacucci Ostini R, Cecchini D, Tognellini R, Reisner Y, Aversa F, Falini B & Velardi A. (2014) HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood, 124, 638–644. [DOI] [PubMed] [Google Scholar]
- Martin GH, Gregoire S, Landau DA, Pilon C, Grinberg-Bleyer Y, Charlotte F, Mege JP, Chatenoud L, Salomon BL & Cohen JL (2013) In vivo activation of transferred regulatory T cells specific for third-party exogenous antigen controls GVH disease in mice. Eur J Immunol, 43, 2263–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, Armand P, Blazar BR, Antin JH, Soiffer RJ & Ritz J. (2013) Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med, 5, 179ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-Hyman C, Muller JT, Loschi M, Thangavelu G, Saha A, Kumari S, Reichenbach DK, Smith MJ, Zhang G, Koehn BH, Lin J, Mitchell JS, Fife BT, Panoskaltsis-Mortari A, Feser CJ, Kirchmeier AK, Osborn MJ, Hippen KL, Kelekar A, Serody JS, Turka LA, Munn DH, Chi H, Neubert TA, Dustin ML & Blazar BR (2018) The vimentin intermediate filament network restrains regulatory T cell suppression of graft-versus-host disease. J Clin Invest, 128, 4604–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver Z, Melenhorst JJ, Wu C, Grim A, Ito S, Cho I, Hensel N, Battiwalla M & Barrett AJ (2013) Donor lymphocyte count and thymic activity predict lymphocyte recovery and outcomes after matched-sibling hematopoietic stem cell transplant. Haematologica, 98, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutis T, van Rijn RS, Simonetti ER, Aarts-Riemens T, Emmelot ME, van Bloois L, Martens A, Verdonck LF & Ebeling SB (2006) Human regulatory T cells control xenogeneic graft-versus-host disease induced by autologous T cells in RAG2−/−gammac−/− immunodeficient mice. Clin Cancer Res, 12, 5520–5525. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH & Negrin RS (2007) In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood, 109, 2649–2656. [DOI] [PubMed] [Google Scholar]
- Noel G, Bruniquel D, Birebent B, DeGuibert S, Grosset JM, Bernard M, Dauriac C, Chevallier P, Lamy-de-la-Chapelle T, Semana G & Brinster C. (2008) Patients suffering from acute graft-versus-host disease after bone-marrow transplantation have functional CD4+CD25hiFoxp3+ regulatory T cells. Clin Immunol, 129, 241–248. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Mera T, Wang L & Faustman DL (2013) Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci Rep, 3, 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst C, Schirutschke H, Ehninger G, Bornhauser M & Platzbecker U. (2007) The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res, 13, 2916–2922. [DOI] [PubMed] [Google Scholar]
- Parmar S, Liu X, Najjar A, Shah N, Yang H, Yvon E, Rezvani K, McNiece I, Zweidler-McKay P, Miller L, Wolpe S, Blazar BR & Shpall EJ (2015) Ex vivo fucosylation of third-party human regulatory T cells enhances anti-graft-versus-host disease potency in vivo. Blood, 125, 1502–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JH, Preijers FW, Woestenenk R, Hilbrands LB, Koenen HJ & Joosten I. (2008) Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS One, 3, e3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini A, Colonna L, Alvarez M, Schneidawind D, Nishikii H, Baker J, Pan Y, Florek M, Kim BS & Negrin RS (2015) Donor Requirements for Regulatory T Cell Suppression of Murine Graft-versus-Host Disease. J Immunol, 195, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini A, Strober W, Moffett C, Baker J, Nishikii H, Alvarez M, Pan Y, Schneidawind D, Meyer E & Negrin RS (2016) TNF-alpha priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood, 128, 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von Boehmer H & Huehn J. (2008) DNA methylation controls Foxp3 gene expression. Eur J Immunol, 38, 1654–1663. [DOI] [PubMed] [Google Scholar]
- Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA & Bluestone JA (2009) Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes, 58, 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R & Barrett AJ (2006) High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood, 108, 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, Steiner B, Berg E, Miehlke S, Bornhauser M, Schneider T, Zeitz M, Stein H, Thiel E, Duchmann R & Uharek L. (2006) Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood, 107, 1717–1723. [DOI] [PubMed] [Google Scholar]
- Riley JL, June CH & Blazar BR (2009) Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity, 30, 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, Hammerling GJ, Markey KA, Koyama M, Clouston AD, Engwerda CR, Hill GR & MacDonald KP (2012) Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood, 119, 5898–5908. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. (2011) Regulatory T cells: history and perspective. Methods Mol Biol, 707, 3–17. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M & Toda M. (2011) Pillars article: immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995. J Immunol, 186, 3808–3821. [PubMed] [Google Scholar]
- Sanchez J, Casano J, Alvarez MA, Roman-Gomez J, Martin C, Martinez F, Gomez P, Serrano J, Herrera C & Torres A. (2004) Kinetic of regulatory CD25high and activated CD134+ (OX40) T lymphocytes during acute and chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol, 126, 697–703. [DOI] [PubMed] [Google Scholar]
- Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y & Kambayashi T. (2012) Cell-autonomous role of TGFbeta and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood, 119, 5575–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI & Negrin RS (2011) Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood, 118, 2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Stempora L, Harvey RD, Kirk AD, Larsen CP, Blazar BR & Kean LS (2014) Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. Am J Transplant, 14, 2691–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanzani M, Martins SL, Saliba RM, St John LS, Bryan S, Couriel D, McMannis J, Champlin RE, Molldrem JJ & Komanduri KV (2004) CD25 expression on donor CD4+ or CD8+ T cells is associated with an increased risk for graft-versus-host disease after HLA-identical stem cell transplantation in humans. Blood, 103, 1140–1146. [DOI] [PubMed] [Google Scholar]
- Steiner D, Brunicki N, Bachar-Lustig E, Taylor PA, Blazar BR & Reisner Y. (2006) Overcoming T cell-mediated rejection of bone marrow allografts by T-regulatory cells: synergism with veto cells and rapamycin. Exp Hematol, 34, 802–808. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Lees CJ & Blazar BR (2002) The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood, 99, 3493–3499. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, June CH, Serody JS & Blazar BR (2004) L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood, 104, 3804–3812. [DOI] [PubMed] [Google Scholar]
- Theil A, Tuve S, Oelschlagel U, Maiwald A, Dohler D, Ossmann D, Zenkel A, Wilhelm C, Middeke JM, Shayegi N, Trautmann-Grill K, von Bonin M, Platzbecker U, Ehninger G, Bonifacio E & Bornhauser M. (2015) Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy, 17, 473–486. [DOI] [PubMed] [Google Scholar]
- Tkachev V, Furlan SN, Watkins B, Hunt DJ, Zheng HB, Panoskaltsis-Mortari A, Betz K, Brown M, Schell JB, Zeleski K, Yu A, Kirby I, Cooley S, Miller JS, Blazar BR, Casson D, Bland-Ward P & Kean LS (2017) Combined OX40L and mTOR blockade controls effector T cell activation while preserving Treg reconstitution after transplant. Sci Transl Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL & Cohen JL (2003) Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest, 112, 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta E, Bessette PH, Silveria SL, Ely LK, Jude KM, Le DT, Holst CR, Coyle A, Potempa M, Lanier LL, Garcia KC, Crellin NK, Rondon IJ & Bluestone JA (2018) A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat Med, 24, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J & Hellmann A. (2009) First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol, 133, 22–26. [DOI] [PubMed] [Google Scholar]
- Ukena SN, Grosse J, Mischak-Weissinger E, Buchholz S, Stadler M, Ganser A & Franzke A. (2011) Acute but not chronic graft-versus-host disease is associated with a reduction of circulating CD4(+)CD25 (high)CD127 (low/-) regulatory T cells. Ann Hematol, 90, 213–218. [DOI] [PubMed] [Google Scholar]
- Veerapathran A, Pidala J, Beato F, Yu XZ & Anasetti C. (2011) Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood, 118, 5671–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtenhuber C, Bucher C, Highfill SL, Koch LK, Goren E, Panoskaltsis-Mortari A, Taylor PA, Farrar MA & Blazar BR (2010) Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease lethality associated with increased regulatory T-cell potency and decreased T effector cell responses. Blood, 116, 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, Blazar BR & Serody JS (2005) Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood, 106, 3300–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, Lineburg KE, Lor M, Teal BE, Raffelt NC, Raju J, Leveque L, Markey KA, Varelias A, Clouston AD, Lane SW, MacDonald KP & Hill GR (2013) Induced regulatory T cells promote tolerance when stabilized by rapamycin and IL-2 in vivo. J Immunol, 191, 5291–5303. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, Forman S & Zeng D. (2008) In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood, 112, 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Liu Y, Liu Y, Liu M, Xiang Z, Lam KT, Lewis DB, Lau YL & Tu W. (2013) Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Sci Transl Med, 5, 168ra169. [DOI] [PubMed] [Google Scholar]
- Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, Bellucci R, Alyea EP, Antin JH, Soiffer RJ & Ritz J. (2005) Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood, 106, 2903–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]


