Abstract
Background:
Preeclampsia is a multiorgan disorder associated with maternal and perinatal morbi-mortality. In Peru, incidence is 10% and accounts for 22% of maternal deaths. Genome and genetic epidemiological studies have found an association between preeclampsia and genetic polymorphisms.
Objective:
To determine the association of the vascular endothelial growth factor (VEGF) +936 C/T and +405 G/C, interleukine-6 (IL-6) -174 G/C, IL-1β-511 C/T, Apo A-1-75 G/A, Apo B-100 2488 C/T (Xbal) polymorphisms with preeclampsia in pregnant Peruvian women.
Methods:
Were included preeclamptic and healthy (control) pregnant women. Maternal blood samples were subjected to DNA extraction, and molecular genetic analysis was conducted using the PCR-RFLP technique and following a specific protocol for each gene. Allele and genotypic frequencies in the cases and controls were compared.
Results:
No association was found between the VEGF+936C/T and VEGF+405 polymorphisms and preeclampsia. The frequencies of the GG genotypes and the G allele of the -174 G/C polymorphism in the IL6 gene in preeclamptic and controls showed significant differences, with higher frequencies in cases. For the -511 C/T polymorphism of the IL-1β gene, no significant differences were found in the frequencies of TT genotypes compared with CT+CC. The genotypes and alleles of the Apo-A1-75 G/A and Apo-B100 Xbal variants showed no significant differences between cases and controls.
Conclusion:
No association was found between the studied genetic markers and preeclampsia. However, in the -174G/C polymorphism of the IL-6 gene, significant differences were found mainly in the GG genotype and G allele.
Key words: Pregnant women, preeclampsia, genetics, polymorphism, genetic, Peru, vascular endothelial growth factor, interleukine-6, genetic polymorphisms.
Remark
| 1) Why was this study conducted? |
| Preeclampsia is a multiorgan disorder that is significantly associated with maternal and perinatal morbidity and mortality. Preeclampsia is defined by the presence of new-onset hypertension and proteinuria in women who are at least 20 weeks pregnant. The etiology of preeclampsia remains unknown; its clinical presentation and dynamics vary, and no method can predict its occurrence. In Peru, preeclampsia incidence is greater than 10%, and it accounts for 22% of maternal deaths. Genome and genetic epidemiologial studies have found an association of preeclampsia and certain gene polymorphisms and variants. In this study, we evaluated the susceptibility gene polymorphisms related to endothelial function, angiogenesis, immunologic and inflammatory processes, and metabolic syndrome in Peruvian preeclamptic women. |
| 2) What were the most relevant results of the study? |
| No association was found between the studied genetic markers and preeclampsia. However, in the -174G/C polymorphism of the IL6 gene, significant differences mainly in the GG genotype and G allele were found, wherein the frequencies were higher in the cases than in the controls. |
| 3) What do these results contribute? |
| This study contributes to our knowledge of the genetic factors associated with preeclampsia, an emergent research topic in Peru. High genetic mixing and other factors may partially explain the conflicting findings for the Peruvian population. |
Introduction
Hypertension is the most frequent medical complication of pregnancy 1, and the most severe clinical presentation of hypertensive disorders of pregnancy is preeclampsia, a condition that is significantly associated with maternal and perinatal morbidity and mortality.
Preeclampsia is the leading cause of maternal death in the western world. It is a multiorgan disorder involving new-onset hypertension (>140/90 mm Hg) and proteinuria in women who are at least 20 weeks pregnant 2. The clinical presentation and dynamics of preeclampsia are variable; for instance, hypertension and proteinuria may not always be present 3. In the absence of proteinuria, preeclampsia diagnosis is based on the occurrence of hypertension accompanied by low platelet count (below 100,000/mL), abnormal liver function (indicated by serum transaminase levels that are twice the normal concentrations), kidney failure (indicated by serum creatinine level exceeding 1.1 mg/dL or by serum creatinine levels that are twice the levels in the absence of other renal diseases), pulmonary edema, or de novo presentation of cerebral or visual alterations 2.
Preeclampsia affects 3% to 8% of pregnant women, depending on the population and region being studied and on the definition of preeclampsia being used 4,5. In Peru, preeclampsia incidence is greater than 10% in various regions 3), and it accounts for 22% of all maternal deaths.
The common factors associated with preeclampsia development in developed countries are obesity, insulin resistance and hyperlipidemia, whereas in developing countries, the associated factors are ethnicity, poor nutritional habits, subclinical infections, and other socioeconomic characteristics 6.
The etiology of preeclampsia remains unknown. However, it has been found that genetic factors cause a defective immune adaptation 7, which in turn leads to inadequate trophoblast invasion and inappropriate placenta development. Abnormal endometrial cytotrophoblast infiltration generates arterial disorders, such as loss of elasticity that affects vascular remodeling and impairs the fetus’s blood supply 8,9. Placental ischemia and hypoxia as well as oxidative stress and endotheliosis then develop 6, compromising the placenta and important maternal organs and systems. Oxidative stress stimulates the syncytiotrophoblast to release proinflammatory cytokines, exosomes, antiangiogenic factors, and cell-free fetal DNA into the maternal circulation 4,10.
Complications can emerge at any point during pregnancy, frequently surreptitiously and severely. Prior to the development of a clinical disease, vasospasm, activation of the coagulation cascade, and reduction in plasma volume occur. There are more harmful effects when preeclampsia appears early, including intrauterine growth retardation (IUGR) and prematurity 3. Moreover, placental senescence is accelerated 11, the concentration of pro-inflammatory cytokines, cell-free DNA, leptin, placental apoptotic debris and soluble fms-like tyrosine kinase 1 (sFLT1) in maternal blood increases, and placental growth factor (PlGF) levels decrease 12.
There is no method that can predict the onset of preeclampsia, and there is no cure for this condition other than the delivery of the fetus and the placenta. Preeclampsia usually resolves soon after delivery. However, epidemiological studies have associated preeclampsia with metabolic, cardiovascular and cerebral disorders 2,13-15 that will appear later in the mother and child, and this association has a great impact on disability and heathcare expenditure eventually 15-17.
Genome and genetic epidemiology studies have found an association between preeclampsia and certain genes 7,18, polymorphisms and other molecular and inflammatory markers 7,8,19,20. Thus, detecting these biomarkers early in gestation will allow us to predict and manage preeclampsia properly.
Several pro- and anti-angiogenic proteins are produced in the placenta from the beginning of pregnancy; these substances play a role in endothelial dysfunction and the risk of preeclampsia 9,20. The most important angiogenic factors 21,22 include the vascular endothelial growth factor (VEGF) 22 and the placental growth factor (PGF) 23. Antiangiogenic substances are abundantly expressed in preeclampsia and cause maternal endothelial cell dysfunction and damage 1,3,24, which have negative consequences to a mother and her fetus. The genes encoding for the important antiangiogenic factors include the soluble tyrosinase-like factor (sFLT-1) gene, the VEGF soluble Fms-like tyrosine kinase -1 (VEGFR-1) 25 gene, and the endothelin-1 gene polymorphisms 26,27.
Due to the high prevalence of preeclampsia in Peru and considering that oxidative stress induces the syncytiotrophoblast to release pro-inflammatory cytokines, exosomes, anti-angiogenic factors, and cell-free fetal DNA into maternal circulation 4,10, we decided to characterize some of these biomarkers in preeclamptic Peruvian women and verify whether these biomarkers can be applied to predict, prevent and manage preeclampsia among Peruvian women. We studied some genes and polymorphisms involved in preeclampsia, including the VEGF +936C/T and +405G/C polymorphisms, interleukin-6 (IL-6) gene polymorphisms, IL-1β gene -511C/T polymorphism 28, and polymorphisms of both the Apo A-1 and Apo B-100 genes, in preeclamptic and healthy Peruvian pregnant women. Our findings increase our knowledge of the genetic factors associated with preeclampsia, an emergent research topic in Peru.
Materials and Methods
This study is an observational associative case-control study performed between 2012 and 2018. The participating institutions were the Institute of Clinical Investigations and the Biochemistry and Nutrition Research Center of the Faculty of Medicine, National University of San Marcos, Lima, Peru. Subjects were recruited from the Hospital Docente Madre-Niño San Bartolomé, a public institution that is managed by the Peruvian Ministry of Health.
A preeclamptic woman was defined as a pregnant woman with a blood pressure of >140/90 mm Hg and proteinuria of ≥300 mg/24 h (>1+ dipstick) according to the International Federation of Gynecology and Obstetrics classification, which was updated in the year 2000 1.
The sampling method was non-probabilistic (for convenience). The inclusion criteria for the preeclampsia group were as follows: pregnant women aged ≥18 years who had been diagnosed with severe preeclampsia in the second half of pregnancy as confirmed by clinical and laboratory data and who had signed an informed consent. Pregnant women without proteinuria or those with chronic hypertension, diabetes, and other medical conditions as well as those with incomplete information were excluded. For the control group, the inclusion criteria were as follows: pregnant women aged ≥18 years who were apparently healthy without preeclampsia and without relevant diseases and who signed the informed consent. Table 1 presents the number of cases and controls studied for each gene polymorphism.
Table 1. Number of preeclamptic women and controls included in the study.
| Gene | Polymorphism | Preeclampsia | Control | Total |
|---|---|---|---|---|
| VEGF | +936 C/T | 45 | 49 | 94 |
| +405 G/C | 39 | 45 | 84 | |
| IL-6 | -174 G/C | 20 | 39 | 59 |
| IL1β | -511 C/T | 49 | 50 | 99 |
| Apo A-1 | -75 G/A | 47 | 45 | 92 |
| Apo B-100 | 2 488 C/T (Xbal) | 47 | 45 | 92 |
We obtained approval for our study protocol from the Ethics Committee of the Faculty of Medicine of the National University of San Marcos and from the ethics committee of the participating hospital. An ad hoc clinical file was filled with mother and newborn data. Written informed consent was obtained from all participants. Blood samples (5 mL) were drawn from the participants’ antecubital vein, and the samples were kept in a refrigerator and then transported to the laboratory. The blood samples were subjected to DNA extraction with the commercial kits used for genotype and allele determination for the investigated genes.
The maternal data entered in the ad hoc file were as follows: age, marital status, height, weight, personal medical history (hypertension, previous preeclampsia, diabetes mellitus, neuropathy, cardiopathy, and metabolic syndrome), family medical history (hypertension, diabetes mellitus, obesity, metabolic syndrome, and other conditions), number of pregnancies, preterm deliveries, low-weight newborns, hemoglobin levels, type of delivery, gestational age at delivery, and hospital stay (in days). The newborn data obtained were as follows: weight, Apgar scores at 1 and 5 min, gestational age, complications (fetal distress, respiratory distress, and perinatal asphyxia), IUGR, prematurity, respiratory distress syndrome, jaundice, infection, fetal death, neonatal death, malformations, and hospital stay (in days).
In the genetic molecular analysis, the PCR-RFLP technique was used, specific protocols was employed for each gene, and adequate laboratory conditions were ensured. The genotypes of the polymorphisms were confirmed by automated Sanger sequencing.
VEGF gene +936C/T and +405G/C polymorphisms
The specific primers F: 5’AAGGAAGAGGAGACTCTGCGCAGAGC3’ and R: 5´TAAATGTATGTATGTGGGTGGGTGTGTCTACAGG3´ were used for the +936C/T amplification, and the NlaIII enzyme was used for digestion according to the protocol of Papazoglou 29. For +405G/C, the primers F: 5’CCGACGGCTTGGGGA GATTGCTC3’ and R: 5’CGGCGGTCACCCCCAAAAGCAG3’ were used for amplification, and the BsmFI enzyme was used for digestion according to the protocol of Banyasz 30 and Garza-Veloz 31.
IL-6 gene -174G/C polymorphism
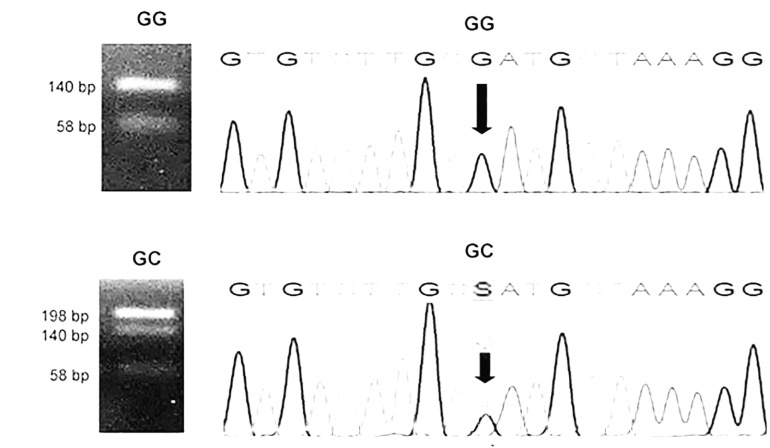
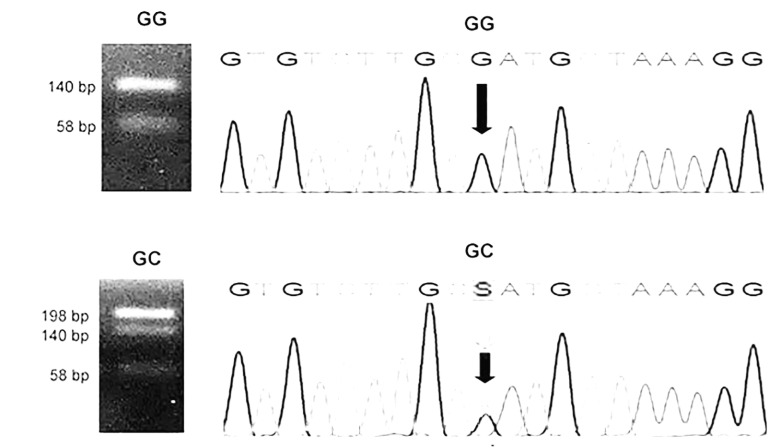
The specific primers F: 5´TGACTTCAGCTTTAC TCTTTGT’3 and R: 5’CTGATTGGAAACCTTATTAGG’3 were used for amplification, and the restriction enzyme SfaNI was used fo digestion according to Berthold 32. The GG and GC genotypes obtained by PCR-RFLP and Sanger sequencing are reported in Figure 1.
Figure 1. Determination of the genotypes of the -174 G/C polymorphism in IL6 gene. Left: Agarose gels with homozygous GG (140 and 58 bp) and heterozygous GC (198, 140 and 58 bp) genotypes as determined by PCR-RFLP with SfaNI as the restriction enzyme 32. Right: Chromatograms obtained by automated Sanger sequencing confirming the GG and CC genotypes (marked with arrows).
IL-1β gene -511C/T polymorphism
The specific primers F: 5′TGGCATTGATCTGGTTCATC3′ and R: 5′GTTTAGGAATCTTCCCACTT3´ were used for amplification, and the restriction enzyme Aval was used for digestion according to Acosta 28.
Apo A-1 gene -75G/A polymorphism
The specific primers F: 5′AGGGACAGAGCTGATCCTTGAACTCTTAAG3′ and R: 5′TTAGGGGACACCTACCCGTCAGGAAGAGCA3′ were used for amplification, and the restriction enzyme Mspl was used for digestion according to Ordovas 33.
Apo B-100 gene 2488C/T (XbaI) polymorphism
The specific primers F: 5′GGAGATATTCAGAAGCTAA3′ and R: 5′GAAGAGCCTGAAGACTGACT3′ were used for amplification, and the restriction enzyme XbaI was used for digestion according to Hu 34.
The data analysis involved the calculation of the allele and genotypic frequencies based on the assumption of the Hardy-Weinberg principle. Moreover, either the chi-square (Ji 2) or he Fisher’s exact test was applied to establish the association between genetic polymorphisms and preeclampsia based on p <0.05 and odds ratio (OR). We used IBM SPSS version 22.0, Arlequin version 3.5.2, and a genetic association software in our analysis.
Results
Adding all the participants of the preeclampsia projects between 2012 and 2017, 450 pregnant women were contacted, 22 of whom were excluded because of the following reasons: unmet inclusion criteria for either group, incomplete data, presence of a relevant disease, clotting of blood samples, unwillingness to participate, or failed sample amplification.
The results of allele and genotype frequencies, for the Hardy-Weinberg equilibrium, and for the association and statistical significance are presented in Table 2.
Table 2. Genetic variants in Peruvian pregnant women with preeclampsia and controls.
| Gene | Genotypes and alleles | Preeclampsia | Controls | OR | 95% CI | p a |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| VEGF +936 C/T | CC | 19 (42.2) | 19 (38.8) | Reference | 0.062 | |
| CT | 12 (26.7) | 23 (46.9) | 0.523 | 0.203-1.341 | ||
| TT | 14 (31.1) | 7 (14.3) | 2.000 | 0.661-6.056 | ||
| C | 50 (55.6) | 61 (62.2) | Reference | 0.434 | ||
| T | 40 (44.4) | 37 (37.8) | 1.319 | 0.736-2.362 | ||
| GG | 7 (17.0) | 15 (33.3) | Reference | 0.256 | ||
| +405 G/C | GC | 27 (69.2) | 24 (53.4) | 2.411 | 0.842-6.904 | |
| CC | 5 (12.8) | 6 (13.3) | 1.786 | 0.403-7.906 | ||
| G | 41 (52.6) | 54 (60.0) | Reference | 0.356 | ||
| C | 37 (47.4) | 36 (40.0) | 1.354 | 0.734-2.498 | ||
| CC | 4 (20.0) | 13 (33.3) | Reference | 0.004 | ||
| CG | 7 (35.0) | 23 (59.0) | 0.989 | 0.243-4.028 | ||
| IL6 -174 G/C | GG | 9 (45.0) | 3 (7.7) | 9.750 | 1.744- 54.525 | |
| C | 15 (37.5) | 49 (62.8) | Reference | 0.011 | ||
| G | 25 (62.5) | 29 (37.2) | 2.816 | 1.281-6.191 | ||
| CC | 30 (61.2) | 29 (58.0) | Reference | 0.946 | ||
| CT | 18 (36.7) | 20 (40.0) | 0.870 | 0.385-1.968 | ||
| IL1B -511 C/T | TT | 1 (2.1) | 1 (2.0) | 0.967 | 0.058-16.192 | |
| C | 78 (79.6) | 78 (78.0) | Reference | 0.863 | ||
| T | 20 (20.4) | 22 (22.0) | 0.909 | 0.460-1.798 | ||
| GG | 13 (27.7) | 12 (26.7) | Reference | 0.832 | ||
| GA | 23 (48.9) | 20 (44.4) | 1.062 | 0.396-2.849 | ||
| APOA1 -75 G/A | AA | 11 (23.4) | 13 (28.9) | 0.781 | 0.254-2.400 | |
| G | 49 (52.1) | 44 (48.9) | Reference | 0.768 | ||
| A | 45 (47.9) | 46 (51.1) | 0.878 | 0.493-1.566 | ||
| X-X- (CC) | 28 (59.6) | 25 (55.6) | Reference | 0.676 | ||
| APOB100 | X- X+ (CT) | 12 (25.5) | 15 (33.3) | 0.714 | 0.282-1.813 | |
| 2488 C/T (XbaI) | X+X+ (TT) | 7 (14.9) | 5 (11.1) | 1.250 | 0.352-4.442 | |
| X- | 68 (72.3) | 65 (72.2) | Reference | 0.883 | ||
| X+ | 26 (27.7) | 25 (27.8) | 0.994 | 0.521-1.896 |
The genotype frequencies of the +936 C/T and +405 G/T variants of the VEGF gene and of the 2488 C/T (XbaI) polymorphism of the APOB100 gene in women with preeclampsia were in Hardy-Weinberg disequilibrium. a According to chi-square test or Fisher’s exact test.
The genotypic frequencies of the VEGF +936C/T and +405G/C polymorphisms in controls were found to be in Hardy-Weinberg equilibrium; however, in cases they were in disequilibrium, indicating the influence of other factors.
No association was found between genotypes and alleles of the VEGF +936 C/T polymorphism and preeclampsia (p= 0.062 and p= 0.434). The differences between genotypic and allelic frequencies of the VEGF +405 polymorphism in cases and controls were not significant (p= 0.256 and p= 0.356). However, we would like to highlight that the proportions of heterozygous GC (69.2%) and C allele (47.4%) were higher in the preeclamptic women than in the control.
As regards the allelic and genotypic frequencies of the IL-6 -174 G/C polymorphism, in Hardy-Weinberg equilibrium, showed different distribution patterns between the preeclamptic women and the controls. Under a codominant model, the GG genotype (OR= 9.750, IC 95%: 1.744-54.525, p= 0.004, with CC genotype as reference) and the G allele (OR= 2.816, IC 95%: 1.281-6.191, p= 0.011, with C allele as reference) are considered to be at risk and with significant differences, wherein the frequencies were higher in the cases than in the controls.
Regarding the -511C/T polymorphism of the IL-1β gene, no significant differences were found in the frequencies of the TT and CT+CC genotypes between the cases and the controls (p >0.946). The homozygous TT was the most frequent genotype (over 50%) in both groups. For the C and T alleles, the differences were not significant (p= 0.863).
The genotypic frequencies of the ApoA-1 -75G/A polymorphism in cases and controls were in Hardy-Weinberg equilibrium, as were the controls for the ApoB100 2488C/T (XbaI) polymorphism; however, preeclamptic pregnant women were in disequilibrium. Overall, genotype and allele frequencies for both ApoA-1 -75G/A and ApoB100 2488C/T (XbaI) polymorphisms, between cases and controls, did not show significant differences (p >0.05) and were not associated with preeclampsia.
Discussion
The cause of preeclampsia remains unknown, and no methods can prevent or treat this disease. Moreover, this obstetric complication may appear unexpectedly in any pregnant woman 3.
An association was observed between preeclampsia and family history of pregnant women, and a genetic factor is considered to be involved in its origin. Until 2012, 178 genes associated with preeclampsia had been described 35. In 2014, from among over 22 million PubMed records, 28,000 articles related to preeclampsia were found, including 729 articles about 535 genes and genetic variants with a “significant” association with preeclampsia 36.
Specific gene polymorphisms, including angiogenic and antiangiogenic factor genes, have been described in pregnant women with preeclampsia. One angiogenic factor is VEGF, which plays a crucial role in vasculogenesis and vascular permeability. It is usually expressed at optimal levels following an adequate blastocyst placentation 37,38. VEGF is genetically regulated; some allelic variations of which are possibly associated with preeclampsia, and some of its polymorphisms function as hypoxia-induced factors that play a role in preeclampsia 37. When placentation is defective, such as in preeclampsia and IUGR, VEGF levels are low 39. A decrease in VEGF levels may result in placental oxidative stress 40. Studies have attempted to determine the relationship between VEGF polymorphisms and preeclampsia 41,42, and some studies have associated these polymorphisms with endothelial dysfunction 43,44, preeclampsia severity 45, or HELLP syndrome 46. The association of some VEGF gene polymorphisms 30, such as +936C/T, with preeclampsia has also been reported 30,31.
However, no association of the VEGF +936C/T and +405G/C polymorphisms with preeclampsia was observed in the studied Peruvian pregnant women (p= 0.062 for +936C/T and p= 0.256 for +405G/C). In the +936C/T polymorphism, the mutant homozygous genotype TT was more frequent in the cases, whereas the heterozygous CT genotype was more frequent in the controls. The differences in the frequencies of C and T alleles in the cases and controls were not significant (p= 0,434), for the genotypes as well, but close to the limits of significance (p=0,062). In terms of the VEGF +405 polymorphism, the proportions of heterozygous GC (69.2%) and C allele (47.4%) were higher in the preeclamptic women than in the controls.
Some studies, such as those of Shim 47 and Papazoglou 29, have reported an association between VEGF polymorphisms and preeclampsia. By contrast, other researchers have found no association of preeclampsia with VEGF +936C/T polymorphism 48; with +813C allele 49; with VEGF rs699947, rs1570360, rs2010963, and rs25648 minor alleles 30; with eNOS and DDAH genes 50; and with VEGF -2578C/A, -634G/C, and 936C/T alleles. The 936C/T allele has been associated only with severe preeclampsia 45. In Latin America, Sandrim et al.51, have found an association of the − C2578A, -1154G, and -634C haplotypes with preeclampsia prevention, and a similar association with the C-2578A allele was found by Cunha et al. 48; both studies were conducted in Brazil. However, in Ecuador, Sandrim’s group has not found such an association with VEGF C2578A and G634C in the same way that Chedraui et al. 44, have not found the said association with the VEGF -2578 C/A, -1498 C/T, -1154 A/G, -634 C/G, and -936C/T polymorphisms 7.
Alterations in inflammatory cytokine and lipid profiles have been associated with the presence and severity of hypertensive disorders of pregnancy 28,52. Cytokines are proteins secreted by innate or adaptive immune cells, many of the functions of which are mediated by cytokines 53. The placenta expresses various pro- and anti-inflammatory cytokines, adipokines, and cytokine-like angiogenic growth factors. However, their production of these markers is altered in preeclampsia, at least partially due to hypoxia. It is postulated that endothelial dysfunction underlies the disease manifestations of preeclampsia 54. Endothelial cell activation seems related to impaired maternal immune response, placental ischemia 55, oxidative stress, and generation of inflammatory cytokines56.
One class of cytokines are ILs, which modify biological responses. The cytokines involved in the pathophysiology of preeclampsia 11,57,58 include IL-6 59, IL-1β 60-62, IL-17, and IL-35 63. Increased stress during pregnancy is a predictor of an elevated production of IL-1β and IL-6 pro-inflammatory cytokines by lymphocytes during the third trimester. This alteration in the cell function of the immune system increases the risk of preeclampsia and preterm delivery 64. Moreover, having a female fetus is associated with low levels of pro-inflammatory IFNγ and IL-12 cytokines in the first trimester and with increased levels of pro-inflammatory IL-1β and TNFβ, anti-inflammatory IL-4r, and regulatory IL-5 and IL-10 cytokines in the second trimester 65. Fetal sex is thus related to the variability in cytokine levels.
IL-6 is a cytokine produced by many innate immune cells, neutrophils, and monocytes/macrophages, and it is expressed during states of cellular stress, such as inflammation, infection, wound, and cancer 66. IL-6 is an important mediator of acute-phase immune response and of trophoblast proliferation, invasion, and differentiation 67,68. Studies have suggested that the IL-6 -174 promoter polymorphism is a major genetic regulator in the etiology of early-onset preeclampsia 69-73. A systematic review that included 73 articles and analyzed 57 unique markers has found that the proinflammatory markers IL-6, IL-8, and tumor necrosis factor alpha have garnered the most support as the potential inflammatory markers for the clinical surveillance of preeclampsia, particularly during the second and third trimesters 58.
However, conflicting results in relation to the role of circulating IL-6 in preeclampsia have been found 69. In Latin America, the Brazilian study conducted by Pinheiro et al. 72, has found an association of the IL-6 -174G/C allele with protection for preeclampsia; however, another Brazilian study conducted by Daher et al. 73, and a Mexican study by Valencia et al. 74, have found no association of this allele with the risk of preeclampsia. Our study on the -174G/C polymorphism of the IL-6 gene showed an association of preeclampsia risk with the GG genotype and the G allele in the preeclamptic Peruvian women. However, these results must be verified in a larger population.
IL-1 is secreted by macrophages, endothelial cells, and some epithelial cells, and it activates endothelial cell inflammation and coagulation. IL-1β gene polymorphisms have been associated with preeclampsia 62,63, preterm birth 75, and recurrent pregnancy loss 76. In their Brazilian study, Leme et al. 77, have found an association of preeclampsia risk with the IL-1β rs1143630 T allele, while Pontillo et al. 78, who also conducted their study in Brazil, have found no association of IL1β rs1143634 with preeclampsia risk, a finding similar to that of other studies 79. The -511 C/T polymorphism in the IL-1β gene promoter region is implicated in the differential production of cytokines. Moreover, it may be associated with the immune inflammatory response in obesity, dyslipidemia, cardiopathy, cancer, infections, and treatment with nutrients and drugs. The IL-1β gene -511C/T polymorphism has also been studied in Peruvian Mestizo, Amazonian, and Andean subpopulations 28, and the T mutant allele associated with an increased cytokine production was frequently observed in these subpopulations. In our study, no significant difference in frequency distribution of the IL-1β gene -511C/T polymorphism TT and CT+CC genotypes between cases (n= 49) and controls (n= 50) was found.
Plasma lipoprotein metabolism is regulated and controlled by the specific apolipoproteins (apo-), constituents of the various lipoprotein classes 80. Apolipoproteins regulate protein metabolism by transporting and redistributing lipids to cells and tissues. Lipoprotein A (LpA) is a low-density lipoprotein (LDL) particle modified with an apolipoprotein A (Apo A-1), the main component of the structural particles of high-density lipoprotein (HDL), which exhibits anti-inflammatory properties, inhibits LDL oxidation, and clears up excess cholesterol from macrophages 81,82. Apo A-1 also protects the trophoblast-endothelial cell integration in the presence of a pro-inflammatory stimulus. Women with preeclampsia have low Apo A-1 levels, which deter their ability to control LDL and inflammation 83,84. Apo B-100 represents the Apo B particles circulating in the body, and it is an LDL. The Apo B-100/Apo A-1 quotient has been proposed as a reliable parameter used to predict atherosclerosis and mortal events resulting from cardiovascular disease that is linked to lipid alterations 85.
Apo A-1 concentrations have been found to increase in normal pregnancy and to decrease in women with preeclampsia 84; thus, Apo A-1 concentrations are an important risk factor for atherosclerosis among preeclamptic women 86. Other researchers have found lower levels of Apo A-1 only in patients with severe preeclampsia 87. Apo B is considered a measure of atherogenic lipoproteins, and it can be used to predict the risk of atherosclerotic cardiovascular disease 88. However, no difference in Apo A-1 and Apo B levels was observed between preeclamptic and normal pregnant women 87,89. In our study on Apo A-1 and Apo B-100 genes, no significant differences in genotypes and alleles were found between the women with severe preeclampsia and the controls.
A higher Apo B/Apo A-1 ratio has been associated with an increased risk of preeclampsia 90. Timur et al. 91, have reported that preeclamptic patients display significantly low Apo A-1 levels and a high Apo B-100/Apo A-1 ratio and that they consider these parameters as useful markers. By contrast, Kharb et al. 92, have found that the serum and cord blood Apo A-1 and Apo B levels were lower in preeclamptic women than in normotensive pregnant women. We have found controversies in the literature regarding the levels of Apo A-1 and Apo B-100 and regarding the possible association of the Apo A-1/Apo B-100 ratio with preeclampsia. Nevertheless, studies have found an association of these gene polymorphisms with cardiovascular disease 93, dyslipidemia 94, osteonecrosis 95, and other disorders.
In this study, the Hardy-Weinberg disequilibrium of the genotype frequencies of the two polymorphisms of the VEGF gene as well as of the Apo B-100 gene in preeclamptic women may indicate population admixture and/or specific characteristics of the patients. However, the lack of association with preeclampsia was corroborated by the Armitage trend test, the result of which is valid even when the frequencies depart from the Hardy-Weinberg equilibrium.
The search for susceptibility genes has led to a drastic increase in the number of published studies associating genetic factors with preeclampsia. However, attempts to replicate the findings of these studies have produced inconsistent results, except for the genes ACE, CTLA4, F2, FV, LPL, and SERPINE1 96.
Conclusions
The present study analyzed polymorphisms related to endothelial function, angiogenesis, immunological and inflammatory processes, and metabolic syndrome in Peruvian preeclamptic women. No association was found between the genetic markers studied and preeclampsia. However, the -174G/C polymorphism in the IL-6 gene presented significant differences mainly for the GG genotype and the G allele, whose frequencies were higher in the cases with respect to the controls; according to OR calculations, they would be risk factors. The limitation of the study of this polymorphism is the number of case samples (n=20), which should be a stimulus for further studies.
The contradictory results of the work can be partially explained by the genetic composition of the Peruvian population. Lima, the Peruvian city where the study was carried out, has a mixed population, characterized by a high Amerindian component, around 70%, and by European, Asian and African ancestry 97. Therefore, genetic ancestry and other variables, such as the sex of the fetus, can be considered in future research.
The present study contributes to a better understanding of the genetics of preeclampsia in Peru. Further research is needed to include larger populations of pregnant women and other Peruvian regions, as well as to comprise additional genes related to preeclampsia, a polygenic disorder.
Funding Statement
Universidad Nacional de San Marcos, as part of 2012-2017 grants from the Vice-Rectorate for Research.
Notes:
Funding: The authors received funding from the Universidad Nacional de San Marcos, as part of 2012-2017 grants from the Vice-Rectorate for Research.
References
- 1.1. Mustafa R, Ahmed S, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012; 2012:105918. doi: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed]; Mustafa R, Ahmed S, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918–105918. doi: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2. American College of Obstetricians and Gynecologists. ACOAG issues updated hypertension guidance, discusses new ACC/AHA criteria. ACOG; 2018. https://www.acog.org/news/news-releases/2018/12/acog-issues-updated-hypertension-guidance ; American College of Obstetricians and Gynecologists . ACOAG issues updated hypertension guidance, discusses new ACC/AHA criteria. ACOG; 2018. https://www.acog.org/news/news-releases/2018/12/acog-issues-updated-hypertension-guidance [Google Scholar]
- 3.3. Pacheco J, Wagner P, Williams MA, Sánchez S. Enfermedades hipertensivas en la gestación. In: Pacheco J. Ginecología, Obstetricia y Reproducción. 2ª Edición. Lima: REP SAC. 2007:1097-130.; Pacheco J, Wagner P, Williams MA, Sánchez S. Pacheco J. Ginecología, Obstetricia y Reproducción. 2ª . Lima: REP SAC; 2007. Enfermedades hipertensivas en la gestación; pp. 1097–1130. [Google Scholar]
- 4.4. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019; 366: l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed]; Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia pathophysiology and clinical implications. BMJ. 2019;366:l2381–l2381. doi: 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 5.5. Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham Jr MW, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). 2016; 130(6): 409-19. doi:10.1042/CS20150702. [DOI] [PMC free article] [PubMed]; Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Jr, Wallace K. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 2016;130(6):409–419. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.6. Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front Physiol. 2014; 5: 372. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed]; Sánchez-Aranguren LC, Prada CE, Riaño-Medina CE, Lopez M. Endothelial dysfunction and preeclampsia role of oxidative stress. Front Physiol. 2014;5:372–372. doi: 10.3389/fphys.2014.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.7. Tomoya MR, de Lima Kaminski V, Bogo Chies JÁ. Genetic variants in preeclampsia: lesson from studies in Latin-American populations. Front Physiol. 2018; 9: 1771. doi: 10.3389/fphys.2018.01771. [DOI] [PMC free article] [PubMed]; Tomoya MR. de Lima Kaminski V.Bogo Chies JÁ Genetic variants in preeclampsia lesson from studies in Latin-American populations. Front Physiol. 2018;9:1771–1771. doi: 10.3389/fphys.2018.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.8. American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 202. Obstet Gynecol. 2019; 133(1): e1-e25. [DOI] [PubMed]; American College of Obstetricians and Gynecologists Gestational hypertension and preeclampsia ACOG Practice Bulletin No. 202. Obstet Gynecol. 2019;133(1):e1–e25. doi: 10.1097/AOG.0000000000003018. [DOI] [PubMed] [Google Scholar]
- 9.9. Williams P, Broughton F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011; 25(4-4): 405-17. DOI: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed]; Williams P, Broughton F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4-4):405–417. doi: 10.1016/j.bpobgyn.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.10. Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: Making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014; 35(Suppl): S20-5. doi:10.1016/j.placenta.2013.12.008. [DOI] [PubMed]; Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture Making sense of pre-eclampsia - two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–S25. doi: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 11.11. Cindrova-Davies T, Fogarty NME, Jones CJP, Kingdom J, Burton GJ. Evidence of oxidative stress-induced senescence in mature, postmature and pathological human placentas. Placenta. 2018; 68: 15-22. doi: 10.1016/j.placenta.2018.06.307. [DOI] [PMC free article] [PubMed]; Cindrova-Davies T, Fogarty NME, Jones CJP, Kingdom J, Burton GJ. Evidence of oxidative stress-induced senescence in mature, postmature and pathological human placentas. Placenta. 2018;68:15–22. doi: 10.1016/j.placenta.2018.06.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.12. Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble FMS-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab 2005; 90: 4895-903. doi:10.1210/jc.2004-1955. [DOI] [PubMed]; Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM. Soluble FMS-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 13.13. Bokslag A, vsn Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016; 102: 47-50. DOI: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed]; Bokslag A, vsn Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 14.14. Gastrich MD, Zinonos S, Bachmann G, Cosgrove NM, Cabrera J, Cheng JQ, et al. Preeclamptic women are at significantly higher risk of future cardiovascular outcomes over a 15-year period. J Women´s Health. 2020; 29(1): 74-83. Doi: 10.1089/jwh.2019.7671. [DOI] [PubMed]; Gastrich MD, Zinonos S, Bachmann G, Cosgrove NM, Cabrera J, Cheng JQ. Preeclamptic women are at significantly higher risk of future cardiovascular outcomes over a 15-year period. J Women´s Health. 2020;29(1):74–83. doi: 10.1089/jwh.2019.7671. [DOI] [PubMed] [Google Scholar]
- 15.15. Aukes AM, De Groot JC, Wiegman MJ, Aarnoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG. 2012; 119(9): 1117-22. doi:10.1111/j.1471-0528.2012.03406.x. [DOI] [PubMed]; Aukes AM, De Groot JC, Wiegman MJ, Aarnoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG. 2012;119(9):1117–1122. doi: 10.1111/j.1471-0528.2012.03406.x. [DOI] [PubMed] [Google Scholar]
- 16.16. McBryde M, Fitzallen GC, Liley HG, Taylor HG, Bora S. Academic outcomes of school-aged children born preterm. A systematic review and meta-analysis. JAMA Network Open. 2020; 3(4): e202027. doi:10.1001/jamanetworkopen.2020.2027. [DOI] [PMC free article] [PubMed]; McBryde M, Fitzallen GC, Liley HG, Taylor HG, Bora S. Academic outcomes of school-aged children born preterm A systematic review and meta-analysis. JAMA Network Open. 2020;3(4):e202027. doi: 10.1001/jamanetworkopen.2020.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.17. Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of preeclampsia in term births with neurodevelopmental disorders in offspring. JAMA Psychiatry. 2020; 77(8):823-829. doi:10.1001/jamapsychiatry.2020.0306. [DOI] [PMC free article] [PubMed]; Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of preeclampsia in term births with neurodevelopmental disorders in offspring. JAMA Psychiatry. 2020;77(8):823–829. doi: 10.1001/jamapsychiatry.2020.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.18. Pacheco J. Del Editor sobre la publicación de una aproximación bioinformática a la genética de la preeclampsia. Re Peru Ginecol Obstet. 2014; 60(2): 105-7. Doi: 10.31403/rpgo.v60i119.; Pacheco J. Del Editor sobre la publicación de una aproximación bioinformática a la genética de la preeclampsia. Re Peru Ginecol Obstet. 2014;60(2):105–107. doi: 10.31403/rpgo.v60i119. [DOI] [Google Scholar]
- 19.19. Sahin H, Gunel T, Benian A, Ucar EA, Guralp O, Kilic A. Genomic and proteomic investigation of preeclampsia. Experim Ther Med. 2015; 10: 711-6. DOI: 10.3892/etm.2015.2509. [DOI] [PMC free article] [PubMed]; Sahin H, Gunel T, Benian A, Ucar EA, Guralp O, Kilic A. Genomic and proteomic investigation of preeclampsia. Experim Ther Med. 2015;10:711–716. doi: 10.3892/etm.2015.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.20. Harmon QE, Engel SM, Wu MC, Moran TM, Luo J, Stuebe AM, et al. Polymorphisms in inflammatory genes are associated with term small for gestational age and preeclampsia. Am J Reprod Immunol. 2014; 71(5): 472-84. doi: 10.1111/aji.12241. [DOI] [PMC free article] [PubMed]; Harmon QE, Engel SM, Wu MC, Moran TM, Luo J, Stuebe AM. Polymorphisms in inflammatory genes are associated with term small for gestational age and preeclampsia. Am J Reprod Immunol. 2014;71(5):472–484. doi: 10.1111/aji.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.21. Karumanchi SA. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension. 2016; 67: 1072-9. doi:10.1161/HYPERTENSIONAHA.116.06421. [DOI] [PubMed]; Karumanchi SA. Angiogenic factors in preeclampsia from diagnosis to therapy. Hypertension. 2016;67:1072–1079. doi: 10.1161/HYPERTENSIONAHA.116.06421. [DOI] [PubMed] [Google Scholar]
- 22.22. Honigberg MC, Cantonwine DE, Thomas AM, Lim KH, Parry SI, McElrath TF. Analysis of changes in maternal circulating angiogenic factors throughout pregnancy for the prediction of preeclampsia. J Perinatol. 2016; 36(3): 172-7. doi: 10.1038/jp.2015.170. [DOI] [PubMed]; Honigberg MC, Cantonwine DE, Thomas AM, Lim KH, Parry SI, McElrath TF. Analysis of changes in maternal circulating angiogenic factors throughout pregnancy for the prediction of preeclampsia. J Perinatol. 2016;36(3):172–177. doi: 10.1038/jp.2015.170. [DOI] [PubMed] [Google Scholar]
- 23.23. Gene. PGF Placental growth factor [Homo sapiens (human)]. Gene ID: 5228, updated on 24-Nov-2020. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=5228 ; Gene PGF Placental growth factor [Homo sapiens (human)]. Gene ID: 5228. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=5228
- 24.24. Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011; 24(10): 1187-207. DOI: 10.3109/14767058.2011.589932 [DOI] [PMC free article] [PubMed]; Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24(10):1187–1207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.25. Roberts JM, Rajakumar A. Preeclampsia and soluble fms-like tyrosine kinase 1. J Clin Endocrinol Metab. 2009; 94(7): 2252-4. doi: 10.1210/jc.2009-0945. [DOI] [PMC free article] [PubMed]; Roberts JM, Rajakumar A. Preeclampsia and soluble fms-like tyrosine kinase 1. J Clin Endocrinol Metab. 2009;94(7):2252–2254. doi: 10.1210/jc.2009-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.26. Barden AE, Herbison CE, Beilin LJ, Michael CA, Walters BN, Van Bockxmeer FM. Association between the endothelin-1 gene Lys198Asn polymorphism blood pressure and plasma endothelin-1 levels in normal and pre-eclamptic pregnancy. J Hypertens. 2001; 19(10): 1775-82. DOI: 10.1097/00004872-200110000-00011 [DOI] [PubMed]; Barden AE, Herbison CE, Beilin LJ, Michael CA, Walters BN, Van Bockxmeer FM. Association between the endothelin-1 gene Lys198Asn polymorphism blood pressure and plasma endothelin-1 levels in normal and pre-eclamptic pregnancy. J Hypertens. 2001;19(10):1775–1782. doi: 10.1097/00004872-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 27.27. Aggarwal PK, Jain V, Srinivasan R, Jha V. Maternal EDN1 G5665T polymorphism influences circulating endothelin-1 levels and plays a role in determination of preeclampsia phenotype. J Hypertens. 2009; 27(10): 2044-50. DOI: 10.1097/HJH.0b013e32832f7f3f [DOI] [PubMed]; Aggarwal PK, Jain V, Srinivasan R, Jha V. Maternal EDN1 G5665T polymorphism influences circulating endothelin-1 levels and plays a role in determination of preeclampsia phenotype. J Hypertens. 2009;27(10):2044–2050. doi: 10.1097/HJH.0b013e32832f7f3f. [DOI] [PubMed] [Google Scholar]
- 28.28. Acosta O, Solano L, Huerta D, Oré D, Sandoval J, Figueroa J, et al. Variabilidad genética de la respuesta inflamatoria. I. Polimorfismo -511 C/T en el gen IL1ß en diferentes subpoblaciones peruanas. An fac med. 2012;73(3):221-5. DOI: 10.15381/anales.v73i3.868.; Acosta O, Solano L, Huerta D, Oré D, Sandoval J, Figueroa J. Variabilidad genética de la respuesta inflamatoria I. Polimorfismo -511 C/T en el gen IL1ß en diferentes subpoblaciones peruanas. An fac med. 2012;73(3):221–225. doi: 10.15381/anales.v73i3.868. [DOI] [Google Scholar]
- 29.29. Papazoglou D, Galazios G, Koukourakis MI, Panagopoulos I, Kontomanolis EN, Papatheodorou K, et al. Vascular endothelial growth factor gene polymorphisms and pre-eclampsia. Mol Hum Reprod. 2004; 10(5):321-4. DOI: 10.1093/molehr/gah048 [DOI] [PubMed]; Papazoglou D, Galazios G, Koukourakis MI, Panagopoulos I, Kontomanolis EN, Papatheodorou K. Vascular endothelial growth factor gene polymorphisms and pre-eclampsia. Mol Hum Reprod. 2004;10(5):321–324. doi: 10.1093/molehr/gah048. [DOI] [PubMed] [Google Scholar]
- 30.30. Bányász I, Szabo S, Bokodi G, Vannay A, Vasarhelyi B, Szabo A, et al. Genetic polymorphisms of vascular endothelial growth factor in severe pre-eclampsia. Mol Hum Reprod. 2006; 12(4): 233-6. [DOI] [PubMed]; Bányász I, Szabo S, Bokodi G, Vannay A, Vasarhelyi B, Szabo A. Genetic polymorphisms of vascular endothelial growth factor in severe pre-eclampsia. Mol Hum Reprod. 2006;12(4):233–236. doi: 10.1093/molehr/gal024. [DOI] [PubMed] [Google Scholar]
- 31.31. Garza-Veloz I, Castruita-De La Rosa C, Cortes-Flores R, Martínez-Gaytan V, Rivera-Muñoz JE, Garcia-Mayorga EA, et al. No association between polymorphisms/haplotypes of the vascular endothelial growth factor gene and preeclampsia. BMC Pregnancy and Childbirth. 2011; 11: 35. doi: 10.1186/1471-2393-11-35. [DOI] [PMC free article] [PubMed]; Garza-Veloz I, Castruita-De La Rosa C, Cortes-Flores R, Martínez-Gaytan V, Rivera-Muñoz JE, Garcia-Mayorga EA. No association between polymorphisms/haplotypes of the vascular endothelial growth factor gene and preeclampsia. BMC Pregnancy and Childbirth. 2011;11:35–35. doi: 10.1186/1471-2393-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.32. Berthold HK, Laudes M, Krone W, Gouni-Berthold I. Association between the interleukin-6 promoter polymorphism -174G/C and serum lipoprotein(a) concentrations in humans. PLoS One. 2011; 6(9): e24719. Doi: 10.1371/journal.pone.0024719 [DOI] [PMC free article] [PubMed]; Berthold H, Laudes M, Krone W, Gouni-Berthold I. Association between the interleukin-6 promoter polymorphism -174G/C and serum lipoprotein(a) concentrations in humans. PLoS One. 2011;6(9):e24719. doi: 10.1371/journal.pone.0024719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.33. Ordovas J, Corella D, Cupples L, Demissie S, Kelleher A, Coltell O, et al. Polyunsaturated fatty acids modulate the effects of the APOA1 G-A polymorphism on HDL-cholesterol concentrations in a sex-specific manner: the Framingham Study. Am J Clin Nutr. 2002; 75(1): 38-46. DOI: 10.1093/ajcn/75.1.38 [DOI] [PubMed]; Ordovas J, Corella D, Cupples L, Demissie S, Kelleher A, Coltell O. Polyunsaturated fatty acids modulate the effects of the APOA1 G-A polymorphism on HDL-cholesterol concentrations in a sex-specific manner the Framingham Study. Am J Clin Nutr. 2002;75(1):38–46. doi: 10.1093/ajcn/75.1.38. [DOI] [PubMed] [Google Scholar]
- 34.34. Hu P, Qin Y, Jing C, Lu L, Hu B, Du P. Effect of apolipoprotein B polymorphism on body mass index, serum protein and lipid profiles in children of Guangxi, China. Ann Hum Biol. 2009; 36(4): 411-20. doi:10.1080/03014460902882475 [DOI] [PubMed]; Hu P, Qin Y, Jing C, Lu L, Hu B, Du P. Effect of apolipoprotein B polymorphism on body mass index, serum protein and lipid profiles in children of Guangxi, China. Ann Hum Biol. 2009;36(4):411–420. doi: 10.1080/03014460902882475. [DOI] [PubMed] [Google Scholar]
- 35.35. Jebbink J, Wolters A, Fernando F, Afink G, van der Post J, Ris-Stalpers C. Molecular genetics of preeclampsia and HELLP syndrome - A review. Biochim Biophys Acta. 2012; 1822(12): 1960-9. DOI: 10.1016/j.bbadis.2012.08.004 [DOI] [PubMed]; Jebbink J, Wolters A, Fernando F, Afink G. van der Post J.Ris-Stalpers C Molecular genetics of preeclampsia and HELLP syndrome - A review. Biochim Biophys Acta. 2012;1822(12):1960–1969. doi: 10.1016/j.bbadis.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 36.36. Triche EW, Uzun A, DeWan AT, Kurihara I, Liu J, Occhiogrosso R, et al. Bioinformatic approach to the genetics of preeclampsia. Obstet Gynecol. 2014; 123(6): 1155- 61. DOI: 10.1097/AOG.0000000000000293 [DOI] [PMC free article] [PubMed]; Triche EW, Uzun A, DeWan AT, Kurihara I, Liu J, Occhiogrosso R. Bioinformatic approach to the genetics of preeclampsia. Obstet Gynecol. 2014;123(6):1155–1161. doi: 10.1097/AOG.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.37. Galazios G, Papazoglou D, Tsikouras P, Kolios G. Vascular endothelial growth factor gene polymorphisms and pregnancy. J Matern Fetal Neonatal Med. 2009; 22(5):371-8. DOI: 10.1080/14767050802645035. [DOI] [PubMed]; Galazios G, Papazoglou D, Tsikouras P, Kolios G. Vascular endothelial growth factor gene polymorphisms and pregnancy. J Matern Fetal Neonatal Med. 2009;22(5):371–378. doi: 10.1080/14767050802645035. [DOI] [PubMed] [Google Scholar]
- 38.38. Gómez CLM. Actualización en la fisiopatología de la preeclampsia. Rev Peru Ginecol Obstet. 2014;60(4):321-32. DOI: 10.31403/rpgo.v60i156; Gómez CLM. Actualización en la fisiopatología de la preeclampsia. Rev Peru Ginecol Obstet. 2014;60(4):321–332. doi: 10.31403/rpgo.v60i156. [DOI] [Google Scholar]
- 39.39. Mateus J. Significancia del desbalance de los factores angiogénicos en preeclampsia. Rev Peru Ginecol Obstet. 2014;60(4):33-44. DOI: 10.31403/rpgo.v60i157; Mateus J. Significancia del desbalance de los factores angiogénicos en preeclampsia. Rev Peru Ginecol Obstet. 2014;60(4):33–44. doi: 10.31403/rpgo.v60i157. [DOI] [Google Scholar]
- 40.40. Kweider N, Fragoulis A, Rosen C, Pecks U, Rath W, Pufe T, et al. Interplay between vascular endothelial growth factor (VEGF) and the nuclear factor erythroid 2-related factor-2 (Nrf2): implications for preeclampsia. J Biol Chem. 2011; 286(50): 42863-72. DOI: 10.1074/jbc.M111.286880 [DOI] [PMC free article] [PubMed]; Kweider N, Fragoulis A, Rosen C, Pecks U, Rath W, Pufe T. Interplay between vascular endothelial growth factor (VEGF) and the nuclear factor erythroid 2-related factor-2 (Nrf2) implications for preeclampsia. J Biol Chem. 2011;286(50):42863–42872. doi: 10.1074/jbc.M111.286880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.41. Song GG, Kim JH, Lee YH. Associations between vascular endothelial growth factor gene polymorphisms and pre-eclampsia susceptibility: a meta-analysis. Immunol Invest. 2013;42(8):749-62. doi: 10.3109/08820139.2013.822394 [DOI] [PubMed]; Song GG, Kim JH, Lee YH. Associations between vascular endothelial growth factor gene polymorphisms and pre-eclampsia susceptibility a meta-analysis. Immunol Invest. 2013;42(8):749–762. doi: 10.3109/08820139.2013.822394. [DOI] [PubMed] [Google Scholar]
- 42.42. Cheng D, Hao Y, Zhou W, Ma Y. Vascular endothelial growth factor +936C/T, -634G/C, -2578C/A, and -1154G/A polymorphisms with risk of preeclampsia: a meta-analysis. PLoS One. 2013; 8(11): e78173. doi: 10.1371/journal.pone.0078173. [DOI] [PMC free article] [PubMed]; Cheng D, Hao Y, Zhou W, Ma Y. Vascular endothelial growth factor +936C/T, -634G/C, -2578C/A, and -1154G/A polymorphisms with risk of preeclampsia a meta-analysis. PLoS One. 2013;8(11):e78173. doi: 10.1371/journal.pone.0078173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.43. Luizon MR, Palei AC, Sandrim VC. Polymorphisms and haplotypes in candidate genes related to angiogenesis and endothelial dysfunction in preeclampsia. J Pregnancy. 2012; 2012: 914704. doi: 10.1155/2012/914704. [DOI] [PMC free article] [PubMed]; Luizon MR, Palei AC, Sandrim VC. Polymorphisms and haplotypes in candidate genes related to angiogenesis and endothelial dysfunction in preeclampsia. J Pregnancy. 2012;2012:914704–914704. doi: 10.1155/2012/914704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.44. Chedraui P, Solis EJ, Bocci G, Gopal S, Russo E, Escobar GS, Hidalgo L, et al. Feto-placental nitric oxide, asymmetric dimethylarginine and vascular endothelial growth factor (VEGF) levels and VEGF gene polymorphisms in severe preeclampsia. J Matern Fetal Neonatal Med. 2013; 26(3): 226-32. doi: 10.3109/14767058.2012.733760 [DOI] [PubMed]; Chedraui P, Solis EJ, Bocci G, Gopal S, Russo E, Escobar GS, Hidalgo L. Feto-placental nitric oxide, asymmetric dimethylarginine and vascular endothelial growth factor (VEGF) levels and VEGF gene polymorphisms in severe preeclampsia. J Matern Fetal Neonatal Med. 2013;26(3):226–232. doi: 10.3109/14767058.2012.733760. [DOI] [PubMed] [Google Scholar]
- 45.45. Procopciuc LM, Caracostea G, Zaharie G, Stamatian F. Maternal/newborn VEGF-C936T interaction and its influence on the risk, severity and prognosis of preeclampsia, as well as on the maternal angiogenic profile. J Matern Fetal Neonatal Med. 2014; 27(17):1754-60. doi: 10.3109/14767058.2014.942625. [DOI] [PubMed]; Procopciuc LM, Caracostea G, Zaharie G, Stamatian F. Maternal/newborn VEGF-C936T interaction and its influence on the risk, severity and prognosis of preeclampsia, as well as on the maternal angiogenic profile. J Matern Fetal Neonatal Med. 2014;27(17):1754–1760. doi: 10.3109/14767058.2014.942625. [DOI] [PubMed] [Google Scholar]
- 46.46. Haram K, Mortensen JH, Nagy B. Genetic aspects of preeclampsia and the HELLP syndrome. J Pregnancy. 2014; 2014: 910751. doi: 10.1155/2014/910751 [DOI] [PMC free article] [PubMed]; Haram K, Mortensen JH, Nagy B. Genetic aspects of preeclampsia and the HELLP syndrome. J Pregnancy. 2014;2014:910751–910751. doi: 10.1155/2014/910751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.47. Shim JY, Jun JK, Jung BK, Kim SH, Won HS, Lee PR, et al. Vascular endothelial growth factor gene +936 C/T polymorphism is associated with preeclampsia in Korean women. Am J Obstet Gynecol. 2007;197(3):271.e1-4. [DOI] [PubMed]; Shim JY, Jun JK, Jung BK, Kim SH, Won HS, Lee PR, et al. Vascular endothelial growth factor gene +936 C/T polymorphism is associated with preeclampsia in Korean women. Am J Obstet Gynecol. 2007;197(3):271.e1–271.e4. doi: 10.1016/j.ajog.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 48.48. Cunha VM, Grecco RL, Paschoini MC, Silva SR, Ruiz MT, Balarin MA. Polimorfismos genéticos do fator de crescimento do endotélio vascular na pré-eclâmpsia. Rev Bras Ginecol Obstet. 2011; 33(7): 158-63. [DOI] [PubMed]; Cunha VM, Grecco RL, Paschoini MC, Silva SR, Ruiz MT, Balarin MA. Polimorfismos genéticos do fator de crescimento do endotélio vascular na pré-eclâmpsia. Rev Bras Ginecol Obstet. 2011;33(7):158–163. doi: 10.1590/s0100-72032011000700007. [DOI] [PubMed] [Google Scholar]
- 49.49. Atis A, Oruc O, Aydin Y, Cetincelik U, Goker N. Vascular endothelial growth factor gene +813CC polymorphism of foetus is associated with preterm labour but not with pre-eclampsia in Turkish pregnant women. Int J Immunogenet. 2012; 39(3): 241-6. [DOI] [PubMed]; Atis A, Oruc O, Aydin Y, Cetincelik U, Goker N. Vascular endothelial growth factor gene +813CC polymorphism of foetus is associated with preterm labour but not with pre-eclampsia in Turkish pregnant women. Int J Immunogenet. 2012;39(3):241–246. doi: 10.1111/j.1744-313X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 50.50. Kim YJ, Park BH, Park H, Jung SC, Pang MG, Ryu HM, et al. No association of the genetic polymorphisms of endothelial nitric oxide synthase, dimethylarginine dimethylaminohydrolase, and vascular endothelial growth factor with preeclampsia in Korean populations. Twin Res Hum Genet. 2008; 11(1): 77-83. DOI: 10.1375/twin.11.1.77 [DOI] [PubMed]; Kim YJ, Park BH, Park H, Jung SC, Pang MG, Ryu HM. No association of the genetic polymorphisms of endothelial nitric oxide synthase, dimethylarginine dimethylaminohydrolase, and vascular endothelial growth factor with preeclampsia in Korean populations. Twin Res Hum Genet. 2008;11(1):77–83. doi: 10.1375/twin.11.1.77. [DOI] [PubMed] [Google Scholar]
- 51.51. Sandrim VC, Palei ACT, Cavalli RC, Araújo FM, Ramos ES, Duarte G, et al. Vascular endothelial growth factor genotypes and haplotypes are associated with pre-eclampsia but not with gestational hypertension. Mol Hum Reprod. 2009; 15: 115-120. doi: 10.1093/molehr/gan076 [DOI] [PubMed]; Sandrim VC, Palei ACT, Cavalli RC, Araújo FM, Ramos ES, Duarte G. Vascular endothelial growth factor genotypes and haplotypes are associated with pre-eclampsia but not with gestational hypertension. Mol Hum Reprod. 2009;15:115–120. doi: 10.1093/molehr/gan076. [DOI] [PubMed] [Google Scholar]
- 52.52. Wang Y, Shi D, Chen L. Lipid profile and cytokines in hypertension of pregnancy: A comparison of preeclampsia therapies. J Clin Hypertens (Greenwich). 2018; 20(2): 394-9. doi:10.1111/jch.13161 [DOI] [PMC free article] [PubMed]; Wang Y, Shi D, Chen L. Lipid profile and cytokines in hypertension of pregnancy A comparison of preeclampsia therapies. J Clin Hypertens (Greenwich) 2018;20(2):394–399. doi: 10.1111/jch.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.53. Abbas AK, Lichtman AH. Inmunología celular y molecular. Madrid, España: Elsevier Science; 2004. 243-74.; Abbas AK, Lichtman AH. Inmunología celular y molecular. Madrid, España: Elsevier Science; 2004. [Google Scholar]
- 54.54. Khong TY, Robertson WB. Spiral artery disease. In: Coulam CB, Faulk WP, McIntyre JA, eds. Immunological obstetrics. New York; Norton. 1992: 492-501.; Khong TY, Robertson WB. Spiral artery disease. In: Coulam CB, Faulk WP, McIntyre JA, editors. Immunological obstetrics. New York: Norton; 1992. pp. 492–501. [Google Scholar]
- 55.55. Taylor RN, Roberts JM. Endothelial cell dysfunction. In: Lindheimer MD, Roberts JM, Cunningham GF, eds. Chesley´s hypertensive disorders in pregnancy, 2nd Ed. Stamford: Appleton & Lange; 1999. 395-429.; Taylor RN, Roberts JM. Endothelial cell dysfunction. In: Lindheimer MD, Roberts JM, Cunningham GF, editors. Chesley´s hypertensive disorders in pregnancy. 2nd . Stamford: Appleton & Lange; 1999. pp. 395–429. [Google Scholar]
- 56.56. Gilbert JS, Ryan MJ, Lamarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol. 2008;294(2):H541-H550. [DOI] [PubMed]; Gilbert JS, Ryan MJ, Lamarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Am J Physiol. 2008;294(2):H541–H550. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 57.57. Raghupathy R. Cytokines as key players in the pathophysiology of preeclampsia. Med Prin Pract. 2013; 22(Suppl 1): 8-19. doi: 10.1159/000354200 [DOI] [PMC free article] [PubMed]; Raghupathy R. Cytokines as key players in the pathophysiology of preeclampsia. Med Prin Pract. 2013;22(Suppl 1):8–19. doi: 10.1159/000354200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.58. Black KD, Horowitz JA. Inflammatory markers and preeclampsia: A systematic review. Nurs Res. 2018;67(3):242-51. doi:10.1097/NNR.0000000000000285 [DOI] [PubMed]; Black KD, Horowitz JA. Inflammatory markers and preeclampsia A systematic review. Nurs Res. 2018;67(3):242–251. doi: 10.1097/NNR.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 59.59. Zhang Z, Gao Y, Zhang L, Jia L, Wang P, Zhang L, et al. Alterations of IL-6, IL-6R and gp130 in early and late onset severe preeclampsia. Hypertens Pregnancy. 2013; 32(3):270-80. doi: 10.3109/10641855.2013.2013.798332 [DOI] [PubMed]; Zhang Z, Gao Y, Zhang L, Jia L, Wang P, Zhang L. Alterations of IL-6, IL-6R and gp130 in early and late onset severe preeclampsia. Hypertens Pregnancy. 2013;32(3):270–280. doi: 10.3109/10641855.2013.2013.798332. [DOI] [PubMed] [Google Scholar]
- 60.60. Lachmeijer AMA, Nosti-Escanilla MP, Bastiaans EB, Sandkuijl LA, Kostense PJ, Aarnoudse JG, et al. Linkage and association studies of IL1B and IL1RN gene polymorphisms in preeclampsia. Hypertens Pregnancy. 2002;21(1):23-38. DOI: 10.1081/PRG-120002907 [DOI] [PubMed]; Lachmeijer AMA, Nosti-Escanilla MP, Bastiaans EB, Sandkuijl LA, Kostense PJ, Aarnoudse JG. Linkage and association studies of IL1B and IL1RN gene polymorphisms in preeclampsia. Hypertens Pregnancy. 2002;21(1):23–38. doi: 10.1081/PRG-120002907. [DOI] [PubMed] [Google Scholar]
- 61.61. Farnaz MF, Afshari JT, Rezaieyazdi Z, Ghomian N. Association of single nucleotide polymorphisms in the human tumor necrosis factor-a and interleukin 1-b genes in patients with pre-eclampsia. Iran J Allergy Asthma Immunol. 2012; 11(3): 224-9. [PubMed]; Farnaz MF, Afshari JT, Rezaieyazdi Z, Ghomian N. Association of single nucleotide polymorphisms in the human tumor necrosis factor-a and interleukin 1-b genes in patients with pre-eclampsia. Iran J Allergy Asthma Immunol. 2012;11(3):224–229. [PubMed] [Google Scholar]
- 62.62. Wang X, Jiang F, Liang Y, Xu L, Li H, Liu Y, et al. Interleukin-1ß-31C/T and -511T/C polymorphisms were associated with preeclampsia in Chinese Han population. PLoS One. 2014; 9(9): e106919. doi: 10.1371/journal.pone.0106919 [DOI] [PMC free article] [PubMed]; Wang X, Jiang F, Liang Y, Xu L, Li H, Liu Y. Interleukin-1ß-31C/T and -511T/C polymorphisms were associated with preeclampsia in Chinese Han population. PLoS One. 2014;9(9):e106919. doi: 10.1371/journal.pone.0106919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.63. Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. Plasma IL-17, IL-35, interferon-?, SOCS3 and TGF-ß levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med. 2014; 27(15): 1513-7. doi: 10.3109/14767058.2013.861415 [DOI] [PubMed]; Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. Plasma IL-17, IL-35, interferon- , SOCS3 and TGF-ß levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med. 2014;27(15):1513–1517. doi: 10.3109/14767058.2013.861415. [DOI] [PubMed] [Google Scholar]
- 64.64. Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007; 21(3): 343-50. doi:10.1016/j.bbi.2006.08.006. [DOI] [PubMed]; Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21(3):343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 65.65. Taylor BD, Ness RB, Klebanoff MA, Tang G, Roberts JM, Hougaard DM, e al. The impact of female fetal sex on preeclampsia and the maternal immune milieu. Pregnancy Hypertens. 2018; 12:53-7. doi: 10.1016/j.preghy.2018.02.009 [DOI] [PMC free article] [PubMed]; Taylor BD, Ness RB, Klebanoff MA, Tang G, Roberts JM, Hougaard DM. e al The impact of female fetal sex on preeclampsia and the maternal immune milieu. Pregnancy Hypertens. 2018;12:53–57. doi: 10.1016/j.preghy.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.66. Choy E, Rose-John S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J Scleroderma Related disorders. J Scleroderma Related Disorders. 2017; 2(2 suppl): S1-S5. Doi: 10.5301/jsrd.5000265; Choy E, Rose-John S. Interleukin-6 as a multifunctional regulator Inflammation, immune response, and fibrosis. J Scleroderma Related disorders. J Scleroderma Related Disorders. 2017;2(2 Suppl):S1–S5. doi: 10.5301/jsrd.5000265. [DOI] [Google Scholar]
- 67.67. LaMarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia: potential therapeutic role for magnesium sulfate. Int J Interferon Cytokine Mediator Res. 2011; 2011(3):59-64. doi:10.2147/IJICMR.S16320 [DOI] [PMC free article] [PubMed]; LaMarca B, Brewer J, Wallace K. IL-6-induced pathophysiology during pre-eclampsia potential therapeutic role for magnesium sulfate. Int J Interferon Cytokine Mediator Res. 2011;2011(3):59–64. doi: 10.2147/IJICMR.S16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.68. Barbosa deLT, Sass N, Mattar R, Moron AF, Torloni MR, Franchim CS, et al. Cytokine gene polymorphisms in preeclampsia and eclampsia. Hypert Res. 2009; 32: 565-9. Doi: 10.1038/hr.2009.58 [DOI] [PubMed]; Barbosa deLT, Sass N, Mattar R, Moron AF, Torloni MR, Franchim CS, et al. Cytokine gene polymorphisms in preeclampsia and eclampsia. Hypert Res. 2009;32:565–569. doi: 10.1038/hr.2009.58. [DOI] [PubMed] [Google Scholar]
- 69.69. Sowmya S, Ramaiah A, Nallari P, Jyothy A, Venkateshwari A. Role of IL-6 -174(G/C) promoter polymorphism in the etiology of early-onset preeclampsia. Inflamm Res. 2015; 64(6): 433-9. doi: 10.1007/s00011-015-0823-z [DOI] [PubMed]; Sowmya S, Ramaiah A, Nallari P, Jyothy A, Venkateshwari A. Role of IL-6 -174(G/C) promoter polymorphism in the etiology of early-onset preeclampsia. Inflamm Res. 2015;64(6):433–439. doi: 10.1007/s00011-015-0823-z. [DOI] [PubMed] [Google Scholar]
- 70.70. Fan DM, Wang Y, Liu XL, Zhang A, Xu Q. Polymorphisms in interleukin-6 and interleukin-10 may be associated with risk of preeclampsia. Genet Mol Res. 2017; 16(1): gmr16018588. doi: 10.4238/gmr16018588 [DOI] [PubMed]; Fan DM, Wang Y, Liu XL, Zhang A, Xu Q. Polymorphisms in interleukin-6 and interleukin-10 may be associated with risk of preeclampsia. Genet Mol Res. 2017;16(1):gmr16018588–gmr16018588. doi: 10.4238/gmr16018588. [DOI] [PubMed] [Google Scholar]
- 71.71. Puppala M, Kalpana VL, Aniradha A, Shusma M, Sudhakar G, Polipalli SK. Association of tumor necrosis factor-alpha and interleukin-6 gene polymorphisms with preeclampsia. Int J Bioassays. 2016; 5(02): 4774.; Puppala M, Kalpana VL, Aniradha A, Shusma M, Sudhakar G, Polipalli SK. Association of tumor necrosis factor-alpha and interleukin-6 gene polymorphisms with preeclampsia. Int J Bioassays. 2016;5(02):4774–4774. [Google Scholar]
- 72.72. Pinheiro MB, Gomes KB, Ronda ARSC, Guimaraes GG, Freitas LG, Teixeira-Carvalho A, et al. Severe preeclampsia: Association of genes polymorphisms and maternal cytokines production in Brazilian population. Cytokine. 2014; 71: 232-7. Doi: 10.1016/j.cyto.2014.10.021 [DOI] [PubMed]; Pinheiro MB, Gomes KB, Ronda ARSC, Guimaraes GG, Freitas LG, Teixeira-Carvalho A. Severe preeclampsia Association of genes polymorphisms and maternal cytokines production in Brazilian population. Cytokine. 2014;71:232–237. doi: 10.1016/j.cyto.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 73.73. Daher S, Sass N, Oliveira LG, Mattar R. Cytokine genotyping in preeclampsia. Am J Reprod Immunol. 2006; 55: 130-135. doi: 10.1111/j.1600-0897.2005.00341.x [DOI] [PubMed]; Daher S, Sass N, Oliveira LG, Mattar R. Cytokine genotyping in preeclampsia. Am J Reprod Immunol. 2006;55:130–135. doi: 10.1111/j.1600-0897.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 74.74. Valencia VEY, Canto-Cetina T, Romero AJF, Coral-Vázquez RM, Canizales-Quinteros S, Coronel A, et al. Analysis of polymorphisms in interleukin-10, interleukin-6, and interleukin-1 receptor antagonist in Mexican-Mestizo women with pre-eclampsia. Genet Test Mol Biomarkers. 2012; 16: 1263-1269. doi: 10.1089/gtmb.2012.0181 [DOI] [PMC free article] [PubMed]; Valencia VEY, Canto-Cetina T, Romero AJF, Coral-Vázquez RM, Canizales-Quinteros S, Coronel A. Analysis of polymorphisms in interleukin-10, interleukin-6, and interleukin-1 receptor antagonist in Mexican-Mestizo women with pre-eclampsia. Genet Test Mol Biomarkers. 2012;16:1263–1269. doi: 10.1089/gtmb.2012.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.75. Schmid M, Haslinger P, Stary S, Leipold H, Egarter C, Grimm C. Interleukin-1 beta gene polymorphisms and preterm birth. Eur J Obstet Gynecol Reprod Biol. 2012; 165(1): 33-6. doi: 10.1016/j.ejogrb.2012.07.013. [DOI] [PubMed]; Schmid M, Haslinger P, Stary S, Leipold H, Egarter C, Grimm C. Interleukin-1 beta gene polymorphisms and preterm birth. Eur J Obstet Gynecol Reprod Biol. 2012;165(1):33–36. doi: 10.1016/j.ejogrb.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 76.76. Nair RR, Khanna A, Singh K. Association of interleukin 1 receptor antagonist (IL1RN) gene polymorphism with recurrent pregnancy loss risk in the North Indian Population and a meta-analysis. Mol Biol Rep. 2014; 41(9): 5719-27. doi: 10.1007/s11033-014-3443-8. [DOI] [PubMed]; Nair RR, Khanna A, Singh K. Association of interleukin 1 receptor antagonist (IL1RN) gene polymorphism with recurrent pregnancy loss risk in the North Indian Population and a meta-analysis. Mol Biol Rep. 2014;41(9):5719–5727. doi: 10.1007/s11033-014-3443-8. [DOI] [PubMed] [Google Scholar]
- 77.77. Leme GLP, Menezes FE, Mendonca C, Barreto I, Alvim-Pereira C, Alvim-Pereira F, et al. Analysis of association of clinical aspects and IL1B tagSNPs with severe preeclampsia. Hypertens Pregnancy. 2016; 35: 112-122. doi: 10.3109/10641955.2015.1116554 [DOI] [PubMed]; Leme GLP, Menezes FE, Mendonca C, Barreto I, Alvim-Pereira C, Alvim-Pereira F. Analysis of association of clinical aspects and IL1B tagSNPs with severe preeclampsia. Hypertens Pregnancy. 2016;35:112–122. doi: 10.3109/10641955.2015.1116554. [DOI] [PubMed] [Google Scholar]
- 78.78. Pontillo A, Reis EC, Bricher PN, Vianna P, Diniz S, Fernandes KS, et al. NLRP1 L155H polymorphism is a risk factor for preeclampsia development. Am J Reprod Immunol. 2015; 73: 577-581. doi: 10.1111/aji.12353 [DOI] [PubMed]; Pontillo A, Reis EC, Bricher PN, Vianna P, Diniz S, Fernandes KS. NLRP1 L155H polymorphism is a risk factor for preeclampsia development. Am J Reprod Immunol. 2015;73:577–581. doi: 10.1111/aji.12353. [DOI] [PubMed] [Google Scholar]
- 79.79. Kang L, Chen C-H, Yu C-H, Chang C-H, Chang F-M. Interleukin-1ß gene is not associated with preeclampsia in Taiwanese. Taiwanese J Obtet Gynecol. 2012; 51(2): 240-4. Doi: 10.1016/j.tjog.2012.04.014 [DOI] [PubMed]; Kang L, Chen C-H, Yu C-H, Chang C-H, Chang F-M. Interleukin-1ß gene is not associated with preeclampsia in Taiwanese. Taiwanese J Obtet Gynecol. 2012;51(2):240–244. doi: 10.1016/j.tjog.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 80.80. Mahley RW, Innerarity TL, Rall Jr SC, Weisgraber KH. Plasma lipoproteins: Apolipoprotein structure and function. J Lipid Res. 1984; 25(12): 1277-94. [PubMed]; Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins Apolipoprotein structure and function. J Lipid Res. 1984;25(12):1277–1294. [PubMed] [Google Scholar]
- 81.81. Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12(Suppl 1):31-7. doi:10.1007/s11789-017-0084-1 [DOI] [PMC free article] [PubMed]; Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12(Suppl 1):31–37. doi: 10.1007/s11789-017-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.82. Oram JF, Yokoyama S. Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J Lipid Res 1996; 37(12):2473-91. [PubMed]; Oram JF, Yokoyama S. Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J Lipid Res. 1996;37(12):2473–2491. [PubMed] [Google Scholar]
- 83.83. Charlton F, Bobek G, Stait-Gardner T, Price WS, Mirabito CKM, Xu B, et al. The protective effect of apolipoprotein in models of trophoblast invasion and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2017; 312(1): R40-R48. doi:10.1152/ajpregu.00331.2016 [DOI] [PubMed]; Charlton F, Bobek G, Stait-Gardner T, Price WS, Mirabito CKM, Xu B. The protective effect of apolipoprotein in models of trophoblast invasion and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2017;312(1):R40–R48. doi: 10.1152/ajpregu.00331.2016. [DOI] [PubMed] [Google Scholar]
- 84.84. Rosing U, Samsioe G, Olund A, Johansson B, Kallner A. Serum levels of apolipoprotein A-I, A-II and HDL-cholesterol in second half of normal pregnancy and in pregnancy complicated by pre-eclampsia. Horm Metab Res. 1989;21(7):376-82. doi: 10.1055/s-2007-1009242 [DOI] [PubMed]; Rosing U, Samsioe G, Olund A, Johansson B, Kallner A. Serum levels of apolipoprotein A-I, A-II and HDL-cholesterol in second half of normal pregnancy and in pregnancy complicated by pre-eclampsia. Horm Metab Res. 1989;21(7):376–382. doi: 10.1055/s-2007-1009242. [DOI] [PubMed] [Google Scholar]
- 85.85. Castillo AI, Armas RNB, Dueñas HA, González GOR, Arocha MC, Castillo GA. Riesgo cardiovascular según tablas de la OMS, el estudio Framingham y la razón apolipoproteína B/apolipoproteína A1. Rev Cubana Invest Biomed. 2010; 29(4): 479-88.; Castillo AI, Armas RNB, Dueñas HA, González GOR, Arocha MC, Castillo GA. Riesgo cardiovascular según tablas de la OMS, el estudio Framingham y la razón apolipoproteína B/apolipoproteína A1. Rev Cubana Invest Biomed. 2010;29(4):479–488. [Google Scholar]
- 86.86. Bayhan G, Koçyigit Y, Atamer A, Atamer Y, Akkus Z. Potential atherogenic roles of lipids, lipoprotein(a) and lipid peroxidation in preeclampsia. Gynecol Endocrinol. 2005;21(1):1-6. doi:10.1080/09513590500097382 [DOI] [PubMed]; Bayhan G, Koçyigit Y, Atamer A, Atamer Y, Akkus Z. Potential atherogenic roles of lipids, lipoprotein(a) and lipid peroxidation in preeclampsia. Gynecol Endocrinol. 2005;21(1):1–6. doi: 10.1080/09513590500097382. [DOI] [PubMed] [Google Scholar]
- 87.87. Zhang H, Zhang Y, Yang F, Lius S, Xu Z, Wang J, et al. Complement component C4A and apolipoprotein A-I in plasmas as biomarkers of the severe, early-onset preeclampsia. Mol Biosyst. 2011;7(8):2470-9. doi:10.1039/c1mb05142c [DOI] [PubMed]; Zhang H, Zhang Y, Yang F, Lius S, Xu Z, Wang J. Complement component C4A and apolipoprotein A-I in plasmas as biomarkers of the severe, early-onset preeclampsia. Mol Biosyst. 2011;7(8):2470–2479. doi: 10.1039/c1mb05142c. [DOI] [PubMed] [Google Scholar]
- 88.88. Carr SS, Hooper AJ, Sullivan DR, Burnett JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. 2019;51(2):148-54. doi:10.1016/j.pathol.2018.11.006 [DOI] [PubMed]; Carr SS, Hooper AJ, Sullivan DR, Burnett JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. 2019;51(2):148–154. doi: 10.1016/j.pathol.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 89.89. Var A, Kuscu NK, Koyuncu F, Uyanik BS, Onur E, Yildirim Y, Oruç S. Atherogenic profile in preeclampsia. Arch Gynecol Obstet. 2003;268(1):45-7. [DOI] [PubMed]; Var A, Kuscu NK, Koyuncu F, Uyanik BS, Onur E, Yildirim Y, Oruç S. Atherogenic profile in preeclampsia. Arch Gynecol Obstet. 2003;268(1):45–47. doi: 10.1007/s00404-002-0317-4. [DOI] [PubMed] [Google Scholar]
- 90.90. Serrano NC, Guio-Mahecha E, Quintero-Lesmes DC, Becerra-Bayona S, Paez MC, et al. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis. 2018; 276:189-94. doi:10.1016/j.atherosclerosis.2018.05.051 [DOI] [PubMed]; Serrano NC, Guio-Mahecha E, Quintero-Lesmes DC, Becerra-Bayona S, Paez MC. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis. 2018;276:189–194. doi: 10.1016/j.atherosclerosis.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 91.91. Timur H, Korkut DH, Kara O, Kirbas A, Inal HA, Gencosmanoglu TG, et al. A study of serum Apo A-1 and Apo B-100 levels in women with preeclampsia. Pregnancy Hypertens. 2016; 6(2):121-5. DOI: 10.1016/j.preghy.2016.04.003 [DOI] [PubMed]; Timur H, Korkut DH, Kara O, Kirbas A, Inal HA, Gencosmanoglu TG. A study of serum Apo A-1 and Apo B-100 levels in women with preeclampsia. Pregnancy Hypertens. 2016;6(2):121–125. doi: 10.1016/j.preghy.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 92.92. Kharb S, Bala J, Nanda S. Markers of obesity and growth in preeclamptic and normotensive pregnant women. J Obstet Gynaecol. 2017;37(5):610-5. doi:10.1080/01443615.2017.1286463 [DOI] [PubMed]; Kharb S, Bala J, Nanda S. Markers of obesity and growth in preeclamptic and normotensive pregnant women. J Obstet Gynaecol. 2017;37(5):610–615. doi: 10.1080/01443615.2017.1286463. [DOI] [PubMed] [Google Scholar]
- 93.93. Bora K, Pathak MS, Borah P, Hussain I, Das D. Association of the apolipoprotein A-I gene polymorphisms with cardiovascular disease risk factors and atherogenic indices in patients from Assam, Northeast India. Balkan J Med Genet. 2017; 20(1):59-70. doi: 10.1515/bjmg-2017-0002 [DOI] [PMC free article] [PubMed]; Bora K, Pathak MS, Borah P, Hussain I, Das D. Association of the apolipoprotein A-I gene polymorphisms with cardiovascular disease risk factors and atherogenic indices in patients from Assam, Northeast India. Balkan J Med Genet. 2017;20(1):59–70. doi: 10.1515/bjmg-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.94. Ou HJ, Huang G, Liu W, Ma XL, Wei Y, Zhou T, et al. Relationship of the APOA5/A4/C3/A1 gene cluster and APOB gene polymorphisms with dyslipidemia. Genet Mol Res. 2015;14(3):9277-90. doi:10.4238/2015.August.10.8 [DOI] [PubMed]; Ou HJ, Huang G, Liu W, Ma XL, Wei Y, Zhou T. Relationship of the APOA5/A4/C3/A1 gene cluster and APOB gene polymorphisms with dyslipidemia. Genet Mol Res. 2015;14(3):9277–9290. doi: 10.4238/2015.August.10.8. [DOI] [PubMed] [Google Scholar]
- 95.95. Chen L, Wei P, Jiang K, Xia XX. Apolipoprotein A1 and neuronal nitric oxide synthase gene polymorphisms and hormone-related osteonecrosis of the femoral head. Eur Rev Med Pharmacol Sci. 2017;21(14):3159-63. [PubMed]; Chen L, Wei P, Jiang K, Xia XX. Apolipoprotein A1 and neuronal nitric oxide synthase gene polymorphisms and hormone-related osteonecrosis of the femoral head. Eur Rev Med Pharmacol Sci. 2017;21(14):3159–3163. [PubMed] [Google Scholar]
- 96.96. Buurma AJ, Turner RJ, Driessen JHM, Mooyaart AL, Schoones JW, Bruijn JA, Bloemenkamp KWM, Dekkers OM, Baelde HJ. Genetic variants in pre-eclampsia: a meta-analysis, Hum Reprod Update. 2013; 19(3):289-303. doi: 10.1093/humupd/dms060 [DOI] [PubMed]; Buurma AJ, Turner RJ, Driessen JHM, Mooyaart AL, Schoones JW, Bruijn JA, Bloemenkamp KWM, Dekkers OM, Baelde HJ. Genetic variants in pre-eclampsia a meta-analysis, Hum Reprod. Update. 2013;19(3):289–303. doi: 10.1093/humupd/dms060. [DOI] [PubMed] [Google Scholar]
- 97.97. Sandoval J, Salazar-Granara A, Acosta O, Castillo-Herrera W, Fujita R, Pena SDJ, et al. Tracing the genomic ancestry of Peruvians reveals a major legacy of pre-Columbian ancestors. J Hum Genet 2013; 58(9):627-34. doi: 10.1038/jhg.2013.73. [DOI] [PubMed]; Sandoval J, Salazar-Granara A, Acosta O, Castillo-Herrera W, Fujita R, Pena SDJ. Tracing the genomic ancestry of Peruvians reveals a major legacy of pre-Columbian ancestors. J Hum Genet. 2013;58(9):627–634. doi: 10.1038/jhg.2013.73. [DOI] [PubMed] [Google Scholar]