Abstract
While the time window for reperfusion after ischemic stroke continues to increase, many patients are not candidates for reperfusion under current guidelines that allow for reperfusion within 24 h after last known well time; however, many case studies report favorable outcomes beyond 24 h after symptom onset for both spontaneous and medically induced recanalization. Furthermore, modern imaging allows for identification of penumbra at extended time points, and reperfusion risk factors and complications are becoming better understood. Taken together, continued urgency exists to better understand the pathophysiologic mechanisms and ideal setting of delayed recanalization beyond 24 h after onset of ischemia.
Keywords: Delayed recanalization, penumbra, perfusion, Stroke, tPA
Introduction: State of current penumbra doctrine
The time window for clinical reperfusion after ischemic stroke continues to increase. Although originally at ∼3 h, it has now been extended to as long as 24 h following known onset of symptoms.1
The ischemic penumbra has been defined with the following criteria: (1) the tissue region must have an increased oxygen extraction fraction; (2) the functional outcome needs to correlate with the concept of penumbra, e.g., acute functional impairment, and subsequent improvement with reperfusion; (3) cerebral blood flow is approximately 18-20 ml/100g per minute compared to <10 ml/100g per minute in the infarct; (4) mismatch between diffusion (DWI) and perfusion weighted imaging (PWI) and the patient’s neurological function.2 Given that ischemia results in cellular ion pump failure, DWI specifically demonstrates these slow diffusion regions of water protons as hyperintensity; therefore, DWI is a marker of ischemia and used to mark the original infarct core.2 In PWI, an intravenous bolus is injected with a contrast agent that transiently reduces its signal as it passes through the plain, and therefore this signal occurs later in ischemic tissue compared to normal tissue.2 The mismatch between DWI and PWI represents salvageable tissue at risk of irreversible damage if reperfusion does not take place.
Reported computed tomography (CT) perfusion thresholds for defining penumbra and core volume vary between studies. Some studies differ by defining penumbra with mean transit time (MTT) versus time-to-maximum (Tmax).3–6 Other studies differ by defining core infarct volume with cerebral blood flow (CBF) versus cerebral blood volume (CBV).5,7–9 Lin et al, 2016 found that CT perfusion, when compared to magnetic resonance imaging (MRI), can most accurately identify ischemic volume and core volume by using a delay time greater than 3 seconds (P = 0.34) and a delay-corrected cerebral blood flow of less than 30% (P = 0.33), respectively. When using limited-coverage CT perfusion, ischemic volume accuracy declined when brain coverage was reduced to 80 mm (P = 0.04); core volume accuracy declined when brain coverage was 40 mm or less (P < 0.001).10 As another example, tissue at risk for infarct occurs with a CBF of 8-22 ml/100g/min (approximately 15-40% of normal); while, ischemic core can be estimated with a CBF of less than 8 ml/100g/min.11
Currently, the most metabolically accurate way to classify and determine the penumbra volume is through positron emission tomography (PET), which can distinguish between at-risk tissue that has an increased rate of oxygen extraction compared to normal tissue. However, due to its limitations in emergency settings, MRI and CT are the current clinical standard for identification of salvageable penumbra.12 CT perfusion imaging is used to calculate an area of reduced cerebral blood volume or reduced cerebral blood flow which estimates the size of the core. CT perfusion is also used to calculate the mean transit time– the length of time it takes for blood to flow from arterioles through capillary beds to venous systems. To estimate the penumbra volume, the volume of central nervous system tissue with delayed mean transit time must be subtracted from the area of reduced cerebral blood volume and/or reduced cerebral blood flow. This mismatch area, if large enough, justifies treatment with revascularization techniques, increasing the likelihood of a favorable outcome.5,7–9,13 Because recanalization in patients with mismatch results in favorable outcome, this implies that the penumbra tissue can survive for hours after stroke onset with only limited perfusion, often by collateral blood vessels.14 However, if the mismatch/penumbra is too small, then the benefits of reperfusion are outweighed by the risks, such as hemorrhage. The axiom that critically hypoperfused tissue progresses to infarct core drives the need for continued refinement of the balance between intervention related risk and the benefit of penumbra targeted intervention.
Reperfusion rationale
In hypoperfusion or extinction of blood flow, tissue survival is the determinant in treatment approaches. Tissue at risk has been shown to survive for hours to days in a salvageable state, regaining function if flow is restored, or losing function if hypoperfusion continues.12 Therefore, as the only FDA approved intervention, clinical trials have focused on the following reperfusion strategies: systemic intravenous tPA tenecteplase, desmoteplase, combined ultrasound-induced mechanical agitation (2 MHz frequency) and systemic tPA, and the use of endovascular multi-modal approaches such as mechanical thrombectomy or thromboaspiration with stent retrievers.15 Although classical stroke intervention targets a 4–6 h window, evidence has shown that lesion volumes may continue to increase if recanalization does not occur. In support, when evaluating the evolution of injury at day one and day 60 from symptom onset, lesion volumes were shown to increase when no recanalization occurred, whereas recanalized groups demonstrated no subsequent lesion growth.16
At the 2016 Stroke Treatment Academic Industry Roundtable (STAIR) IX meeting that discussed the current state of endovascular therapy for large vessel stroke, note was made of the need to prioritize investigation of adjuvant therapies and evaluate the potential for expansion of patient endovascular therapy eligibility. Current weaknesses in stroke response include underutilization of thrombectomy and in other cases triage of patients to the most appropriate initial facility. Furthermore, when compared to ST-segment elevation myocardial infarction, endovascular stroke response is slower, suggesting room for improvement. One method of improvement could be development of newer, more effective thrombectomy devices. Also, co-administered therapies with thrombectomy, such as early neuroprotectants, hypothermia, and stem cells, show promise for stroke treatment.17
Many limitations exist in monotherapies in the acute time window of ischemic stroke, and with the expansion of the time-window for reperfusion due to more effective thrombectomy devices, new opportunities for stroke therapies are emerging. Individuals from academia and industry who convened at the STAIR X meeting concluded that cerebroprotective therapies in combination with reperfusion will improve the efficacy of stroke treatment and may be the next step in stroke intervention. Savitz et al. discuss potential cytoprotection therapies in three main contextes: one, prehospital cytoprotection to increase the percentage of reperfusion eligible patients arriving at a thrombectomy center; two, in hospital pre-thrombectomy cytoprotection which may reduce the deleterious consequences of ischemia and reperfusion; and three, post-thrombectomy cytoprotection treatment that can target reperfusion injury and delayed cell death.18 Treatments targeting the whole neurovascular unit which have already passed safety profiles in phase II or III clinical trials may be better candidates in the aforementioned scenarios.18
Despite the risk of hemorrhagic transformation and vasogenic edema following recanalization, endovascular thrombectomy (EVT) has been proven effective in patients with proximal large artery occlusions beyond the acute time window.19 Evaluating the MR CLEAN trial, Kimberly et al. reported that early reperfusion and recanalization via EVT reduced brain edema.20 However, additional studies are needed to understand the association between reperfusion and brain edema at recanalization outside the acute time-window.
Given that there is a wide range for prognosis after reperfusion, literature has supported the development of a more standardized system of image-based criteria for patient selection undergoing revascularization in acute and extended time-windows.14,21–23 Rationale for altering recanalization guidelines in stroke patients is supported by the observed benefits from delayed recanalization (outside 6 h) in patients with a small completed infarct and a large area of perfusion mismatch– resulting in minimal intracerebral hemorrhage compared to patients that undergo recanalization of mostly infarcted tissue.21 Leonard et al. reported that recruitment of collateral channels significantly limited infarct growth from reaching maximum expansion independent of recanalization subgroup.24
Even though collateral circulation is one way to sustain the ischemic penumbra, the effectiveness of leptomeningeal collaterals is dynamic and variable between stroke patients; congenital differences contribute to a spectrum of anomalous primary and secondary collaterals supplied by the Circle of Willis and leptomeningeal vessels.24,25 For this reason, two explanations exist to explain why delayed collateral recruitment leads to worsened outcomes in non-perfused patients: one, when both maximal vasodilation in the ischemic area and collateral recruitment in the adjacent regions becomes inadequate for sustaining cellular survival, the ischemic tissue undergoes “venous steal”– a collateral failure phenomenon that results in the expansion of the ischemic core; on the other hand, autoregulatory failure in the ischemic vascular territory may lead to damage from increased hyperperfusion accompanied by a higher incidence of bleeding.24,26 Taken together, delayed collateral recruitment occurs efficaciously after recanalization and can be detrimental without it.24
In multicenter clinical trials, infarct volume correlates moderately with standard clinical measures of stroke outcome. Likely, the most important factor disrupting this correlation is infarct location.27 In acute ischemic stroke patients with anterior circulation proximal artery occlusions, final infarct volume (FIV) was shown to be a surrogate endpoint as it significantly correlated to 3-month functional outcome and survival. In detail, FIV was associated with functional outcomes (modified Rankin Scale) and survival, in which a FIV of 50 cm3 displayed reliability in distinguishing good versus poor outcome, whereas a FIV of 90 cm3 was explicit for poor outcome.28 In patients with acute ischemic MCA stroke, infarct volume (0.2 to 187 cm3) was significantly correlated with short-term outcomes [length of hospitalization (r = 0.67)] and long-term outcomes [Glasgow Outcome Scale (r = 0.68), Barthel Index (r = 0.67), and outcome status (r = 0.65)] (P < 0.001).29
Delayed reperfusion: Research/cases
In part due to limitations in available therapies, endogenously spontaneous recanalization has fortuitously demonstrated efficacy at atypical timepoints following ischemic stroke. For example, Vang et al. reported that if spontaneous recanalization was observed within 24 h, it resulted in better central motor conduction times in 87% of the patients; furthermore, if spontaneous recanalization occurred after 24 h, better central motor conduction times were also observed in 62% of patients.30 Therefore, although earlier recanalization shows higher efficacy, patients still report a significant benefit at delayed time-points (Table 1 and Figure 1). With the option for no recanalization versus delayed recanalization, evidence suggests that delayed recanalization may be the better option.
Table 1.
Literature search on delayed recanalization (>24 h).
| Author (Year) | Affected vessel | Occlusion duration | Successful recanalization rate, % (N) | Outcome |
|---|---|---|---|---|
| Zhang et al.39 | ICA | 17–120 days | 100% (30) | mRS score of 2.5 ± 0.6 at 3 months |
| Ishihara et al.78 | ICA | 22 days | 100% (1) | Preoperative mini-mental score improved from 23/30 to 28/30 |
| Thomas et al.79 | ICA | 0.75 months | 100% (2) | Asymptomatic at 30 days |
| Kao et al.80 | ICA | 3.5 ± 3.5 months | 73% (22) | Recanalized patients were asymptomatic at 6 months |
| Lin et al.81 | ICA | 7.9 ± 10.9 months | 65% (35) | No change in NIHSS at baseline, 1, and 3 month follow up |
| Bhatt et al.82 | ICA | 9 months | 100% (1) | Neurologically intact at 9 months |
| Terada et al.83 | ICA | 5.1 ± 3.6 months | 93% (14) | Neurological symptoms were transiently aggravated but returned to baseline after 30 days |
| Shojima et al.84 | ICA | 19.4 ± 7.3 months | 88% (7) | Recanalized patients were asymptomatic |
| Iwata et al.85 | ICA | 4 ± 0.8 months | 100% (4) | Pre-CAS CVR was impaired at 3 months |
| Namba et al.86 | ICA | 4.3 ± 4.2 months | 77.2% (8) | 64% (7) had new DWI hyperintensities |
| Rostambeigi et al.87 | ICA | 1 month | 100% (1) | Asymptomatic at 3 months |
| Fan et al.88 | ICA | 21 ± 6.7 months | 89% (16) | Improvement in cognitive function of ≥8 on the MoCA at 1, 3, and 6 months |
| Bigliardi et al.89 | ICA | 3 months | 62% (85) | The patient regained autonomy at 3 and 6 months |
| Hasan et al.40 | ICA | 2.4 ± 3 months | 69% (22) | 65% (20) experienced improved neurological outcome at 2–6 months |
| Zhang et al.39 | ICA | 0.5-4 months | 100% (30) | mRs 2.5 ± 0.6 at 3 months |
| Vang et al.30 | MCA | >24 h | 38% (21) Spontaneous Recanalization | 62% (13) had improved CMCT |
| Ma et al.36 | MCA | ∼60 days | 100% (2) | Asymptomatic |
| Zheng et al.37 | MCA | 15–29 days | 95% (21) | 86% achieved a mRS of 0–2 |
| Yamamoto et al.32 | PCA | NA | 100% (1) | Resolution of homonymous hemianopia |
| Zhao et al.38 | BA | >2 days | 81% (17) | 77% achieved a mRS score of 0–3 |
| Grigoriadis et al.42 | BA | 50 h | 100% (1) | Fully restored neurologic function |
| Cognard et al.44 | BA | 36 h | 100% (1) | Complete recovery |
| Cross et al.45 | BA | 1–79 h | 50% (10) | 20% (4) mRS of 0–2 and Barthel index of 95–100 |
| Xavier et al.73 | MCA, VBJ, ICA | 10–169 h | NA | 92% (11) achieved a TIMI ≥2 |
| Abou-Chebl et al.21 | MCA, ICA, tandem ICA/MCA, VBJ | 18.6 ± 16.0 h | 85% (21) | No difference in ICH rate; 56% mRS 0–2; 86% TIMI 2-3 |
| Ishibashi et al.77 | MCA, ACA | 25–54 h | 100% (5) | 50% (2) mRS of 0 in MCA; 100% (1) mRS of 0 in ACA |
Note: Continuous variables listed as mean ± standard deviation or range. Counts listed as percent (number).
CMCT: central motor conduction time; TIMI: thrombolysis in myocardial infarction; ACA: anterior cerebral artery; ICA: internal carotid artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; VBJ: vertebrobasilar junction; BA: basilar artery; mRS: modified Rankin Score; MoCA: Montreal Cognitive Assessment; Pre-CAS CVR: pre-cerebral artery stenting cerebral vascular reserve; NA: not available.
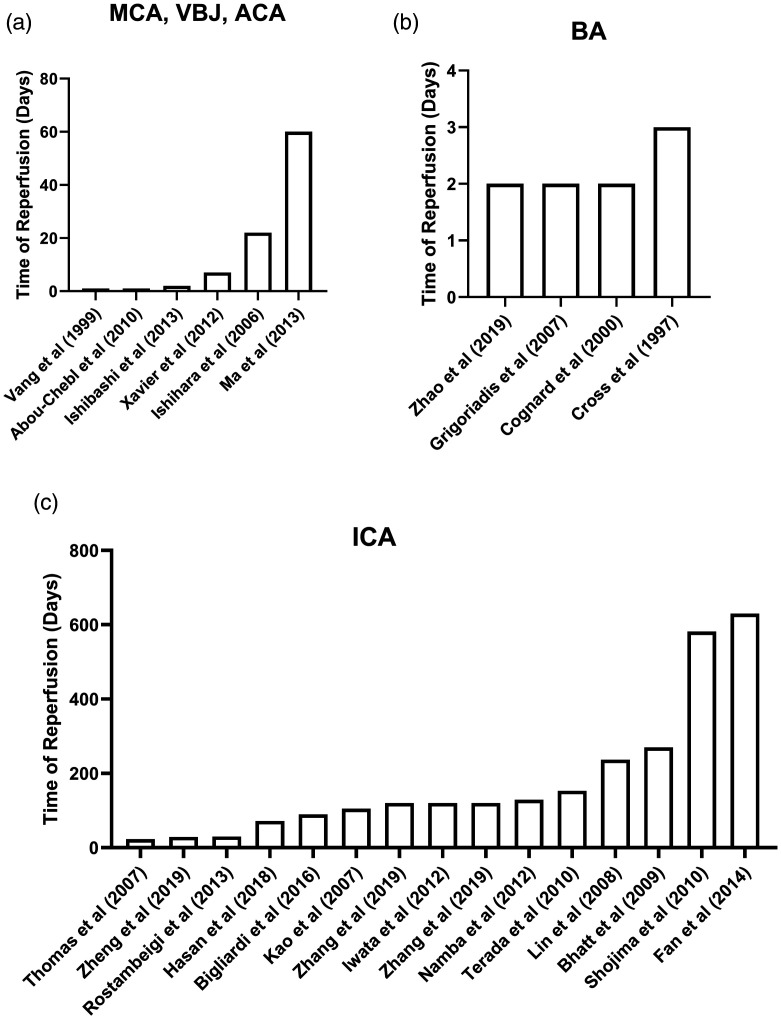
Figure 1.
Time of reperfusion in stroke per study. Histogram of the highest reported time of reperfusion in days for each stroke study discussed: (a) ICA (internal carotid artery), MCA (middle cerebral artery), and VBJ (vertebrobasilar junction); (b) BA = basilar artery; (c) ICA (internal carotid artery).
Because the number of patients receiving recanalization after stroke is low with the largest variable being delayed admission times, a more lenient time window for intervention along with a multi-modal therapeutic approach using stent placement, a thrombectomy device, and/or combination treatment in the acute posterior circulation may lead to higher recanalization rates, favorable outcomes, and rescue of the penumbra.31 In an example of an acute isolated posterior cerebral artery occlusion, Yamamoto et al. reported that endovascular therapy reduced hyperintensity on DWI and improved the homonymous hemianopia. This is somewhat surprising as it suggests that ischemic penumbra may have some overlap with the predicted region of irreversible ischemia on DWI imaging.32
Conversely, the most significant prognostic indicator arguing against recanalization focuses on mortality rates. In a clinical study on the efficacy and safety of mechanical thrombectomy (MT) in elderly patients, no significant difference was seen between those treated within and those treated beyond 8 h (39 vs. 20%, p = 0.1), paralleling the results from the recently completed DAWN study which focused on MT up to 24 h after symptom onset.23 Similarly, others have shown that the mortality rate in patients greater than 80 treated with mechanical thrombectomy beyond 8 h of symptom onset was not significantly higher than patients treated within 8 h of symptom onset (56% versus 32.4%, p = 0.055); although not statistically significant, the mortality number was certainly higher among the group with an initial moderate disability compared to the group with mild disability.33 Because of the small sample size, the lack of significance in mortality rates may be attributed to a statistical type-2 error, and additional studies are needed with a higher power to establish if mortality rates are significantly increased due to recanalization outside of 8 h from stroke onset.
If recanalization procedures fail or are not an option due to delayed admission times, generally poorer outcomes, as expected, are reported. In contrast, even with delayed intervention, improved outcomes have been reported. In evaluating early versus late recanalization at 7 days after MCA occlusion, the final infarct volume was 80 cm3 in patients with recanalization under 6 h, 93 cm3 in those recanalized beyond 6 h, and 194 cm3 in those without recanalization.34 Anecdotally in a case study, a 55-year old male suffered global aphasia and right side hemiplegia, resulting from a left proximal MCA occlusion, and after intravenous thrombolysis, the NIHSS improved from 11 to 4 points.35 However, after 7 h, the NIHSS scores increased from 4 to 13 points, and endovascular thrombectomy was performed to remove the remaining clot around 11 h after symptom onset, resulting in an unexpected NIHSS score of 3 at discharge and a modified Rankin Scale score of 0 at 3 months.35 Therefore, failed recanalization after administration of intravenous rtPA may be salvaged by immediate EVT of the large artery occlusion and result in a more favorable outcome compared to patients without intervention.
In two case reports of patients with chronic right MCA occlusion presenting with a 2-month history of TIA of left-sided weakness, recanalization was successful in both via stenting: one patient remained asymptomatic at the 29-month follow-up, while the other patient developed symptomatic in-stent restenosis at 12-months which was resolved with repeat angioplasty.36 In 22 patients, stenting in non-acute symptomatic atherosclerotic MCA total occlusion resulted in ∼95% successful recanalization at 15–29 days after symptom onset, of which ∼86% achieved a favorable outcome (mRS score of 0–2).37
In 21 patients with basilar artery occlusion (BAO), endovascular recanalization performed beyond 48 h from time last known well resulted in successful recanalization in 81%. At 3-months, one death occurred (pneumonia). No recurrent cases of TIA or stroke ensued, and 77% of patients demonstrated a favorable outcome (mRS scores 0-3). Time between initial symptoms and treatment ranged from 13 to 365 days, while time between image-documented BAO and treatment ranged from 6 to 43 days. This report included 21 patients who were endovascularly recanalized at >2 days (a medium time to treatment of 27 days). Of note, the time between initial symptoms and recanalization was 365 days for 2 patients. Of these two patients, recanalization improved mRS for one of two patients (mrS score improved from 3 to 0 in 90 days after treatment). Although proposed but not quantified, favorable outcomes in delayed reperfusion time points (especially 365 days) may depend on the variable extent of collateralization in each patient and his/her ability to maintain blood flow to the penumbra.38
Evaluating a hybrid operation (endarterectomy and carotid stenting) to recanalize internal carotid artery occlusions, this hybrid procedure implemented at an average of 41 days (ranging from 17-120 days) after symptom onset resulted in 100% recanalization and mRS scores improved from 3.4 ± 0.6 at admission to 2.5 ± 0.6 at 3-months from symptom onset.39
In evaluating internal carotid artery strokes, Hasan et al. recommend a classification system on occlusion type to better evaluate the efficacy of endovascular therapy: type A, a tapered occlusion with supraclinoid reconstitution of the ICA; type B, an abrupt occlusion with a supraclinoid reconstitution; type C, a completely absent ICA from the bifurcation with supraclinoid reconstitution; and type D, a completely absent ICA from the bifurcation without supraclinoid reconstitution.40 Using these classifications of stroke subgroups, in a review of literature on chronically occluded internal carotid artery patients, Zanaty et al. reported that reperfusion (ranging from 15-days to 840-days) via endovascular technique (ET) or hybrid surgery (HS, i.e., ET plus carotid endarterectomy) resulted in the following efficacy: in type A and B, recanalization rates were 95% with a 14% complication rate; in type C, ET resulted in a recanalization rate of 46% with a complication rate of 46%, while HS resulted in a recanalization rate of 88% with a complication rate of 14%; and in type D, a recanalization rate of 29% with a 30% complication rate.41 In successful recanalization, intervention sustained the penumbra, normalized mean transit time, and improved Montreal cognitive assessment scores.
Investigating atypical delayed recanalization timepoints, in a case study of a 6-year old boy with acute BAO, complete clinical recovery was achieved after recanalization at 50 h from symptom onset, and one month after the procedure, the patient’s strength in both arms and legs fully recovered.42 Although adults with basal artery occlusion incur a mortality rate up to 90%, outlying examples of adolescent patients treated with delayed-recanalization, in the temporal window of 2043 to 36 h,44 report markedly favorable outcomes. This subgroup offers unique insights into potential interventional targets. For this reason, research is needed to better understand such cases where late recanalization results in an ironic positive outcome. Also, recanalization in adults with BAO was successful in 50% of patients, 60% of which survived and 30% of which had good neurological outcomes; two patients received intervention at 72 and 79 h after BAO and surprisingly reported a Modified Rankin Score of 2 at three months after intervention.45
Taken together, recanalization has been shown effective at different timepoints following occlusion in stroke; the variable and in some cases high efficacy of delayed recanalization merits further investigation to understand optimized interventional targets for specific occlusion-types and sub-populations. Recanalization up to 72 h and beyond, depending on the stroke type, has shown efficacy and improved functional outcome in animal models as well as patients. In addition to the DAWN46 and DEFUSE47 trials supporting the extension of recanalization to 24 h, mounting evidence from case studies and basic science research has supported the re-evaluation of interventions even beyond a 24 h window.
Variables affecting reperfusion
Numerous variables dictate the rate of successful reperfusion intervention. For instance, if the duration of reperfusion procedures extended past 60 min, patients had significantly higher mortality and disability rates compared to patients in whom recanalization was performed in less than 60 min.48 The time window for intervention with tPA is in part dependent on the evolution of clot lysability; initially, low levels of thrombin form a porous fibrin scaffold, which is easily dissolved by tPA. Then, after fibrin accumulation and increased crosslinking via factor XIII (typically after 150 min from symptom onset), the thrombus stabilizes and creates a compact interface, less porous and more resilient to tPA.49 Therefore, MT and other methods of recanalization may be prefered outside of the acute time frame for IV tPA. Not only does pathophysiology depend on the age of a clot but also on the origin. For example, when evaluating recanalization rates in different types of thrombotic/embolic ischemia, independent of the organ, coronary artery thrombi were recanalized at a rate of 90% compared to 52% in other stroke subtypes (large artery and undefined origin occlusions).50 Generally, the standard criteria for thrombolysis exclude patients with unclear timing of stroke onset, but using positive perfusion-diffusion mismatch and absence of well-developed fluid-attenuated inversion recovery changes of acute diffusion lesions, Cho et al. reported that reperfusion, either via intravenous tPA or mechanical clot disruption coupled with urokinase (<6 h), resulted in no detectable differences in rates of recanalization at 5-days, early neurological improvement, 3-month modified Rankin Scale scores, and symptomatic ICH compared to clear time of onset stroke subjects.51
When recanalization cannot be achieved, the OPTIMAL study, a prospective trial evaluating the penumbra inside 12 h from symptom onset, reported that clinical outcome is dependent on the early improvement in penumbra perfusion from collateral vessels.52 Along these lines, high arterial blood pressure, a product of cardiac output and systemic vascular resistance, was shown to reduce infarction volume and improve functional outcome because penumbra perfusion from collaterals was increased compared to patients with lower blood pressure.53 During arterial occlusion, collateral vasculature is recruited, and if sufficient flow is established, flow to the penumbra is restored, thereby reducing the final size of the infarct core. On this basis, if cardiac output is optimized to increase collateral flow to the penumbra, patients potentially may achieve a more favorable outcome.52 For example, in a randomized pilot study, Hillis et al. reported that pharmacologically induced blood pressure elevation, increased collateral flow, and subsequently improved outcome.53 Also, in a case report, increasing blood pressure to enhance collateral flow has shown efficacy in the preservation of the penumbral brain tissue for over several days.54 Taken together, in addition to clot lysis treatments, stroke interventions need to continue to target increased collateral perfusion to reduce ischemic damage.
With 25-50% of patients presenting with hyperglycemia, some of which ironically are not diabetic and have hyperglycemia caused by cortisol and norepinephrine release, Alvarez-Sabin et al. report a significant negative correlation between neurological improvement after recanalization and elevated glucose levels; however, elevated glucose has limited effect on outcomes in patients with delayed recanalization or no recanalization.55 In literature, hyperglycemia may exacerbate infarct injury by increased intracellular acidosis, inflammation, mitochondrial dysfunction, BBB permeability, and bioenergetic failure in the penumbra.55,56
With the risk of negative outcomes after recanalization, researchers have noted that one third of patients in the ER did not improve once recanalization therapy was initiated within 3 h from symptom onset, and four-fifths of this cohort persisted with low neurological scores at 24 h.57 However, by 3-months, recanalized groups with a poor early treatment response reported favorable outcomes, suggesting that the brain may have been “stunned” in the acute phase following ischemia, which may be attributed to strokes of greater severity than imaging estimates, no-reflow phenomenon with or without persisting distal occlusion, proximal re-occlusion, and/or reperfusion injury.57
Of the 30% of patients that are admitted to the emergency department within the acute therapeutic window of 3 h, 7% receive rt-PA and 1·6% receive endovascular therapy.58 Some of the disparity in stroke intervention is due to atypical manifestations, known as “stroke chameleons.” For example, in a retrospective analysis of patients diagnosed with ischemic stroke or TIA, 25-30% of patients with a final diagnosis of ischemic stroke or TIA did not receive the correct admitting diagnosis. Furthermore, most of these patients were misdiagnosed with disorders such as altered skin sensation, alteration of consciousness, or dysarthria, and would be later corrected with more accurate and thorough neurological assessment and neuroimaging.58 Thus, improved time to diagnosis could benefit from further discussion and research of these “stroke chameleons.”
Patient response to thrombectomy is in part the result of fast versus slow expansion of the ischemic core. Variables affecting the rate at which ischemic core expands (fast versus slow) are still poorly understood; however, collateral flow likely plays a critical but incompletely quantified contribution.17 MRI perfusion weighted imaging (PWI) and time to peak (TTP) maps in a dog model support a slow versus fast stroke evolution model. Voxel wise analysis of PWI images at 30 minutes after stroke is able to predict slow versus fast stroke evolution. Slow stroke evolution was defined as less than 50% of final infarct volume at 4 hours, and fast stroke evolution was defined as greater than 50% of final infarct volume at 2 hours. Interestingly, there was no difference in sex, age, weight, or vital signs between slow versus fast evolving stroke groups; although, stroke evolution rate is likely at least in part related to degree of collateralization. The ability to predict variability in infarct evolution will aid in randomization of future stroke studies.59
Dangers of reperfusion: Edema and hemorrhage
In order to accept the paradigm of delayed recanalization beyond the conventional window, risk of intracerebral hemorrhage as well as baseline disability must be considered. Studies have shown endovascular therapy to have a higher rate of success and a lower rate of intracerebral hemorrhage than the conventional intravenous t-PA therapy. For example, 1 week after endovascular intervention for large vessel occlusion in patients originally presenting >8 h after stroke symptom onset, 70% of patients showed a reduction of >4 points in NIHSS scores.60 And, with the presence of a large perfusion mismatch and small ischemic core, a fifty-five patient study– 34 early presenting (3.4 ± 1.6 h) and 21 late presenting (18.6 ± 16.0 h)– reported that delayed time to treatment did not increase ICH, 8.8% early versus 9.5% late (P = 1.0).21 In addition to these findings, in randomized trials of MT (excluding patients over the age of 80 years with a premorbid disability), Slawski et al. evaluated the effects of acute and delayed recanalization and reported a 90-day mortality rate of 38.5% in patients with pre-existing morbidity, slightly up compared to mortality rates in patients without pre-existing morbidity, 28% and 27.5% in the HERMES and STRATIS, respectively.33 Also, when assessing patients treated within 8 h after symptom onset (n = 71) compared to those treated beyond 8 h after symptom onset (n = 25), no detectable differences were noted in the rate of favorable outcome or mortality rate at 90 days between these two groups.33 Given variability in the outcomes of numerous examples of delayed recanalization, optimizing and refinement of standard age range, classification of pre-existing morbidity, and time to treatment may help to optimize post-intervention outcomes.
Given that recanalization may exacerbate injury, a study evaluated the time course of reperfusion associated HT and found that acute recanalization resulted in a hemorrhagic transformation rate of ∼20% compared to ∼53% in delayed recanalization.61 In this study, several classifications of reperfusion induced hemorrhages were used: HI1 as small petechiae, HI2 as more confluent petechiae, PH1 as hematoma involving ≤30% of the infarcted area with some mild space-occupying effect, and PH2 as hematoma involving >30% of the infarcted area with significant mass effect or clot remote from the infarcted area. Of note, HI1-HI2 patients had lower infarct volumes and modified Rankin Scale scores compared to both PHI-PH2 and those without HT.61 When evaluating acute vs late recanalization, hemorrhagic transformation occurred in 43.7% of patients receiving therapy, and in patients with acute recanalization and subsequent hemorrhagic transformation, ∼90% were HI1-HI2, whereas 20% were PH1-PH2. In contrast, in patients with hemorrhagic transformation after late recanalization, only 11% were H1-H2, while 80% were PH1-PH2.61 Because of the potential risk, hemorrhagic transformation due to recanalization requires close monitoring of patients, and given a dearth of efficacious pharmacological therapies, better delineated and/or new therapies, possibly even combination treatments, targeting hemorrhagic transformation are needed to better attenuate some of these deleterious outcomes.
Imaging modalities in stroke
Since hemorrhagic transformation (HT) remains a significant etiology for ischemia-reperfusion injury, studies have suggested that TTP mapping on admission may identify patients at risk of HT after ischemic stroke. For example, when evaluating computing tomography perfusion (CTP) as a predictor for HT in patients receiving reperfusion therapy (ie IV rt-TPA), Shinoyama et al. reported the ability to categorize reperfusion candidates into two groups: high-HT risk and low-HT risk.62 In the low-HT risk group, no TTP map-defect was observed, possibly due to an increased presence of collaterals, increasing their tolerance to ischemia and/or reperfusion injury.62 Also, with delayed recanalization outside the 6 h window after stroke onset, Renu et al. reported that parenchymal hematoma was predicted by CBV and CBF values 2.5% below mean CBV and CBF values.63 But a higher-powered sample size is needed to validate these relationships. Dual-energy CT allows for distinction between contrast staining, suggestive of BBB disruption and brain hemorrhage. Since BBB disruption drives HT and edema formation, dual-energy CT showed strong associations between post-endovascular therapy BBB damage and poor clinical outcomes, as well as contrast staining and delayed HT.64
In a study evaluating recanalization outside the 4.5 h window, investigators reported that delayed recanalization via PCT-guided intravenous rtPA resulted in similar recanalization rates to patients treated inside the acute window; moreover, both hemorrhagic transformation rates and favorable outcomes were also similar among groups.65 Taken together, compared to MRI, PCT is efficient and readily available, and its parameters, such as CBV, MTT, CBF maps, can be easily evaluated; however, this comes at the cost of potentially less brain coverage, increased radiation exposure, and less sensitivity for the detection of minor strokes or stroke mimics.65
Hyperintensity on DWI occurs after a reduction in the apparent diffusion coefficient of water (ADC) and represents a restriction in the diffusional movement of water, resulting from ion pump failure.66 As a complementary technique, PWI displays information about the perfusion of the infarct core as well as the surrounding brain tissue.66 Because PWI-DWI mismatch may not precisely predict the volume and evolution of the penumbra, a retrospective study on patients with acute stroke and a PWI-DWI mismatch demonstrated that TTP-Tmax perfusion delay mismatch volume and FLAIR vascular hyperintensity (FVH) were also associated with the final infarct volume.67 Although some patients exhibit spontaneous resolution of neurological deficits, a significant subpopulation (8 out of 50 [16%]) experienced subsequent worse outcomes; Alexandrov et al. report that transcranial doppler and angiography imaging at the time of admission on all potential stroke patients may identify and confirm patients with a potential occlusion or stenosis independent of neurological symptoms.25 Taken together, evaluation of occlusion and/or stenosis type and size are important factors to address when attempting delayed recanalization in combination with neuroprotectants.
When transcortical collateralization is robust, recanalization may significantly improve outcome and reduce infarct size when administered within 8 h of symptom onset.68 Standard angiograms may be used to confirm robust leptomeningeal collateralization in patients that may benefit from delayed recanalization. For instance, when retrograde leptomeningeal collateral flow is unable to compensate for antegrade perfusion loss, physicians may use angiography to monitor for progressive collateral failure, resulting in infarct expansion.69 Favorable outcome after delayed recanalization may be attributed to the presence of congenital collaterals compared to unfavorable outcome in patients with limited collaterals. With recanalization rates ranging from 30% to 60% of patients in the first 6 to 24 h after t-PA treatment, continuous transcranial doppler monitoring may be considered as a tool for real-time assessment of recanalization, and since the clearance of rtPA is very rapid, e.g., only 1% of the drug remains after 15 min of infusion, additional interventions may be needed if the clot remains after the initial recanalization attempt.70
Since thrombus length is an important determinant of vessel recanalization using intravenous rTPA, literature has shown that thrombus length of >8 mm (evaluated using a sliced non-contrast CT and contrast enhanced CT (80 seconds post CTA contrast injection)) resulted in a < 1% rate of recanalization.71 Because CT angiography can overestimate thrombus length, delayed phase contrast enhanced CT may be used to more accurately predict thrombus length.71 This data supports the use of thrombus size to help guide the decision for intervention. In evaluating pre-thrombolysis arteriograms, recanalization graded via a modified Thrombolysis in Myocardial Infarction flow grade (mTIMI 2–3) was achieved in ∼95% of patients with a clot outline sign– a slow antegrade contrast opacification distal to the thrombus.72 This association may provide essential information in acute thrombolytic therapy.
Given that PET studies have reported salvageable penumbral tissue several days after stroke onset, delayed recanalization at 10-168 h via stent placement (six balloon mounted stents, five Wingspan and one Enterprise self-expanding intracranial stent), was achieved in 92% of patients and resulted in favorable outcomes in 60% of patients (mRS ≤ 2 at 90 days).54,73 Thus many patients may benefit from evaluation and consideration for recanalization at later time-points than the current precedent. In a qualitative clinical pilot study evaluating intracranial stenting for acute ischemic stroke beyond 8 h from symptom onset, Xavier et al. reported a recanalization rate of 91.7% after stenting compared to 35% and 70% intravenous thrombolytic recanalization rates in Prolyse in Acute Cerebral Thromboembolism (PROACT)-II and multi-MERCI trials, respectively.73 Stent-supported angioplasty, therefore, is an alternative approach compared to clot retrieval and thrombolytic strategies in delayed-recanalization treatment of penumbral tissue. In support of this conclusion in severe acute ischemic stroke patients with large intracranial vessel occlusions in the anterior circulation outside the 4.5 h rtPA window, thrombectomy via Penumbra System and Solitaire devices reached a successful recanalization rate of ∼94%, of which 37% reached a favorable (mRS score 0–2) functional outcome, and 54% reached an acceptable (mRS score 0–3) functional outcome by 3 months.74
In a study evaluating the combination of PET/MRI, Werner et al. report that without significant delays in MRI diagnostic routines and without compromising standard MRI diagnosis, hybrid imaging of both PET and MRI imaging provide complementary information which may more accurately identify salvageable tissue.75 With the increasing window of opportunity for reperfusion, a more flexible window for time to treatment may allow hybrid imaging (PET/MRI) to identify ideal candidates with volumes of penumbra large enough to justify intervention at delayed time points, thereby providing the necessary information to balance between intervention related risk and the benefit of reperfusion.
Clinical combination treatments
In a randomized, open-labeled, blinded endpoint clinical trial, patients with internal carotid artery or middle cerebral artery occlusions were treated with the combination of fingolimod and alteplase at 3-6 h, and after treatment, greater perfusion, lesion reduction, and suppression of infarction volume were observed compared to the alteplase treated group.23 Due to the significant impact of neuroinflammation in stroke, a clinical trial has started evaluating the efficacy of fingolimod in conjunction with mechanical thrombectomy for patients with proximal large vessel occlusion within 6 h after symptom onset.76 Alternatives to typical tPA inhibitors have also been investigated. In 4 patients, argatroban, an inhibitor of thrombin, was also shown to effectively induce 100% recanalization when administered >24 hours, ranging from 25–54 hours after symptom onset.77 Therefore, other treatment options in combination with thrombolysis and/or surrogate treatments may improve outcome after stroke and could potentially have a more granular effect on apoptosis, BBB disruption, and neuroinflammation.
Future directions
In conclusion, outside of reperfusion interventions, thousands of treatment agents and numerous clinical trials have failed due to the pathophysiological complexity of stroke. This review highlights significant findings outside of the conventional reperfusion window in narrative fashion (Table 2); future research can focus on systematic review to evaluate functional improvement after stroke. With current clinical trials extending the reperfusion window up to 24 h, the research in this review supports even further investigation into delayed-reperfusion time-points ranging from 2 days to as long as 26 months after infarct depending on stroke type. Given the complex cascade of inflammation, apoptosis, edema, and BBB disruption after stroke, investigation of combination approaches coupled with reperfusion may also be an important direction for stroke management research.
Table. 2.
Advances in knowledge.
| Tissue at risk has been shown to survive for hours to days in a salvageable state, regaining function if flow is restored, or losing function if hypoperfusion continues. |
| Recanalization up to 72 h and beyond, depending on the stroke type, has shown efficacy and improved functional outcome in animal models as well as patients. |
| Further refining standard imaging thresholds and utilizing more novel modalities such as PET/MRI may help to optimize post-intervention outcomes. |
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by an NIH grant (JHZ, NS081740).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions: Richard Camara, Nathanael Matei, and John H. Zhang drafted the manuscript. Richard Camara and Nathanael Matei drafted the table. All authors critically revised the manuscript for intellectual content and approved the manuscript in its final form.
ORCID iD: John H Zhang https://orcid.org/0000-0002-4319-4285
References
- 1.Colasuonno M, Palange AL, Aid R, et al. Erythrocyte-inspired discoidal polymeric nanoconstructs carrying tissue plasminogen activator for the enhanced lysis of blood clots. ACS Nano 2018; 12: 12224–12237. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Kang D-W, Ahn JS, et al. Imaging of the ischemic penumbra in acute stroke. Korean J Radiol 2005; 6: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivot J-M, Mlynash M, Thijs VN, et al. Optimal tmax threshold for predicting penumbral tissue in acute stroke. Stroke 2009; 40: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bivard A, Spratt N, Levi C, et al. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain J Neurol 2011; 134: 3408–3416. [DOI] [PubMed] [Google Scholar]
- 5.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke 2006; 37: 979–985. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BCV, Christensen S, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke 2012; 43: 2648–2653. [DOI] [PubMed] [Google Scholar]
- 7.Kamalian S, Kamalian S, Maas MB, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke 2011; 42: 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bivard A, McElduff P, Spratt N, et al. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc Dis Basel Dis 2011; 31: 238–245. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BCV, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011; 42: 3435–3440. [DOI] [PubMed] [Google Scholar]
- 10.Lin L, Bivard A, Krishnamurthy V, et al. Whole-brain CT perfusion to quantify acute ischemic penumbra and core. Radiology 2016; 279: 876–887. [DOI] [PubMed] [Google Scholar]
- 11.Baron JC.Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis Basel Dis 2001; 11 Suppl 1: 2–8. [DOI] [PubMed] [Google Scholar]
- 12.Grotta J.Timing of thrombolysis for acute ischemic stroke: ‘timing is everything’ or ‘everyone is different. Ann N Y Acad Sci 2012; 1268: 141–144. [DOI] [PubMed] [Google Scholar]
- 13.Shi F, Gong X, Liu C, et al. Acute stroke: prognostic value of quantitative collateral assessment at perfusion CT. Radiology 2019; 290: 181510. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, Straka M, Kemp S, et al.; DEFUSE 2 Study Investigators. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012; 11: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsivgoulis G, Katsanos AH, Alexandrov AV.Reperfusion therapies of acute ischemic stroke: potentials and failures. Front Neurol 2014; 5: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pialat J-B, Wiart M, Nighoghossian N, et al. Evolution of lesion volume in acute stroke treated by intravenous t-PA. J Magn Reson Imaging JMRI 2005; 22: 23–28. [DOI] [PubMed] [Google Scholar]
- 17.Jovin TG, Albers GW, Liebeskind DS; STAIR IX Consortium. Stroke treatment academic industry roundtable: the next generation of endovascular trials. Stroke 2016; 47: 2656–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savitz SI, Baron J-C, Fisher M, STAIR X Consortium. Stroke treatment academic industry roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke 2019; 50: 1026–1031–1031. [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet Lond Engl 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 20.Kimberly WT, Dutra BG, Boers AMM, et al.; for the MR CLEAN Investigators. Association of reperfusion with brain edema in patients with acute ischemic stroke. JAMA Neurol 2018; 75: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou-Chebl A.Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke 2010; 41: 1996–2000. [DOI] [PubMed] [Google Scholar]
- 22.Jovin TG, Liebeskind DS, Gupta R, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke 2011; 42: 2206–2211. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Chen X, Luo Y, et al. Potential molecular targets of peroxynitrite in mediating blood–brain barrier damage and haemorrhagic transformation in acute ischaemic stroke with delayed tissue plasminogen activator treatment. Free Radic Res 2018; 52: 11–12. [DOI] [PubMed] [Google Scholar]
- 24.Yeo LLL, Paliwal P, Low AF, et al. How temporal evolution of intracranial collaterals in acute stroke affects clinical outcomes. Neurology 2016; 86: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexandrov AV, Felberg RA, Demchuk AM, et al. Deterioration following spontaneous improvement : sonographic findings in patients with acutely resolving symptoms of cerebral ischemia. Stroke 2000; 31: 915–919. [DOI] [PubMed] [Google Scholar]
- 26.Pranevicius O, Pranevicius M, Pranevicius H, et al. Transition to collateral flow after arterial occlusion predisposes to cerebral venous steal. Stroke 2012; 43: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saver JL, Johnston KC, Homer D, et al. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS investigators. Stroke 1999; 30: 293–298. [DOI] [PubMed] [Google Scholar]
- 28.Engelter ST, Provenzale JM, Petrella JR, et al. Infarct volume on apparent diffusion coefficient maps correlates with length of stay and outcome after Middle cerebral artery stroke. Cerebrovasc Dis 2003; 15: 188–191. [DOI] [PubMed] [Google Scholar]
- 29.Yoo AJ, Chaudhry Zeshan A, Nogueira RG, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke 2012; 43: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 30.Vang C, Dunbabin D, Kilpatrick D.Effects of spontaneous recanalization on functional and electrophysiological recovery in acute ischemic stroke. Stroke 1999; 30: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Liang C, Shen C, et al. The effects of pharmaceutical thrombolysis and multi-modal therapy on patients with acute posterior circulation ischemic stroke: results of a one center retrospective study. Int J Surg Lond Surg 2017; 39: 197–201. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Ohshima T, Sato M, et al. A case of acute isolated posterior cerebral artery occlusion successfully treated with endovascular clot aspiration. NMC Case Rep J 2017; 4: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slawski DE, Salahuddin H, Shawver J, et al. Mechanical thrombectomy in elderly stroke patients with mild-to-moderate baseline disability. Intervent Neurol 2018; 7: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humpich M, Singer OC, Du Mesnil de Rochemont R, et al. Effect of early and delayed recanalization on infarct pattern in proximal middle cerebral artery occlusion. Cerebrovasc Dis 2006; 22: 51–56. [DOI] [PubMed] [Google Scholar]
- 35.Chang Y-M, Huang C-Y, Su H-C, et al. Delayed endovascular thrombectomy in a patient suffering from stroke in progression after intravenous thrombolytic therapy. Acta Neurol Taiwanica 2018; 27: 18–21. [PubMed] [Google Scholar]
- 36.Ma N, Mo D-P, Gao F, et al. Endovascular recanalization for chronic symptomatic middle cerebral artery total occlusion. J Neurointerv Surg 2013; 5: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng M, Song Y, Zhang J, et al. Endovascular recanalization of non-acute symptomatic middle cerebral artery total occlusion and its short-term outcomes. Front Neurol 2019; 10: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao W, Zhang J, Song Y, et al. Endovascular recanalization for symptomatic subacute to chronic atherosclerotic basilar artery occlusion. Front Neurol 2019; 10: 1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang K, Gao B-L, Zhao T-Y, et al. Hybrid operation to revascularize long-segment occluded internal carotid artery prevent further ischemic events. Neuroradiology 2019; 61: 217–224. [DOI] [PubMed] [Google Scholar]
- 40.Hasan D, Zanaty M, Starke RM, et al. Feasibility, safety, and changes in systolic blood pressure associated with endovascular revascularization of symptomatic and chronically occluded cervical internal carotid artery using a newly suggested radiographic classification of chronically occluded cervical internal carotid artery: pilot study. J Neurosurg 2018; 130: 1–10. [DOI] [PubMed] [Google Scholar]
- 41.Zanaty M, Roa JA, Jabbour PM, et al. Recanalization of the chronically occluded internal carotid artery: review of the literature. World Neurosurg X 2020; 5: 100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grigoriadis S, Gomori JM, Grigoriadis N, et al. Clinically successful late recanalization of basilar artery occlusion in childhood: what are the odds? Case report and review of the literature. J Neurol Sci 2007; 260: 256–260. [DOI] [PubMed] [Google Scholar]
- 43.Kirton A, Wong JH, Mah J, et al. Successful endovascular therapy for acute basilar thrombosis in an adolescent. Pediatrics 2003; 112: e248–e251. [DOI] [PubMed] [Google Scholar]
- 44.Cognard C, Weill A, Lindgren S, et al. Basilar artery occlusion in a child: “clot angioplasty” followed by thrombolysis. Childs Nerv Syst 2000; 16: 496–500. [DOI] [PubMed] [Google Scholar]
- 45.Cross DT, Moran CJ, Akins PT, et al. Relationship between clot location and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol 1997; 18: 1221–1228. [PMC free article] [PubMed] [Google Scholar]
- 46.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 47.Albers GW, Lansberg MG, Kemp S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke off Stroke 2017; 12: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiotta AM, Vargas J, Turner R, et al. The golden hour of stroke intervention: effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointervent Surg 2014; 6: 511–516. [DOI] [PubMed] [Google Scholar]
- 49.Kim YD, Nam HS, Kim SH, et al. Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke 2015; 46: 1877–1882. [DOI] [PubMed] [Google Scholar]
- 50.Cho K-H, Lee DH, Kwon SU, et al. Factors and outcomes associated with recanalization timing after thrombolysis. Cerebrovasc Dis Basel Dis 2012; 33: 255–261. [DOI] [PubMed] [Google Scholar]
- 51.Cho A-H, Sohn S-I, Han M-K, et al. Safety and efficacy of MRI-based thrombolysis in unclear-onset stroke. A preliminary report. Cerebrovasc Dis 2008; 25: 572–579. [DOI] [PubMed] [Google Scholar]
- 52.Fuhrer H, Günther A, Zinke J, et al. Optimizing cardiac out-put to increase cerebral penumbral perfusion in large middle cerebral artery ischemic lesion – OPTIMAL study. Front Neurol 2017; 8: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hillis AE, Ulatowski JA, Barker PB, et al. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis 2003; 16: 236–246. [DOI] [PubMed] [Google Scholar]
- 54.Verro P, Chow M.Prolonged dependence of ischemic penumbra on induced hypertension. Neurologist 2009; 15: 296–297. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez-Sabín J, Molina CA, Ribó M, et al. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke 2004; 35: 2493–2498. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Yan J, Shi H.Hyperglycemia as a risk factor of ischemic stroke. J Drug Metab Toxicol 2013; 4: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexandrov AV, Hall CE, Labiche LA, et al. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke 2004; 35: 449–452. [DOI] [PubMed] [Google Scholar]
- 58.Chompoopong P, Rostambeigi N, Kassar D, et al. Are We overlooking stroke chameleons? A retrospective study on the delayed recognition of stroke patients. Cerebrovasc Dis Basel Dis 2017; 44: 83–87. [DOI] [PubMed] [Google Scholar]
- 59.Shazeeb MS, King RM, Brooks OW, et al. Infarct evolution in a large animal model of middle cerebral artery occlusion. Transl Stroke Res 2020; 11: 468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janjua N, El-Gengaihy A, Pile-Spellman J, et al. Late endovascular revascularization in acute ischemic stroke based on clinical-diffusion mismatch. AJNR Am J Neuroradiol 2009; 30: 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molina CA, Alvarez-Sabín J, Montaner J, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 2002; 33: 1551–1556. [DOI] [PubMed] [Google Scholar]
- 62.Shinoyama M, Nakagawara J, Yoneda H, et al. Initial ‘TTP Map-Defect’ of computed tomography perfusion as a predictor of hemorrhagic transformation of acute ischemic stroke. Cerebrovasc Dis Extra 2013; 3: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renú A, Laredo C, Tudela R, et al. Brain hemorrhage after endovascular reperfusion therapy of ischemic stroke: a threshold-finding whole-brain perfusion CT study. J Cereb Blood Flow Metab off J Tab 2017; 37: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Renú A, Amaro S, Laredo C, et al. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: dual-energy computed tomographic study. Stroke 2015; 46: 673–679. [DOI] [PubMed] [Google Scholar]
- 65.García-Bermejo P, Calleja AI, Pérez-Fernández S, et al. Perfusion computed tomography-guided intravenous thrombolysis for acute ischemic stroke beyond 4.5 hours: a case-control study. Cerebrovasc Dis 2012; 34: 31–37. [DOI] [PubMed] [Google Scholar]
- 66.Neumann-Haefelin T, Wittsack H-Jrg, Wenserski Frank, et al. Diffusion- and perfusion-weighted MRI. Stroke 1999; 30: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 67.Kim S, Kang M, Choi S, et al. Mismatch of delayed perfusion volume between TTP and tmax map of perfusion MRI. Clin Imaging 2016; 40: 63–67. [DOI] [PubMed] [Google Scholar]
- 68.Ringelstein EB, Biniek R, Weiller C, et al. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology 1992; 42: 289–298. [DOI] [PubMed] [Google Scholar]
- 69.Liebeskind DS, Kim D, Starkman S, et al. Collateral failure? Late mechanical thrombectomy after failed intravenous thrombolysis. J Neuroimaging off J Uroimaging 2010; 20: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribo M, Alvarez-Sabín J, Montaner J, et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke 2006; 37: 1000–1004. [DOI] [PubMed] [Google Scholar]
- 71.Mortimer AM, Little DH, Minhas KS, et al. Thrombus length estimation in acute ischemic stroke: a potential role for delayed contrast enhanced CT. J Neurointervent Surg 2014; 6: 244–248. [DOI] [PubMed] [Google Scholar]
- 72.Christoforidis GA, Mohammad Y, Avutu Bet al. Arteriographic demonstration of slow antegrade opacification distal to a cerebrovascular thromboembolic occlusion site as a favorable indicator for intra-arterial thrombolysis. AJNR Am J Neuroradiol 2006; 27: 1528–1531. [PMC free article] [PubMed] [Google Scholar]
- 73.Xavier AR, Tiwari A, Purai N, et al. Safety and efficacy of intracranial stenting for acute ischemic stroke beyond 8 h of symptom onset. J Neurointerv Surg 2012; 4: 94–100. [DOI] [PubMed] [Google Scholar]
- 74.Sallustio F, Koch G, Di Legge S, et al. Intra-arterial thrombectomy versus standard intravenous thrombolysis in patients with anterior circulation stroke caused by intracranial arterial occlusions: a single-center experience. J Stroke Cerebrovasc Dis off J Natl Stroke Assoc 2013; 22: e323-331–e331. [DOI] [PubMed] [Google Scholar]
- 75.Werner P, Saur D, Zeisig V, et al. Simultaneous PET/MRI in stroke: a case series. J Cereb Blood Flow Metab off J Tab 2015; 35: 1421–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S, Zhou Y, Zhang R, et al. Rationale and design of combination of an immune modulator fingolimod with alteplase bridging with mechanical thrombectomy in acute ischemic stroke (FAMTAIS) trial. Int J Stroke 2017; 12: 906–909. [DOI] [PubMed] [Google Scholar]
- 77.Ishibashi H, Koide M, Obara S, et al. High-dose argatroban therapy for stroke: novel treatment for delayed treatment and the recanalization mechanism. J Stroke Cerebrovasc Dis off J S 2013; 22: 656–660. [DOI] [PubMed] [Google Scholar]
- 78.Ishihara H, Sakai N, Kuroiwa T, et al. Percutaneous transluminal angioplasty and stenting for chronic total occlusion of intracranial carotid artery: a case report. Interv Neuroradiol 2006; 12: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas AJ, Gupta R, Tayal AH, et al. Stenting and angioplasty of the symptomatic chronically occluded carotid artery. AJNR Am J Neuroradiol 2007; 28: 168–171. [PMC free article] [PubMed] [Google Scholar]
- 80.Kao H-L, Lin M-S, Wang C-S, et al. Feasibility of endovascular recanalization for symptomatic cervical internal carotid artery occlusion. J Am Coll Cardiol 2007; 49: 765–771. [DOI] [PubMed] [Google Scholar]
- 81.Lin M-S, Lin L-C, Li H-Y, et al. Procedural safety and potential vascular complication of endovascular recanalization for chronic cervical internal carotid artery occlusion. Circ Cardiovasc Interv 2008; 1: 119–125. [DOI] [PubMed] [Google Scholar]
- 82.Bhatt A, Majid A, Kassab M, et al. Chronic total symptomatic carotid artery occlusion treated successfully with stenting and angioplasty. J Neuroimaging off J Uroimaging 2009; 19: 68–71. [DOI] [PubMed] [Google Scholar]
- 83.Terada T, Okada H, Nanto M, et al. Endovascular recanalization of the completely occluded internal carotid artery using a flow reversal system at the subacute to chronic stage. J Neurosurg 2010; 112: 563–571. [DOI] [PubMed] [Google Scholar]
- 84.Shojima M, Nemoto S, Morita A, et al. Protected endovascular revascularization of subacute and chronic total occlusion of the internal carotid artery. AJNR Am J Neuroradiol 2010; 31: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iwata T, Mori T, Tajiri H, et al. Long-term angiographic and clinical outcome following stenting by flow reversal technique for chronic occlusions older than 3 months of the cervical carotid or vertebral artery. Neurosurgery 2012; 70: 82–90; discussion 90. [DOI] [PubMed] [Google Scholar]
- 86.Namba K, Shojima M, Nemoto S.Wire-Probing technique to revascularize subacute or chronic internal carotid artery occlusion. Interv Neuroradiol 2012; 18: 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rostambeigi N, Khatri R, Hassan AE, et al. Duplex ultrasound assisted endovascular revascularization of chronic internal carotid artery occlusion: technical note. J Vasc Interv Neurol 2013; 6: 42–46. [PMC free article] [PubMed] [Google Scholar]
- 88.Fan Y-L, Wan J-Q, Zhou Z-W, et al. Neurocognitive improvement after carotid artery stenting in patients with chronic internal carotid artery occlusion: a prospective, controlled, Single-Center study. Vasc Endovascular Surg 2014; 48: 305–310. [DOI] [PubMed] [Google Scholar]
- 89.Bigliardi Guido, Dell'Acqua MariaLuisa, Vallone Stefano, et al. Opening the unopenable”: endovascular treatment in a patient with three months’ internal carotid artery occlusion and hemispheric symptomatic hypoperfusion. J Stroke Cerebrovasc Dis 2016; 25: 2016–2018. [DOI] [PubMed] [Google Scholar]