Abstract
Previous studies have investigated whether migraine is a circulatory disorder, as migraineurs are at heightened risk of cerebrovascular disease. However, in most cases, systemic vascular function was evaluated, which may not reflect abnormalities in the cerebral circulation. Therefore, we aimed to determine whether cerebrovascular function differs between migraineurs and controls. A systematic literature search was conducted across three electronic databases to search for studies that compared cerebrovascular function in migraineurs to controls. Where applicable, meta-analyses were used to determine standardised mean differences (SMD) between migraineurs and controls. Seventy articles were identified, 40 of which contained quantitative data. Meta-analyses showed pulsatility index (PI) was higher (SMD = 0.23; 95%CI = 0.05 to 0.42, P = 0.01) and cerebrovascular responsiveness (CVR) to hypercapnia was lower (SMD=−0.34; 95%CI=−0.67 to −0.01, P = 0.04) in the posterior circulation of migraineurs, particularly those without aura. The meta-analyses also indicated that migraineurs have higher resting mean blood flow velocity in both anterior (SMD = 0.14; 95%CI = 0.05 to 0.23, P = 0.003) and posterior circulations (SMD = 0.20; 95%CI = 0.05 to 0.34, P = 0.007). Compared to healthy controls, migraineurs have altered cerebrovascular function, evidenced by elevated PI (representing arterial stiffness) and impaired CVR to hypercapnia (representing cerebral vasodilator function). Future studies should investigate whether improvement of cerebrovascular function is able to alleviate migraine.
Keywords: Cerebral blood flow, cerebrovascular function, cerebrovascular responsiveness, neurovascular coupling, pulsatility index
Introduction
Migraine is a primary headache disorder associated with significant physical, psychosocial and emotional disability.1 Estimated to affect one in 10 people worldwide, migraine is characterised by a severe, throbbing, often unilateral headache that can last for up to three days.2 A typical migraine attack can be divided into four main phases, viz. prodromal, aura, headache and postdromal. However, not all migraineurs experience every phase of a typical migraine attack.3 There are two main types of migraine: migraine with aura and migraine without aura, the latter subtype being the more common of the two.4 Additionally, migraine can be subdivided into two categories based on the frequency of migraine attacks: chronic migraine and episodic migraine. Chronic migraine is characterised by headaches occurring on 15 or more days per month, whereas episodic migraine is characterised by headaches that occur on 14 or fewer days per month.5
Migraine pathophysiology
Despite its high prevalence, the pathophysiology of migraine is yet to be completely elucidated. A current hypothesis of migraine involves activation of the trigeminovascular system (TVS), comprising sensory neurons that originate from the trigeminal ganglion and innervate blood vessels of the meninges (such as the middle meningeal artery) and cerebral arteries (such as those that form the Circle of Willis).6 Activation of the TVS is believed to trigger the release of vasoactive neuropeptides (namely substance P, neurokinin A, pituitary adenylate-cyclase activating peptide and calcitonin gene-related peptide) from trigeminal nerve fibres onto cerebral and meningeal blood vessels, subsequently resulting in transient vasodilation, plasma protein extravasation and acute neurogenic inflammation of the vessels. The neurogenic inflammation and vasodilation of cerebral and meningeal blood vessels may trigger mechanical and chemical stimulation of neighbouring nociceptors, resulting in migraine pain and the further release of vasodilatory and inflammatory neuropeptides; thus, perpetuating the migraine pain cycle.6,7 Activation of the TVS is central to the current theory of migraine; however, what causes the initial activation in migraine is unknown.
Endothelial dysfunction and migraine
There has been conflicting evidence as to whether migraine is associated with endothelial dysfunction. A crucial function of the endothelium is to regulate the vasomotor tone; if compromised, both vasodilatation and vasoconstriction may be impaired.8 To see whether endothelial dysfunction is associated with migraine, Sacco et al.9 reviewed studies that examined systemic endothelial function, as measured by flow-mediated dilatation, endothelial progenitor cells and arterial tonometry. The findings from these studies were inconsistent, with reports of lower, higher or no difference in systemic endothelial function of migraineurs when compared to that of controls.9 However, systemic vascular abnormalities may not necessarily reflect cerebrovascular pathology.10–12 Recently, Ornello et al.13 investigated whether migraine was associated with poor cerebrovascular function; however, findings from their narrative review, which assessed cerebrovascular reactivity to hypercapnia or hypocapnia, were conflicting. As such, it is still not clear if migraineurs have abnormalities of cerebrovascular function, which could partially account for their heightened risk of cerebrovascular diseases, such as stroke. Hence, the aim of this systematic review and meta-analysis is to determine whether migraine is associated with altered cerebrovascular function by summarising the findings of original studies that have compared cerebrovascular function in migraineurs to that of controls.
Methods
Search strategy and inclusion/exclusion criteria
A systematic literature search was conducted using three electronic databases (EMBASE, Medline and Web of Science) to search for full text, original studies that compared cerebrovascular function in migraineurs to controls until July 2020. The following search terms were used: “migraine” or “headache” AND “brain” or “cerebral” AND “endothelial” or “blood flow”. Other inclusion criteria were studies conducted in adult humans, studies conducted in the migraine-free period and studies published in English. Studies that assessed cerebrovascular function in migraineurs and controls as part of an intervention trial were included if they provided baseline cerebrovascular function data. Additionally, the reference lists of relevant studies were scanned to identify any studies that may have been missed during the initial search. Studies were excluded if they assessed cerebrovascular function during a migraine attack, if they did not compare cerebrovascular function in migraineurs to controls and/or if they were not published in English. Abstracts, case reports, letters, notes, reviews and surveys were also excluded.
Data extraction and analysis
Data extraction was performed by one investigator using a standardised data extraction form. Data extracted from the articles that met the eligibility criteria for the qualitative analysis comprised the study population and migraine subtype(s), the blood vessel(s) or brain region(s) where cerebrovascular function was assessed, whether cerebrovascular function was assessed at rest or under dynamic conditions, the metric used to assess cerebrovascular function and key findings. Data extracted from the articles that met the eligibility criteria for the quantitative analysis included the mean and standard deviation for each relevant measure of cerebrovascular function and the sample size of the migraineur and control groups.
Quality assessment
A modified Newcastle-Ottawa scale (NOS) for cross-sectional studies was used to assess and evaluate the methodological quality of studies included in the meta-analyses. The maximum score for the modified NOS was nine where scores between 0 and 3, 4 and 6 and 7 and 9 were categorised as low, moderate and high quality respectively.
Data analysis and statistical methods
Meta-analyses of data were performed where applicable using a random-effects model in Review Manager (RevMan) for Windows, version 5.4.0 (The Cochrane Collaboration, 2020) to determine standardised mean differences (SMD) between migraineurs and controls. To be included in the meta-analyses, studies had to provide sufficient data in order for the SMD of the cerebrovascular function metric between migraineurs and controls to be calculated. As cerebrovascular function was assessed in many different cerebral blood vessels and brain regions, relevant measures of cerebrovascular function were grouped into the anterior and/or posterior circulations or similar brain regions for meta-analyses, where applicable. The I2 statistic was used to estimate the heterogeneity between studies, where values of 25%, 50% and 75% represent low, medium and high heterogeneity, respectively.14 Publication bias was assessed through visual examination of the funnel plots and Egger’s regression test was used for meta-analyses that included 10 or more studies. Duval and Tweedie’s trim-and-fill method was used to estimate any additional studies for meta-analyses that were found to have publication bias.15 Comprehensive meta-analysis (CMA Software, Version 3.3.070) was used for the Egger’s regression test and Duval and Tweedie’s trim-and-fill. For all analyses, a P value of <0.05 was considered statistically significant.
Subgroup analysis
We performed subgroup analyses for the different subtypes of migraine, which included migraine with aura, migraine without aura, chronic migraine and episodic migraine. Additionally, subgroup analysis was performed for a mixed subgroup for studies that did not perform separate analyses for migraineurs based on their aura status or frequency of migraine.
Results
Study selection
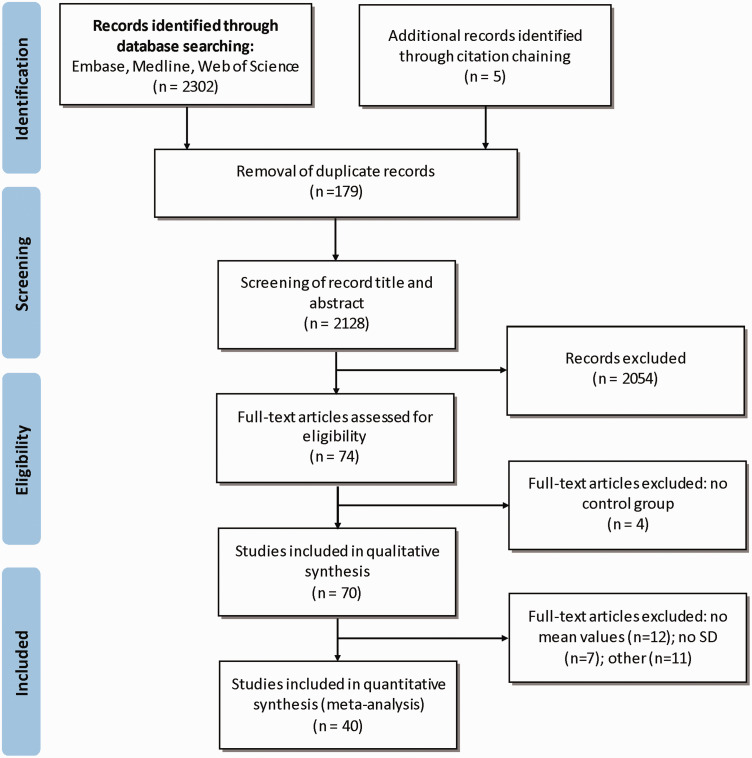
The database search and other sources yielded 2307 articles (see Figure 1). After duplicates were removed, 2128 articles remained and were subsequently screened for relevance by examining the article title and abstract. In total, 2054 articles were excluded after screening for relevancy leaving 74 full text articles to be assessed for eligibility. Four articles did not meet the inclusion criteria, as there was no control group. In total, 70 articles met the eligibility criteria and were included in the qualitative analysis and 40 articles were included in the quantitative analysis.
Figure 1.
PRISMA flow diagram of selection of studies for quantitative and qualitative analysis.
Study characteristics
Characteristics of each study are summarised in Table 1. Fifty-five studies identified from the literature search used Transcranial Doppler (TCD) ultrasound to measure cerebral blood flow velocity (CBFV) in resting and/or dynamic conditions. Cerebral arteries that were assessed using TCD ultrasound included those that form the anterior circulation, viz. anterior cerebral artery (ACA) and middle cerebral artery (MCA) and those that form the posterior circulation, viz. basilar artery (BA), posterior cerebral artery (PCA) and vertebral artery (VA). Three studies also assessed cerebrovascular function of the internal carotid artery (ICA).
Table 1.
Cerebrovascular function in migraineurs compared to controls during the migraine free period.
| Study | Population and numbers | Area of assessment | Assessment | Key findings | NOS |
|---|---|---|---|---|---|
| TCD Studies | |||||
| Altamura et al.51 | Total = 129MA = 56 (46 F/10M) Stroke = 20 (9 F/11M) Controls = 53 (42 F/11M) | Unilateral MCA (left) Unilateral PCA (right) | CVR to BHT(30s breath hold) | MA had a higher BHI compared to controls (and stroke patients). | 8 |
| Lee et al.56 | Total = 353MX = 248 (201 F/47M) Controls = 105 (86 F/19M) | BABilateral MCA Bilateral PCA | CVR to BHT (30 s breath hold) | MX had a lower BHI compared to controls. | 8 |
| Altamura et al.87 | Total = 70MA = 39 (34 F/5M) Stroke = 15 (8 F/7M) Controls = 16 (8 F/8M) | Unilateral MCA (left) Unilateral PCA (right) | CVR to BHT (30s breath hold) | MA had a higher BHI compared to controls (and stroke patients). | 5 |
| Gollion et al.52 | Total = 44MA = 22 (18 F/4M) Controls = 22 (17 F/5M) | Unilateral MCA (right) | CVR to BHT (30s breath hold) | No difference. | 8 |
| Cerebral autoregulation (Dx, Mx, phase, gain) | No difference. | ||||
| Karacay Ozkalayci et al.17 | Total = 75MA = 10 (MD) MO = 30 (MD) TTH = 10 (MD) Controls = 25 (17 F/8M) | Bilateral ACA BABilateral MCA Bilateral PCA Bilateral VA Bilateral MCA | Resting MBFV | No difference. | 7 |
| Podgorac et al.16 | Total = 273MA = 100 (71 F/29M) MO = 70 (61 F/9M) TTH = 38 (21 F/17M) CH = 35 (10 F/25M) Controls = 30 (13 F/17M) | CVR to BHT(30s breath hold) | MA had a higher BHI compared to controls. | 8 | |
| Resting MBFV | No difference. | ||||
| Choi et al.18 | Total = 84EM = 28 (24 F/4M) RCVS = 28 (24 F/4M) Controls = 28 (25 F/3M) | BABilateral MCABilateral PCA | CVR to BHT (30 s breath hold) | MD. | 8 |
| Resting MBFV | MD. | ||||
| Gonzalez-Quintanilla et al.53 | Total = 113CM = 35 (31 F/4M) EM = 37 (27 F/10M) Controls = 41 (29 F/12M) | BABilateral MCA | CVR to BHT(breath hold time not specified) | CM and EM had a lower BHI compared to controls. | 7 |
| Akgun et al.19 | Total = 60CM = 31 (MD) Controls = 29 (MD) | Bilateral MCA Bilateral PCA | CVR to BHT (30s breath hold) | CM had a lower BHI compared to controls. | 6 |
| Resting MBFV | No difference. | ||||
| Fabjan et al.20 | Total = 52MX = 29 (21 F/8M) Controls = 23 (13 F/10M) | Unilateral MCA (left) Unilateral PCA (right) | Neurovascular coupling (photic stimulation) | MD. | 7 |
| Resting MBFV | No difference. | ||||
| Rajan et al.12 | Total = 89MX = 45 (36 F/9M) Controls = 44 (31 F/13M) | BABilateral MCA Bilateral PCA | CVR to BHT (30s breath hold) | MX had a lower BHI in BA, left PCA and right PCA compared to controls. | 7 |
| Resting MBFV | No difference. | ||||
| Karadas et al.21 | Total = 40MA = 20 (15 F/5M) Controls = 20 (16 F/4M) | Bilateral MCABilateral PCA | CVR to BHT (100s breath hold) | MA had a higher BHI in the MCA and PCA compared to controls. | 8 |
| Resting MBFV | MA had a higher MBFV in the MCA and PCA compared to controls. | ||||
| Quirico et al.22 | Total = 30MO = 16 (MD) Controls = 14 (MD) | Unilateral MCA (right) | Resting MBFV | No difference. | N/A |
| Reinhard et al.23 | Total = 69MA = 17 (12 F/5M) MO = 17 (13 F/4M) Controls = 35 (26 F/9M) | Unilateral MCA (left) Unilateral PCA (right) | Resting MBFV, PI | No difference. | 7 |
| Cerebral autoregulation (phase, gain, Dx) | MA had higher Dx than controls in the MCA. | ||||
| Min et al.25 | Total = 36MA = 22 (14 F/8M) Controls = 14 (9 F/5M) | Bilateral PCA | Neurovascular coupling (20 s photic stimulus, then 20 s rest for 10 cycles) | No difference. | 6 |
| Resting MBFV | No difference. | ||||
| Perko et al.24 | Total = 60MA = 20 (16 F/4M)MO = 20 (16 F/4M) Controls = 20 (16 F/4M) | Unilateral MCA (right) Unilateral PCA (left) | CVR to L-arginine infusion (assessed 30 min after infusion) | MX had a lower CVR in the PCA. | 8 |
| Resting MBFV | No difference. | ||||
| Sousa et al.58 | Total = 284MX = 228 (189 F/39) Controls = 56 (31 F/25M) | Bilateral MCA | CVR to BHT(30s breath hold) | MX had a higher BHI compared to controls. | N/A |
| Wallasch et al.70 | Total = 120MX = 60 (50 F/10M) Controls = 60 (MD) | Bilateral MCA | Cerebral autoregulation (CVR to the Valsalva manoeuvre) | MX had a higher CVR to the Valsalva manoeuvre compared to controls. | N/A |
| El-Khawas et al.26 | Total = 41MX = 27 (23 F/4M) Controls = 14 (9 F/5M) | Bilateral MCA Bilateral PCA | CVR to hyperventilation (3-min hyperventilation) | MX had a lower CRI. | 9 |
| Resting MBFV | No difference. | ||||
| Wolf et al.67 | Total = 110MA = 70 (70 F/0M) Controls = 40 (30 F/10M) | Bilateral PCA | Neurovascular coupling (photic stimulation) | MX had a higher VEFR ((MBFV during stimulation – MBFV at rest)/MBFV at rest×100) compared to controls. | 8 |
| Zaletel et al.68 | Total = 70MA = 14 (MD) MO = 16 (MD) Controls = 40 (21 F/19M) | Unilateral MCA (left) Unilateral PCA (right) | Neurovascular coupling (photic stimulation) | MA and MO had a higher VEFR compared to controls. | N/A |
| Arjona et al.28 | Total = 131MO = 36 (26 F/10M) TTH = 51 (32 F/19M) Controls = 44 (25 F/19M) | Bilateral MCA | Resting MBFV, PI | No difference. | 8 |
| CVR to BHT (15-45 s breath hold) | No difference. | ||||
| Reinhard et al.27 | Total = 94MX = 19 (14 F/5M) Controls = 75 (33 F/42M) | Bilateral MCA | Resting MBFV | No difference. | 7 |
| Cerebral autoregulation (Dx, Mx, phase, gain) | No difference. | ||||
| MüLler and Marziniak69 | Total N = 55MX = 22 (19 F/3M) Controls = 33 (15 F/18M) | Bilateral MCA | Cerebral autoregulation (gain) | MX had a lower gain compared to controls. | N/A |
| Zaletel et al.47 | Total = 60MX = 30 (20 F/10M) Controls = 30 (22 F/8M) | Unilateral MCA (left) Unilateral PCA (right) | Neurovascular coupling (70 s photic stimulation stimulus, 30 s rest) | MX had a higher VEFR compared to controls. | N/A |
| Resting MBFV | No difference. | ||||
| Backer et al.64 | Total = 20 MX = 10 (MD) Controls = 10 (MD) | Unilateral MCA (left) Unilateral PCA (right) | Neurovascular coupling (photic stimulation) | MX had an exaggerated increase in CBFV at the beginning of the photic stimulation and a delayed decrease to baseline following photic stimulation compared to controls. | N/A |
| Nedeltchev et al.29 | Total = 38 MA = 19 (10 F/9M) Controls = 19 (8 F/11M) | Unilateral MCA (right) Unilateral PCA (left) | Neurovascular coupling (photic stimulation) (10 s stimulus, then 10 s rest for 10 cycles) | MA had a higher MBFV during photic stimulation compared to controls. | 7 |
| Resting MBFV | No difference. | ||||
| Silvestrini et al.57 | Total = 45MA = 15 (15 F/0M) MO = 15 (15 F/0M) Controls = 15 (15 F/0M) | BAUnilateral MCA (left or right side chosen at random) | CVR to BHT (30s breath hold) | MA had a lower BHI in BA compared to controls (and MO). | 8 |
| Dora et al.30 | Total = 31MO = 20 (18 F/2M) Controls = 11 (9 F/2M) | Bilateral MCA | CVR to BHT (30 s breath hold) | MO had a higher BHI compared to controls. | 8 |
| Resting MBFV, PI | No difference. | ||||
| Thomaides et al.31 | Total = 60MA = 45 (36 F/9M) Controls = 15 (12 F/3M) | Bilateral MCA | Resting MBFV | No difference. | 7 |
| Dora et al.32 | Total = 33MO = 23 (22 F/1M) Controls = 10 (9 F/1M) | Bilateral MCA | CVR to BHT (30s breath hold) | MO had a higher BHI compared to controls. | N/A |
| Resting MBFV, PI | No difference. | ||||
| Backer et al.65 | Total = 38MX= 19 (MD) Controls = 19 (MD) | Unilateral MCA (left) Unilateral PCA (right) | Neurovascular coupling (3 rounds of 57 s photic stimulation, 57 s rest) | MX had a higher initial increase of CBFV following the onset than controls in MCA and PCA. | N/A |
| Chernyshev et al.33 | Total = 69RSM = 25 (23 F/2M) LSM = 25 (23 F/2M) Controls =19 (9 F/10M) | BA Bilateral MCA | Resting MBFV, PI | RSM had higher MBFV in the BA and higher PI in the right MCA compared to LSM and controls. | 9 |
| Fiermonte et al.34 | Total = 42MA = 9 (4 F/5M) MO = 12 (8 F/4M) Controls = 21 (MD) | Bilateral MCA | CVR to BHT (breath hold time not specified) and hyperventilation (3-min hyperventilation) | MA had a higher CRI to hyperventilation compared to controls. | 6 |
| Resting MBFV | MA and MO had higher MBFV compared to controls. | ||||
| Heckmann et al.36 | Total = 45MA = 15 (11 F/4M) MO = 15 (11 F/4M) Controls = 15 (8 F/7M) | MCA (unclear if unilateral or bilateral) | Cerebral autoregulation (CVR to physical stress) | MA and MO had a lower ΔRI to physical stress compared to controls. | 7 |
| Resting MBFV, RI | No difference. | ||||
| Kastrup Aet al.35 | Total = 50MX = 20 (16 F/4M) Controls = 30 (17 F/13M) | Bilateral ACA Bilateral MCABilateral PCA | CVR to inhalation of 5%CO2 (duration not stated) | MX had higher CRI in the left ACA, right MCA and right PCA. | 8 |
| Resting MBFV | MX had higher MBFV in the right ACA and MCA compared to controls. | ||||
| Kastrup A et al.55 | Total = 40MX = 20 (17 F/3M) Controls = 20 (MD) | Bilateral MCA | CVR to inhalation of 5%CO2 (before metoprolol treatment initiated) | No difference. | N/A |
| Totaro et al.37 | Total = 90 MA = 30 (26 F/4M) MO = 30 (28 F/2M) Controls = 30 (27 F/3M) | Bilateral MCA (unclear if unilateral or bilateral) | CVR to inhalation of 5%CO2 (1–2 min inhalation), and hyperventilation (duration not stated) | MO had a lower CRI to CO2 inhalation compared to controls. | 7 |
| Resting MBFV | No difference. | ||||
| Silvestrini et al.38 | Total = 45MA = 15 (11 F/4M) MO = 15 (13 F/2M) Controls = 15 (MD) | Bilateral MCA | CVR to BHT (30s breath hold) | No difference. | 8 |
| Resting MBFV | No difference. | ||||
| Valikovics et al.60 | Total = 31MX = 12 (MD) Controls = 19 (MD) | Bilateral MCA | CVR to acetazolamide infusion | No difference | 7 |
| Fiermonteet al.40 | Total = 45MA = 15 (9 F/6M) MO = 15 (10 F/5M) Controls = 15 (8 F/7M) | Bilateral MCA | CVR to hyperventilation (3-min hyperventilation) | MA had a higher CRI than controls. | 7 |
| Resting MBFV, PI | MA and MO had lower PI than controls. | ||||
| Piccini et al.39 | Total = 27MO = 10 (7 F/3M) TTH = 10 (8 F/2M) Controls = 7 (5 F/2M) | MCA (unclear if unilateral or bilateral) | Resting MBFV, PI | No difference. | 7 |
| Thomsen et al.62 | Total = 50MA = 8 (MD) MO = 12 (MD) Controls = 30 (25F/5M) | Bilateral MCA | CVR to hyperventilation (90 s hyperventilation) | MA had a higher CRI, whereas MO had lower CRI compared to controls difference. | 7 |
| Abernathy et al.41 | Total = 220MX = 182 (141 F/41M) Controls = 38 (29 F/9M) | BABilateral ACABilateral ICABilateral MCABilateral PCABilateral VA | Resting MBFV | MX had higher MBFV compared to controls. | 8 |
| Anzola et al.43 | Total = 60MX = 30 (22 F/8M) Controls = 30 (18 F/12M) | Bilateral MCA | Resting MBFV | No difference. | 5 |
| CVR to hyperventilation (1-min hyperventilation) and the BHT (30 s breath hold) | MX had higher CVR to hyperventilation and lower CVR to hypercapnia compared to controls. | ||||
| Rieke et al.44 | Total = 50MX = 30 (24 F/6M) Controls = 20 (15 F/5M) | Unilateral MCA (ipsilateral to migraine) | CVR to inhalation of CO2 (% of CO2 not stated) | MX had lower CVR compared to controls. | N/A |
| Resting MBFV, PI | MX had higher resting MBFV compared to controls. | ||||
| Thomsen et al.42 | Total = 43MO = 17 (16 F/1M) TTH = 9 (8 F/1M) Controls = 17 (16 F/1M) | MCA(unclear if unilateral or bilateral) | Resting MBFV (before nitric oxide infusion) | No difference. | 8 |
| Haring and Aichner 46 | Total = 60MA = 17 (MD) MO = 23 (MD) Controls = 20 (14 F/6M) | Bilateral ACABilateral MCABilateral PCA | Resting MBFV | No difference. | 8 |
| Thie et al.66 | Total = 23MX = 11 (MD) Controls = 12 (MD) | Unilateral MCA (left) Unilateral PCA (left) | Neurovascular coupling (cognitive and photic stimulation) | MX had higher CVR to photic stimulation, observation of complex images and cognitive tasks compared to controls. | N/A |
| Totaro et al.45 | Total = 132MA = 44 (39 F/5M) Controls = 88 (MD) | BABilateral ACABilateral MCA Bilateral PCA | Resting MBFV, PI | Migraineurs had higher PI in the MCA compared to controls. | 7 |
| Harer et al.54 | Total = 82IMX = 32 (MD) CMX = 18 (MD) Controls = 32 (MD) | MCA (ipsilateral and contralateral to migraine) | CVR to inhalation of 5%CO2 | MX had higher CRI compared to controls. | N/A |
| Zwetsloot et al.63 | Total = 65MO = 48 (MD) Controls = 17 (10 F/7M) | BAUnilateral MCA | CVR to hyperventilation (1-min hyperventilation) | No difference. | 7 |
| Thomas et al.59 | Total = 20MX = 10 (7 F/3M) Controls =10 (3 F/7M) | Bilateral MCA | CVR to inhalation of 5%CO2 (3–6 min inhalation) and hyperventilation (3-min hyperventilation) | MX had higher CVR to inhalation of 5%CO2 compared to controls. | N/A |
| fMRI Studies | |||||
| Hu et al.73 | Total = 48CM = 24 (15 F/9M) Controls = 24 (15 F/9M) | Angular gyrus Parietal gyrus (inferior, superior) Marginal gyrus (superior) Occipital gyrus (superior) | Neurovascular coupling (cognitive stimuli) | CM had lower CVR in the left inferior parietal gyrus, superior marginal gyrus and angular gyrus but a higher CVR in the right superior occipital gyrus and superior parietal gyrus. | N/A |
| Chen et al.78 | Total = 30EM = 15 (11 F/4M) Controls = 15 (11 F/4M) | Grey matterSuperior temporal gyrus (left hemisphere) | Resting CBF | EM had higher CBF in the left superior temporal gyrus compared to controls. | N/A |
| Hodkinson et al.72 | Total = 34MO = 17 (12 F/5M) Controls = 17 (MD) | Primary somatosensory cortex | Resting CBF | MO had higher regional CBF compared to controls. | N/A |
| Loehrer et al.74 | Total = 2489MX = 456 (365 F/91M)Controls =2033 (100 F/1933M) | BABilateral ICA | Resting CBF | MX had higher CBF in the BA and a higher parenchymal CBF compared to controls. | N/A |
| Zhang et al.75 | Total = 170 MA = 56 (45 F) MO = 60 (48 F) Controls = 54 (39 F) | GlobalGrey matter White matter | Resting CBF | No difference. | N/A |
| Datta et al.71 | Total = 75MA = 25 (21 F/4M) MO = 25 (21 F/4M) Controls = 25 (21 F/4M) | Primary visual cortex | Resting CBF | No difference. | N/A |
| Arkink et al.76 | Total = 45MA = 12 (12 F/0M) MO = 17 (17 F/0M) Controls = 16 (16 F/0M) | Frontal gyrus Temporal gyrus Postcentral gyrus | Resting CBF | MA had higher CBF in the frontal gyrus (medial) but lower CBF in the temporal gyrus (inferior) and postcentral gyrus. MO had higher CBF in the temporal gyrus (inferior, middle) and lower CBF in the frontal gyrus (inferior). | N/A |
| Ances et al.77 | Total = 10MM = 5 (5F/0M)Controls = 5 (5F/0M) | Occipital lobe | Neurovascular coupling (photic stimulation)Resting CBF | MM had a greater increase in cerebral perfusion in response to photic stimulation compared to controls during the mid-follicular phaseNo difference. | N/A |
| NIRS Studies | |||||
| Schytz et al.81 | Total = 24MO = 12 (12 F/0M) Controls = 12 (12 F/0M) | Frontal cortex | Neurovascular coupling (cognitive stimuli): Stroop task | No difference. | N/A |
| Akin et al.80 | Total = 12MX = 6 (5 F/1M) Controls = 6 (4 F/2M) | Frontal cortex | CVR to BHT (20s to 30 s breath hold) | MX had lower ID and R amplitudes of Hb, tHb and oxygen concentrations; therefore lower CVR. | N/A |
| Vernieri et al.48 | Total = 37MA = 16 (13 F/3M) Controls = 21 (16 F/5M) | Bilateral MCAFrontotemporal region | CVR to inhalation of 7%CO2 (90s inhalation) | MA had higher CVR compared to controls. | 5 |
| Resting MBFV | No difference. | ||||
| SPECT Studies | |||||
| Cheng et al.85 | Total = 34MX = 19 (13 F/6M) Controls = 15 (9 F/6M) | Frontal lobe Occipital lobe Parietal lobeTemporal lobe | Resting CBF | MX had lower parenchymal CBF in the frontal, temporal, left parietal and right occipital lobes. | N/A |
| Meyer et al.82 | Total = 64MX = 37 (25 F/12M) Controls = 27 (11 F/16M) | Global Grey matter (cortical, subcortical) White matter | Resting CBF | No difference. | N/A |
| CVR to oral acetazolamide | MX had higher CVR compared to controls. | ||||
| Mirza et al.86 | Total = 61MX = 44 (42 F/2M) Controls = 17 (15 F/2M) | Frontal lobe (inferior, superior) Occipital lobe Parietal lobeTemporal lobe | Resting CBF | MX had lower CBF in the superior frontal and occipital lobes. | N/A |
| Facco et al.83 | Total = 151MA = 20 (15 F/5M) MO = 50 (41 F/9M) TTH = 21 (17 F/4M)Controls = 60 (35 F/25M) | Homologous regions of both the left and right cerebral hemispheres | Resting CBF | MA had lower posterior CBF and lower mean CBF compared to controls. MO had higher mean CBF compared to controls. | N/A |
| Levine et al.84 | Total = 47MA = 15 (MD) MO = 12 (MD) Controls = 20 (MD) | Homologous regions of both the left and right cerebral hemispheres (10 anterior probes, 6 posterior probes). | Resting CBF | MA and MO had lower posterior CBF compared to controls and lower mean CBF compared to controls. | N/A |
| Benedittis49 | Total = 62MA = 35 (27 F/8M) MO = 13 (7 F/6M) Controls = 14 (8 F/6M) | Bilateral ICABilateral MCABilateral VA Frontal lobeOccipital lobeParietal lobeTemporal lobe | Resting CBF, MBFV | No difference. | N/A |
ACA: anterior cerebral artery; BA: basilar artery; BHT: breath hold task; CBFV: cerebral blood flow velocity; CRI: cerebral reactivity index; CVR: cerebrovascular responsiveness; ICA: internal carotid artery; LSM: left-sided migraineurs; MA: migraineurs with aura; MBFV; mean blood flow velocity; MCA: middle cerebral artery; MD: missing data; MM: menstrual migraineurs; MO: migraineurs without aura; MX: mix of migraineurs with and without aura; NOS: Newcastle-Ottawa scale; PCA: posterior cerebral artery; PI: pulsatility index;: RI: resistive index; RSM: right-sided migraineurs; TCD: transcranial Doppler; TTH: tension type headache; VA: vertebral artery; VEFR: visually evoked cerebral blood flow response.
Other methods that were used to assess cerebrovascular function included the following: functional magnetic resonance imaging (fMRI) which was used by eight studies; near infrared spectrometry (NIRS) which was used by three studies and single-photon emission computerized tomography (SPECT) which was used by six studies. The main regions of the brain where cerebrovascular function was assessed included the frontal lobe, occipital lobe, parietal lobe, temporal lobe, primary somatosensory cortex, primary visual cortex, postcentral gyrus and the frontotemporal region. Two studies assessed the cerebrovascular function in grey matter, white matter and globally.
Cerebrovascular function assessed using TCD ultrasound
Mean blood flow velocity
Thirty-five studies were identified from the literature search that compared resting mean blood flow velocity (MBFV) in migraineurs to controls;12,16–49 30 studies assessed MBFV in the anterior circulation.12,19–24,26–32,34–49 All but four studies found no significant difference in MBFV in the anterior circulation between migraineurs and controls; Abernathy et al., Fiermonte et al., Karadas et al. and Kastrup et al. found migraineurs to have higher MBFV in the anterior circulation.21,34,35,41
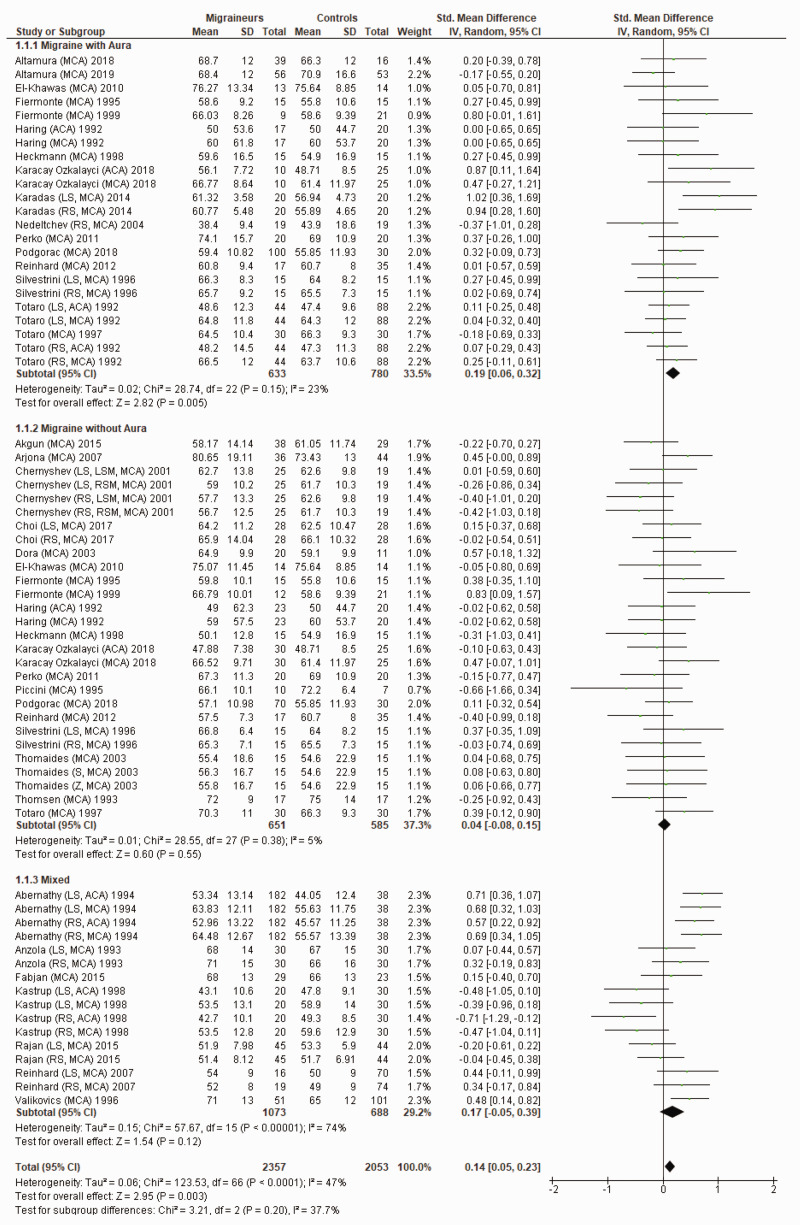
Figure 2 shows a forest plot of mean differences in MBFV in the anterior circulation between 67 migraine subgroups (n = 2357) and their respective controls (n = 2053). Heterogeneity between comparisons was low (I2=47%) and MBFV (SMD = 0.14; 95%CI 0.05 to 0.23; P = 0.003) was significantly higher in migraineurs.
Figure 2.
Forest plot of 67 quantitative comparisons of mean blood flow velocity in the anterior circulation in migraineurs versus controls taken from 30 studies. ACA: anterior cerebral artery; LS: left side of cerebral artery; MCA: middle cerebral artery; RS: right side of cerebral artery.
Sixteen studies compared MBFV in the posterior circulation of migraineurs and controls.12,19–21,23–26,29,33,35,41,45–47,49 Abernathy et al., Chernyshev et al. and Karadas et al. found migraineurs to have higher MBFV in the posterior circulation compared to controls;21,33,41 the remaining 13 studies were not able to detect a significant difference in MBFV between migraineurs and controls.12,19,20,23–26,29,35,45–47,49
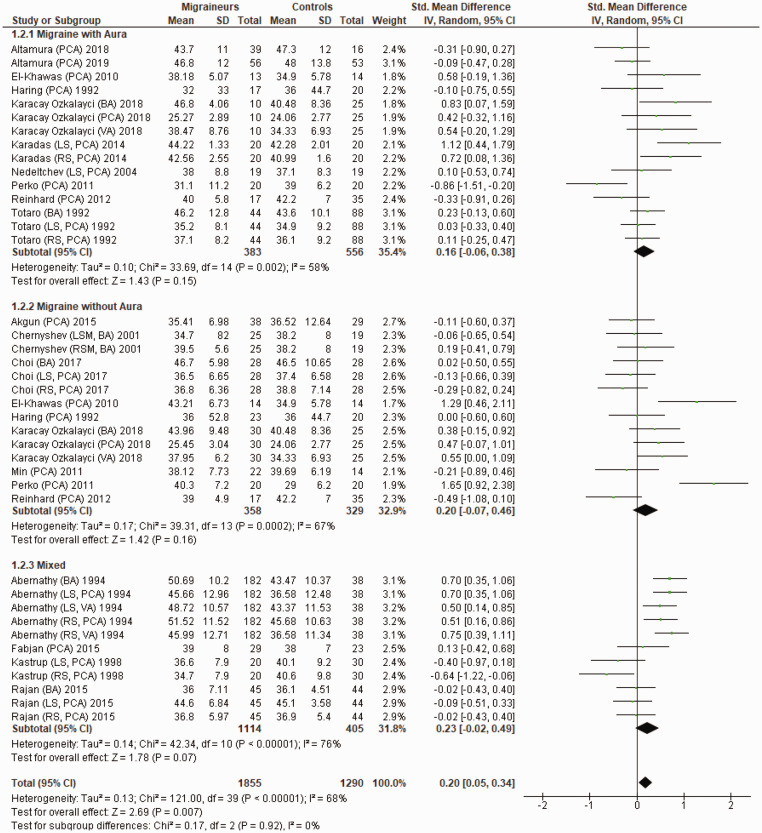
Figure 3 shows a forest plot of mean differences in MBFV in the posterior circulation between 39 migraine subgroups (n = 1855) and their respective controls (n = 1290). Heterogeneity between comparisons was moderate (I2=68%) and MBFV was significantly higher in migraineurs (SMD = 0.20; 95%CI 0.05 to 0.34; P = 0.007).
Figure 3.
Forest plot of 40 quantitative comparisons of mean blood flow velocity in the posterior circulation in migraineurs versus controls taken from 18 studies. BA: basilar artery; LS: left side of cerebral artery; PCA: posterior cerebral artery; RS: right side of cerebral artery; VA: vertebral artery.
Pulsatility index
The pulsatility index (PI) is used as a measure of stiffness of a blood vessel; a higher PI correlates with a stiffer blood vessel. PI is calculated as follows: PI = (maximum BFV-minimum BFV)/MBFV.
Twelve studies were identified from the literature search that used TCD ultrasound to compare PI in the anterior circulation of migraineurs and controls.16,17,23,28,30,32,33,39,40,44–46 Nine of these studies found no significant difference in PI between migraineurs and controls.16,17,23,28,30,32,39,44,46 Chernyshev et al. and Totaro et al. found migraineurs to have significantly higher PI in the anterior circulation compared to controls;33,45 Fiermonte et al. were the only study to find migraineurs to have lower PI than controls.40
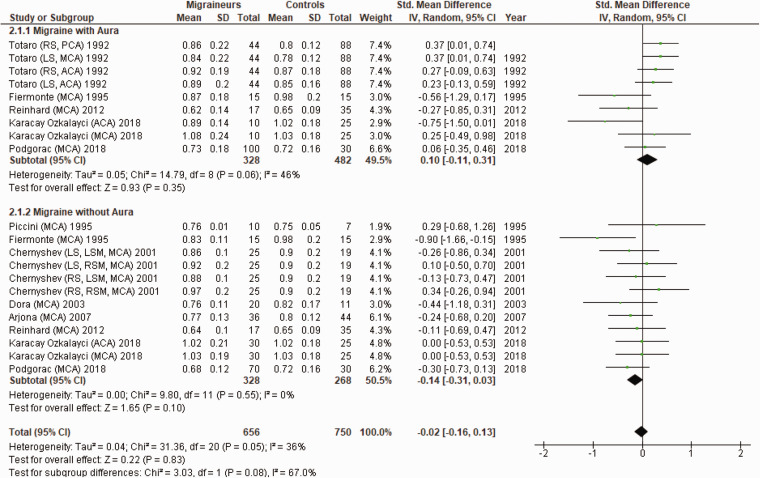
Figure 4 shows a forest plot of mean differences in PI in the anterior circulation between 21 migraine subgroups (n = 656) and their respective controls (n = 750). Heterogeneity between comparisons was low (I2=36%) and there was no significant difference in PI between migraineurs and controls (SMD=−0.02; 95%CI −0.16 to 0.13; P = 0.83).
Figure 4.
Forest plot of 21 quantitative comparisons of pulsatility index in the anterior circulation in migraineurs versus controls taken from nine studies. ACA: anterior cerebral artery; LS: left side of cerebral artery; LSM: left-sided migraineur; MCA: middle cerebral artery; RS: right side of cerebral artery; RSM, right-sided migraineur.
Five studies compared PI in the posterior circulation of migraineurs to controls; no significant difference in PI between migraineurs and controls was reported.17,23,33,45,46
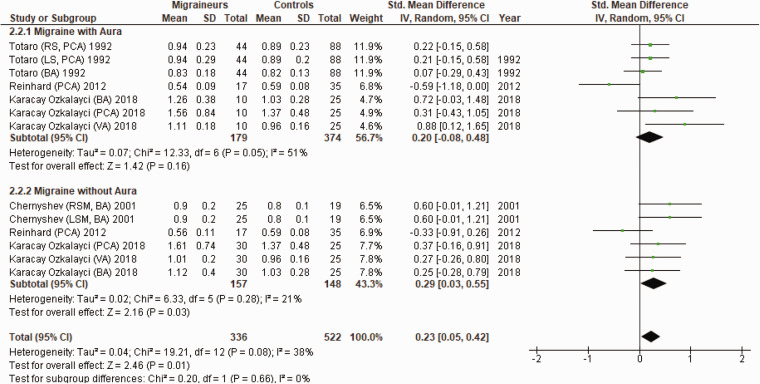
Figure 5 shows a forest plot of mean differences in PI in the posterior circulation between 13 migraine subgroups (n = 336) and their respective controls (n = 522). Heterogeneity between comparisons was low (I2=38%) and PI was significantly higher in migraineurs (SMD = 0.23; 95%CI 0.05 to 0.42; P = 0.01).
Figure 5.
Forest plot of 13 quantitative comparisons of pulsatility index in the posterior circulation in migraineurs versus controls taken from four studies. BA: basilar artery; LSM; left-sided migraineur; MCA: middle cerebral artery; PCA: posterior cerebral artery; RSM: right-sided migraineur; VA: vertebral artery.
Cerebrovascular responsiveness to hypercapnia
Twenty-six studies compared cerebrovascular responsiveness (CVR) to hypercapnia in the anterior and posterior circulations of migraineurs with that of controls.12,16,18,19,21,24,28,30,32,34,35,37,38,43,44,50–60 Various methods were used to induce hypercapnia: the breath-holding task (BHT), inhalation of carbogen gas, infusion of acetazolamide and infusion of L-arginine. Fourteen studies used the BHT to induce hypercapnia, wherein participants hold their breath for a specified duration, which results in an increase in arterial CO2 and subsequent vasodilation of cerebral blood vessels. The breath holding index (BHI) is a measure of CVR to the BHT, which is calculated by dividing the percentage increase of CBFV by the duration of the BHT. Some studies measured CVR by calculating a cerebral reactivity index (CRI) in which the change in CBFV from basal conditions is divided by the change in end tidal CO2. Other studies calculated CVR by calculating the percentage increase of CBFV during stimulation as follows: CVR (%) = (peak BFV during stimulation – resting CBFV)/resting CBFV) × 100.
Twenty-six studies compared CVR to hypercapnia in the anterior circulation between migraineurs and controls with mixed results; 10 studies found migraineurs to have higher CVR to hypercapnia,16,21,30,32,35,50,51,54,58,59 five studies found migraineurs to have lower CVR to hypercapnia19,37,43,44,56 and nine studies found no difference in CVR to hypercapnia in the anterior circulation between migraineurs and controls.12,24,28,34,38,52,55,60 In Gonzalez-Quintanilla et al. and Choi et al., the comparison of CVR to hypercapnia between migraineurs and controls was unclear.18,53
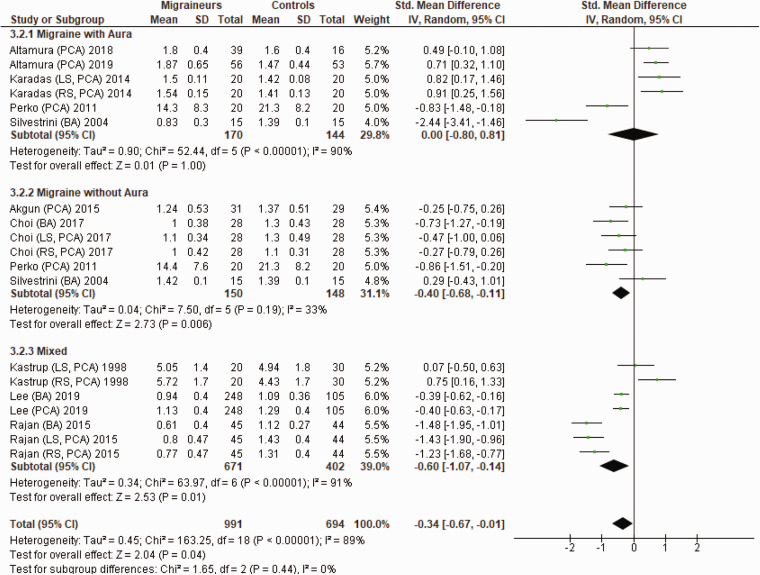
Figure 6 shows a forest plot of mean differences in CVR to hypercapnia in the anterior circulation between 32 migraine subgroups (n = 1166) and their respective controls (n = 937). Heterogeneity between comparisons was high (I2=85%) and there was no significant difference in CVR to hypercapnia (SMD = 0.11; 95%CI −0.13 to 0.35; P = 0.37) between migraineurs and controls.
Figure 6.
Forest plot of 32 quantitative comparisons of cerebrovascular responsiveness to hypercapnia in the anterior circulation in migraineurs versus controls taken from 18 studies. ACA: anterior cerebral artery; CM: chronic migraineurs; EM: episodic migraineur; LS: left side of cerebral artery; MCA; middle cerebral artery; RS: right side of cerebral artery.
Eleven studies compared CVR to hypercapnia in the posterior circulation between migraineurs and controls.12,18,19,21,24,35,50,51,53,56,57 Four studies found migraineurs to have higher CVR to hypercapnia in the posterior circulation21,35,50,51 and five studies found migraineurs to have lower CVR to hypercapnia in the posterior circulation.12,19,24,56,57
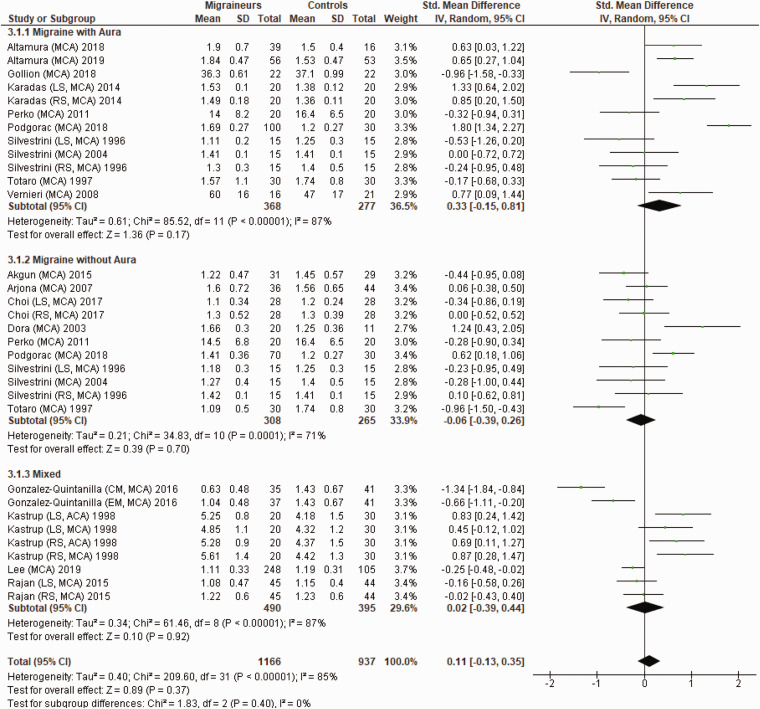
Figure 7 shows a forest plot of mean differences in CVR to hypercapnia in the posterior circulation between 19 migraine subgroups (n = 991) and their respective controls (n = 694). Once again, heterogeneity between comparisons was high (I2=89%). The CVR to hypercapnia in the posterior circulation was significantly lower (SMD=−0.34; 95%CI −0.67 to −0.01; P = 0.04) in migraineurs.
Figure 7.
Forest plot of 19 quantitative comparisons of cerebrovascular responsiveness to hypercapnia in the posterior circulation in migraineurs versus controls taken from 10 studies. BA: basilar artery; LS: left side of cerebral artery; PCA: posterior cerebral artery; RS: right side of cerebral artery.
Cerebrovascular responsiveness to hypocapnia
Hypocapnia is believed to measure the cerebral vasoconstrictor response.61 Eight studies compared CVR to hypocapnia in the anterior circulation in migraineurs to controls with varying results. All eight studies used hyperventilation to stimulate hypocapnia.26,34,37,40,43,59,62,63 Fiermonte et al.34,40 found migraineurs with aura to have a higher CRI than controls, while in migraineurs without aura it was similar to controls. Thomsen et al.62 similarly found migraineurs with aura to have a higher CRI to hypocapnia but found migraineurs without aura to have a lower CRI to hypocapnia than controls. El-Khawas et al.26 found migraineurs to have lower CRI than controls. Anzola et al., Thomas et al., Totaro et al. and Zwetsloot et al. all found migraineurs and controls to have similar CVR to hypocapnia.37,43,59,63
Figure 8 shows a forest plot of mean differences in CVR to hypocapnia in the anterior circulation between nine migraine subgroups (n = 157) and their respective controls (n = 195). Heterogeneity between comparisons was high (I2=74%) and there was no significant difference in CVR to hypocapnia (SMD = 0.01; 95%CI −0.43 to 0.46; P = 0.95) between migraineurs and controls.
Figure 8.
Forest plot of nine quantitative comparisons of cerebrovascular responsiveness to hypocapnia in the anterior circulation in migraineurs versus controls taken from five studies. MCA: middle cerebral artery.
Neurovascular coupling
During neurovascular coupling, the MBFV in the cerebral artery or brain region of interest is measured while participants are shown a stimulus that provokes increases in neural activity, such as a cognitive task (e.g. Stroop task) or photic stimulation task (e.g. checkerboard pattern reversal). Changes in MBFV relative to basal conditions is used to calculate neurovascular coupling.25
Eight studies compared neurovascular coupling during photic stimulation in the posterior circulation between migraineurs and controls.20,25,29,47,64–68 Studies found migraineurs to have higher neurovascular coupling during photic stimulation compared to controls: Thie et al.66 in migraineurs; Nedeltchev et al.29 in migraineurs with aura; Zaletel et al.47,68 in migraineurs and migraineurs with aura; Wolf et al.67 in migraineurs with aura. Min et al.25 was the only study to find that neurovascular coupling was similar participants with migraine and controls. Both studies by Backer et al.64,65 found migraineurs to have a higher initial increase of CBFV than controls following the onset of photic stimulation.
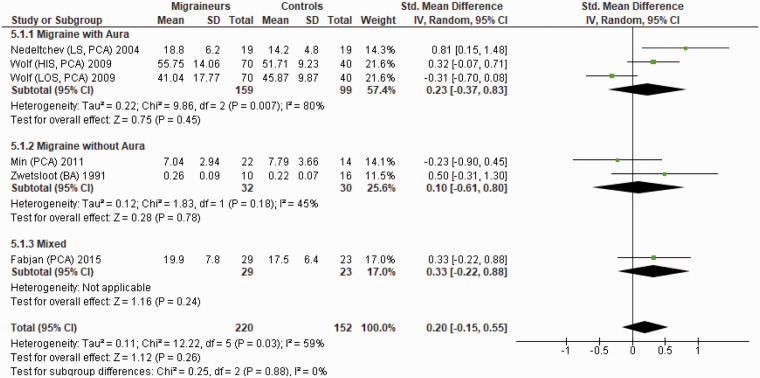
Figure 9 shows a forest plot of mean differences in neurovascular coupling during photic stimulation in the posterior circulation between six migraine subgroups (n = 220) and their respective controls (n = 152). Heterogeneity between comparisons was moderate (I2= 59%) and there was no significant difference in the neurovascular coupling (SMD = 0.20; 95%CI −0.15 to 0.55) between migraineurs and controls (P = 0.26).
Figure 9.
Forest plot of six quantitative comparisons of neurovascular coupling during photic stimulation in the posterior circulation in migraineurs versus controls taken from five studies. BA: basilar artery; HIS: higher side; LOS: lower side; LS: left side of cerebral artery; PCA: posterior cerebral artery.
Cerebral autoregulation
During cerebral autoregulation, cerebral blood flow is maintained constant despite fluctuations of cerebral perfusion pressure. Six studies compared cerebral autoregulation in the anterior circulation of migraineurs to controls.23,27,36,52,69,70 Different metrics and indexes were used to assess cerebral autoregulation, which included gain, phase, Mx and Dx. Gain measures the amount of mean arterial blood pressure (MABP) variation that is transferred onto MBFV; a lower gain is associated with a more efficient cerebral autoregulation. Phase reflects the time delay between MABP and MBFV signals; a positive phase value indicates that cerebral autoregulation is intact, whereas a negative phase indicates impaired cerebral autoregulation. Mx is used to measure the correlation between MABP and MBFV; cerebral autoregulation is deemed as optimal when values of Mx are close to 0, whereas Mx values close to 1 indicate impaired cerebral autoregulation. Dx is used to measure the degree of correlation between diastolic MABP and diastolic MBFV; the higher the Dx, the poorer the cerebral autoregulation. Most studies found no difference in phase, gain, Dx or Mx between migraineurs and controls.27,52 However, Wallasch et al.69 found migraineurs to have a lower gain than controls.
Wallasch et al. used the Valsalva manoeuvre, a breathing exercise that results in a rapid increase of arterial blood pressure, to assess cerebral autoregulation between migraineurs and controls. Wallasch et al.70 found migraineurs to have a higher cerebral auto-regulatory response to the Valsalva manoeuvre, compared to controls, indicating poorer cerebral autoregulation.
The meta-analyses revealed no significant difference in phase (SMD = 0.13; 95%CI −0.11 to 0.36; P = 0.29), gain (SMD=−0.21; 95%CI −0.43 to 0.01; P = 0.06), Mx (SMD = 0.05; 95%CI −0.34 to 0.44; P = 0.80) and Dx (SMD = 0.29; 95%CI −0.08 to 0.66; P = 0.12) between migraineurs and controls in the anterior circulation (Supplemental Figures 1 to 4).
Reinhard et al. was the only study to compare the cerebral autoregulation of migraineurs to controls in the posterior circulation; no difference in phase, gain, Dx or Mx between migraineurs and controls was found.23
Cerebrovascular function assessed using fMRI
Resting cerebral blood flow
Eight studies used fMRI to assess the resting CBF of migraineurs to compare to controls.71–78
Hodkinson et al.72 compared CBF in the primary somatosensory cortex of migraineurs to controls. They found migraineurs without aura to have higher resting CBF in the primary somatosensory cortex when compared to controls.
Datta et al.71 compared CBF in the primary visual cortex between migraineurs and controls and found no difference in CBF. Additionally, Ances et al.77 found no difference in resting CBF in the occipital lobe when menstrual migraineurs were compared to controls.
Zhang et al.75 compared the grey, global and white matter CBF of migraineurs to controls and found no difference between the two groups. Similarly, Chen et al.78 found there to be no difference in grey matter CBF between migraineurs and controls.
Neurovascular coupling
Hu et al.73 used fMRI to assess cerebrovascular function; they found migraineurs to have lower neurovascular coupling during cognitive stimulation (Stroop task) than controls in the left inferior parietal gyrus, left superior marginal gyrus and left angular gyrus but higher neurovascular coupling in the right superior occipital gyrus and right superior parietal gyrus.
Cerebrovascular function assessed by NIRS
Three studies were identified from the systematic search that used NIRS to assess the cerebrovascular function of migraineurs to compare to controls.
Cerebrovascular responsiveness to hypercapnia
Akin et al. and Vernieri et al. assessed CVR to hypercapnia in the frontal cortex/frontotemporal region with conflicting findings; Akin et al. found migraineurs to have lower CVR to hypercapnia, whereas Vernieri et al. found migraineurs to have higher CVR to hypercapnia.48,79
Neurovascular coupling
Schytz et al.80 used NIRS to assess neurovascular coupling during the Stroop task in the frontal cortex and found no difference between migraineurs without aura and controls.
Cerebrovascular function assessed by SPECT
Six studies used SPECT to assess the resting CBF of migraineurs and controls.49,81–85 Cheng et al., De Benedittis et al. and Mirza et al. compared the resting CBF in the frontal, occipital, parietal and temporal lobes of migraineurs to controls.49,84,85 De Benedittis et al. found no difference in resting CBF between migraineurs and controls, whereas Cheng et al. and Mirza et al. both found migraineurs to have lower CBF in the frontal and occipital lobes.49,84,85 Cheng et al. additionally found migraineurs to have lower CBF in the parietal and occipital lobes.84 Meyer et al. compared the CBF of grey, white and global matter of migraineurs to controls and found there to be no difference in CBF between the two groups.81 Facco et al. and Levine et al. compared CBF in the posterior territory of migraineurs to controls and both found migraineurs to have lower posterior CBF than controls.82,83
Subgroup analysis
For all outcomes, subgroup analyses revealed no significant difference between migraine subgroups (migraine with aura, migraine without aura and mixed; chronic migraineurs and episodic migraineurs) (Supplemental Table 1(a) and (b), Supplemental Figures 5 to 8). However, the migraine with aura subgroup was the only subgroup where MBFV in the anterior circulation was found to be significantly higher than controls (SMD = 0.19; 95%CI 0.06 to 0.32, P = 0.005). Additionally, the PI in the posterior circulation was found to be significantly higher than controls in the migraine without aura subgroup only (SMD = 0.29; 95%CI 0.03 to 0.55, P = 0.03); this finding was not significant in the migraine with aura subgroup (SMD = 0.20; 95%CI −0.08 to 0.48, P = 0.16). The subgroup analyses also revealed that CVR to hypercapnia in the posterior circulation was significantly lower in migraineurs than controls for all subgroups (migraine without aura, mixed and episodic migraineurs) except the chronic migraine and migraine with aura subgroups.
Risk of bias
All studies included in the meta-analyses were of moderate or high methodological quality; scores for all studies were between 5 and 9 points on the NOS (see Table 1).
Publication bias
There was no evidence of publication bias upon visual examination of funnel plots for the meta-analyses for MBFV and CVR to hypercapnia in the anterior and posterior circulations (see Supplemental Figure 9). However, the P value for the Egger’s regression test for the meta-analysis for PI in the anterior circulation suggested that there was publication bias (Supplemental Table 2). Duval and Tweedie’s trim-and-fill estimated an additional six studies to the right of the mean and a revised point estimate of 0.22 (−0.04 to 0.24) for PI in the anterior circulation.
Discussion
Summary of findings
Our systematic search identified 70 articles that compared the cerebrovascular function of migraineurs with that of controls. This review and meta-analysis builds on the findings of a recent narrative review by Ornello et al. where the cerebrovascular function of migraineurs, as measured by TCD ultrasound and NIRS, was compared to controls. To the best of our knowledge, this is the first meta-analysis to investigate whether migraineurs and controls have differing cerebrovascular function. Similar to the review by Ornello et al., the qualitative findings of fMRI, NIRS, SPECT and TCD ultrasound studies included in this present review were inconsistent and did not provide a clear indication as to whether the cerebrovascular function of migraineurs differed to controls.13 However, our meta-analyses of TCD ultrasound studies were able to provide a better indication as to whether migraineurs have differing cerebrovascular function to controls as meta-analyses revealed migraineurs to have higher resting MBFV, higher PI and lower CVR to hypercapnia. Our findings of higher resting MBFV in migraineurs were evident in both the anterior and posterior cerebral circulations, whereas our findings of higher PI and lower CVR to hypercapnia were restricted to the posterior circulation. The revised point estimate after the trim-and-fill method revealed no significant difference for PI in the anterior circulation between migraineurs and controls. Subgroup analysis revealed higher resting MBFV in the anterior circulation to be more evident in migraineurs with aura, whereas higher PI and lower CVR to hypercapnia in the posterior circulation were found to be more evident in migraineurs without aura. Additionally, episodic migraineurs were found to have significantly lower CVR to hypercapnia in the posterior circulation.
Cerebrovascular responsiveness to hypercapnia
CVR assesses the ability of cerebral blood vessels to dilate and constrict in response to metabolic stimuli (i.e. arterial CO2). High concentrations of arterial CO2 (hypercapnia) stimulate increased production of nitric oxide (a potent vasodilator) from endothelial cells, whereas low arterial CO2 concentrations (hypocapnia) result in decreased concentrations of endothelial-derived nitric oxide.86,87 Therefore, CVR to hypercapnia assesses endothelial dysfunction by measuring the ability of cerebral endothelial cells to produce nitric oxide to facilitate cerebral vasodilation. Our finding of lower CVR to hypercapnia in migraineurs reflects a lower cerebrovascular dilator capacity in the posterior circulation of migraineurs. Lower cerebral vasodilator capacity has previously been associated with an increased risk of cerebrovascular disease (i.e. haemorrhagic and ischaemic stroke). King et al.88 found lower CVR to hypercapnia in patients with asymptomatic and symptomatic carotid artery stenosis to be associated with a higher risk of both ischaemic and haemorrhagic stroke. Interestingly, migraine has also been associated with an increased risk of cerebrovascular disease.89–92 A meta-analysis by Mahmoud et al.90 revealed migraineurs to have an increased risk of ischaemic stroke, haemorrhagic stroke and myocardial infarction compared to controls. Additionally, meta-analyses by Sacco et al.89 and Spector et al.91 have also found migraineurs to have an increased risk of haemorrhagic stroke and ischaemic stroke respectively when compared to controls. Therefore, that raises a question of whether the association between migraine and stroke is partially attributable to altered cerebrovascular function as represented by lower cerebral vasodilator capacity than controls. However, previous studies have predominantly found migraineurs with aura to have a higher risk of cerebrovascular disease but have been unable to confirm this association in migraineurs without aura. This is particularly interesting as our subgroup analysis revealed a significant difference in lower CVR to hypercapnia in the posterior circulation of migraineurs without aura but not migraineurs with aura. However, findings in the migraine with aura subgroup may have been limited by the high statistical heterogeneity.
It is plausible that our finding of higher cerebral arterial stiffness, as represented by higher PI in migraineurs reflects pathological changes that may underlie the lower CVR to hypercapnia in the posterior cerebral arteries. Once again, our finding of higher PI in the posterior circulation of migraineurs was significantly higher in the migraineurs without aura subgroup but not the migraineurs with aura subgroup; high statistical heterogeneity in the migraineurs with aura subgroup may have limited the findings. Additionally, our finding of higher resting MBFV in migraineurs may also be associated with the lower CVR to hypercapnia; the higher resting MBFV may dampen the ability of cerebral blood vessels to dilate which may make the brain more susceptible to ischemia in times where an increase of cerebral blood flow is urgently required.93
Cerebral autoregulation
Cerebral autoregulation describes the process whereby cerebral blood flow is maintained constant over a wide range of cerebral perfusion pressure, often from 50 to 150 mmHg in healthy adults.86 Cerebral autoregulation may be compromised in conditions such as postural hypotension or essential hypertension, where cerebral perfusion pressures exceed the limits of cerebral autoregulation, thereby increasing an individual’s risk of ischemia or hyperemia.86,87 Thus, an impairment of cerebral autoregulation in migraineurs may partially explain their increased risk of ischaemic stroke.
This review identified only six studies that compared cerebral autoregulation between migraineurs and controls; qualitative and quantitative analyses of autoregulation in the anterior and posterior circulations revealed no clear difference between migraineurs and controls. Interestingly, Reinhard et al. reported that migraineurs with aura have poorer autoregulation in the cerebellar circulation than controls.23 Further studies are needed to confirm whether autoregulation, particularly in posterior and cerebellar circulations, is compromised in migraineurs and whether this is associated with an increased risk of stroke.
Neurovascular coupling
Neurovascular coupling describes a process wherein cerebral blood flow is increased in local brain regions to meet the demands of higher neuronal activity.87 Therefore, impaired or abnormal neurovascular coupling may result in inadequate blood supply to areas of the brain that need it. Qualitative findings in our review indicate that neurovascular coupling during photic stimuli is greater in migraineurs than controls, in agreement with the narrative review by Ornello et al.,13 yet our meta-analyses reveal no difference in neurovascular coupling during photic stimuli between migraineurs and controls. However, statistical heterogeneity was high and due to the small number of studies included in the meta-analysis of neurovascular coupling, we were unable to test for risk of publication bias with Egger’s regression. Nonetheless, the lower CVR to hypercapnia and higher PI in the posterior circulation of migraineurs suggest that their neurovascular coupling may also differ from controls. If future studies confirm that migraine is associated with impaired neurovascular coupling, interventions that improve neurovascular coupling may help to improve migraine symptomatology.
Localisation of altered cerebrovascular function to the posterior circulation
The localisation of higher resting MBFV, higher PI and lower CVR to hypercapnia in the posterior circulation of migraineurs suggests that the posterior circulation is more susceptible to variations in cerebrovascular function than the anterior circulation. This increased susceptibility to altered cerebrovascular function may be attributable to differences in the anatomical structure of the circle of Willis in migraineurs. Cucchiara et al.94 and Bugnicourt et al.95 found that migraineurs were more likely to have an incomplete circle of Willis compared to controls, particularly in the posterior circulation.95 Additionally, Cucchiara et al.94 reported that anatomical variants of the circle of Willis in the posterior circulation were associated with more hemispheric CBF asymmetries than the anterior circulation. Therefore, it is plausible that differences in the anatomical structure of cerebral arteries in the posterior circulation may affect cerebral haemodynamics and consequently affect factors such as shear stress in a way that contributes to endothelial dysfunction; both abnormally high and low shear stress has been associated with endothelial dysfunction.96 Further studies are needed to confirm this association and to investigate whether hemispheric asymmetries of CBF in migraineurs with an incomplete circle of Willis are associated with unilateral migraine.
Proposed migraine pathophysiology and altered cerebrovascular function
It has been hypothesised that repeated episodes of migraine may lead to altered cerebrovascular function through repeated exposure to neurogenic inflammation, plasma protein extravasation and the release of vasoactive neuropeptides during migraine; therefore, abnormalities of cerebrovascular function may be expected to be more evident in chronic migraineurs than episodic migraineurs.12,24 However, our findings of significantly lower CVR to hypercapnia in the posterior circulation in episodic migraineurs but chronic migraineurs do not support this theory. Nevertheless, the number of comparisons and studies included in the separate meta-analyses for chronic and episodic migraineurs was small; there were only three comparisons from one study by Choi et al.18 in the subgroup analysis of episodic migraineurs. Further studies are needed to determine whether episodic migraineurs and chronic migraineurs differ in their cerebrovascular function. Additionally, some evidence suggests that altered cerebral vasodilator capacity may be involved in the pathophysiology of migraine through the initiation of cortical spreading depression (CSD) and subsequent pain pathways.97 CSD is a process that is believed to be responsible for producing aura symptoms in some migraineurs. During CSD, neurones spontaneously depolarise, resulting in excitation of neuronal and glial activity that is shortly followed by suppression of neuronal and glial activity.24 Interestingly, the depolarisation of neurons during CSD originates in posterior parts of the brain and spreads anteriorly; which may suggest that alterations in the cerebrovascular function of posterior brain regions may be involved in initiating CSD. Additionally, animal studies have shown that CSD can lead to neurogenic inflammation and plasma protein extravasation of cerebral blood vessels,98 possibly though activation of the TVS. Therefore, alterations of cerebrovascular function may trigger migraine via CSD, and CSD may in turn further alter cerebrovascular function via neurogenic inflammation. Future studies are needed to further investigate the potential relationships between cerebrovascular function, CSD, activation of the TVS and neurogenic inflammation.
Combined oral contraceptives and cerebrovascular function
The use of combined oral contraceptives (COCs) (i.e. contraceptives that contain both oestrogen and progesterone) in migraineurs may be associated with an increased risk of ischaemic stroke.99,100 Whilst endogenous oestrogens may lower blood pressure, exogenous oral oestrogens tend to raise blood pressure in women, albeit only marginally.101–103 There is currently a paucity of research on the effects of COCs on cerebrovascular function, particularly cerebral autoregulation, in migraineurs. If COCs are shown to affect alter or impair cerebral autoregulation, this may render female migraineurs more susceptible to ischaemic stroke if their cerebral perfusion pressure falls below their cerebral autoregulation limits. Further studies are needed to investigate the effect of COCs on cerebrovascular function in migraineurs.
Migraine prophylactic medications and cerebrovascular function
Beta-blockers (e.g. propranolol, atenolol), calcium channel blockers (e.g. nimodipine) and angiotensin II receptor blockers (e.g. candesartan) are vasoactive anti-hypertensive medications104,105 that have been shown to reduce the risk of stroke in patients with hypertension compared to placebo.106 They are also used for migraine prophylaxis but there is limited evidence on effects of these medications on cerebrovascular function in migraineurs. Beta-blockers are less effective than calcium channel blockers and angiotensin II receptor blockers for reducing the risk of cerebrovascular disease in patients with hypertension.107 While calcium channel blockers and angiotensin II receptor blockers are direct-acting vasodilators, beta-blockers lower blood pressure by reducing cardiac output; they may in fact inhibit endothelium-dependent vasodilatation. This difference in vasoactive properties may explain why they are not as effective at reducing risk of cerebrovascular disease. Additionally, in a recent meta-analysis, Webb et al. found that vasodilators such as calcium channel blockers were able to significantly increase CVR to hypercapnia and reduce PI when compared to non-vasodilator medications (such as beta blockers) or placebo. This is particularly interesting, given our findings of lower CVR to hypercapnia and higher PI in migraineurs compared to controls.107 However, findings from the meta-analyses by Webb et al. may have been limited by the heterogeneous populations and heterogeneous drug classes. Nonetheless, future studies should investigate whether the use of vasodilator medications in migraine prophylaxis is associated with improvements in cerebrovascular function and reduced risk of cerebrovascular disease, i.e. stroke, in migraineurs.
Limitations
We acknowledge that this systematic review and meta-analyses may have some limitations. Firstly, whilst every effort was made to capture all articles of relevance for this review and meta-analysis, some articles may have inadvertently been missed. Secondly, 30 of the 70 studies identified were not able to be included in the meta-analyses due to incomplete or incomparable data, which meant that the findings from the meta-analysis for each measure of cerebrovascular function might have been over- or under-estimated. Thirdly, all the studies included in the meta-analyses used TCD ultrasound to assess cerebrovascular function; however, TCD ultrasound is limited by its inability to assess cerebrovascular function in specific brain regions due to its low spatial resolution.108 Therefore, we are unable to identify specific brain regions associated with the cerebrovascular abnormalities detected by the meta-analyses. Fourthly, findings from this review may be limited by the heterogeneity between studies. All the meta-analyses, except PI in the posterior circulation and the subgroup analyses for chronic and episodic migraineurs, had high statistical heterogeneity. It is interesting to note that statistical heterogeneity was often higher in the migraine with aura subgroup than other subgroups; this high heterogeneity may have been due to differences in aura status (rare aura vs. frequent aura). Statistical heterogeneity remained high after subgroup analysis, suggesting that there may be additional contributors to heterogeneity such as differences in the vascular risk profile (i.e. smoking, type 2 diabetes mellitus and hypertension) of participants included in the studies. Smoking, diabetes and hypertension have been shown to impair cerebrovascular function.109 Most studies excluded participants with these vascular risk factors. However, the studies that did not adjust analyses for the vascular risk profile of migraineurs may have reported poorer cerebrovascular function that was associated with their vascular risk factors as opposed to purely migraine. Gender may also be another source of heterogeneity between the studies included in this review and meta-analysis. Women are three times more likely to suffer from migraine compared to men. This gender disparity may be influenced by the fluctuation of female sex hormones, particularly oestrogen, throughout a woman’s reproductive life and especially during menstruation. More than 50% of female migraineurs experience migraine attacks without aura that occur when oestrogen concentrations rapidly decrease prior to menstruation or during ovulation. These migraines are classified as menstrual migraines.110 Additionally, in a study by Nevo et al.,111 cerebrovascular function, as assessed by MBFV and cerebrovascular resistance, was found to change between different phases of the menstrual cycle. Therefore, the cerebrovascular function of women included in this review may have been influenced by their menstrual cycle phase (i.e. follicular, ovulation or luteal phase). Additionally, the use of COCs may also be a source of heterogeneity as COCs may increase resting MBFV and therefore may have affected the resting MBFV findings.
Laterality of cerebrovascular function assessment may be another source of heterogeneity; some studies assessed cerebrovascular function unilaterally, others bilaterally. Approximately 15% of migraineurs report migraine pain to occur on the same side for at least 90% of their migraine attacks.112 Therefore, migraineurs who experience these ‘side-locked’ migraines may have differences in cerebrovascular function between the right and left hemispheres. Most studies did not report whether the migraineurs in their study experienced lateralised migraine and therefore did not compare the migraine pain side to the non-migraine pain side. Additionally, we were not able to conduct a separate analysis for studies that compared the cerebrovascular function of the migraine pain side to the non-migraine pain side and therefore we may have not captured the complete picture of the cerebral haemodynamics in migraineurs.
Conclusion
This systematic review and meta-analysis was conducted to investigate whether migraine is associated with altered cerebrovascular function. Despite high statistical heterogeneity, findings from the meta-analyses indicate that migraineurs have altered cerebrovascular function, as represented by a higher resting MBFV, higher PI and lower CVR to hypercapnia in the posterior circulation and higher resting MBFV in the anterior circulation. The higher PI and lower CVR to hypercapnia in the posterior circulation were particularly evident in migraineurs without aura, whereas higher resting MBFV in the anterior circulation was particularly evident in migraineurs with aura. Our findings of altered cerebrovascular function being predominantly restricted to the posterior circulation suggest that the posterior circulation of migraineurs may be more susceptible to alterations in cerebrovascular function, possibly due to anatomical variations. Additionally, a complex relationship may exist between altered cerebrovascular function in the posterior cerebral circulation and CSD that partially underlies the pathophysiology of migraine. Future studies should investigate relationships between cerebrovascular function (particularly in the posterior circulation) and migraine and investigate whether normalisation or amelioration of cerebrovascular function is able to alleviate migraine. Additionally, future studies should also investigate the effect of vasoactive migraine prophylactic medications on cerebrovascular function in migraineurs and whether these medications are able to reduce vascular risk in migraineurs.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20964344 for Profiling cerebrovascular function in migraine: A systematic review and meta-analysis by Jemima SA Dzator, Peter RC Howe and Rachel HX Wong in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X20964344 for Profiling cerebrovascular function in migraine: A systematic review and meta-analysis by Jemima SA Dzator, Peter RC Howe and Rachel HX Wong in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Organisation WH. Headache disorders, https://www.who.int/news-room/fact-sheets/detail/headache-disorders (2016, accessed 1 September 2020).
- 2.Woldeamanuel YW, Cowan RP.Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci 2017; 372: 307–315. [DOI] [PubMed] [Google Scholar]
- 3.Victoria So. Headache – migraine, https://www.betterhealth.vic.gov.au/health/ConditionsAndTreatments/headache-migraine (2018, accessed 1 September 2020)
- 4.Medicine USNLo. Migraine, https://medlineplus.gov/migraine.html (2018, accessed 1 September 2020)
- 5.Society IH. 1.3 Chronic migraine, 2019.
- 6.May A, Goadsby PJ.The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab 1999; 19: 115–127. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra R.Understanding migraine: potential role of neurogenic inflammation. Ann Indian Acad Neurol 2016; 19: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci 2013; 9: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacco S, Ripa P, Grassi D, et al. Peripheral vascular dysfunction in migraine: a review. J Headache Pain 2013; 14: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pretnar-Oblak J, Sabovic M, Zaletel M.Associations between systemic and cerebral endothelial impairment determined by cerebrovascular reactivity to L-arginine. Endothelium 2007; 14: 73–80. [DOI] [PubMed] [Google Scholar]
- 11.Perko D, Pretnar-Oblak J, Šabovič M, et al. Associations between cerebral and systemic endothelial function in migraine patients: a post-hoc study. BMC Neurol 2011; 11: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajan R, Khurana D, Lal V.Interictal cerebral and systemic endothelial dysfunction in patients with migraine: a case-control study. J Neurol Neurosurg Psychiatry 2015; 86: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 13.Ornello R, Frattale I, Caponnetto V, et al. Cerebral vascular reactivity and the migraine-stroke relationship: a narrative review. J Neurol Sci 2020; 414: 116887. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duval S, Tweedie R.Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 16.Podgorac A, Petrusic I, Radojicic A, et al. Breath holding index in episodic primary headaches. VSP 2018; 75: 347–351. [Google Scholar]
- 17.Karacay Ozkalayci S, Nazliel B, Batur Caglayan HZ, et al. Cerebral blood flow velocity in migraine and chronic tension-type headache patients. J Pain Res 2018; 11: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi HA, Mj L, Chung CS.Cerebral endothelial dysfunction in reversible cerebral vasoconstriction syndrome: a case-control study. J Headache Pain 2017; 18: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akgun H, Tasdemir S, Ulas UH.Reduced breath holding index in patients with chronic migraine. Acta Neurol Belg 2015; 115: 323–327. [DOI] [PubMed] [Google Scholar]
- 20.Fabjan A, Bajrovic FF, Musizza B, et al. Study of neurovascular coupling during cold pressor test in patients with migraine. Cephalalgia 2015; 35: 692–701. [DOI] [PubMed] [Google Scholar]
- 21.Karadas O, Gul HL, Ozturk B, et al. The effects of topiramate therapy on cerebral metabolism in migraine with aura patients. Turk Neurosurg 2014; 24: 704–709. [DOI] [PubMed] [Google Scholar]
- 22.Quirico PE, Allais G, Ferrando M, et al. Effects of the acupoints PC 6 neiguan and LR 3 taichong on cerebral blood flow in normal subjects and in migraine patients. Neurol Sci 2014; 35: 129–S133. [DOI] [PubMed] [Google Scholar]
- 23.Reinhard M, Schork J, Allignol A, et al. Cerebellar and cerebral autoregulation in migraine. Stroke 2012; 43: 987–993. [DOI] [PubMed] [Google Scholar]
- 24.Perko D, Pretnar-Oblak J, Sabovic M, et al. Cerebrovascular reactivity to l-arginine in the anterior and posterior cerebral circulation in migraine patients. Acta Neurol Scand 2011; 124: 269–274. [DOI] [PubMed] [Google Scholar]
- 25.Min JH, Kwon HM, Nam H.The effect of propranolol on cerebrovascular reactivity to visual stimulation in migraine. J Neurol Sci 2011; 305: 136–138. [DOI] [PubMed] [Google Scholar]
- 26.El-Khawas HM, Shalash AS, Said AM, et al. Multimodal visual functions and cerebrovascular reactivity in migraine patients between attacks. Egypt J Neurol Psychiatr Neurosurg 2010; 47: 655–664. [Google Scholar]
- 27.Reinhard M, Wehrle-Wieland E, Roth M, et al. Preserved dynamic cerebral autoregulation in the middle cerebral artery among persons with migraine. Exp Brain Res 2007; 180: 517–523. [DOI] [PubMed] [Google Scholar]
- 28.Arjona A, de Torres LAP, Serrano-Castro PJ, et al. A transcranial Doppler study in interictal migraine and tension-type headache. J Clin Ultrasound 2007; 35: 372–375. [DOI] [PubMed] [Google Scholar]
- 29.Nedeltchev K, Arnold M, Schwerzmann M, et al. Cerebrovascular response to repetitive visual stimulation in interictal migraine with aura. Cephalalgia 2004; 24: 700–706. [DOI] [PubMed] [Google Scholar]
- 30.Dora B, Balkan S, Tercan E.Normalization of high interictal cerebrovascular reactivity in migraine without aura by treatment with flunarizine. Headache 2003; 43: 464–469. [DOI] [PubMed] [Google Scholar]
- 31.Thomaides T, Karagounakis D, Spantideas A, et al. Transcranial Doppler in migraine attacks before and after treatment with oral zolmitriptan or sumatriptan. Headache 2003; 43: 54–58. [DOI] [PubMed] [Google Scholar]
- 32.Dora B, Balkan S.Exaggerated interictal cerebrovascular reactivity but normal blood flow velocities in migraine without aura. Cephalalgia 2002; 22: 288–290. [DOI] [PubMed] [Google Scholar]
- 33.Chernyshev OY, Vein AM, Mathew NT, et al. Blood flow velocity and pulsatility index differences in patients with unilateral migraine. Headache 2001; 41: 704–709. [DOI] [PubMed] [Google Scholar]
- 34.Fiermonte G, Annulli A, Pierelli F.Transcranial Doppler evaluation of cerebral hemodynamics in migraineurs during prophylactic treatment with flunarizine. Cephalalgia 1999; 19: 492–496. [DOI] [PubMed] [Google Scholar]
- 35.Kastrup A, Thomas C, Hartmann C, et al. Cerebral blood flow and CO2 reactivity in interictal migraineurs: a transcranial Doppler study. Headache 1998; 38: 608–613. [DOI] [PubMed] [Google Scholar]
- 36.Heckmann JG, Hilz MJ, Katalinic A, et al. Myogenic cerebrovascular autoregulation in migraine measured by stress transcranial Doppler sonography. Cephalalgia 1998; 18: 133–137. [DOI] [PubMed] [Google Scholar]
- 37.Totaro R, Marini C, De Matteis G, et al. Cerebrovascular reactivity in migraine during headache-free intervals. Cephalalgia 1997; 17: 191–194. [DOI] [PubMed] [Google Scholar]
- 38.Silvestrini M, Matteis M, Troisi E, et al. Cerebrovascular reactivity in migraine with and without aura. Headache 1996; 36: 37–40. [DOI] [PubMed] [Google Scholar]
- 39.Piccini P, Pavese N, Palombo C, et al. Transcranial doppler ultrasound in migraine and tension-type headache after apomorphine administration – double-blind crossover versus placebo study. Cephalalgia 1995; 15: 399–403. [DOI] [PubMed] [Google Scholar]
- 40.Fiermonte G, Pierelli F, Pauri F, et al. Co2 reactivity in migraine with aura and without aura – a transcranial doppler study. Acta Neurol Scand 1995; 92: 166–169. [DOI] [PubMed] [Google Scholar]
- 41.Abernathy M, Donnelly G, Kay G, et al. Transcranial Doppler sonography in headache-free migraineurs. Headache 1994; 34: 198–203. [DOI] [PubMed] [Google Scholar]
- 42.Thomsen LL, Iversen HK, Brinck TA, et al. Arterial supersensitivity to nitric-oxide (nitroglycerin) in migraine sufferers. Cephalalgia 1993; 13: 395–399. [DOI] [PubMed] [Google Scholar]
- 43.Anzola GP, Magoni M, Dalla Volta G.Abnormal photoreactivity in lctal migraine – reversal by sumatriptan. Headache 1993; 33: 417–420. [DOI] [PubMed] [Google Scholar]
- 44.Rieke K, Gallen CC, Baker L, et al. Transcranial doppler ultrasound and magnetoencephalography in migraine. J Neuroimaging 1993; 3: 109–114. [DOI] [PubMed] [Google Scholar]
- 45.Totaro R, Matteis GD, Marini C, et al. Blood-flow in migraine with aura – a transcranial Doppler sonography study. Headache 1992; 32: 446–451. [DOI] [PubMed] [Google Scholar]
- 46.Haring HP. and Aichner F. Hemodynamic-findings in migraine patients on transcranial Doppler sonography. Wien Klin Wochen 1992; 104: 620–625. [PubMed] [Google Scholar]
- 47.Zaletel M, Strucl M, Bajrovi FF, et al. Coupling between visual evoked cerebral blood flow velocity responses and visual evoked potentials in migraneurs. Cephalalgia 2005; 25: 567–574. [DOI] [PubMed] [Google Scholar]
- 48.Vernieri F, Tibuzzi F, Pasqualetti P, et al. Increased cerebral vasomotor reactivity in migraine with aura: an autoregulation disorder? A transcranial Doppler and near-infrared spectroscopy study. Cephalalgia 2008; 28: 689–695. [DOI] [PubMed] [Google Scholar]
- 49.Benedittis D.G. CBF changes during headache-free periods and spontaneous/induced attacks in migraine with and without aura: a TCD and SPECT comparison study. J Neurosurg Sci 1999; 43: 141–147. [PubMed] [Google Scholar]
- 50.Altamura C, Paolucci M, Brunelli N, et al. Migraineurs with aura present an increased cerebral hemodynamics in the anterior and posterior circulation compared with stroke patients and controls. Neurol Sci 2018; 39: 101–102. [DOI] [PubMed] [Google Scholar]
- 51.Altamura C, Paolucci M, Brunelli N, et al. Right-to-left shunts and hormonal therapy influence cerebral vasomotor reactivity in patients with migraine with aura. PLoS One 2019; 14: e0220637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gollion C, Nasr N, Fabre N, et al. Cerebral autoregulation in migraine with aura: a case control study. Cephalalgia 2019; 39: 635–640. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Quintanilla V, Toriello M, Palacio E, et al. Systemic and cerebral endothelial dysfunction in chronic migraine. A case-control study with an active comparator. Cephalalgia 2016; 36: 552–560. [DOI] [PubMed] [Google Scholar]
- 54.Harer C, von Kummer R. CEREBROVASCULAR co2 reactivity in migraine – assessment by transcranial Doppler ultrasound. J Neurol 1991; 238: 23–26. [DOI] [PubMed] [Google Scholar]
- 55.Kastrup A, Thomas C, Hartmann C, et al. No effect of prophylactic treatment with metoprolol on cerebrovascular CO2 reactivity in migraineurs. Cephalalgia 1998; 18: 353–357. [DOI] [PubMed] [Google Scholar]
- 56.Lee MJ, Cho S, Woo S-Y, et al. Paradoxical association between age and cerebrovascular reactivity in migraine: a cross-sectional study. J Neurol Sci 2019; 398: 204–209. [DOI] [PubMed] [Google Scholar]
- 57.Silvestrini M, Baruffaldi R, Bartolini M, et al. Basilar and middle cerebral artery reactivity in patients with migraine. Headache 2004; 44: 29–34. [DOI] [PubMed] [Google Scholar]
- 58.Sousa A, Gc Coelho R, Teixeira R.Cerebrovascular reactivity in migraineurs. Headache Med 2011; 2: 10–12. [Google Scholar]
- 59.Thomas TD, Harpold GJ, Troost BT.Cerebrovascular reactivity in migraineurs as measured by transcranial Doppler. Cephalalgia 1990; 10: 95–99. [DOI] [PubMed] [Google Scholar]
- 60.Valikovics A, Olah L, Fulesdi B, et al. Cerebrovascular reactivity measured by transcranial Doppler in migraine. Headache 1996; 36: 323–328. [DOI] [PubMed] [Google Scholar]
- 61.Raichle ME, Plum F.Hyperventilation and cerebral blood flow. Stroke 1972; 3: 566–575. [DOI] [PubMed] [Google Scholar]
- 62.Thomsen LL, Iversen HK, Olesen J.Increased cerebrovascular pCO2 reactivity in migraine with aura – a transcranial Doppler study during hyperventilation. Cephalalgia 1995; 15: 211–215. [DOI] [PubMed] [Google Scholar]
- 63.Zwetsloot CP, Caekebeke JFV, Odink J, et al. Vascular reactivity during migraine attacks – a transcranial Doppler study. Headache 1991; 31: 593–595. [DOI] [PubMed] [Google Scholar]
- 64.Backer M, Hammes M, Sander D, et al. Changes of cerebrovascular response to visual stimulation in migraineurs after repetitive sessions of somatosensory stimulation (acupuncture): a pilot study. Headache 2004; 44: 95–101. [DOI] [PubMed] [Google Scholar]
- 65.Backer M, Sander D, Hammes MG, et al. Altered cerebrovascular response pattern in interictal migraine during visual stimulation. Cephalalgia 2001; 21: 611–616. [DOI] [PubMed] [Google Scholar]
- 66.Thie A, Carvajal-Lizano M, Schlichting U, et al. Tests of cerebrovascular reactivity in migraine – a transcranial Doppler study. J Neurol 1992; 239: 338–342. [DOI] [PubMed] [Google Scholar]
- 67.Wolf ME, Jager T, Bazner H, et al. Changes in functional vasomotor reactivity in migraine with aura. Cephalalgia 2009; 29: 1156–1164. [DOI] [PubMed] [Google Scholar]
- 68.Zaletel M, In M.Neurovascular coupling during ageing. Zdravniski Vestnik Slovenian Med J 2008; 77: II65–II70. [Google Scholar]
- 69.MüLler M, Marziniak M.The linear behavior of the system middle cerebral artery flow velocity and blood pressure in patients with migraine – lack of autonomic control? Stroke 2005; 36: 1886–1890. [DOI] [PubMed] [Google Scholar]
- 70.Wallasch TM, Beckmann P, Kropp P.Cerebrovascular reactivity during the valsalva maneuver in migraine, tension-type headache and medication overuse headache. Funct Neurol 2011; 26: 223–227. [PMC free article] [PubMed] [Google Scholar]
- 71.Datta R, Aguirre GK, Hu S, et al. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia 2013; 33: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hodkinson DJ, Veggeberg R, Wilcox SL, et al. Primary somatosensory cortices contain altered patterns of regional cerebral blood flow in the interictal phase of migraine. PLoS One 2015; 10: e0137971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu B, Yu Y, Dai YJ, et al. Multi-modal MRI reveals the neurovascular coupling dysfunction in chronic migraine. Neuroscience 2019; 419: 72–82. [DOI] [PubMed] [Google Scholar]
- 74.Loehrer E, Vernooij MW, van der Lugt A, et al. Migraine and cerebral blood flow in the general population. Cephalalgia 2015; 35: 190–198. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Q, Datta R, Detre JA, et al. White matter lesion burden in migraine with aura may be associated with reduced cerebral blood flow. Cephalalgia 2017; 37: 517–524. [DOI] [PubMed] [Google Scholar]
- 76.Arkink EB, Bleeker EJW, Schmitz N, et al. Cerebral perfusion changes in migraineurs: a voxelwise comparison of interictal dynamic susceptibility contrast MRI measurements. Cephalalgia 2012; 32: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ances BM, Detre JA.Perfusion changes with photic stimulation during two phases of the menstrual cycle: a pilot study comparing controls and true menstrual migraine patients. Cephalalgia 2003; 23: 907–913. [DOI] [PubMed] [Google Scholar]
- 78.Chen ZY, Chen XY, Liu MY, et al. Evaluation of gray matter perfusion in episodic migraine using voxel-wise comparison of 3D pseudo-continuous arterial spin labeling. J Headache Pain 2018; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akin A, Bilensoy D, Emir UE, et al. Cerebrovascular dynamics in patients with migraine: near-infrared spectroscopy study. Neurosci Lett 2006; 400: 86–91. [DOI] [PubMed] [Google Scholar]
- 80.Schytz HW, Ciftci K, Akin A, et al. Intact neurovascular coupling during executive function in migraine without aura: interictal near-infrared spectroscopy study. Cephalalgia 2010; 30: 457–466. [DOI] [PubMed] [Google Scholar]
- 81.Meyer JS, Terayama Y, Konno S, et al. Age-related cerebrovascular disease alters the symptomatic course of migraine. Cephalalgia 1998; 18: 202–208. [DOI] [PubMed] [Google Scholar]
- 82.Facco E, Munari M, Baratto F, et al. Regional cerebral blood flow (rCBF) in migraine during the interictal period. Different rCBF patterns in patients with and without aura. Cephalalgia 1996; 16: 161–168. [DOI] [PubMed] [Google Scholar]
- 83.Levine SR, Welch KMA, Ewing JR, et al. Asymmetric cerebral blood-flow patterns in migraine. Cephalalgia 1987; 7: 245–248. [DOI] [PubMed] [Google Scholar]
- 84.Cheng MH, Wen SL, Zhou HJ, et al. Evaluation of headache and regional cerebral flood flow in patients with migraine. Clin Nucl Med 2013; 38: 874–877. [DOI] [PubMed] [Google Scholar]
- 85.Mirza M, Tutuş A, Erdoğan F, et al. Interictal SPECT with tc-99m HMPAO studies in migraine patients. Acta Neurol Belg 1998; 98: 190–194. [PubMed] [Google Scholar]
- 86.Altamura C, Paolucci M, Vernieri F.Migraine, endothelium, hemodynamics. Neurol Sci 2018; 39: 87–89. [DOI] [PubMed] [Google Scholar]
- 87.Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, et al. Cerebral autoregulation-oriented therapy at the bedside a comprehensive review. Anesthesiology 2017; 126: 1187–1199. [DOI] [PubMed] [Google Scholar]
- 88.King A, Serena J, Bornstein NM, et al. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke 2011; 42: 1550–1555. [DOI] [PubMed] [Google Scholar]
- 89.Sacco S, Ornello R, Ripa P, et al. Migraine and hemorrhagic stroke: a meta-analysis. Stroke 2013; 44: 3032–3038. [DOI] [PubMed] [Google Scholar]
- 90.Mahmoud AN, Mentias A, Elgendy AY, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018; 8: e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med 2010; 123: 612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sacco S, Kurth T.Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep 2014; 16: 524. [DOI] [PubMed] [Google Scholar]
- 93.Bush AM, Borzage MT, Choi S, et al. Determinants of resting cerebral blood flow in sickle cell disease. Am J Hematol 2016; 91: 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cucchiara B, Wolf RL, Nagae L, et al. Migraine with aura is associated with an incomplete circle of willis: results of a prospective observational study. PLoS One 2013; 8: e71007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bugnicourt JM, Garcia PY, Peltier J, et al. Incomplete posterior circle of Willis: a risk factor for migraine? Research submission. Headache 2009; 49: 879–886. [DOI] [PubMed] [Google Scholar]
- 96.Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling. Molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007; 49: 2379–2393 [DOI] [PubMed] [Google Scholar]
- 97.Murinova N, Krashin DL, Lucas S.Vascular risk in migraineurs: interaction of endothelial and cortical excitability factors. Headache 2014; 54: 583–590. [DOI] [PubMed] [Google Scholar]
- 98.Cui Y, Kataoka Y, Watanabe Y.Role of cortical spreading depression in the pathophysiology of migraine. Neurosci Bull 2014; 30: 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sacco S, Merki-Feld GS, Ae KL, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and reproductive health (ESC). J Headache Pain 2017; 18: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ornello R, Canonico M, Merki-Feld GS, et al. Migraine, low-dose combined hormonal contraceptives, and ischemic stroke in young women: a systematic review and suggestions for future research. Expert Rev 2020; 20: 313–317. [DOI] [PubMed] [Google Scholar]
- 101.Nichols M, Robinson G, Bounds W, et al. Effect of four combined oral contraceptives on blood pressure in the pill-free interval. Contraception 1993; 47: 367–376. [DOI] [PubMed] [Google Scholar]
- 102.Abramson BL, Melvin RG.Cardiovascular risk in women: focus on hypertension. Can J Cardiol 2014; 30: 553–559. [DOI] [PubMed] [Google Scholar]
- 103.Barton M, Meyer MR.Postmenopausal hypertension: mechanisms and therapy. Hypertension 2009; 54: 11–18. [DOI] [PubMed] [Google Scholar]
- 104.Chen GJ, Yang MS.The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: a meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PLoS One 2013; 8: e57854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu GC, Cheng JW, Zhu KM, et al. A systematic review of angiotensin receptor blockers in preventing stroke. Stroke 2009; 40: 3876–3878. [DOI] [PubMed] [Google Scholar]
- 106.Dahlof B.Prevention of stroke: new evidence. Eur Heart J 2009; 11: F33–F38. [Google Scholar]
- 107.Webb AJS.Effects of vasodilating medications on cerebral haemodynamics in health and disease: systematic review and meta-analysis. J Hypertension 2019; 37: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leoni RF, Mazzetto-Betti KC, Silva AC, et al. Assessing cerebrovascular reactivity in carotid steno-occlusive disease using MRI BOLD and ASL techniques. Radiol Res Pract 2012; 2012: 268483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Silvestrini M, Troisi E, Matteis M, et al. Effect of smoking on cerebrovascular reactivity. J Cereb Blood Flow Metab 1996; 16: 746–749. [DOI] [PubMed] [Google Scholar]
- 110.Sacco S, Ricci S, Degan D, et al. Migraine in women: the role of hormones and their impact on vascular diseases. J Headache Pain 2012; 13: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nevo O, Soustiel JF, Thaler I.Cerebral blood flow is increased during controlled ovarian stimulation. Am J Physiol 2007; 293: H3265–H3269. [DOI] [PubMed] [Google Scholar]
- 112.Loder E, Weizenbaum E, Giddon D.Migraine pain location and measures of healthcare use and distress: an observational study. Pain Res Manag 2018; 2018: 6157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X20964344 for Profiling cerebrovascular function in migraine: A systematic review and meta-analysis by Jemima SA Dzator, Peter RC Howe and Rachel HX Wong in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-2-jcb-10.1177_0271678X20964344 for Profiling cerebrovascular function in migraine: A systematic review and meta-analysis by Jemima SA Dzator, Peter RC Howe and Rachel HX Wong in Journal of Cerebral Blood Flow & Metabolism