Abstract
Ectomycorrhizal fungi (ECMF) can develop the resistance of host plants to heavy metal stress. However, little is known about the response of ECMF to heavy metal exposure. In this study, the growth and physiological indices of Lepista sordida under Cd and Cu stress were studied. The growth of L. sordida on PDA medium under Cd and Cu stress was observed using scanning electron microscopy (SEM). After the addition of Cd and Cu to the medium, the mycelium started twisting, breaking, sticking together, and even dissolving. In the control group, a good and luxuriant mycelium growth of L. sordida along with the numerous clamp connections was observed. The mycelial biomass decreased with increasing concentrations of heavy metals in a liquid medium. The catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), and ascorbate peroxidase (APX) activities were also investigated, and the results showed that the Cd and Cu treatments caused a significant increase in the antioxidant enzyme activities. The contents of soluble protein, soluble sugar, and free proline in L. sordida were investigated, and it was found that the contents initially increased and then decreased with the increasing concentrations of Cd and Cu. However, the content of malondialdehyde (MDA) increased with the increasing concentrations of Cd and Cu. In conclusion, the present study provides a theoretical basis for the better utilization of Ectomycorrhizal fungal resources for the remediation of soil contaminated with heavy metal.
Keywords: Ectomycorrhizal fungi (ECMF), Lepista sordida, Cd and Cu stress, Pure culture
Introduction
Since the beginning of the 21st century, there has been a rapid increase in industrial development and production. This has also been accompanied by the unreasonable disposal of household waste. Heavy metal contamination of soil has become a severe environmental problem worldwide (Ruttens et al., 2006), attracting much attention from the scientific community (Granero & Domingo, 2002). The rapid industrialization of mining, smelting, lead batteries, and other industries, and the use of sewage to irrigate farmland have led to serious heavy metal contamination in the soil. Cadmium (Cd) and copper (Cu) are the most common heavy metal contaminants. Cd contamination exceeded the standard by 7.0 percent, ranking Cd first among all the pollutants (Liu, Wu & Zhang, 2019). Pollution of Cd and Cu in the soil leads to excessive levels of heavy metals in the soil, with the pollution of Cd and Cu in agricultural and forestry production increasingly at serious levels (Wang et al., 2020). Heavy metal pollution in the soil is stealthy, long-term, accumulative, and irreversible. Some heavy metal elements, such as Cd and Cu, are easy to accumulate in plants, especially food crops, and harm human health through dietary exposure. In this context, remediation of soil contaminated by heavy metals becomes necessary (Wageh, Hitoshi & Waleed, 2019; Silvia, Olga & Alfredo, 2018). Among the various soil remediation methods, bioremediation is one of the most important (Selosse, Baudoin & Vandenkoornhuyse, 2004).
Mycorrhizal fungi can not only promote plant growth but also protect the plant against heavy metal stress (Wu et al., 2020; Chen et al., 2019; Meier et al., 2011; Moora et al., 2011; Gadd, 2007). Many species of fungi inhabit the forest ecosystems and can resist heavy metal stress (Li et al., 2020; Li et al., 2012; Mandyam & Jumpponen, 2005). However, their mechanisms of resistance to heavy metal stress are unclear. Thus, the ability of mycorrhizal fungi to withstand heavy metal stress needs to be investigated extensively.
Ectomycorrhizal fungi (ECMF) are an important group of fungi, which are ubiquitous in many forest ecosystems (Yin et al., 2017; Yin et al., 2018). These fungi can increase the nutrient levels in the soil to the levels required by plants for their growth and also help encounter adverse conditions to survive the environmental stress (Mucha et al., 2006; Edda et al., 2010; Sharma, Rajak & Pandey, 2010). ECMF coexist with plant roots, promote plant growth, and even affect the soil microenvironment. Thus, these fungi play an important role in the relationship between plants and the environment (Regvar et al., 2010). Ma (2013) studied the effect of ECMF on the ability of Populus × canescens to absorb and tolerate Cd, and found that ECMF inoculation could improve the resistance of P. × canescens to Cd and Cu, so that the plants could grow better.
However, the mechanism by which ECMF resist heavy metal stress is not clear. Relevant studies have shown that ECMF can promote plant growth and enhance plant resistance to heavy metals so that the host plant can grow better under severe stress (Zhan, Li & Jiang, 2019; Zhang, Hu & Yan, 2019; Andrade-Linares et al., 2011; Abbott, Robson & Boer, 1984).
Heavy metal stress can induce the production of a large number of reactive oxygen species (ROS), which can induce biological damage. Fungi can adopt various mechanisms to resist the toxicity of heavy metals. It is an important intracellular defense mechanism to secrete antioxidant enzymes to reduce the physiological toxicity caused by heavy metals (Yan et al., 2017). For instance, ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) are involved in reducing heavy metal stress and resisting ROS. POD, SOD, and CAT are important ROS-scavenging systems. SOD is the first barrier for cells to resist the stress caused by ROS, which can then be converted into H2O2, which has a relatively weak oxidation effect. Then, H2O2 can be decomposed into H2O by POD and CAT (Li et al., 2015). There are many studies on the physiological responses of host plants to heavy metals. However, little is known about the role of antioxidant enzymes, such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD), in reducing heavy metal stress and resisting reactive oxygen species (ROS) (Grataõ et al., 2005; Hou et al., 2007; Zhang et al., 2007).
Osmotic regulatory substances, including soluble sugar, soluble protein, free proline, and malondialdehyde (MDA), are the products of membrane lipid peroxidation, which can develop the potential of the lining cells and osmotic pressure in the cells after exposure to environmental stress (Yin et al., 2018). Therefore, the changes occurring in the osmotic regulatory substances in the ECM fungal cells under heavy metal stress can be used to evaluate the level of environmental stress (Yin et al., 2020).
At present, there is still a lack of basic research data on the development of mycorrhizal agents suitable for heavy metal-contaminated soil, with quantitative research on the stress resistance of Ectomycorrhizal bacteria reported rarely. The present study involved, from the perspective of Lepista Sordida’s growth and physiological response, the simulation of the response of two Ectomycorrhizal fungi under heavy metal (Cd2+ and Cu2+) stress, to provide a theoretical basis for the development of mycorrhizal agents for the remediation of soil polluted with heavy metals.
This study aimed to quantify the growth and physiological responses of ECMF to different Cd and Cu concentrations through the analysis of the antioxidant enzyme activities (APX, CAT, POD, and SOD) and osmotic adjustment substances (soluble sugar, soluble protein, MDA, and free proline). The results of this study can provide a basis for the bioremediation of heavy metal-contaminated soil and the utilization of Ectomycorrhizal fungal resources.
Material and Methods
Organism
The fungal strain HLXM obtained from the Liaoning Poplar Research Institute was used in the present study. The strain was grown for ten days on the PDA medium at a pH of around 6.8, as described by Brundrett et al. (1996).
Molecular biology verification of the taxonomic status of strain
PCR amplification was performed using the ITS1 and ITS4 primers (ITS1, 5′-TCCGTAGGTGAACCTGCGG-3′;ITS4, 5′-TCCTCCGCTTATTATTGATATGC-3′), synthesized by Shanghai Bio-Chemical Co. LTD. The PCR reaction system was: 10 × PCR buffer 5 µL, dNTPS 5 µL, ITS1 primer 5 µL, ITS4 primer 5 µL, Taq enzyme 0.75 µL, DNA template 2.5 µL, ddH2O 26.75 µL, and total volume 50 µL. The PCR reaction conditions were: pre-denaturation at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing at 56 °C for 45 s, elongation at 72 °C for 2 min, 30 cycles, and supplementation at 72 °C for 10 min. The non-pure PCR products were directly sequenced, and the sequencing results were Blast analyzed in the DNA database in GenBank to determine their classification status. Meanwhile, the sequence was submitted to obtain the gene login number, and the adjacency method in MEGA5.0 software was used to construct the phylogenetic tree for the ITS region (ITS1+ 5.8s +ITS2) for phylogenetic relationship analysis.
Effects of Cd and Cu stress on strain growth
The strain was cut with a sterile puncher (φ = 10 mm) and then cultured on solid PDA medium supplemented with different concentrations of Cd and Cu (0, 0.1, 0.2, 0.3, 0.4, and 0.5 mmolL−1). The diameter of the mycelium was measured every five days using the cross-sectional method. The state of mycelium of L. sordida was determined by scanning electron microscopy (SEM). The strain was grown in a liquid medium containing Cd and Cu for one week. The aliquot was then filtered to retain the hyphae. After oven-drying the mycelium at 80 °C for 10 h, the hyphae were weighed to measure the dry weight of L. sordida.
Antioxidant enzyme activities of strain
The CAT and POD enzymes were extracted by mixing 0.1 g of hypha in 10 mL of 50 mmol L−1 phosphoric acid buffer solution, pH 7.0, followed by centrifuging the mixture at 10,000 rpm for 20 min at 4 °C. The activities of both CAT and POD in the supernatant were analyzed. The SOD and APX enzymes were extracted using 0.1 g of hypha in 10 mL of 50 mmol L−1 phosphoric acid buffer solution, pH 7.8. The extract was centrifuged at 10,000 rpm for 20 min at 4 °C, and the activities of total SOD and APX in the supernatant were analyzed.
Commercial kits obtained from Jiancheng, Nanjing, China were used to measure the activities of antioxidant enzymes according to the respective specifications and calculation formulas. These experiments were repeated three times.
Determination of the contents of osmotic adjustment substances
The content of soluble protein was determined following the method described by Christos, Konstantinos & George (2008). One hundred mg of Coomassie Brilliant Blue (CBB) G-250 100 mg L−1 reagent was added to 50 mL of 95% ethanol and mixed until dissolved. One hundred mL of 15 molL−1 H3PO4 was added to 500 mL of distilled H2O and mixed well. Hyphae (0.5 g) were ground in 10 mL distilled water and centrifuged at 10,000 rpm for 5 min. Finally, 1 mL supernatant was added to 5 mL CBB reagent and mixed well. The absorbance was recorded at 595 nm. The experiment was repeated three times.
The content of soluble sugars was analyzed using the anthrone method described by Yemm & Willis (1954). The fine powder of hyphae (about 100 mg) was homogenized in 3 mL of 80% ethanol, followed by incubation in an ultrasonic bath at 80 °C for 30 min. After centrifugation (6000 g, 25 °C, 10 min), the supernatant was collected. The pellet was extracted as above, and the supernatant was collected and combined with the previous one. After adding 2 mL of the anthrone reagent to the supernatant, the mixture was heated in boiling water for 7 min. After the mixture was cooled to room temperature, the absorbance of the mixture was recorded spectrophotometrically at 620 nm. The experiment was repeated three times.
Malondialdehyde (MDA) levels were measured as described by Wasowicz, Jean & Peratz (1993). About 0.2 g of hyphae fresh tissues were placed in a tube containing 1 mL distilled water. After the addition of 1 mL of 29 mmol L−1 acetic acid solution, the samples were placed in a water bath and heated for 1 h at 95-−100 °C. After the samples were cooled under running water, 25 µL 5 mol L−1 HCl was added, and the reaction was stopped by adding 3.5 mL of n-butanol and agitating for 5 min. After centrifugation (10,000 rpm for 5 min), the separated butanol phase was removed, and the fluorescence was measured in a spectrofluorometer (Shimadzu-RF-5000, Kyoto, Japan) using 525 nm for excitation and 547 nm for emission. The experiment was repeated three times.
Proline in the hyphae tissues was analyzed using the method described by Bates, Waldren & Teare (1973). About 0.5 g of frozen hyphae fresh tissue was ground in liquid N2. The powder was then mixed into 1 mL of aqueous sulfosalicylic acid (3%, w/v). This solution was then mixed with an equal volume of glacial acetic acid and ninhydrin reagent and heated at 95 °C for 1 h. The reaction was terminated by placing the container in an ice bath for 15 min, and the reaction solution was mixed with 2 mL of toluene. The absorbance of the chromophore was measured at 520 nm in a UV–VIS spectrophotometer (HACH DR/4000; model 48000, HACH Co., Loveland, Colorado, USA) and compared with the absorbance of a standard. The experiment was repeated three times.
Statistical analysis
Data processing was performed using Excel 2010, and one-way analysis of variance (ANOVA) was performed using GraphPad Prism 7.0. Multiple comparison tests for different treatments were conducted using Duncan’s multiple range test with the significance levels (α) of 0.05, 0.01, and 0.001. The charts were drawn using GraphPad Prism 7.0.
Results
Molecular biological verification of the taxonomic status of strain
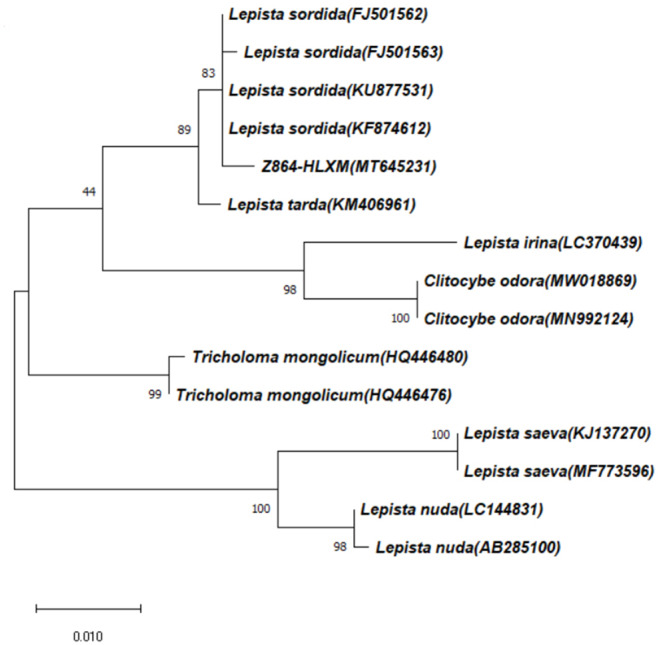
After determining the ITS sequence of the strain and verifying its ITS classification status, the length of the ITS sequence PCR product of the strain was determined to be 643 bp. MEGA5.0 software adjacency method was used to construct the phylogenetic tree, and the sequence similarity rate between the strain and Lepista Sordida was up to 99.69%. The sequence was submitted to GenBank to obtain the gene login number: MT645231 (https://www.ncbi.nlm.nih.gov/nuccore/MT645231) (Fig. 1).
Figure 1. Genetic evolutionary tree of strain.
Morphological observation of L. sordida under Cd and Cu stress
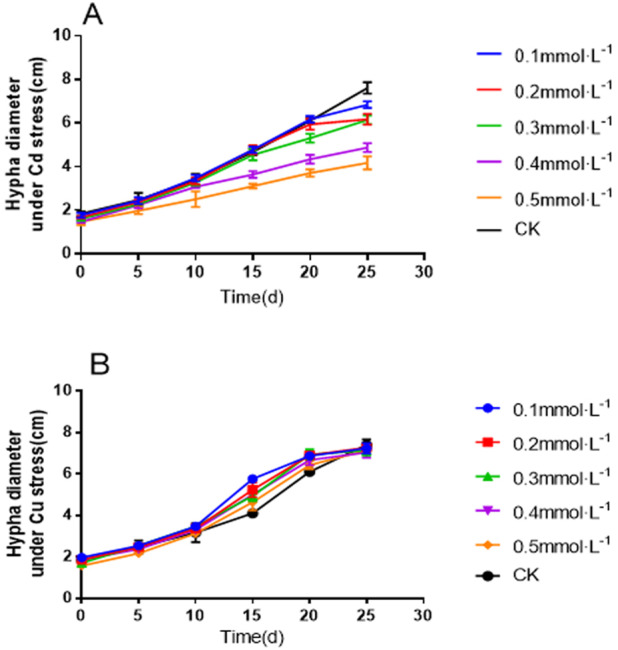
The growth of L. sordida on the PDA medium supplemented with Cd and Cu was monitored for 25 days. There were considerable changes in the hyphal diameter in the media containing different Cd concentrations compared to the control (0 mmol L−1) (Fig. 2A). However, mycelial growth was not significantly inhibited under the effect of heavy metals (Fig. 2B).
Figure 2. Twenty-five-day-old hyphal diameter of L. sordida on a PDA medium: (A) Mycelial growth curve under Cd stress; (B) Mycelial growth curve under Cd stress.
With increasing Cd concentration, the diameter of the colonies gradually decreased, and the growth also slowed down. At the end of the experiment (25 days), the colonies in the control group had covered the whole Petri dish (Fig. 3A), while those in the Cd stress group (Figs. 3B–3F) had not covered the Petri dishes. When the concentration of Cd increased from 0.1 to 0.5 mmol L−1, the diameter of the colony decreased successively, and the growth also slowed down gradually. The mycelial colony gradually became sparse.
Figure 3. The growth of L. sordida exposed to different concentrations of Cd for 25 days.
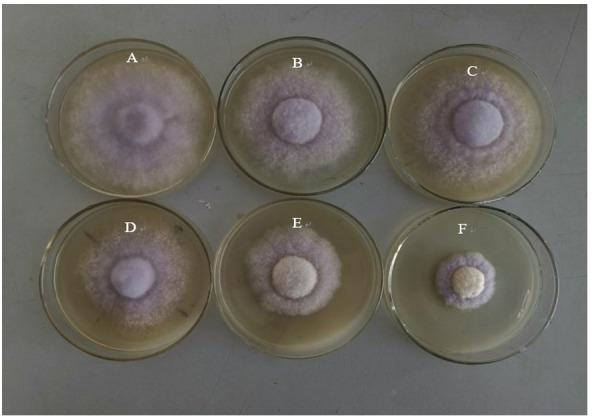
(A) The growth of L. sordida with no Cd stress; (B) The growth of L. sordida on PDA medium with 0.1 mmol L−1 of Cd; (C) The growth of L. sordida on PDA medium with 0.2 mmol L−1 of Cd; (D) The growth of L. sordida on PDA medium with 0.3 mmol L−1 of Cd; (E) The growth of L. sordida on PDA medium with 0.4 mmol L−1 of Cd; (F) The growth of L. sordida on PDA medium with 0.5 mmol L−1 of Cd.
The difference is that the increasing Cu concentration did not result in a significant decrease in the colony diameter. At the end of the experiment (25 days), although the colonies in the control group had covered the whole Petri dish (Fig. 4A), those in the Cu stress group (Figs. 4B–4F) had not covered the Petri dishes. However, when the concentration of Cu increased from 0.1 to 0.5 mmol L−1, the decrease in the colony diameter was not significant. The mycelial colony gradually became sparse. The results showed that L. sordida could successfully resist the stress caused by heavy metals.
Figure 4. The growth of L. sordida exposed to different concentrations of Cu for 25 days.
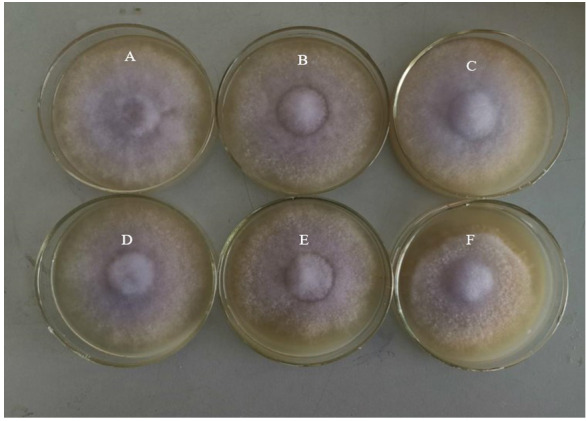
(A) The growth of L. sordida with no Cu stress; (B) The growth of L. sordida on PDA medium with 0.1 mmol L−1 of Cu; (C) The growth of L. sordida on PDA medium with 0.2 mmol L−1 of Cu; (D) The growth of L. sordida on PDA medium with 0.3 mmol L−1 of Cu; (E) The growth of L. sordida on PDA medium with 0.4 mmol L−1 of Cu; (F) The growth of L. sordida on PDA medium with 0.5 mmol L−1 of Cu.
The morphological changes in the mycelium of L. sordida were observed by SEM. Cadmium (Cd) caused conspicuous changes in the hyphal morphology (Fig. 5). The control group presented profound and luxuriant mycelium growth, with numerous clamp connections (Fig. 5A). A bright contrast was observed between the control group (Figs. 5B–5F) and the Cd stress group, with the observation of twisting of individual hyphae under the influence of Cd (Fig. 5C) and the breaking of certain mycelia (indicated by the arrow) (Figs. 5D–5E). When the concentration of Cd was 0.5 mmolL−1, the mycelium started dissolving, as indicated by the arrow (Fig. 5F).
Figure 5. Growing status of L. sordida on PDA medium under Cd stress.
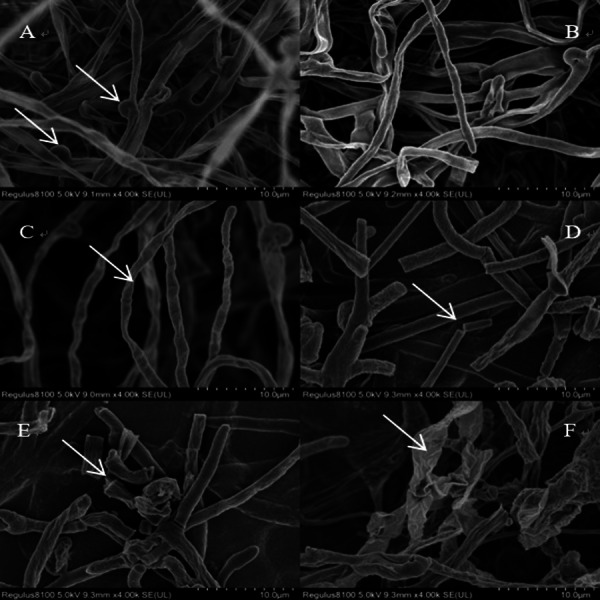
SEM of the hyphae from the colony edge of L. sordida. After adding Cd to the medium, the mycelium will twist and break, stick to each other and even dissolve. Control group mycelial growth is good, the mycelium is luxuriant with full of clamp connections. (A) Hyphae from the 0 mmol L−1; (B) Hyphae from a colony treated with 0.1 mmol L−1 of Cd; (C-F) Mycelial special morphology of L. sordida under Cd stress from 0.2 mmol L−1 to 0.5 mmol L−1.
Copper (Cu), at particularly high concentrations, caused conspicuous changes in the hyphal morphology (Fig. 6F). When the concentration increased from 0 to 0.2 mmolL−1 in the Cu stress groups, the mycelium growth was profound and luxuriant, with numerous clamp connections, as indicated by the arrow (Figs. 6A–6C). There was a high contrast between the control group and the Cu stress group, with a twisting of the intertwined hyphal strands under the effect of Cu (indicated by the arrow) (Figs. 6D–6F). When the concentration of Cu was 0.5 mmol L−1, the mycelium started breaking and dissolving, as indicated by the arrow (Fig. 6F).
Figure 6. Growing status of L. sordida on PDA medium under Cu stress.
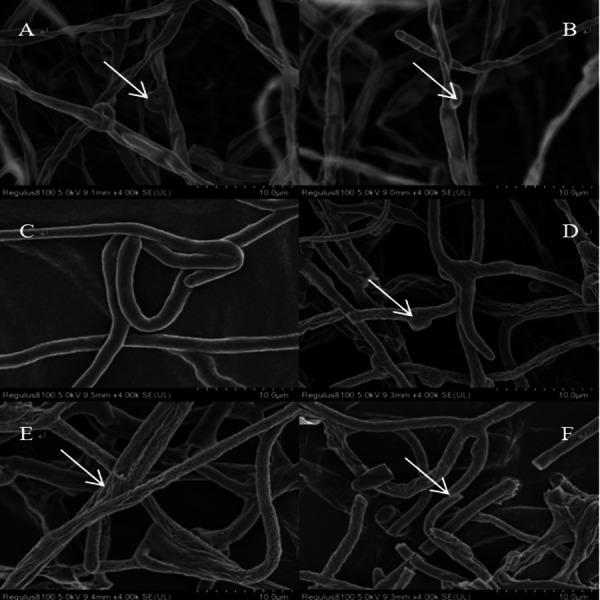
SEM of the hyphae from the colony edge of L. sordida. After adding Cu to the medium, the mycelium will twist and break, sunken. Control group mycelium growth is good, mycelium is luxuriant with full of clamp connections. (A) Hyphae from the 0 mmol L−1; (B) Hyphae from a colony treated with 0.1 mmol L−1 of Cu; (C–F) Mycelial special morphology of L. sordida under Cu stress from 0.2 mmol L−1 to 0.5 mmol L−1.
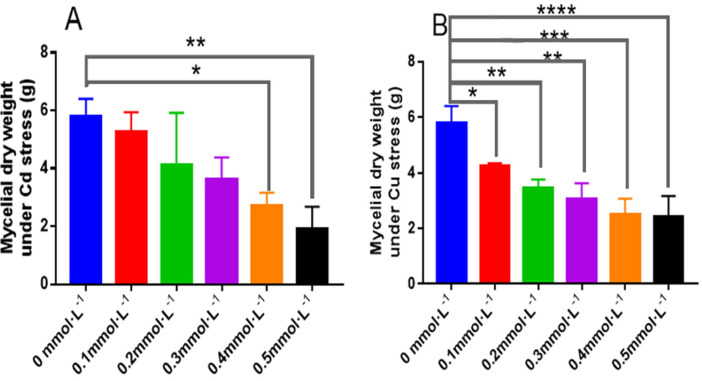
The growth of L. sordida in the liquid medium containing Cd was observed. There were remarkable differences in the mycelial dry weight under Cd and Cu stress. At higher concentrations of Cd and Cu (0.4–0.5 mmol L−1), the mycelial dry weight was significantly different from that in the control group (P < 0.05 and P < 0.01, respectively) (Fig. 7A). The results showed that L. sordida could tolerate low concentrations of heavy metals.
Figure 7. The biomass of L. sordida treated with Cd and Cu for 10 days.
(A) The biomass of L. sordida under Cd stress; (B) the biomass of L. sordida under Cu stress.
Antioxidant enzyme activities of L. sordida under Cd and Cu stress
As shown in Fig. 8, When the Cd concentrations were 0.2 and 0.3 mmolL−1, the activities of CAT in L. sordida were 66% and 233% higher than those in the control, with significant differences between them (P < 0.05 and P < 0.0001, respectively) (Fig. 8A). Similarly, when the Cu concentrations were 0.3 and 0.4 mmol L−1, the activities of CAT in L. sordida were 100% and 56% higher than those in the control, with significant differences between the groups (P < 0.001 and P < 0.05, respectively) (Fig. 8B). The effect of Cd on the activity of POD in L. sordida was more significant than that on its CAT activity (Fig. 8C). Furthermore, the POD activity in L. sordida exposed to 0.4 mmol L−1 was significantly different (P < 0.05) from that of the control (Fig. 8D). One explanation for the inconsistently varied pattern of POD under Cd and Cu stress could be that Cu2+ is a trace element for this organism and, therefore, a low concentration of Cu2+ would not exert a great impact on the organism, while a higher concentration of Cu2+ would exert a greater impact on the growth and physiological metabolism of the organism. Nonetheless, the specific reasons for this require further investigation.
Figure 8. The responses of CAT and POD to the toxicity of Cd and Cu for 25 days.
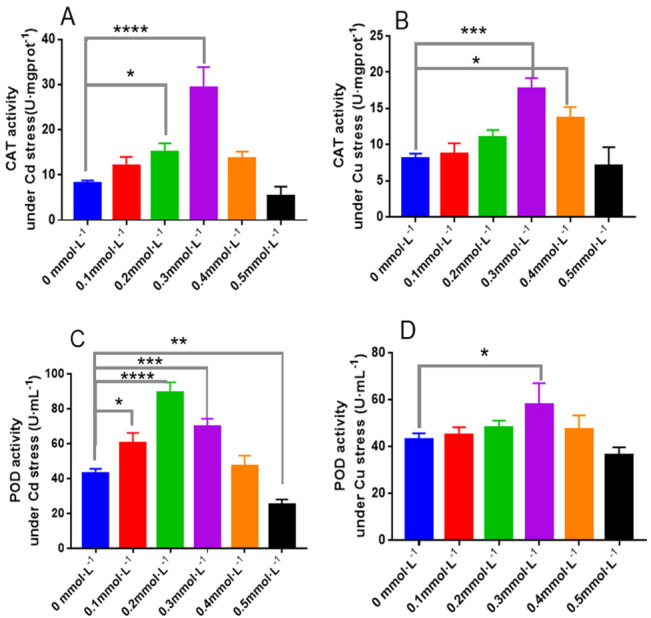
(A) The activity of CAT under Cd stress; (B) the activity of CAT under Cd stress; (C) the activity of POD under Cu stress; (D) the activity of POD under Cu stress. * indicate significant differences (P < 0.05) assessed by Duncan’s test. ** was P < 0.01; ***was P < 0.001; **** was P < 0.0001. Data are means ± SE (n = 3).
As shown in Fig. 9, when the Cd concentrations were 0.2 and 0.3 mmol L−1, the activities of SOD in L. sordida were 100% and 45.5% higher than those in the control, respectively. Similarly, when the Cu concentrations were 0.2 and 0.3 mmol L−1, the activities of SOD were 82% and 91% higher than those in the control, with significant differences (P < 0.05 and P < 0.001), respectively, between the treatments (Fig. 9B). The effect of Cd on the activity of APX in L. sordida was more significant than that on its SOD activity (Fig. 9C). Moreover, the POD activity in L. sordida exposed to 0.2 mmol L−1 was significantly different (P < 0.01) from that of the control (Fig. 9D).
Figure 9. The responses of SOD and APX to the toxicity of Cd and Cu for 25 days.
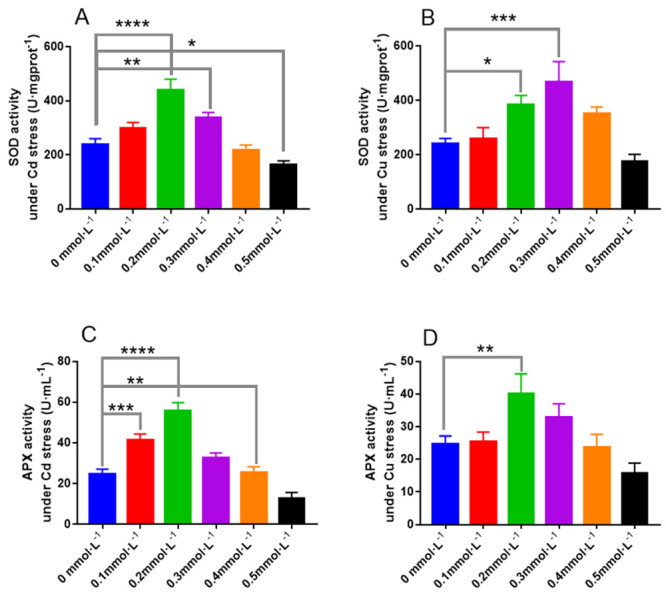
(A) The activity of SOD under Cd stress; (B) the activity of SOD under Cd stress; (C) the activity of APX under Cu stress; (D) the activity of APX under Cu stress. * indicate significant differences (P < 0.05) assessed by Duncan’s test. ** was P < 0.01; ***was P < 0.001; **** was P < 0.0001. Data are means ± SE (n = 3).
The contents of osmotic adjustment substances in L. sordida under Cd and Cu stress
The contents of soluble sugar and protein in L. sordida under Cd and Cu stress presented in Fig. 10 indicate that the addition of Cd and Cu could affect the soluble sugar and protein contents in L. sordida. Higher Cd concentrations, particularly 0.1 and 0.2 mmolL−1, increased the soluble sugar and protein contents (Fig. 10A). Similarly, when the concentration of Cu was between 0.1 and 0.3 mmol L−1, the contents of soluble sugar in L. sordida were 71%, 57%, and 86% higher, respectively, than those in the control, with significant differences between the groups (P < 0.01, P < 0.05, P < 0.01) (Fig. 10B). The effect of Cu on the content of soluble protein in L. sordida was more significant than on the control (Fig. 10D).
Figure 10. The responses of soluble sugar and protein to the toxicity of Cd and Cu for 25 days.
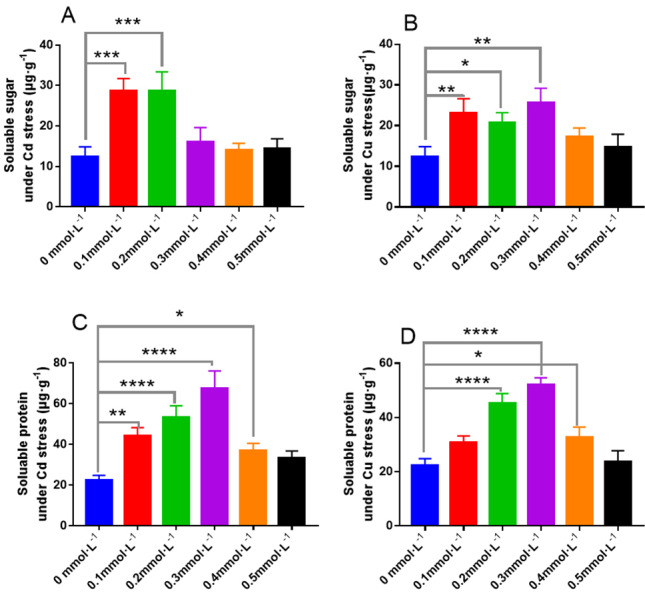
(A) The content of soluble sugar under Cd stress; (B) the content of soluble sugar under Cd stress; (C) the content of soluble protein under Cu stress; (D) the content of soluble protein under Cu stress. * indicate significant differences (P < 0.05) assessed by Duncan’s test. ** was P < 0.01; *** was P < 0.001; **** was P < 0.0001. Data are means ± SE (n = 3).
Figure 11 demonstrates the changes in the malondialdehyde (MDA) levels and free proline content in L. sordida as a result of the addition of Cd and Cu to the medium. Higher Cd concentrations, particularly 0.3–0.5 mmol L−1, increased the MDA content significantly (with significance differences of P < 0.01, P < 0.0001, and P < 0.0001, respectively) (Fig. 11A). Similarly, when the Cu concentration ranged from 0.3 to 0.5 mmol L−1, the contents of soluble sugar in L. sordida were 50%, 150%, and 225% higher, respectively, than those in the control, with significant differences (P < 0.05, P < 0.0001, P < 0.0001) between the groups (Fig. 11B). The effects of Cd and Cu on the content of free proline in L. sordida were more significant compared to those on the control (Figs. 11C and 11D).
Figure 11. The responses of MDA and free proline to the toxicity of Cd and Cu for 25 days.
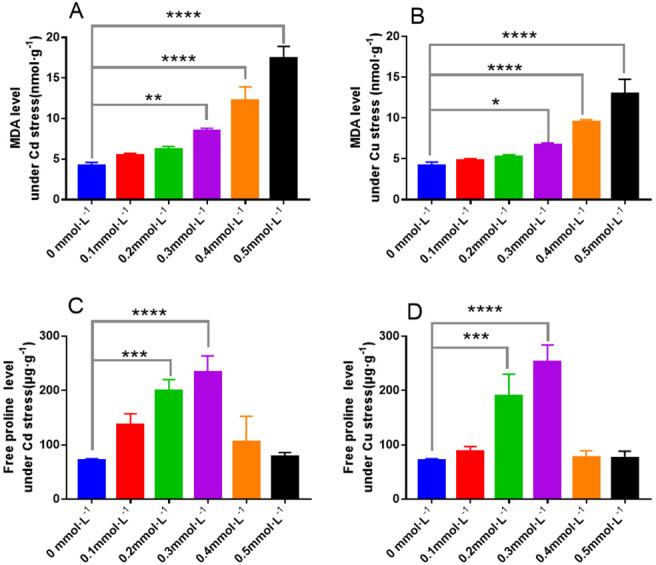
(A) The content of MDA under Cd stress; (B) the content of MDA under Cd stress; (C) the content of free proline under Cu stress; (D) the content of free proline under Cu stress. * indicate significant differences (P < 0.05) assessed by Duncan’s test. ** was P < 0.01; *** was P < 0.001; **** was P < 0.0001. Data are means ± SE (n = 3).
Discussion
Soil remediation using a combination of plants and microorganisms has become a research hotspot nowadays (Chaney, 1983; Jamal, Ayub & Usman, 2002; Hu, Lin & Wang, 2009; Jack, Jose & Joanne, 2010). In recent years, scholars have attempted to use woody plants with more abundant biomass for restoration and exploration. Zhang, Chai & Wang (2013) used the mycorrhizal fungi Pinus tabulaeformis for the remediation of soil contaminated with Cd and suggested that inoculation with heat-resistant proteins secreted by exogenous mycorrhizal fungi could significantly improve the fixation capacity of heavy metals in the rhizosphere of P. tabulaeformis. It was found that ECM could protect the aboveground parts of birch seedlings under high concentrations of Cu and Cd. It is noteworthy that understanding the mechanisms of heavy metal tolerance in mycorrhizal fungi is important for improving the soil using mycorrhizal fungi.
In this study, Cd and Cu stress were observed to significantly affect the growth of mycorrhizal fungi. After the addition of Cd and Cu to the medium, mycelium started twisting, breaking, and finally dissolved. The mycelial growth in the control group was profound, luxuriant, and with numerous clamp connections. Moreover, the mycorrhizal fungi had better growth at low concentrations of heavy metals. The results showed that ECMF had a certain degree of tolerance to heavy metals and could grow normally in the presence of heavy metals.
When the concentration of Cu increased from 0.1 to 0.5 mmol L−1, the decrease in the colony diameter was not significant (Fig. 4). However the mycelial dry weight under Cd stress was significantly different from that in the control group in Fig. 7B. The reason may be that the toxicity of the two heavy metal ions is different. Cd is a highly toxic heavy metal, while Cu is a micronutrient element of organisms, and when the concentration is too high, it will cause adverse effects on the growth of organisms, or even toxic effects. However, Cd is different. Cd itself is a highly toxic heavy metal element. Under the regulation of Cd, it will cause great harm to organisms.
CAT, POD, SOD, and APX are the protective enzymes occurring widely in animals, plants, and microorganisms (Yan et al., 2017). When in coordination with each other, they can maintain the dynamic balance between the production and removal of oxygen free radicals in the cells, remove ROS produced due to environmental stress, reduce the oxidative stress caused by ROS in cells, and thus prevent the toxicity of oxygen free radicals (Halliwell & Gutteridge, 2007). The activities of antioxidant enzymes in L. sordida were analyzed to study the responses of anti-reactive oxygen capacity to Cd and Cu stress. The toxic effects of Cd and Cu appeared to be related to the production of ROS and could cause cell rupture. Therefore, the degree of destruction of the ectomycorrhizal cell under Cd and Cu stress can be represented by the changes in the antioxidant enzyme activities and osmotic regulatory substances (Baldrian, 2003; Bai, Harvey & McNeil, 2003; Halliwell & Gutteridge, 2007). In this study, the antioxidant enzyme activities in L. sordida increased when exposed to 0.2 and 0.3 mmol L−1 Cu and Cd. However, upon exposure to 0.4 and 0.5 mmol L−1 of these metals, the antioxidant enzyme activities decreased. APX, POD, and SOD had the highest activities at the Cd2+ andCu2+ concentrations of was 0.2 mmol L−1. CAT activity reached its maximum value at a metal concentration of 0.3 mmol L−1, indicating that the reaction time of CAT for ROS induction was longer than that of the other three enzymes. The reason might be the effect of high concentrations of Cd and Cu, which resulted in cell death in L. sordida. The results showed that the fungus L. sordida can grow normally under heavy metal stress, has a certain degree of tolerance to heavy metals, can resist the ROS, and remove oxygen radicals and their products via the production of antioxidant enzymes and increasing their activities (Halliwell & Gutteridge, 2007). These results were similar to those reported by Hegedu et al. (2007).
Soluble sugar and soluble protein play the most crucial role in osmotic regulation in organisms. Under environmental stress, the contents of soluble sugar and soluble protein can increase and develop the potential of the biological lining cells, followed by an increase in the osmotic pressure of cells to resist adverse environments. In this study, the changes in the contents of soluble protein and soluble sugar were similar to those of the antioxidant enzyme activities. This indicates that ECMF can increase the osmotic pressure of the cells as well as their resistance to Cd and Cu stress by increasing the soluble sugar and soluble protein contents in the cells exposed to Cd and Cu stress.
In this paper, the content of MDA in the fungus L. sordida exposed to Cd and Cu stress was higher than in that in the control. However, when the concentrations of heavy metals were 0.1 and 0.2 mmol L−1, the MDA contents were not significantly different (P > 0.05) from those in the control group, indicating that the low concentrations of Cd and Cu had no significant effects on the Ectomycorrhizal cells.
The content of MDA increased with the increase of heavy metal concentration, indicating the aggravation of heavy metal ion damage to L. sordida cells.These results corresponded with the previous increase in soluble protein and soluble sugar content to varying degrees, indicating that heavy metal ions had a certain destructive effect on L. sordida cells.However, under low concentration of heavy metals stress, the MDA content of L. sordida cells was not significantly affected, indicating that L. sordida had a certain tolerance to heavy metals.
The increase in the free proline content in organisms is a physiological and biochemical response to stress. In this study, free proline content in the fungus was higher in Cd and Cu treatment groups compared to the control group, regardless of Cd and Cu stress, and it followed a pattern similar to that of the antioxidant enzyme activities. When the concentrations of heavy metals were 0.1 and 0.2 mmol L−1, the free proline content in ECMF was not significantly different (P > 0.05) from that of the control group, indicating that low concentrations of Cd and Cu had no significant effect on the cells of ECMF.
In conclusion, the results of the present study can provide a theoretical basis for the better utilization of ECM fungal resources for the remediation of soil contaminated with heavy metals.
Conclusion
Lepista sordida, an Ectomycorrhizal (ECM) fungus, can resist against Cd and Cu. The fungus L. sordida was observed for its growth, antioxidant enzyme activities, and osmotic regulation. These indicators can reflect strong resistance to heavy metal stress in L. sordida. Besides, this study provides necessary data for further investigations aiming for better utilization of Ectomycorrhizal fungal resources for the remediation of heavy metal-contaminated soil.
Supplemental Information
Funding Statement
The research was supported by the National Natural Science Foundation of China (31800542). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yin Dachuan conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Qi Jinyu analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The sequence is available at GenBank: https://www.ncbi.nlm.nih.gov/nuccore/MT645231.
Data Availability
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.
References
- Abbott, Robson & Boer (1984).Abbott L, Robson A, Boer G. The effect of phosphorus on the formation of hyphae in soil by vesicular mycorrhizal fungus, Glomus fasciculatum. New Phytologist. 1984;97:437–446. doi: 10.1111/j.1469-8137.1984.tb03609.x. [DOI] [Google Scholar]
- Andrade-Linares et al. (2011).Andrade-Linares DR, Grosch R, Restrepo S, Krumbein A, Franken P. Effects of dark septate endophytes on tomato plant performance. Mycorrhiza. 2011;21(5):413–422. doi: 10.1007/s00572-010-0351-1. [DOI] [PubMed] [Google Scholar]
- Bai, Harvey & McNeil (2003).Bai ZH, Harvey LM, McNeil B. Oxidative stress in submerged cultures of fungi. Critical Reviews in Biotechnology. 2003;23:267–302. doi: 10.1080/07388550390449294. [DOI] [PubMed] [Google Scholar]
- Baldrian (2003).Baldrian P. Interactions of heavy metals with white-rot fungi. Enzyme and Microbial Technology. 2003;32:78–91. doi: 10.1016/S0141-0229(02)00245-4. [DOI] [Google Scholar]
- Bates, Waldren & Teare (1973).Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Brundrett et al. (1996).Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research Monograph, Canberra. 1996. p. 32.
- Chaney (1983).Chaney RL. Plant uptake of inorganic waste constituents. In: Parr JF, editor. Land treatment of hazardous wastes. No yes Data Corporation; Park Ridge: 1983. pp. 50–76. [Google Scholar]
- Chen et al. (2019).Chen BD, Zhang X, Wu SL, Li L, Ram A. The Role of Arbuscular Mycorrhizal Fungi in Heavy Metal Translocation, Transformation and Accumulation in the Soil-Plant Continuum: Underlying Mechanisms and Ecological Implications. Rock and Mineral Analysis. 2019;38(1):1–25. [Google Scholar]
- Christos, Konstantinos & George (2008).Christos DG, Konstantinos G, George Z. Mechanism of Coomassie brilliant blue G-250 binding to proteins: a hydrophobic assay for nanogram quantities of proteins. Analytical and Bioanalytical Chemistry. 2008;391:391–403. doi: 10.1007/s00216-008-1996-x. [DOI] [PubMed] [Google Scholar]
- Edda et al. (2010).Edda S, Oddsdottir ES, Eilenberg J, Sen R, Halldorsson G. The effects of insect pathogenic soil fungi and ectomycorrhizal inoculation of birch seedlings on the survival of Otiorhynchus larvae. Agricultural and Forest Entomology. 2010;12:319–324. [Google Scholar]
- Gadd (2007).Gadd GM. Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycological Research. 2007;111:3–49. doi: 10.1016/j.mycres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Granero & Domingo (2002).Granero S, Domingo JL. Levels of metals in soils of Alcala’ de Henares, Spain: Human health risks. Environment International. 2002;28:159–164. doi: 10.1016/S0160-4120(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Grataõ et al. (2005).Grataõ PL, Polle A, Lea PJ, Azevedo RA. Making the life of heavy metalstressed plants a little easier. Functional Plant Biology. 2005;32:481–494. doi: 10.1071/FP05016. [DOI] [PubMed] [Google Scholar]
- Halliwell & Gutteridge (2007).Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford University Press; Oxford: 2007. p. 440 p. [Google Scholar]
- Hegedu et al. (2007).Hegedu SN˝, Emri T, Szila’gyi J, Kara’nyi Z, Nagy I. Effect of heavy metals on the glutathione status in different ectomycorrhizal Paxillus involutus strains. World Journal of Microbiology and Biotechnology. 2007;23:1339–1343. doi: 10.1007/s11274-007-9368-9. [DOI] [Google Scholar]
- Hou et al. (2007).Hou WH, Chen X, Song GL, Wang QH, Chang CC. Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor) Plant Physiology and Biochemistry. 2007;45:62–69. doi: 10.1016/j.plaphy.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hu, Lin & Wang (2009).Hu JL, Lin XG, Wang JH. Arbuscular mycorrhizal fungus enhances crop yield and P-uptake of maize (Zea mays L.): a field case study on a sandy loam soil as affected by long-term P-deficiency fertilization. Soil Biology & Biochemistry. 2009;41:2460–2465. doi: 10.1016/j.soilbio.2009.09.002. [DOI] [Google Scholar]
- Jack, Jose & Joanne (2010).Jack AA, Jose RP, Joanne TE. Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM fechniques. Environmental and Experimental Botany. 2010;68:139–148. doi: 10.1016/j.envexpbot.2009.08.009. [DOI] [Google Scholar]
- Jamal, Ayub & Usman (2002).Jamal A, Ayub N, Usman M. Arbuscular mycorrhizal fungi enhance zinc and nickel up take from contaminated soil by soybean and lentil. International Journal Phytorem. 2002;4:205–221. doi: 10.1080/15226510208500083. [DOI] [Google Scholar]
- Li et al. (2015).Li DQ, Chen GK, Zheng H, Li HS, Li XB. Effects of cadmium on growth and antioxidant enzyme activities of two kidney bean (Phaseolus vulgaris L.) cultivars. Journal of Agro Environment Science. 2015;34(2):221–226. [Google Scholar]
- Li et al. (2012).Li HY, Li DW, He CM, Zhou ZP, Mei T. Diversity and heavy metal tolerance of endophytic fungi from six dominant plant species in a Pb-Zn mine wasteland in China. Fungal Ecology. 2012;5:309–315. doi: 10.1016/j.funeco.2011.06.002. [DOI] [Google Scholar]
- Li et al. (2020).Li XR, Mi YD, Wei Y, Zhou M. Research progress on applications of arbuscular mycorrhizal fungi-plant symbiotic system in remediation of heavy metals contaminated soil. 2020;40(5):14–18. [Google Scholar]
- Liu, Wu & Zhang (2019).Liu XY, Wu CY, Zhang GC. Study on heavy metal pollution and control of vegetables. Journal of Anhui Agricultural Sciences. 2019;47(15):10–12. [Google Scholar]
- Ma (2013).Ma YL. Master Thesis. 2013. The ectomycorrhizal fungi Paxillus involutus increased the absorption and tolerance of heavy metal Cd in Populus×canescens. [Google Scholar]
- Mandyam & Jumpponen (2005).Mandyam K, Jumpponen A. Seeking the elusive function of the rootcolonising dark septate endophytic fungi. Studies in Mycology. 2005;53:173–189. doi: 10.3114/sim.53.1.173. [DOI] [Google Scholar]
- Meier et al. (2011).Meier S, Azcon R, Cartes P, Borie F, Cornejo P. Alleviation of Cu toxicity in Oenothera picensis by copper-adapted arbuscular mycorrhizal fungi and treated agrowaste residue. Applied Soil Ecology. 2011;48:117–124. doi: 10.1016/j.apsoil.2011.04.005. [DOI] [Google Scholar]
- Moora et al. (2011).Moora M, Berger S, Davison J, Opik M, Bommarco R, Bruelheide H. Alien plants associate with widespread generalist arbuscular mycorrhizal fungal taxa: evidence from a continental-scale study using massively parallel 454 sequencing. Journal of Biogeography. 2011;38:1305–1317. doi: 10.1111/j.1365-2699.2011.02478.x. [DOI] [Google Scholar]
- Mucha et al. (2006).Mucha J, Dahm H, Strzelczyk E, Werner A. Synthesis of enzymes connected with mycoparasitism by ectomycorrhizal fungi. Archives of Microbiology. 2006;185:69–77. doi: 10.1007/s00203-005-0068-2. [DOI] [PubMed] [Google Scholar]
- Regvar et al. (2010).Regvar M, Likar M, Piltaver A, Kugonič N, Smith JE. Fungal community structure under goat willows (Salix caprea L.) growing at metal polluted site: the potential of screening in a model phytostabilisation study. Plant Soil. 2010;330:345–356. doi: 10.1007/s11104-009-0207-7. [DOI] [Google Scholar]
- Ruttens et al. (2006).Ruttens A, Mench M, Colpaert JV, Boisson J, Carleer R. Phytostabilization of a metal contaminated sandy soil. I: Influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environmental Pollution. 2006;144:524–532. doi: 10.1016/j.envpol.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Selosse, Baudoin & Vandenkoornhuyse (2004).Selosse M-A, Baudoin E, Vandenkoornhuyse P. Symbiotic microorganisms, a key for ecological success and protection of plants. Comptes Rendus Biologies. 2004;327:639–648. doi: 10.1016/j.crvi.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Sharma, Rajak & Pandey (2010).Sharma R, Rajak RC, Pandey AK. Evidence of antagonistic interactions between rhizosphere and mycorrhizal fungi associated with Dendrocalamus strictus (Bamboo) Journal of Yeast and Fungal Research. 2010;1:112–117. [Google Scholar]
- Silvia, Olga & Alfredo (2018).Silvia M, Olga P, Alfredo S. Assessment of metal levels in foodstuffs from the region of valencia (Spain) Toxicology Reports. 2018;5:654–670. doi: 10.1016/j.toxrep.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wageh, Hitoshi & Waleed (2019).Wageh SD, Hitoshi C, Waleed REG. Determination of polycyclic aromatic hydrocarbon content in heattreated meat retailed in egypt: Health risk assessment, benzo [a] pyrene induced mutagenicity and oxidative stress in human colon(Caco-2) cells and protection using rosmarinic and ascorbic acids. Food Chemistry. 2019;290:114–124. doi: 10.1016/j.foodchem.2019.03.127. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang BJ, Jiang L, Pan B, Lin Y. Acute toxicity of single and compound pollution of Cu2+, Pb2+ and two herbicides to earthworm. Agrochemicals. 2020;59(6):425–429. [Google Scholar]
- Wasowicz, Jean & Peratz (1993).Wasowicz W, Jean N, Peratz A. Optimized steps in fluorometric determination of thiobarbituric acid reactive substances in serum; importance of extraction pH and influence of sample preservation and storage. Clinical Chemistry. 1993;38(12):2522–2526. [PubMed] [Google Scholar]
- Wu et al. (2020).Wu X, Fang B, Yue XM, Zou JW, Chen YH, Su NN, Cui J. The research progress of vegetable Cd contamination and physiological blocking agents of Cd. Journal of Nanjing Agricultural University. 2020;43(6):988–997. [Google Scholar]
- Yan et al. (2017).Yan XF, Zheng CY, Li KY, Wan FH, Wang JP. The effect of Cd (II) on antioxidant enzymes cctivity of Beauveria bassiana. Journal of Environmental Entomology. 2017;39(5):992–999. [Google Scholar]
- Yemm & Willis (1954).Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2017).Yin DC, Qi JY, Deng JF, Du H, Deng X. Effects of ectomycorrhizal cooperating with exogenous calcium on Pinus sylvestris var. mongolica growth. China Environmental Science. 2017;37(6):2295–2304. [Google Scholar]
- Yin et al. (2020).Yin DC, Saiyaremu HF, Song RQ, Qi JY, Deng X, Deng JF. Efects of an ectomycorrhizal fungus on the growth and physiology of Pinus sylvestris var. mongolica seedlings subjected to saline–alkali stress. The Journal of Forestry Research. 2020;31(3):781–788. doi: 10.1007/s11676-019-01007-7. [DOI] [Google Scholar]
- Yin et al. (2018).Yin DC, Song RQ, Qi JY, Deng X. Ectomycorrhizal fungus enhances drought tolerance of Pinus sylvestris var. mongolica seedlings and improves soil condition. The Journal of Forestry Research. 2018;29(6):1775–1788. doi: 10.1007/s11676-017-0583-4. [DOI] [Google Scholar]
- Zhan, Li & Jiang (2019).Zhan FD, Li B, Jiang M. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers] grown in a lead-zinc mine wasteland. International Journal of Phytoremediation. 2019;21(9):849–856. doi: 10.1080/15226514.2019.1577353. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2007).Zhang FQ, Wang YS, Lou ZP, Dong JD. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza) Chemosphere. 2007;67:44–50. doi: 10.1016/j.chemosphere.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Zhang, Hu & Yan (2019).Zhang XF, Hu ZH, Yan TX. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicology and Environmental Safety. 2019;171:352–360. doi: 10.1016/j.ecoenv.2018.12.097. [DOI] [PubMed] [Google Scholar]
- Zhang, Chai & Wang (2013).Zhang YW, Chai LW, Wang DW. Effects of inoculation of ectomycorrhizal fungi under Cu and Cd stress on the ability of heat-resistant protein to hold heavy metal in rhizosphere of Pinus tabulaeformis. Environmental Sciences. 2013;35(3):1169–1171. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available in the Supplemental Files.