Abstract
Objective
To evaluate the effects of personalized exposure in vivo on level of physical activity and quality of life in patients with painful diabetic neuropathy.
Design
Randomized, single-case, ABC design.
Subjects
Twelve patients with painful diabetic neuropathy, age > 18 years, diabetes mellitus type II, Clinical Neurological Examination score > 5, Diabetic Neuropathy Symptom Score ≥ 1 and Douleur Neuropathique 4 Questions score ≥ 3.
Methods
The treatment consists of an Intensive screening, followed by an 8-week exposure in vivo intervention specifically adapted to the needs/risks of patients with painful diabetic neuropathy, and 6-months follow-up. Outcome measures included daily and non-daily measures of physical activity, quality of life, metabolic parameters, disability, depression, general and painful diabetic neuropathy-related anxiety, pain intensity and pain catastrophizing.
Results
Due to high drop-out rates (n = 6 during screening, n = 2 during treatment, n = 1 after treatment), only 3 participants completed the study. Slight, but non-significant, changes in physical activity and disability were observed. In quality of life, no changes were observed.
Conclusion
Analysis of the reasons for the high drop-out rate indicate that exposure in vivo may have added value in patients with painful diabetic neuropathy only for those patients: (i) whose daily life functioning is impaired mainly by the painful diabetic neuropathy; (ii) in whom painful diabetic neuropathy-related fears are exaggerated and irrational; (iii) in whom specific activities evoke the painful diabetic neuropathy-related fears; (iv) whose spouse and healthcare providers are involved in the treatment; and (v) who are willing to change their daily behaviour. Further research is needed into this subject.
LAY ABSTRACT
Painful diabetic neuropathy places a high burden on patients’ physical and emotional wellbeing. Patients with painful diabetic neuropathy may have several fears related to diabetes and pain (e.g. fear of pain, fear of falling), which can limit their physically activity in daily life. This study investigated the effects of a personalized rehabilitation treatment, exposure in vivo, which aimed to help people with painful diabetic neuropathy to overcome their fears, so that they could become more active in daily life and improve their quality of life. Slight improvements in physical activity and disability were seen. There were no changes in quality of life. The results of this study should be interpreted with caution, as there was a large number of drop-outs.
Key words: rehabilitation, diabetic neuropathy, neuralgia, quality of life, exercise
Diabetic neuropathy (DN) is present in 50% of all chronic diabetic patients and is a major cause of morbidity and mortality (1). Up to 25% of diabetic patients develop painful diabetic neuropathy (PDN), characterized by pain, paraesthesia and sensory loss (1).
PDN is associated with a decrease in levels of physical activity (PA) (2). This is alarming, since reduced mobility can lead to dependence on others, restrictions in daily and social activities, depression, and decreased quality of life (QoL) (3). Depression, in its turn, is related to poor glycaemic control and the development of pressure ulcers (PUs) (4). Pain can be enhanced by negative feelings, again leading to less PA and diminished QoL, creating a vicious circle (5). Patients with PDN may develop anxiety and fears, such as fear of pain, fear of falling and fear of hypoglycaemia, thereby avoiding PA (6, 7). Anxiety is common in patients with diabetes (20–32%) (8, 9). Anxiety can also play an important role in the maintenance of the consequences of the PDN (5, 6). It seems plausible that overall QoL of patients with PDN might successfully be improved only if comorbid anxiety and negative emotions are optimally screened, diagnosed and treated (5, 10).
Current care for patients with PDN, based largely on pharmacotherapy and physical training, seems to be insufficient to increase PA and to regain normal daily functioning. Dropout rates from physical exercise programmes are high; up to 45%, due to PUs, overuse injuries, and lack of motivation (11, 12). Fears may contribute to this dropout. An interdisciplinary therapeutic approach, focusing on more than reduction of pain alone, is recommended (13, 14).
In order to restore QoL and improve functioning in daily life, it would be beneficial to integrate the knowledge obtained from populations with other pain syndromes into the field of PDN, since patients with chronic pain frequently share the comorbidities depression and fear and, as a consequence, disability. Research on the fear-avoidance model (FAM) succeeded in identifying the disabling role of specific fears, such as fear of movement, fear of pain or fear of (re)injury. This pain-related fear can lead to avoidance behaviour and hypervigilance to pain-related stimuli (15). Previous research has confirmed that the FAM may also be applicable in patients with PDN (5, 10, 16). Patients with PDN may have several fears related to diabetes and pain (e.g. kinesiophobia, fear of falling), which might be important predictors of levels of physical and social activities (16). In addition, PDN has shown to be associated with catastrophic thinking, which in turn can lead to a perceived decline in PA, increased disability, and lower QoL (5). Based on the FAM, a cognitive behavioural therapy, exposure in vivo treatment (EXP) was developed, which appears to be successful in breaking this vicious circle (17).
This pilot study aimed to investigate whether EXP could also be effective in patients with PDN. It was hypothesized that, through targeting specific fears, a reduction in the perceived harmfulness of activities would occur, leading to higher level of PA and improved QoL in patients with PDN.
METHODS
Population and procedure
The study was performed at Adelante Centre of Expertise in Rehabilitation and Audiology, Maastricht University Medical Centre (MUMC+), the Netherlands, where a diversity of patients with chronic pain are successfully treated with EXP (18). It was also embedded in the Diabetes Centre and movement laboratories of MUMC+, where patients were invited to participate. Furthermore, an invitational letter was sent to patients with PDN who had participated in our previous research (5, 10, 16, 19, 20). An advertisement was placed on the website of the patient organization (Dutch Diabetes Foundation). The study was approved by the Medical Ethics Committee MUMC+ (reg. no. 163024) and registered at clinicaltrials.gov (NCT03066570).
Inclusion criteria
Inclusion criteria were: age > 18 years, diabetes mellitus type II; Diabetic Neuropathy Symptom Score (DNS) ≥ 1; Douleur Neuropathique 4 Questions (DN4) ≥ 3; and standardized Clinical Neurological Examination (CNE) > 5. Previous research has shown that EMG and CNE scores resulted in the same diagnosis of PDN in patients with diabetes mellitus type II compared with using the above-mentioned criteria (21).
Exclusion criteria
Exclusion criteria were: patients with lower limb morbidities other than PDN (e.g. peripheral arterial disease, severe osteo-arthritis); other diseases causing pain in the feet and/or damage to the peripheral nervous system (e.g. ulcers); other disease that may cause limitations in PA (e.g. severe cardiopulmonary disease); and/or patients who received cognitive behavioural therapy within the last 6 months.
Design
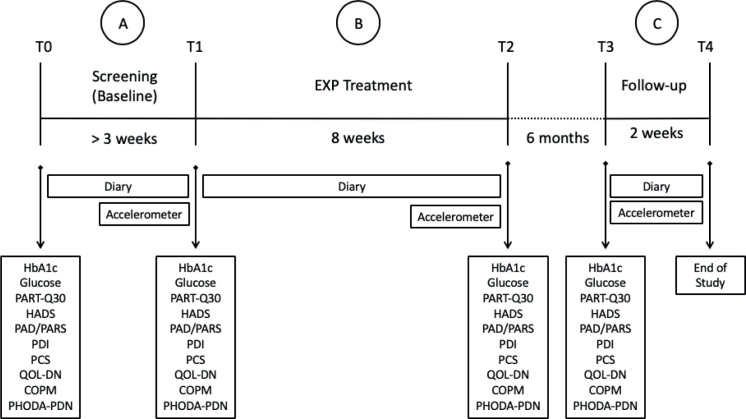
A pilot study using a randomized replicated sequential single-case experimental ABC design (SCED) with multiple measurements was performed (22). T0 represents the first consultation by the rehabilitation physician, in which rehabilitation goals were determined and inclusion criteria checked. A baseline measurement period of at least 3 weeks was then started (period A, T0–T1) in which patients underwent extensive screening. Patients were then randomly assigned to an 8-week treatment (period B, T1–T2). After 6 months, there was a follow-up period of 2 weeks (period C, T3–T4). Fig. 1 illustrates the study design.
Fig. 1.
Study design. PARTQ-30: Painful Diabetic Neuropathy Anxiety Rasch-Transformed Questionnaire; HADS: Hospital Anxiety and Depression Scale; PAD: Perceived Activity Decline; PARS: Physical Activity Rating Scale; PDI: Pain Disability Index; PCS: Pain Catastrophizing Scale; Norfolk QOL-DN: Norfolk Quality of Life Questionnaire-Diabetic Neuropathy; COPM: Canadian Occupational and Performance Scale; PHODA-PDN: Photograph-series Of Daily Activities – Painful Diabetic Neuropathy version.
In this SCED ABC design, the sequencing of phases is fixed so the randomization cannot be applied to the treatment order. One feature that can be randomly determined without distorting the treatment order is the moment of phase change, e.g. ABBBC, AABBBC, AAABBBC. Therefore, the starting point of the intervention (T1) was determined at random, using the waiting list for regular treatments. Repeated measurements (diary and questionnaires) took place in each phase (A, B and C). No blinding was possible for participants or members of the rehabilitation team, since all participants received the EXP treatment. Data analysts were blinded, as they were not involved in the treatment. Subjects who withdrew from the study, received follow-up according to care as usual, and were asked to complete all the questionnaires at the remaining time-points.
Intervention
The EXP in this study was designed specifically for patients with PDN who experienced PDN-related fears and wanted to improve their level of activity and QoL. It was adapted to the needs and risks of patients with PDN (potential risks for injury, PUs and/or hypoglycaemia, etc.). Treatment was based on the results of our earlier qualitative and quantitative studies, and PDN-specific screening tools, such as the Painful Diabetic Neuropathy Anxiety Rasch-Transformed Questionnaire (PART-Q30) (19), and Photograph-series of Daily Activities, PDN version (PHODA-PDN) were constructed. Furthermore, blood glucose levels were measured pre- and post-treatment in insulin-dependent patients.
The EXP treatment consisted of 3 parts; intake by rehabilitation physician, an extensive interdisciplinary 1-day screening, and an 8-week EXP treatment. During the intake session, the rehabilitation physician took a full medical history and assessed the patient’s current PDN-related complaints, risks and medication. The interdisciplinary screening consisted of behavioural analysis by the psychologist, observation during activities, and physical examination by the physical therapist, goal identification by the occupational therapist, a team meeting with the physician and all therapists, and an educational session for the patient by all team members. The 8-week EXP-programme consisted of 2 1-h sessions of EXP per week, in which thoughts and beliefs about the subject’s fears and bodily sensations were challenged. Then, patients were encouraged to increase the level of PA and apply their newly learned associations in new situations. The full protocol of this EXP treatment has been published elsewhere (20).
Data collection
Data on age, sex, duration of complaints, insulin treatment, use of pain medication, pain intensity, metabolic parameters, anxiety, depression, PA and disability were collected. To check whether EXP increased PA by decreasing PDN-related fears, daily and non-daily measures (at T0–T4) were used.
Non-daily measures
Primary outcome measures
The primary outcome measures were as follows:
Physical activity and perceived activity decline
Self-reported PA was measured using the Physical Activity Rating Scale (PARS), consisting of 20 daily activities (24). On a 5-point Likert scale (0–4), patients scored how often they had performed these activities in the past 2 weeks. To estimate perceived activity decline (PAD), patients indicated whether they would have performed each specific activity of the PARS more often (yes/no) if they had not experienced PDN-related pain. The PAD scale has shown good internal consistency and reliability (25).
Quality of life
QoL was measured using the 35-item Norfolk Quality of Life Questionnaire, Diabetic Neuropathy Version (Norfolk-QOL-DN), a self-administered questionnaire designed to capture and quantify the perceived impact of diabetic neuropathy on QoL. Low score indicates good QoL (26). Reliability has shown to be good for most domains (26).
Secondary outcome measures
The secondary outcome measures were:
Metabolic parameters
Blood samples were taken to assess insulin, glucose, HbA1c and HbA1c%.
Pain intensity
Pain intensity was measured using a visual analogue scale (VAS), ranging from 0 to 10.
Disability
The 7-item Pain Disability Index (PDI) investigated the magnitude of the self-reported disability in different situations, e.g. work, leisure-time, activities of daily living and sports (27). The PDI has shown to be internally reliable (27).
Anxiety and depression
These were measured using the Hospital Anxiety and Depression Scale (HADS), a self-report scale that consists of 7 items on depression symptoms (HADS-D) and 7 items on anxiety symptoms (HADS-A) (28). The HADS has been shown to have adequate reliability and validity (29).
Fear reduction
Overall PDN-related fear was measured using the Painful Diabetic Neuropathy Anxiety Rasch-Transformed 30-item questionnaire (PART-Q30), which encompasses various domains of PDN-related anxieties and fears. Higher scores indicate more presence of fears (19). The PART-Q30 has shown to explain approximately one-third of disability and almost half of QoL reduction, as experienced by patients with PDN. The personal separation index of the PART-Q30 has been shown to be good (19).
Pain catastrophizing
To measure negative thoughts and beliefs during actual or anticipated painful experiences, the Dutch version of the validated 13-item Pain Catastrophizing Scale (PCS) was used (30). Psychometric properties of the PCS have shown to be adequate (30, 31).
Identification of PDN-specific fears and activities
A list of fear-eliciting activities was made using the PHODA-PDN version (20) with 8 additional photographs to assess the following PDN-related fears; hypoglycaemia, falling, amputation, pain, exhaustion, injury, social isolation, and loss of identity (10, 16). The PHODA-PDN was used in 2 phases. First, the patient identified which PDN-related fear he/she experienced using the 8 additional pictures. Next, the identified pictures were paired with pictures of activities from the original PHODA (e.g. walking up a slope induces/activates fear of hypoglycaemia). In this way, the team could determine during which activity the PDN-related fear occurred (e.g. walking on uneven ground elicited fear of falling).
Rehabilitation goals
The Canadian Occupational Performance Measure (COPM) was used to assess perceived limitations in personally relevant activities and participation, aiding the goal formulation process (32).
A full description of the PHODA-PDN and COPM are published elsewhere (20).
Daily measures
To check whether the intervention modified PDN-related fear, pain catastrophizing and/or pain experience, participants completed 16 questions in an electronic diary. Participants received daily e-mails asking them to fill in the diary. It consisted of 1 question concerning pain intensity VAS (1–10), 10 questions derived from the PART-Q30 questionnaire, and 2 personalized questions based on the PHODA-PDN and COPM (highest scores taken at baseline). Here, participants scored how often they had performed the activity (PHODA-PDN and COPM) and how satisfied they were about the way they could perform this activity (COPM). An example of the diary questions is shown in Table I.
Table I.
Diary questions
| Questions | Never | Always | |
|---|---|---|---|
| 1. How much nerve pain in the feet do you experience at this moment? | 1–10 | ||
| 2. I am afraid of injuring myself accidentally. | 1 | 2 | 3 |
| 3. I would not have this much pain if there was not something potentially dangerous going on in my body. | 1 | 2 | 3 |
| 4. I think that if my pain gets too severe, it will never decrease. | 1 | 2 | 3 |
| 5. My fatigue has put my body at risk for the rest of my life. | 1 | 2 | 3 |
| 6. How often have you have worried about not recognizing/realizing I am having a reaction because of low blood sugar? | 1 | 2 | 3 |
| 7. I try to avoid activities that cause pain. | 1 | 2 | 3 |
| 8. I will stop any activity as soon as I sense pain is coming. | 1 | 2 | 3 |
| 9. As soon as pain increases, I take medication to reduce it. | 1 | 2 | 3 |
| 10. How concerned are you that you will fall while walking up or down a slope? | 1 | 2 | 3 |
| 11. How concerned are you that you will fall while reaching for something above your head or on the ground? | 1 | 2 | 3 |
| 12. Today, how often have you performed activity X (fearful activity identified by PHODA-PDN)? | 1–10 | ||
| 13. A. Today, how often have you performed activity Y (desired activity identified by COPM)? | 1–10 | ||
| B. How satisfied are you about the execution of this activity Y (desired activity identified by COPM)? | 1–10 |
COPM: Canadian Occupational and Performance Scale; PHODA-PDN: Photograph-series of Daily Activities – Painful Diabetic Neuropathy version
Statistical analyses
Baseline characteristics are presented as means (standard deviation; SD) or percentages. Results of questionnaires for PA, depression, fears and QoL on T0–T4 are displayed in tables. Due to the small sample size, no statistical analyses could be performed on these data.
Daily measures were first presented graphically and next interpreted for trends, followed by a randomization test for SCEDs, based on the random determination of the moments of phase change (23). In SCEDs, the single-case experiments (SCEs) are replicated one after another, hereby demonstrating the external validity of the effects. These replicated SCEs may be considered as multiple studies that can be combined using meta-analytical procedures (33). The randomization tests in this study used the difference between means as test statistics. Because EXP (B) was expected to be superior to baseline (A), the null hypothesis that there was no differential effect for any of the measurement times was tested using a randomization test on the differences between B and A. The follow-up period (C) was expected to be superior to A and should not change in relation to B; therefore, differences between C and A were also tested.
The outcome variables of the diaries were combined in themes, for which means were calculated; pain intensity (item 1), fear of injury (FOI, items 2–4), fear of exhaustion/hypoglycaemia (items 5, 6), avoidance behaviour (items 7–9), fear of falling (items 10, 11), PHODA (item 12), COPM (items 13A, 13B). Next, the themes were measured systematically over time in each phase (A–B and A–C). Because replicated SCEs in this study provided independent tests of the same null hypothesis, the directional p-values of these test were combined by calculating the sum of the p-values and comparing this sum with all other sums that arose under the general null hypothesis; if the null hypothesis is true, the p-value is a random draw from a uniform [0,1] distribution (23). The results of the diaries are presented visually in running medians (batch size 4, averaged by pairs). The analyses were performed using the online Shiny SCDA web app (https://tamalkd.shinyapps.io/scda) by Kumar De and Onghena, KU Leuven, Belgium.
RESULTS
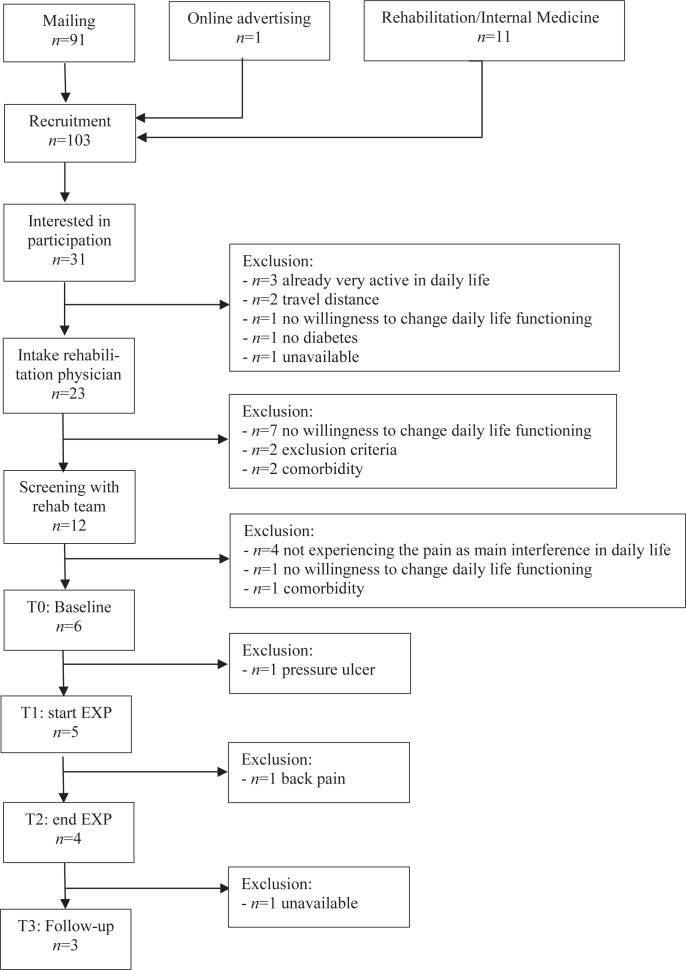
A total of 103 people were invited to participate in this study, of whom 31 expressed interest. After an informative phone call from our research team, 23 participants who potentially fulfilled the inclusion criteria were invited to the intake session with the rehabilitation physician in order to determine final inclusion. Eleven subjects were excluded due to the following factors: unwillingness to change their daily life functioning (n = 7), exclusion criteria (n = 2), and comorbidity (n = 2). The 12 remaining participants were admitted to interdisciplinary screening, which resulted in further exclusion of 6 participants due to not experiencing pain as the main interference in daily life functioning (n = 4), no willingness to change daily life functioning (n = 1), and comorbidity (n = 1). A full overview of the inclusion procedure and reasons for exclusion is given in Fig. 2.
Fig. 2.
Flow chart of inclusion procedure. EXP: exposure in vivo; T0, T1, T2, T3, T4: timepoints T0-T4.
A total of 6 participants started the baseline period (A); 5 started the treatment period (B); 4 completed the treatment; and a final number of 3 participants completed the follow-up period (C). The baseline characteristics of the 6 participants who started the treatment are shown in Table II.
Table II.
Baseline characteristics
| Participant number | 1 | 2 | 3 | 4 | 5 | 6 | Mean (SD) |
|---|---|---|---|---|---|---|---|
| Age, years | 63.7 | 51.7 | 68.1 | 56.3 | 45.6 | 75.4 | 60.1 (11.0) |
| Sex | Male | Female | Male | Male | Male | Female | |
| Duration of neuropathic pain complaints, years | 5 | – | 3 | 5 | 5 | 5 | 4.6 (0.9) |
| Intensity neuropathic pain (min–max. VAS) | 4–8 | 4–8 | 2–7 | 4–7 | 7–7 | 3–7 | 3–8 |
| Current pain intensity (VAS) | 6 | 2 | 3 | 7 | 7 | 1 | 4.3 (2.7) |
| Medication use | Pregabalin | Pregabalin | None | None | Amitriptyline | Gabapentin | – |
| Glucose, mmol/L | 4.7 | 13.3 | 9.1 | 9.2 | 10.1 | 8 | 9.1 (2.8) |
| Insulin, mU/L | 90 | 152 | 104 | 280 | 152 | 107 | 147.5 (69.9) |
| HbA1c, mmol/mol | 47 | 54 | 56 | 78 | 50 | 48 | 55.5 (11.5) |
| HbA1c, % | 6.5 | 7.1 | 7.3 | 9.3 | 6.7 | 6.6 | 7.3 (1.1) |
| PDI | – | 28 | 20 | 43 | 40 | 47 | 35.6 (11.2) |
| PARS | – | 14 | 27 | 13 | 21 | 11 | 17.2 (6.6) |
| PAD | – | 6 | 7 | 8 | 4 | 8 | 6.6 (1.7) |
| HADS-A | 11 | 13 | 1 | 6 | 10 | 17 | 10.3 (2.7) |
| HADS-D | 8 | 9 | 4 | 7 | 10 | 15 | 10.5 (1.4) |
| PARTQ-30 | 47 | 64 | 32 | 43 | 49 | 67 | 50.3 (13.2) |
| PCS | 27 | 50 | 8 | 25 | 32 | 30 | 28.7 (13.5) |
| Norfolk QoL -DN | 57 | 64 | 18 | 71 | 68 | 87 | 60.8 (20.2) |
PDI: Pain Disability Index; PARS: Physical Activity Rating Scale; PAD: Perceived Activity Decline; HADS-A: Hospital Anxiety and Depression Scale –Anxiety Subscale; HADS-D: Hospital Anxiety and Depression Scale –Depression Subscale; PARTQ-30: PCS: Pain Catastrophizing Scale; Painful Diabetic Neuropathy Anxiety Rasch-Transformed Questionnaire; Norfolk QOL-DN: Norfolk Quality of Life Questionnaire-Diabetic Neuropathy; SD: standard deviation.
In the next paragraphs, data for participants 3 (P3), 4 (P4) and 5 (P5) are presented, as these are the only participants who completed the full treatment procedure and all measurements. Participants 1 and 2 dropped out after the intake session. Participant 6 finished the treatment, but did not return questionnaires at T1–T3. Due to the small sample size, the data presented are mostly descriptive. For the diaries, pvalues are given for the combined items, as described earlier.
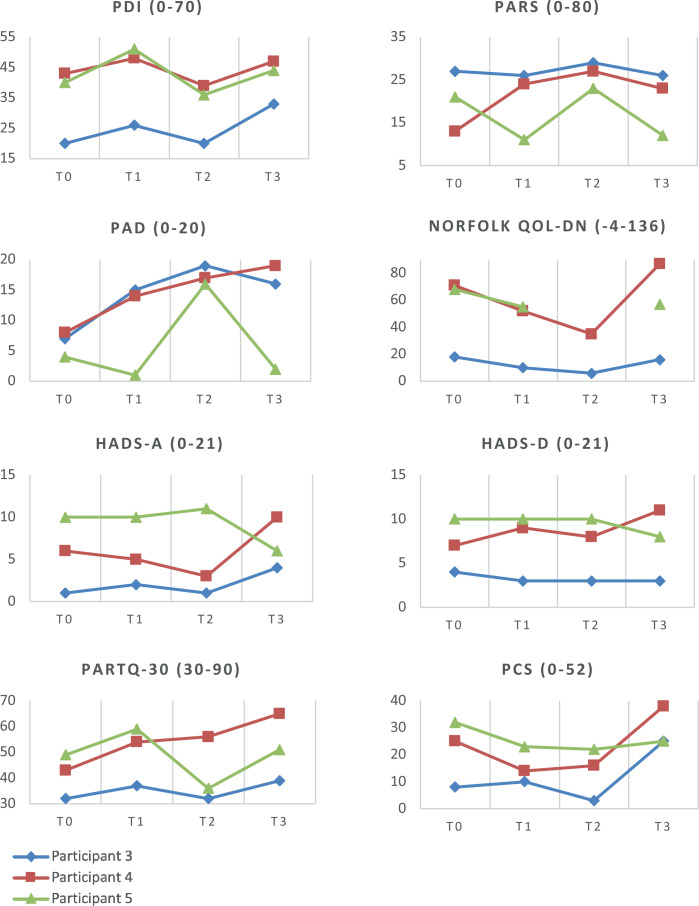
Physical activity and quality of life
For PA, PAD and QoL, all measures (PARS, PAD and Norfolk-QOL-DN) showed great variability amongst the 3 participants, with no clear trends. For these measures, no cut-off values for a minimal clinically important change (MCIC) are available. All participants deteriorated in terms of disability (PDI) between T0 and T1, followed by an improvement back to baseline after treatment (T2), and a deterioration at follow-up (T3). Here, MCIC (8.5–9.5) was reached in P3 at T2–T3, in P4 at T1–T2, and in P5 at T0–T1 and T1–T2.
Anxiety, depression and catastrophizing
P3 reported no signs of depression or anxiety at all time-points (HADS-A and HADS-D < 8). P4 scored only mild complaints on the HADS-A (8–10) at T3, and on the HADS-D at all time-points. P5 had mild complaints on HADS-A at T1–T2 that had improved on T3, while the mild complaints on HADS-D persisted at all time-points. The PARTQ-30 questionnaire showed a great variability amongst all participants without clear trends. For PARTQ-30 no cut-off values/MCIC are available. The presence of catastrophizing is defined as a PCS score of > 30, which was observed only in P4 at T3 (PCS 38) and in P5 at T0 (PCS 32). In addition, no clear trend could be identified throughout T0–T3.
All data from the questionnaires are shown in Fig. 3.
Fig. 3.
Results of questionnaires about physical activity, quality of life (QoL), depression and fears. Participants 3, 4 and 5. PDI: Pain Disability Index; PARS: Physical Activity Rating Scale; PAD: Perceived Activity Decline; Norfolk QOL-DN: Norfolk Quality of Life Questionnaire-Diabetic Neuropathy: HADS-A: Hospital Anxiety and Depression Scale –Anxiety Subscale; HADS-D: Hospital Anxiety and Depression Scale – Depression Subscale; PARTQ-30: Painful Diabetic Neuropathy Anxiety Rasch-Transformed Questionnaire; PCS: Pain Catastrophizing Scale.
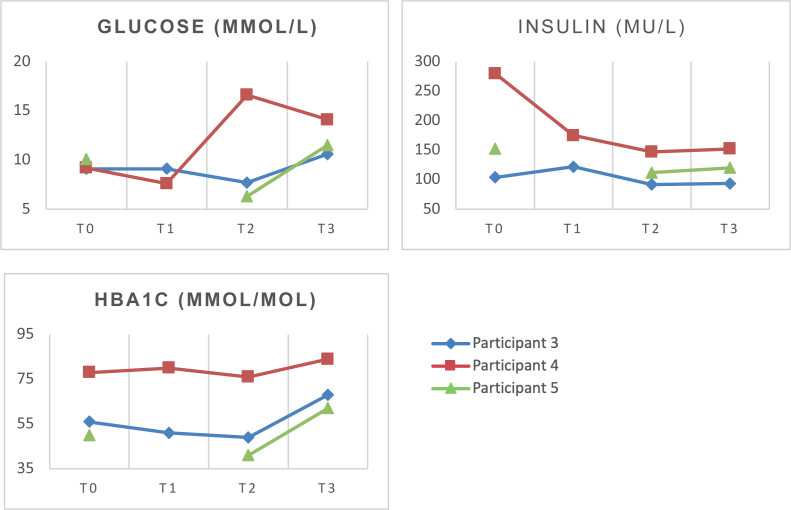
Glucose regulation
For P3, the glucose and insulin levels were relatively similar in period A (T0–T1) and showed a decrease after the treatment (T2). In the follow-up period C (T3), the glucose levels increased compared with the end of period B (T2), whereas the insulin levels remained stable. HbA1c levels were stable throughout baseline and treatment; however, they showed an increase at the follow-up measurement.
For P4, a different trend was observed. The glucose and insulin levels decreased in period A (T0–T1), and had increased after the treatment (period B, T2) and remained stable after the follow-up period C (T3). The HbA1c levels were stable across all time-points.
For P5, glucose, insulin levels and HbA1c levels at T1 were not available, due to non-compliance. All values showed a decrease between T0 and T2 and an increase in the follow-up period C (T3).
Data for the metabolic parameters are shown in Fig. 4 .
Fig. 4.
Results of metabolic parameters. Missing values for T1 for participant 5 on all parameters.
Diaries
Visual analyses
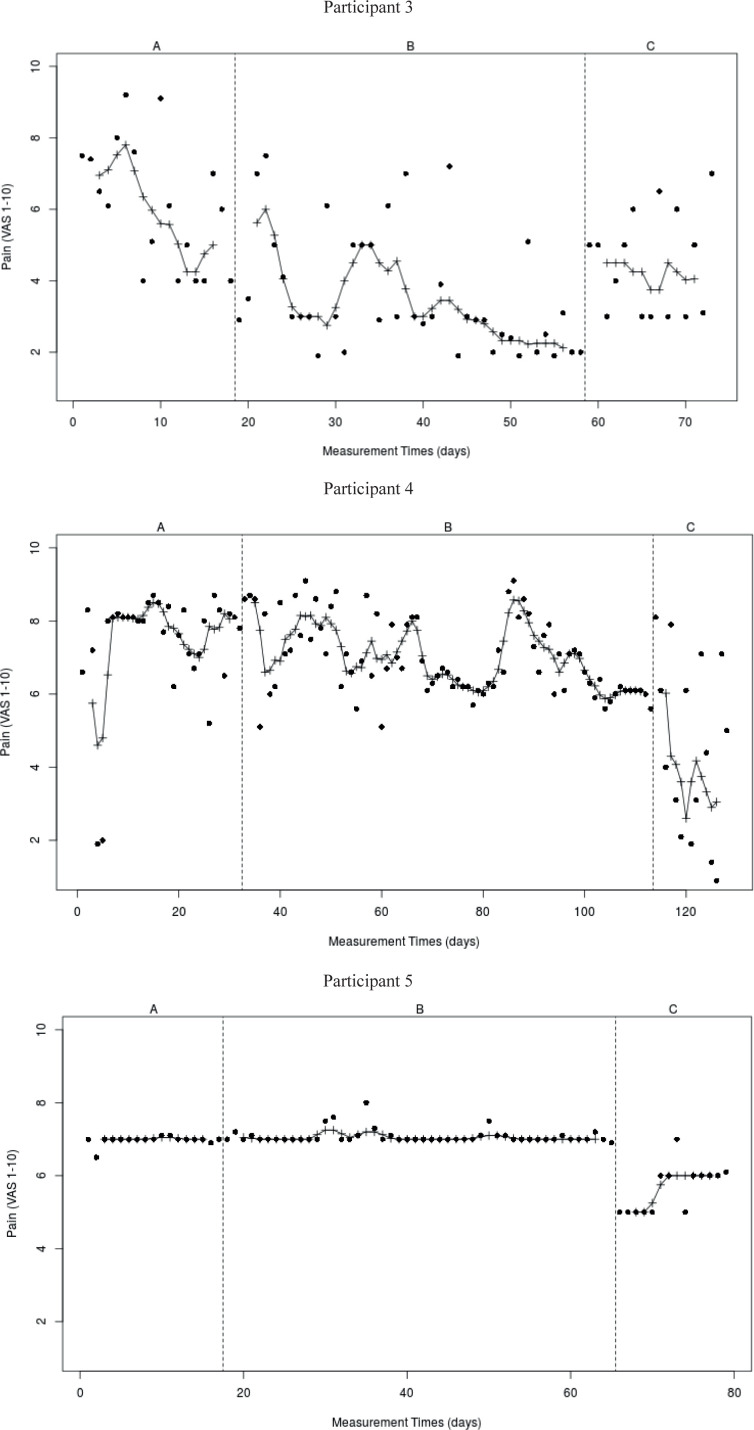
Overall, no clear trends could be identified for the individual items in all 3 participants. Only in P3, a decreasing trend was observed for question 1 (pain at this moment) in period A (8–4) and B (6–2), with a higher stable level in period C (4–6). P4 and P5 showed no changes in pain intensity over periods A and B, with scores fluctuating between 6 and 9 (P4) and stable scores of approximately 7 (P5), respectively. P4 showed a decrease in pain in period C from 6 to 3, while P5 fluctuated between 5 and 6. The graphs for question 1 are shown in Fig. 5.
Fig. 5.
Visual analyses of data on item “Pain at this moment” (VAS 1-10)
For questions 2–11, large floor effects were observed in all participants, with little to no variation. For the personalized questions (questions 12, 13A and 13B), only some variation was observed in P3. Here, for question 12, values between 4 and 8 in period A, 2–6 in period B, and 2–8 in period C (PHODA-PDN; walk down the stairs) were observed. Questions 13A and 13B (COPM performance and satisfaction; walk for 1 h) showed large variability, with a decrease during treatment phase (period B) and an increase in the follow-up period (period A 0–6; period B 2–6, period C 4–8, respectively). For the other participants, no variability could be observed in periods A, B and C for the personalized questions (data not shown).
Statistical analysis of daily measures
The changes between baseline and treatment (A–B) and between baseline and follow-up (A–C) of the diary measures were not statistically significant on an individual basis, nor in the combined themes (Table III).
Table III.
Statistical analyses of diary questions grouped in categories.
| Participant 3 | Participant 4 | Participant 5 | |
|---|---|---|---|
| Pain intensity | |||
| Baseline-EXP | 0.194 | 0.945 | 0.949 |
| Baseline-FU | 0.403 | 0.787 | 0.795 |
| Fear of movement | |||
| Baseline-EXP | 0.222 | 0.252 | 0.154 |
| Baseline-FU | 0.333 | 0.252 | 0.179 |
| FOH/exhaustion | |||
| Baseline-EXP | 0.056 | 1.000 | 0.333 |
| Baseline-FU | 0.056 | 1.000 | 0.372 |
| Avoidance | |||
| Baseline-EXP | 0.181 | 1.000 | 0.128 |
| Baseline-FU | 0.528 | 1.000 | 0.154 |
| Fear of falling | |||
| Baseline-EXP | 0.236 | 0.323 | 0.218 |
| Baseline-FU | 0.222 | 0.252 | 0.218 |
| PHODA-PDN | |||
| Baseline-EXP | 0.972 | 0.472 | 0.987 |
| Baseline-FU | 0.556 | 0.354 | 0.564 |
| COPM | |||
| Baseline-EXP | 0.662 | 0.299 | 0.218 |
| Baseline-FU | 0.225 | 0.441 | 0.218 |
p-values for each participant on the diary data between baseline – exposure in vivo (EXP) and baseline – follow-up (FU) for the aggregated independent variables of pain intensity, fear of hypoglycaemia (FOH)/exhaustion, avoidance, fear of falling, Photograph-series Of Daily Activities – Painful Diabetic Neuropathy version (PHODA-PND) and Canadian Occupational Performance Measure (COPM), respectively.
DISCUSSION
In this pilot study, it was hypothesized that EXP treatment, through targeting specific fears, would lead to a reduction in the perceived harmfulness of activities, resulting in a higher level of PA and improved QoL in patients with PDN. The study describes the results of 3 participants who completed the full study procedure. The results are heterogeneous for most outcomes, such as PA, depression, fears, QoL, and metabolic parameters, and, due to the small sample, it is difficult to determine the effectiveness of EXP treatment. Nonetheless, some valuable lessons can be learned from the findings and drop-out rates of this study.
The study was designed around the hypothesis that the FAM is also applicable to a subgroup of patients with PDN, as was supported by the results of previous studies, in which we identified PDN-related fears that could be challenged with EXP (5, 10, 16, 19, 20). However, during the study some unexpected, yet significant, differences were encountered compared with experiences of EXP in other pain conditions. These differences can be related to the aetiology and multimodality of the underlying disease (diabetes mellitus) in which many different healthcare providers are involved, the aetiology of the pain itself (neuropathic vs musculoskeletal) and/or the possible underlying cognitive processes in coping with the pain. These topics are discussed below.
Diabetes mellitus is a common multidimensional condition, daily management of which is burdensome, and long-term complications occur frequently. Up to 25% of patients with diabetes mellitus develop PDN, which has debilitating effects on daily life, both physical and mental (3, 5). Despite the high prevalence of patients with PDN with anxiety-related complaints, difficulties were experienced in recruiting candidates for the current study and a large number of drop-outs occurred. The most important reason for dropping out after screening by the physician or treatment team, was subject’s unwillingness to change daily life functioning (38%) or not experiencing pain as the main interference in daily life (19%). This was unexpected, as the participants were specifically recruited based on their experienced burden of the pain. There seems to be a discrepancy in how individuals perceive their pain and disability (high burden), vs the willingness/readiness to participate in a rehabilitation programme that addresses these problems. A possible reason could be that the diabetes mellitus-related comorbidity is so predominant, that patients do not allocate PDN first priority, or patients may simply do not believe/realize that their perception and burden of the complaints can be altered. Also, other internal or external personal factors (e.g. personality traits, role of healthcare providers, spouse) may play a role in this. Based on our experiences, we believe that more research should be done on the hierarchical experienced burden of all aspects of diabetes mellitus and its comorbidities (“What is the most important diabetes mellitus-related problem that limits your daily life functioning at this moment?”). Ideally, this information should be paired with (qualitative) data from diabetes mellitus patients on: (i) which of these experienced burdens they want to learn to cope with (willingness to change), (ii) what they are now lacking in order to be able to tackle these problems (readiness to change/empowerment), (iii) and in which way or form they feel healthcare could meet their needs in order to achieve their goals.
EXP aims to increase physical ability and QoL by establishing a new, positive association between previously expected negative irrational outcomes during a specific activity (15). EXP is most powerful when the discrepancy between the real-life situation and the irrational feared consequences is large (15). The difficulty with PDN-related fears is that the evaluation of a fear is not always irrational and some level of concern can be considered appropriate and adaptive; e.g. being afraid of falling when having balance impairments, or being (hyper)vigilant to develop PUs when having little to no sensation in the feet and being told to check for PUs, etc. (5, 16). To date, no tool is available that can help clinicians to identify the difference between a rational and an irrational fear. We also believe that EXP can only be effective when the fears are the foremost reason for the experienced disability in daily life. If there are other reasons, such as significantly debilitating (co)morbidity, EXP alone may not succeed in improving physical functioning. In the current study, there were 5 drop-outs due to comorbidity (back pain, cardiopulmonary problems, pressure ulcer).
Furthermore, it is known that diabetes mellitus is a complex condition that requires attention to many more aspects of one’s health than pain alone. Patients encounter many healthcare providers over short periods of time, and almost all of them have a mainly somatic (bio) approach to the various diabetes mellitus-related issues. There is a continuous focus on glucose regulation, skin care, dietary restrictions, and prevention/treatment of hypertension and/or dyslipidaemia. When multiple healthcare providers are involved, this could result in non-compatible or even contradictory advice being given regarding PA, glucose management and/or the management of skin problems. EXP can only be powerful and successful at diminishing pain-related fears if the patient can be convinced that PA is not harmful. Adequate medical counselling about individual possibilities regarding PA is essential to create this awareness. As soon as other healthcare providers, spouses, friends or relatives advise adversely, uncertainty may (re-)occur, and the effect of EXP can be diminished. This mechanism could be an explanation for the discrepancies in improved PA levels combined with higher PAD scores during treatment, and the worsening of almost all outcome variables in in the follow-up period in P4.
There seems to be a difference in how patients cope with chronic musculoskeletal pain compared with neuropathic pain. Studies in various chronic musculoskeletal pain conditions have shown that factors such as psychological flexibility (PF) can play an important role in relation to well-being and daily functioning (37, 38). In PDN, however, only low correlations between PF and functional impairment, depression severity and depression impact were seen, whereas relatively higher correlations between the pain itself and functional impairment were found (39). Pain severity generally appeared to play a more important role in relation to daily functioning in PDN, than psychological factors did (39). These findings confirm our previous work, in which pain severity was shown to be the main predictor for disability and QoL, rather than various fears (5), and could also be an explanation for the discrepancies found in this study regarding how one perceives the pain and its disability, vs the willingness/readiness to do something about it. Another study demonstrated that, while the neuropathic pain in PDN did contribute to depression, unsteadiness was the symptom with the strongest, cumulative effect on depression. The patients’ perception of their unsteadiness appeared to be an adequate indicator of the actual balance impairment (14). Future interventions that aim to improve PA in PDN, should take into account balance impairments and patients’ perception of unsteadiness, in addition to the above-mentioned bio-psychological factors.
Study limitations
A limitation of this study that should be addressed is that the PHODA-PDN is different from the PHODA. The original PHODA is designed to identify activities that evoke fear of (additional) injury. In EXP, these activities are challenged and the fears will be diminished when the patient experiences that they can perform the activity without the occurrence of the feared consequence. However, in previous qualitative studies, we found that the most frequent PDN-related fears (fear of falling, fear of hypoglycaemia, etc.) were not associated with specific activities. To overcome this issue, we altered the procedure by using the PHODA-PDN in 2 phases (step 1: identification of type of fear; step 2: pairing this fear to a specific situation or activity). This adaptation of the PHODA may have affected the effectiveness of EXP, as EXP was originally designed to challenge feared activities, rather than fears itself. Therefore, more research on the PHODA-PDN is needed. Another limitation was the way in which patients were recruited, as most participants were approached by their own physician and did not apply to enter the study themselves.
Conclusion
In summary, the effectiveness of EXP for restoring QoL and physical wellbeing in patients with PDN was not confirmed by this study. Despite the overlap of concepts within the FAM that were well-established in a variety of other chronic pain conditions, this study revealed that there seem to be some other significant factors involved in patients with PDN. Given the lessons learned, we believe that EXP may have potential added value only for patients with PDN in whom: (i) daily life functioning is mostly impaired by PDN, and not by other (co)morbidities; (ii) PDN-related fears (feared consequences) that cause these impairments are exaggerated and irrational; (iii) specific activities can be identified that evoke the PDN-related fears; (iv) spouses and other healthcare providers are involved in the treatment; and (v) there is a willingness to participate in a rehabilitation programme that addresses PDN-related fears in daily life situations. Further research is needed into to elucidate this subject.
ACKNOWLEDGEMENTS
This work was partially supported by a grant from the Dutch Diabetes Foundation.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev 2011; 27: 629–638. [DOI] [PubMed] [Google Scholar]
- 2.van Sloten TT, Savelberg HH, Duimel-Peeters IG, Meijer K, Henry RM, Stehouwer CD, et al. Peripheral neuropathy, decreased muscle strength and obesity are strongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Res Clin Pract 2011; 91: 32–39. [DOI] [PubMed] [Google Scholar]
- 3.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006; 29: 1518–1522. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez JS, Vileikyte L, Ulbrecht JS, Rubin RR, Garrow AP, Delgado C, et al. Depression predicts first but not recurrent diabetic foot ulcers. Diabetologia 2010; 53: 2241–2248. [DOI] [PubMed] [Google Scholar]
- 5.Geelen CC, Smeets R, Schmitz S, van den Bergh JP, Goossens M, Verbunt JA. Anxiety affects disability and quality of life in patients with painful diabetic neuropathy. Eur J Pain 2017; 21: 1632–1641. [DOI] [PubMed] [Google Scholar]
- 6.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008; 31: 2108–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lascar N, Kennedy A, Hancock B, Jenkins D, Andrews RC, Greenfield S, et al. Attitudes and barriers to exercise in adults with type 1 diabetes (T1DM) and how best to address them: a qualitative study. PLoS One 2014; 9: e108019.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins MM, Corcoran P, Perry IJ. Anxiety and depression symptoms in patients with diabetes. Diabet Med 2009; 26: 153–161. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Fisher KJ, Harmer P, McAuley E, Wilson NL. Fear of falling in elderly persons: association with falls, functional ability, and quality of life. J Gerontol B Psychol Sci Soc Sci 2003; 58: 283–290. [DOI] [PubMed] [Google Scholar]
- 10.Geelen CC, Kindermans HP, van den Bergh JP, Verbunt JA. Perceived physical activity decline as a mediator in the relationship between pain catastrophizing, disability, and quality of life in patients with painful diabetic neuropathy. Pain Practice 2016; 17: 320–328. [DOI] [PubMed] [Google Scholar]
- 11.Praet SF, van Rooij ES, Wijtvliet A, Boonman-de Winter LJ, Enneking T, Kuipers H, et al. Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2008; 51: 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melai T, Schaper NC, Ijzerman TH, de Lange TL, Willems PJ, Lima Passos V, et al. Lower leg muscle strengthening does not redistribute plantar load in diabetic polyneuropathy: a randomised controlled trial. J Foot Ankle Res 2013; 6: 41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geerts M, Landewe-Cleuren SA, Kars M, Vrijhoef HJ, Schaper NC. Effective pharmacological treatment of painful diabetic neuropathy by nurse practitioners: results of an algorithm-based experience. Pain Med 2012; 13: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 14.Vileikyte L, Crews RT, Reeves ND. Psychological and bio-mechanical aspects of patient adaptation to diabetic neuropathy and foot ulceration. Curr Diab Rep 2017; 17: 109.. [DOI] [PubMed] [Google Scholar]
- 15.den Hollander M, de Jong JR, Volders S, Goossens ME, Smeets RJ, Vlaeyen JW. Fear reduction in patients with chronic pain: a learning theory perspective. Expert Rev Neurother 2010; 10: 1733–1745. [DOI] [PubMed] [Google Scholar]
- 16.Kanera IM, van Laake-Geelen CC, Ruijgrok JM, Goossens MEJB, de Jong JR, Verbunt JA, et al. A qualitative investigation into living with diabetic peripheral neuropathic pain: patient’s experiences, beliefs, and fears. Psychol Health 2019; 34: 84–105. [DOI] [PubMed] [Google Scholar]
- 17.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. Graded exposure in vivo in the treatment of pain-related fear: a replicated single-case experimental design in four patients with chronic low back pain. Behav Res Ther 2001; 39: 151–166. [DOI] [PubMed] [Google Scholar]
- 18.den Hollander M, Goossens M, de Jong J, Ruijgrok J, Oosterhof J, Onghena P, et al. Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. Pain 2016; 157: 2318–2329. [DOI] [PubMed] [Google Scholar]
- 19.Geelen CC, Brouwer BA, Hoeijmakers JG, Faber CG, Merkies IS, Verbunt JA. Painful diabetic neuropathy Anxiety Rasch-Transformed Questionnaire (PART-Q30 ). J Peripher Nerv Syst 2016; 21: 96–104. [DOI] [PubMed] [Google Scholar]
- 20.van Laake-Geelen C, Smeets R, van Meulenbroek T, den Hollander M, Goossens M, Verbunt J. Rehabilitation treatment ‘exposure in vivo’ in patients with painful diabetic neuropathy: development of a treatment protocol. JRM-CC 2019; 2: 1000015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer JW, Bosma E, Lefrandt JD, Links TP, Smit AJ, Stewart RE, et al. Clinical diagnosis of diabetic polyneuropathy with the diabetic neuropathy symptom and diabetic neuropathy examination scores. Diabetes Care 2003; 26: 697–701. [DOI] [PubMed] [Google Scholar]
- 22.Onghena P, Edgington ES. Randomization tests for restricted alternating treatments designs. Behav Res Ther 1994; 32: 783–786. [DOI] [PubMed] [Google Scholar]
- 23.Onghena P, Edgington ES. Customization of pain treatments: single-case design and analysis. Clin J Pain 2005; 21: 56–68; discussion 69–72. [DOI] [PubMed] [Google Scholar]
- 24.Vercoulen JH BE, Swanink CM, Galama JM, Jongen PJ, Hommes O, Van der Meer JW, Bleijenberg G. Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J Psychiatr Res 1997; 31: 661–673. [DOI] [PubMed] [Google Scholar]
- 25.Verbunt JA. Reliability and validity of the PAD questionnaire: a measure to assess pain-related decline in physical activity. J Rehabil Med 2008; 40: 9–14. [DOI] [PubMed] [Google Scholar]
- 26.Vinik EJ, Hayes RP, Oglesby A, Bastyr E, Barlow P, Ford-Molvik SL, et al. The development and validation of the Norfolk QOL-DN, a new measure of patients’ perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 2005; 7: 497–508. [DOI] [PubMed] [Google Scholar]
- 27.Tait RC, Chibnall JT, Krause S. The Pain Disability Index: psychometric properties. Pain 1990; 40: 171–182. [DOI] [PubMed] [Google Scholar]
- 28.Turk DC, Dworkin RH, Trudeau JJ, Benson C, Biondi DM, Katz NP, et al. Validation of the Hospital Anxiety and Depression Scale in patients with acute low back pain. J Pain 2015; 16: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 29.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan, Bishop, Pivik. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995; 7: 524. [Google Scholar]
- 31.Severeijns R, van den Hout MA, Vlaeyen JW, Picavet HSJ. Pain catastrophizing and general health status in a large Dutch community sample. Pain 2002; 99: 367–376. [DOI] [PubMed] [Google Scholar]
- 32.Wressle E, Lindstrand J, Neher M, Marcusson J, Henriksson C. The Canadian Occupational Performance Measure as an outcome measure and team tool in a day treatment programme. Disabil Rehabil 2003; 25: 497–506. [DOI] [PubMed] [Google Scholar]
- 33.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the UK. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soer R, Reneman MF, Vroomen PC, Stegeman P, Coppes MH. Responsiveness and minimal clinically important change of the Pain Disability Index in patients with chronic back pain. Spine (Phila Pa 1976) 2012; 37: 711–715. [DOI] [PubMed] [Google Scholar]
- 35.Olssøn I, Mykletun A, Dahl AA. The Hospital Anxiety and Depression Rating Scale: a cross-sectional study of psychometrics and case finding abilities in general practice. BMC Psychiatry 2005; 5: 46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 2000; 23: 351–365. [DOI] [PubMed] [Google Scholar]
- 37.McCracken LM, Gauntlett-Gilbert J, Vowles KE. The role of mindfulness in a contextual cognitive-behavioral analysis of chronic pain-related suffering and disability. Pain 2007; 131: 63–69. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Norton S, Almarzooqi S, McCracken LM. Preliminary investigation of self-as-context in people with fibromyalgia. Br J Pain 2017; 11: 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kioskli K, Winkley K, McCracken L. Might psychological flexibility processes and Acceptance and Commitment Therapy (ACT) apply in adults with painful diabetic neuropathy? A cross-sectional survey. J Contextual Behav Sci 2019; 13: 66–73. [Google Scholar]