Abstract
Background –
We have previously shown that the presence of dual muscular coronary sinus (CS) to left atrial (LA) connections, coupled with rate-dependent unidirectional block in one limb, is associated with atrial fibrillation (AF) induction. This study sought to examine whether ablation of distal CS to LA connections at a first AF ablation reduces arrhythmia recurrence during follow-up.
Methods –
In this single center, randomized, controlled trial, 35 consecutive patients with drug refractory AF undergoing first time ablation between August, 2018 and August, 2019, were randomly assigned to (1) standard ablation (pulmonary vein [PV] isolation and non-PV trigger ablation), versus (2) standard ablation plus elimination of distal CS to LA connections targeting the earliest LA activation during distal CS pacing with a deca-polar catheter placed with its proximal electrode at the ostium. Change of the local CS atrial electrogram and LA activation sequence to early activation of the LA septum or roof during distal CS pacing were the endpoint for CS-LA connection elimination.
Results –
Thirty patients completed 6 months study follow-up (15 patients in each group). Demographic characteristics including age and AF persistence were similar in both groups. After a mean follow-up of 170±22 days, there were 7 atrial arrhythmia recurrences in the standard group and 1 recurrence in the CS-LA connection elimination group (46.7% vs 6.7%, HR 0.12, P=0.047).
Conclusions –
Elimination of distal CS to LA connections reduced atrial arrhythmia recurrences compared to standard PV isolation and non-PV trigger ablation in patients undergoing a first AF ablation procedure in a small randomized study.
Keywords: atrial fibrillation, coronary sinus, pulmonary vein isolation, non-PV triggers
Journal Subject Terms: Electrophysiology, Catheter Ablation and Implantable Cardioverter-Defibrillator
Graphical Abstract
Introduction
Electrical isolation of pulmonary vein (PV) triggers from the left atrium (LA) is the cornerstone strategy of atrial fibrillation (AF) ablation.1–3 The importance of identification and ablation of non-PV triggers is increasingly recognized and has improved AF ablation outcomes.3–6 However, overall outcomes for AF ablation remain unsatisfactory. Worldwide, one year post AF ablation efficacy remains limited at 70% after a single procedure in paroxysmal and 60% in non-paroxysmal AF patients.7, 8 Previous studies have revealed anatomic connections between the LA and coronary sinus (CS) musculature.9, 10 Moreover, we have demonstrated that rate-dependent unidirectional block in one limb of dual muscular connections between CS and LA, is associated with AF/flutter induction.11 The association is mediated by single or multiple reentry beats between the CS and LA that trigger AF. In the present study, we sought to prospectively examine the efficacy of distal CS and LA muscular connection elimination for suppression of recurrent atrial arrhythmias.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
The study is a single center, randomized, controlled trial, enrolling drug-refractory symptomatic patients who presented for first time AF ablation at the Hospital of the University of Pennsylvania between August 2018 and August 2019 (PRECAF ClinicalTrials.gov number: NCT03646643). Based upon alpha 0.05, power 0.8, 1:1 randomization, and estimated relative risk of 14.4 for AF susceptibility with rate dependent CS-LA conduction block,11 we anticipated a minimum sample size of 6 patients per group would be necessary to detect a difference using the log rank test. Additional patients were recruited to account for potential losses to follow up and a smaller detectable difference. Patients were randomly assigned to (1) standard ablation (PV isolation and non-PV trigger ablation), versus (2) standard ablation plus prospective elimination of distal CS to LA connection. Documented or induced atrial flutter during the procedure was also ablated in both groups. All patients provided institutional review board-approved written informed consent and were blinded to treatment assignment.
Transseptal Access, Mapping and Ablation
The standard mapping and AF ablation techniques at the Hospital of the University of Pennsylvania have been previously described.6 In brief, intravenous heparin was administered to achieve an activated clotting time of >350 seconds before trans-septal access. Under the guidance of fluoroscopy, two long 8.5 Fr. Trans-septal sheaths (Agilis and SL-1, St. Jude Medical, St. Paul, Minnesota) were advanced over a long guidewire to the superior vena cava and a flushed BRK trans-septal needle (St. Jude Medical) was introduced into the Agilis/SL-1 sheaths. Then, the BRK needle was utilized to puncture through the atrial septum and the whole system was advanced into the LA under fluoroscopy and intra-cardiac echocardiography (ICE, 8 Fr., AcuNav, Biosense Webster, Diamond Bar, California) monitoring. Patients that presented in AF were cardioverted before mapping. Electroanatomic maps (EAM) were created with the Carto 3 system (Biosense Webster Inc., Diamond Bar, CA) using multi-polar PentaRay (20 electrodes with 2-6-2 mm spacing) or Lasso (Circular 20-electrodes) catheters during distal coronary sinus pacing. Pacing was performed at the minimum output that captured the distal CS, to avoid direct capture of adjacent LA myocardium. Mapping was performed with an equal distribution of points (Figure 1A). Intracardiac echocardiography (ICE), orthogonal fluoroscopy, and electrogram characteristics were used to confirm adequate contact and reliable mapping during distal CS pacing. Radiofrequency ablation was performed using a 3.5mm open-irrigated Thermocool, Thermocool Smarttouch or Thermocool Smarttouch SF (Biosense Webster Inc.) with wide antral PV isolation (Figure 1B) together with ablation of any spontaneous or inducible non-PV triggers in both groups.3, 12 Bidirectional PV entrance and exit block was confirmed and adenosine administration was used to survey acute PV reconnection with further ablation as necessary. Non-PV triggers were targeted when identified following PV isolation in both groups using (1) isoproterenol infusion (starting at 3 mcg and incrementing every 3–5 minutes to 6, 12, and 20 mcg), and (2) cardioversion of spontaneous AF or AF induced by left or right atrial pacing. Previously documented typical atrial flutter and/or induced atrial flutters were also ablated in both groups. CS to LA connection ablation targeted the earliest activated LA focus during distal CS pacing with a deca-polar catheter (2-8-2mm interelectrode distance) placed with its proximal electrode at the CS ostium and adjacent to the atrial septum (Figure 1C), which was confirmed by the fluoroscopy and ICE. Radiofrequency energy was delivered at 40 watt or less, for 30 seconds or less and titrated down using standard criteria at the discretion of the proceduralist (Figure 1D). Change of the local CS atrial electrogram and LA activation sequence to early activation of the LA septum or roof during distal CS pacing were the endpoint for elimination of CS to LA connection (Figure 2). Stimulation for induction of AF with pacing cycle length from 250 to 180 ms or 2:1 capture was performed from proximal coronary sinus, distal coronary sinus and at the site of left atrial to CS connection following PV isolation, before and after ablation of the connection.
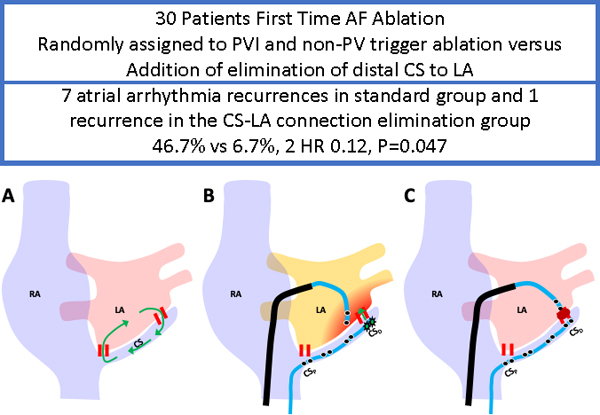
Figure 1.

Standard AF Ablation with Prospective Elimination of Connection between Distal Coronary Sinus and Left Atrium. Figure 1A: Left atrial (LA) electroanatomical voltage map acquired during distal coronary sinus (CS) pacing with normal left atrial substrate is displayed; Figure 1B: the standard LA pulmonary antrum circumferential ablation to achieve pulmonary vein isolation; Figure 1C: LA activation map with earliest atrial activation at lateral wall of LA during distal CS pacing; Figure 1D: Ablation lesions targeting at the distal CS to LA connection.
Figure 2.
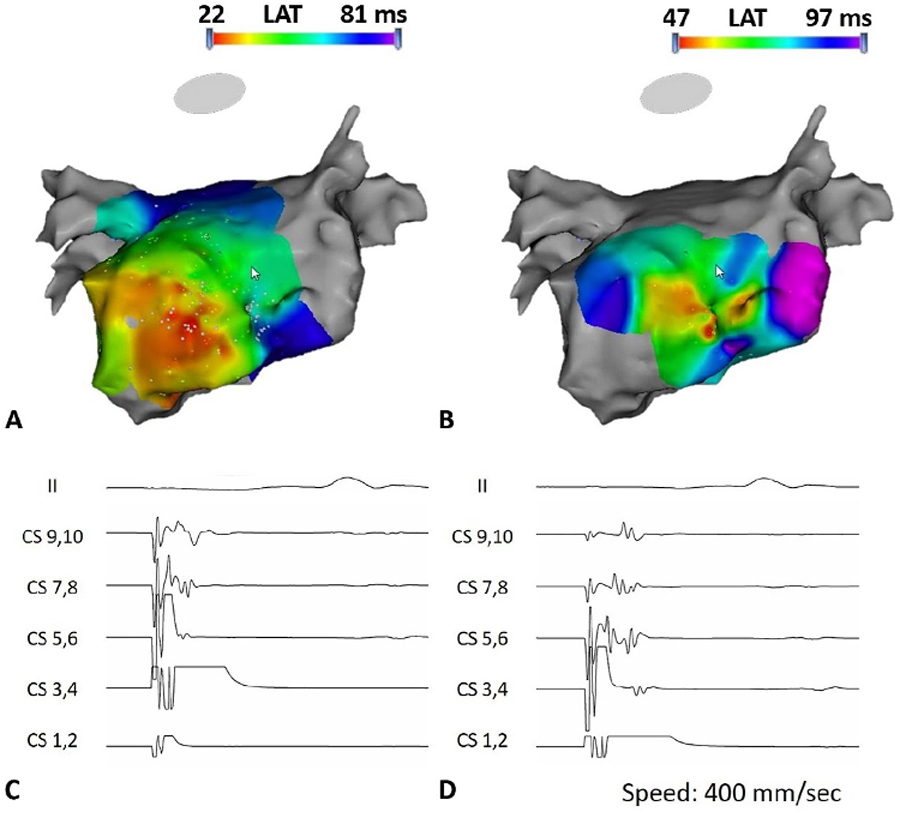
Endpoint of the Elimination of Distal Coronary Sinus to Left Atrium Connection. Figure 2A: the LA activation map before elimination of distal CS to LA connection during distal CS pacing; Figure 2B: the LA activation map after elimination of distal CS to LA connection, which demonstrates the change of earliest atrial activation site from lateral wall of LA toward septum; Figure 2C: the local atrial electrogram recorded on the CS catheter during distal CS pacing before ablation at distal CS to LA connection, showing parallel near- and far-field activation of the CS and LA, in a distal to proximal direction. Figure 2D: the change of local atrial electrogram activation recorded on the CS catheter following elimination of the CS to LA connection, showing nearfield activation of the CS itself distal to proximal, followed by pivot at the proximal CS, where the LA activates and then proximal to distal activation of the LA.
Study Endpoint and Clinical Follow-up
Insertable cardiac monitor implantation was performed per physician discretion. Regular clinic visits at 6 weeks and 6 months post-ablation were arranged with 12 lead electrocardiogram recordings at each session for all patients. A 30-day monitor during the first month after ablation, and symptom prompted 3- to 30-day monitors following the first month, were provided to all patients without insertable monitors. Time to first atrial arrhythmia episode was recorded as the primary outcome. The endpoint was defined as an episode of atrial fibrillation, atrial flutter, or atrial tachycardia lasting >30 seconds in duration following the 90-day post-ablation blanking period.
Statistical Analysis
Continuous variables are expressed as mean and standard deviation and categorical variables as count and percentage. The independent samples Student t test, Fisher exact test or χ2 test were used to compare differences across groups, as appropriate. Atrial arrhythmia recurrence at follow-up was reported with a time-to-event analysis with censoring on November 20th, 2019, and survival curves were created utilizing the Kaplan–Meier method with differences between groups compared with the log-rank test. All analyses were performed using SPSS version 24 (SPSS Inc., IBM Corp., Chicago, IL).
Results
Demographic characteristics
This study enrolled 35 patients, 30 of whom completed follow-up at our center (15 in each group, 47% female, mean age 65.6 ± 7.2 years). The baseline characteristics are summarized in Table 1 and were similar between groups.
Table 1.
Baseline Clinical Data of the Overall Study Population and According to the Ablation Strategy
| Variable | Overall (n=30) |
Standard Procedure (n=15) | Standard Procedure plus Prospective Elimination of CS-LA connection (n=15) |
p value |
|---|---|---|---|---|
| Clinical data | ||||
| Age, year | 65.6±7.2 | 66.7±6.1 | 64.6±8.3 | 0.4 |
| Female sex, n (%) | 14 (46.7) | 9 (60) | 5 (33.3) | 0.1 |
| Body weight, kilogram | 93.8±21.2 | 90.6±24.4 | 97.0±17.6 | 0.4 |
| Persistent atrial fibrillation, n (%) | 13 (43.3) | 7 (46.7) | 6 (40) | 0.7 |
| Congestive Heart Failure, n (%) | 5 (16.7) | 2 (13.3) | 3 (20) | 0.6 |
| Hypertension, n (%) | 21 (70) | 12 (80) | 9 (60) | 0.2 |
| Diabetes mellitus, n (%) | 6 (20) | 5 (33.3) | 1 (6.7) | 0.07 |
| Prior stroke history or systemic embolism, n (%) | 6 (20) | 2 (13.3) | 4 (26.7) | 0.4 |
| Obstructive sleep apnea, n (%) | 10 (33.3) | 4 (26.7) | 6 (40) | 0.4 |
| Chronic obstructive pulmonary disease, n (%) | 1 (3.3) | 1 (6.7) | 0 | 1 |
| Coronary artery disease, n (%) | 4 (13.3) | 2 (13.3) | 2 (13.3) | 1 |
| Hyperlipidemia, n (%) | 13 (43.3) | 5 (33.3) | 8 (53.3) | 0.3 |
| Chronic kidney disease, n (%) | 5 (16.7) | 3 (20) | 2 (13.3) | 0.6 |
| CHA2DS2-VASc score | 2.6±1.6 | 3.0±1.4 | 2.1±1.7 | 0.1 |
| Failed antiarrhythmic drugs | 1.1±0.9 | 0.9±0.8 | 1.4±0.9 | 0.1 |
| Echocardiographic data | ||||
| LVEF, % | 56.6±11.6 | 56.6±10.2 | 56.5±13.4 | NS |
Impact of Prospective Elimination of CS-LA Connection on Atrial Arrhythmia Recurrence
The endpoint of distal CS to LA connection elimination was achieved in all patients randomized to the corresponding arm using the study protocol and without need for intra-CS ablation. After a mean follow-up of 170±22 days, 8 (27%) patients experienced atrial arrhythmia recurrences. The distal CS to LA connection elimination group experienced fewer recurrences (6.7%) compared to the standard group (46.7%), which translated to an 88% lower hazard of recurrence compared to the standard group (HR 0.12, CI 0.02–0.97, P=0.047). The Kaplan-Meier survival curve of atrial arrhythmia recurrences is shown in Figure 3.
Figure 3.
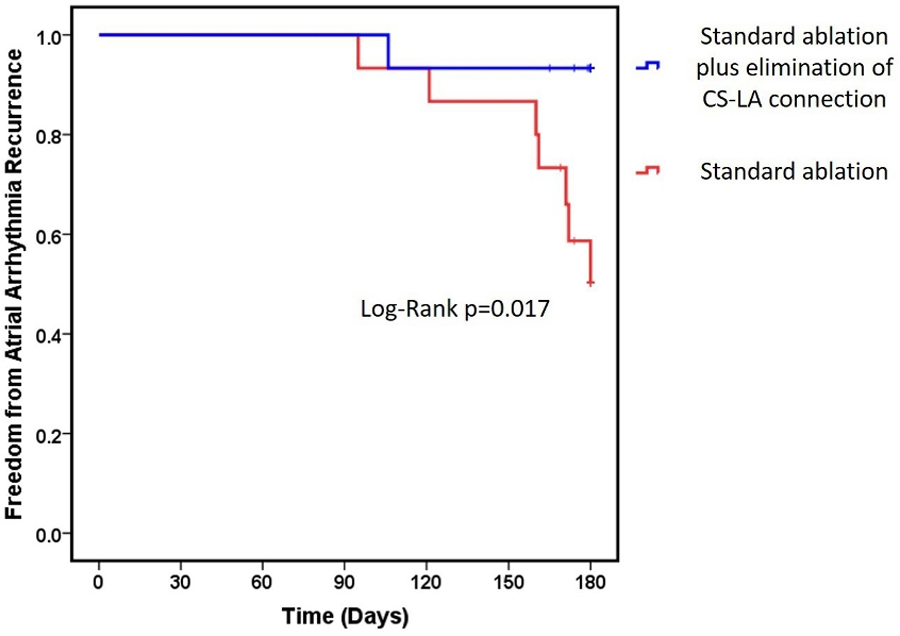
Kaplan–Meier Survival Curve of Atrial Arrhythmia Recurrences. Kaplan–Meier survival curve showing freedom from atrial arrhythmia recurrences in patients received prospective elimination of distal coronary sinus to left atrium connection in addition to standard procedure compared with patients performed pulmonary vein isolation plus non-pulmonary vein triggers ablation.
Aside from the randomized assignment to ablation strategies, univariate predictors of atrial arrhythmia recurrence after ablation are summarized in Table 2. Patients with persistent AF had a trend towards higher recurrence of atrial arrhythmias (HR 4.52, CI 0.91 – 22.46, P=0.07). Procedural data is demonstrated in Table 3. One patient randomized to the standard group had atrial premature complexes from the distal coronary sinus and received ablation at that site without efforts to fully isolate the LA to CS connection. There was no significant difference in ablation time between two groups of PV isolation (27.4 ± 8.9 vs 27.2 ± 7.3 mins, P=0.9). The mean ablation time required to eliminate the CS to LA connection was 4.4 ± 3.2 mins. There was no significant difference among methods of rhythm monitoring between groups (insertable cardiac monitor: 7 [47%] vs 5 [33%], P=0.5; 30-day wearable monitor at 6 months: 4 [50%] vs 5 [50%], P=1; symptoms prompted ECG/event monitor (3 days): 4[50%] vs 5[50%], P=1).
Table 2.
Univariate Analysis of Clinical and Procedural Variables Affecting Atrial Arrhythmia Recurrence by 6-month Follow-Up
| Univariate Analysis | |||
|---|---|---|---|
| Variable | HR | 95% CI | p value |
| Clinical data | |||
| Age, year | 1 | 0.91–1.10 | 1 |
| Female sex | 1.84 | 0.44–7.74 | 0.4 |
| Body weight | 1 | 0.99–1.02 | 0.7 |
| Persistent atrial fibrillation | 4.52 | 0.91–22.46 | 0.07 |
| Congestive Heart Failure | 0.72 | 0.09–5.85 | 0.8 |
| Hypertension | 0.59 | 0.14–2.52 | 0.5 |
| Diabetes mellitus | 1.44 | 0.29–7.13 | 0.7 |
| Prior stroke history or systemic embolism | 1.18 | 0.24–5.89 | 0.8 |
| Obstructive sleep apnea | 0.61 | 0.12–3.04 | 0.6 |
| Chronic obstructive pulmonary disease | NA | NA | NA |
| Coronary artery disease | NA | NA | NA |
| Hyperlipidemia | 0.63 | 0.15–2.65 | 0.5 |
| Chronic kidney disease | 0.56 | 0.07–4.59 | 0.6 |
| CHA2DS2-VASc score | 0.97 | 0.62–1.50 | 0.9 |
| Failed antiarrhythmic drugs | 1.46 | 0.73–2.94 | 0.3 |
| Echocardiographic data | |||
| LVEF, % | 0.98 | 0.93–1.04 | 0.6 |
| Procedural data | |||
| Distal CS to LA connection Elimination added to Standard PV isolation & non-PV triggers ablation | 0.12 | 0.02–0.97 | 0.047 |
NA: non-applicable
Table 3.
Comparison of Procedural Data between Standard Procedure and Prospective CS to LA connection ablation
| Overall (n=30) |
Standard Procedure (n=15) |
Standard Procedure plus Prospective Elimination of CS-LA connection (n=15) |
p value | |
|---|---|---|---|---|
| Distal coronary sinus mapping | 23 (79.3) | 8 (57.1) | 15 (100) | 0.004 |
| Pulmonary vein isolation only | 8 (26.7) | 8 (53.8) | 0 | 0.001 |
| Posterior wall isolation | 3 (10) | 2 (13.3) | 1 (6.7) | 0.5 |
| Non-pulmonary vein triggers ablation | 4 (13.8) | 4 (13.8) | 0 | 0.03 |
| Cavotricuspid isthmus ablation | 8 (26.7) | 2 (13.3) | 6 (40) | 0.1 |
| No. of PVI lesions | 89.2±28.1 | 92.7±36.9 | 85.8±15.9 | 0.5 |
| Ablation time of PVI (mins) | 27.3±8.0 | 27.4±8.9 | 27.2±7.3 | 0.9 |
| No. of prophylactic CS-LA connection elimination | 5.7±7.3 | 0 | 11.3±6.5 | <0.001 |
| Ablation time of CS-LA connection elimination (mins) | 2.2±3.2 | 0 | 4.4±3.2 | <0.001 |
| Insertable Cardiac Monitor | 12 (40%) | 7 (46.7) | 5 (33.3) | 0.5 |
PVI: pulmonary vein isolation; CS-LA: coronary sinus-left atrium
Procedural Complication and Readmission
There was no procedural-related complication clearly attributed to the experimental strategy of distal CS to LA connection elimination in this study. One patient was re-admitted with pericarditis and underwent pericardiocentesis for drainage of 200 ml serous pericardial effusion 16 days following ablation, which included PV isolation (35 minutes RF time), mitral isthmus line for induced peri-mitral flutter (10 minutes RF time), and distal CS to LA connection elimination (1.5 minutes RF time). Following pericardiocentesis, the patient recovered and was discharged on anticoagulation and without further sequela.
Discussion
To the best of our knowledge, this is the first study to assess the effectiveness of distal CS to LA connection elimination in first time AF ablation patients. Our study demonstrates a remarkable reduction of atrial arrhythmia recurrences utilizing this novel ablation strategy compared to standard PV isolation with non-PV trigger ablation.
These findings are biologically plausible given prior work that demonstrated an association between AF/flutter induction and rate-dependent unidirectional block in one limb of dual muscular connections between CS and LA, mostly in the distal CS.11 A study by Oral et al. reported electrical disconnection of the CS from the left atrium in 9 of 22 patients with inducible AF after PV isolation. Following elimination of CS to LA connection, only 3 patients had persistent inducible sustained AF.13 Haïssaguerre et al. have also reported CS ablation endocardially and/or epicardially in cases of persistent AF despite PV isolation resulting in prolongation of AF cycle length and AF termination in 35% of patients.14 Given our prior observation of the importance of rate dependent unidirectional block in the distal CS to LA connection for acute arrhythmia induction,11 we simplified CS lesions to target the distal CS to LA connection from inside the LA. Complete CS isolation, for which extensive ablations are required, was neither required nor sought. Following this strategy, we noted no clear signal for increased risk (such as increased rates of mitral annular flutter) compared to the standard procedure and a strong signal for improved outcome.
Amongst patients with atrial arrhythmia recurrence in our study, 6 (75%) had baseline persistent AF. Of these, 3 had AF recurrence, 2 had atypical atrial flutter mixed with AF, and 1 had dominant atypical atrial flutter with low burden of AF (< 0.1%). However, AF persistence prior to ablation did not reach statistical significance as a predictor of outcome. It is conceivable that the association would have reached statistical significance with a larger study sample size. A recent multicenter report of long-term AF ablation outcomes among 3,466 patients in Europe7 demonstrated higher recurrence after PV isolation in the non-paroxysmal AF group (39%) compared to the paroxysmal AF group (29%), and 42% after PV isolation with additional ablations in both groups. It concluded that additional lesions to PVI did not result in improved outcomes. We agree that extensive additional lesion sets appear to be unhelpful or even pro-arrhythmic.8 However, exploration of limited targets with electrophysiological and mechanistic importance is necessary to advance the field.
AF ablation is now routinely performed for symptomatic, drug-refractory AF patients; however, recurrence rates remain high at up to 30% by 1-year follow-up for paroxysmal AF and even higher in non-paroxysmal AF.7, 8 Substantial costs, financial and more importantly emotional and physical, are paid for recurrent AF related electrical cardioversions, ablations, and rehospitalizations in daily practice. Better ablation outcomes are necessary both for paroxysmal and non-paroxysmal AF patients. This study showed considerable improvement in AF ablation outcome by addition of a limited lesion set to the current standard procedure. Future studies are needed to ascertain whether the type of atrial fibrillation, paroxysmal or persistent, affects the benefit of performing distal CS-LA connection ablation.
Study Limitations
The current study has several limitations. First, this was a single-center pilot study with a relatively small sample size. However, randomization of patient assignment resulted in balanced patient groups and the effect size was substantial resulting in a clear outcome improvement in the experimental arm. Second, 40% of patients underwent insertable cardiac monitor implantation. However, the distribution of insertable monitors was balanced across randomization groups. Of patients without insertable monitors, 56% had 30-days wearable monitor pre-ablation, during the first month after ablation, and at 6 months, and 44% had follow-up for detection of atrial arrhythmia recurrences based upon symptom or pulse check prompted monitors and ECGs during clinic visits. Therefore, it is possible that some episodes of asymptomatic paroxysmal AF may have been missed. Although a strong signal was observed between the randomized groups, this is a relatively small single center study without standardized long-term monitoring for AF recurrence. A larger multi-center randomized study with rigorous monitoring for silent recurrent AF and long-term follow up will be necessary to refine these results.
Conclusions
Prospective elimination of distal CS to LA connections added to standard PV isolation and non-PV trigger ablation reduced atrial arrhythmia recurrences in patients referred for a first AF ablation procedure. Distal CS to LA connection elimination holds promise as an adjunct to PV isolation and warrants further study with a multicenter randomized design.
What Is Known?
The coronary sinus has its own musculature which is electrically insulated from left atrial musculature but conducts to and from the latter through anatomic connections proximally and distally.
Prior studies indicate the involvement of coronary sinus to left atrial connections in stable macro-reentrant atrial tachycardia and flutter or unstable single or multi-beat reentry with degeneration to atrial fibrillation.
Rate-dependent distal coronary sinus to left atrial conduction block is associated with atrial fibrillation induction in drug-refractory atrial fibrillation patients undergoing ablation.
What the Study Adds?
Ablation of distal coronary sinus to left atrial connections reduces atrial arrhythmia recurrences compared to standard pulmonary vein isolation and non-pulmonary vein trigger ablation in patients undergoing a first atrial fibrillation ablation procedure.
Sources of Funding:
Dr. Kuo is supported by Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program, No. 106-V-A-009. The study was also funded by a Biosense Webster Grant to Dr Nazarian and the Koegel Family EP Research Fund. Dr. Nazarian’s research laboratory is supported by NIH grants R01HL116280 and R01HL142893, which indirectly supported this research. The contents do not necessarily represent the views of the National Institutes of Health.
Nonstandard Abbreviations and Acronyms
- PV
Pulmonary vein
- LA
Left atrium
- CS
Coronary sinus
- PRECAF
Prospective Elimination of Distal Coronary Sinus to Left Atrial Connection for Atrial Fibrillation Ablation Randomized Controlled Trial
- EAM
electroanatomic map
- ICE
intracardiac echocardiography
Footnotes
Registration - ClinicalTrials.gov; Unique Identifier: NCT03646643
Disclosures: Dr. Nazarian is a consultant for CardioSolv and Circle CVI and has given Lectures for Circle CVI; he serves as a principal investigator for research funding from Biosense Webster, ImriCor, Siemens, and the US National Heart lung and Blood Institute. The University of Pennsylvania Conflict of Interest Committee manages all commercial arrangements. The other authors report no conflicts of interest.
References
- 1.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–86. [DOI] [PubMed] [Google Scholar]
- 3.Santangeli P, Zado ES, Hutchinson MD, Riley MP, Lin D, Frankel DS, Supple GE, Garcia FC, Dixit S, Callans DJ, et al. Prevalence and distribution of focal triggers in persistent and long-standing persistent atrial fibrillation. Heart Rhythm. 2016;13:374–82. [DOI] [PubMed] [Google Scholar]
- 4.Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–83. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Di Biase L, Trivedi C, Mohanty S, Bai R, Mohanty P, Gianni C, Santangeli P, Horton R, Sanchez J, et al. Importance of non-pulmonary vein triggers ablation to achieve long-term freedom from paroxysmal atrial fibrillation in patients with low ejection fraction. Heart Rhythm. 2016;13:141–9. [DOI] [PubMed] [Google Scholar]
- 6.Dixit S, Lin D, Frankel DS, Marchlinski FE. Catheter ablation for persistent atrial fibrillation: antral pulmonary vein isolation and elimination of nonpulmonary vein triggers are sufficient. Circ Arrhythm Electrophysiol. 2012;5:1216–23; discussion 1223. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt B, Brugada J, Arbelo E, Laroche C, Bayramova S, Bertini M, Letsas KP, Pison L, Romanov A, Scherr D, et al. Ablation strategies for different types of atrial fibrillation in Europe: results of the ESC-EORP EHRA Atrial Fibrillation Ablation Long-Term registry. Europace. 2019. [DOI] [PubMed]
- 8.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 9.Chauvin M, Shah DC, Haissaguerre M, Marcellin L, Brechenmacher C. The anatomic basis of connections between the coronary sinus musculature and the left atrium in humans. Circulation. 2000;101:647–52. [DOI] [PubMed] [Google Scholar]
- 10.Saremi F, Thonar B, Sarlaty T, Shmayevich I, Malik S, Smith CW, Krishnan S, Sanchez-Quintana D, Narula N. Posterior interatrial muscular connection between the coronary sinus and left atrium: anatomic and functional study of the coronary sinus with multidetector CT. Radiology. 2011;260:671–9. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Marine JE, Li JB, Zghaib T, Ipek EG, Sinha S, Spragg DD, Ashikaga H, Berger RD, Calkins H, et al. Association of Rate-Dependent Conduction Block Between Eccentric Coronary Sinus to Left Atrial Connections With Inducible Atrial Fibrillation and Flutter. Circ Arrhythm Electrophysiol. 2017;10:e004637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm. 2017;14:1087–1096. [DOI] [PubMed] [Google Scholar]
- 13.Oral H, Ozaydin M, Chugh A, Scharf C, Tada H, Hall B, Cheung P, Pelosi F, Knight BP, Morady F. Role of the coronary sinus in maintenance of atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:1329–36. [DOI] [PubMed] [Google Scholar]
- 14.Haissaguerre M, Hocini M, Takahashi Y, O’Neill MD, Pernat A, Sanders P, Jonsson A, Rotter M, Sacher F, Rostock T, et al. Impact of catheter ablation of the coronary sinus on paroxysmal or persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:378–86. [DOI] [PubMed] [Google Scholar]


