Abstract
Coronavirus disease 2019 (COVID-19) has swept through the world with millions of cases and hundreds of thousands of deaths. COVID-19-associated coagulopathy has been recognized as the major cause of morbidity and mortality. To the best of our knowledge, a majority of the cases of coagulopathy have been reported in patients with moderate-to-severe COVID-19 and limited to observations during the recovery/postcytokine storm state. Herein, we report a case series of two patients with COVID-19 who developed pulmonary embolism in the late phase of the disease. This raised the hypothesis that the risk of hypercoagulability in patients with COVID-19 can persist until the recovery phase, which would warrant a follow-up with D-dimer and fibrinogen trending, as well as postdischarge thromboprophylaxis for at least 2 weeks during the recovery phase.
Keywords: Anticoagulation, coronavirus disease 2019, hypercoagulable, pulmonary embolism, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
The novel coronavirus, which was first discovered in January 2020, has swept through the world and reached a global pandemic. The International Committee on Taxonomy of Viruses has designated the disease as coronavirus disease 2019 (COVID-19). COVID-19-associated coagulopathy has been increasingly recognized as the major cause of increasing morbidity and mortality. The pathogenesis of hypercoagulability observed in COVID-19 is poorly understood. Classically, hypercoagulability is observed with the disturbance of the Virchow's triad – endothelial injury, venous stasis, and a hypercoagulable state. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the beta-CoV responsible for COVID-19, has been reported to cause endothelial injury by direct invasion. Moreover, Begbie et al. reported that an endotheliitis can occur in the setting of cytokine storm mediated by interleukin 6 or other acute phase reactants.[1] A prothrombotic state has also been reported in severe patients with COVID-19 with elevations of Factor VIII, fibrinogen and prothrombic microparticles.[2,3] Nonetheless, most studies on COVID-19 hypercoagulability describe patients with severe COVID-19, with relatively fewer patients being in the recovery phase. Herein, we report a case series of two patients who developed pulmonary embolism (PE) in the late phase of COVD-19. This raised the hypothesis that, after viral pneumonitis/pneumonia and/or cytokine storm, there is a period of persistent hypercoagulability, which would warrant a follow-up with D-dimer and fibrinogen trending as well as consideration of thromboprophylaxis after discharge until the patient can fully ambulate.
CASE SERIES
Case 1
A 65-year-old male with no known past medical history presented to the emergency room with shortness of breath. He tested positive for COVID-19 about 2 weeks earlier after he experienced nonproductive cough and fever. He was not hospitalized and was sent home on hydroxychloroquine and azithromycin for 5 days, which he had completed. He denied any symptoms of chest discomfort, dizziness, and gastrointestinal symptoms. He denied a history of smoking or recreational drug use. His body mass index (BMI) was 23.43 kg/m2. Initial vitals on admission were stable, afebrile, and saturating well on room air and ambulation. Electrocardiogram showed normal sinus rhythm, normal axis, and no ST-T waves abnormalities. Chest X-ray showed bilateral peripheral consolidations. Initial laboratory showed white blood cells of 7.2 × 10 × 3/uL with absolute neutrophil count of 5.4 × 10 × 3/uL and absolute lymphocyte count of 0.9 × 10 × 3/uL, D-dimer 9225 ng/ml, fibrinogen 534 mg/dL, C-reactive protein (CRP) 9.6 mg/dL, ferritin 584 ng/mL, lactate dehydrogenase (LDH) 302 U/L, procalcitonin < 0.05 ng/mL, and creatinine kinase 70 U/L. Computed tomography angiogram (CTA) showed pulmonary emboli in the right upper and lower lobe pulmonary arteries, bilateral peripheral consolidations, and bilateral hilar and mediastinal lymphadenopathy [Figure 1]. He was then started on therapeutic enoxaparin 1 mg/kg two times a day for the treatment of PE. Echocardiogram did not show any right heart strain. He was clinically stable and was discharged on rivaroxaban 15 mg two times a day for 21 days and 20 mg daily for 3–6 months.
Figure 1.
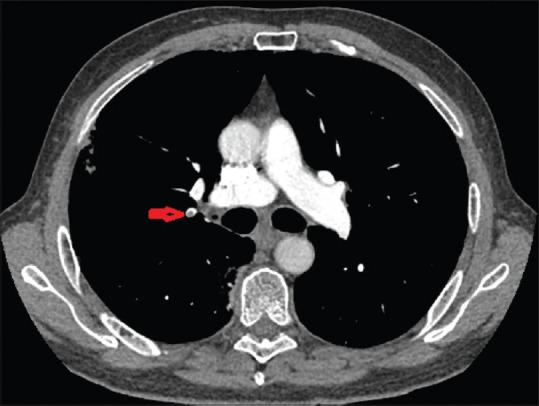
Computed tomography angiogram showed pulmonary emboli in the right upper lobe pulmonary arteries
Case 2
An 85-year-old female with a past medical history of asthma, coronary artery disease, Type II diabetes mellitus, dyslipidemia, and hypertension presented to the emergency room with generalized weakness, nonproductive cough, shortness of breath, fevers, and chills for the past 1 week. She had two episodes of diarrhea before admission with mild abdominal pain and reduced appetite. She lives in a senior citizens home. She denied any sick contact or recent travel. She used to smoke for 25 years but has quit 18 years ago. Her BMI was 41.13 kg/m2. Initial vital signs on admission showed a temperature of 101.7°F, heart rate of 99 beats/min, respiratory rate of 20 breaths/min, blood pressure of 116/48 mmHg, and oxygen saturation at 88% on room air. Electrocardiogram showed normal sinus rhythm, right bundle branch block, and QTc 508 ms (<470 ms). Chest X-ray showed hazy opacities within the right upper lobe and left lower lobe, suspicious for pneumonia. Initial laboratory showed white blood cells of 6.9 × 10 × 3/uL (4.4–11 10 × 3/uL) with absolute neutrophil count of 4.0 × 10 × 3/uL and absolute lymphocyte count of 1 × 10 × 3/uL, D-dimer 1110 ng/ml (0–500 ng/ml), CRP 7.8 mg/dL (0–0.8 mg/dL), ferritin 296 ng/ml (11–307 ng/mL), LDH 401 U/L (122–222 U/L), procalcitonin < 0.05 ng/ml (0–0.5 ng/mL), lactic acid 1.0 mmol/L (0–2 mmol/L), creatinine kinase 203 U/L (38–176 U/L), and fibrinogen 484 mg/dL (200–393 mg/dL). Her nasopharyngeal swab was positive for SARS-CoV-2 reverse transcription-polymerase chain reaction. She received oxygen therapy, hydroxychloroquine, and doxycycline for 5 days, and inflammatory markers improved. She was on enoxaparin 40 mg daily for deep venous thrombosis prophylaxis. On Day 7 of admission (day 14 of symptoms), her oxygen requirement increased, she was placed on a nonrebreather mask, and her D-dimer increased to 16,195 ng/ml. She became tachycardic with a heart rate of 158 beats/min with mild chest pain; CTA showed a pulmonary embolus involving the segmental branch of the left upper lobe pulmonary artery [Figure 2]. Anticardiolipin antibodies IgM and IgG were 21 – indeterminate (IgM 0–14 GPL U/mL and IgG 0–12 MPL U/mL). She was started on therapeutic enoxaparin 1 mg/kg two times a day with decrease in her D-dimer and clinical improvement. Her saturation was 88% on room air and 94% on 3L nasal cannula by day 14 of admission. She was discharged with apixaban, and home oxygen with outpatient follow-up.
Figure 2.
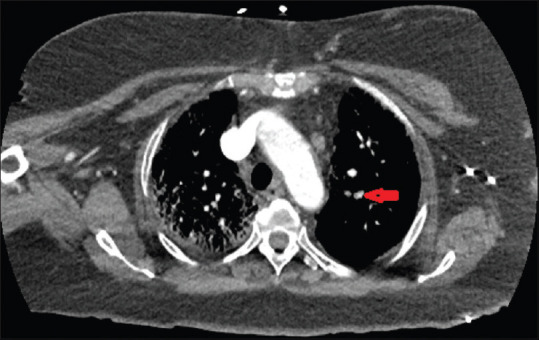
Computed tomography angiogram which showed a pulmonary embolus involving the segmental branch of the left upper lobe pulmonary artery
DISCUSSION
SARS-CoV-2 is a novel virus that causes respiratory tract infection, which was first identified in Wuhan, China, in the end of 2019. It rapidly spread across China and worldwide to become a global pandemic. It was found that approximately 20% of patients with COVID-19 had severe coagulation abnormalities. Almost all severe and critically ill COVID-19 infection showed major coagulation disorders.[4,5,6]
COVID-19 coagulopathy is a newly defined phenomenon. Patients with COVID-19 tend to have coagulopathy characterized by high D-dimers, fibrinogen, and fibrin degradation products.[5] Some severe patients with COVID-19 develop disseminated intravascular coagulation, which characterized by thrombocytopenia (platelet count < 50 × 109/L), prolongation of the prothrombin time (PT) and activated partial thromboplastin time (aPTT), significant elevation of D-dimer, and decreased fibrinogen (<1.0 g/L) as per the International Society on Thrombosis and Hemostasis criteria.[7]
Diagnosing PE in patient with COVID-19 is very challenging because symptoms such as low-grade fever, cough, and dyspnea are nonspecific for PE. Clinical symptoms that raise suspicion and should prompt diagnostic testing include acute onset of tachycardic or hypotension, worsening respiratory status, hemoptysis, and electrocardiography with S1Q3T3 and/or new right bundle branch block as well as signs and symptoms of deep vein thrombosis (DVT).[8] In cases where diagnostic tests such as CTA or V/Q scan are not available, bilateral compression ultrasonography, echocardiography, or point-of-care ultrasonography may be done for diagnostic purpose.[9] The use of CTA is reserved for patients with a high index of suspicion due to the risk of contrast-induced nephropathy in patients with COVID-19. The use of hydration to reduce that risk in patients with acute kidney injury is advisable.[8]
Patients with COVID-19 tend to have markedly elevated coagulation parameters; the American Society of Hematology (ASH) recommended that prophylactic dose of low-molecular-weight heparin is recommended for all patients without contraindication.[9] In patients with documented PE, therapeutic anticoagulation is initiated as untreated PE will worsen the outcome. The empiric use of a therapeutic dose of anticoagulation in patients with COVID-19 without PE/DVT still remains controversial. All patients who started with therapeutic anticoagulation should be given a minimum course of 3 months of the regimen.[9]
In this case series, both patients presented with symptoms of acute onset of shortness of breath and significantly elevated D-Dimer in the recovery phase, which raised the suspicion of PE. The elevation of D-dimer is nonspecific and may be elevated in other causes such as secondary infection, myocardial infarction, renal failure, or coagulopathy.[9] Chen et al. 2020 reported a statistically significant difference in the incidence of PE in patients with higher D-dimer levels.[4] Abnormal coagulation parameters are associated with more severe disease and death in COVID-19 patients.[7] In patients with severe COVID-19, the PT and D-dimer levels were significantly higher, but there was no significant difference observed in platelet and APTT between severe and mild patients.[10]
Surprisingly, our patients presented with PE in the late phase of the disease (day 13–14 of symptoms), after the viral pneumonia and the cytokine storm resolved. Moreover, one of them was already receiving prophylactic anticoagulation before developing PE. This suggests the novel observation that PE/DVT will not only occur in the severe patients with COVID-19 but also that risk will persist even after the pulmonary and/or hyperinflammation phase has resolved. Numerous reports have been published documenting the importance of anticoagulation during the pulmonary and cytokine storm state but rarely highlighting the persistence of hypercoagulable state during the recovery period. The severe endothelial dysfunction, driven by the cytokine storm and associated hypoxemia, which leads to thromboembolic complications, could persist even during the recovery phase. It was also hypothesized that alveolar viral damage is followed by an inflammatory reaction and microvascular pulmonary thrombosis. The multiple organ failure and death may be due to the progressive endothelial thromboinflammatory syndrome involving the microvascular bed of the brain and other vital organs.[11] Figure 3 illustrates the proposed pathophysiology of the hypercoagulable state observed in patients with COVID-19 during the different phases of the illness. Based on the data available and our observation, the hypercoagulable state begins early during the pulmonary and cytokine storm (hyperinflammatory phase) and persists during the recovery phase. We believe that early anticoagulation is paramount at the time of admission to the hospital to suppress the thrombophilic state. It is also important to address the cytokine-mediated hyperinflammation, which promotes endothelial cell activation and endothelial dysfunction, which may potentiate a prothrombotic state.
Figure 3.
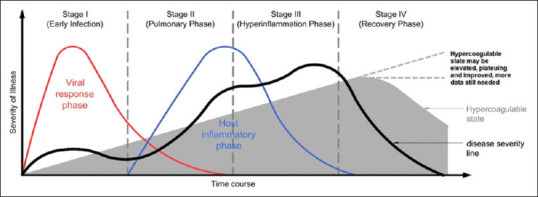
Proposed pathophysiology of the hypercoagulable state observed in patients with COVID-19 during the different phases of the illness (staging system was proposed and adopted from Siddiqi and Mehra[10])
There are increasing data that COVID-19 causes an immunodysregulation which causes platelets, coagulation factors, and innate immune effector systems (such as monocyte-derived macrophages [Mø], polymorphonuclear neutrophils, and the complement system) to interact to promote thrombotic activity in a process termed immunothrombosis (also known as thromboinflammation).[12] Hence, in addition to anticoagulation, early administration of immunomodulator therapy (complement inhibitors, cytokine inhibitors) during the hyperinflammation phase can curb this immunothrombotic activity.[13,14,15] With our clinical observation, there may be a need to follow-up the patients with COVID-19 and trend their D-dimer to monitor PE/DVT. A recent study showed that there was no statistically significant difference in survival or time-to-mechanical ventilation between patients who received anticoagulation versus no anticoagulation or antiplatelet therapy. There may be a role of using “therapeutic dose” anticoagulation as thromboprophylaxis during hospitalization for severe COVID-19 infections.[16] Paranjpe et al. studied 786 COVID patients who received systemic anticoagulation, and the intervention appeared to correlate with improved survival within the subset of patients who were on mechanical ventilation. There was a slightly higher rate of hemorrhage in the group that received “therapeutic dose” thromboprophylaxis during hospitalization.[17]
However, it is important to note that the ASH recommends the use of prophylaxis anticoagulation for those who have not had confirmed venous thromboembolism (VTE) unless in patients with high-risk probability and confirmatory test was not able to be performed. There is still a paucity of evidence in the medical literature with regard to optimal anticoagulation intensity and duration. Until we have more definitive guidelines, we will have to individualize anticoagulation decisions based on coagulation parameters and the patient's overall clinical risk for thrombosis.
CONCLUSION
These case studies highlighted the increased risk of VTE in COVID-19 patients during the hyperinflammatory phase, which persists until the recovery phase. It is important to have close follow-up and do serial monitoring D-dimer and fibrinogen to allow early identification of clinical symptoms related to PE/DVT, so an early use of therapeutic anticoagulation can be initiated prevent worsened outcome in these patients. More data are still needed on the initiation, duration of empiric therapeutic anticoagulation, and follow-up in these groups of patients.
Statement of ethics
Patients have given written informed consent to publish the cases including publication of images.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published, and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Research quality and ethics statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting, and reproducibility guidelines set forth by the EQUATOR Network. The authors also attest that this clinical investigation was determined to not require the institutional review board/ethics committee review, and the corresponding protocol/approval number is not applicable. We also certify that we have not plagiarized the contents in this submission and have done a plagiarism check.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Begbie M, Notley C, Tinlin S, Sawyer L, Lillicrap D. The factor VIII acute phase response requires the participation of NFkappaB and C/EBP. Thromb Haemost. 2000;84:216–22. [PubMed] [Google Scholar]
- 2.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–42. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranucci M, Ballotta A, Di Dedda U, Bayshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–51. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–20. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N, Li D, Wang X, and Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–7. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouhezamin MR, Haseli S. Diagnosing pulmonary thromboembolism in COVID-19: A stepwise clinical and imaging approach. Acad Radiol. 2020;27:896–7. doi: 10.1016/j.acra.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Society of Hematology. COVID-19 and coagulopathy: Frequently Asked Questions. [Last accessed May 30th, 2020]. Available from: https://www.hematology.org/covid-19/covid-19-and-coagulopathy .
- 10.Xiong M, Liang X, Wei YD. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Br J Haematol. 2020;189:1050–2. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–7. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaertner F, Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol. 2016;28:561–9. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 14.Delabranche X, Helms J, Meziani F. Immunohaemostasis: A new view on haemostasis during sepsis. Ann Intensive Care. 2017;7:117. doi: 10.1186/s13613-017-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–18. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 16.Tremblay D, van Gerwen M, Alsen M, Thibaud S, Kessler AJ, Venugopal S, et al. Impact of anticoagulation prior to COVID-19 infection: A propensity score-matched cohort study. Blood. 2020;136:144–7. doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients With COVID-19. J Am Coll Cardiol. 2020;76:122–4. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


