Abstract
Context:
Influenza infection in pregnancy causes 4%–8% case fatality and five times more perinatal mortality. Influenza is a major contributor to mortality in developing countries; however, the morbidity has largely been underestimated. Public health interventions for prevention are also lacking.
Aims:
This study aimed to determine the seasonality of influenza in pregnant Indian women and to estimate the maternal and perinatal morbidity after treatment with oseltamivir.
Settings and Design:
This was a prospective observational cohort study, conducted in a tertiary hospital.
Subjects and Methods:
Pregnant women with ILI (influenza-like illness) were recruited into Cohort 1 (polymerase chain reaction [PCR] positive) and Cohort 2 (PCR negative). Gestational age-matched asymptomatic controls formed Cohort 3. Women in Cohort 1 received oseltamivir for 5 days. The incidence of small-for-gestational age (SGA) and preterm birth were the primary outcomes. Maternal and neonatal morbidity formed the secondary outcomes.
Statistical Analysis:
Unmatched (Cohort 1 and 2) and matched analysis (Cohort 1 and 3) were done. Student's t-test and Chi-square test were used to compare between variables.
Results:
Year-round incidence of influenza was recorded. Severe illness was more in Cohort 1 compared to Cohort 2 (36.2% vs. 6.3%; P < 0.001). SGA was comparable in all the cohorts (13%). Preterm birth (7.8% vs. 3.3%; P < 0.08; relative risk-2.75) was considerably high in Cohort 1. Secondary maternal and neonatal outcomes were similar between the groups.
Conclusion:
Influenza in pregnancy showed year-round incidence and increased maternal and neonatal morbidity despite treatment with oseltamivir. We suggest the need for newer interventions to curtail the illness in pregnancy.
Keywords: Influenza, influenza like illness, pregnancy, preterm birth, small for gestational age
INTRODUCTION
Influenza virus is an important respiratory pathogen that causes increased morbidity and mortality among pregnant women, a risk well documented in the 1918 global influenza pandemic and 2009 H1N1 pandemic.[1] Severe maternal illness has also been associated with low birth weight and increased risk of preterm birth.[2]
South East Asia[3] and equatorial regions have been implicated as the source of many new strains of influenza that circulate globally.[4] The virus is a major contributor to mortality in these low-income countries; however, the morbidity has largely been underestimated. Awareness campaigns and health interventions to curtail influenza infections are also lacking. Continued and enhanced surveillance in the tropics is hence warranted to monitor both the disease load and the impact of interventions. The WHO Strategic Advisory Group of Experts on immunization has given highest priority for pregnant women to receive influenza immunization.[5] However, in India, vaccine uptake among these women is extremely poor.[6] In addition, the lack of disease burden data among pregnant Indian women has reduced the attention toward influenza prevention as a public health priority. Hence, the objectives of our study were to determine the seasonality of influenza and to estimate its influence on maternal and perinatal morbidity after treatment with oseltamivir. The data would be valuable for planning further preventive strategies.
SUBJECTS AND METHODS
Procedure
This prospective observational cohort study was conducted in Christian Medical College (CMC), Vellore, a 2700-bedded tertiary referral center in South India from November 1, 2015, to October 31, 2017. All pregnant women who attended the antenatal clinic or admitted to the obstetric wards with influenza-like illness (ILI) were eligible for participation. Enrollment was done after obtaining written informed consent. ILI was defined as fever ≥38°C in the presence of either cough or sore throat reported by women. Women with gestational hypertension, pregestational diabetes, hyperthyroidism, and severe anemia were excluded. Gestational age was confirmed from antenatal ultrasound reports. After clinical examination (included recordings of pulse rate, respiratory rate, blood pressure, oxygen saturation, and auscultation of lung fields), nasal and oropharyngeal swabs were collected, transported appropriately in cold containers, and tested for influenza virus using a standardized polymerase chain reaction (PCR) for all women with ILI. Because the clinical picture was strongly suggestive of respiratory illness, investigations (blood and urine cultures) to rule out other causes of fever were done only for patients requiring admission. PCR results were obtained within 3 days based on which pregnant women with ILI were grouped into two cohorts – influenza PCR positive cohort and PCR negative ILI cohort. In addition, one gestational-age matched asymptomatic pregnant woman seen in the same week was selected as control for every woman who presented with PCR-positive influenza. The group with PCR negative ILI was considered the first control group (Cohort 2), while the healthy pregnant women constituted the second control group (Cohort 3). Antitussives, gargles, bronchodilators, or antibiotics, as needed, were given to all women with signs of ILI. In addition, women with PCR positive influenza were started on oral antiviral drug oseltamivir (75 mg twice daily for 5 days) within 72 h of presentation.[7] Those who were admitted to the wards/intensive care unit (ICU) were considered to have severe illness.[8] After discharge, they were provided routine clinic based antenatal care. All participants were followed up through pregnancy, childbirth, and the postnatal period. The infant's weight, length, head circumference, Apgar scores, and perinatal complications were recorded immediately after birth. The infants were followed up at 2 and 4 weeks after birth either through postnatal checks or telephonic interviews to assess their health status. The primary outcomes were the incidence of small-for-gestational age (SGA) and preterm births. The secondary outcomes assessed were obstetric complications in the mother and perinatal morbidity.
Definition of outcomes
SGA was defined as an infant with birth weight lower than the tenth centile of the sex-specific and gestational age-specific INTERGROWTH-21st birth weight standard.[9] Preterm delivery was defined as birth before 37 weeks of gestation. The secondary perinatal outcomes included Apgar score < 6 at 5 min, blood culture-positive neonatal sepsis, respiratory distress syndrome (RDS), admission to neonatal ICU (NICU) for preterm care and pregnancy loss defined as any abortion or birth of a nonviable fetus after 22 weeks of gestation. Perinatal data of twins and those who did not give birth in our institution were excluded from the analysis. Maternal data [Tables 1 and 2] from all women at the time of infection were included to assess the morbidity. However, data on obstetric complications could be collected only from women who gave birth in our hospital.
Table 1.
Demographic variables
| Variable | Cohort I (n=174), n (%)Mean±SD | Cohort II(n=302), n (%)Mean±SD | Pa | Cohort III (n=174), n (%)Mean±SD | Pb |
|---|---|---|---|---|---|
| Age (years) | 25.6±4.4 | 25.9±4.1 | 0.350 | 25.9±4.3 | 0.382 |
| BMI | |||||
| ≤25 | 94 (54.0) | 143 (47.4) | 0.161 | 100 (57.5) | 0.517 |
| >25 | 80 (46.0) | 159 (52.6) | 74 (42.5) | ||
| Gravidity | |||||
| Primi gravida | 78 (44.8) | 148 (49.0) | 0.379 | 87 (50.0) | 0.334 |
| Multi gravida | 96 (55.2) | 154 (51.0) | 87 (50.0) | ||
| Previous pregnancy loss | |||||
| No | 168 (966) | 289 (95.7) | 0.646 | 165 (94.8) | 0.428 |
| Yes | 6 (3.4) | 13 (4.3) | 9 (5.2) | ||
| Residence | |||||
| Urban | 122 (70.1) | 193 (63.9) | 0.168 | 111 (63.8) | 0.210 |
| Rural | 52 (29.9) | 109 (36.1) | 63 (36.2) | ||
| Annual income (INR) | |||||
| Median (IQR) | 120,000 (77,250-200,000) | 160,000 (85,000-245,000) | 0.004 | 180,000 (110,000-250,000) | 0.001 |
| GA at presentation with ILI (weeks) | |||||
| <13 | 5 (2.9) | 33 (10.9) | <0.001 | 8 (4.6) | 0.694 |
| 13-28 | 47 (27.0) | 113 (37.4) | 47 (27.0) | ||
| >28 | 122 (70.1) | 156 (51.7) | 119 (68.4) |
BMI: Body mass index, GA: Gestational age, ILI: Influenza-like illness, SD: Standard deviation
Table 2.
Clinical characteristics and severity of illness in the mothers at the time of infection/recruitment
| Variable | Cohort I (n=174), n (%)Mean±SD | Cohort II (n=302), n (%)Mean±SD | Pa | Cohort III (n=174), n (%)Mean±SD | Pb |
|---|---|---|---|---|---|
| Pulse rate | 101.9±14.5 | 93.6 ±11.1 | <0.001 | 78.9±5.9 | <0.001 |
| Respiratory rate | 23.1± 6.7 | 21.9±5.3 | 0.039 | 20.3±4.1 | <0.001 |
| Systolic BP | 105·6±10.5 | 105.4±10.1 | 0.814 | 107.0±8.4 | 0.175 |
| Diastolic BP | 67.8±10.0 | 67.9±8.5 | 0.879 | 69.8±8.4 | 0.049 |
| Oxygen saturation | 98·6±0.8 | 98.8±0.8 | 0.86 | 99.4±1.6 | <0.001 |
| Severity of illness | |||||
| OP care | 111 (63.8) | 283 (93.7) | <0.001 | 174 (100.0) | <0.001 |
| IP and ICU care | 63 (36.2) | 19 (6.3) | - |
BP: Blood pressure, OP: Outpatient, IP: Inpatient, ICU: Intensive care unit, SD: Standard deviation
Sample size calculation
The expected SGA rates were 25% in the influenza group and 10% in the noninfluenza group. To show a 15% statistically significant difference in proportions with unequal allocation of 1:2, α = 0.01, β = 0.1, and dropout rate of 20%, the required sample sizes were 166 women with influenza and 332 women with ILI, respectively. To compare the SGA rates of women with influenza and healthy controls (8% SGA rate), with equal allocation of 1:1, the required sample size was 166.
Ethical approval was obtained from the Institutional Review Boards of CMC, Vellore on August 27, 2014 (IRB No. 9014[OBSERVE]), and Cincinnati Children's Medical Centre, USA (Study ID: 2014-7670), who funded the study and developed the study protocol.
Statistical methods
The data were abstracted in a predesigned case report form and entered in EpiData software, version 3.1, Odense, Denmark. Categorical variables were presented as frequencies and percentages. Continuous variables were presented as mean (standard deviation) or median (interquartile range) as appropriate. Continuous variables were compared between groups using Student's t-test and categorical variables were compared using Chi-square test. Unmatched analysis (Cohorts 1 and 2) and matched analysis (Cohorts 1 and 3) were done as appropriate to compare between the cohorts. Relative risks (RRs) with 95% confidence interval (CI) were calculated. The significance level was fixed at 5% level.
RESULTS
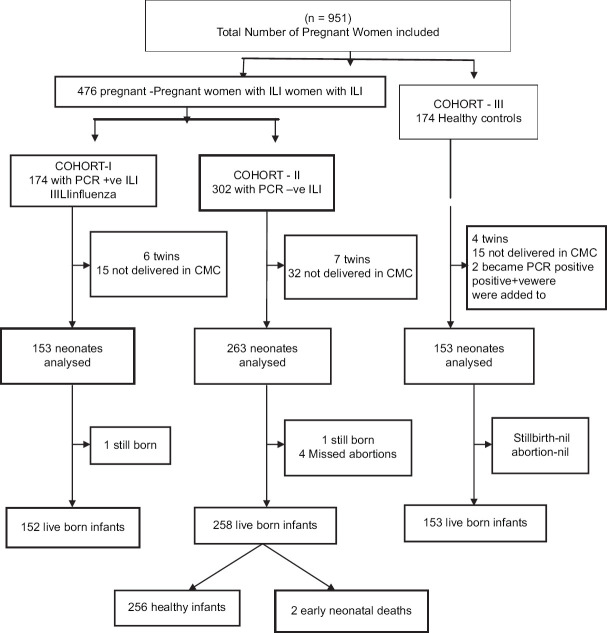
During the study period, on an average, 8455 women attended the antenatal clinics every month. A total of 29,635 live births were registered during the same period, with an average of 1234 births per month. Figure 1 describes the flow of participants into the study. A total of 650 pregnant women were included in the study. Of the 476 women with ILI, 174 were influenza PCR positive (Cohort 1) and 302 were influenza PCR negative (Cohort 2).174 gestational age-matched healthy pregnant women were recruited into Cohort 3. Among the 174 women with influenza, 49 (28%) were positive for human influenza virus Type A (H1N1), 93 (54%) for A (H3N2), and 32 (18%) for influenza B. After excluding 6 sets of twins and 14 infants who were born elsewhere, Cohort 1 had 153 live born infants. In Cohort 2, data from 7 sets of twins and 32 infants born elsewhere were excluded. Among the 263 pregnancy outcomes, there were four missed abortions, one stillbirth, and 258 live births. Among the 174 gestational age-matched healthy pregnant women recruited into Cohort 3, two later developed influenza. They were removed from Cohort 3 and added to Cohort 1. In their place, two new gestational age-matched controls were enrolled in Cohort 3 but not at the same time as the recruitment of cases. In Cohort 3, data from 4 sets of twins and 15 born elsewhere were excluded from the analysis. One hundred and fifty-three infants were born healthy in this cohort. The data from a total of 570 birth outcomes registered in our hospital have been included in the analysis.
Figure 1.
Influenza study flow chart
Figure 2 shows the incidence rate of PCR-positive influenza during the study period from November 2015 to October 2017. The peak incidence has occurred during late monsoon and winter months. An epidemic range incidence had been recorded during September–October 2017. During the summer months (March–August), the reports of influenza were very low though there was year-round incidence. AH3 showed peak infection rates during the winters of 2015 and 2017, whereas AH1 and B subtypes had prevailed from 2016 November to February 2017 [Figure 3].
Figure 2.
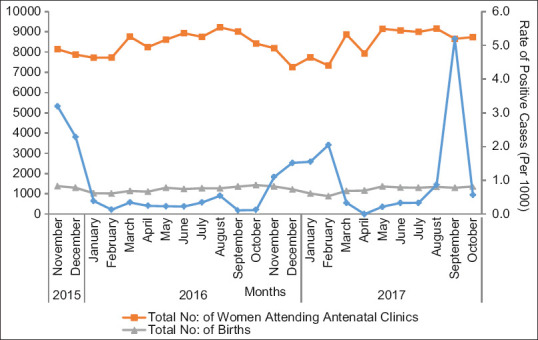
Line diagram plotting total number of women attending antenatal clinics, total number of births (primary axis), and incidence rate of influenza (secondary axis)
Figure 3.
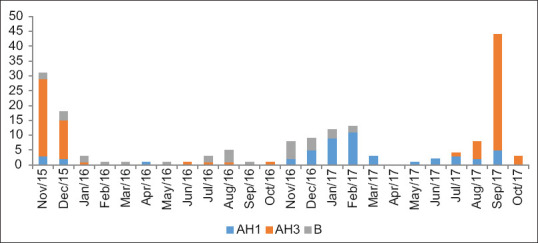
Strains of influenza viruses detected by polymerase chain reaction over the study period
Data from Cohort 1 were compared separately with Cohort 2 and Cohort 3. Demographic features [Table 1] of the mothers in the three groups were comparable with respect to age, body mass index, gravidity, place of residence, and previous adverse pregnancy outcomes. Annual income was significantly lower among women with influenza compared to the negative group (P = 0.004) and healthy controls (P < 0.001). The influenza infection rates steadily increased with advancing gestational age.
The symptoms of ILI included fever, coryza, and dyspnea. The incidence of tachycardia (pulse rate > 100 beats/min), tachypnea (respiratory rate > 20 breaths/min), and hypoxia (oxygen saturation < 90%), assessed as per our hospital protocol, was significantly more (P < 0·001) among women with influenza [Table 2]. Significantly higher proportion of women in Cohort 1 had severe illness requiring inpatient and ICU care compared to PCR-negative women (36.2% vs. 6.3%, P < 0.001). The expenditure toward treatment, ₹15,432 (~$ 216) for an average of 5 days, was three times higher for women with severe illness compared to women who had outpatient-based care. On an average, each woman who had outpatient care had to spend ₹4392 (~$ 62). This included the cost of influenza PCR, antiviral drugs, and physician fees.
Mean birth weight, head circumference, and length of the neonate were comparable between the cohorts [Table 3]. The primary neonatal outcomes analyzed are listed in Table 4. The incidence of SGA was similar between the cohorts. Twenty babies (13·1%) in the influenza PCR-positive group were SGA compared to 33 (12.8%, P = 0.9, RR – 1.01) in the PCR-negative ILI group and 20 (13%, P = 0.9, RR– 1.12) among the healthy controls. The incidence of preterm birth was also similar among the women with acute respiratory infection (7.8% vs. 8·9%, P = 0.72). However, there was a trend toward more preterm births in Cohort 1 compared to the healthy controls in Cohort 3 (7.8% vs. 3.3%; P = 0.08), but the difference was not statistically significant.
Table 3.
Characteristics of neonates at birth
| Variable | Cohort I (n=153)Mean±SD, n (%) | Cohort II (n=258)Mean±SD, n (%) | P | Cohort III (n=153) Mean±SD, n (%) | P |
|---|---|---|---|---|---|
| Head circumference (cm) | 33.5±1.30 | 33.7±1.40 | 0.135 | 33.6±1.2 | 0.324 |
| Length (cm) | 48.12±2.3 | 48·00±2.7 | 0.648 | 48.2±2.0 | 0.742 |
| Gender | |||||
| Male | 84 (54.9) | 127 (49.2) | 0.266 | 70 (45.7) | 0.108 |
| Female | 69 (45.1) | 131 (50.8) | 83 (54.3) | ||
| Mean birth weight (kg) | 2.96±0.4 | 2.95±0.5 | 0.782 | 2.97± 0.43 | 0.817 |
SGA: Small for gestational age, SD: Standard deviation
Table 4.
Primary outcomes
| Variable | Cohort I (n=153), n (%) | Cohort II (n=258), n (%) | P | RRCI | Cohort III (n=153), n (%) | P | RRCI |
|---|---|---|---|---|---|---|---|
| SGA | |||||||
| Yes | 20 (13.1) | 33 (12.8) | 0·946 | 1.010.69-1.47 | 20 (13.0) | 0.899 | 1.120.58-2.15 |
| No | 133 (86.9) | 224 (87.2) | 133 (87.0) | ||||
| Preterm (<37 weeks) | 12 (7.8) | 23 (8.9) | 0·724 | 0.920.57-1.48 | 5 (3.3) | 0.086 | 2.750.88-8.64 |
| Term | 141 (92.2) | 235 (91.0) | 148 (96.7) |
SGA: Small for gestational age, RR: Relative risks, CI: Confidence interval
There was one unexplained term stillbirth in Cohort 1 3 weeks after the mother had recovered from influenza infection. Cohort 2 also had a stillbirth due to abruption at 24 weeks of gestation which occurred 4 weeks after the mother had recovered from ILI. The overall pregnancy loss (including miscarriage) was lower among women with influenza (0.6%) compared to PCR-negative ILI (1.9%). No birth defects or neonatal deaths occurred in Cohort 1. The secondary neonatal outcomes are listed in Table 5. Cohort 1 had one baby with Apgar score < 6 at 5 min, four cases of neonatal sepsis and five babies with RDS. There was a trend toward increased preterm care requirement in this group compared to healthy controls (RR 2.75; P = 0.1). Obstetrical complications such as polyhydramnios (0.7% vs. 1%; P = 1.0; RR-0.74) and oligohydramnios (3.3% vs. 1.9%; P = 0.3; RR – 1.44) were very few. None of these women in Cohort 1 had abruptio placentae or placenta previa, hence no tests of statistical significance could be done. Overall, there were no statistically significant differences in the secondary outcomes.
Table 5.
Secondary outcomes
| Variable | Cohort I(n=153), n (%) | Cohort II (n=258), n (%) | P | RRCI | Cohort III (n=153), n (%) | P | RRCI |
|---|---|---|---|---|---|---|---|
| Apgar score | |||||||
| <6 at 5 min | 1 (0.7) | 2 (0.8) | 1.000 | 0.890.18-4.47 | 1 (0.7) | 1.000 | 1.000.06-15.99 |
| ≥6 at 5 min | 152 (99.3) | 256 (99.2) | 152 (99.3) | ||||
| RDS/sepsis | 9 (5.8) | 11 (4.2) | 0.432 | 0.810.49-1.33 | 6 (4.0) | 0.427 | 1.500.53-4.21 |
| No complications | 144 (94.2) | 247 (95.8) | 147 (96.0) | ||||
| Preterm care in NICU up to 4 weeks after birth | |||||||
| Yes | 9 (5.9) | 12 (4.7) | 0.573 | 1.170.7-1.94 | 4 (2.7) | 0.167 | 2.250.69-7.31 |
| No | 144 (94.1) | 246 (95.3) | 149 (97.3) |
RDS: Respiratory distress syndrome, NICU: Neonatal intensive care unit, RR: Relative risks, CI: Confidence interval
DISCUSSION
Our study highlights the potential use of clinical data to assess the health impacts of influenza in pregnant women a target population for vaccination. We identified 174 laboratory confirmed maternal influenza infections in Vellore (South India) during the period of 2015–2017. Influenza contributed to 36% of the acute respiratory infections in the observed pregnant women. Seventy percent were affected during the third trimester and 36% had severe illness which required inpatient care. There were more preterm births in the infected women (RR – 2.75) requiring prolonged NICU care.
The influenza viruses that contribute mainly to the disease burden in humans are influenza A and B. Currently, there are two major subtypes of influenza A in circulation among humans, A/H3N2 and A/H1N1. In India, influenza A/H3N2 was the major strain prior to the emergence of H1N1pdm09. 2013 witnessed the co-circulation of A/H3N2 with A/H1N1pdm09.[10] In Vellore, A/H1N1pdm09 prevailed in 2009, 2010, and 2012; A/H3N2 in 2011 and 2013; and influenza B in 2012. The changing trend continued during our study period with H3N2 peaks in 2015 and 2017 and H1N1 in 2016. Though earlier studies from South India have reported greater prevalence of A/H1N1pdm09 among pregnant women, later studies have attributed this to greater circulation of this strain.[11] Our findings are in concordance with Koul et al., with more H3N2 infections (49%) as compared to A/H1N1 (27%) and influenza B (18%).
Patterns of influenza vary in tropical and subtropical areas.[12] Countries closer to equator have year-round incidence, whereas those further away show monsoon peaks. Previous Indian studies have reported seasonal and pandemic patterns.[13] The seasonality would depend on latitude, rainfall, humidity, indoor crowding, and increased viral survival in winter months.[14] Three major patterns of circulation have been seen in India-winter peak in Srinagar (January–April); during the monsoon from June to October in Delhi, Kolkata, Nagpur, and Alappuzha; late monsoon-related peaks in Chennai and Vellore (September to December).[15] A year-round incidence of all the common strains was observed in our study with late monsoon and winter peaks (September to February). This would suggest a greater load of disease seen in this study compared to the previous studies, which have supported seasonal or pandemic patterns.[13]
The risk for infection was found to increase with advancing gestational age.[16] Liu et al. reported that 9.1% of the cases occurred in the first trimester, 29.8% in the second trimester, and 47% in the third trimester.[17] Previous reports from India suggest that four out of five pregnant women who developed respiratory failure were in the third trimester.[11] Our findings are consistent with 70% being infected in the third trimester. During the study period, maternal mortality in our institution ranged from 48/100,000 livebirths (2016) to 55/100,000 live births (2017). Only one death was due to H1N1 infection. This woman could not be included in our study as she was directly admitted to ICU without prior admission to obstetric ward. However, we found that influenza caused six times more maternal morbidity with a significant proportion developing severe illness (P < 0.001) and one-third requiring inpatient care (63 out of 174). Previous studies have reported higher morbidity with 92% hospitalization rates,[18] 73% ICU admission rates, high rates of pneumonia (75%), and maternal mortality (25%–70%).[19] However, such high rates could be an overestimation as majority were hospital-based studies and did not include community cases.[20] In addition there could have been obstetric concerns prompting hospitalization. However, a recent community-based study has reported drop in hospitalization rate to 18% and case fatality rate to 4%–8%.[21]
Many studies of maternal influenza reported significant impact on the fetus with 5-fold increase in perinatal mortality[22] and 3-fold increase in preterm birth.[23] Preterm birth rates reported from Western countries were lower (4%–25%)[24] compared to India (20%–33%).[13] The risk was more with severe maternal illness (odds ratio [OR] 3.2; 95% CI 2.4–4.0),[25] whereas studies based on a wider range of illness did not find any increased risk (OR 1.2; 95% CI 1.03-1.27).[26] We found a lower rate of preterm birth compared to the previous Indian studies, but it was still two times more in women with influenza (7.8% vs. 3.3%; P = 0.08; RR – 2.75) compared to healthy controls. Supporting evidence comes from the higher rate of NICU admission for preterm care (RR – 2.25). Incidence of SGA was similar among the three cohorts (RR – 1.1), which is again comparable to previous reports (2.8% to 15.3%; OR: 1.24)[24] However, studies on more severe illness have reported higher odds of SGA (OR: 1.66-2.35; 95% CI: 1.03–5.36).[27]
Fetal mortality reported from India ranges from 5.5% to 33%.[13] We found low fetal loss rate (0.4%) compared to an earlier study from the same hospital (5%; 1 in 20).[28] Incidence of RDS and sepsis was also comparable between the groups (P = 0.4). There were no neonatal deaths among the infected women. The better perinatal outcome could be attributed to less maternal morbidity after treatment with oseltamivir and higher standards of neonatal care. Lower maternal mortality could also be attributed to similar causes. Data on the effectiveness and safety of the drug are limited, though isolated reports are reassuring.[29] In addition, none of the women reported any adverse effects of oseltamivir necessitating stoppage of treatment. Still, the maternal and neonatal morbidity are higher compared to healthy women. Hence, primary prevention would be important as the next step to curtail the ailment. Our study was not designed to evaluate the effect of vaccination. However, it was estimated that a woman would spend far less for influenza vaccination (₹1239; ~$17) compared to outpatient care (₹4392; ~$62) or inpatient care (₹ 15,432; ~$216). Add on would be the costs of neonatal care. The economic burden related to the treatment of severe maternal illness (36.2% vs. 6.3%, P < 0.001) and advanced neonatal care (5.9% vs. 2.7%; P = 0.1; RR – 2.25) can prove challenging to developing nations.
The results of this study are relevant to India and other tropical countries in order to understand the burden of influenza and plan preventive strategies. The two sets of controls and matched analysis provide highly objective data. Both mild and severe cases of influenza were included, which makes the data more applicable to the general population. The study found year-round disease burden and a trend toward increased maternal and neonatal morbidity even after treatment with antiviral drug. Hence, more interventions would be needed to curtail the disease in pregnancy. The study has the limitation of being restricted to hospital based cases which could overestimate the morbidity. Community-based studies with bigger sample sizes would give a true picture of the morbidity.
CONCLUSION
The study has shown year-round incidence and increased disease burden from influenza infection in India. We found reduced maternal and neonatal mortality but morbidity was comparatively higher even after treatment with oseltamivir. This study highlights the need for newer public health interventions toward primary prevention to curtail morbidity due to influenza in pregnancy. Large community-based studies are needed to evaluate the impact of the infection at the grass-root level.
Financial support and sponsorship
The study was funded by the Cincinnati Children's Research Foundation (Study ID: 2014-7670).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We thank the patients and their families for providing consent and assisting with the present study, despite the suffering they have endured. We would also like to thank the health professional from the virology department of CMC, Vellore, for providing help in viral PCR analysis. We are extremely grateful to Matthews Mathai, Chair in Maternal and Newborn Health, Department of International Public Health, Liverpool School of Tropical Medicine, for his valuable inputs and critical revision of the manuscript. We also thank Nandhini. K and project staff Alice Rani for research assistance.
REFERENCES
- 1.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr. 2010;2:RRN1153. doi: 10.1371/currents.RRN1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Liu ZW, Lu YP, Li TY, Liang XJ, Arck PC, et al. A systematic review and meta-analysis of influenza a virus infection during pregnancy associated with an increased risk for stillbirth and low birth weight. Kidney Blood Press Res. 2017;42:232–43. doi: 10.1159/000477221. [DOI] [PubMed] [Google Scholar]
- 3.Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): A global comparative review. PLoS One. 2013;8:e54445. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedford T, Riley S, Barr IG, Broor S, Chadha M, Cox NJ, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523:217–20. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz JR, Neuzil KM, Ahonkhai VI, Gellin BG, Salisbury DM, Read JS, et al. Translating vaccine policy into action: A report from the Bill & Melinda Gates Foundation consultation on the prevention of maternal and early infant influenza in resource-limited settings. Vaccine. 2012;30:7134–40. doi: 10.1016/j.vaccine.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Bhaskar E, Thobias S, Anthony S, Kumar V, Navaneethan Vaccination rates for pandemic influenza among pregnant women: An early observation from Chennai, South India. Lung India. 2012;29:232–5. doi: 10.4103/0970-2113.99105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–8. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta AA, Kumar VA, Nair SG, K Joseph F, Kumar G, Singh SK. Clinical profile of patients admitted with swine-origin influenza a (H1N1) virus infection: An experience from a tertiary care hospital. J Clin Diagn Res. 2013;7:2227–30. doi: 10.7860/JCDR/2013/5657.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 10.Chadha MS, Potdar VA, Saha S, Koul PA, Broor S, Dar L, et al. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PLoS One. 2015;10:e0124122. doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koul PA, Bali NK, Mir H, Jabeen F, Ahmad A. Influenza illness in pregnant Indian women: A cross-sectional study. Infect Dis Obstet Gynecol. 2016;2016:1248470. doi: 10.1155/2016/1248470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha S, Chadha M, Al Mamun A, Rahman M, Sturm-Ramirez K, Chittaganpitch M, et al. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and South-Eastern Asia. Bull World Health Organ. 2014;92:318–30. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalerao-Gandhi A, Chhabra P, Arya S, Simmerman JM. Influenza and pregnancy: A review of the literature from India. Infect Dis Obstet Gynecol. 2015;2015:867587. doi: 10.1155/2015/867587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamerius JD, Shaman J, Alonso WJ, Bloom-Feshbach K, Uejio CK, Comrie A, et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9:e1003194. doi: 10.1371/journal.ppat.1003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koul PA, Broor S, Saha S, Barnes J, Smith C, Shaw M, et al. Differences in influenza seasonality by latitude, northern India. Emerg Infect Dis. 2014;20:1723–6. doi: 10.3201/eid2010.140431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim ML. 2009/H1N1 infection in pregnancy association with adverse perinatal outcomes. Evid Based Nurs. 2012;15:11–2. doi: 10.1136/ebn.2011.100205. [DOI] [PubMed] [Google Scholar]
- 17.Liu SL, Wang J, Yang XH, Chen J, Huang RJ, Ruan B, et al. Pandemic influenza a (H1N1) 2009 virus in pregnancy. Rev Med Virol. 2013;23:3–14. doi: 10.1002/rmv.1712. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 19.Mathur S, Dubey T, Kulshrestha M, Agarwal H, Mathur G, Mathur A, et al. Clinical profile and mortality among novel influenza a (H1N1) infected patients: 2009-2010 Jodhpur, Rajasthan pandemic. J Assoc Physicians India. 2013;61:627–32. [PubMed] [Google Scholar]
- 20.Mertz D, Geraci J, Winkup J, Gessner BD, Ortiz JR, Loeb M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: A systematic review and meta-analysis of observational studies. Vaccine. 2017;35:521–8. doi: 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regan AK, Moore HC, Sullivan SG, De Klerk N, Effler PV. Epidemiology of seasonal influenza infection in pregnant women and its impact on birth outcomes. Epidemiol Infect. 2017;145:2930–9. doi: 10.1017/S0950268817001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaan N, Amzallag S, Laskov I, Cohen Y, Fried M, Lessing JB, et al. Maternal and neonatal outcome of pregnant women infected with H1N1 influenza virus (swine flu) J Matern Fetal Neonatal Med. 2012;25:130–2. doi: 10.3109/14767058.2011.562569. [DOI] [PubMed] [Google Scholar]
- 23.Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. UKOSS. Perinatal outcomes after maternal 2009/H1N1 infection: National cohort study. BMJ. 2011;342:d3214. doi: 10.1136/bmj.d3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fell DB, Savitz DA, Kramer MS, Gessner BD, Katz MA, Knight M, et al. Maternal influenza and birth outcomes: Systematic review of comparative studies. BJOG. 2017;124:48–59. doi: 10.1111/1471-0528.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieto-Pascual L, Arjona-Berral JE, Marín-Martín EM, Muñoz-Gomariz E, Ilich I, Castelo-Branco C. Early prophylactic treatment in pregnant women during the 2009-2010 H1N1 pandemic: Obstetric and neonatal outcomes. J Obstet Gynaecol. 2013;33:128–34. doi: 10.3109/01443615.2012.740526. [DOI] [PubMed] [Google Scholar]
- 26.Håberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–40. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naresh A, Fisher BM, Hoppe KK, Catov J, Xu J, Hart J, et al. A multicenter cohort study of pregnancy outcomes among women with laboratory-confirmed H1N1 influenza. J Perinatol. 2013;33:939–43. doi: 10.1038/jp.2013.110. [DOI] [PubMed] [Google Scholar]
- 28.Pramanick A, Rathore S, Peter JV, Moorthy M, Lionel J. Pandemic (H1N1) 2009 virus infection during pregnancy in South India. Int J Gynaecol Obstet. 2011;113:32–5. doi: 10.1016/j.ijgo.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Dunstan HJ, Mill AC, Stephens S, Yates LM, Thomas SH. Pregnancy outcome following maternal use of zanamivir or oseltamivir during the 2009 influenza A/H1N1 pandemic: A national prospective surveillance study. BJOG. 2014;121:901–6. doi: 10.1111/1471-0528.12640. [DOI] [PubMed] [Google Scholar]