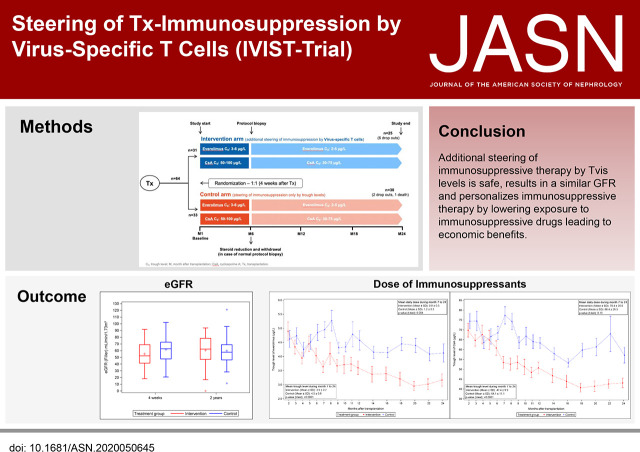
Significance Statement
Pharmacokinetic monitoring of immunosuppressive drugs after transplantation is associated with risk of excessive or inadequate immunosuppression. In this randomized, controlled trial involving 64 pediatric kidney recipients, the control and intervention groups both received trough-level monitoring of immunosuppressants; the intervention group also received additional guidance from measuring virus-specific T cell levels, which have been shown to be a marker of the individual intensity of immunosuppression. Patients in the intervention group had lower daily doses and trough levels of cyclosporin A and everolimus. Two years after transplantation, eGFR of the intervention group was comparable with that of the control group. These findings indicate that additional steering of immunosuppressive therapy by virus-specific T cell levels seems to be safe and may help in personalizing dosing and reducing exposure to immunosuppressive drugs.
Keywords: pediatric kidney transplantation, kidney transplantation, immunosuppression, lymphocytes
Visual Abstract
Abstract
Background
Pharmacokinetic monitoring is insufficient to estimate the intensity of immunosuppression after transplantation. Virus-specific T cells correlate with both virus-specific and general cellular immune defense. Additional steering of immunosuppressive therapy by virus-specific T cell levels might optimize dosing of immunosuppressants.
Methods
In a multicenter, randomized, controlled trial, we randomized 64 pediatric kidney recipients to a control group with trough-level monitoring of immunosuppressants or to an intervention group with additional steering of immunosuppressive therapy by levels of virus-specific T cells (quantified by cytokine flow cytometry). Both groups received immunosuppression with cyclosporin A and everolimus in the same target range of trough levels. Primary end point was eGFR 2 years after transplantation.
Results
In the primary analysis, we detected no difference in eGFR for the intervention and control groups 2 years after transplantation, although baseline eGFR 1 month after transplantation was lower in the intervention group versus the control group. Compared with controls, patients in the intervention group received significantly lower daily doses of everolimus and nonsignificantly lower doses of cyclosporin A, resulting in significantly lower trough levels of everolimus (3.5 versus 4.5 µg/L, P<0.001) and cyclosporin A (47.4 versus 64.1 µg/L, P<0.001). Only 20% of patients in the intervention group versus 47% in the control group received glucocorticoids 2 years after transplantation (P=0.04). The groups had similar numbers of donor-specific antibodies and serious adverse events.
Conclusions
Steering immunosuppressive therapy by virus-specific T cell levels in addition to pharmacokinetic monitoring seems safe, results in a similar eGFR, and personalizes immunosuppressive therapy by lowering exposure to immunosuppressive drugs, likely resulting in lower drug costs.
Clinical Trial registry name and registration number:
IVIST trial, https://www.clinicaltrialsregister.eu/ctr-search/search?query=2009-012436-32 and ISRCTN89806912
Kidney transplantation is the preferred treatment for pediatric patients with ESKD.1 To avoid graft rejections, lifelong immunosuppressive therapy is necessary, which is associated with the risk of serious bacterial and viral complications. Moreover, the side effects of immunosuppressive medication include nephrotoxicity, arterial hypertension, new-onset diabetes mellitus, dyslipidemia, bone mineral disorders, growth impairment, malignancies, and delayed sexual maturation.2–7 Consequently, it is crucial to achieve the optimal individual balance between over- and underimmunosuppression and thereby, avoid unnecessary exposure to immunosuppressive drugs. However, diagnostic and prognostic markers to assess the individual intensity of immunosuppression are missing. Monitoring of immunosuppressive therapy is most often performed on immunosuppressant blood levels that mirror the pharmacokinetics but not the pharmacodynamics.8 Therefore, other potential methods to determine immune function and grade of immunosuppression, such as analysis of the Torque-Teno virus load9 or virus-specific T cells (Tvis),10 are currently evaluated. Tvis control virus replication and have been shown to correlate with virus-specific as well as general cellular immune defense, which represents the individual’s susceptibility to infections.10 Accordingly, they may serve as an indicator of the intensity of immunosuppression as well as a prognostic marker for virus-induced diseases after transplantation.10–14
The randomized IVIST trial has been designed to investigate the hypothesis that additional steering of immunosuppressive therapy by Tvis levels optimizes dosing of immunosuppressive therapy15 (effect-related drug monitoring), resulting in a personalized treatment with improved clinical courses on the basis of fewer viral infections and possible changes in exposure to immunosuppressive drugs.
Methods
Trial Oversight
The detailed methodology of the IVIST trial has been published previously.15 In brief, this was a 2-year, multicenter, open-label, randomized, controlled trial that included 64 pediatric kidney transplant recipients (aged 0–16 years at the time of randomization). In the initial study protocol, the study was planned to include pediatric kidney and liver recipients from Hannover Medical School. In 2011, in the early stages of the study, two amendments were implemented. The first amendment restricted the study population to kidney-transplanted children because of the lack of experience with everolimus in pediatric liver recipients; the second amendment permitted the extension to multicenter recruitment because of lower recruitment rates than expected at Hannover Medical School. The study was, therefore, performed at four study sites in Germany (Hannover, Cologne, Hamburg, and Rostock). The trial protocol was approved by the scientific ethics committees of the participating university hospitals. The trial was performed in accordance with the principles of the Declaration of Helsinki. The authors assume responsibility for the accuracy and completeness of the data and analyses, as well as for the fidelity of the trial and this report to the protocol.
Patients
Full inclusion and exclusion criteria have been published previously.15 Kidney transplant recipients aged 0–16 years were eligible for the trial if they received a first or second transplantation. Key exclusion criteria were (1) history of malignancy (despite post-transplant lymphoproliferative disease) and (2) highly sensitized patients (panel reactive antibodies >50%).
Trial Procedures
Four weeks after transplantation, pediatric kidney recipients were randomized (1:1) either to the control group with conventional steering of immunosuppressants according to trough levels or to the intervention group with additional steering by the levels of Tvis against adenovirus (ADV), cytomegalovirus (CMV), and herpes simplex virus (HSV) at weeks 4, 6, 8, 10, and 12 after transplantation and then monthly until month 12 followed by measurements every 2 months until month 24 (Figure 1). The randomization was stratified for study site and CMV prophylaxis.
Figure 1.
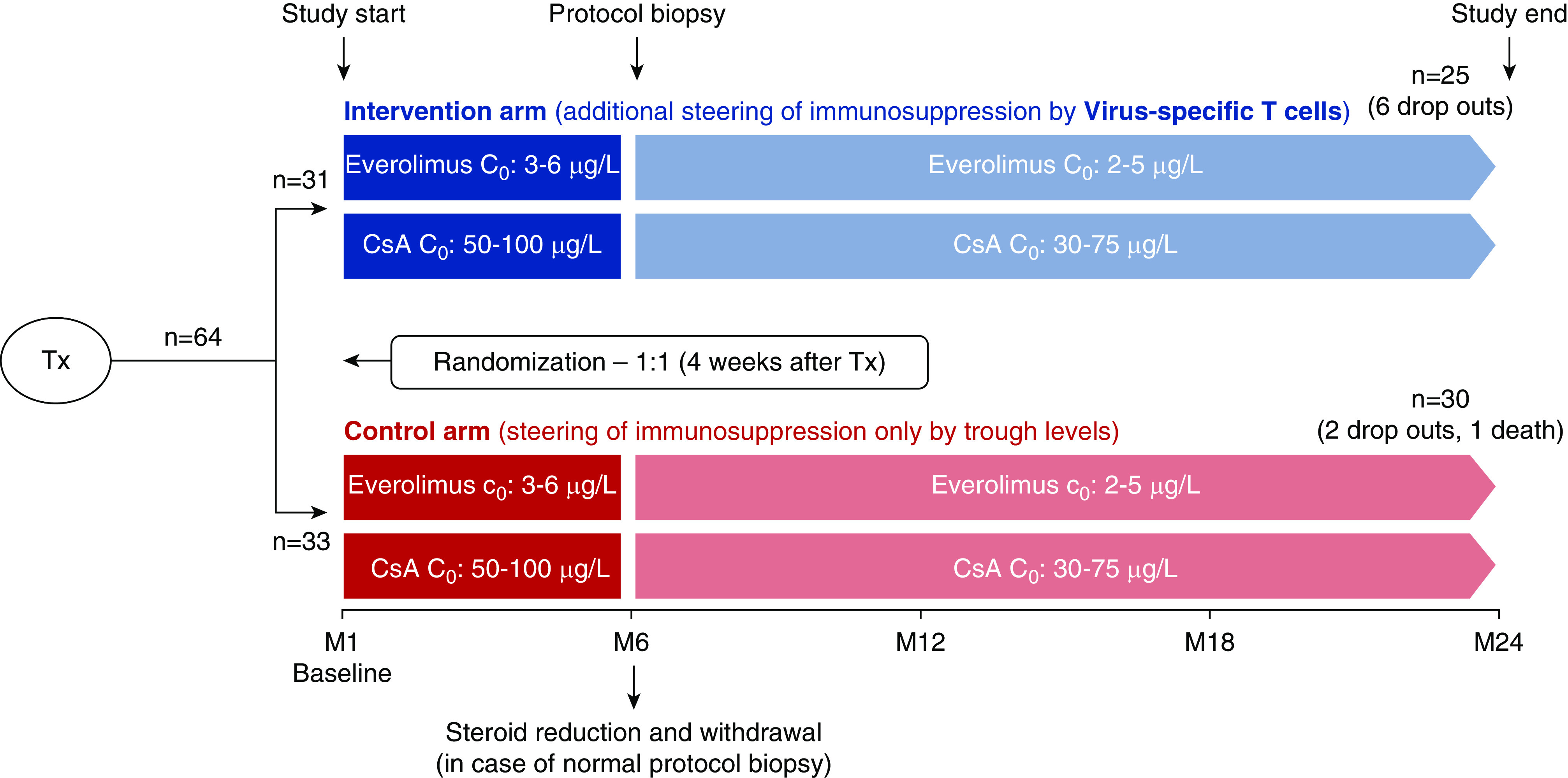
Study design. C0, trough level; M, month after transplantation; CsA, cyclosporin A; Tx, transplantation.
Initially, all kidney transplant recipients received an immunosuppressive therapy comprising cyclosporin A (CsA) and glucocorticoids, including an induction therapy with basiliximab. Four weeks after transplantation, everolimus was initiated at a dose of 1.6 mg/m2 per day in two doses16 and adjusted to achieve a target trough level of 3–6 µg/L. Simultaneously, CsA dose was halved and adjusted to achieve a target trough level of 50–100 µg/L. After month 6, target trough levels were reduced to 30–75 µg/L for CsA and to 2–5 µg/L for everolimus (Figure 1).
For the intervention group, in addition to trough-level monitoring of immunosuppressants, the levels of CD4 Tvis (cells per microliter) were measured by intracellular cytokine staining followed by flow cytometric analysis as described below.17 In case of low Tvis numbers (below the lower threshold value of two cells per microliter), the dose of immunosuppressive drugs was decreased by 10%–15%. If the trough levels of CsA or everolimus reached the lower target levels given above, no adaptation of CsA or everolimus doses according to Tvis number was performed. Tvis-based increase of immunosuppression was performed in individual patients if all Tvis levels were elevated more than ten cells per microliter. In the control group, immunosuppressive medications were only steered conservatively by trough levels according to the standard protocol given above.
As per protocol, low-dose glucocorticoids were withdrawn at month 9 after transplantation if the protocol biopsy at month 6 showed no acute rejection BANFF ≥ IA, independent of prior rejection episodes.
Acute rejections were not specified as a reason for study discontinuation. The study was only stopped for patients in whom the immunosuppressive regimen was changed by the investigator.
Analysis of Virus-Specific T Cells
Virus-specific CD4 T cells against CMV, ADV, and HSV were analyzed separately at the same time. The analysis of virus-specific CD4 T cells was performed in four steps from heparinized whole blood (starting not later than 24 hours after blood withdrawal). For measurement of these three types of Tvis, a total amount of 3.15 ml whole blood was needed, including one positive control and the respective negative controls.
Stimulation. The blood samples were stimulated in vitro by viral antigens for 6 hours at 6% CO2 and 37°C. As virus-specific stimuli, manufactured CMV-, ADV-, or HSV-infected cells and cell culture medium (concentrated and purified) were used (Serion KBR Antigen Cytomegalovirus [1130], Adenovirus [1121], and Herpes Simplex Virus 1/2 [1154]; Institute Virion/Serion GmbH, Wuerzburg, Germany). For each blood sample, the virus-specific stimulations were combined with a positive control analysis and the respective negative control analyses. As negative control antigen, the blood samples were stimulated with noninfected cells extracted with alkaline buffer (concentrated and purified; Serion KBR Control Antigen Cytomegalovirus [2130], Adenovirus [2121], and Herpes Simplex Virus 1/2 [2154]; Institute Virion/Serion GmbH); the positive control was performed using 2.5 µg/ml Staphylococcus aureus enterotoxin B (Sigma-Aldrich). The stimulations were carried out for a total of 6 hours in the presence of 1 µg/ml costimulatory antibodies against CD28 and CD49d (BD Biosciences, Heidelberg, Germany), maximizing the detection of T cells. This antigen-specific stimulation led to induction of intracellular cytokine production, such as IFNγ. For the last 4 hours of stimulation, 10 µg/ml brefeldin A (Sigma-Aldrich) was added to inhibit granule secretion of cytokines and cause intracellular accumulation of cytokines.
Fixation. After the stimulation procedure, the cells were treated with 2 mM EDTA for 15 minutes to disrupt cell-cell interaction. Afterward, erythrocytes were lysed (FACS Lysing solution; BD, Heidelberg, Germany), and leukocytes were fixed.
Immunostaining. At first, the leukocytes were permeabilized by 0.1% Saponin (Sigma-Aldrich). Subsequently, the activated T cells were immunostained using saturating amounts of fluorescent antibodies against CD4, CD69, and IFNγ (all antibodies from BD).
Flow cytometry. The percentage of fluorescence-marked lymphocytes was measured on a BD FACSCalibur using BD FACS CellQuest Pro software. Hence, virus-specific CD4 T cells were identified as frequency of CD69-positive and IFNγ-positive CD4 T cells.
The percentage of virus-specific CD4 T cells against CMV, ADV, and HSV was calculated by subtraction of the result obtained by the respective negative control. Absolute numbers of virus-specific CD4 T cells were quantified by multiplication of the absolute number of lymphocytes (on the basis of the differential blood count analyzed in parallel) with the percentage of virus-specific CD4 T cells because the absolute cell number per microliter blood better represents the individual virus-specific cellular immunity compared with the percentage of Tvis (i.e., in patients with lymphopenia).
The method was performed in our own laboratory and has been used in clinical practice in our center for several years.
Outcomes
The primary end point was the eGFR defined by Filler and coworkers18 2 years after transplantation calculated using the formula 91.62×(1/serum cystatin C [milligrams per liter]).18 For sensitivity analyses, the CKiD Schwartz formula [39.1×(height [meters]/serum creatinine [milligrams per deciliter])0.516×(1.8/serum cystatin C [milligrams per liter])0.294×(30/BUN [milligrams per deciliter])0.169×(height [meters]/1.4)0.188(×1.099 if a man)], the current standard for the calculation of eGFR, was analyzed in addition.19 Secondary end points were occurrence and number of acute rejections, development of donor-specific antibodies (Luminex single-antigen assay MFI>1000 was defined as positive), viral infections, adverse events (AEs), and serious adverse events (SAEs), as well as immunosuppressant doses and trough levels.
Statistical Methods
Sample Size Estimation
The primary hypothesis of the study was to demonstrate that the eGFR 24 months after kidney transplantation was higher in the intervention group. Sample size calculations are described in detail elsewhere.15 Basically, a mean eGFR difference between the treatment groups of at least 7.5 ml/min per 1.73 m2 was considered as relevant, assuming an SD of 15 ml/min per 1.73 m2. Under these assumptions, the total sample size of 64 patients in this study would have led to a power of 50% to reject the null hypothesis of no difference between treatment groups using a two-sided independent samples t test at a significance level of 5%.
Patient Demographics/Further Baseline Characteristics
Demographic and further baseline characteristics, including transplant background information, were analyzed descriptively using summary statistics for numeric variables and absolute and relative frequencies for categorical variables.
Primary Analyses
Confirmatory analysis of the primary end point was performed on the intention-to-treat (ITT) population, including all randomized patients. The primary analysis model was an analysis of covariance including the eGFR (defined by Filler and coworkers18) 24 months after transplantation as the dependent variable and treatment strategy (intervention versus control group), baseline eGFR, CMV prophylaxis (yes/no), and study site as independent factors. The estimate as well as the two-sided 95% confidence interval (95% CI) for the difference in means (intervention group minus control group) was calculated for the eGFR.
Handling of missing values was implemented as prespecified in the study protocol. The necessity for dialysis, graft loss, and renal-related death were defined as treatment failures. In this case, the eGFR value after 2 years was set to zero. Death from any cause except graft loss was not defined as a treatment failure. In this case, missing values were replaced using last observation carried forward (LOCF).
Patients were under routine surveillance by the transplant center. Patients who withdrew consent or could not further participate were asked to consent to the use of routinely taken eGFR values for the originally planned follow-up visits. If it was not possible to receive the eGFR value 24 months after transplantation, the missing value was replaced using LOCF.
In addition, descriptive statistics for the eGFR are presented by treatment group at baseline and 24 months after transplantation, as well as for change from baseline.
Sensitivity analyses were performed for alternative definitions of eGFR and on the following populations.
Population A. Modified ITT population including only patients with complete information of the primary end point.
Population B. Per protocol population including patients who completed the treatment regimen according to the study protocol.
Population C. Per protocol population including all randomized patients. Missing eGFR values were replaced by the value of the last compliant visit.
The course of eGFR during the study is depicted in mean error plots where missing values were not replaced.
Secondary Analyses
All secondary end points were evaluated in an explorative manner. Therefore, no adjustment for multiplicity has been performed. Two-sided P values and 95% CIs were assessed descriptively.
Because only one patient died due to drowning and only one patient experienced graft loss, the analyses of these two end points using Kaplan–Meier curves and log rank tests were not conducted.
The analyses of the doses and trough levels of the study medications and the oral therapy with glucocorticoids (ATC: H02AB) were performed on patients who completed the treatment regimen according to the study protocol (population B). Summary statistics (mean and SD) are presented for the mean trough levels and mean daily dose per meter squared of everolimus and CsA from month 7 until month 24 after transplantation. Intervention and control groups were compared using two-sided t tests. The number of patients who received an oral therapy with glucocorticoids 2 years after transplantation was analyzed using a two-sided chi-squared test.
The frequency and type of rejections were analyzed separately for protocol biopsy and biopsy of cause. The number of patients with at least one rejection diagnosed in biopsy of cause was compared between the treatment groups using a two-sided chi-squared test. The number of rejections per patient was analyzed using a Poisson regression, including the number of rejections per patient as the dependent variable and the treatment group as the independent variable. The time to first rejection observed in biopsy of cause was compared between the treatment groups using the Kaplan–Meier method and two-sided log rank test. All analyses of rejections were performed in the ITT population. Patients without any documented information on a rejection are counted as no rejection.
The analyses of the occurrence of virus DNAemia were on the basis of the results of PCR. Virus DNA greater than or equal to the cutoff value of 1000 copies per milliliter was defined as a DNAemia. The numbers of patients who experienced a virus DNAemia or biopsy-proven BK polyomavirus–associated nephropathy during the study were presented and compared between the treatment groups using the two-sided chi-squared test or Fisher exact tests. Furthermore, the time to first Epstein–Barr virus (EBV) DNAemia was compared between the treatment groups using the Kaplan–Meier method and the two-sided log rank test. All analyses of virus events were performed in the ITT population.
Course of eGFRs is displayed using mean error plots by visit for all eGFR values of the ITT population that were compiled during a regular study visit. Missing values were not replaced. Course of eGFR was additionally analyzed in the per protocol population (population B).
Safety Evaluation
Safety and tolerability analyses were performed on the safety population, which comprises all patients who received at least one dose of study medication. In this study, all patients received the study medications; consequently, the ITT and the safety population are identical.
The assessment of safety was mainly on the basis of the occurrence of AEs. AEs are summarized by presenting the number and percentage of patients having any AE or SAE and compared by the two-sided chi-squared test or Fisher exact tests. AEs were coded according to MedDRA (Version: V22.0 1903 e) and are presented by system organ class.
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute).
Patient and Public Involvement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Results
Patients Characteristics
From April 2010 to April 2017, a total of 64 pediatric kidney recipients were enrolled at four study sites; 31 patients were randomized to the intervention group, and 33 patients were randomized to the control group (Figure 2).
Figure 2.
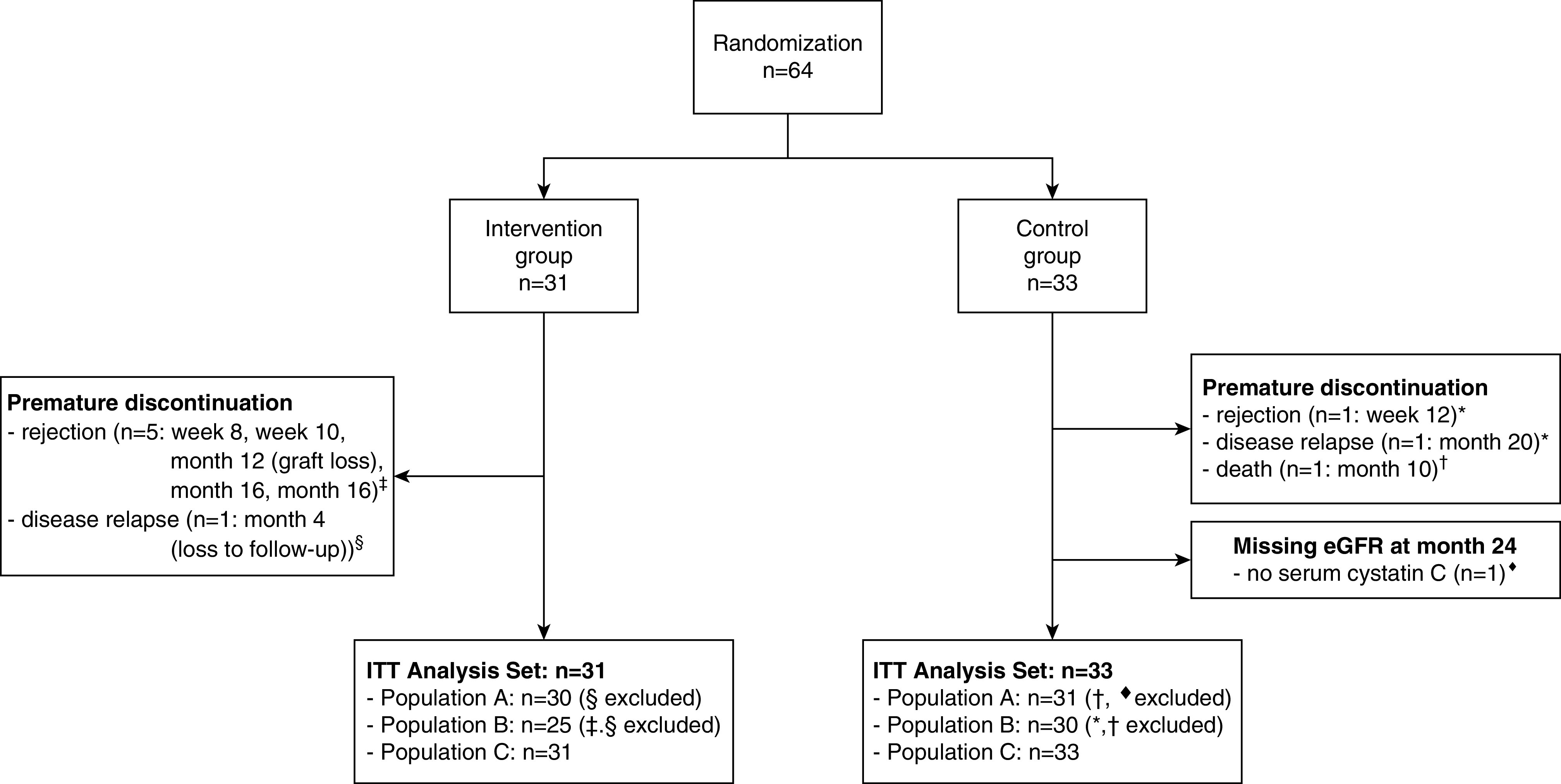
Study flow chart.
Overall, the two groups were balanced with respect to baseline characteristics, except for race and proportion of living donors (Table 1). All Asian patients (n=7) were in the intervention group, and twice as many living donors (n=10) were in the control group. Because of the complex logistical procedures, most of the patients (85.9%) where randomized only at one study site (site 1, Medical School Hannover) (Table 1). Unfortunately, there was a competing trial (the CRADLE study) for pediatric kidney recipients at that time, resulting in the low inclusion numbers in our IVIST trial by the other centers. Underlying diseases, HLA mismatches, and specific donor characteristics are reported in Table 1.
Table 1.
Baseline characteristics
| Baseline Characteristics | Intervention, n=31 | Control, n=33 | Total, n=64 |
|---|---|---|---|
| Recipient | |||
| Age, yr | |||
| Mean ± SD | 11.03±4.05 | 10.65±4.31 | 10.83±4.16 |
| Range [minimum to maximum] | [1.58–16.17] | [1.75–16.42] | [1.58–16.42] |
| Sex (girls) | 13 (41.9%) | 14 (42.4%) | 27 (42.2%) |
| EBV-IgG positivity | 18 (58.1%) | 17 (51.5%) | 35 (54.7%) |
| CMV-IgG positivity | 12 (38.7%) | 11 (33.3%) | 23 (35.9%) |
| CMV high-risk constellationa | 10 (32.3%) | 9 (27.3%) | 19 (29.7%) |
| CMV prophylaxis | 12 (38.7%) | 12 (36.4%) | 24 (37.5%) |
| Donor | |||
| Age, yr | |||
| Mean ± SD | 22.69±17.06 | 24.22±19.52 | 23.48±18.24 |
| Range [minimum to maximum] | [0–50] | [0–53] | [0–53] |
| Sex (women) | 16 (51.6%) | 14 (42.4%) | 30 (46.9%) |
| CMV-IgG positivity | 19 (61.3%) | 15 (45.5%) | 34 (53.1%) |
| Graft ischemia time, h | |||
| Mean ± SD | 11.48±5.53 | 11.50±7.29 | 11.49±6.45 |
| Range [minimum to maximum] | [1.83–21.75] | [2.27–27.50] | [1.83–27.50] |
| Living donor | 5 (16.1%) | 10 (30.3%) | 15 (23.4%) |
| Study site | |||
| Site 1 | 27 (87.1%) | 28 (84.8%) | 55 (85.9%) |
| Site 2 | 2 (6.5%) | 1 (3.0%) | 3 (4.7%) |
| Site 3 | 2 (6.5%) | 3 (9.1%) | 5 (7.8%) |
| Site 4 | 0 (0.0%) | 1 (3.0%) | 1 (1.6%) |
| Race | |||
| White | 24 (77.4%) | 33 (100.0%) | 57 (89.1%) |
| Asian | 7 (22.6%) | 0 (0.0%) | 7 (10.9%) |
| Etiology of kidney disease | |||
| CAKUT | 9 (29.0%) | 11 (33.3%) | 20 (31.3%) |
| ARPKD | 2 (6.5%) | 2 (6.1%) | 4 (6.3%) |
| Nephronophthisis | 8 (25.8%) | 4 (12.1%) | 12 (18.8%) |
| Nephropathic cystinosis | 2 (6.5%) | 3 (9.1%) | 5 (7.8%) |
| FSGS | 2 (6.5%) | 3 (9.1%) | 5 (7.8%) |
| HUS | 2 (6.5%) | 3 (9.1%) | 5 (7.8%) |
| Congenital nephrotic syndrome | 1 (3.2%) | 2 (6.1%) | 3 (4.7%) |
| Unknown origin | 1 (3.2%) | 0 (0.0%) | 1 (1.6%) |
| Other | 4 (12.9%) | 5 (15.2%) | 9 (14.1%) |
| HLA mismatches at locus A | |||
| 0 | 12 (38.7%) | 9 (27.3%) | 21 (32.8%) |
| 1 | 16 (51.6%) | 18 (54.6%) | 34 (53.1%) |
| 2 | 3 (9.7%) | 6 (18.2%) | 9 (14.1%) |
| HLA mismatches at locus B | |||
| 0 | 6 (19.4%) | 4 (12.1%) | 10 (15.6%) |
| 1 | 11 (35.5%) | 18 (54.6%) | 29 (45.3%) |
| 2 | 14 (45.2%) | 11 (33.3%) | 25 (39.1%) |
| HLA mismatches at locus DR | |||
| 0 | 9 (29.0%) | 9 (27.3%) | 18 (28.1%) |
| 1 | 16 (51.6%) | 20 (60.6%) | 36 (56.3%) |
| 2 | 6 (19.4%) | 4 (12.1%) | 10 (15.6%) |
CAKUT, congenital anomalies of the kidney and urinary tract; ARPKD, autosomal recessive polycystic kidney disease; HUS, hemolytic uremic syndrome.
CMV-IgG–negative recipient with CMV-IgG–positive donor.
Primary Analyses
Twenty-five patients in the intervention group and 30 patients in the control group completed the 23-month study period (per protocol population; population B). In the intervention group, six patients prematurely dropped out because of changes to the immunosuppressive regimen (one due to relapse of underlying disease and five due to rejection, which led to graft loss in one patient caused by nonadherence). In the control group, one patient died because of drowning in a bathtub,20 and two patients prematurely discontinued the study because of a change to the immunosuppressive regimen (one due to rejection and one due to relapse of the underlying disease) (Figure 2).
In the intervention group, one dropout patient was lost to follow-up because of refusal of consent to use of eGFR values after study discontinuation. In the control group, the cystatin C value at month 24 was missing for one patient. For these two patients and the deceased patient, the missing eGFR value (Filler and coworkers,18 CKiD19) was replaced using LOCF (Figure 2).
In the primary analysis model, no difference between intervention and control group was observed for the eGFR (Filler and coworkers18) 2 years after transplantation (adjusted mean difference: 1.7; 95% CI, −10.2 to 13.6; P=0.77) (Table 2). Similar results were obtained using different eGFR calculations (Tables 2 and 3). All sensitivity analyses in the different populations were in line with the primary analysis (Table 2). However, the intervention group had a lower eGFR at study start (4 weeks after transplantation). The descriptive analysis showed an increase of eGFR (Filler and coworkers18) from 55.0±17.5 (study start) to 60.7±22.8 ml/min per 1.73 m2 (study end) in the intervention group and no substantial change in the control group (from 61.5±21.3 to 59.6±22.3 ml/min per 1.73 m2) (Table 3). The respective unadjusted mean difference of ΔGFR from study start to study end between both groups (intervention minus control) was 7.5±27.6 ml/min per 1.73 m2 (Table 3). Whereas the observed absolute mean difference is in line with the assumptions in the sample size planning, the observed variability of eGFR was almost two times larger than originally assumed. This could be one explanation why the results of the analyses for the mean difference were not statistically significant. The respective post hoc power calculation with the observed SD results in a lower power of 19%.
Table 2.
Results of primary and sensitivity analyses of eGFR at month 24
| Intervention, Mean (95% CI) | Control, Mean (95% CI) | Intervention Minus Control, Mean Diff (95% CI) | P Value | |
|---|---|---|---|---|
| ITT population | ||||
| eGFR (Filler and coworkers18) | 61.8 (45.3 to 78.2) | 60.1 (44.1 to 76.0) | 1.7 (−10.2 to 13.6) | 0.77 |
| eGFR (CKiD19) | 69.2 (53.1 to 85.3) | 68.8 (53.4 to 84.2) | 0.4 (−11.2 to 12.0) | 0.95 |
| Population A | ||||
| eGFR (Filler and coworkers18) | 62.7 (45.8 to 79.7) | 61.49 (44.8 to 78.1) | 1.2 (−11.2 to 13.6) | 0.84 |
| eGFR (CKiD19) | 70.0 (53.4 to 86.6) | 70.24 (54.2 to 86.3) | −0.2 (−12.3 to 11.8) | 0.97 |
| Population B | ||||
| eGFR (Filler and coworkers18) | 66.2 (50.7 to 81.7) | 60.8 (46.0 to 75.6) | 5.4 (−6.4 to 17.3) | 0.36 |
| eGFR (CKiD19) | 74.1 (59.2 to 89.0) | 69.1 (55.1 to 83.1) | 5.1 (−6.3 to 16.4) | 0.38 |
| Population C | ||||
| eGFR (Filler and coworkers18) | 61.9 (46.7 to 77.2) | 59.4 (44.5 to 74.2) | 2.6 (−8.4 to 13.6) | 0.64 |
| eGFR (CKiD19) | 69.6 (54.6 to 84.6) | 68.2 (53.9 to 82.6) | 1.4 (−9.4 to 12.2) | 0.80 |
Displayed are means and mean differences (Mean Diffs) with respective 95% CIs and P values from the analysis of covariance model including baseline eGFR, CMV prophylaxis, and study site.
Table 3.
Results of descriptive analyses of eGFR
| Intervention, Mean ± SD | Control, Mean ± SD | Intervention Minus Control, Mean Diff ± SD | P Valuea | |
|---|---|---|---|---|
| eGFR (Filler and coworkers18) | ||||
| BSL | 55.0±17.5 | 61.5±21.3 | −6.4±19.6 | 0.19 |
| Month 24 | 60.7±22.8 | 59.6±22.3 | 1.1±22.5 | 0.85 |
| Difference from BSLb | 5.6±21.3 | −1.9±32.5 | 7.5±27.6 | 0.28 |
| eGFR (CKiD19) | ||||
| BSL | 62.7±15.8 | 66.6±22.1 | −3.9±19.3 | 0.42 |
| Month 24 | 64.4±23.6 | 65.1±21.1 | −0.7±22.4 | 0.89 |
| Difference from BSLb | 1.7±22.0 | −1.4±29.9 | 3.2±26.4 | 0.63 |
Mean Diff, mean difference; BSL, baseline.
P values from two-sided independent t tests.
eGFR at month 24 minus eGFR at baseline.
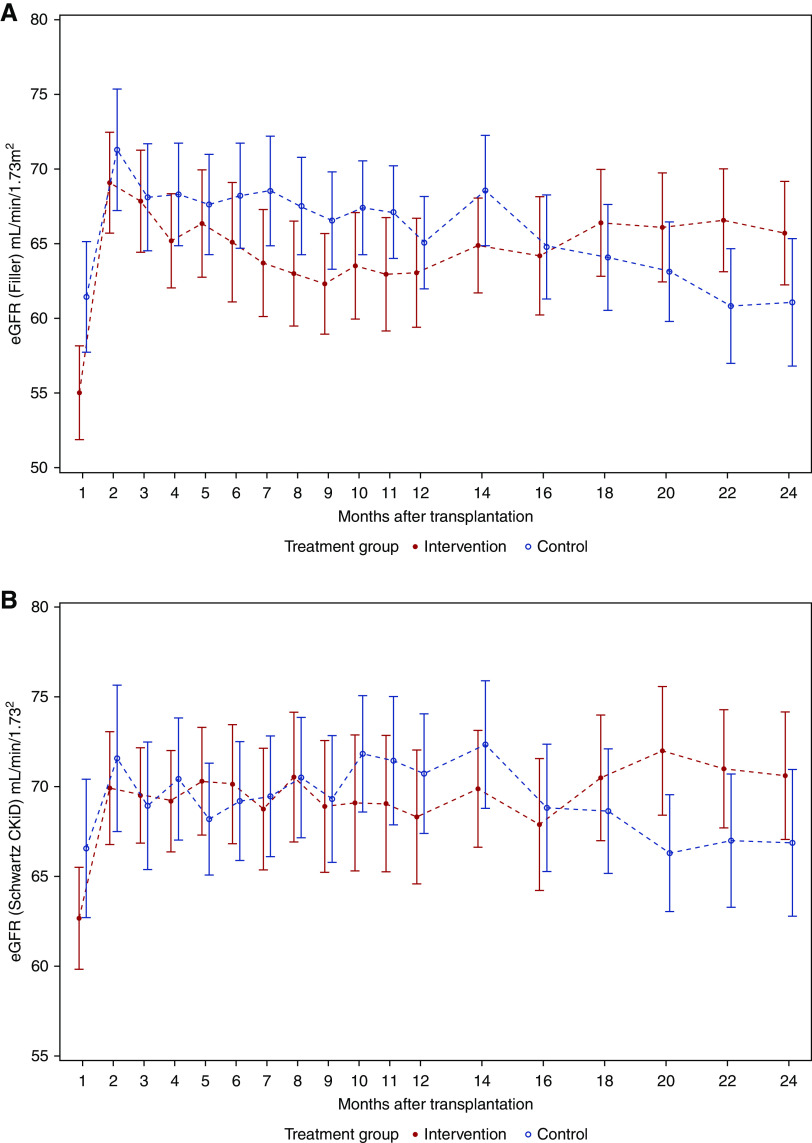
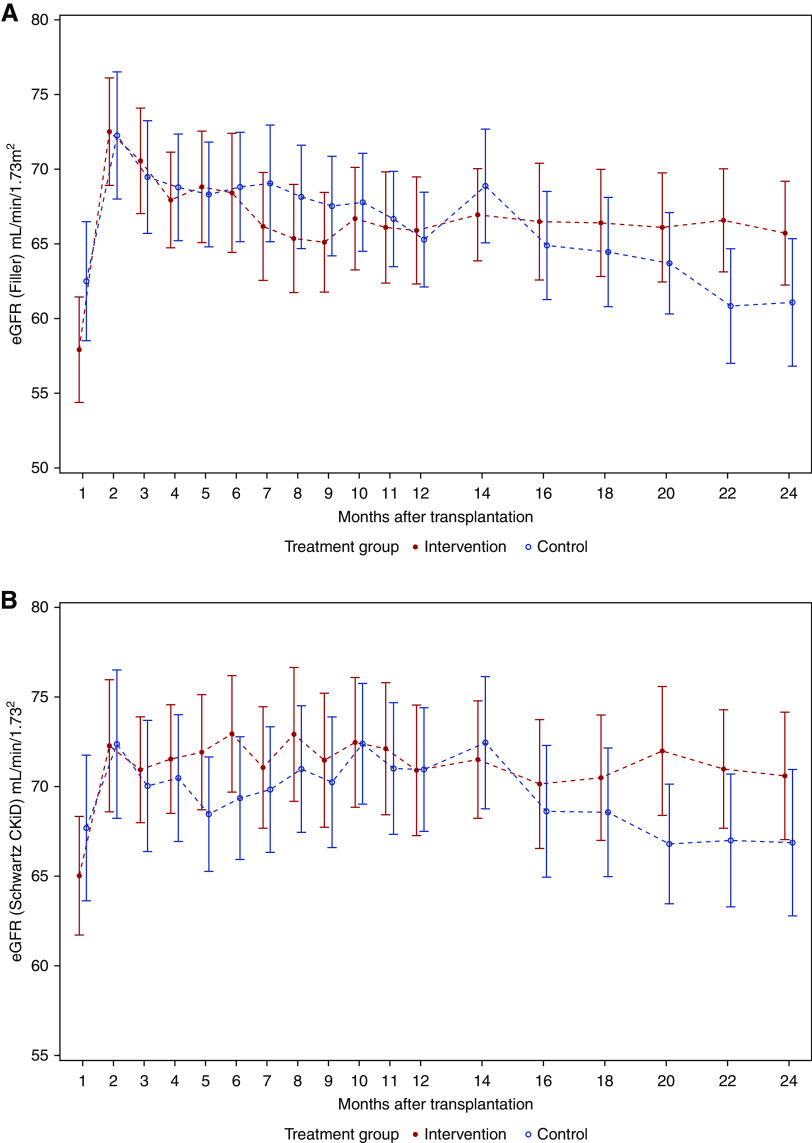
During the first post-transplant year, the eGFR curves ran nearly in parallel, whereas after month 14, we found an increasing divergence in favor of the intervention group (Figure 3). Changes in eGFR course of the ITT population were partly on the basis of dropout patients. For comparison, the course of eGFR in the per protocol population (population B) was additionally analyzed, showing that the eGFR was stable in the intervention group but decreasing in the control group during the second post-transplant year (Figure 4).
Figure 3.
In the first post-transplant year, the eGFR curves of the IIT population ran nearly in parallel whereas after month 14, an increasing divergence in favor of the intervention group was found. Mean error plots for eGFR in the ITT population (n=64) from month 1 to month 24 after transplantation defined by (A) Filler and coworkers18 and (B) Schwartz CKiD.19 All regular visits of all randomized patients (up to regular or premature study end) were included in the analyses. Missing values were not replaced.
Figure 4.
The course of eGFR in the per-protocol population was stable in the intervention group but decreasing in the control group during the second post-transplant year. Mean error plots for eGFR in the per protocol population (n=55) from month 1 to month 24 after transplantation defined by (A) Filler and coworkers18 and (B) Schwartz CKiD.19 All regular visits of all patients who completed the treatment regimen according to the study protocol were included in the analyses. Missing values were not replaced.
Secondary Analyses
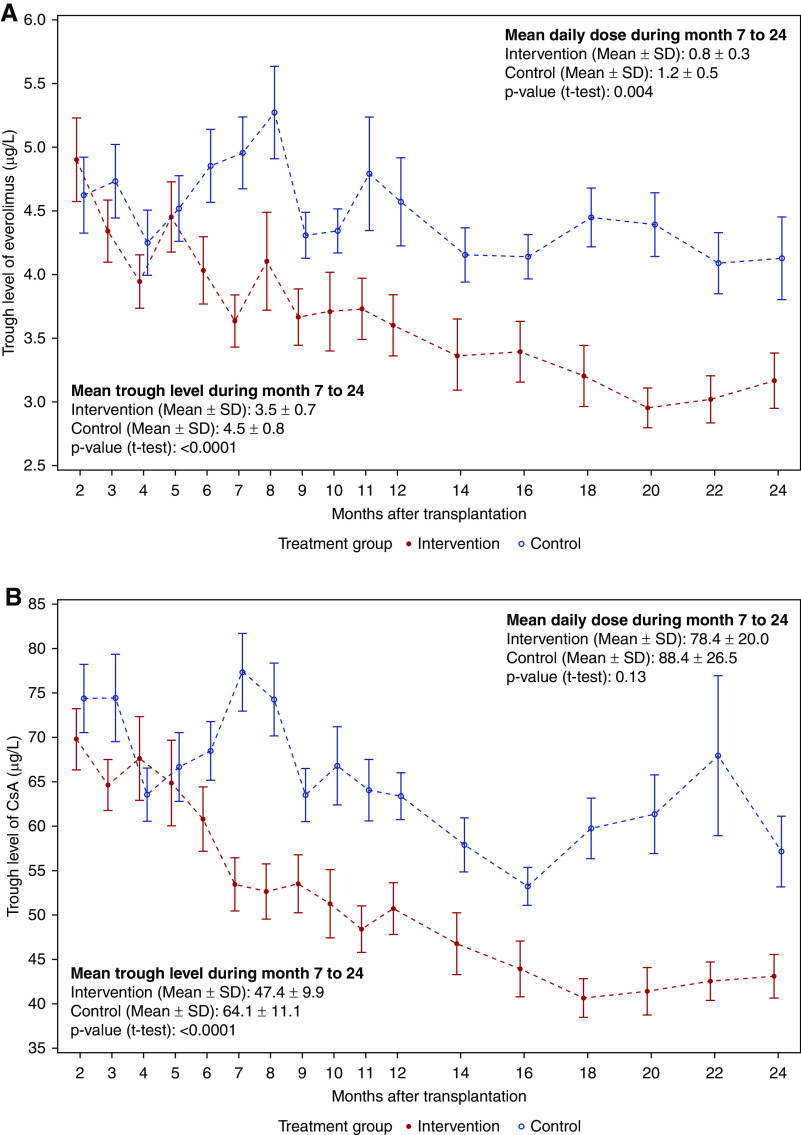
Analysis of dose and trough levels of study medication was performed in population B, including 55 patients who completed the treatment regimen according to the study protocol. In the intervention group, significantly lower trough levels (micrograms per liter) of everolimus (3.5±0.7 versus 4.5±0.8, t test, P<0.001) and CsA (47.4±9.9 versus 64.1±11.1, t test, P<0.001) were found during months 7–24 (Figure 5) as well as significantly lower mean daily doses (milligrams per meter squared) of everolimus (0.8±0.3 versus 1.2±0.5, t test, P=0.004) and numerically lower daily doses of CsA (78.4±20.0 versus 88.4±26.5, t test, P=0.13). In the intervention group, dose reductions of CsA and everolimus caused by Tvis levels were performed in 28 of 31 patients (median, four; range, zero to ten). Tvis-based dose increases were implemented in only two children. Additional granular information and analyses of the number and direction of immunosuppression dose adjustments made for both groups are given in Supplemental Table 1.
Figure 5.
Since month 6, trough levels of Everolimus and CsA were continiously lower in the intervention group compared to the control group. Mean error plots for trough levels of (A) everolimus and (B) CsA. All patients who completed the treatment regimen according to the study protocol were included in the analyses (n=55, per protocol population).
Two years after transplantation, more patients were free of glucocorticoid treatment in the intervention group compared with the control group (80% versus 53%, chi-squared test, P=0.04).
In total, 34 biopsies of cause were performed on 26 patients (14 biopsies on ten patients in the intervention group and 20 biopsies on 16 patients in the control group). In the intervention group, 11 acute rejection episodes (including borderline findings) were documented compared with 19 rejections in the control group. BANFF grades IIB and III only occurred in the control group (Table 4). During the first 2 years after transplantation, fewer patients in the intervention group developed biopsy-proven acute rejections (including borderline findings): 29% versus 49% (chi-squared test, P=0.11) (Figure 6, Table 4). In the first year after transplantation, acute rejection rates were 16% versus 36% (chi-squared test, P=0.07) including borderline findings and 13% versus 27% (chi-squared test, P=0.15) without borderline findings in the intervention group versus control group (Figure 6, Table 4).
Table 4.
Occurrence of viral events, donor-specific antibodies, and acute rejections
| Intervention, n=31 | Control, n=33 | Total, n=64 | P Value | |
|---|---|---|---|---|
| Viral events | ||||
| CMV DNA ≥1000 copies per mla | 2 (6.5%) | 2 (6.1%) | 4 (6.3%) | >0.99 |
| ADV DNA ≥1000 copies per mla | 1 (3.3%) | 1 (3.0%) | 2 (3.1%) | >0.99 |
| HSV DNA ≥1000 copies per ml | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | — |
| EBV DNA ≥1000 copies per ml | 7 (22.6%) | 14 (42.4%) | 21 (32.8%) | 0.09 |
| BKPyVANa | 2 (6.5%) | 1 (3.0%) | 3 (4.7%) | 0.61 |
| Donor-specific antibodies | 8 (25.8%) | 9 (27.3) | 17 (26.6%) | 0.89 |
| No. of patients with ≥1 acute rejection (proven by biopsy of cause) | ||||
| Within 12 mo after transplantation | ||||
| Including borderline | 5 (16.1%) | 12 (36.4%) | 17 (26.6%) | 0.07 |
| Without borderline | 4 (12.9%) | 9 (27.3%) | 13 (20.3%) | 0.15 |
| Within 24 mo after transplantation | ||||
| Including borderline | 9 (29.0%) | 16 (48.5%) | 25 (39.1%) | 0.11 |
| Without borderline | 8 (25.8%) | 11 (33.3%) | 19 (29.7%) | 0.51 |
| All biopsies of cause: type of acute rejection episodes | ||||
| Borderline | 1 | 7 | 8 | |
| Banff type IA | 3 | 2 | 5 | |
| Banff type IB | 4 | 7 | 11 | |
| Banff type IIA | 3 | 1 | 4 | |
| Banff type IIB | 0 | 1 | 1 | |
| Banff type III | 0 | 1 | 1 |
—, not applicable; BKPyVAN, BK polyomavirus–associated nephropathy.
P values from Fisher exact tests.
Figure 6.
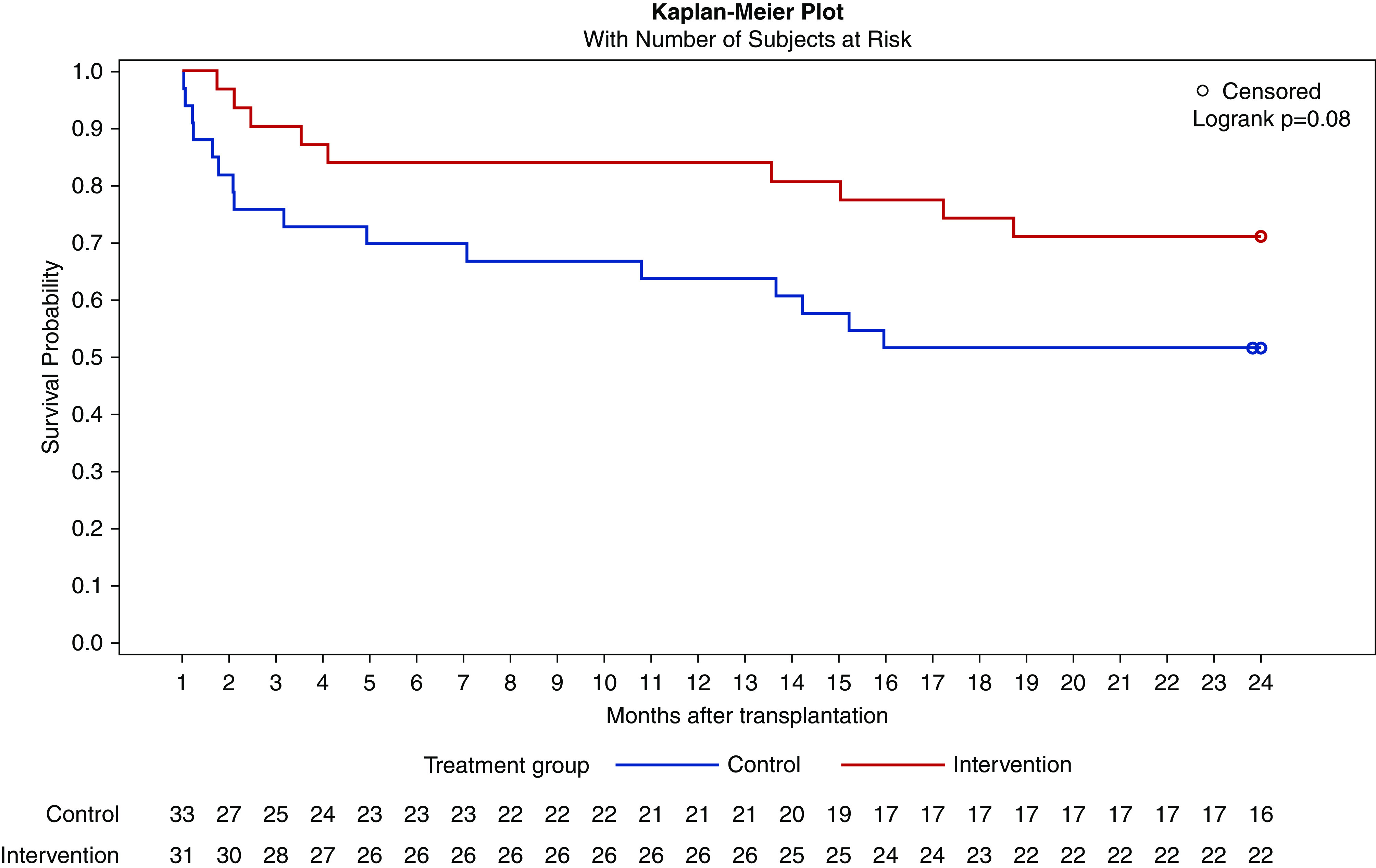
Kaplan–Meier curve for time to first acute rejection (including borderline findings) proven by biopsy of cause with number of subjects of risk. There is a tendency for a higher risk of acute rejections in the control group.
At month 6 after transplantation, a protocol biopsy was performed on 51 patients without substantial histologic differences between either group (Supplemental Table 2).
De novo donor-specific antibodies were detected in eight patients of the intervention and in nine patients of the control group by Luminex single-antigen assay (Table 4).
There were no differences between either treatment group for CMV DNAemia (>1000 copies per milliliter) and biopsy-proven BK polyomavirus-associated nephropathy, whereas EBV DNAemia (>1000 copies per milliliter) occurred in numerically more patients from the control group (42% versus 23%, chi-squared test, P=0.09), predominantly starting during the first 6 months after transplantation (Figure 7, Table 4). Post-transplant lymphoproliferative disease was not diagnosed in either group.
Figure 7.
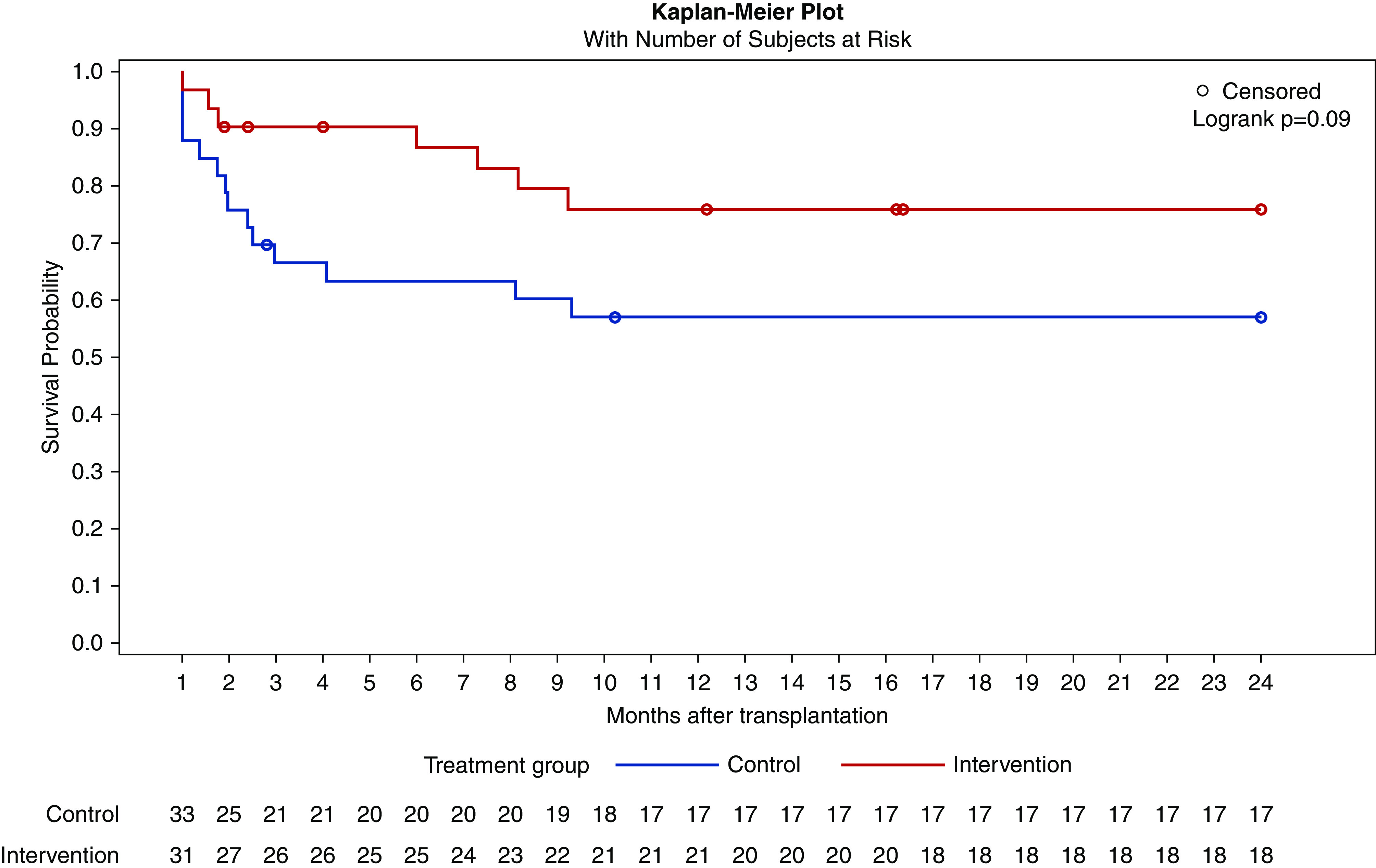
Kaplan–Meier curve for time to first EBV DNAemia with number of subjects of risk. EBV DNAemia occurred in numerically more patients from the control group, predominantly starting during the first 6 months after transplantation.
Safety Analyses
The overall numbers of AEs (947 versus 967) and SAEs (168 versus 157) of the intervention and control groups, respectively, were comparable (Supplemental Tables 3 and 4). No new safety signals were detected.
Discussion
With this randomized, controlled trial, we have demonstrated that additional steering of immunosuppressive therapy by individual Tvis levels personalizes pediatric post-transplant care and thereby, leads to reduced exposure to immunosuppressive drugs. These findings were combined with a numerically lower risk of EBV DNAemia and acute rejections (proven by biopsies of cause) and with stabilization of graft function without the detection of any additional safety signals. Graft function and rejection rate were comparable with other current and published pediatric kidney transplant studies.2,21
At the time of sample size calculation, it was assumed that there would be no significant difference between the two treatment groups with regard to the baseline eGFR at study start (4 weeks after transplantation), meaning that the baseline eGFR should be balanced between the two groups through randomization. If that had been true, it would have made no difference whether the absolute eGFR at month 24 or the change from baseline to month 24 was compared between the two groups. However, eGFR at study start was actually unbalanced between the two groups with the control group superior to the intervention group, although this difference was not statistically significant due to the limited sample size. Taking into account that the eGFR at study start would be a prognostic factor for the eGFR at follow-up, we thought the analysis of month 24 adjusted for month 1 would be more adequate from both the statistical and clinical aspects than to compare only eGFR at study end.
As prespecified in the study protocol, immunosuppressive therapy was reduced in the patients of the intervention group in case of low numbers of Tvis. This procedure resulted in a lower total exposure to CsA, everolimus, and glucocorticoids in the intervention group. Interestingly, the number of acute rejections did not increase despite reduced immunosuppressive therapy but was even numerically lower than in the control group and comparable with other current trials in pediatric kidney transplantation.2,21 Consequently, Tvis measurements allowed the identification of those patients with overimmunosuppression receiving more immunosuppressive therapy than needed to prevent rejections. With regard to the progressively increasing divergence of eGFR in favor of the intervention group, it could be hypothesized that reduced cumulative exposure to immunosuppressive drugs is associated with lower drug-specific side effects like nephrotoxicity, dyslipidemia, and arterial hypertension, thereby improving graft and possibly patient survival during long-term follow-up. The apparent increase of eGFR in the intervention group is at least partly caused by withdrawal of dropout patients, but the analysis of the per protocol population confirmed that the eGFR of the control group was decreasing, whereas eGFR in the intervention group was stable, an effect most probably caused by reduced exposure to immunosuppressive drugs. A 5-year follow-up of our patients is planned to determine if the long-term benefits of early reductions in immunosuppression exposure in the Tvis-guided group need more time to accrue than was allotted in this study.
Considering the general dilemma of optimal dosing of immunosuppressive therapy, post-transplant measurements of Tvis levels could be applied in other transplant cohorts, such as adults or recipients of other solid organs, to detect those patients who would profit from a reduction in immunosuppressive therapy without an increased risk of rejection.
The reduced use of immunosuppressants in our patients would also improve economics by reducing drug costs (25% for everolimus and 11% for CsA). Total yearly costs for prescriptions in Germany would decrease from a mean of €16.716 to €12.992 in a pediatric patient using the mean doses of immunosuppressants in this study. This reduction would only be counterbalanced by the costs of Tvis determination (€88 per measurement in our laboratory) of €352 in the case of four measurements per year per patient, which would probably be sufficient in routine care. If the method would theoretically be expanded to the 100,000 kidney recipients who are transplanted yearly worldwide with 15 years of organ survival, medication costs could globally be reduced by around €7.5 billion, taking into account the higher doses needed in adult recipients.
This is the first randomized, controlled trial using effect-related steering of immunosuppressive therapy. Other markers, such as the “ImmuKnow” assay22 or the measurement of Torque-Teno virus load,9 have been evaluated retrospectively in this regard with variable results, but they have never been used in a prospective trial. Previously, Tvis measurements have been shown to improve management of post-transplant viral infections (for example, in CMV10,23 or BK polyomavirus infection11,13,14), but the value as a diagnostic marker of overimmunosuppression has not been hitherto assessed.
Any pediatric cohort is at increased risk of viral infections and EBV-associated post-transplant lymphoproliferative disease compared with adult kidney transplant recipients due to its more frequent naïve status for EBV and other viruses.7 In our trial, no post-transplant lymphoproliferative disease was diagnosed, but the numerically higher number of patients with EBV DNAemia in the control group may be associated with an elevated risk. It is known that the risk for post-transplant lymphoproliferative disease is related more to donor-recipient EBV immune status than to absolute levels of EBV DNAemia, but regarding the comparable pretransplant EBV-IgG positivity in both treatment groups, the numerically higher incidence of EBV DNAemia in the control group could favor the development of EBV-associated complications. Because of the very low incidence of CMV DNAemia and BK polyomavirus–associated nephropathy in our trial, no difference between the groups could be detected. Therefore, reduction of symptomatic post-transplant CMV infections, which was one of the secondary end points, could not be achieved. This might be on the basis of the fact that mammalian target of rapamycin inhibitor–based immunosuppression, such as everolimus (which was used in our trial), leads to lower numbers of viral complications.24–26 However, it might be speculated that, especially in patients receiving standard immunosuppression with tacrolimus and mycophenolate mofetil, the general risk for viral complications, primarily by CMV, EBV, and BK polyomavirus, could be reduced by Tvis-based steering of immunosuppressive therapy, resulting in lower exposure to immunosuppressants.
Overall, the safety of the additional Tvis-based steering of immunosuppressive therapy was in line with earlier studies in pediatric kidney transplantation using mammalian target of rapamycin inhibitors,16,21,27 and no new or unexpected safety signals were identified. The number of de novo donor-specific antibodies was comparable with the 3-year data of the most recent trial in pediatric kidney transplantation.28 The number of study discontinuations was very low in both treatment groups, which can be interpreted as a sign that this therapy regimen is highly practicable outside the study setting.
The experiences and results from our trial provide pilot data for further clinical investigations in the area of immune assay–guided tailoring of immunosuppression. It provides a rationale for taking the results forward in larger trials, probably in adult kidney recipients with the aim of identifying overimmunosuppressed patients so as to reduce immunosuppressive medication. A longer study period (e.g., 3 years) could lead to more significant results, as the IVIST trial showed an increasing divergence of eGFR in the second post-transplant year. In a future larger randomized, controlled trial, with a focus on prevention of overimmunosuppression, it would be sufficient to measure Tvis levels followed by adoption of immunosuppression less frequently (i.e., every 3 months). Using our Tvis-based steering for a standard immunosuppressive regimen consisting of tacrolimus and mycophenolate mofetil, which is associated with higher rates of viral infections, could help to get clear answers to the question of whether Tvis-guided reduction of immunosuppressive therapy can reduce the burden of viral diseases. In a larger adult population, it would also be more feasible to see a possible positive influence on the incidence of post-transplant malignancy.
Our study is limited by the relatively low number of patients. The final analysis showed that the trial was underpowered to ultimately demonstrate the primary hypothesis despite appropriate power calculation in the study plan due to higher variability of the results as expected. However, even international trials in pediatric transplantation do not provide much higher patient numbers, and we extended the inclusion time for as long as was feasible. Especially in pediatric patients, long-term graft survival as well as avoidance of infectious and drug-based complications is crucial for psychomotoric development, quality of life, and socioeconomic conditions, taking into account the lifelong burden of immunosuppressive therapy. In our trial, only one immunosuppressive regimen, consisting of a calcineurin inhibitor, an antiproliferative drug, and low-dose glucocorticoids, was used. We cannot prove that similar results could be achieved with other immunosuppressive regimens. However, the same strategy had already been successfully implemented in routine use in our center with an immunosuppressive regimen consisting of tacrolimus and mycophenolate mofetil.
In conclusion, additional steering of immunosuppressive therapy by Tvis levels in pediatric kidney transplant recipients is safe, personalizes dosing of immunosuppressants by reducing exposure to immunosuppressive therapy, and thereby, economizes transplant medicine. It results in similar outcomes compared with standard trough level–based steering of immunosuppressive therapy.
Disclosures
T. Ahlenstiel-Grunow received travel grants from Alexion, Astellas, and Novartis; received lecture fees from Novartis; and reports other interests/relationships with Deutsche Gesellschaft für Nephrologie, Deutsche Gesellschaft für Kinder- und Jugendmedizin, Deutsche Transplantationsgesellschaft, Gesellschaft für Pädiatrische Nephrologie, and International Pediatric Transplant Association. J. Oh received research support from Chiesi; lecture fees from Alexion and Chiesi; advisory fees from Alexion, Alynylam, Amgen, Boehringer Ingelheim, Chiesi, Horizon, Neovii, Novartis, Recordati, and Sanofi; and travel grants from Neovii and is scientific advisor to or has membership with Pediatric Nephrology. L. Pape reports receiving research support from Chiesi and Novartis and lecture fees from Alexion, Chiesi, and Novartis, as well as travel grants from Neovii. C. Taylan reports honoraria from Sanofi 670 Euro. L.T. Weber reports receiving research support from Chiesi; receiving lecture fees from Novartis; receiving advisory fees by Alexion; receiving travel grants from Astellas; receiving honoraria from Alexion Pharmaceuticals, Alnylam Pharmaceuticals, Chiesi GmbH, Orphan Europe, and Pfizer Pharma GmbH; scientific advisor or membership as Der Nephrologe Member of Alexion advisory board “aHUS,” a member of Chiesi advisory board “Cystinosis,” with Clinical Nephrology, as Treasurer of the German Society for Pediatric Nephrology, with Monatsschrift Kinderheilkunde, as an Editorial Board member for Pediatric Nephrology; and other interests/relationships with Chrokokids, Deutsche Gesellschaft für Nephrologie, Deutsche Transplantationsgesellschaft, European Society of Pediatric Nephrology/International Pediatric Nephrology Association, Gesellschaft für Pädiatrische Nephrologie, International Pediatric Transplant Association, and Nephrokids. All remaining authors have nothing to disclose.
Funding
This study was supported by Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research of Germany) grant 01EO0802 and unrestricted grants from Novartis.
Supplementary Material
Acknowledgments
This study could not have been carried out without the dedicated work of our colleagues Dr. Lena Brunkhorst, Dr. Jens Drube, Dr. Doris Franke, Dr. Kerstin Froede, Dr. Michaela Gessner, Dr. Imke Hennies, Dr. Nele Kirsten Kanzelmeyer, Dr. Martin Kreuzer, Dr. Ulrike Mayer, and Dr. Sarah Wente-Schulz (patient aquisition); student Carolin Klages (data analysis); our biologist Nadja Borsum; and technicians Ina Ruhl and Beate Eberle (technical assistance).
The funders had no influence on the design or conduct of the trial and were not involved in data collection or analysis, in the writing of the manuscript, or in the decision to submit it for publication.
T. Ahlenstiel-Grunow, A. Großhennig, and L. Pape designed the trial; T. Ahlenstiel-Grunow and L. Pape obtained research funding; J. Oh, R. Schild, H. Staude, C. Taylan, and L.T. Weber included patients in the trial; R. Sabau and C. Schröder were in charge of pharmacovigilance; A. Großhennig and X. Liu performed statistical analyses; M. Verboom evaluated data on donor-specific antibodies; T. Ahlenstiel-Grunow, A. Großhennig, X. Liu, and L. Pape wrote the first draft of this paper, which was critically revised by all coauthors; and all authors read and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050645/-/DCSupplemental.
Supplemental Table 1. Analysis of the Tvis-based and non–Tvis-based dose adjustments of immunosuppressants.
Supplemental Table 2. Results of protocol biopsy.
Supplemental Table 3. Summary of (serious) adverse events.
Supplemental Table 4. System organ classes of serious adverse events.
References
- 1.McDonald SP, Craig JC; Australian and New Zealand Paediatric Nephrology Association: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Grenda R, Watson A, Trompeter R, Tönshoff B, Jaray J, Fitzpatrick M, et al.: A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: The TWIST study. Am J Transplant 10: 828–836, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Franke D, Thomas L, Steffens R, Pavičić L, Gellermann J, Froede K, et al.: Patterns of growth after kidney transplantation among children with ESRD. Clin J Am Soc Nephrol 10: 127–134, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Zheng Y, Liu L, Fu Q, Li J, Huang Q, et al.: Steroid avoidance or withdrawal regimens in paediatric kidney transplantation: A meta-analysis of randomised controlled trials. PLoS One 11: e0146523, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsampalieros A, Knoll GA, Molnar AO, Fergusson N, Fergusson DA: Corticosteroid use and growth after pediatric solid organ transplantation: A systematic review and meta-analysis. Transplantation 101: 694–703, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schober T, Framke T, Kreipe H, Schulz TF, Großhennig A, Hussein K, et al.: Characteristics of early and late PTLD development in pediatric solid organ transplant recipients. Transplantation 95: 240–246, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Naicker D, Reed PW, Ronaldson J, Kara T, Wong W, Prestidge C: Nationwide conversion to generic tacrolimus in pediatric kidney transplant recipients. Pediatr Nephrol 32: 2125–2131, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Rezahosseini O, Drabe CH, Sørensen SS, Rasmussen A, Perch M, Ostrowski SR, et al.: Torque-Teno virus viral load as a potential endogenous marker of immune function in solid organ transplantation. Transplant Rev (Orlando) 33: 137–144, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Sester M, Leboeuf C, Schmidt T, Hirsch HH: The “ABC” of virus-specific T cell immunity in solid organ transplantation. Am J Transplant 16: 1697–1706, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Ahlenstiel-Grunow T, Pape L: Diagnostics, treatment, and immune response in BK polyomavirus infection after pediatric kidney transplantation. Pediatr Nephrol 35: 375–382, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Ahlenstiel-Grunow T, Pape L: Immunosuppression, BK polyomavirus infections, and BK polyomavirus-specific T cells after pediatric kidney transplantation. Pediatr Nephrol 35: 625–631, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Ahlenstiel-Grunow T, Sester M, Sester U, Hirsch HH, Pape L: BK polyomavirus-specific T cells as a diagnostic and prognostic marker for BK polyomavirus infections after pediatric kidney transplantation. Transplantation 104: 2393–2402, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Ahlenstiel-Grunow T, Pape L: Virus-specific T cells in pediatric renal transplantation [published online ahead of print March 27, 2020]. Pediatr Nephrol 10.1007/s00467-020-04522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlenstiel-Grunow T, Koch A, Großhennig A, Frömke C, Sester M, Sester U, et al.: A multicenter, randomized, open-labeled study to steer immunosuppressive and antiviral therapy by measurement of virus (CMV, ADV, HSV)-specific T cells in addition to determination of trough levels of immunosuppressants in pediatric kidney allograft recipients (IVIST01-trial): Study protocol for a randomized controlled trial. Trials 15: 324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pape L, Offner G, Kreuzer M, Froede K, Drube J, Kanzelmeyer N, et al.: De novo therapy with everolimus, low-dose ciclosporine A, basiliximab and steroid elimination in pediatric kidney transplantation. Am J Transplant 10: 2349–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Sester M, Sester U, Gärtner B, Heine G, Girndt M, Mueller-Lantzsch N, et al.: Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation 71: 1287–1294, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Sharma AP, Yasin A, Garg AX, Filler G: Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol 6: 1599–1608, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al.: Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82: 445–453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todt M, Rothämel T, Klintschar M, Pape L: Death of a 5-year-old boy in the bath. Arterial hypertension and left ventricular hypertrophy in steroid-resistant nephrotic syndrome. Rechtsmedizin 23: 472–476, 2013 [Google Scholar]
- 21.Tönshoff B, Ettenger R, Dello Strologo L, Marks SD, Pape L, Tedesco-Silva H Jr., et al.: Early conversion of pediatric kidney transplant patients to everolimus with reduced tacrolimus and steroid elimination: Results of a randomized trial. Am J Transplant 19: 811–822, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Liu X, Lu P, Han Z, Tao J, Wang J, et al.: Performance of the ImmuKnow assay in differentiating infection and acute rejection after kidney transplantation: A meta-analysis. Transplant Proc 46: 3343–3351, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al.; The Transplantation Society International CMV Consensus Group: The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 102: 900–931, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Höcker B, Schneble L, Murer L, Carraro A, Pape L, Kranz B, et al.: Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: An international CERTAIN registry study. Transplantation 103: 1224–1233, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Höcker B, Zencke S, Pape L, Krupka K, Köster L, Fichtner A, et al.: Impact of everolimus and low-dose cyclosporin on cytomegalovirus replication and disease in pediatric renal transplantation. Am J Transplant 16: 921–929, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Berger SP, Sommerer C, Witzke O, Tedesco H, Chadban S, Mulgaonkar S, et al.; TRANSFORM investigators: Two-year outcomes in de novo renal transplant recipients receiving everolimus-facilitated calcineurin inhibitor reduction regimen from the TRANSFORM study. Am J Transplant 19: 3018–3034, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Pape L, Ahlenstiel T: mTOR inhibitors in pediatric kidney transplantation. Pediatr Nephrol 29: 1119–1129, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Tonshoff B, Tedesco H, Ettenger R, Christian M, Bjerre A, Dello Strologo L, et al.: Three-year outcomes from the CRADLE study in de novo pediatric kidney transplant recipients receiving everolimus with reduced tacrolimus and early steroid withdrawal [published online ahead of print May 14, 2020]. Am J Transplant 10.1111/ajt.16005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.