Significance Statement
Exposure to an air pollutant, fine particulate matter (PM2.5), is an important environmental risk factor for cardiopulmonary disease. However, minimal data exist on how exposure to high PM2.5 levels, such as in areas of mainland China, may affect CKD risk. In their analysis of data from a large survey of Chinese adults, the authors demonstrate significant associations between long-term exposure to high ambient levels of PM2.5 and an increased risk of CKD prevalence and albuminuria. These associations were significantly stronger in urban areas, among males, among individuals <65 years, and among those with comorbidities. The study’s findings offer insight for target population protection and provide evidence for policy makers and public health practices to reduce the CKD risk associated with ambient PM2.5 pollution.
Keywords: chronic kidney disease, prevalence, ambient PM2.5, long-term exposure
Visual Abstract
Abstract
Background
Fine particulate matter (PM2.5) is an important environmental risk factor for cardiopulmonary diseases. However, the association between PM2.5 and risk of CKD remains under-recognized, especially in regions with high levels of PM2.5, such as China.
Methods
To explore the association between long-term exposure to ambient PM2.5 and CKD prevalence in China, we used data from the China National Survey of CKD, which included a representative sample of 47,204 adults. We estimated annual exposure to PM2.5 before the survey date at each participant’s address, using a validated, satellite-based, spatiotemporal model with a 10 km×10 km resolution. Participants with eGFR <60 ml/min per 1.73 m2 or albuminuria were defined as having CKD. We used a logistic regression model to estimate the association and analyzed the influence of potential modifiers.
Results
The 2-year mean PM2.5 concentration was 57.4 μg/m3, with a range from 31.3 to 87.5 μg/m3. An increase of 10 μg/m3 in PM2.5 was positively associated with CKD prevalence (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.22 to 1.35) and albuminuria (OR, 1.39; 95% CI, 1.32 to 1.47). Effect modification indicated these associations were significantly stronger in urban areas compared with rural areas, in males compared with females, in participants aged <65 years compared with participants aged ≥65 years, and in participants without comorbid diseases compared with those with comorbidities.
Conclusions
These findings regarding the relationship between long-term exposure to high ambient PM2.5 levels and CKD in the general Chinese population provide important evidence for policy makers and public health practices to reduce the CKD risk posed by this pollutant.
Ambient fine particulate matter (PM2.5) pollution has emerged as a major environmental and public-health concern worldwide, with staggering levels of attributable morbidity and mortality demonstrated by numerous epidemiologic studies.1 However, most studies have focused on the health effects for the cardiovascular and respiratory system, and there is far less evidence for the effects on kidney disease.
CKD represents a serious, global, public-health challenge. The Global Burden of Disease Study 2017 estimated that all-age deaths from CKD increased by 33.7% in 2017 compared with 2007, and ranked as the 16th leading cause of death worldwide.2
The most advanced stage of CKD, ESKD, requires costly dialysis or transplantation and results in reduced quality of life and enormous socioeconomic burden. Furthermore, CKD is regarded as an important risk factor for cardiovascular disease (CVD).3,4 It has been reported that the cardiovascular mortality rate is much higher in patients with CKD than in those with normal kidney function.
Ambient PM2.5 is suggested to be a novel potential environmental risk factor for CKD.5 However, studies on the relationships between ambient PM2.5 exposure and risk of CKD are still limited,6 and almost all of the evidence comes from countries or regions with low levels of PM2.5.7–15 The only available evidence is limited to the elderly, such as US veterans,7,8 those enrolled in the US Medicare program (aged ≥65 years), or cohorts9–12 or populations in a single city or region,5,10,11,15 where PM2.5 levels are much lower than those in mainland China. Minimal data exist on the association between PM2.5 and the risk of CKD in areas with high levels of PM2.5, and epidemiologic studies in areas with high PM2.5 pollution should be a research priority.16
Considering ambient PM2.5 pollution is an environmental issue worldwide, especially in China, where high levels of PM2.5 remain a tremendous challenge,17 and CKD prevalence has rapidly increased in recent years,18 the associations between long-term ambient PM2.5 exposure and the risk of CKD in the general Chinese population were investigated in this study. This study used a representative sample from the China National Survey of CKD,19 and used a validated, satellite-based spatiotemporal model for estimation of long-term ambient PM2.5 exposure.20 Furthermore, the relationships between ambient PM2.5 exposure and albuminuria were also explored, because albuminuria is a well-established kidney damage indicator that reflects the high risk of progression or poor prognosis of CKD.21,22 The results of this study will contribute to evidence of the relationship between high levels of ambient PM2.5 and CKD risk in the general population.
Methods
Study Population and Health Measurements
A multistage, stratified, sampling method was used to obtain a representative sample of the general population aged ≥18 years in China. In the China National Survey of CKD from September 2009 to September 2010, 13 provinces from different regions were selected by probability-proportional-to-size sampling.
A questionnaire was distributed to the participants to collect information, including urban or rural area residence, demographic status (e.g., age, sex, family income, and education), personal and family health history (e.g., hypertension, diabetes, and kidney disease), lifestyle factors (e.g., smoking and alcohol consumption), comorbidities (e.g., diabetes mellitus or hypertension), history of CVD (including myocardial infarction or stroke), nephrotoxic medications, and medical insurance. Anthropometric measurements, including weight and height, were obtained by the study investigators and staff members using standard instruments.
The eGFR was measured from venipuncture blood samples, and the eGFR was calculated using an equation developed by adapting the Modification of Diet in Renal Disease equation on the basis of data from Chinese patients with CKD.23 Albuminuria was measured with immunoturbidimetric tests. The urinary albumin-creatinine ratio (milligrams per gram creatinine) was calculated. Participants with a urinary albumin-creatinine ratio ≥30 mg/g were defined as having albuminuria. Participants with an eGFR <60 ml/min per 1.73 m2 or albuminuria were defined as having CKD, according to the 2012 Clinical Practice Guideline for the Evaluation and Management of CKD.24
A training program was provided for all study investigators, and a strict quality-control procedure was performed during the whole process. The details of the survey have been described elsewhere.19 The ethics committee of Peking University First Hospital approved the study. All the participants provided written informed consent before data collection.
Ambient PM2.5 Exposure Assessment
Annual exposure to PM2.5 before the survey date was assessed at each participant’s address, which was geocoded into latitude and longitude data. The participants in the survey were defined as residents who lived in the investigated region for at least 1 year. Considering the survey design in this study and the main exposure metric used in a previous study,13 the annual average PM2.5 concentration before the survey date and the annual average for the previous year (2-year mean PM2.5) was calculated as the primary exposure variable in our main analysis. A validated, spatiotemporal model that combined ground-level PM2.5 monitoring data measured in China and satellite-derived data were used to estimate ambient PM2.5 concentrations.20
Daily, average, ground PM2.5 concentrations in 2013 in China were obtained from the China Environmental Monitoring Center. Satellite, remote-sensing, aerosol optical depth (AOD) data retrieved by the Moderate Resolution Imaging Spectroradiometer (http://modis.gsfc.nasa.gov), which was launched by the US National Aeronautics and Space Administration, were used in this study.
A 0.1°×0.1° grid (approximately 10 km×10 km) was created for data integration and model development. Ground PM2.5 data from multiple monitors in each grid cell were averaged. The percentage of forest cover and urban areas in each grid cell and the daily total counts for Moderate Resolution Imaging Spectroradiometer fire spots for each grid cell, using a 75-km radius buffer, were calculated. Finally, all of the variables in 2013 were matched by grid cell and day of year for model fitting.
A two-stage statistical model was developed to calibrate the spatiotemporal relationships between PM2.5 and AOD.
The first-stage, linear, mixed-effects model included day-specific random intercepts, slopes for AOD, and season-specific random slopes for meteorologic variables. The dependent variable in the first-stage model was the daily PM2.5 concentration.
The second-stage, generalized, additive model was constructed as follows:
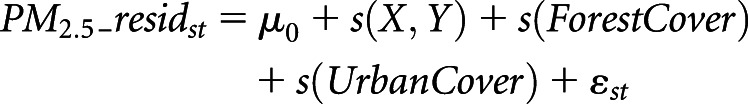 |
In this equation, PM2.5_residst is the residual from the first-stage model in grid cell s on day t; μ0 is the intercept term; s(X, Y) is the smooth term of the coordinates of the centroid of grid cell s; s(ForestCover) and s(UrbanCover) are the smooth functions of the percentage of forest cover and urban areas for grid cell s, respectively; and εst is the error term.
The historical daily ambient PM2.5 concentrations (2005–2010) in China were estimated using the two-stage model developed on the basis of 2013 data, assuming that the daily relationship between PM2.5 and AOD was constant for the same day of year in each year. The full-model fitting R2 value of the historical PM2.5 predictions on monthly and seasonal scales were 0.73 and 0.79, respectively,20 suggesting that PM2.5 predictions are accurate representations of the ground measurements, with relatively low bias, and can serve as reasonable exposure estimates to study the health effects of long-term PM2.5 exposure in China. This study used the predicted PM2.5 concentrations from a study by Ma et al.20 in exactly the same study period and study area. This model was validated to minimize bias in assessment of long-term PM2.5 concentrations.
Statistical Analyses
We used a logistic regression model to estimate the associations between increases in the annual average PM2.5 exposure and the dichotomous outcomes of CKD and albuminuria, with adjustment for covariates in the following equation:
In this model, Yi is the status of CKD or albuminuria (yes or no), b0 is the intercept, x1–xp are the covariates, b1–bp are the coefficients of the covariates, β is the coefficient (log odds ratio [OR]) of PM2.5. Model covariates included urban or rural area, age (as a continuous variable), sex (male or female), body mass index (BMI; as a continuous variable), current smoker (yes or no), alcohol consumption (never, one to three times a month to three to five times a week, almost once a day), education (≥ high-school education or < high-school education), family income index (low income, middle income, and high income), history of CVD (yes or no), diabetes mellitus (yes or no), hypertension (yes or no), nephrotoxic medications (yes or no), and medical insurance (yes or no). Study province was adjusted as a factor. The OR and 95% confidence interval were calculated to estimate the associations between ambient PM2.5 exposure and the prevalence of CKD and albuminuria.
Spline functions were widely used to characterize the exposure-response curves of the estimate effects of air pollution in environmental epidemiologic studies,25 and they can flexibly capture the linear or nonlinear relationship between environmental exposure and health outcomes. Thus, the linear variable PM2.5 was replaced by cubic regression spline functions of PM2.5 to explore the possible curve shape between long-term PM2.5 exposure and the prevalence of CKD and albuminuria in our analysis.
Analysis of the within- and between-city analysis was performed using a logistic regression model for the associations of CKD prevalence and albuminuria with PM2.5 exposure. For PM2.5 exposure, the site average PM2.5 and individual PM2.5 exposure minus the site mean PM2.5 were used as between-site and within-site exposure variables, respectively.26
In addition, models were stratified by urban or rural area residence, sex (male or female), age (≥65 years and <65 years), current smoker (yes or no), and in participants with a normal weight (BMI<25 kg/m2) or those who were overweight (BMI≥25 kg/m2). The influences of comorbidities, including diabetes mellitus, hypertension, and CVD history, were also estimated. Effect modification analysis was performed by adding an interaction terms between PM2.5 and the testing variable in the full model.10
Sensitivity Analysis
Sensitivity analyses were performed to examine whether the results were robust to changes in the parameters, including using the 1-year mean PM2.5 as an exposure assessment and controlling for different covariates in the models, such as adding the medical insurance status (yes or no) as a potential confounder in the model.
All of the analyses were performed using R software (version 3.1.2; R Core Team), and statistical significance was defined as a two-sided P value of <0.05. A Bonferroni-corrected P value of 0.006 was used as the significance threshold for the eight interaction analyses.
Results
In total, 55,550 study participants aged ≥18 years in China were invited to participate in the China National Survey of CKD, and 47,204 participants who completed the questionnaire and health examination were enrolled in the study, with a response rate of 93% (Supplemental Figure 1).
The 2-year mean PM2.5 concentration, on the basis of AOD data, was 57.4 μg/m3 (with an SD of 15.6 μg/m3) at participants’ addresses, with a range from 31.3 to 87.5 μg/m3 (Figure 1). The study sites were distributed in both urban and rural areas (Supplemental Figure 2), and the 2-year mean PM2.5 concentration was 58.3 μg/m3 (SD of 17.5 μg/m3) in urban areas, which was higher than the mean concentration of 53.9 μg/m3 (SD of 12.0 μg/m3) in rural areas. The percentage of study participants in each PM2.5 level was shown in Supplemental Table 1. Table 1 shows the baseline characteristics of the study population, stratified by the 2-year mean PM2.5 concentration.
Figure 1.

The spatial distribution of the AOD-derived, 2-year mean PM2.5 concentration show obvious difference in the study region in China between 2007 and 2010. AOD, aerosol optical depth.
Table 1.
Baseline characteristics of the study population stratified above and below 2-yr mean PM2.5 concentrations in China between 2007 and 2010
| Characteristic | 2-Yr Mean PM2.5 | |
|---|---|---|
| ≤2-Yr Mean Concentration | >2-Yr Mean Concentration | |
| Number of participants (%) | 25,188 (53.36) | 22,016 (46.64) |
| CKD, n (%) | ||
| Yes | 3205 (12.72) | 3108 (14.12) |
| eGFR (mL/min per 1.73 m2), mean±SD | 101.04±26.69 | 101.40±28.28 |
| eGFR category, n (%) | ||
| ≥60 ml/min per 1.73 m2 | 24,563 (97.52) | 21,456 (97.46) |
| <60 ml/min per 1.73 m2 | 625 (2.48) | 560 (2.54) |
| uACR (mg/g), mean (range) | 6.67 (3.24–13.58) | 6.60 (2.91–13.77) |
| uACR category, n (%) | ||
| <30 mg/g | 22,430 (89.05) | 19,302 (87.67) |
| ≥30 mg/g | 2758 (10.95) | 2714 (12.33) |
| Age (yr), mean±SD | 49.71±15.54 | 49.47±14.83 |
| Sex, n (%) | ||
| Male | 10,445 (41.47) | 9703 (44.07) |
| Female | 14,743 (58.53) | 12,313 (55.93) |
| Education, n (%) | ||
| < High-school education | 14,372 (57.24) | 11,763 (53.53) |
| ≥ High-school education | 10,737 (42.76) | 10,213 (46.47) |
| Family income, n (%) | ||
| Low income | 6388 (36.02) | 5843 (26.57) |
| Middle income | 10,324 (58.21) | 12,860 (58.47) |
| High income | 1022 (5.77) | 3290 (14.96) |
| Region, n (%) | ||
| Urban | 13,629 (54.11) | 11,716 (53.22) |
| Rural | 11,559 (45.89) | 10,300 (46.78) |
| BMI (kg/m2), mean±SD | 23.86±3.66 | 23.91±3.72 |
| BMI category, n (%) | ||
| <25 kg/m2 | 17,048 (68.41) | 13,370 (60.73) |
| ≥25 kg/m2 | 7871 (31.59) | 8582 (38.98) |
| Smoking, n (%) | ||
| Noncurrent smokers | 19,748 (78.4) | 16,362 (74.32) |
| Current smokers | 5440 (21.6) | 5654 (25.68) |
| Alcohol consumption, n (%) | ||
| Never | 19,728 (78.32) | 16,046 (72.88) |
| One to three times a month to three to five times a week | 3641 (14.46) | 4235 (19.24) |
| Almost once a day | 1819 (7.22) | 1735 (7.88) |
| History of CVD, n (%) | ||
| Yes | 641 (2.90) | 579 (2.86) |
| Diabetes mellitus, n (%) | ||
| Yes | 1752 (6.96) | 1736 (7.89) |
| Hypertension, n (%) | ||
| Yes | 8364 (33.45) | 8240 (37.52) |
| Nephrotoxic medication, n (%) | ||
| Yes | 915 (3.63) | 621 (2.82) |
There are missing values for some of the variables (numbers that do not add up to the total because of missing values), and the percentage of each category was calculated excluding the missing data. uACR, urinary albumin creatinine ratio.
An increase of 10 μg/m3 in the 2-year mean ambient PM2.5 was positively associated with the prevalence of CKD, with an OR of 1.28 (95% CI, 1.22 to 1.35). In addition, a significant association was also found for albuminuria, with an OR of 1.39 (95% CI, 1.32 to 1.47). The results were robust to changes in parameters in the models (Table 2).
Table 2.
Estimated effects of an increase of 10 μg/m3 in 2-yr mean PM2.5 exposure on CKD and albuminuria in China between 2007 and 2010
| Models | CKD | Albuminuria | ||||
|---|---|---|---|---|---|---|
| N | OR (95% CI) | P Value | N | OR (95% CI) | P Value | |
| Model 1a | 47,204 | 1.28 (1.22 to 1.35) | <0.001 | 47,204 | 1.39 (1.32 to 1.47) | <0.001 |
| Model 2b | 47,204 | 1.29 (1.23 to 1.26) | <0.001 | 47,204 | 1.40 (1.32 to 1.48) | <0.001 |
Model 1: age, sex, education, family income, and urban/rural areas were adjusted in the model.
Model 2: model 1 plus health-related factors, including BMI, smoking history, alcohol consumption, history of CVD, diabetes mellitus, hypertension, and nephrotoxic medications were adjusted in the model.
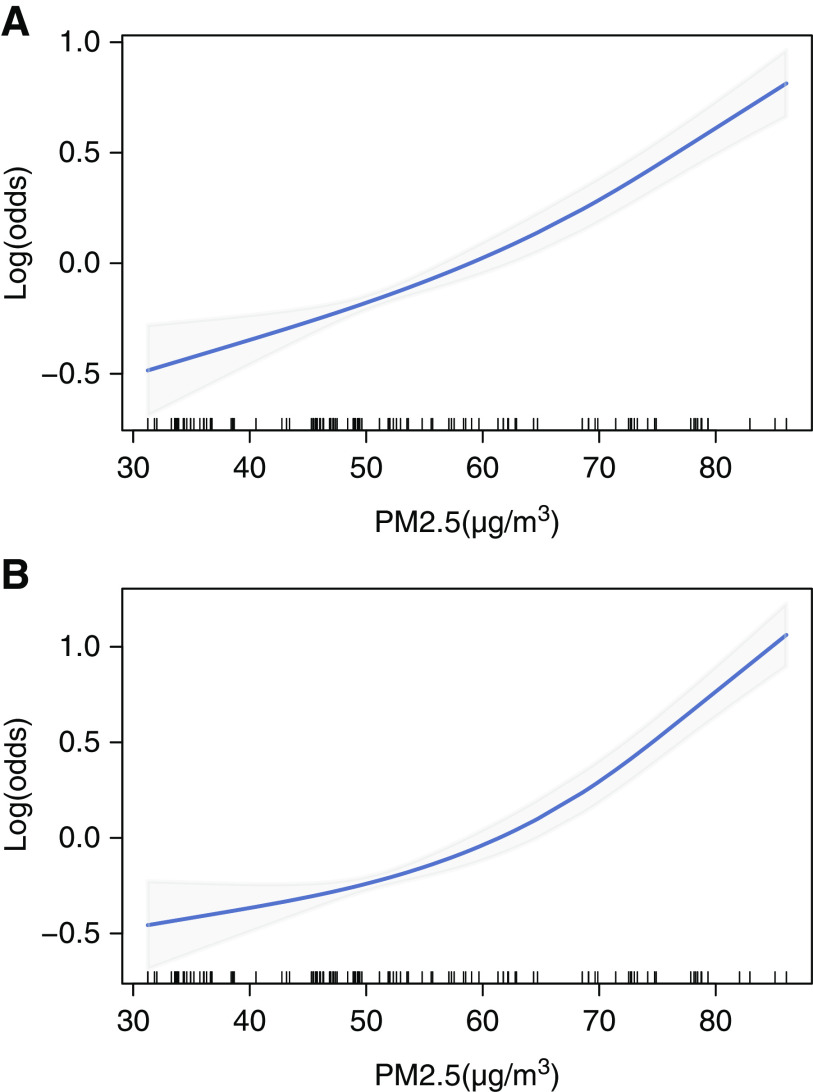
There were increasing trends in concentration-response curves for long-term PM2.5 exposure and the prevalence of CKD and albuminuria, and the curves suggest a generally positive, linear relationship between PM2.5 exposure and CKD risk (Figure 2). Furthermore, the risk started to increase at a relatively low concentration of PM2.5 that was well below the grade-2 criteria set by the Chinese ambient air-quality standards (35 μg/m3).
Figure 2.
The linear exposure-response curves of ambient PM2.5 exposure and the prevalence of CKD and albuminuria in China between 2007 and 2010. The PM2.5 exposure level was calculated as the 2-year mean concentration. (A) CKD; (B) albuminuria.
The difference in the PM2.5 effects on CKD prevalence between urban and rural areas was analyzed, and a significantly higher effect was found in the urban areas (OR, 1.27; 95% CI, 1.21 to 1.34) than in rural areas (OR, 1.17; 95% CI, 1.07 to 1.27) (interaction P=0.004).
Effect modification of ambient PM2.5 exposure and CKD prevalence showed that the estimated effects were significantly higher in males (OR, 1.34; 95% CI, 1.26 to 1.41) than in females (OR, 1.27; 95% CI, 1.20 to 1.34) (interaction P=0.005), and significantly higher in people <65 years (OR, 1.34; 95% CI, 1.27 to 1.41) than in older people (OR, 1.17; 95% CI, 1.11 to 1.25) (interaction P<0.001).
Furthermore, the association was stronger in participants without diabetes than in participants with diabetes. Specifically, the multivariable-adjusted OR was 1.31 (95% CI, 1.25 to 1.38) in participants without diabetes, which was higher than that of 1.20 (95% CI, 1.12 to 1.28) in participants with diabetes (interaction P=0.002). The associations also showed a stronger trend in participants without hypertension or CVD history than in those with hypertension or CVD history, although no statistical significance was found.
The results for the associations between ambient PM2.5 exposure and albuminuria showed the same trend as that for between ambient PM2.5 and the prevalence of CKD, except the association was significantly higher in participants without CVD history (OR, 1.40; 95% CI, 1.32 to 1.48) than in those with CVD history (OR, 1.29; 95% CI, 1.20 to 1.39) (interaction P=0.001) (Table 3).
Table 3.
The associations between long-term PM2.5 exposure and CKD and albuminuria in participants with different characteristics in China between 2007 and 2010
| Characteristic | Number of Eligible Participants | CKD | Albuminuria | ||
|---|---|---|---|---|---|
| OR (95% CI) | Interaction P Value | OR (95% CI) | Interaction P Value | ||
| Region | |||||
| Urban | 25,345 | 1.27 (1.21 to 1.34) | 0.004 | 1.37 (1.29 to 1.45) | 0.002 |
| Rural | 21,859 | 1.17 (1.07 to 1.27) | 1.23 (1.12 to 1.36) | ||
| Sex | |||||
| Male | 20,148 | 1.34 (1.26 to 1.41) | 0.005 | 1.45 (1.37 to 1.54) | 0.001 |
| Female | 27,056 | 1.27 (1.20 to 1.34) | 1.36 (1.29 to 1.44) | ||
| Age | |||||
| ≥65 yr | 7915 | 1.17 (1.11 to 1.25) | <0.001 | 1.25 (1.17 to 1.34) | <0.001 |
| <65 yr | 39,289 | 1.34 (1.27 to 1.41) | 1.44 (1.36 to 1.53) | ||
| BMI | |||||
| ≥25 kg/m2 | 16,453 | 1.28 (1.21 to 1.35) | 0.30 | 1.39 (1.31 to 1.47) | 0.49 |
| <25 kg/m2 | 30,418 | 1.31 (1.24 to 1.38) | 1.41 (1.33 to 1.49) | ||
| Smoking status | |||||
| Noncurrent smoker | 36,110 | 1.28 (1.22 to 1.35) | 0.14 | 1.39 (1.31 to 1.47) | 0.19 |
| Current smoker | 11,094 | 1.33 (1.25 to 1.41) | 1.43 (1.34 to 1.53) | ||
| Diabetes mellitus | |||||
| No | 43,671 | 1.31 (1.25 to 1.38) | 0.002 | 1.38 (1.31 to 1.46) | 0.11 |
| Yes | 3488 | 1.20 (1.12 to 1.28) | 1.32 (1.23 to 1.42) | ||
| Hypertension | |||||
| No | 30,357 | 1.30 (1.24 to 1.36) | 0.03 | 1.42 (1.34 to 1.51) | 0.02 |
| Yes | 16,604 | 1.25 (1.18 to 1.31) | 1.36 (1.28 to 1.45) | ||
| History of CVD | |||||
| No | 41,114 | 1.30 (1.23 to 1.36) | 0.03 | 1.40 (1.32 to 1.48) | 0.001 |
| Yes | 1220 | 1.23 (1.16 to 1.32) | 1.29 (1.20 to 1.39) | ||
Model 2 adjustment (as illustrated in the Table 2 footnote) was used for the stratified analyses. The 2-yr mean PM2.5 concentrations were used in the models. The analysis was performed for an increase of 10 μg/m3 in PM2.5.
The within-city effects were significant, whereas the between-city effects were nonsignificant (Supplemental Table 2), suggesting no significant differences in the magnitude of risk within major metropolitan areas were found in our study. Sensitivity analyses showed the results were robust to adjustment for potential confounders in the model (Supplemental Table 3), and the association of the 1-year mean PM2.5 with the prevalence of CKD and albuminuria showed the same trend as the 2-year mean PM2.5 (Supplemental Figure 3, Supplemental Tables 4 and 5).
Discussion
We found that long-term exposure to ambient PM2.5 was associated with an increased risk of CKD and albuminuria in the general Chinese population. The results were robust after adjustment for multiple covariates and individual-level risk factors, including age, sex, BMI, smoking, alcohol consumption, education, family income, urban or rural area residence, comorbidities (including diabetes mellitus, hypertension, and CVD history), nephrotoxic medication use, and medical insurance status. Although the effect estimates of ambient PM2.5 were modest compared with other traditional risk factors, such as CVD, considering its ubiquitous existence, PM2.5 could have a substantial effect on CKD risk in the general population. Furthermore, increasing trends in exposure-response curves were found between PM2.5 and CKD prevalence and albuminuria, with the risk increasing at PM2.5 concentrations below the current PM2.5 standards in China. These results may lead to a greater impetus to encourage public-health efforts to offer greater protection to the general population in lowering the risk of CKD associated with ambient PM2.5 exposure, and provides evidence for stricter air quality control of ambient PM2.5.
In this study, we used the estimated ground-level PM2.5 from satellite-retrieved AOD data, which is a promising and new method that has rapidly advanced in recent years.27,28 Satellite-based, spatiotemporal models have the potential to fill the spatiotemporal PM2.5 gaps left by ground monitors with high-quality predictions, and the model applied in our study was validated to provide reliable historical PM2.5 concentration estimates in China.20 The 2-year mean PM2.5 concentration was 57.4 μg/m3, which was relatively higher than that in previous studies, and had a broad range from 31.3 to 87.5 μg/m3.
Current literature on ambient PM2.5 exposure and CKD remain relatively limited. The available studies have mainly been conducted in countries or regions with low levels of PM2.5, and the results have been inconsistent.7–15 For example, a positive association was observed between county-level PM2.5 concentrations and CKD diagnoses in adults ≥65 years old enrolled in the US Medicare program, with an adjusted prevalence ratio of 1.03 (95% CI, 1.02 to 1.05) for an increase of 4 μg/m3 in PM2.5.9 Another study demonstrated that an increase of 10 μg/m3 in the annual average PM2.5 was associated with an increased incidence of CKD in United States veterans with a hazard ratio of 1.27 (95% CI, 1.17 to 1.38).8 Each increase of 1 μg/m3 in the annual average PM2.5 concentration was associated with a significantly higher risk of incident CKD in a community-based cohort in the United States (hazard ratio, 1.05; 95% CI, 1.01 to 1.10).10 Nevertheless, a study conducted in elderly residents in Taipei city found an interquartile range increase in PM2.5 was not significantly associated with CKD prevalence, with an OR of 1.01 (95% CI, 0.96 to 1.06).5
The effect estimation of the OR between long-term exposure to ambient PM2.5 and CKD prevalence was 1.28 (95% CI, 1.22 to 1.35) for every increase of 10 μg/m3 in PM2.5 in our study, suggesting the association of CKD risk with PM2.5 may vary geographically.29 However, the results may not be directly comparable, because the average concentrations of ambient PM2.5 in the aforementioned studies (ranging from 5.0 to 27.1 μg/m3) were much lower than the average level of PM2.5 in our study (57.4 μg/m3). Thus, our results add to the current evidence on the associations between high levels of PM2.5 exposure and the increased risk of CKD prevalence in the general population. In addition, we found a significant association between PM2.5 and the prevalence of albuminuria, which is a well-established indicator of kidney damage in the prognosis of CKD.21,22 Nevertheless, a previous study did not find an association between chronic PM2.5 exposure and albuminuria, which was probably due to the low mean PM2.5 (16.5 μg/m3).11
There are plausible biologic mechanisms linking PM2.5 exposure and increased CKD risk. The kidney is a vascularized organ, susceptible to vascular dysfunction.30 Experimental evidence has shown that exposure to diesel exhaust particles, which are a major source for urban ambient PM2.5, exacerbated vascular damage in rats31 and facilitated progression to tubular damage.32 In addition, diesel exhaust particles also induced nephrotoxicity in vitro and in vivo through autophagy, endoplasmic-reticulum stress, and apoptosis in kidney tissues.33 Furthermore, various chemical components in PM2.534 could have adverse consequences on renal function through inflammation,35 oxidative stress,36 and endothelial dysfunction,37 thus contributing to progressive, cumulative renal injury and increased risk of CKD over a long time.38,39 Nevertheless, research on renal toxicity of ambient PM2.5 exposure remains limited, and the underlying mechanisms need to be further elucidated.
The association of PM2.5 exposure with CKD risk was significantly higher in urban areas than in rural areas. Previous studies also found that higher urbanicity may lead to an additional increase in the health risk associated with particulate matter exposure.40,41 The following factors might be responsible for this disparity. First, the average concentration of PM2.5 was higher in urban areas than in rural areas. Second, source and compositional differences have been reported between rural and urban PM2.5.42,43 For instance, PM2.5 in urban areas has larger contributions from traffic emission and coal combustion, whereas PM2.5 in rural areas has larger contributions from biomass burning and road dust.43,44 Different sources lead to disparity in the PM2.5 composition, and these sources might contribute to the urban and rural disparity in CKD risk associated with PM2.5 exposure. The findings suggest the potential PM2.5 effects on CKD risk may differ between urban and rural areas, which needs to be further explored in future studies.
The results in our study showed the association between PM2.5 exposure and the prevalence of CKD and albuminuria was higher in males than in females, which was possibly due to more outdoor time in males (236 min/d) than in females (209 min/d) and could result in longer exposure to ambient PM2.5 in males in China,45 thus increasing the adverse influence of ambient PM2.5 exposure.
Furthermore, the associations were significantly stronger in the young (<65 years) than in the elderly (≥65 years). The effects of PM2.5 on CKD prevalence were significantly higher in participants without diabetes than in those with diabetes, whereas the effects on albuminuria were significantly higher in participants without CVD history than in those with CVD history. These results were consistent with the results of some previous studies. For instance, Mehta et al.7 also reported that the inverse association between PM2.5 and renal function, as reflected by eGFR, was significantly stronger in participants without diabetes than in participants with diabetes, and was stronger in participants without coronary heart disease than in those with coronary heart disease. In our study, individuals who were elderly or had comorbidities were more likely to take medications, such as antihypertensive drugs and angiotensin receptor blockers. The medication-use proportions were 4.82%, 3.91%, and 8.20% in the elderly, those with diabetes, and those with CVD history, respectively, which were relatively higher than the proportions of 2.94%, 3.20%, and 2.96%, respectively, in the young, those without diabetes, and those without CVD history. Medication use may attenuate the oxidative stress and vasoconstrictive effects of PM2.5 exposure,46 and the age or comorbidity status may dominate the main effect on renal dysfunction, thus reducing their susceptibility to the adverse renal effects of PM2.5. If the relationship between PM2.5 and CKD risk is truly independent, it would manifest more strongly in groups with lower baseline risk, such as younger individuals and individuals without diabetes or CVD history.47 Nevertheless, further studies to elucidate the susceptibility of the population to kidney disease related to ambient PM2.5 exposure are warranted.
To the best of our knowledge, this is the first study to comprehensively evaluate long-term high levels of ambient PM2.5 exposure on CKD risk in Chinese adults, on the basis of the China National Survey of CKD, using a standard, multistage, stratified sampling method and strict quality-control procedures. The response rate of the participants was 93% in this study. This rate was relatively high compared with previous studies.5,14 Thus, this analysis could provide convincing results and could be extrapolated to the general Chinese population. In addition, a major strength of this study is that it was a nationwide survey in a developing country with high levels of ambient PM2.5 and, thus, provided us with a unique opportunity to explore the exposure-response relationship between high levels of ambient PM2.5 exposure and CKD prevalence and albuminuria. Our results will contribute to evidence of a relationship between high levels of PM2.5 exposure and the risk of CKD. Third, this study revealed a significantly higher effect in urban areas than in rural areas, which provides insights for target PM2.5 pollution control. Furthermore, the use of a validated, spatiotemporal model to assess PM2.5, and the adjustment for multiple covariates and individual-level risk factors, strengthened the credibility of the results in our study.
Our study also had several limitations. First, causal inferences between long-term ambient PM2.5 exposure and the prevalence of CKD could not be made because this study was cross-sectional. Second, single measurements of indicators were used to define CKD, which may have led to misclassification of CKD due to acute kidney disease or other diseases. Nevertheless, repeated measurements were poorly feasible in such a large-scale, national survey. Third, we did not evaluate the effects of gaseous pollutants. Nevertheless, ambient PM2.5 and gaseous pollutants are generally highly correlated with each other, and the lack of data on gaseous pollutants is unlikely to affect our conclusion. In addition, considering that PM2.5 is a heterogeneous mixture,48 the integration of information about the relative distribution of major PM2.5 components into health risk assessments of CKD needs to be investigated in future studies. This study did not account for some potentially important confounders, including indoor air pollution and second-hand smoke, due to data availability. Furthermore, the relatively low resolution of the model of a 10-km grid in our study would make the exposure assessment nondifferential, probably leading to an underestimation of the potential effects.49
In conclusion, this study assessed the long-term effects of high levels of ambient PM2.5 on CKD risk in the general Chinese population and revealed a significant association between ambient PM2.5 and the prevalence of CKD and albuminuria. A significantly higher effect was found in urban areas than in rural areas. In addition, the risk started to increase at PM2.5 concentrations well below the Chinese ambient air quality standards, suggesting that air quality control should be more stringent in China. Furthermore, the association was stronger in younger participants than in older participants, and in participants without comorbidities than in those with comorbidities, providing novel insights for target population protection. These findings offer important evidence to inform policy makers and public-health practices in lowering the risk of CKD associated with exposure to ambient PM2.5 pollution.
Disclosures
J. Wang reports being a member of a speakers bureau for Boehringer-Ingelheim. L. Zhang reports being a scientific advisor or member of the American Journal of Kidney Disease and receiving research funding from AstraZeneca. M. Zhao reports being an executive member of the Asian Pacific Society of Nephrology; receiving honoraria from the Asian Pacific Society of Nephrology, Chinese Medical Association, Chinese Society of Nephrology, and International Society of Nephrology; being the vice president of the Chinese Society of Internal Medicine and the Chinese Society of Nephrology; and having consultancy agreements with Roche. All remaining authors have nothing to disclose.
Funding
This study was supported by National Natural Science Foundation of China grants 91846101, 81771938, 81301296, 81502780, and 81900665; Beijing Nova Programme Interdisciplinary Cooperation Project grant Z191100001119008; the Ministry of Science and Technology of the People’s Republic of China grants 2016YFC1305400, 2017YFC0211600, and 2019YFC2005000 (under the National Key R&D Program); Chinese Academy of Medical Sciences (CAMS) grants 2019RU023 (under the Research Unit) and 2019-I2M-5-046 (under the CAMS Innovation Fund for Medical Sciences); and Peking University grants BMU20160466, BMU2018JI012, and BMU2019JI005 (under the University of Michigan Health System–Peking University Health Science Center Joint Institute for Translational and Clinical Research), Baidu Fund 2019BD017, BMU2018MX020, PKU2017LCX05, and PKU2019LCXQ008.
Supplementary Material
Acknowledgments
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Dr. Luxia Zhang, Dr. Shaowei Wu, Dr. Minghui Zhao, and Dr. Xinbiao Guo conceptualized the study; Dr. Jinwei Wang and Dr. Yang Liu were responsible for data curation; Dr. Guoxing Li was responsible for formal analysis; Dr. Luxia Zhang, Dr. Shaowei Wu, and Dr. Jing Huang were responsible for funding acquisition; Dr. Minghui Zhao was responsible for investigation; Dr. Guoxing Li and Dr. Shaowei Wu were responsible for methodology; Dr. Guoxing Li was responsible for software; Dr. Luxia Zhang and Dr. Shaowei Wu provided supervision; Dr. Guoxing Li, Dr. Jing Huang, and Dr. Jinwei Wang were responsible for visualization; Dr. Jing Huang and Dr. Jinwei Wang wrote the original draft; and Dr. Luxia Zhang, Dr. Shaowei Wu, and Dr. Guoxing Li reviewed and edited the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Road Ahead for Research on Air Pollution and Kidney Disease,” on pages 260–262.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040517/-/DCSupplemental.
Supplemental Table 1. The percentage of study participants in each PM2.5 level in China between 2007 and 2010.
Supplemental Table 2. Within-city effects and between-city effects of ambient PM2.5 exposure on CKD and albuminuria in China between 2007 and 2010.
Supplemental Table 3. Estimated effects of an increase of 10 μg/m3 in 2-year mean PM2.5 exposure on CKD and albuminuria in China between 2007 and 2010 when controlling for different potential confounders.
Supplemental Table 4. Estimated effects of an increase of 10 μg/m3 in 1-year mean PM2.5 exposure on CKD and albuminuria in China between 2007 and 2010.
Supplemental Table 5. The associations between 1-year mean PM2.5 exposure on CKD and albuminuria in participants with different characteristics in China between 2007 and 2010.
Supplemental Figure 1. CONSORT diagram of study participants recruitment in the analysis.
Supplemental Figure 2. Distribution of the study sites in both urban and rural areas. Note: Triangle indicates the urban sites, and circular indicates the rural sites.
Supplemental Figure 3. Exposure-response curves of 1-year mean PM2.5 exposure and the prevalence of chronic kidney disease (A) and albuminuria (B) in China between 2007 and 2010.
References
- 1.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al.: Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med 381: 705–715, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators: Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet 393: e44, 2019 10.1016/S0140-6736(19)31049-9]. Lancet 392: 1736–1788, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al.: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Webster AC, Nagler EV, Morton RL, Masson P: Chronic kidney disease. Lancet 389: 1238–1252, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Chen SY, Chu DC, Lee JH, Yang YR, Chan CC: Traffic-related air pollution associated with chronic kidney disease among elderly residents in Taipei City. Environ Pollut 234: 838–845, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Ran J, Yang A, Sun S, Han L, Li J, Guo F, et al.: Long-term exposure to ambient fine particulate matter and mortality from renal failure: A retrospective cohort study in Hong Kong, China. Am J Epidemiol 189: 602–612, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Mehta AJ, Zanobetti A, Bind MA, Kloog I, Koutrakis P, Sparrow D, et al.: Long-term exposure to ambient fine particulate matter and renal function in older men: The Veterans Administration Normative Aging Study. Environ Health Perspect 124: 1353–1360, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z: Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 29: 218–230, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bragg-Gresham J, Morgenstern H, McClellan W, Saydah S, Pavkov M, Williams D, et al.; Centers for Disease Control and Prevention CKD Surveillance System: County-level air quality and the prevalence of diagnosed chronic kidney disease in the US Medicare population. PLoS One 13: e0200612, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum MF, Surapaneni A, Stewart JD, Liao D, Yanosky JD, Whitsel EA, et al.: Particulate matter and albuminuria, glomerular filtration rate, and incident CKD. Clin J Am Soc Nephrol 15: 311–319, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neill MS, Diez-Roux AV, Auchincloss AH, Franklin TG, Jacobs DR Jr., Astor BC, et al.: Airborne particulate matter exposure and urinary albumin excretion: The Multi-Ethnic Study of Atherosclerosis. Occup Environ Med 65: 534–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver AM, Wang Y, Wellenius GA, Young B, Boyle LD, Hickson DA, et al.: Long-term exposure to ambient air pollution and renal function in African Americans: The Jackson Heart Study. J Expo Sci Environ Epidemiol 29: 548–556, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan TC, Zhang Z, Lin BC, Lin C, Deng HB, Chuang YC, et al.: Long-term exposure to ambient fine particulate matter and chronic kidney disease: A cohort study. Environ Health Perspect 126: 107002, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YR, Chen YM, Chen SY, Chan CC: Associations between long-term particulate matter exposure and adult renal function in the Taipei metropolis. Environ Health Perspect 125: 602–607, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SY, Ju SW, Lin CL, Hsu WH, Lin CC, Ting IW, et al.: Air pollutants and subsequent risk of chronic kidney disease and end-stage renal disease: A population-based cohort study. Environ Pollut 261: 114154, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Shaffer RM, Sellers SP, Baker MG, de Buen Kalman R, Frostad J, Suter MK, et al.: Improving and expanding estimates of the global burden of disease due to environmental health risk factors. Environ Health Perspect 127: 105001, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Pan X, Guo X, Li G: Health impact of China’s air pollution prevention and control action plan: An analysis of national air quality monitoring and mortality data. Lancet Planet Health 2: e313–e323, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Romanowski K, Clark EG, Levin A, Cook VJ, Johnston JC: Tuberculosis and chronic kidney disease: An emerging global syndemic. Kidney Int 90: 34–40, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al.: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Ma Z, Hu X, Sayer AM, Levy R, Zhang Q, Xue Y, et al.: Satellite-based spatiotemporal trends in PM2.5 concentrations: China, 2004– 2013. Environ Health Perspect 124: 184–192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al.; Chronic Kidney Disease Prognosis Consortium: Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorriz JL, Martinez-Castelao A: Proteinuria: Detection and role in native renal disease progression. Transplant Rev (Orlando) 26: 3–13, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al.: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Available at: https://kdigo.org/guidelines/ckd-evaluation-and-management/. Accessed November 4, 2020 [Google Scholar]
- 25.Samoli E, Analitis A, Touloumi G, Schwartz J, Anderson HR, Sunyer J, et al.: Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect 113: 88–95, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al.: Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356: 447–458, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Waller LA, Lyapustin A, Wang Y, Liu Y: 10-year spatial and temporal trends of PM2.5 concentrations in the southeastern US estimated using high-resolution satellite data. Atmos Chem Phys 14: 6301–6314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian L, Zeng Q, Dong WT, Guo Q, Wu ZT, Pan XC, et al.: Addressing the source contribution of PM2.5 on mortality: An evaluation study of its impacts on excess mortality in China. Environ Res Lett 12: 104016, 2017 [Google Scholar]
- 29.Bowe B, Artimovich E, Xie Y, Yan Y, Cai M, Al-Aly Z: The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: A modelling study. BMJ Glob Health 5: e002063, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lue SH, Wellenius GA, Wilker EH, Mostofsky E, Mittleman MA: Residential proximity to major roadways and renal function. J Epidemiol Community Health 67: 629–634, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Suleimani YM, Al Mahruqi AS, Al Za’abi M, Shalaby A, Ashique M, Nemmar A, et al.: Effect of diesel exhaust particles on renal vascular responses in rats with chronic kidney disease. Environ Toxicol 32: 541–549, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Yan YH, Chou CC-K, Wang JS, Tung CL, Li YR, Lo K, et al.: Subchronic effects of inhaled ambient particulate matter on glucose homeostasis and target organ damage in a type 1 diabetic rat model. Toxicol Appl Pharmacol 281: 211–220, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Hsu YH, Chuang HC, Lee YH, Lin YF, Chen YJ, Hsiao TC, et al.: Traffic-related particulate matter exposure induces nephrotoxicity in vitro and in vivo. Free Radic Biol Med 135: 235–244, 2019 [DOI] [PubMed] [Google Scholar]
- 34.Huang RJ, Zhang Y, Bozzetti C, Ho KF, Cao JJ, Han Y, et al.: High secondary aerosol contribution to particulate pollution during haze events in China. Nature 514: 218–222, 2014 [DOI] [PubMed] [Google Scholar]
- 35.He M, Ichinose T, Ito T, Toriba A, Yoshida S, Kaori S, et al.: Investigation of inflammation inducing substances in PM2.5 particles by an elimination method using thermal decomposition. Environ Toxicol 34: 1137–1148, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Xu MX, Ge CX, Qin YT, Gu TT, Lou DS, Li Q, et al.: Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic Biol Med 130: 542–556, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Wilker EH, Ljungman PL, Rice MB, Kloog I, Schwartz J, Gold DR, et al.: Relation of long-term exposure to air pollution to brachial artery flow-mediated dilation and reactive hyperemia. Am J Cardiol 113: 2057–2063, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataria A, Trasande L, Trachtman H: The effects of environmental chemicals on renal function. Nat Rev Nephrol 11: 610–625, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perticone F, Maio R, Perticone M, Sciacqua A, Shehaj E, Naccarato P, et al.: Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation 122: 379–384, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Bravo MA, Ebisu K, Dominici F, Wang Y, Peng RD, Bell ML: Airborne fine particles and risk of hospital admissions for understudied populations: Effects by urbanicity and short-term cumulative exposures in 708 U.S. counties. Environ Health Perspect 125: 594–601, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng RD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, et al.: Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA 299: 2172–2179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cazier F, Genevray P, Dewaele D, Nouali H, Verdin A, Ledoux F, et al.: Characterisation and seasonal variations of particles in the atmosphere of rural, urban and industrial areas: Organic compounds. J Environ Sci (China) 44: 45–56, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Deng F, Wei H, Huang J, Wang X, Hao Y, et al.: Association of cardiopulmonary health effects with source-appointed ambient fine particulate in Beijing, China: A combined analysis from the Healthy Volunteer Natural Relocation (HVNR) study. Environ Sci Technol 48: 3438–3448, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Yang W, Xie S, Zhang Z, Hu J, Zhang L, Lei X, et al.: Characteristics and sources of carbonaceous aerosol across urban and rural sites in a rapidly urbanized but low-level industrialized city in the Sichuan Basin, China. Environ Sci Pollut Res Int 26: 26646–26663, 2019 [DOI] [PubMed] [Google Scholar]
- 45.Ministry of Environmental Protection of the People’s Republic of China: Exposure Factors Handbook of Chinese Population, Beijing, China, China Environmental Science Press, 2013, pp 270 [Google Scholar]
- 46.Ghelfi E, Wellenius GA, Lawrence J, Millet E, Gonzalez-Flecha B: Cardiac oxidative stress and dysfunction by fine concentrated ambient particles (CAPs) are mediated by angiotensin-II. Inhal Toxicol 22: 963–972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanderWeele TJ, Knol MJ: Interpretation of subgroup analyses in randomized trials: Heterogeneity versus secondary interventions. Ann Intern Med 154: 680–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Zhang Z, van Donkelaar A, Bai L, Martin RV, Lavigne E, et al.: Understanding the joint impacts of fine particulate matter concentration and composition on the incidence and mortality of cardiovascular disease: A component-adjusted approach. Environ Sci Technol 54: 4388–4399, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Guo Y, Li G, Zhang Y, Westerdahl D, Jin X, et al.: Spatiotemporal analysis for the effect of ambient particulate matter on cause-specific respiratory mortality in Beijing, China. Environ Sci Pollut Res Int 23: 10946–10956, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.