Significance Statement
Although proximal tubular secretion is the primary mechanism of kidney drug elimination, kidney drug dosing strategies are on the basis of eGFR. In a study of 54 participants with or without CKD, measurements of tubular secretory clearance of endogenous secretory solutes predicted the elimination of avidly secreted medications, furosemide and penciclovir. However, GFR’s predictive accuracy (measured by iohexol clearance) resembled that of the secretory solute clearances for predicting furosemide and penciclovir clearance, and the addition of secretory solute clearances yielded only small improvements in predictive accuracy. These findings demonstrate that secretory clearance measurements predict kidney drug elimination but also suggest a tight linkage between GFR and tubular secretory clearance in stable outpatients. This provides some reassurance that GFR is a useful surrogate for secretory drug clearance for drug dosing in such individuals.
Keywords: kidney medication clearance, glomerular filtration rate, secretory solutes clearances, furosemide, penciclovir
Visual Abstract
Abstract
Background
Although proximal tubular secretion is the primary mechanism of kidney drug elimination, current kidney drug dosing strategies are on the basis of eGFR.
Methods
In a dedicated pharmacokinetic study to compare GFR with tubular secretory clearance for predicting kidney drug elimination, we evaluated stable outpatients with eGFRs ranging from 21 to 140 ml/min per 1.73 m2. After administering single doses of furosemide and famciclovir (metabolized to penciclovir), we calculated their kidney clearances on the basis of sequential plasma and timed urine measurements. Concomitantly, we quantified eight endogenous secretory solutes in plasma and urine using liquid chromatography-tandem mass spectrometry and measured GFR by iohexol clearance (iGFR). We computed a summary secretion score as the scaled average of the secretory solute clearances.
Results
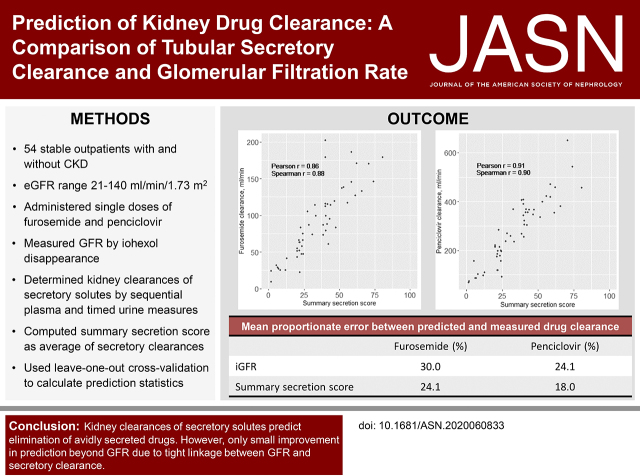
Median iGFR of the 54 participants was 73 ml/min per 1.73 m2. The kidney furosemide clearance correlated with iGFR (r=0.84) and the summary secretion score (r=0.86). The mean proportionate error (MPE) between iGFR-predicted and measured furosemide clearance was 30.0%. The lowest MPE was observed for the summary secretion score (24.1%); MPEs for individual secretory solutes ranged from 27.3% to 48.0%. These predictive errors were statistically indistinguishable. Penciclovir kidney clearance was correlated with iGFR (r=0.83) and with the summary secretion score (r=0.91), with similar predictive accuracy of iGFR and secretory clearances. Combining iGFR with the summary secretion score yielded only modest improvements in the prediction of the kidney clearance of furosemide and penciclovir.
Conclusions
Secretory solute clearance measurements can predict kidney drug clearances. However, tight linkage between GFR and proximal tubular secretory clearance in stable outpatients provides some reassurance that GFR, even when estimated, is a useful surrogate for predicting secretory drug clearances in such patients.
The kidneys play a central role in eliminating prescribed medications and their metabolites from the circulation. The accurate dosing of kidney medications is necessary for reducing the risks of treatment failures and adverse drug events.1 Impaired kidney function is a major risk factor for preventable medication-related hospital admissions.2–10 The kidneys clear administered drugs from the circulation by two distinct mechanisms: glomerular filtration and proximal tubular secretion. Of these, secretion is the primary mechanism of kidney drug elimination.11,12 Hundreds of drugs and their metabolites are fully or partially cleared by tubular secretion, including antivirals (e.g., tenofovir, famciclovir), antibiotics (e.g., penicillins, cephalosporins), diuretics (e.g., furosemide), and antidiabetes agents (e.g., metformin). Secretion is capable of eliminating protein-bound drugs that are inefficiently filtered due to the size and charge selectivity of the glomerular basement membrane. Moreover, tubular secretory clearance can greatly exceed the GFR, providing a highly efficient mechanism for eliminating retained solutes and drugs.13
Despite the primacy of tubular secretion in kidney drug handling, current drug dosing strategies are on the basis of estimates of GFR (or creatinine clearance) under the assumption that secretion and filtration are tightly linked within an individual. Yet, tubular secretion is a physiologically different process from filtration that may be specifically affected by competition for secretory transporters, alterations in cellular energy generation, and disproportionate tubulointerstitial injury across disease etiologies. In animal models of CKD, the expression of tubular organic anion transporters (OATs) is reduced, possibly as a result of tubulointerstitial fibrosis or as an adaptive response to excess uremic solutes.14–16 The assumption that GFR represents a reliable proxy of kidney drug elimination was challenged in a review of published pharmacokinetic data, which identified differences between GFR-predicted and actual kidney elimination for 48% of evaluated drugs.17
Few studies have empirically determined relationships between measured tubular secretory clearance and kidney drug elimination. We conducted a pharmacokinetic profiling study of two avidly secreted medications in 54 individuals with a wide range of kidney function. We determined the kidney clearances of eight endogenous secretory solutes by measuring their concentrations in sequential plasma and timed urine samples, and we measured GFR by iohexol disappearance (iGFR). We then compared tubular secretory clearance and iGFR as predictors of kidney drug elimination.
Methods
Study Population
We designed the Proximal Tubular Clearance of Renal Medications study to investigate the role of tubular secretory solute clearance in kidney drug pharmacokinetics. Between 2017 and 2019, study personnel recruited 58 individuals by electronic medical record screening of outpatient primary care and nephrology clinics at the University of Washington (UW), community advertisements, and review of the Kidney Research Institute research registry. Participants were selected on the basis of categories of eGFRs to include a wide range of kidney function, including normal and CKD.
Exclusion criteria included age <18 years; receipt of any form of RRT; current use of the study medications; allergy to the study medications; use of cimetidine, probenecid, or digoxin; and a history of nephrotic syndrome or cirrhosis (a full list of exclusions is in Supplemental Table 1). We further excluded four participants for whom reliable intravenous access could not be established for the scheduled blood collections. The institutional review board at UW approved the study protocol. All participants provided informed consent.
Measurements of Secretory Solute Clearance
We previously identified a set of endogenous solutes suspected to be eliminated primarily by proximal tubular secretion on the basis of one or more of the following characteristics: affinity for proximal tubular OAT1/3, elevated plasma concentrations following transporter knockout in experimental models, a high degree of protein binding, relatively low diurnal variation in plasma, and/or kidney clearances that exceed GFR or creatinine clearance.18–20 Herein, we estimated the kidney clearances of these solutes by measuring their concentrations in sequential plasma samples and a concomitant supervised daytime urine collection. Upon arrival at the study center, participants provided a spot urine void, which marked the beginning of the timed urine collection but was excluded from the timed collection itself. Participants then voided throughout the remainder of the study visit, and the time of the last void was recorded (mean collection time 9.8±1.2 hours). During this same period, coordinators collected and processed blood samples for secretory solute measurements at times 0, 60, 300, and 480 minutes from an indwelling intravenous catheter. Participants were provided three standard meals and ample fluids throughout the study visit.
We quantified plasma and urine concentrations of endogenous solutes using our previously published methods.21,22 Plasma samples underwent solid-phase extraction (Phree phospholipid removal plate; Phenomenex) after precipitation in organic solvent. Urine samples went through two consecutive solid-phase extractions (HLB or MCX µElution plates; Waters). We reconstituted dried extracts in 80 µl of 5% acetonitrile/0.2% formic acid in H2O followed by filtration through a large-pore filter plate (MSBVN1210; Millipore) to remove particulates before being analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS; Shimadzu and Sciex). We normalized data to internal standard peak areas on the basis of stable isotope-labeled solutes.
Calibration was achieved using a single-point calibration approach. The concentrations of each solute in the single-point calibrators (pooled human serum and urine) were previously quantified by standard addition of purified spiking solutions, which were characterized using quantitative nuclear magnetic resonance. Five replicates of single-point calibrators were included on each plate. Intra- and interassay coefficients of variation were generally low (Supplemental Table 2).
We calculated the kidney clearance of each endogenous secretory solute as
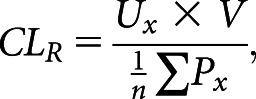 |
where Ux represents the concentration of solute in the supervised daytime urine collection (nanograms per milliliter), V represents the timed urine flow rate (milliliters per minute), n is the number of plasma measurements (49 participants provided four measurements and five participants provided three), and Px represents solute concentration in plasma (nanograms per milliliter).
Measurements of Drug Pharmacokinetics
At the beginning of each study visit, coordinators administered a single 5-mg intravenous dose of furosemide (Hospira). Syringes were weighed pre- and postadministration to precisely calculate the administered furosemide dosage, which was then used to calculate its kidney clearance.
Furosemide is highly protein bound (>95%) and eliminated primarily via secretory transporters in the proximal tubules. Coordinators simultaneously provided participants with a single 125-mg oral dose of famciclovir (Macleods Pharma). Orally administered famciclovir has high bioavailability and is rapidly metabolized by the liver to penciclovir, the active form of the drug, which is efficiently eliminated by proximal tubular secretion.23–27 All study medications were acquired, stored, and dispensed by the Northwest Kidney Center Pharmacy.
Coordinators collected and processed blood specimens from the indwelling catheter for measurements of furosemide and penciclovir concentrations at 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 300, 480, and 600 minutes after drug administration. At the end of the study visit, participants were instructed to complete an overnight urine collection and to return to the study center the following morning to provide a final (1440-minute) blood sample.
We quantified plasma and urine concentrations of each medication using LC-MS/MS at the UW Department of Pharmaceutics Pharmacokinetics Laboratory (Supplemental Material). Briefly, plasma and urine samples were mixed with internal standards of known concentrations. The internal standards for furosemide and penciclovir were probenecid and labeled d4-penciclovir, respectively. Calibration samples were prepared by combining drug-free plasma or urine samples with internal standards and working standard solutions. Working standard solutions were prepared with 0.6 ng/µl furosemide in methanol for furosemide plasma and urine samples, 1.0 ng/µl penciclovir for penciclovir plasma samples, and 160 ng/µl penciclovir for penciclovir urine samples. Samples were vortexed and centrifuged, and supernatant was then injected into the LC-MS/MS system.
The area under the concentration-time curve (AUC) for each medication was calculated using the linear up/log down trapezoidal rule, and any portion of the curve extrapolated to infinity was calculated by fitting a monoexponential, biexponential, or triexponential decay, whichever fitted best to the observed data, to the terminal portion of the curve. For the calculation of furosemide AUC, we back extrapolated the curve to time 0. This method incorporated drug exposure before the first blood sample was taken (15 minutes after administration), which usually constitutes a large portion of it. We calculated the kidney clearance of each medication as
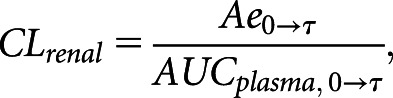 |
where Ae0→τ represents the amount of drug recovered in the timed urine sample (milligrams). The plasma AUC (milligrams per milliliter × minute) was calculated over the same time interval. For furosemide, we used the daytime urine collection from 0 to 10 hours and blood samples over the matched time interval to calculate the urine recovery and plasma AUC010 hours of furosemide because the 1440-minute plasma furosemide concentration was undetectable or below the lower limit of quantification for the majority of participants. For penciclovir, by contrast, we used the 0- to 10-hour and the 10- to 24-hour urine collections (supervised daytime collection plus overnight sample) and included the 1440-minute blood sample to calculate urine recovery and the plasma AUC024 hours of penciclovir.
Measurements of Iohexol Clearance
At the start of each study visit, coordinators administered a single 5-ml intravenous bolus of iohexol (Omnipaque; 647 mg/ml). Syringes were weighed pre- and postadministration to calculate the administered dosage. Coordinators collected blood samples at 120, 180, 240, and 600 minutes postadministration for iohexol measurements. Plasma iohexol concentrations were quantified by LC-MS/MS at the University of Minnesota Advanced Research and Diagnostic Laboratory.28 We calculated iohexol clearance using the slope-intercept method in conjunction with the Brochner–Mortensen equation.29 This method provides good accuracy and reliability and does not require plasma samples collected at very short intervals immediately after iohexol administration.30,31
Statistical Analyses
We expressed all clearances in milliliters per minute without standardization for body surface area. We summarized correlations among the kidney clearances of each study medication, secretory solutes, and iGFR using scatterplots, Pearson correlation, and Spearman correlation. We used Fisher transformation to compare correlations of drug clearances with iGFR and secretory clearances.32,33 This method works well for highly correlated data retrieved from the same sample. We computed a summary measure of secretory clearance by standardizing the kidney clearance of each secretory solute to a common 0–100 scale:
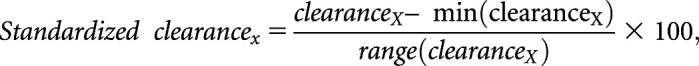 |
where clearanceX represents the kidney clearance of solute X, min(clearanceX) represents the minimum clearance value in the distribution of this study, and range(clearanceX) represents the difference between the maximum and minimum values. We then calculated the summary secretion score as the average of the eight standardized clearances.
We constructed linear regression models to quantify associations between the kidney clearance of each study medication (dependent variable) and either secretory solute clearances or GFR (independent variables). We used leave-one-out crossvalidation to estimate the mean absolute error (MAE) and the mean proportionate error (MPE). The MAE and MPE can be interpreted as the average absolute or proportionate difference between model-predicted and measured drug clearance, respectively. Both MAE and MPE measure the amount of errors in prediction models. Lower MAE and MPE values indicate greater predictive accuracy. MAE itself is more directly interpretable, as it shares the same unit of the variable being predicted (milliliters per minute in this study). However, MAE weighs different observations proportionately to their absolute values.
For example, a prediction error of 50 ml/min in furosemide clearance in a person with a true clearance of 100 ml/min weighs twice as much in the calculation of MAE than an error of 25 ml/min in another person with a true clearance of 50 ml/min. In comparison, MPE, as a percentage, is much less influenced by extremely high or low values—the error of both persons in the above example is 50% and weighs exactly the same in the calculation of MPE.
We compared the predictive accuracy of univariate iGFR and secretory clearance models by computing the difference in MAE or MPE between these models with 95% confidence intervals (95% CIs) and P values (for the correction of multiple comparisons) derived using a bootstrap approach with 500 iterations.34 We then assessed the combined prediction of iGFR plus secretory solute clearance by comparing models that included only iGFR with models that included iGFR plus each secretory clearance measure. In sensitivity analyses, we assessed the MAE and MPE of models that included eGFR as a single predictor. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation using a spot creatinine concentration from blood samples collected at baseline before drug administration.35 We then used the same method as in the primary analysis to calculate root mean square error (RMSE) and the percentage of predicted values that differed by >30% from measured drug clearances (1-P30) as additional metrics of predictive accuracy. Lower RMSE and 1-P30 values also indicate better predictive accuracy. We used the Hommel method to correct for multiple comparisons (ten comparisons in Tables 2 and 3 and nine comparisons in Table 4).36 Analyses were performed using Stata/IC 14.2 (StataCorp. 2015, Stata Statistical Software: Release 14; College Station, TX) and RStudio 3.6.3 (R Core Team 2017, Vienna, Austria).
Table 2.
Predictive accuracy of GFR and secretory solute clearances for predicting kidney furosemide clearance
| Predictor | MAE between Predicted and Measured Drug Clearance, ml/min | Difference in MAE Comparing iGFR with Secretory Clearance, ml/min (95% CI)a | MPE between Predicted and Measured Drug Clearance, % | Difference in MPE Comparing iGFR with Secretory Clearance, % (95% CI)a |
|---|---|---|---|---|
| iGFR | 21.5 | — | 30.0 | — |
| eGFRcreatinine | 22.4 | −0.9 (−5.6 to 3.5) | 33.5 | −3.5 (−12.9 to 3.7) |
| Secretion score | 17.2 | 4.3 (−1.1 to 9.1) | 24.1 | 5.9 (−2.1 to 14.0) |
| Individual clearance | ||||
| Pyridoxic acid | 18.9 | 2.6 (−2.9 to 7.0) | 27.3 | 2.7 (−6.8 to 10.1) |
| Isovalerylglycine | 25.7 | −4.2 (−11.4 to 2.2) | 42.5 | −12.5 (−26.9 to 1.1) |
| Tiglylglycine | 27.0 | −5.5 (−12.9 to 1.1) | 43.8 | −13.8 (−32.2 to 1.9) |
| Kynurenic acid | 21.0 | 0.5 (−6.2 to 6.3) | 33.3 | −3.3 (−16.8 to 9.2) |
| Xanthosine | 28.3 | −6.8 (−14.3 to 0.1) | 48.0 | −18.0 (−35.9 to −1.3) |
| Cinnamoylglycine | 20.9 | 0.6 (−5.1 to 5.3) | 29.2 | 0.8 (−8.2 to 9.7) |
| Indoxyl sulfate | 20.6 | 0.9 (−4.0 to 5.6) | 31.1 | −1.1 (−10.4 to 9.0) |
| p-cresol sulfate | 20.0 | 1.5 (−3.3 to 5.7) | 29.5 | 0.5 (−5.9 to 8.5) |
iGFR was measured by iohexol disappearance. eGFR was on the basis of serum creatinine concentrations from the 2009 Chronic Kidney Disease Epidemiology Collaboration equation. MAE, mean absolute error; MPE, mean proportionate error.
The 95% CIs were derived using leave-one-out cross validation with bootstrap with 500 iterations. Positive values indicate greater agreement for secretory solute clearances, and negative values indicate greater agreement for iGFR. None of the differences remained significant after correction for multiple comparisons using the Hommel method.
Table 3.
Predictive accuracy of GFR and secretory solute clearances for predicting kidney penciclovir clearance
| Predictor | MAE between Predicted and Measured Drug Clearance, ml/min | Difference in MAE Comparing iGFR with Secretory Clearance, ml/min (95% CI)a | MPE between Predicted and Measured Drug Clearance, % | Difference in MPE Comparing iGFR with Secretory Clearance, % (95% CI)a |
|---|---|---|---|---|
| iGFR | 53.9 | — | 24.1 | — |
| eGFRcreatinine | 67.5 | −13.6 (−25.7 to 0.1) | 29.8 | −5.7 (−11.9 to 0.3) |
| Secretion score | 41.1 | 12.8 (−1.8 to 25.1) | 18.0 | 6.1 (−1.3 to 12.9) |
| Individual clearance | ||||
| Pyridoxic acid | 51.0 | 2.9 (−11.6 to 16.6) | 22.0 | 2.1 (−5.8 to 12.7) |
| Isovalerylglycine | 54.5 | −0.6 (−16.7 to 18.3) | 26.7 | −2.6 (−11.2 to 8.5) |
| Tiglylglycine | 66.7 | −12.8 (−29.2 to 6.3) | 31.1 | −7.0 (−16.1 to 5.6) |
| Kynurenic acid | 57.0 | −3.1 (−17.2 to 14.6) | 25.8 | −1.7 (−11.6 to 9.4) |
| Xanthosine | 77.6 | −23.7 (−40.9 to −8.3) | 38.4 | −14.3 (−23.1 to −4.2)b |
| Cinnamoylglycine | 60.3 | −6.4 (−20.8 to 7.8) | 27.0 | −2.9 (−10.2 to 6.0) |
| Indoxyl sulfate | 61.7 | −7.8 (−19.8 to 5.1) | 29.2 | −5.1 (−11.7 to 1.2) |
| p-cresol sulfate | 49.2 | 4.7 (−7.4 to 18.2) | 22.0 | 2.1 (−3.8 to 6.5) |
iGFR was measured by iohexol disappearance. eGFR was on the basis of serum creatinine concentrations from the 2009 Chronic Kidney Disease Epidemiology Collaboration equation. MAE, mean absolute error; MPE, mean proportionate error.
The 95% CIs were derived using leave-one-out cross validation with bootstrap with 500 iterations. Positive values indicate greater agreement for secretory solute clearances, and negative values indicate greater agreement for iGFR.
Statistically significant adjusted correction for multiple comparisons using the Hommel method.
Table 4.
Prediction of kidney drug clearances combining iGFR with secretory solute clearances
| Predictor | Furosemide | Penciclovir | ||
|---|---|---|---|---|
| MAE between Predicted and Measured Drug Clearance, ml/min | Difference in MAE Comparing iGFR Only with iGFR Plus Secretory Clearance, ml/min (95% CI)a | MAE between Predicted and Measured Drug Clearance, ml/min | Difference in MAE Comparing iGFR Only with iGFR Plus Individual Secretory Clearance, ml/min (95% CI)a | |
| iGFR | 21.5 | — | 53.9 | — |
| Secretion score | 17.4 | 4.1 (0.1 to 9.1) | 39.9 | 14.0 (3.4 to 25.0) |
| Individual clearance | ||||
| Pyridoxic acid | 18.1 | 3.4 (0.2 to 7.5) | 46.6 | 7.3 (0.8 to 17.2) |
| Isovalerylglycine | 20.7 | 0.8 (−0.7 to 4.4) | 43.0 | 10.9 (0.7 to 24.9) |
| Tiglylglycine | 21.4 | 0.1 (−0.8 to 3.3) | 48.3 | 5.6 (−1.9 to 15.9) |
| Kynurenic acid | 18.3 | 3.2 (−0.4 to 7.5) | 44.4 | 9.5 (0.9 to 19.9) |
| Xanthosine | 20.5 | 1.0 (−0.4 to 3.7) | 52.1 | 1.8 (−1.9 to 7.5) |
| Cinnamoylglycine | 19.7 | 1.8 (−0.9 to 5.7) | 53.3 | 0.6 (−4.8 to 9.6) |
| Indoxyl sulfate | 18.6 | 2.9 (0.2 to 6.3) | 50.8 | 3.1 (−1.2 to 9.9) |
| p-cresol sulfate | 19.4 | 2.1 (−0.2 to 5.3) | 46.1 | 7.8 (0.6 to 18.1) |
iGFR was measured by iohexol disappearance. eGFR was on the basis of serum creatinine concentrations from the 2009 Chronic Kidney Disease Epidemiology Collaboration equation. MAE, mean absolute error; MPE, mean proportionate error.
The 95% CIs were derived using leave-one-out cross validation with bootstrap with 500 iterations. Positive values indicate greater agreement for secretory solute clearances, and negative values indicate greater agreement for iGFR. None of the differences were significant after correction for multiple comparisons using the Hommel method.
Results
Study Population and Characteristics
The 54 study participants were characterized by a mean age of 55 ±15 years; 33% were women, 33% were Black, and there was a median iGFR of 73 ml/min per 1.73 m2 (interquartile range [IQR], 48–91 ml/min per 1.73 m2) (Table 1). There were 23 participants (43%) with an iGFR<60 ml/min per 1.73 m2 (12 with iGFR 45–60 ml/min per 1.73 m2 and 11 with iGFR<45 ml/min per 1.73 m2). Thirty-three percent of participants were using statins, and 39% were using an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. The kidney clearances of six of the eight secretory solutes under evaluation were higher than iGFR (Supplemental Table 2). Pearson correlations between individual secretory solute clearances and iGFR ranged from 0.69 for xanthosine clearance to 0.83 for pyridoxic acid clearance. The summary secretion score was strongly correlated with iGFR (Pearson correlation coefficient of 0.85). Spearman correlations for these associations were similar (Supplemental Table 2). Plasma concentrations of the individual secretory solutes did not meaningfully change following administration of the study medications (Supplemental Table 3).
Table 1.
Characteristics of participants in the Proximal Tubular Clearance of Renal Medications study
| Characteristics | |
|---|---|
| iGFR, ml/min per 1.73 m2a | 73 (48–91) |
| <45 | 11 (20) |
| 45–60 | 12 (22) |
| 60–90 | 17 (31) |
| ≥90 | 14 (26) |
| Age, yr | 55±15 |
| Women | 18 (33) |
| Race | |
| White | 34 (63) |
| Black | 18 (33) |
| Other | 2 (4) |
| Body mass index, kg/m2 | 29±6 |
| Education categories | |
| Less than high school | 2 (4) |
| High school graduate | 11 (20) |
| Some college | 16 (30) |
| College graduate or higher | 25 (46) |
| Current smoker | 14 (26) |
| History of diabetes | 6 (11) |
| History of cardiovascular disease | 7 (13) |
| Systolic BP, mm Hg | 134±20 |
| Laboratory measurements | |
| 24-h urine albumin, mg/da,b | 13 (5–68) |
| 24-h urine albumin >300 mg/db | 9 (17) |
| Serum albumin, g/dl | 4.1±0.3 |
| Serum calcium, mg/dl | 9.0±0.4 |
| Serum bicarbonate, mEq/L | 22.7±3.4 |
| Hemoglobin, g/dl | 13.5±1.7 |
| Medications | |
| Insulin | 3 (6) |
| Statin | 18 (33) |
| ACEi/ARB | 21 (39) |
| Thiazide diuretic | 3 (6) |
Continuous variables are mean ± SD; categorical variables are N (%). ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Median (IQR).
Urine albumin collected during supervised daytime urine collection standardized to 24 hours.
Prediction of Furosemide Pharmacokinetics
The median kidney clearance of furosemide was 83 ml/min (IQR, 51–117 ml/min). Kidney furosemide clearance was strongly correlated with iGFR and with the summary secretion score (Figure 1) (Pearson correlation =0.84 and 0.86, respectively). These correlations were not significantly different from each other (P=0.52). Using iGFR as a single predictor, the MAE between model-predicted and measured furosemide clearance was 21.5 ml/min (Table 2). Expressed as proportionate difference, the MPE between iGFR-predicted and measured furosemide clearance was 30.0%. MAE and MPE values were slightly higher for the corresponding model of eGFR. The lowest MAE and MPE values were observed for the summary secretion score (4.3 ml/min and 5.9% lower than iGFR, respectively). However, predictions of kidney furosemide elimination on the basis of the summary secretion score and individual secretory solute clearances were not statistically significant from those of iGFR. Analyses of RMSE and 1-P30 also showed statistically similar accuracy of iGFR and secretory solute clearances for the prediction of kidney furosemide clearance (Supplemental Tables 4 and 5).
Figure 1.
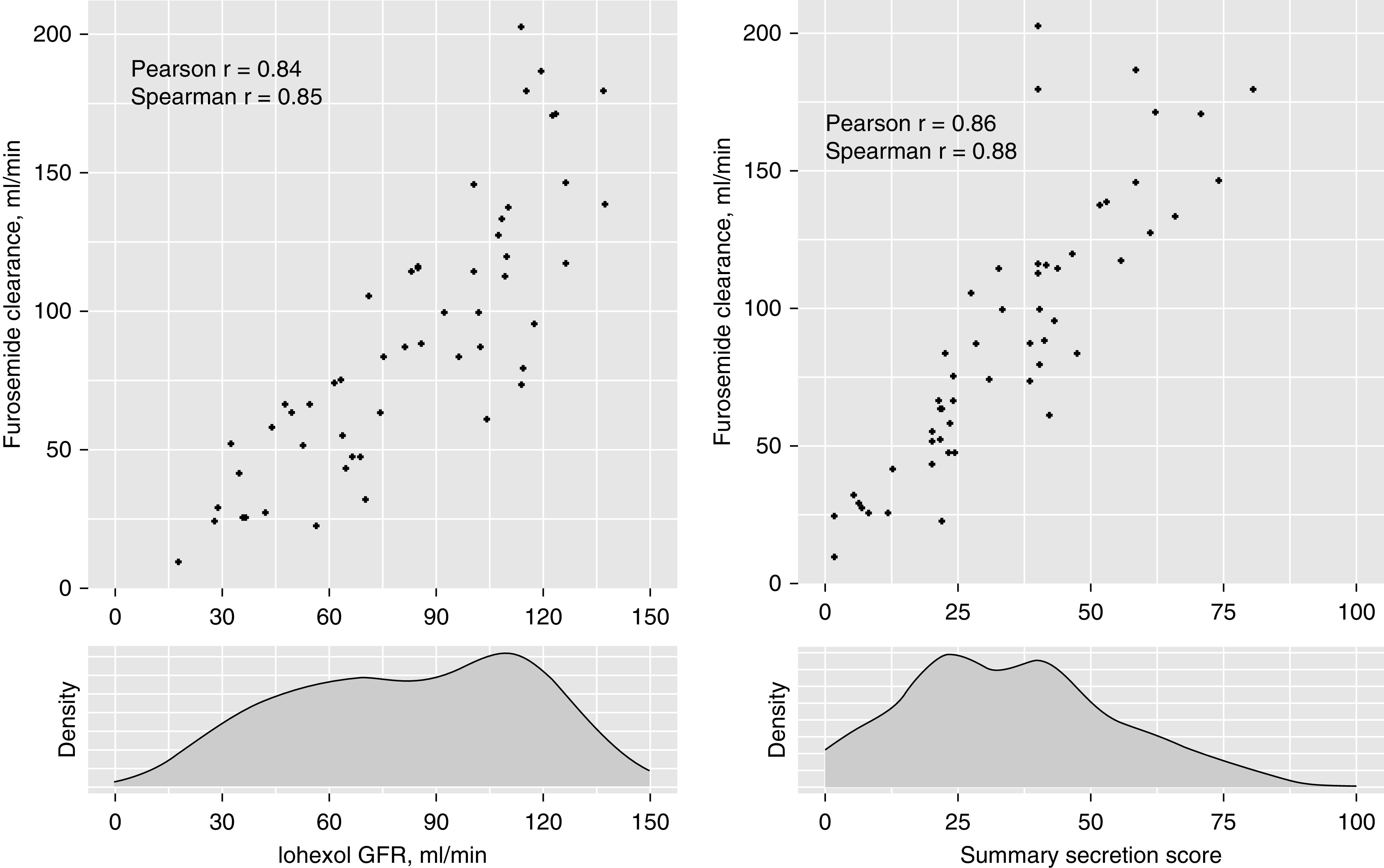
Strong associations of iGFR and summary secretion score with kidney furosemide clearance. P values for all correlations are <0.001. The correlations of furosemide clearance with iGFR and the summary secretion score were not significantly different from each other (Pearson: P=0.52; Spearman: P=0.27).
Prediction of Penciclovir Pharmacokinetics
The median kidney clearance of penciclovir was 269 ml/min (IQR, 177–368 ml/min), which was 3.3 times higher than iGFR on average. Correlations of penciclovir clearance with iGFR and the summary secretion score were 0.83 and 0.91, respectively (Figure 2) (P value for the test of difference in correlations =0.007). The MAE between iGFR-predicted and measured kidney penciclovir clearance was 53.9 ml/min; the corresponding MPE for this model was 24.1% (Table 3). Modestly higher MAE and MPE values for penciclovir clearance were observed using eGFR as a single predictor. The summary secretion score yielded the lowest observed MAE and MPE for penciclovir clearance. However, these prediction statistics were not statistically different from those of iGFR.
Figure 2.
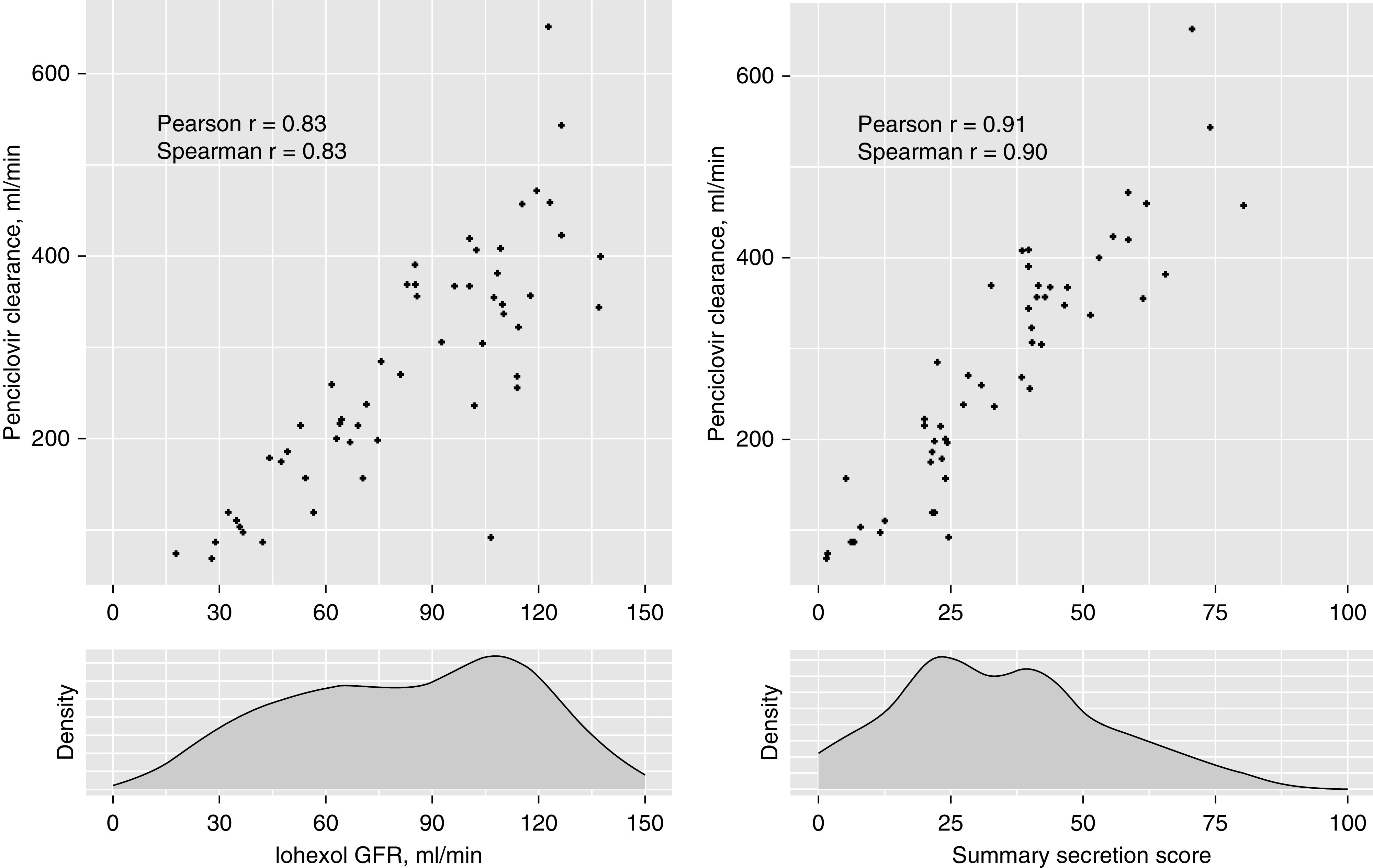
Strong associations of iGFR and summary secretion score with kidney penciclovir clearance. P values for all correlations are <0.001. The correlations of penciclovir clearance with iGFR and the summary secretion score were significantly different from each other (Pearson: P=0.007; Spearman: P=0.02).
Combining iGFR with Secretory Solute Clearances
Adding the summary secretion score to the iGFR model (Table 4) reduced the MAE by 4.1 ml/min for furosemide clearance (95% CI, +0.1 to +9.1 ml/min) and by 14.0 ml/min for penciclovir clearance (95% CI, +3.4 to +25.0 ml/min). The addition of all of the individual secretory solutes to the iGFR model yielded numerically small improvements in the prediction of kidney drug clearances that were not statistically significant after correction for multiple comparisons. Further addition of age, sex, and race to the iGFR plus summary secretion score models yielded no further improvement in predictive accuracy for either drug clearance.
Discussion
In summary, we found iGFR and the kidney clearances of secretory solutes to each be strongly correlated with the kidney elimination of two avidly secreted drugs among stable outpatients with and without CKD. A summary measure of kidney secretory clearance demonstrated numerically greater accuracy for predicting the clearances of furosemide and penciclovir; however, the observed differences were numerically small and not statistically significant. A modest numerical improvement in predicting the kidney clearance of both drugs was also achieved by combining the summary secretion score with iGFR. These findings obtained from an empirical pharmacokinetic study provide some reassurance that GFR, even when estimated, is a useful surrogate for secretory drug clearance in healthy persons and stable patients with CKD. The results also suggest cautious optimism for future improvements in kidney drug dosing strategies by incorporating measurements of tubular secretory clearance.
The close correlation among iGFR, secretory solute clearances, and the kidney elimination of two avidly secreted drugs suggests a tight linkage between glomerular filtration and tubular secretory clearance in stable persons with and without CKD. This result was somewhat surprising given physiologic differences in the underlying mechanisms of glomerular filtration and tubular secretion. Although both pathways are governed by hemodynamic conditions, filtration is primarily passive and determined by size and charge selectivity of the glomerular basement membrane. In contrast, the secretion of organic solutes and drugs involves remote sensing of increased plasma solutes levels and upregulation of secretory transporters by tubular epithelial cells, coordinated uptake by specific transporters on the basolateral cell surface, pericellular transport, and active secretion into the urine against a chemical gradient. Central to these secretory processes is efficient cellular energy generation via mitochondrial respiration, which may be differentially affected by pathologic processes. Contrasts between GFR and tubular secretory clearance may also arise from different kidney disease etiologies,21,22 competition for cellular transporters by endogenous solutes and other drugs, and genetic variation in the transporters. For example, an intergenic polymorphism between OAT1/3 modifies the effect of hydrochlorothiazide on BP, and a nonsynonymous polymorphism in organic cation 2 (OCT2) is associated with higher kidney metformin clearance.37,38 In addition, differences in tubular secretory clearances have also been observed between men and women and among different racial groups.13
As our study included only 33 persons with CKD due to heterogenous etiologies, we cannot exclude the possibility of greater dissociation between GFR and tubular secretory clearance for specific causes of CKD or in the setting of AKI, which may preferentially affect tubular functions. It is also possible that distinctions between GFR and secretory drug clearance may be greater for cationic drugs. For example, the clearance of S-pindolol, which is avidly secreted via OCT2 transporters, was only weakly correlated with GFR measured by 51Cr-EDTA clearance in a previous pharmacokinetic study.39
These results provide empirical evidence that tubular secretory clearance can be estimated from endogenous solutes. The solutes selected for this study are substrates of OAT1/3 transporters and are either highly protein bound and/or exhibit kidney clearances that exceed GFR. For example, kynurenic acid is >95% bound to serum albumin, including in persons with advanced CKD, suggesting minimal glomerular filtration, is efficiently cleared by the kidneys at rates that exceed GFR, and was strongly correlated with the kidney elimination of furosemide and penciclovir in this study. Nonetheless, the clearances of these solutes require timed urine collections, which remain cumbersome to obtain in clinical practice, and plasma concentrations of endogenous secretory solutes exhibit diurnal variation, which reduces precision in estimating their clearance. Further refinement in methods for estimating tubular secretory clearance on the basis of endogenous solutes could advance this important area.
The two medications selected for evaluation in this study are avidly secreted by the proximal tubules. Furosemide circulates bound to serum albumin (>95%), minimizing filtration,40 and is primarily eliminated by active secretion via OAT1/3 transporters.11 We administered furosemide intravenously to avoid individual differences in oral bioavailability, which range from 0.37 to 0.83 in healthy subjects and from 0.43 to 0.76 in patients with CKD.40 Famciclovir, an oral prodrug of penciclovir, is a nucleoside analog that inhibits herpes simplex virus DNA polymerase. Orally administered famciclovir undergoes rapid metabolism to penciclovir in the liver with a consistent oral bioavailability of approximately 60%.41 Although the protein binding of penciclovir is low (<20%), the drug is avidly secreted, primarily via OAT2, with a kidney clearance that greatly exceeds GFR.24,42 We observed a median penciclovir clearance of 269 ml/min, which was 3.3 times higher than iGFR in this study.
The primary strengths of this study are the detailed procedures used to measure kidney drug pharmacokinetics, GFR, and tubular secretory clearances with high accuracy and precision. We calculated secretory clearances using time-averaged plasma concentrations of target solutes measured at multiple time points to reduce the effect of diurnal variation and urine concentrations from a supervised timed collection. We quantified the solutes of interest using targeted mass spectrometry assays with labeled internal standards and external calibrators that have been developed for this purpose. Plasma concentrations of the selected solutes were unchanged by the administration of furosemide and penciclovir, suggesting that the small doses of these drugs administered in this study were below transporter thresholds. Another strength is the inclusion of participants with a wide range of GFR and secretory solutes clearances—from healthy participants to those with CKD. Several important weaknesses deserve comment. The relatively small sample size and self-reported cause of kidney disease preclude assessment of kidney drug clearances for specific etiologies of CKD or among patients with severely reduced kidney function. The small sample size also limited study power to detecting relatively large differences between GFR and secretory clearances. We selected furosemide and penciclovir as prototypical secreted drugs; however, GFR and secretory clearance may have different relative effects on the elimination of other drugs, particularly cationic drugs that utilize the OCT2 pathway. Some furosemide is metabolized into a glucuronide metabolite, which we were unable to measure reliably due to its instability.
In summary, we found relatively similar accuracy of iGFR and tubular secretory solute clearances for predicting the kidney elimination of two avidly secreted drugs. Some improvement in the prediction of furosemide clearance was achieved by combining secretory solute clearance measurements with iGFR, suggesting possible future applications of secretory clearance measurements to refine kidney drug dosing. These results also demonstrate the feasibility of estimating tubular secretory clearance using endogenous secretory solutes. Such measurements could be applicable to the drug development process, particularly for the evaluation of new drugs that are highly protein bound and avidly secreted. Future studies to evaluate other secreted drugs, particularly those that utilize different transporters, with a larger sample size and in persons with different etiologies of CKD are needed to extend these study findings.
Disclosures
I. de Boer reports consultancy agreements with Boehringer-Ingelheim, Cyclerion Therapeutics, George Clinical, Goldfinch Bio, and Ironwood Pharmaceuticals; honoraria from the National Institutes of Health; and scientific advisor or membership as Associate Editor of Contemporary Clinical Trials, Clinical Practice Guideline Co-Chair of Kidney Disease Improving Global Outcomes, Deputy Editor of CJASN, and Editorial Advisory Board Member of American Family Physician. J. Himmelfarb reports consultancy agreements with Akebia Therapeutics, Chinook Therapeutics, Maze Therapeutics, Pfizer, RenalytixAI, and Seattle Genetics; honoraria from various academic institutions for invited lectures; scientific advisor or membership with BMC Medicine (Editorial Board), CJASN (Editorial Board), and Nature Reviews Nephrology (Scientific Advisory Board); and other interests/relationships as Founder, President, and Board Member of AKTIV-X Technologies, Inc. and research grant support from Northwest Kidney Centers. A. Hoofnagle reports ownership interest in Seattle Genetics; research funding from Waters; patents and inventions with SISCAPA Assay Technologies; scientific advisor or membership with Clinical Chemistry (Associate Editor); and other interests/relationships as expert witness for Kilpatrick, Stockton, LLC, and Townsend. B. Kestenbaum reports honoraria from Medscape Inc., Reatta Pharmaceuticals, and Sanofi Inc. B. Phillips reports current employment with Agilent Technologies. C. Yeung reports consultancy agreements with Nortis Inc. and honoraria from the National Institutes of Health. L. Zelnick reports consultancy agreements with Microsoft Research. All remaining authors have nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health grant R01 DK107931 and National Institute of General Medical Sciences, National Institutes of Health grant R01 GM121354. This work was also partially supported by a Northwest Kidney Centers unrestricted fund.
Supplementary Material
Acknowledgments
We thank Ms. Rucille Montenegro for her dedicated work on this study.
Dr. Bryan Kestenbaum and Dr. Catherine Yeung designed the study; Dr. Yan Chen, Dr. Bryan Kestenbaum, Dr. Laura Shireman, and Dr. Catherine Yeung conducted the literature search; Mr. Calder Brauchla, Dr. Yan Chen, Dr. Andrew Hoofnagle, Ms. Linda Manahan, Mr. Brian Phillips, Dr. Laura Shireman, and Dr. Catherine Yeung collected data; Mr. Calder Brauchla, Dr. Yan Chen, Dr. Andrew Hoofnagle, Dr. Bryan Kestenbaum, Mr. Brian Phillips, Dr. Laura Shireman, Dr. Catherine Yeung, and Dr. Leila Zelnick analyzed data; all authors interpreted data; Mr. Calder Brauchla, Dr. Yan Chen, Dr. Andrew Hoofnagle, Dr. Bryan Kestenbaum, and Dr. Laura Shireman drafted the manuscript; and all authors revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060833/-/DCSupplemental.
Supplemental Material. Methods.
Supplemental Table 1. Exclusion criteria of the PROCLAIM study.
Supplemental Table 2. Kidney clearances and laboratory characteristics of secretory solutes.
Supplemental Table 3. Plasma concentration of secretory solutes during study visits.
Supplemental Table 4. Root mean square error of kidney clearance of medications predicted by iGFR or secretory solutes clearances.
Supplemental Table 5. The 1-P30 of kidney clearance of medications predicted by iGFR or secretory solutes clearances.
References
- 1.Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM: Clinical pharmacokinetics in kidney disease: Fundamental principles. Clin J Am Soc Nephrol 13: 1085–1095, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM; HARM Study Group: Frequency of and risk factors for preventable medication-related hospital admissions in The Netherlands. Arch Intern Med 168: 1890–1896, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Cantú TG, Ellerbeck EF, Yun SW, Castine SD, Kornhauser DM: Drug prescribing for patients with changing renal function. Am J Hosp Pharm 49: 2944–2948, 1992 [PubMed] [Google Scholar]
- 4.Hu KT, Matayoshi A, Stevenson FT: Calculation of the estimated creatinine clearance in avoiding drug dosing errors in the older patient. Am J Med Sci 322: 133–136, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Long CL, Raebel MA, Price DW, Magid DJ: Compliance with dosing guidelines in patients with chronic kidney disease. Ann Pharmacother 38: 853–858, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Papaioannou A, Clarke JA, Campbell G, Bédard M: Assessment of adherence to renal dosing guidelines in long-term care facilities. J Am Geriatr Soc 48: 1470–1473, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Pillans PI, Landsberg PG, Fleming AM, Fanning M, Sturtevant JM: Evaluation of dosage adjustment in patients with renal impairment. Intern Med J 33: 10–13, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Sheen SS, Choi JE, Park RW, Kim EY, Lee YH, Kang UG: Overdose rate of drugs requiring renal dose adjustment: Data analysis of 4 years prescriptions at a tertiary teaching hospital. J Gen Intern Med 23: 423–428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong NA, Jones HW: An analysis of discharge drug prescribing amongst elderly patients with renal impairment. Postgrad Med J 74: 420–422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yap C, Dunham D, Thompson J, Baker D: Medication dosing errors for patients with renal insufficiency in ambulatory care. Jt Comm J Qual Patient Saf 31: 514–521, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM: Renal transporters in drug development. Annu Rev Pharmacol Toxicol 53: 503–529, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Nigam SK: What do drug transporters really do? Nat Rev Drug Discov 14: 29–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Zelnick LR, Wang K, Hoofnagle AN, Becker JO, Hsu CY, et al.; CRIC Study Investigators: Kidney clearance of secretory solutes is associated with progression of CKD: The CRIC study. J Am Soc Nephrol 31: 817–827, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masereeuw R, Mutsaers HAM, Toyohara T, Abe T, Jhawar S, Sweet DH, et al.: The kidney and uremic toxin removal: Glomerulus or tubule? Semin Nephrol 34: 191–208, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Naud J, Michaud J, Beauchemin S, Hébert M-J, Roger M, Lefrancois S, et al.: Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos 39: 1363–1369, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Torres AM, Mac Laughlin M, Muller A, Brandoni A, Anzai N, Endou H: Altered renal elimination of organic anions in rats with chronic renal failure. Biochim Biophys Acta Mole Basis Dis 1740: 29–37, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Chapron A, Shen DD, Kestenbaum BR, Robinson-Cohen C, Himmelfarb J, Yeung CK: Does secretory clearance follow glomerular filtration rate in chronic kidney diseases? Reconsidering the intact nephron hypothesis. Clin Transl Sci 10: 395–403, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush KT, Wu W, Lun C, Nigam SK: The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem 292: 15789–15803, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, et al.: A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24: 1330–1338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Zelnick LR, Chen Y, Hoofnagle AN, Watnick T, Seliger S, et al.: Alterations of proximal tubular secretion in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 15: 80–88, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Zelnick LR, Hoofnagle AN, Chen Y, de Boer IH, Himmelfarb J, et al.: Differences in proximal tubular solute clearance across common etiologies of chronic kidney disease [published online ahead of print July 25, 2019]. Nephrol Dial Transplant 10.1093/ndt/gfz144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, et al.: Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther 308: 1021–1029, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Vapurcuyan A, Shahidullah M, Aleksunes LM, Pelis RM: Expression of organic anion transporter 2 in the human kidney and its potential role in the tubular secretion of guanine-containing antiviral drugs. Drug Metab Dispos 40: 617–624, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Crumpacker C: The pharmacological profile of famciclovir. Semin Dermatol 15[Suppl 1]: 14–26, 1996 [PubMed] [Google Scholar]
- 26.Oh SW, Han SY: Loop diuretics in clinical practice. Electrolyte Blood Press 13: 17–21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boike SC, Pue MA, Freed MI, Audet PR, Fairless A, Ilson BE, et al.: Pharmacokinetics of famciclovir in subjects with varying degrees of renal impairment. Clin Pharmacol Ther 55: 418–426, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Schmit DJ, Carroll LJ, Eckfeldt JH, Seegmiller JC: Verification of separate measurement procedures where analytical determinations influence the clinical interpretation of GFR: Iohexol quantitation by HPLC and LC-MS/MS. Clin Biochem 67: 16–23, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Bröchner-Mortensen J: A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 30: 271–274, 1972 [DOI] [PubMed] [Google Scholar]
- 30.Delanaye P, Ebert N, Melsom T, Gaspari F, Mariat C, Cavalier E, et al.: Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 1. How to measure glomerular filtration rate with iohexol? Clin Kidney J 9: 682–699, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming JS, Zivanovic MA, Blake GM, Burniston M, Cosgriff PS; British Nuclear Medicine Society: Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun 25: 759–769, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Fisher RA: Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 10: 507–521, 1915 [Google Scholar]
- 33.Steiger JH: Tests for comparing elements of a correlation matrix. Psychol Bull 87: 245–251, 1980 [Google Scholar]
- 34.Efron B, Tibshirani RJ: An Introduction to the Bootstrap, Washington, DC, CRC Press, 1994 [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hommel G: A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 75: 383–386, 1988 [Google Scholar]
- 37.Chen Y, Li S, Brown C, Cheatham S, Castro RA, Leabman MK, et al.: Effect of genetic variation in the organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet Genomics 19: 497–504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han YF, Fan XH, Wang XJ, Sun K, Xue H, Li WJ, et al.: Association of intergenic polymorphism of organic anion transporter 1 and 3 genes with hypertension and blood pressure response to hydrochlorothiazide. Am J Hypertens 24: 340–346, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Putt TL, Duffull SB, Schollum JB, Walker RJ: GFR may not accurately predict aspects of proximal tubule drug handling. Eur J Clin Pharmacol 70: 1221–1226, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Ponto LLB, Schoenwald RD: Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I). Clin Pharmacokinet 18: 381–408, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Gill KS, Wood MJ: The clinical pharmacokinetics of famciclovir. Clin Pharmacokinet 31: 1–8, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Pue M, Benet L: Pharmacokinetics of famciclovir in man. Antivir Chem Chemother 4: 47–55, 1993 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.