Significance Statement
Increasing evidence suggests that patients have lingering subclinical damage after AKI, despite clinical recovery as defined by serum creatinine levels. Recent reports from clinical studies found that women with a history of AKI have poor maternal and fetal outcomes during pregnancy, suggesting that subclinical injury leaves these patients unable to cope with increased renal demands of pregnancy. In this study, which sought to establish an experimental animal model that recapitulates these findings, the authors demonstrate that female Sprague Dawley rats that experience biochemical resolution of surgically induced renal ischemia-reperfusion injury subsequently developed renal insufficiency during pregnancy and intrauterine growth restriction. This novel model may serve as a useful preclinical tool to address the critical gap in knowledge regarding the mechanisms by which AKI predisposes to adverse pregnancy outcomes.
Keywords: ischemia-reperfusion, intrauterine growth, animal model, acute kidney injury, pregnancy
Abstract
Background
Recent clinical studies report that women with a history of AKI have an increased incidence of maternal and fetal adverse outcomes during pregnancy, despite fully recovering renal function prior to conception. The mechanisms contributing to such adverse outcomes in pregnancy after AKI are not yet understood.
Methods
To develop a rodent model to investigate fetal and maternal outcomes in female animals with a history of AKI, we used ischemia-reperfusion injury as an experimental model of AKI in female Sprague Dawley rats. The 12-week-old animals underwent warm bilateral ischemia-reperfusion surgery involving clamping of both renal arteries for 45 minutes or sham surgery (control). Rats were allowed to recover for 1 month prior to mating. Recovery from ischemia-reperfusion injury was confirmed by measurements of plasma creatinine and urinary protein excretion. We assessed maternal and fetal outcomes during late pregnancy on gestational day 20.
Results
After recovery from ischemia-reperfusion injury, compared with healthy sham-surgery controls, dams exhibited pregnancy-induced renal insufficiency with increases in plasma creatinine and urea, along with increased urinary protein excretion. Additionally, recovered ischemia-reperfusion dams experienced worse fetal outcomes compared with controls, with intrauterine growth restriction leading to higher rates of fetal demise and smaller pups.
Conclusions
In this rat model, despite biochemical resolution of ischemia-reperfusion injury, subsequent pregnancy resulted in maternal renal insufficiency and significant impairments in fetal growth. This mirrors findings in recent reports in the clinical population, indicating that this model may be a useful tool to further explore the alterations in kidney function after AKI in women.
AKI is the sudden loss of kidney function and the most common cause of organ dysfunction in hospitalized patients.1 The incidence of AKI is increasing in the United States,2,3 which is of critical concern given that the mortality rates associated with AKI can be as high as 62%.4 Although AKI studies typically focus on older adults, recent studies report that AKI is also common in younger patients in the intensive care unit, occurring in approximately 40% of those 16–25 years old and approximately 47% of those 26–35 years old.5 AKI is diagnosed by a rise in serum creatinine, with current treatment focused on returning renal function to baseline levels via fluid volume expansion, diuretics, or RRT.6 However, even after serum creatinine levels return to baseline values in these patients, there is increasing evidence of long-lasting subclinical injury.6–8 Renal functional reserve (RFR) is the capacity of the kidney to increase GFR in response to a physiologic stressor. Reductions in RFR have been reported 3 months after AKI, despite clinical recovery as measured by serum creatinine.9 This chronic deficit in renal function leaves patients vulnerable to any additional stress placed on the kidney.
One patient population that is particularly vulnerable to a second stressor after AKI is young women due to the significant rise in renal demands during pregnancy. Pregnancy requires significant alterations in renal hemodynamics, as GFR increases approximately 50% throughout pregnancy and renal plasma flow increases approximately 80% in midpregnancy.10 This increased renal demand results in a decrease in serum creatinine and urea in healthy, pregnant women.11 Women who are not able to adapt to this increase in renal function, including women with CKD, have high rates of poor pregnancy outcomes, including miscarriage and preeclampsia.12
Recent clinical studies reported that women with a history of AKI, despite normal renal function prior to conception, have higher rates of adverse maternal and fetal outcomes during pregnancy, including preeclampsia, gestational hypertension, small for gestational age babies, and poor neonatal outcomes.13,14 The mechanisms contributing to these adverse outcomes of pregnancy in women after AKI are not yet understood, and a critical barrier to research in this field is a lack of preclinical studies examining pregnancy outcomes after recovery from AKI.
The goal of this study was to develop an animal model of pregnancy after AKI using renal ischemia-reperfusion (IR) injury as an experimental model of AKI. Despite recovery of normal renal function prior to pregnancy, recovered ischemia-reperfusion (r-IR) dams had significantly smaller pups and higher rates of fetal demise than control (CON) dams. r-IR dams also exhibited signs of renal insufficiency during pregnancy, including elevations in plasma creatinine and urea. This novel animal model can serve as a useful tool to further explore the mechanisms by which previous AKI events can affect subsequent pregnancy outcomes.
Methods
Animals
Sprague Dawley rats were purchased from Envigo (Indianapolis, IN). All rats were maintained on normal rodent chow (Teklad 2918; Envigo Teklad, Indianapolis, IN) and water ad libitum. Rats were housed in a temperature- (20–26°C) and humidity-controlled (40%–70%) facility on a 12-hour light-dark cycle. All experiments were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals15 and were approved and monitored by the Augusta University Institutional Animal Care and Use Committee.
Warm Bilateral Renal IR Injury
At 12 weeks of age, female Sprague Dawley rats were randomized to receive either warm bilateral renal IR injury or surgical sham (n=8–9). Briefly, rats were anesthetized with approximately 2% isoflurane, and left and right kidneys were accessed by flank incisions. The renal artery was isolated from the renal vein and nerve, and both arteries were clamped with microserrefines (Fine Science Tools, Foster City, CA). The second clamp was applied within 2 minutes of the first clamp. After 45 minutes of ischemia, the clamps were removed, and blood flow was restored to both kidneys. Body temperature was monitored throughout the surgery and ischemic period via rectal probe, and body temperature was maintained at 37°C by a heated table and ultraviolet heating lamp. Sham surgeries were performed in the CON group; briefly, both renal arteries were isolated as in the IR group, but the clamps were not applied. All animals were exposed to similar duration of isoflurane anesthesia, and all animals received slow release Buprenorphine (1.2 mg/kg subcutaneously) as an analgesic and were carefully monitored after surgery.
Mating
One month after recovery from IR or control sham surgery, timed breeding was performed with nonlittermate Sprague Dawley males in half of the Sprague Dawley females. The remaining animals were not mated and served as virgin CONs. This study included a total of four groups in order to have a surgical control to determine the effect of IR and a virgin CON to determine the effect of pregnancy: (1) CON virgins, (2) CON pregnant, (3) r-IR virgins, and (4) r-IR pregnant. Daily vaginal smears were performed to detect pregnancy in the mated rats, with presence of sperm on the slide indicative of gestational day 1 (GD1) of pregnancy.
Telemetry Studies
Continuous BP measurements were collected in a separate set of female Sprague Dawley rats. Briefly, female Sprague Dawley rats were implanted with mouse telemeters (Data Sciences International, St. Paul, MN) via the femoral artery at 10 weeks of age, making a small pocket in the lower abdominal cavity for the transmitter body while under isoflurane anesthesia. Following 10 days of recovery, rats were then randomized to receive either warm bilateral renal IR injury or sham surgery as described above (n=3). All telemetry rats were mated following 1 month of recovery. Data are presented as the average mean arterial pressure (MAP) per day. Area under the curve was also calculated during pregnancy in r-IR and CON dams as a measure of the total pressure load in pregnancy from GD1 to GD20.
Saline Challenge Studies
In order to better determine the ability of the kidney to respond to an increase in demand, a separate set of rats underwent a saline challenge (as adapted from ref. 16). Briefly, female Sprague Dawley rats (11 weeks of age, n=5) were injected intraperitoneally with 5% body wt of warm sterile isotonic saline while under isoflurane anesthesia. Rats were then immediately placed in metabolic cages for 4 hours for urine collection. Urine volume was collected, and results were calculated as a percentage of the volume of the saline injected (urine volume/saline injection volume).
Plasma Volume Measurement
Plasma volume was measured in CON and r-IR rats during late pregnancy on GD20 using the Evans blue dilution method.17 Studies were also performed in age-matched CON and r-IR virgin female Sprague Dawley rats. Briefly, the femoral artery and vein were cannulated with polyethylene 50 tubing while under isoflurane anesthesia. A 500-μl sample was taken from the arterial catheter to serve as a blank control for the assay. Then, 250 μl of Evans blue (0.3 mg/ml; Sigma, St. Louis, MO) was injected into the venous catheter, followed by 200 μl of sterile isotonic saline to ensure all of the dye was delivered. Additional 300-μl blood samples were taken after 5 and 10 minutes of Evans blue injection from the arterial catheter. The concentration of the Evans blue in the plasma was then measured on an Epoch microplate reader (Biotek, Winooski, VT). Plasma volume was calculated from the known quantity of the dye injected and the concentration of the dye in the plasma using the following formula: 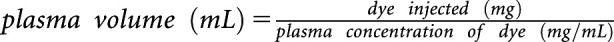 .
.
Urinary and Blood Measurements
A subset of rats in the pregnancy group was placed in metabolic cages for 24-hour urine collection at baseline prior to surgery, after 1 month of recovery (30 days after surgery), and during late pregnancy (GD19–GD20, n=6). Urine collections were also performed in the virgin IR and sham CONs at baseline prior to surgery, after 1 month of recovery (30 days after surgery), and prior to termination of the study (n=5). Blood samples were collected via the tail vein prior to surgery, 24 hours after surgery, and during late pregnancy (GD20). Urinary protein excretion was measured by Bradford Assay (Bio-Rad, Hercules, CA). Urinary albumin excretion was measured by the commercially available Albumin Blue fluorescent assay (Active Motif, Inc., Carlsbad, CA) according to the manufacturer’s instructions. Plasma urea was determined by commercially available QuantiChrom Urea Assay (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions. Creatinine was measured in the blood and urine via commercially available QuantiChrom Creatinine Assay (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions. Creatinine clearance was calculated by the following formula:
 |
Uterine Artery Resistance Index
Power Doppler velocimetry measurements were performed on anesthetized (approximately 1.5% isoflurane) pregnant dams at an imaging station with a Vevo 770 unit (FUJIFILM VisualSonics). Rats were placed on a platform supine, and hair was removed on the abdomen via depilatory cream. Measurements were taken during midpregnancy (GD14) and late pregnancy (GD18) using a 30-Hz transducer (RMV 710B) and an insonating angle <30°. To determine uterine artery resistance index (UARI), peak systolic flow velocity (PSV) and end diastolic flow velocity were analyzed using VisualSonics software, and UARI was calculated using the following formula: UARI = (PSV − end diastolic flow velocity)/PSV. UARI was measured in three different locations along the uterine artery in each rat, and the mean UARI was calculated for each dam. To limit potential effects of isoflurane on the fetuses, rats were under anesthesia for a maximum of 15 minutes.
Tissue Collection and Fetal Biometrics
Rats were euthanized during late pregnancy on GD20 under isoflurane anesthesia. A blood sample was taken from the abdominal aorta. The numbers of viable and resorbed fetuses were recorded from each uterine horn, and placental weight, fetus weight, and fetus length were recorded for each fetus. Fetal data are reported as the average of all pups in a litter. Fetal demise was calculated as the number of fetal resorptions (nonviable fetuses) divided by the total number of all pups (viable and nonviable).
Statistical Analyses
All data are presented as mean ± SEM. Maternal data are presented as n=1 dam, where each dot on the graph represents the data from one dam. For the fetal data, all pups in the same litter were averaged together, and data are presented as n=1 litter. Statistical analyses were performed by t test, one-way ANOVA, or two-way ANOVA, as indicated for each graph, using GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA). Means were considered significantly different if P<0.05.
Results
Renal IR Results in Renal Injury That Resolves within 1 Month under Normal Physiologic Conditions
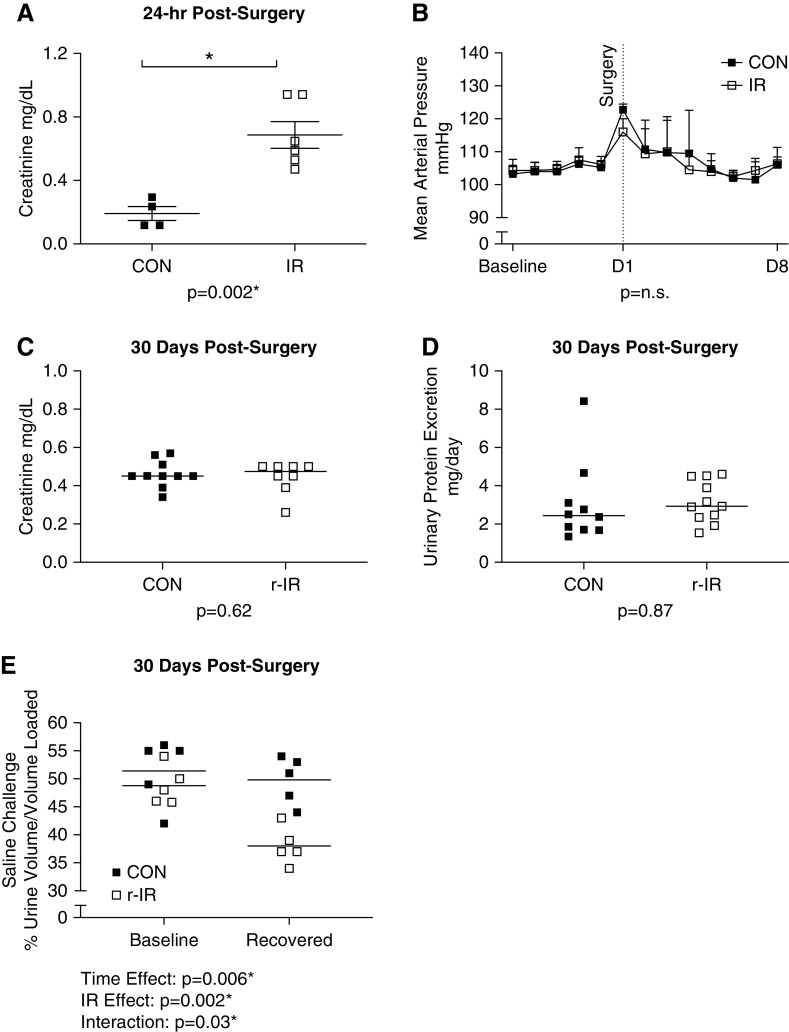
Renal IR injury resulted in a two- to three-fold increase in plasma creatinine compared with sham CONs 24 hours after surgery (Figure 1A). MAP was measured by telemetry in a separate set of rats. Surgical intervention resulted in an acute increase in MAP in both IR and CON (Figure 1B), although there was no difference in MAP between the groups, and MAP returned to baseline within 3 days of surgery. After 1 month of recovery, renal function returned to baseline in the IR group (r-IR) and was comparable with CON as measured by both plasma creatinine (Figure 1C) and urinary protein excretion (Figure 1D). A saline challenge was performed prior to surgery and after 30 days of recovery to further assess renal function post-IR. Prior to surgery, there was no difference between groups in the ability to increase renal function in response to saline challenge (Figure 1E). However, despite plasma creatinine and urinary protein excretion returning to CON levels, renal function in the r-IR rats did not adapt in response to the saline bolus compared with CON rats.
Figure 1.
Renal IR results in renal injury that resolves within 1 month without affecting BP. (A) Plasma creatinine was measured by colorimetric assay 24 hours after surgery in IR and CON rats (n=4–6). (B) MAP was measured via telemetry over a 5-day baseline period and then for 1 week after surgery in IR and CON rats (n=3). (C) Plasma creatinine was measured by colorimetric assay after 1 month of recovery in r-IR and CON rats from a blood sample from the tail vein (n=4–6). (D) Urinary protein excretion was measured by Bradford assay after 1 month of recovery in r-IR and CON rats (n=4–6). (E) Urine volume was measured in response to a saline challenge in r-IR and CON rats prior to surgery and after 30 days of recovery (n=5). Data were analyzed by (A, C, and D) t test, (B) repeated measures one-way ANOVA within groups and t test between groups, and (E) two-way ANOVA. *P<0.05.
Dams That Have Recovered Renal Function after IR Have Pregnancy-Induced Renal Injury and Renal Insufficiency Compared with CON Dams
In a healthy pregnancy, plasma creatinine decreases compared with virgin CONs as observed in CON rats on GD20 (Figure 2A). However, in pregnant rats following recovery from IR, plasma creatinine was elevated compared with virgin CONs. Similarly, plasma urea decreased in CON dams but increased in r-IR dams in late pregnancy compared with CON and r-IR virgin CONs (Figure 2B). Urinary creatinine increased during pregnancy in both r-IR and CON dams compared with virgin CONs. Creatinine clearance was calculated as a surrogate measure of GFR using creatinine from the plasma and urine during late pregnancy (GD19–GD20). Creatinine clearance increased in CON dams compared with virgin CONs as expected, but did not increase during pregnancy in r-IR dams (Figure 2D). r-IR dams also exhibited a greater increase in urinary protein excretion (Figure 2E) and urinary albumin excretion (Figure 2F) during late pregnancy compared with virgin CONs and CON dams. Interestingly, r-IR dams also had a blunted increase in plasma volume during late pregnancy on GD20, compared with the robust increase in plasma volume seen in the CON dams compared with CON virgins (Figure 2G). There were no observed differences in food or water consumption in the r-IR dams compared with CON dams during late pregnancy from GD19 to GD20 (Table 1).
Figure 2.
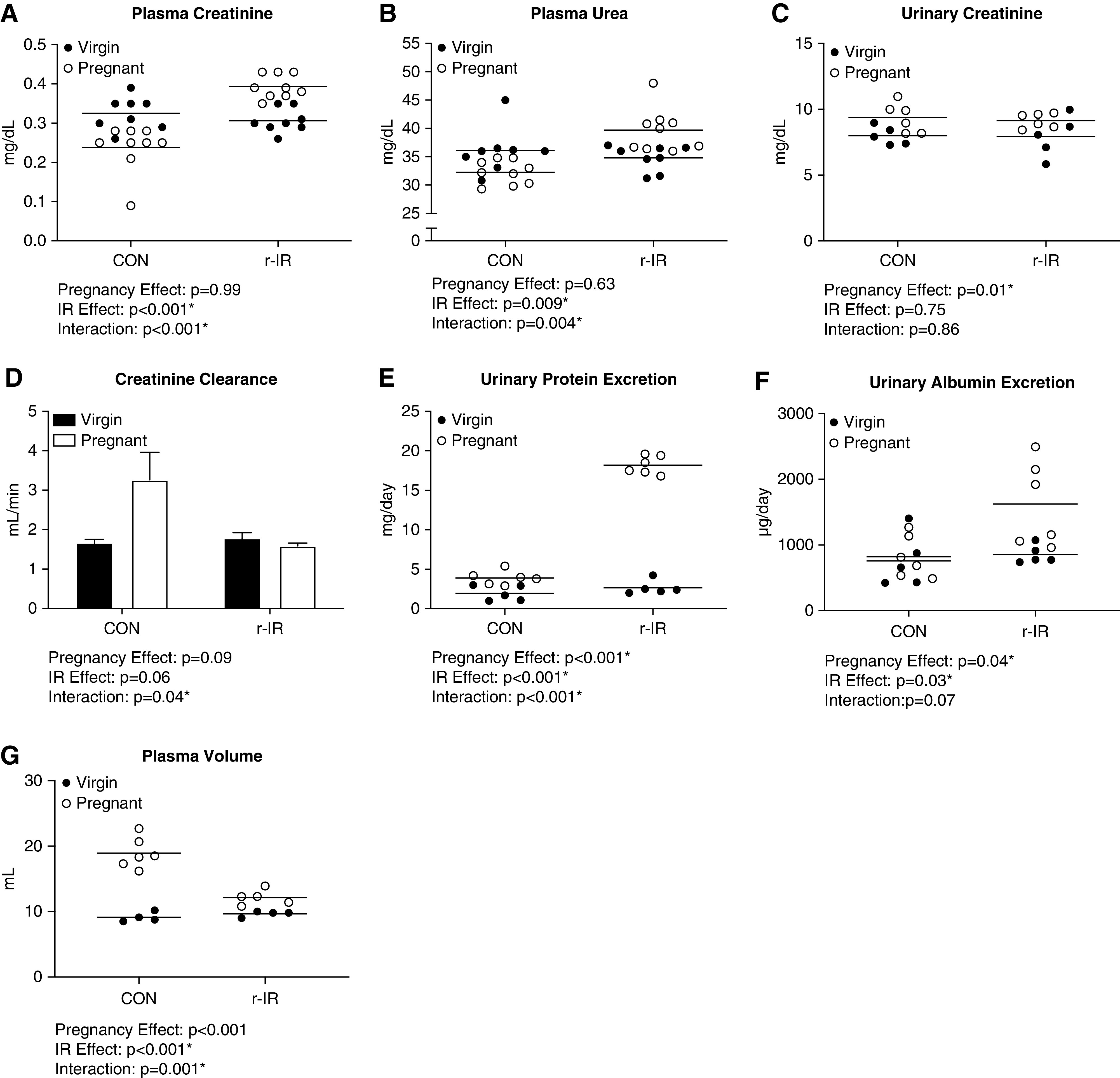
Dams that have recovered renal function after IR have pregnancy-induced renal injury and renal insufficiency compared with CON dams. (A) Plasma creatinine was measured by colorimetric assay during late pregnancy on GD20 in r-IR and CON dams and age-matched virgin CONs (n=8–9). (B) Plasma urea was measured by colorimetric assay during late pregnancy on GD20 in r-IR and CON dams and age-matched virgin CONs (n=8–9). (C) The 24-hour urinary creatinine excretion was measured by colorimetric assay during late pregnancy from GD19 to GD20 in r-IR and CON dams and age-matched virgin r-IR and CONs (n=5–6). (D) Creatinine clearance was calculated during late pregnancy in r-IR and CON dams and age-matched virgin CONs (n=5–6). (E) Urinary protein excretion was measured via Bradford assay during late pregnancy on GD19–GD20 in r-IR and CON dams and age-matched virgin CONs (n=5–6). (F) Urinary albumin excretion was measured by fluorescent assay during late pregnancy on GD19–GD20 in r-IR and CON dams and age-matched virgin CONs (n=5–6). (G) Plasma volume was measured by Evans blue dilution during late pregnancy on GD20 in CON and r-IR dams and age-matched virgin CONs (n=4–6). Data were analyzed by two-way ANOVA. *P<0.05.
Table 1.
Metabolic data for CON and r-IR pregnant rats during late pregnancy from GD19 to GD20, with age-matched virgin CONs
| Experimental Groups | Body Weight, g | Urine, ml | Food, g | Water Intake, ml |
|---|---|---|---|---|
| CON virgin | 260.4±1.5 | 10.5±2.6 | 13.3±1.0 | 23.6±4.7 |
| CON pregnant | 341.5±8.5 | 25.5±4.3 | 18.85±2.7 | 45.3±4.6 |
| r-IR virgin | 272.8±2.8 | 10.6±2.4 | 14.5±1.56 | 26.3±3.9 |
| r-IR pregnant | 335.3±5.1 | 13.9±2.0 | 20.6±0.8 | 39.7±1.6 |
| IR effect | P=0.59 | P=0.08 | P=0.40 | P=0.71 |
| Pregnancy effect | P=0.01 | P=0.01 | P=0.01 | P=0.01 |
| Interaction | P=0.11 | P=0.07 | P=0.89 | P=0.30 |
Data are presented as mean ± SEM and analyzed by two-way ANOVA.
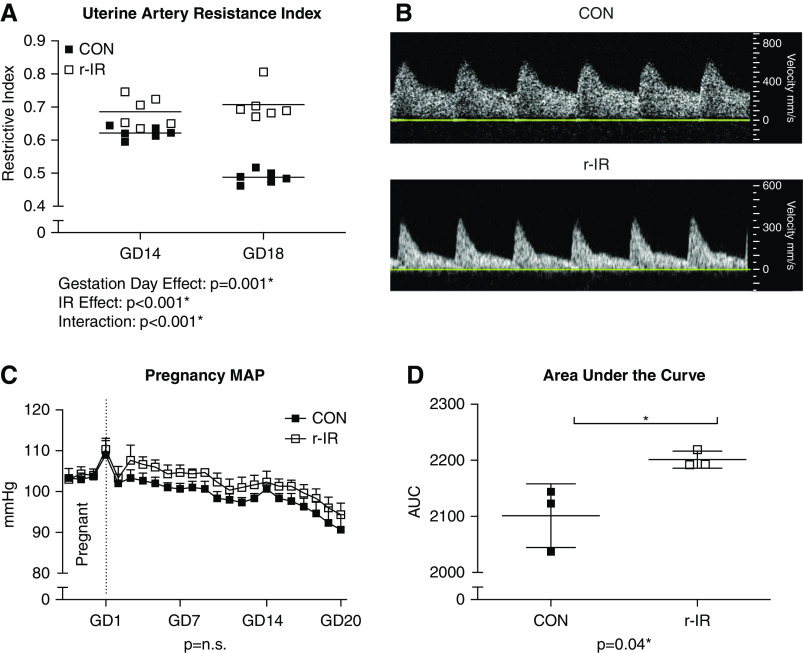
Dams That Have Recovered from IR Have Increased UARI and a Higher BP Load during Pregnancy Compared with CON Dams
UARI is typically high during midpregnancy but decreases during late pregnancy due to systemic vasodilation in healthy pregnancy, as observed in CON dams (Figure 3A). In contrast, UARI remains elevated during late pregnancy in rats following recovery from IR. The difference in UARI is easily visualized in the representative Doppler waveforms obtained during late pregnancy in CON (Figure 3B, upper panel) and r-IR rats (Figure 3B, lower panel). Despite elevated UARI, r-IR dams did have the expected decrease in MAP during pregnancy, and the difference in MAP was not significantly different than CON at any given time point (Figure 3C). However, r-IR dams exhibit a higher MAP than CON dams throughout pregnancy, resulting an increase in total pressure load as calculated by area under the curve (Figure 3D).
Figure 3.
Dams that have recovered from IR have increased UARI and higher BP loads during pregnancy compared with CON dams. (A) UARI was measured via Doppler ultrasound on GD14 and GD18 during midpregnancy and late pregnancy in r-IR dams and CON dams (n=6). (B) Representative waveforms of uterine artery resistance in (upper panel) CON and (lower panel) r-IR dams during late pregnancy on GD18. (C) MAP was measured via telemetry for a baseline period 3 days prior to pregnancy and then throughout pregnancy in r-IR and CON dams (n=3). (D) Area under the curve analysis for MAP throughout pregnancy for each r-IR and CON dam (n=3). Data were analyzed by (A) two-way ANOVA, (C) repeated measures one-way ANOVA within groups and t test between groups, and (D) t test. *P<0.05.
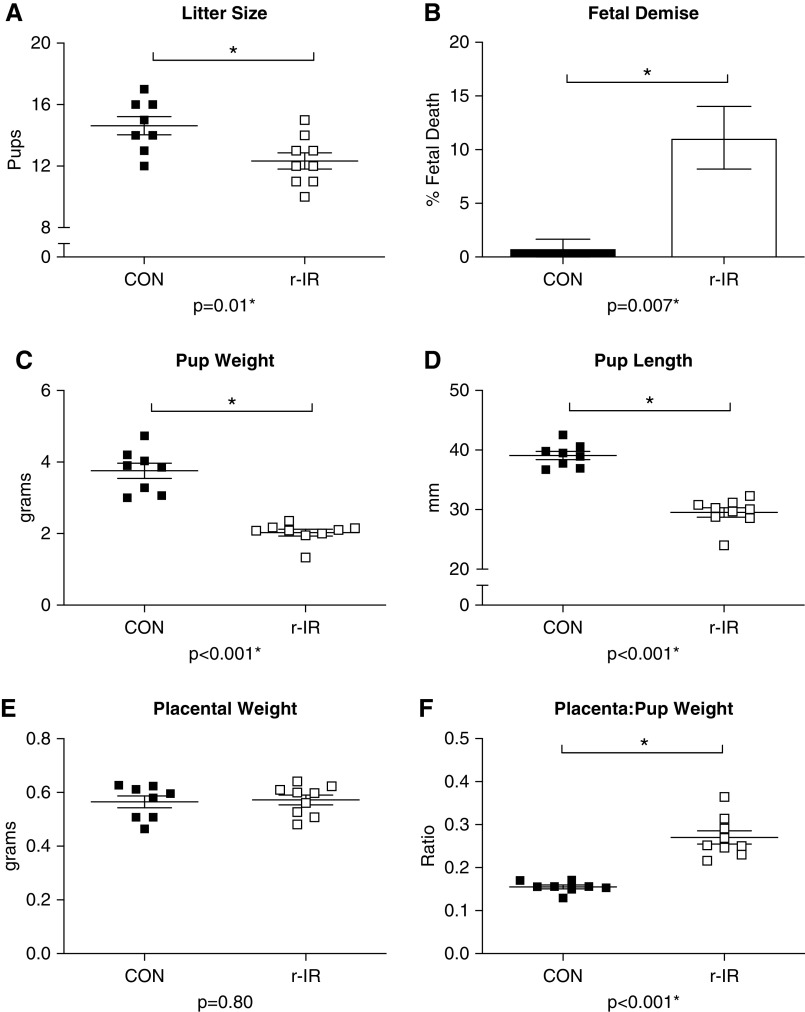
Dams That Have Recovered from IR Have Fetal Growth Restriction and Higher Rates of Fetal Demise than Sham Dams
r-IR dams had significantly fewer pups than CON dams (Figure 4A). These smaller litter sizes can partially be attributed to an increased rate of fetal demise in the r-IR dams, with approximately 11% of all pups dying in utero in the r-IR dams (Figure 4B) compared with only one instance of fetal demise in CON dams. In addition to having fewer pups, r-IR dams also had significantly smaller pups as seen by a decrease in average pup weight (Figure 4C) and pup length (Figure 4D) compared with CON dams. Despite having smaller pups, placental weight was similar in r-IR and CON dams (Figure 4E), resulting in a significant increase in the placenta-pup weight ratio (Figure 4F).
Figure 4.
Dams that have recovered from IR have fetal growth restriction and higher rates of fetal demise than CON dams. (A) The number of pups per litter was counted on GD20 during late pregnancy in r-IR and CON dams (n=8–9 dams). (B) The number of resorptions (nonviable fetuses) in the uterus of each dam was recorded on GD in r-IR and CON dams (n=8–9 dams). (C) Average pup weight per litter was recorded in grams on GD20 in r-IR and CON dams (n=8–9 dams). (D) Average pup length from crown to rump was recorded per litter in millimeters on GD20 in r-IR and CON dams (n=8–9 dams). (E) Average placenta weight per litter was recorded in grams on GD20 in r-IR and CON dams (n=8–9 dams). (F) Placenta-pup weight ratio was calculated and presented as average per litter on GD20 (n=8–9 dams). Data were analyzed by t test. *P<0.05.
Discussion
Pregnancy is a unique physiologic state with increased renal demands as plasma volume expansion occurs early in pregnancy. In healthy women, the kidney is able to sufficiently adapt to this increase in renal function. Recent clinical studies report that women with a history of AKI have poor maternal and fetal outcomes in pregnancy, despite having normal renal function prior to conception.13,14 In order to better understand the mechanisms by which AKI predisposes these women to adverse pregnancy events, this study aimed to develop an animal model of pregnancy after recovery from AKI. The major findings of this study were that after recovery from IR, pregnant rats exhibit (1) pregnancy-induced renal insufficiency, (2) elevated UARI, and (3) poor fetal outcomes.
RFR is the capacity of the kidney to increase GFR in response to a physiologic stressor, such as the increased plasma volume expansion in pregnancy. RFR has also been proposed to be a more accurate indicator of renal recovery after AKI than serum creatinine, as patients may have normal serum creatinine values yet reduced RFR.18 RFR is critically important during pregnancy, as renal demand is drastically increased, with a 50% increase in GFR and 80% increase in renal plasma flow.19 Typically, this rise in kidney function during pregnancy is not a problem in healthy young women with uncompromised RFR, but it has recently been proposed that adverse pregnancy outcomes in women with mild renal dysfunction may be due to a deficit in RFR.20 Additionally, pregnancies complicated by hypertension21 or preexisting CKD12 are characterized by a failure of renal function to increase during pregnancy. In this study, despite having normal renal function prior to pregnancy under basal conditions as determined by serum creatinine levels 1 month after IR, rats had pregnancy-induced renal dysfunction, with elevations in plasma creatinine and urea. Additionally, rats subjected to a saline challenge 30 days after recovery from IR were unable to adapt their renal function to excrete the saline load compared with CON rats. These data suggest IR results in RFR deficits that do not affect standard measures of baseline renal function but are unmasked by physiologic stress, such as pregnancy or a saline challenge. Although women are protected from the long-term effects of IR injury relative to men,22 previous studies have not investigated the effect of pregnancy following IR injury.
In addition to the increased renal demands of pregnancy, the vasculature also undergoes substantial remodeling to accommodate the increased blood flow to the uterus. Peripheral vascular resistance decreases in healthy pregnancy, and maternal uterine arteries undergo outward hypertrophic remodeling to allow increased blood flow to the placenta.23 Uteroplacental blood flow can be assessed noninvasively via Doppler ultrasound by measuring UARI.24 In a healthy pregnancy, UARI decreases during the second trimester as uterine arteries vasodilate to supply blood to the placenta.25 However, in pregnancies complicated by intrauterine growth restriction (IUGR), this resistance remains elevated, leading to a decrease in blood flow to the placenta and a decrease of oxygen and nutrient delivery to the fetus.26,27 Elevated UARI is one of the earliest and most reliable predictors of IUGR in the clinical population.28 In this study, UARI was elevated in rats after recovery from IR during both midpregnancy and late pregnancy compared with sham pregnant rats. This failure of the uterine artery to dilate suggests that there is maternal vascular dysfunction present and indicates restriction of blood flow and nutrient supply to the growing fetuses. This is further supported by the fetal data, with the pups from IR dams being significantly smaller than their CON counterparts.
IUGR is a significant problem in pregnancy, as it not only affects fetal growth and increases the risk of fetal death29 but also, results in fetal programming that can have lifelong effects.30–33 Numerous clinical and basic science studies have reported a link between IUGR and high rates of hypertension, diabetes, and metabolic disorders in adulthood.33–35 In this study, fetal outcomes were significantly impaired in IR dams compared with pups from CON dams, as measured by the decrease in pup weight and high rate of fetal demise. There is also evidence of placental inefficiency in this study, as placentas from r-IR dams were of similar size to CON dams, despite the significant decrease in the pup size. Abnormal placentation in this model may serve to compensate for the inadequate plasma volume expansion, and further studies are required to fully elucidate the mechanisms underlying these changes in placentation and plasma volume during pregnancy. Understanding the mechanism by which renal IR induces IUGR in pregnancy is important not only to improve maternal health but also, to improve the fetal outcomes.
One limitation of this model is that we do not report hypertension in pregnancy as was seen in the clinical studies,13,14 although there is an increase in overall BP load. Preeclampsia and gestational hypertension were commonly observed in the clinical studies of women with a history of AKI. Despite not developing overt hypertension in this study, the renal insufficiency induced by pregnancy alone after IR resulted in poor maternal and fetal outcomes that have been shown clinically to negatively affect overall health long term.
In this study, we demonstrate that IR injury prior to conception negatively affects maternal and fetal outcomes, despite the fact that kidney function apparently returns to baseline prior to pregnancy. Many of the findings in this study mirror what has been reported in the clinical literature,13,14 including poor fetal growth and decreased survival. We propose that this model will serve as a useful tool to better understand the long-term consequences of AKI on renal function and the role of kidney function on pregnancy outcomes.
Disclosures
M.W. Brands reports research funding from National Institutes of Health R01. J.C. Sullivan reports being a scientific advisor or member with Central Savannah River Area American Heart Association Board of Directors. The remaining author has nothing to disclose.
Funding
This study was supported by National Heart, Lung, and Blood Institute grants HL127091 and HL150281.
Acknowledgments
E.E. Gillis and J.C. Sullivan contributed to experimental design; M.W. Brands and E.E. Gillis performed the experiments; M.W. Brands and E.E. Gillis collected and analyzed data; E.E. Gillis drafted the manuscript and made the figures; and M.W. Brands, E.E. Gillis, and J.C. Sullivan edited and approved the final submission.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Animal Model of Pregnancy after Acute Kidney Injury Mirrors the Human Observations,” on pages 259–260.
References
- 1.Doyle JF, Forni LG: Acute kidney injury: Short-term and long-term effects. Crit Care 20: 188, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavkov ME, Harding JL, Burrows NR: Trends in hospitalizations for acute kidney injury—United States, 2000-2014. MMWR Morb Mortal Wkly Rep 67: 289–293, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawhney S, Fraser SD: Epidemiology of AKI: Utilizing large databases to determine the burden of AKI. Adv Chronic Kidney Dis 24: 194–204, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CH, Fan PC, Chang MY, Tian YC, Hung CC, Fang JT, et al.: Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PLoS One 9: e109649, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuhrman DY, Kane-Gill S, Goldstein SL, Priyanka P, Kellum JA: Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16-25 years of age treated in an adult intensive care unit. Ann Intensive Care 8: 26, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al.: Acute disease quality initiative workgroup 16. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initaitive (ADQI) 16 worksgroup. Nat Rev Nephrol 13: 241–257, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Kellum JA, Sileanu FE, Bihorac A, Hoste EAJ, Chawla LS: Recovery after acute kidney injury. Am J Respir Crit Care Med 195: 784–791, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, et al.; ADQI XIII Work Group: Progression after AKI: Understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol 27: 687–697, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husain-Syed F, Ferrari F, Sharma A, Hinna Danesi T, Bezerra P, Lopez-Giacoman S, et al.: Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol Dial Transplant 34: 308–317, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Dunlop W: Serial changes in renal haemodynamics during normal human pregnancy. Br J Obstet Gynaecol 88: 1–9, 1981 [DOI] [PubMed] [Google Scholar]
- 11.Fischer MJ: Chronic kidney disease and pregnancy: Maternal and fetal outcomes. Adv Chronic Kidney Dis 14: 132–145, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Williams D, Davison J: Chronic kidney disease in pregnancy. BMJ 336: 211–215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangren JS, Wan Md Adnan WAH, Powe CE, Ecker J, Bramham K, Hladunewich MA, et al.: Risk of preeclampsia and pregnancy complications in women with a history of acute kidney injury. Hypertension 72: 451–459, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangren JS, Powe CE, Ankers E, Ecker J, Bramham K, Hladunewich MA, et al.: Pregnancy outcomes after clinical recovery from AKI. J Am Soc Nephrol 28: 1566–1574, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Research Council : Guide for the Care and Use of Laboratory Animals, 8th Ed., Washington, DC, National Academies Press, 2011 [Google Scholar]
- 16.Lu X, Rudemiller NP, Privratsky JR, Ren J, Wen Y, Griffiths R, et al.: Classical dendritic cells mediate hypertension by promoting renal oxidative stress and fluid retention. Hypertension 75: 131–138, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fekete A, Sasser JM, Baylis C: Chronic vasodilation produces plasma volume expansion and hemodilution in rats: Consequences of decreased effective arterial blood volume. Am J Physiol Renal Physiol 300: F113–F118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Mucino MJ, Ronco C: Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127: 94–100, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Cheung KL, Lafayette RA: Renal physiology of pregnancy. Adv Chronic Kidney Dis 20: 209–214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koratala A, Kazory A: Renal functional reserve and pregnancy outcomes. Kidney Int 92: 768, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Lopes van Balen VA, van Gansewinkel TAG, de Haas S, Spaan JJ, Ghossein-Doha C, van Kuijk SMJ, et al.: Maternal kidney function during pregnancy: Systemic review and meta-analysis. Ultrasound Obstet Gynecol 54: 297–307, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima-Posada I, Portas-Cortés C, Pérez-Villalva R, Fontana F, Rodríguez-Romo R, Prieto R, et al.: Gender differences in the acute kidney injury to chronic kidney disease transition. Sci Rep 7: 12270, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osol G, Mandala M: Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudinger BJ, Giles WB, Cook CM: Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. Br J Obstet Gynaecol 92: 39–45, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Gómez O, Figueras F, Fernández S, Bennasar M, Martínez JM, Puerto B, et al.: Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet Gynecol 32: 128–132, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ghi T, Contro E, Youssef A, Giorgetta F, Farina A, Pilu G, et al.: Persistence of increased uterine artery resistance in the third trimester and pregnancy outcome. Ultrasound Obstet Gynecol 36: 577–581, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Roberts LA, Ling HZ, Poon LC, Nicolaides KH, Kametas NA: Maternal hemodynamics, fetal biometry and Doppler indices in pregnancies followed up for suspected fetal growth restriction. Ultrasound Obstet Gynecol 52: 507–514, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Coleman MA, McCowan LM, North RA: Mid-trimester uterine artery Doppler screening as a predictor of adverse pregnancy outcome in high-risk women. Ultrasound Obstet Gynecol 15: 7–12, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Figueras F, Gratacós E: Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn Ther 36: 86–98, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, et al. : Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 121: 2427–2436, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Barker DJ, Osmond C, Law CM: The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health 43: 237–240, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Kris-Etherton PM, Hartman TJ: Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J 18: 1423–1432, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Fagerberg B, Bondjers L, Nilsson P: Low birth weight in combination with catch-up growth predicts the occurrence of the metabolic syndrome in men at late middle age: The Atherosclerosis and Insulin Resistance study. J Intern Med 256: 254–259, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Gluckman PD, Hanson MA: Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatr Res 56: 311–317, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Seferovic MD, Goodspeed DM, Chu DM, Krannich LA, Gonzalez-Rodriguez PJ, Cox JE, et al. : Heritable IUGR and adult metabolic syndrome are reversible and associated with alterations in the metabolome following dietary supplementation of 1-carbon intermediates. FASEB J 29: 2640–2652, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]