Abstract
Membranous nephropathy (MN) occurs due to deposition of immune complexes along the subepithelial region of glomerular basement membrane. Two previously identified target antigens for the immune complexes, PLA2R (identified in 2009) and THSD7A (in 2014), account for approximately 60% of all MN, both primary and secondary. In the remaining MN, target antigens were unknown. Use of laser microdissection and mass spectrometry enabled identification of new “antigens.” This approach led to the identification of four novel types of MN: exotosin 1 (EXT1)– and exotosin 2 (EXT2)–associated MN, NELL1-associated MN, Sema3B-associated MN, and PCDH7-associated MN. Each of these represents a distinct disease entity, with different clinical and pathologic findings. In this review, the structure of the proteins and the clinical and pathologic findings of the new types of MN are discussed. The role of mass spectrometry for accurate diagnosis of MN cannot be overemphasized. Finally, any classification of MN should be made on the basis of the antigens that are detected. Further studies are required to understand the pathophysiology, response to treatment, and outcomes of these new MNs.
Keywords: kidney biopsy, membranous nephropathy, nephrotic syndrome
The diagnosis of membranous nephropathy (MN) is made on the basis of finding bright granular Ig staining along the glomerular basement membrane (GBM) on immunofluorescence (IF) microscopy and subepithelial electron-dense deposits on electron microscopy. Sometimes, the kidney biopsy specimen findings suggest association with a secondary cause, most commonly an autoimmune disease. MN is thus traditionally divided into primary MN, for patients that have no disease association, or secondary MN, for patients that have a disease association, such as an autoimmune disease, infection, malignancy, or drug toxicity.
The recognition that an autoimmune response to an antigen is responsible for MN was first shown in an animal model in 1959.1 However, it was not until 2009 that a causal antigen was identified, when a groundbreaking study identified M-type phospholipase A2 receptor 1 (PLA2R) as the target antigen in primary MN.2 This was followed, in 2014, by the identification of a second antigen, thrombospondin type 1 domain–containing 7A (THSD7A) in primary MN.3 PLA2R-associated and THSD7A-associated MN accounted for approximately 70% and 1%–5% of patients with primary MN, respectively.4
Following these discoveries, many renal biopsy laboratories started staining specimens for PLA2R. Thus, on the basis of positive or negative PLA2R staining on the kidney biopsy specimen, MN is diagnosed as PLA2R-positive or PLA2R-negative MN. THSD7A is quite rare, and only a few specialized laboratories perform THSD7A staining. No new antigens were discovered after 2014, although approximately 40% (including both primary and secondary) of the MN biopsy specimens are PLA2R negative. The importance of finding the remaining antigens cannot be overemphasized, because MN associated with specific antigens likely represent distinct diseases, each with different clinical courses, pathology findings, and responses to treatment.
Recently, an approach using laser microdissection and tandem mass spectrometry (MS/MS) has enabled detection of novel proteins in glomerular diseases, including identification of new types of amyloidosis.5 Thus, early experiments involved performing laser microdissection of PLA2R-positive MN glomeruli, followed by mass spectrometry, to determine whether PLA2R could be detected in the biopsy specimens. MS/MS easily detected high spectral counts of PLA2R in the patients with PLA2R-positive MN. Further, these initial studies suggested that other novel proteins and potential antigens could also be detected using MS/MS. Thus, we dissected glomeruli from PLA2R-negative MN and analyzed the data in an attempt to detect novel proteins/antigens by MS/MS analysis. In this review, we focus on the “new” antigens discovered by MS/MS using the Mayo Clinic cohort, findings subsequently validated by French, Belgian, and Italian cohorts.
MS/MS identifies approximately 1500–2000 proteins in glomerular extracts. It also allows for semiquantitative measurement of proteins and uses spectral counts to compare their relative abundance. Most of these proteins have housekeeping functions, and many are present in low total spectral counts. The basic premise in finding a new antigen or antigens has been to identify a unique protein with high spectral counts in PLA2R-negative MN that is absent (or present in low spectral counts) in PLA2R-positive MN, and in control patients, such as patients with IgA nephropathy, diabetes, FSGS, zero-time transplant biopsies, and other conditions. Once a unique protein was identified, we performed immunohistochemistry (IHC), using an antibody to the unique protein, and looked for the presence of the membranous (granular) staining pattern along the GBM. This was really the litmus test in identifying the putative antigen. Some upregulated proteins in MN that appeared to be unique failed to show the granular GBM staining of MN; some were present in control patients or did not stain at all. Amid the tedious task of ruling out the nonspecific staining of many proteins, we detected four unique proteins in PLA2R-negative MN. After the discovery of a unique MN antigen, we screened a large number of patients with PLA2R-negative MN by IHC to find additional patients with MN with the specific antigen and performed MS/MS in each case to confirm the IHC findings. We then used confocal microscopy to colocalize the antigen and IgG, and performed Western blot analysis on serum samples to detect circulating antibodies to the “antigen.”
The first novel proteins and putative antigens were exostosin 1/exostosin 2 (EXT1/EXT2). EXT1/EXT2 were the most common among the unique proteins in the initial MS/MS cohort of PLA2R-negative MN and are present in secondary (autoimmune) MN.6 This was followed by discovery of neural EGF-like-1 protein (NELL-1), which was the second most common MN antigen detected in the MS/MS cohort.7 The third novel antigen, not common but unique, was semaphorin 3B (Sema3B).8 Lastly, protocadherin 7 (PCDH7), another novel antigen that was recently detected in PLA2R-negative MN,9 is the third most common novel protein, after EXT1/EXT2 and NELL1, in our MS/MS cohort of PLA2R-negative MN. Further studies are required to confirm the prevalence of these novel proteins and putative antigens in MN.
The New Membranous Nephropathies
EXT1/EXT2-Associated MN
Clinical Findings
The mean age of patients with EXT1/EXT2-MN was 35.7 (SD ±13.4) years. EXT1/EXT2-MN was predominantly present in women (at a ratio of 4:1) (Table 1). Most importantly, EXT1/EXT2-MN was associated with autoimmune findings, such as being positive for antinuclear antibody, anti–double-stranded DNA antibodies, anti–Sjögren syndrome–related antigen A or B antibodies, or anti-ribonucleoprotein antibodies. Most patients have an underlying autoimmune disease, such as SLE or mixed connective tissue disease. Approximately 30%–35% of patients with membranous (class V) lupus nephritis are positive for EXT1/EXT2, whereas the remaining are negative (A. Ravindran et al., unpublished observations). In addition, a subset of EXT1/EXT2-MN may coexist with class III/IV lupus nephritis. On the other hand, patients with pure proliferative lupus nephritis (class II, III, IV) are negative for EXT1 and EXT2. Other causes of secondary MN—such as infections, malignancy, and drugs—were not present in EXT1/EXT2-associated MN.
Table 1.
Clinical and pathologic findings
| MN | Mean Age (yr) | Sex (M: F) | Laboratory Findings | Disease Association | Serum Antibody | LM | IF | Complement | IgG Subtype | EM |
|---|---|---|---|---|---|---|---|---|---|---|
| EXT1/EXT2-MN (n=26) | 36 | 1:4 | ANA, dsDNA, SSA, SSB, others | Autoimmune diseases: lupus, MCTD/ | No | Proliferative features may be present | IgG, IgA/IgM | C3, C1q | IgG1 | SE, ME + SU +/− TRI |
| NELL1-MN (n=34) | 63 | 1:1 | Negative | Malignancy | Yes (nonreducing) | Nonproliferative | IgG | C3 | IgG1 | SE, segmental deposits |
| Sema3B-MN (n=11) | 7a; 36b | 6:4 | Negative | Family history | Yes | Nonproliferative | IgG, TBM + | C3 | IgG1 | SE, TRI, TBM + |
| PCDH7-MN (n=10) | 61 | 3:1 | Negative | None | Yes | Nonproliferative | IgG | −/Trace | IgG1, IgG4 | SE |
The numbers in parenthesis represent the total patients with each specific type of MN that were part of the original studies. M, male; F, female; LM, light microscopy; EM, electron microscopy; ANA, anti-nuclear antibody; dsDNA, double-stranded DNA; SSA, anti–Sjögren syndrome–related antigen A; SSB, anti–Sjögren syndrome–related antigen B; MCTD, mixed connective tissue disease; SE, subepithelial; ME, mesangial; SU, subendothelial; TRI, tubuloreticular inclusions.
Age in children.
Age in young adults.
Kidney Biopsy Specimen Findings
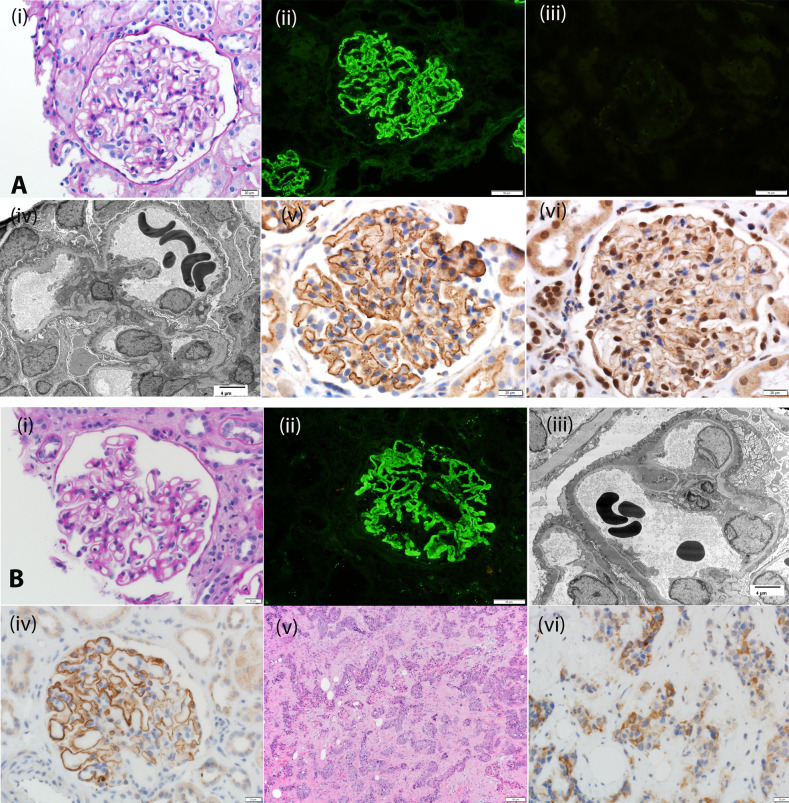
Kidney biopsy specimen findings exhibit features of secondary MN. Light microscopy shows thickened GBM; mesangial proliferative or even endocapillary proliferative GN may be present. Typically, IF microscopy shows bright granular GBM staining for IgG, C1q, and C3; IgA and IgM may be present too. IgG subtyping shows dominant IgG1 staining. Electron microscopy reveals the subepithelial GBM deposits. In addition, mesangial deposits and subendothelial deposits may also be present, depending on the class of lupus nephritis. Tubuloreticular inclusions are often present in endothelial cells. IHC shows bright granular GBM staining for both EXT1 and EXT2 (Figure 1A), with EXT1 tending to be slightly brighter than EXT2. Both EXT1 and EXT2 staining coexist; we have not seen any patient with only EXT1 or only EXT2 staining. There may be podocyte nuclear staining. The mesangium is negative for EXT1 and EXT2. There is no tubular basement membrane (TBM) staining for EXT1/EXT2, even when tubular deposits are present.
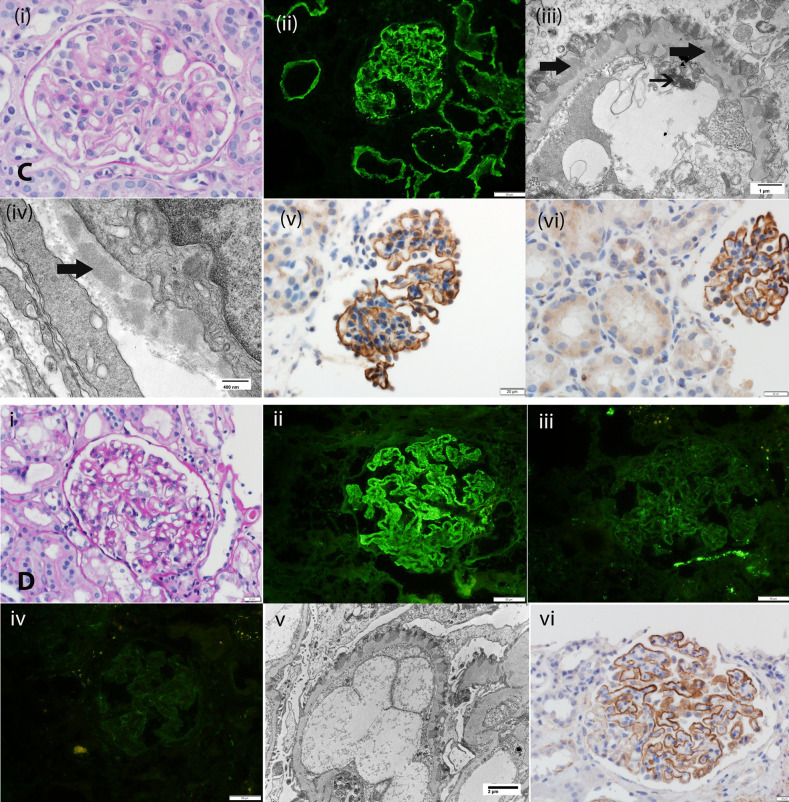
Figure 1.
Biopsy specimen findings of EXT1/EXT2-, NELL1-, Sema3B-, and PCDH7-associated MN. (A) EXT1/EXT2-associated MN: (i) Periodic acid–Schiff stain showing thickened glomerular capillary walls. Original magnification, ×40. (ii) IF microscopy showing bright C3 staining along glomerular capillary walls; (iii) IF microscopy is negative for PLA2R; (iv) electron microscopy showing subepithelial electron dense deposits. Original magnification, ×2900. IHC showing (v) EXT1 and (vi) EXT2 staining along the GBM. (B) NELL1-associated MN: (i) Periodic acid–Schiff stain showing thickened glomerular capillary walls. Original magnification, ×40. (ii) IF microscopy showing bright C3 staining along glomerular capillary walls; (iii) electron microscopy showing subepithelial electron dense deposits. Original magnification, ×2900. (iv) IHC showing NELL1 staining along the glomerular capillary walls; (v) hematoxylin and eosin stain showing squamous cell carcinoma; (vi) IHC showing that the tumor cells are positive for NELL1. (C) Sema3B-associated MN: (i) Periodic acid–Schiff stain showing thickened glomerular capillary walls. Original magnification, ×40. (ii) IF microscopy showing bright IgG staining along the GBM and TBM. Original magnification, ×20. (iii) Electron microscopy showing subepithelial electron dense deposits along GBM and tubuloreticular inclusions in endothelial cells. Original magnification, ×11,000. (iv) Electron microscopy showing TBM electron dense deposits. Original magnification, ×30,000. (v) IHC showing Sema3B staining along the GBM. Original magnification, ×40. (vi) IHC showing Sema3B staining along GBM but negative staining along the TBM. Original magnification, ×20. Arrows point to tubular basement membrane deposits. (D) PCDH7-associated MN: (i) Periodic acid–Schiff stain showing thickened glomerular capillary walls. Original magnification, ×40. (ii) IF microscopy showing bright IgG staining along the GBM. Original magnification, ×240. (iii) IF microscopy showing trace staining for C3, and (iv) negative staining for C1q. (v) Electron microscopy showing subepithelial electron dense deposits alongGBM. Original magnification, ×6800. (vi) IHC showing PCDH7 staining along the GBM. Original magnification, ×40.
Serum Antibodies
In our initial experiments, we could not detect circulating anti-EXT1/EXT2 antibodies by Western and native blotting analysis in nonreducing conditions. Hence, EXT1/EXT2 cannot be considered as MN antigens at this time. It is possible that the antibodies were not detected for two reasons. First, the serum antibody may not recognize the epitopes on recombinant EXT1/EXT2 proteins, because the antibodies may be against specific epitopes of truncated EXT proteins (EXT proteins in serum are truncated) that are not present in the recombinant EXT1/EXT2 protein. It is also possible that the serum antibodies are present in such a low titer that the glomerulus acts as a sink.
The Proteins
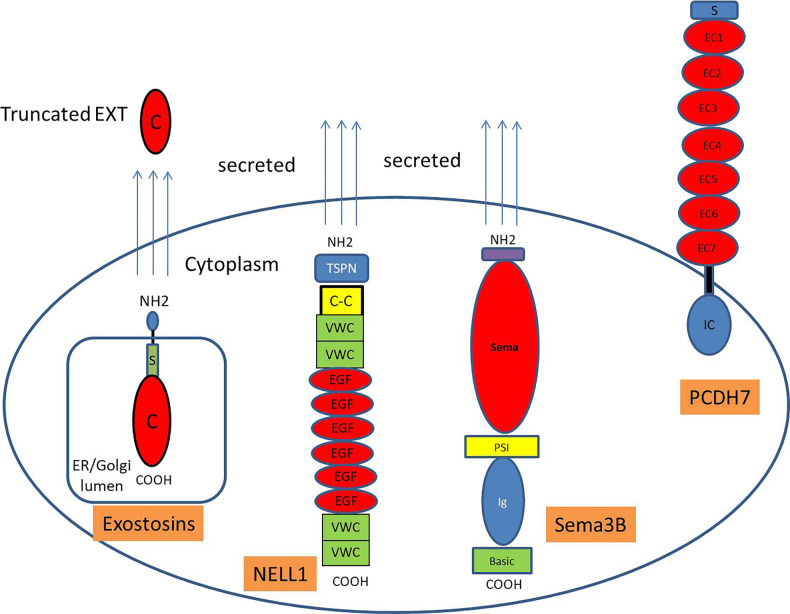
EXTs are glycosyltransferases that are responsible for the synthesis of the heparan-sulfate backbone that add glycosaminoglycan residues to the core protein, resulting in the generation of complex polysaccharides.10,11 They are transmembrane proteins that are localized to the endoplasmic reticulum and that distribute to the Golgi apparatus, where biosynthesis of the heparan sulfates occur (Figure 2). EXT proteins have a short amino-terminal cytoplasmic tail; a single transmembrane domain; a stem region; and a long, globular catalytic carboxy-terminal (C-terminal) domain within the Golgi lumen.12,13 Like other glycosyltransferases, EXTs are secreted into the extracellular medium in a truncated form.14 The EXT proteins are well conserved, especially in their C-terminal regions. There are five genes that encode the EXT proteins: EXT1, EXT2, EXTL1, EXTL2, and EXTL3. EXT1 and EXT2 show structural similarities, and EXT1 (86 kDa) and EXT2 (82 kDa) can exist as heterodimers and act as copolymerases in the elongation of the heparin-sulfate chain.15 The heterodimer of EXT1/EXT2 also has increased stability and activity.16 This is the likely reason that both EXT1 and EXT2 (the heterodimer form) are found together in EXT1/EXT2-associated MN.6 Except for EXTL1, the EXT proteins are ubiquitously expressed in various mammalian tissues, including podocytes. Mutations in EXT1 and EXT2 are associated with the autosomal-dominant disorder hereditary multiple exostoses, which is one of the most common inherited skeletal disorders.11,17 FSGS in a patient with multiple exostoses has been described.18
Figure 2.
Schematic representation of the MN proteins. Exostosins are transmembrane proteins in the Golgi apparatus that have a short amino-terminal cytoplasmic tail (NH2), a single transmembrane domain, a stem/stalk region (S), and a long globular catalytic C-terminal domain (C) within the Golgi lumen. Exostosins are secreted as truncated (C) proteins. NELL1 is a secreted protein and is characterized by an amino-terminal TSP-1-like (TSPN) domain, a coiled-coil (C-C) domain, two von Willebrand factor type C (VWC) domains, six EGF-like domains (E), and two VWC domains. Sema3B is a secreted protein with large sema domain region, plexin-semaphorin-integrin (PSI) domain, Ig domain, and short C-terminal basic domain. PCDH7 is a transmembrane protein with a signal (S) peptide, seven extracellular (EC) domains, a single pass transmembrane domain (black bar), and an intracellular (IC) cytoplasmic domain. COOH, carboxyl group; ER, endoplasmic reticulum.
Clinical Implications
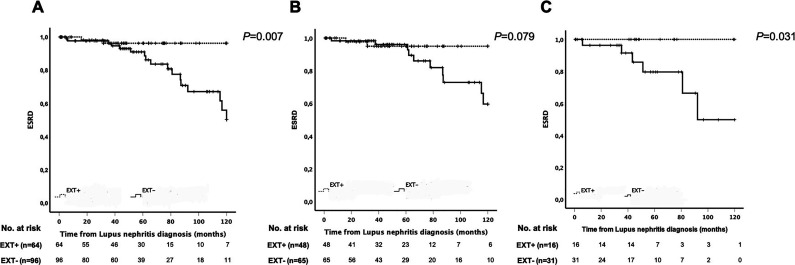
Most importantly, EXT1/EXT2-associated MN is present in patients with autoimmune disease, such as lupus and mixed connective tissue disorders. Because the circulating antibodies have yet to be detected, EXT1/EXT2 may be a biomarker for autoimmune MN and not an antigen. Further studies are required to confirm these findings. In rare cases, EXT1/EXT2-associated MN may be present even before development of the autoimmune disease. Our preliminary studies suggest that EXT1/EXT2-positive lupus membranous nephritis shows favorable biopsy specimen findings and clinical outcomes compared with EXT1/EXT2-negative lupus membranous nephritis, both with and without a proliferative class III/IV lupus nephritis component (Figure 3) (A. Ravindran et al., unpublished observations).
Figure 3.
Cumulative incidence of ESKD in patients with lupus membranous nephritis (LMN). Kaplan–Meier plots of the cumulative incidence of ESKD over 10 years. (A) EXT1/EXT2-positive and EXT1/EXT2-negative LMN (including class III/IV lupus nephritis [LN]): 64 EXT+ versus 96 EXT−; two versus 18 events; time to event, 116 versus 101 months; P=0.007. (B) EXT1/EXT2-positive and EXT1/EXT2-negative pure class V LMN (with no class III/IV LN): 48 EXT+ versus 65 EXT−; two versus 11 events; time to event, 115 versus 104 months; P=0.08. (C) EXT1/EXT2-positive and EXT1/EXT2-negative class V LMN + class III/IV LN: 16 EXT+ versus 31 EXT−; zero versus seven events; time to event in EXT−, 96 months; P=0.03. EXT1/EXT-positive represented by dotted lines and EXT1/EXT2-negative represented by solid lines. Plots courtesy of Dr. Aishwarya Ravindran and Dr. Marta C. Moura.
NELL1-Associated MN
Clinical Findings
The mean age of patients with NELL1-associated MN is 63.1 (SD ±10.4) years, with an almost equal male/female ratio. In a subgroup of patients with NELL1-associated MN, a coexisting malignancy may be present.
Kidney Biopsy Specimen Findings
Kidney biopsy specimens in NELL1-associated MN show features of MN with thickened GBM, and bright IgG and C3 along the GBM. The glomeruli are nonproliferative. IgG subtyping reveals predominantly IgG1. An interesting finding on IF microscopy and electron microscopy is the segmental GBM distribution of the immune deposits in a subset of the biopsy specimens.7,19 IHC with an anti-NELL1 antibody confirms the diagnosis, showing granular deposits along the GBM with absence of mesangial and TBM staining (Figure 1B). Furthermore, in patients with segmental deposits, the NELL1 staining corresponds to the segmental IgG and subepithelial deposits.
Serum Antibodies
Antibodies to NELL1 are present in patients with NELL1-associated MN. The antibodies are not detected under reducing conditions, suggesting that NELL1 autoantibody recognizes conformation-dependent epitopes.
The Protein
NELL1 is a gene named after its similarity to a gene called Nel that is strongly expressed in neural tissue encoding a protein with EGF-like repeats (Figure 2).20 NELL1 is a cytoplasmic, 90-kDa protein kinase C–binding protein containing a secretory signal peptide, amino-terminal thrombospondin-1–like molecule (TSPN), coiled-coil domain, four von Willebrand–type domains, and six EGF-like repeats. The thrombospondin-1-like is the heparin-binding domain, and the EGF repeats are the protein kinase C–binding domains.21 NELL1 is highly expressed in osteoblasts and promotes bone regeneration. The C-terminal region of NELL1 mediates osteoblastic cell adhesion through integrin α3β2.22,23 NELL1 is overexpressed in patients with craniosynostosis, one of the common congenital craniofacial deformities, where it is specifically upregulated within prematurely fusing sutures.24,25 In the kidney, NELL1 expression is higher in tubules; it is barely detectable in the glomeruli, although 5%–25% of glomerular cells express NELL1 at the mRNA level.26,27
Clinical Implications
In most cases, NELL1-associated MN appears as a primary MN. Malignancy may be present in a subgroup of NELL1-associated MN. The incidence of malignancy varies from 10% to 33%, depending on demographics.28 The MN may even precede the detection of the malignancy. Serum antibodies to NELL1 are detected in NELL1-associated MN. Further studies are required to determine the pathogenicity of NELL1 antibody and to correlate the antibody titers with the clinical course of the disease.
Sema3B-Associated MN
Clinical Findings
Sema3B-associated MN is primarily present in the pediatric population (comprising 73% of cases), with a smaller proportion of cases in young adults (27%). The mean age of the pediatric patients is 6.9 (SD ±6.8) years, and the mean age of the adult patients is 36.3 (SD ±7.2) years. Nearly half of Sema3B-associated MN cases are detected in children <2 years. Because MN is primarily a disease of adults, Sema3B-associated MN is rare and accounts for 1%–3% of all MN. However, in the pediatric age group, it accounts for approximately 15% of MN cases. It is present more often in male children, with a male/female ratio of 6.3:3.7.
Kidney Biopsy Specimen Findings
Light microscopy shows thickened GBM, and IF microscopy shows bright IgG and C3 along the GBM. However, interestingly, in the pediatric patients <2 years, there is also IgG staining along the TBM. IgG subtyping reveals predominantly IgG1. IHC using an anti-Sema3B antibody confirms the diagnosis, showing granular deposits along the GBM (Figure 1C). The TBM deposits in patients <2 years are negative for Sema3B. Electron microscopy shows subepithelial deposits and also shows the TBM deposits in such patients, who also have tubuloreticular inclusions in endothelial cells.
Serum Antibodies
Antibodies to Sema3B are present in patients with Sema3B-associated MN. These antibodies are detected under reducing conditions; this suggests that Sema3B autoantibody may recognize a cryptic epitope that is unmasked by disruption of disulfide bonds, and that an event is required to disrupt the disulfide bonds and expose the epitope.
The Protein
Semaphorins are a group of secreted and transmembrane/membrane-bound proteins containing a conserved extracellular semaphorin (sema) domain of about 500 amino acids that is characterized by highly conserved cysteine residues.29–31 The sema domain is the critical component through which semaphorins mediate their effects. The first semaphorins were identified as proteins that guide neuronal axons to their targets. Since then, >20 semaphorins have been identified and they are classified into eight subclasses. Class I and IV–VII are membrane associated (I, IV, V, and VI are transmembrane; VII is membrane bound), whereas class II, III, and VIII are secreted. Sema3B (83 kDa) is a secreted protein with a sema domain, a plexin-semaphorin-integrin domain, an Ig domain, and a basic domain (Figure 2). The semaphorin 3 family and their receptors have been detected in endothelial cells, podocytes, and tubular epithelial cells.29,32
Clinical Implications
Sema3B-assocated MN is present in children and young adults, some of whom have a family history—thus raising the possibility of a genetic basis of the disease. Also, because Sema3B-associated MN is present in children, it can be misdiagnosed as steroid-resistant nephrotic syndrome if a kidney biopsy is not performed. Finally, clinical response was varied in the small series of Sema3B-associated MN, and a subset of patients required calcineurin inhibitors, rituximab, or even cyclophosphamide to achieve remission.
PCDH7-Associated MN
Clinical Findings
The mean age of PCDH7-associated MN patients is 61 (SD ±11.7) years.9 Males are more frequently affected, with a male/female ratio of 3:1. Autoimmune disease and infections are not present. A single patient out of 12 (8.3%) with PCDH7-associated MN had an associated prostate carcinoma.
Kidney Biopsy Specimen Findings
Light microscopy shows thickened GBM, IF microscopy shows bright IgG staining along the GBM, and electron microscopy shows subepithelial GBM deposits. Mesangial deposits are not present. Interestingly, C1q and C3 staining are minimal or absent on IF microscopy. IgG subtypes show either predominantly IgG1 or IgG4. IHC with an anti-PCDH7 antibody confirms the diagnosis, showing granular deposits along the GBM (Figure 1D).
Serum Antibodies
Antibodies to PCDH7 are present in PCDH7-associated MN.
The Protein
Cadherins are a large group of transmembrane proteins on the cell surface that mediate cell-cell recognition and adhesion.33 They have a common structural domain called the extracellular cadherin (EC) domain that consists of approximately 110 amino acids; most EC domains have conserved calcium-binding sites. The name cadherin is thus derived from the calcium-dependent adhesive function of these proteins. The cadherins are further classified into subfamilies on the basis of the number and arrangement of EC domains. Thus, the cadherins are subdivided into classic (type-I) cadherins and closely related (type-II) cadherins, desmosomal cadherins, and protocadherins.34 Protocadherins have six to seven EC repeats that have low sequence EC similarities and a divergent cytoplasmic domain compared with classic cadherins. PCDH7 is a 116-kDa glycosylated protocadherin with seven EC repeats (Figure 2). The exact function of PCDH7 is unknown, but it likely plays a role in cell signaling.35
Clinical Implications
PCDH7 is mostly present in older patients. An important finding both on kidney biopsy specimens and mass spectrometry is that complement activation is minimal or absent. Some of the patients in our cohort went into spontaneous remission without immunosuppressive treatment. Further studies are needed to determine whether PCDH7-associated MN belongs to a group of MN that undergoes spontaneous remission and has good outcomes.
Mass Spectrometry for Diagnosis of MN
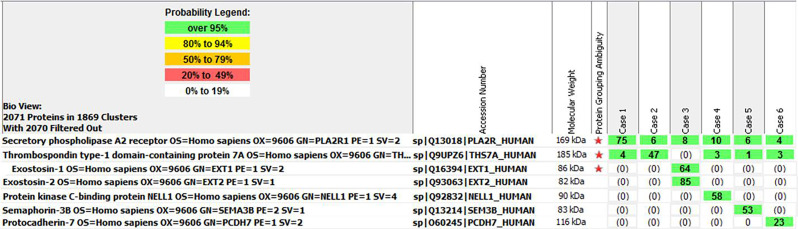
MS/MS typing of the antigens is made on the basis of detection of the unique MN-associated protein in the dissected glomeruli. Thus, in a typical case, moderate to high spectral counts of the specific antigen are detected, whereas findings for the other MN antigens are negative (Figure 4). Baseline spectral counts of PLA2R and THSD7A may be present in MN not associated with PLA2R and THSD7A. When one antigen is detected, other antigens are generally not present. We have only encountered a single case of dual NELL1- and PLA2R-associated MN on the basis of MS/MS only (unpublished data).
Figure 4.
Representative mass spectrometry and detection of antigens in MN. Case 1 is from a biopsy specimen of PLA2R-associated MN, case 2 from THSD7A-associated MN, case 3 from EXT1/EXT2-associated MN, case 4 from NELL1-associated MN, case 5 from Sema3B-associated MN, and case 6 from PCDH7-associated MN. Numbers in green boxes represent spectral counts of MS/MS matches to a respective protein. Red star indicates shared amino acid sequences among proteins.
MS/MS was critical in the identification of the new antigens in MN. As the list of MN antigens grows, use of MS/MS of biopsied kidney may be an alternative to IHC/IF to identify the antigen. Use of MS/MS has several advantages over IHC/IF. First, it offers a “one-stop” method to identify an MN antigen versus performing an IHC/IF stain for each specific antigen. Second, IF/IHC of less common MN antigens may be difficult to establish in most clinical laboratories. Third, additional new antigens can be identified with MS/MS. Fourth, this technique is less prone to staining interpretation error. And finally, MS/MS is likely a more sensitive and specific test for detecting the antigen. We have identified PLA2R-positive MN on the basis of MS/MS in patients that were negative on routine IF (unpublished data). Further studies are needed to confirm these findings.
The disadvantages of the use of MS/MS include the need for a specialized laboratory to establish MS/MS methodology. In addition, turnaround time for testing may be longer than for IF/IHC, at least initially. However, as experience in methodology grows, the antigen in MN can likely be typed within 3–4 days. The MS/MS approach in MN is reminiscent of amyloid detection and typing but can also be typed by IF/IHC. However, MS/MS is now the gold standard for confirmation of difficult amyloid cases and for detection of various, less common, amyloid types.36
Other MN Proteins in the Pipeline
MS/MS studies have detected other unique proteins in a smaller (1%–3%) subset of PLA2R-negative MN. These proteins are under active investigation at this time.
Other Potential MN Antigens
Two new potential antigens have been recently described. Neural cell adhesion molecule 1 has been detected in both primary MN and membranous lupus nephritis.37 Our MS/MS data also confirms the finding of neural cell adhesion molecule 1 in approximately 2%–4% of patients with primary MN and membranous lupus nephritis (unpublished data). Another potential antigen, high temperature requirement protein A1 has also been recently described in MN.38 Additional studies are required to confirm these findings.
Proposed Classification of MN
The historical basis of classification of MN into primary and secondary MN is that the etiology is unknown in primary MN, whereas, in secondary MN, the condition is associated with various diseases even though the etiology is unknown. The discovery of antigens associated with MN makes this classification debatable, because different antigens may be present in a primary setting, that is, with no detectable secondary disease. Thus, MN associated with PLA2R, THSD7A, NELL1, Sema3B, or PCDH7 may be present with no underlying disease association—that is, present as a primary MN. On the other hand, some MN antigens may be associated with a secondary disease, such as PLA2R-, THSD7A-, or NELL1-associated MN linked with a malignancy, and EXT1/EXT2-, Sema3B-, or even PLA2R-associated MN linked with an autoimmune disease. It is also recognized that some of the underlying diseases in secondary MN may be coincidental, and that treatment of the secondary disease, such as resection of a tumor, do not always correlate with outcomes of the MN.
It is also likely that each specific antigen-associated MN is distinctive with regard to pathophysiology, clinical and laboratory findings, disease associations, response to treatment, and outcomes. Further studies are required to confirm these differences between the various types of MN on the basis of the antigen detected. PLA2R-associated MN is the prototype of MN, where the disease course and response to treatment can be followed by determining the antibody titers to PLA2R,39 sometimes regardless of the disease association. Additional studies are required to determine whether a similar approach can be used to detect and follow the respective antibody titers in THSD7A-, NELL1-, and Sema3B-associated MN, and probably PCDH7-associated MN as well. However, to be able to do this, the classification of the MN should involve the underlying antigen.
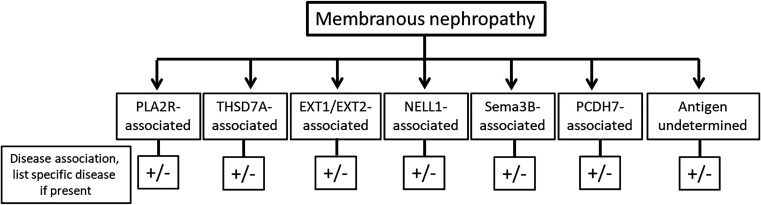
At the same time, an underlying secondary disease association in MN cannot be overlooked. Searching for an association with a disease or a toxic substance—such as a malignancy, infections such as hepatitis, or an adverse effect from a drug—is warranted in MN, because the search may indeed yield a positive disease association. An initial basic proposal would be to classify MN on the basis of the antigen detected (Figure 5), and, if present, on a disease association. MN-associated antigens can be expanded as new antigens are discovered, while the terminology “undetermined” can be used in MN cases where the antigen is not known. An example would be PLA2R-associated MN with no associated disease, EXT1/EXT2-associated MN with class V lupus nephritis, NELL1-associated MN with NELL1-expressing malignancy, MN with undetermined antigen associated with hepatitis or nonsteroidal anti-inflammatory drug use, and so on. When a secondary disease is identified, it may help direct therapy for MN—with treatment of both MN and secondary disease separately, or addressing the secondary disease alone as treatment of the MN.
Figure 5.
Proposed classification of MN. MN is classified on the basis of the antigen detected. In cases where none of the known antigens are detected, the terminology “undetermined” should be used. The disease association should be given if present, not present, or if not known.
Finally, with the identification of the new antigens, the classification of MN into PLA2R-positive and PLA2R-negative MN is outdated. It can be argued that MN is just a pattern of glomerular injury, and that PLA2R-, THSD7A-, EXT1/EXT2-, NELL1-, Sema3B-, and PCDH7-associated MN are different diseases that have a common “membranous” pattern of injury. This is similar to other glomerular diseases that have different etiologies but present with a common pattern of injury, such as membranoproliferative GN and FSGS.40,41
Conclusions
Four new antigens have been recently identified in MN. These include EXT1/EXT2, NELL1, Sema3B, and PCDH7. The clinicopathologic findings in these new types of antigen-associated MN appear distinctive. However, antibodies to EXT1/EXT2 have not yet been detected, and, as such, these proteins are best called putative antigens at present. Further studies are required to confirm these findings.
Disclosures
S. Sethi reports receiving honoraria from teaching, grand rounds, lectures, and reviewing slides for a study for Novartis.
Funding
None.
Acknowledgments
The discovery of new MN antigens would not be possible without my daily interactions and discussions with my friend, colleague, and collaborator, Dr. Fernando Fervenza. Most of our studies have been done together and this one is no different. I would like to thank Benjamin Madden and Cristine Charlesworth of the Proteomics Core, Mayo Clinic for performing mass spectrometry analysis. I would like to thank my collaborators Dr. Pierre Ronco and Dr. Hanna Debiec at Sorbonne Université/Tenon Hopital for their friendship; discussions; and performing validation studies, confocal analysis, and Western blot analysis. I would like to thank LouAnn Gross and Vivian Negron of the Pathology Research Core, Mayo Clinic, for performing the IHC studies. Thank you to Dr. Lilian M. P. Palma for critically reading the manuscript. Thanks to our resident Dr. Aishwarya Ravindran and visiting research fellow Marta Rodrigues Casal Moura for analysis of the clinical data. Finally, I would like to thank my colleagues in nephrology and renal pathology at the Mayo Clinic for their support and daily interactions.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Heymann W, Hackel DB, Harwood S, Wilson SG, Hunter JL: Production of nephrotic syndrome in rats by Freund’s adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med 100: 660–664, 1959 [DOI] [PubMed] [Google Scholar]
- 2.Beck LH Jr., Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couser WG: Primary membranous nephropathy. Clin J Am Soc Nephrol 12: 983–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S, Theis JD: Pathology and diagnosis of renal non-AL amyloidosis. J Nephrol 31: 343–350, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al.: Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 30: 1123–1136, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al.: Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97: 163–174, 2020 [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, et al.: Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 98: 1253–1264, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Negron V, et al.: Protocadherin-7 associated membranous nephropathy. Presented at the 2020 American Society of Nephrology Kidney Week, online, October 22–25, 2020 [DOI] [PMC free article] [PubMed]
- 10.Busse-Wicher M, Wicher KB, Kusche-Gullberg M: The exostosin family: Proteins with many functions. Matrix Biol 35: 25–33, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Ahn J, Lüdecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, et al.: Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat Genet 11: 137–143, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Duncan G, McCormick C, Tufaro F: The link between heparan sulfate and hereditary bone disease: Finding a function for the EXT family of putative tumor suppressor proteins. J Clin Invest 108: 511–516, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick C, Duncan G, Goutsos KT, Tufaro F: The putative tumor suppressors EXT1 and EXT2 form a stable complex that accumulates in the Golgi apparatus and catalyzes the synthesis of heparan sulfate. Proc Natl Acad Sci U S A 97: 668–673, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulson JC, Colley KJ: Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem 264: 17615–17618, 1989 [PubMed] [Google Scholar]
- 15.Busse M, Kusche-Gullberg M: In vitro polymerization of heparan sulfate backbone by the EXT proteins. J Biol Chem 278: 41333–41337, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Busse M, Feta A, Presto J, Wilén M, Grønning M, Kjellén L, et al.: Contribution of EXT1, EXT2, and EXTL3 to heparan sulfate chain elongation. J Biol Chem 282: 32802–32810, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Cook A, Raskind W, Blanton SH, Pauli RM, Gregg RG, Francomano CA, et al.: Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet 53: 71–79, 1993 [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts IS, Gleadle JM: Familial nephropathy and multiple exostoses with exostosin-1 (EXT1) gene mutation. J Am Soc Nephrol 19: 450–453, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kudose S, Santoriello D, Debiec H, Canetta PA, Bomback AS, Stokes MB, et al.: The clinicopathologic spectrum of segmental membranous glomerulopathy [published online ahead of print June 27, 2020]. Kidney Int 10.1016/j.kint.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 20.Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, et al.: New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn 203: 212–222, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Kuroda S, Oyasu M, Kawakami M, Kanayama N, Tanizawa K, Saito N, et al. : Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun 265: 79–86, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hasebe A, Tashima H, Ide T, Iijima M, Yoshimoto N, Ting K, et al.: Efficient production and characterization of recombinant human NELL1 protein in human embryonic kidney 293-F cells. Mol Biotechnol 51: 58–66, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Hasebe A, Nakamura Y, Tashima H, Takahashi K, Iijima M, Yoshimoto N, et al.: The C-terminal region of NELL1 mediates osteoblastic cell adhesion through integrin α3β1. FEBS Lett 586: 2500–2506, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Ting K, Vastardis H, Mulliken JB, Soo C, Tieu A, Do H, et al.: Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res 14: 80–89, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Carpenter D, Bokui N, Soo C, Miao S, Truong T, et al.: Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development. J Bone Miner Res 18: 2126–2134, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, et al.: Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics 38: 273–276, 1996 [DOI] [PubMed] [Google Scholar]
- 27.The Human Protein Atlas: NELL1. Available at: www.proteinatlas.org/ENSG00000165973-NELL1/tissue/kidney. Accessed November 26, 2020
- 28.Caza T, Hassen S, Dvanajscak Z, Kuperman M, Edmondson R, Herzog C, et al.: NELL1 is a target antigen in malignancy-associated membranous nephropathy [published online ahead of print August 20, 2020]. Kidney Int 10.1016/j.kint.2020.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan F, Villegas G, Teichman J, Mundel P, Tufro A: Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int 69: 1564–1569, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Alto LT, Terman JR: Semaphorins and their signaling mechanisms. In: Semaphorin Signaling: Methods and Protocols, edited by Terman JR, New York, Springer New York, 2017, pp 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazdani U, Terman JR: The semaphorins. Genome Biol 7: 211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A: Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int 73: 733–740, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Brasch J, Harrison OJ, Honig B, Shapiro L: Thinking outside the cell: How cadherins drive adhesion. Trends Cell Biol 22: 299–310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morishita H, Yagi T: Protocadherin family: Diversity, structure, and function. Curr Opin Cell Biol 19: 584–592, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Halbleib JM, Nelson WJ: Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20: 3199–3214, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A: Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Caza T, Hassen S, Kuperman M, Sharma S, Dvanajscak Z, Arthur J, et al.: Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis [published online ahead of print October 9, 2020]. Kidney Int 10.1016/j.kint.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Rabadi L, Caza T, Avillach C, Rodan AR, Williams B, Abraham J, et al.: High Temperature Recombinant Protein A1 (HTRA1): A novel antigen in membranous nephropathy. Presented at the 2020 American Society of Nephrology Kidney Week, online, October 22–25, 2020
- 39.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 421–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis—a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 41.De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC: Differentiating primary, genetic, and secondary FSGS in adults: A clinicopathologic approach. J Am Soc Nephrol 29: 759–774, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]