Significance Statement
In this prospective study of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in a cohort comprising all patients receiving RRT in Belgium’s Flanders region, the authors investigated whether such patients are especially vulnerable to the infection. After adjusting for age and taking into account SARS-CoV-2 under-reporting in the general population, risk of the infection did not appear to be severely increased among patients on RRT. Although mortality rates among patients on hemodialysis with SARS-CoV-2 infection were high, the authors did not find an overall excess mortality among the hemodialysis population during the epidemic wave because mortality was balanced by lower than expected mortality among patients without SARS-CoV-2 infection. These findings highlight the importance of correctly delineating the referral population and appropriately adjusting for sources of bias when reporting incidence and mortality of SARS-CoV-2 infection.
Keywords: hemodialysis, kidney transplantation, clinical epidemiology, mortality, virology, COVID-19
Visual Abstract
Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection disproportionally affects frail, elderly patients and those with multiple chronic comorbidities. Whether patients on RRT have an additional risk because of their specific exposure and complex immune dysregulation is controversial.
Methods
To describe the incidence, characteristics, and outcomes of SARS-CoV-2 infection, we conducted a prospective, multicenter, region-wide registry study in adult patients on RRT versus the general population from March 2 to May 25, 2020. This study comprised all patients undergoing RRT in the Flanders region of Belgium, a country that has been severely affected by coronavirus disease 2019 (COVID-19).
Results
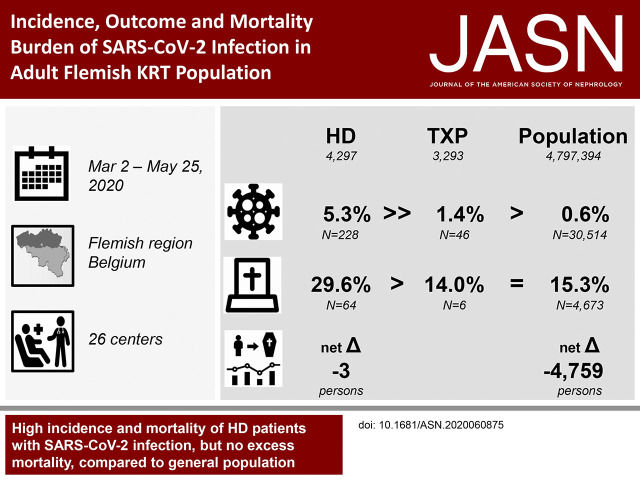
At the end of the epidemic wave, crude and age-standardized cumulative incidence rates of SARS-CoV-2 infection were 5.3% versus 2.5%, respectively, among 4297 patients on hemodialysis, and 1.4% versus 1.6%, respectively, among 3293 patients with kidney transplants (compared with 0.6% in the general population). Crude and age-standardized cumulative mortality rates were 29.6% versus 19.9%, respectively, among patients on hemodialysis, and 14.0% versus 23.0%, respectively, among patients with transplants (compared with 15.3% in the general population). We found no excess mortality in the hemodialysis population when compared with mean mortality rates during the same 12-week period in 2015–2019 because COVID-19 mortality was balanced by lower than expected mortality among uninfected patients. Only 0.18% of the kidney transplant population died of SARS-CoV-2 infection.
Conclusions
Mortality associated with SARS-CoV-2 infection is high in patients on RRT. Nevertheless, the epidemic’s overall effect on the RRT population remained remarkably limited in Flanders. Calculation of excess mortality and age standardization provide a more reliable picture of the mortality burden of COVID-19 among patients on RRT.
Older age, frailty, and chronic comorbid disease are prognostic factors of poor outcome in the event of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1–3 Patients on RRT and patients on maintenance hemodialysis in particular, therefore, are especially vulnerable. In addition, rapid spread of the virus may be facilitated by the collectivity of hemodialysis wards, waiting rooms, and transport modalities and by indirect contact transmission through environmental surfaces in dialysis rooms. Patients on hemodialysis characteristically have an intense health care exposure tantamount to a high risk of nosocomial transmission by asymptomatic health care workers or external visitors in the hospital.4 Part of the elderly patient population on dialysis lives in nursing homes, where transmission from asymptomatic residents is known to contribute to extensive spread of the infection.5 As such, they may function as bidirectional vectors of infection between the dialysis unit and nursing facility. Further, a substantial proportion of patients on hemodialysis may remain asymptomatic6 or have a paucisymptomatic early disease course,7,8 delaying diagnosis and quarantining. Although patients on peritoneal dialysis and kidney transplant recipients do not share the specific transmission risks of patients on hemodialysis, health care contacts in these patients are also substantial.
Coronavirus disease 2019 (COVID-19) is characterized by a wide spectrum of disease manifestations, ranging from asymptomatic carriership to critical respiratory insufficiency, multiorgan failure, and death, resulting from macrophage activation syndrome and cytokine storm.9 Whether the complex immune dysregulation caused by the uremic syndrome in patients on dialysis and by the use of immunosuppressive therapy in transplant recipients translates into differences in susceptibility, disease course, and outcome in these populations is presently unknown.
Several case reports and case series on COVID-19 in patients on RRT have been published.7,8,10–18 However, a systematic assessment of epidemiologic data in a well-defined RRT population compared with the referral and general population has not been reported. The Kidney Registry of the Dutch-speaking Belgian Society of Nephrology (NBVN) is particularly well suited for this purpose because all patients undergoing RRT in Flanders are registered in an online database, and the population of the Flemish region is well defined. In this prospective, multicenter, region-wide registry study, we describe the incidence, characteristics, and outcome of SARS-CoV-2 infection in patients on RRT compared with the general population.
Methods
Study Design and Setting
Flanders is the densely populated Dutch-speaking northern region of Belgium, counting 6,589,069 inhabitants and a population density of 484/km2.19 The NBVN Kidney Registry, acting on request of the Federal Ministry of Health, prospectively collects data of all patients starting dialysis or undergoing kidney transplantation. All 26 nephrology divisions in Flanders contribute to this registry (Supplemental Table 1). Since 2001, demographic and clinical data at RRT initiation and treatment modality switch along with date and cause of death are recorded in an online database, with 100% coverage of the RRT population and quarterly validation checks. Ethics approval for the NBVN Kidney Registry was obtained in all participating centers, and all registered patients provided informed consent. As COVID-19 is interpreted as a comorbid disease and this study fits the term of outcome study as defined in the data transfer agreement, the study stays within the boundaries of the General Data Protection Regulation and was approved by the Scientific Committee of the NBVN Kidney Registry.
Participants, Data Sources, and Variables
All adult patients (aged ≥25 years) on maintenance RRT between Monday March 2 and Monday May 25, 2020, were included in this analysis. Demographic data, comorbid conditions, and date and cause of death were extracted from the NBVN database. In patients diagnosed with COVID-19 within this 12-week time frame, indication for diagnostic testing (clinical symptoms or screening program), diagnostic procedure (clinical evaluation, chest computed tomography [CT] scan, or nasopharyngeal swab), outcome (death or recovery), and number of days in hospital were additionally collected. Epidemiologic data on the SARS-CoV-2 infection in the inhabitants of the Flemish region aged ≥25 years were obtained from Sciensano, the Belgian Institute for Public Health.20 Sciensano receives a daily report of the SARS-CoV-2 tests performed by all laboratories and of the number of confirmed/suspected cases and deaths in all hospitals and nursing homes.
SARS-CoV-2 infection was defined as (1) detection of SARS-CoV-2 RNA in a nasopharyngeal swab specimen with quantitative real-time RT-PCR (cycle threshold value <40), or in case of negative RT-PCR, (2) a chest CT scan with a high level of suspicion (COVID-19 Reporting and Data System [CO-RADS] score of greater than or equal to four)21 in combination with suggestive clinical signs (fever, new-onset respiratory symptoms), as specified by the Belgian Institute for Public Health.22 Although testing was initially prompted by a suggestive clinical history and symptoms, starting from April 10 new cases could also be detected via the obligatory screening program of the Belgian National Security Council in nursing home residents and all hospitalized patients. A few dialysis units additionally screened the entire dialysis population.
Mortality was defined as death in a patient recently diagnosed with SARS-CoV-2 infection, either symptomatic or not, irrespective of whether the cause of death could be directly linked to COVID-19. Excess mortality was defined as the number of deaths above expectation from mortality rates of previous years (2015–2019).
Mitigation Policies
All dialysis centers followed the screening and quarantining recommendations issued by the National Security Council23 to minimize disease transmission among patients and personnel. Transplant recipients received written information about preventive measures and were instructed to report suggestive symptomatology to their treating physician at the earliest opportunity. Transplant activity was ceased on March 19 and resumed on May 10. Social distancing rules and restrictions within the frame of the general lockdown were imposed on all inhabitants of Belgium. Between March 14 and March 18, schools, workplaces, and public places were closed. De-escalation of these restrictions was gradually initiated as of April 13 but was still minimal on May 25.
Statistical Methods
Descriptive statistics were used to outline baseline demographic and clinical characteristics, cumulative incidence of infection, and cumulative mortality. The age distribution of both patients on hemodialysis and transplant recipients was substantially different from that of the general population, hence indicating the necessity of age standardization. Because the number of events in both RRT groups was relatively small, leading to rather imprecise age-specific rates, we used the indirect method of standardization with the age structure of the general population as reference. The standardized mortality ratio (SMR) was calculated as the ratio of observed to the expected number of deaths. The calculation of their 95% confidence intervals (95% CIs) and P values was done according to the Mid-P exact method.24 A 95% CI not including the null value of one indicates a significant excess or deficit mortality. Proportions were compared using the chi-squared test and the Fisher exact test in case the assumptions for the former test were violated. To study whether patient’s characteristics and SARS-CoV-2 infection were synergistically related to cumulative mortality, log-linear models were fit consecutively, including apart from main effects, the interaction between SARS-CoV-2 infection status and the different patient characteristics. Interaction terms were statistically evaluated in the model taking into account an α-level of 0.10. Expected cumulative mortalities for the year 2020 were obtained by averaging cumulative mortalities observed in the years 2015–2019. Because a small number of patients remained in the hospital and patient-specific outcomes of these were unknown at the time of data cutoff, we censored the data regarding their outcomes as of the time of our analysis. In general, an α-level of 0.05 was adopted to conclude statistical significance. All analyses were carried out using SAS (release 9.4).
Results
Participants
On March 2, 2020, a total number of 7764 patients aged ≥25 years were receiving maintenance RRT in Flanders, of which 4158 were treated with hemodialysis, 313 were treated with peritoneal dialysis, and 3293 had a functioning kidney transplant. During the 12-week observation period from March 2 to May 25, 139 new patients on maintenance hemodialysis and 16 new patients on peritoneal dialysis were additionally included. Before and after the temporary shutdown of the transplant activity (March 19 to May 10), two and five kidney transplantations, respectively, were performed. Overall, 5.3% of patients on hemodialysis are nursing home residents (197 of 3700; in 597 patients, data on residence were not available), in particular women aged >85 years.
Incidence of SARS-CoV-2 Infection
By May 25, 2020, a total of 5.31% (228 of 4297) of patients on hemodialysis, 1.82% (six of 329) of patients on peritoneal dialysis, and 1.40% (46 of 3293) of transplant recipients were diagnosed with SARS-CoV-2 infection compared with 0.64% (30,514 of 4,797,394) of the population aged ≥25 years in the Flemish region (Table 1). As the absolute number of patients on peritoneal dialysis with SARS-CoV-2 infection was limited, these patients were excluded from further detailed analysis. In patients on hemodialysis and patients with transplants, cumulative incidence of SARS-CoV-2 infection over the study period was more or less equally distributed across the different age categories, whereas in the general population, it was strikingly associated with older age (Table 1). After age standardization, cumulative incidence remained significantly higher in patients on hemodialysis (2.54%) and transplant recipients (1.60%) than in the general population (Table 2). SARS-CoV-2 infection occurred more often in women than in men. The sex difference became even more pronounced after age standardization in patients on hemodialysis and transplant recipients (for the latter, not statistically significant so) (Table 2).
Table 1.
Cumulative incidence of SARS-CoV-2 infection in adult patients on hemodialysis, kidney transplant recipients, and the general population on May 25, 2020: Crude
| Population | Patients on Hemodialysis | Kidney Transplant Recipients | General Population | |||
|---|---|---|---|---|---|---|
| Positive/Total | % | Positive/Total | % | Positive/Total | % | |
| Total | 228/4297 | 5.31 | 46/3293 | 1.40 | 30,514/4,797,394 | 0.64 |
| Age, yr | ||||||
| 25–44 | 5/191 | 2.62 | 6/445 | 1.35 | 6755/1,647,317 | 0.41 |
| 45–64 | 38/739 | 5.14 | 24/1476 | 1.63 | 8950/1,818,562 | 0.49 |
| 65–74 | 44/917 | 4.80 | 11/990 | 1.11 | 2963/690,167 | 0.43 |
| 75–84 | 82/1519 | 5.40 | 4/345 | 1.16 | 4828/439,557 | 1.10 |
| 85+ | 59/931 | 6.34 | 1/37 | 2.70 | 7018/201,791 | 3.48 |
| P value across age | P=0.26 | P=0.78 | P<0.001 | |||
| Sex | ||||||
| Men | 123/2620 | 4.69 | 26/2007 | 1.30 | 11,337/2,343,718 | 0.48 |
| Women | 105/1677 | 6.26 | 20/1286 | 1.56 | 19,177/2,453,676 | 0.78 |
| P-value men versus women | P=0.03 | P=0.54 | P<0.001 | |||
Table 2.
Cumulative incidence of SARS-CoV-2 infection in adult patients on hemodialysis, kidney transplant recipients, and the general population on May 25, 2020: Age standardized
| Population | General Population | Patients on Hemodialysis | Kidney Transplant Recipients | ||
|---|---|---|---|---|---|
| % (95% CI) | Versus Population | % (95% CI) | Versus Population | ||
| Total | 0.64% | 2.54 (2.23 to 2.89) | P<0.001 | 1.60 (1.18 to 2.11) | P<0.001 |
| Men | 0.48% | 1.98 (1.65 to 2.35) | P<0.001 | 1.24 (0.82 to 1.78) | P<0.001 |
| Women | 0.78% | 3.21 (2.63 to 3.86) | P<0.001 | 2.00 (1.26 to 3.04) | P<0.001 |
| Men versus women | P<0.001 | P=0.03 | P=0.55 | ||
In patients on RRT with confirmed SARS-CoV-2 infection, testing was prompted by suggestive symptoms in 77% (167 of 228 patients on hemodialysis, three of six patients on peritoneal dialysis, and 45 of 46 kidney transplant recipients), and it was part of a nephrology division– or nursing home–initiated screening program in the remaining 23%. The diagnosis was established by the detection of SARS-CoV-2 RNA in respiratory samples in 95% (218 of 228 patients on hemodialysis, six of six patients on peritoneal dialysis, and 42 of 46 kidney transplant recipients) and by suggestive clinical presentation combined with a high probability on chest CT scan (CO-RADS score of greater than or equal to four) in the remaining 5%.
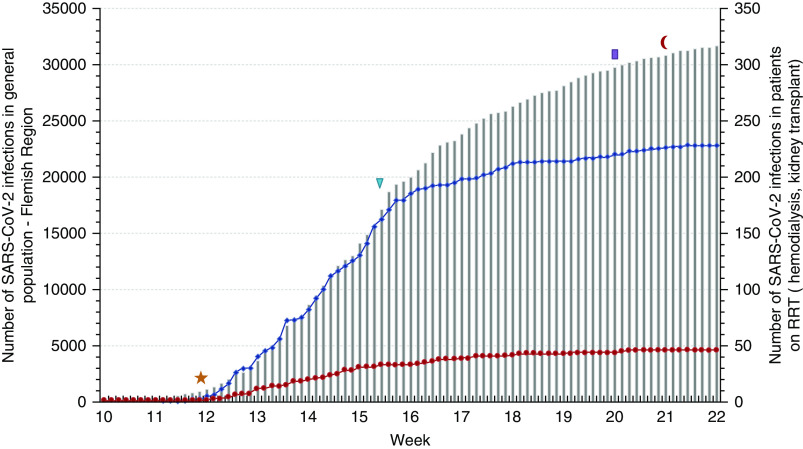
The dynamic of the epidemic in the RRT population paralleled that in the general population during the initial phase, but the flattening of the curve was achieved more rapidly (Figure 1). Patients with SARS-CoV-2 were spread unevenly across Flanders, but the regional distribution was similar in patients on RRT and in the general population (Supplemental Figure 1). The proportion of affected patients on hemodialysis varied from 0% to 16% (median 4%) among the 26 nephrology divisions in Flanders.
Figure 1.
Cumulative incidence of SARS-CoV-2 infection. Cumulative incidence of SARS-CoV-2 infection in the adult general population (gray bars, y axis left), adult patients on hemodialysis (blue line, y axis right), and adult kidney transplant recipients (red line, y axis right) in the Flemish region from March 2 to May 25, 2020. Implementation of general lockdown (yellow star, March 14), start of routine screening of all nursing home inhabitants (blue triangle, April 8), initial phase of the exit strategy with reopening of commercial activity (purple bar, May 11), and partial reopening of schools and resumption of daily life and gatherings (red crescent, May 18) are shown. Patients on peritoneal dialysis are not shown.
Within the RRT population, infection was not associated with age. Patients with diabetic nephropathy were more likely to be diagnosed with SARS-CoV-2 infection, and patients with glomerular disease and “miscellaneous” diagnoses were less likely so. As comorbid factors, diabetes and obesity strongly increased the odds of SARS-CoV-2 infection. Cardiovascular disease at RRT initiation was not significantly associated with the risk of infection. A shorter total RRT vintage ensued in an increased risk of SARS-CoV-2 infection in patients on hemodialysis. The proportion of patients on hemodialysis residing in a nursing home was significantly higher in those infected than in those not infected with SARS-CoV-2 (Table 3).
Table 3.
Characteristics of adult patients on RRT with and without SARS-CoV-2 infection
| Population | Patients on Hemodialysis, n=4297 | Kidney Transplant Recipients, n=3293 | ||||
|---|---|---|---|---|---|---|
| COVID-19 Positive, % (No.) | COVID-19 Negative, % (No.) | P Value | COVID-19 Positive, % (No.) | COVID-19 Negative, % (No.) | P Value | |
| Total no. | 228 | 4069 | 46 | 3247 | ||
| Age, yr | P=0.26 | P=0.78 | ||||
| 25–44 | 2.2 (5) | 4.6 (186) | 13.0 (6) | 13.5 (439) | ||
| 45–64 | 16.7 (38) | 17.2 (701) | 52.2 (24) | 44.7 (1452) | ||
| 65–74 | 19.3 (44) | 21.5 (873) | 23.9 (11) | 30.2 (979) | ||
| 75–84 | 36.0 (82) | 35.3 (1437) | 8.7 (4) | 10.5 (341) | ||
| 85+ | 25.9 (59) | 21.4 (872) | 2.2 (1) | 1.1 (36) | ||
| Sex | P=0.03 | P=0.55 | ||||
| Men | 53.9 (123) | 61.4 (2497) | 56.5 (26) | 61.0 (1981) | ||
| Women | 46.1 (105) | 38.6 (1572) | 43.5 (20) | 39.0 (1266) | ||
| Diagnosis | ||||||
| ARF | 1.3 (3) | 2.5 (100) | P=0.37 | 0.0 (0) | 0.9 (30) | P>0.99 |
| Cardiorenal syndrome | 5.7 (13) | 7.2 (292) | P=0.51 | 0.0 (0) | 0.2 (7) | P>0.99 |
| ADPKD | 6.1 (14) | 5.2 (212) | P=0.54 | 13.0 (6) | 19.9 (647) | P=0.35 |
| Type 1 diabetes | 5.3 (12) | 2.0 (83) | P=0.004 | 0.0 (0) | 5.7 (184) | P=0.11 |
| Type 2 diabetes | 25.0 (57) | 18.7 (762) | P=0.02 | 4.3 (2) | 4.8 (157) | P>0.99 |
| Hereditary diseases | 2.2 (5) | 1.2 (47) | P=0.20 | 4.3 (2) | 3.8 (124) | P=0.70 |
| Glomerular diseases | 8.3 (19) | 14.6 (593) | P=0.008 | 37.0 (17) | 28.3 (918) | P=0.19 |
| Hypertension/RVD | 27.2 (62) | 23.5 (958) | P=0.23 | 8.7 (4) | 8.5 (275) | P=0.79 |
| TID | 8.8 (20) | 9.8 (400) | P=0.73 | 19.6 (9) | 14.6 (474) | P=0.40 |
| Miscellaneous | 2.6 (6) | 6.1 (250) | P=0.03 | 0.0 (0) | 3.8 (125) | P=0.42 |
| Unknown | 7.5 (17) | 9.1 (372) | P=0.48 | 13.0 (6) | 9.4 (306) | P=0.44 |
| Comorbid conditions | ||||||
| Diabetes mellitus | 51.3 (117) | 39.8 (1618) | P<0.001 | 10.9 (5) | 14.0 (451) | P=0.67 |
| CVD | 57.2 (123) | 55.7 (2053) | P=0.31 | NA | NA | |
| BMI≥30 kg/m2 | 37.3 (81) | 28.2 (1047) | P=0.002 | NA | NA | |
| Nursing home residence | 11.7 (24/205) | 4.9 (173/3495) | P<0.001 | NA | NA | |
| Total RRT vintage | P=0.008 | P=0.003 | ||||
| 0–6 mo | 13.2 (30) | 8.2 (333) | 2.2 (1) | 0.1 (4) | ||
| 7–12 mo | 8.8 (20) | 9.5 (387) | 0.0 (0) | 0.5 (16) | ||
| 1.0–2.4 yr | 21.1 (48) | 26.4 (1074) | 8.7 (4) | 3.1 (100) | ||
| 2.5–4.9 yr | 26.8 (61) | 25.9 (1054) | 6.5 (3) | 10.8 (352) | ||
| 5.0–9.9 yr | 23.7 (54) | 18.9 (769) | 23.9 (11) | 27.4 (891) | ||
| 10+ yr | 6.6 (15) | 11.1 (452) | 58.7 (27) | 58.0 (1884) | ||
Hereditary diseases excludes patients with ADPKD. Cardiovascular disease was defined as presence of at least one of the following at the start of RRT: congestive heart failure, ischemic heart disease, peripheral vascular disease, cerebrovascular disease. Total RRT vintage was calculated from the first day of any form of RRT. ADPKD, autosomal dominant polycystic kidney disease; RVD, renal vascular disease; TID, tubulointerstitital disease; CVD, cardiovascular disease; NA, not available; BMI, body mass index.
Outcome of Patients with SARS-CoV-2 Infection
None of the patients on RRT were lost to follow-up. The final outcome of the SARS-CoV-2 infection was not yet known in 12 patients on hemodialysis and three transplant recipients at closure of the database. Overall, 64% (138 of 228 patients on hemodialysis, four of six patients on peritoneal dialysis, and 38 of 46 kidney transplant recipients) were admitted at some point during the disease course, whereas the remaining 36% of patients on RRT continued as outpatients for the duration of their illness.
Crude cumulative mortality rate in SARS-CoV-2–infected patients on hemodialysis was 29.6% (64 of 216), and it was 14.0% (six of 43) in transplant recipients compared with 15.3% (4673 of 30,514) in the general population (Table 4). Two of the six affected patients on peritoneal dialysis died. Age-standardized cumulative mortality rates (95% CI) were 19.9% (95% CI, 15.4% to 25.2%) in patients on hemodialysis (versus 15.3% in the general population; P=0.04) and 23.0% (95% CI, 9.3% to 47.9%) in transplant recipients (versus 15.3% in the general population; P=0.31). As only six of the total group of 3293 patients died, the absolute mortality risk in kidney transplant recipients was 0.18%.
Table 4.
Cumulative mortality of SARS-CoV-2 infection in adult patients on hemodialysis, kidney transplant recipients, and the general population on May 25, 2020
| PopulationC | Patients on Hemodialysis | Kidney Transplant Recipients | General Population | |||
|---|---|---|---|---|---|---|
| Death/Total | % | Death/Total | % | Death/Total | % | |
| Total | 64/216a | 29.6 | 6/43b | 14.0 | 4673/30,514 | 15.3 |
| Age, yr | ||||||
| 25–44 | 0/5 | 0.0 | 0/6 | 0.0 | 16/6755 | 0.2 |
| 45–64 | 6/38 | 15.8 | 2/22 | 9.1 | 299/8950 | 3.3 |
| 65–74 | 13/41 | 31.7 | 2/11 | 18.2 | 589/2963 | 19.9 |
| 75–84 | 24/77 | 31.2 | 2/4 | 50.0 | 1277/4828 | 26.4 |
| 85+ | 21/55 | 38.2 | 0/0 | 0.0 | 2492/7018 | 35.5 |
| P value across age | P=0.10 | P<0.001 | ||||
| Sex | ||||||
| Men | 42/117 | 35.9 | 2/26 | 7.7 | 2100/11,337 | 18.5 |
| Women | 22/99 | 22.2 | 4/17 | 23.5 | 2573/19,177 | 13.4 |
| P value men versus women | P=0.04 | P<0.001 | ||||
No final outcome data in 12 patients on hemodialysis.
No final outcome data in three kidney transplant recipients.
Older age significantly increased mortality in the general population but not in patients on hemodialysis and kidney transplant recipients (Table 4). Patients diagnosed through a screening program had a better outcome than those in whom diagnosis was prompted by suggestive symptoms (Supplemental Table 2), whereas there was no obvious effect of the type of the diagnostic procedure on outcome (Supplemental Table 3).
Cumulative mortality was higher in patients on RRT who were diagnosed with SARS-CoV-2 infection than in patients who were not: for patients on hemodialysis (64 of 216; 29.6% versus 144 of 4069; 3.5%; P<0.001), patients on peritoneal dialysis (two of six; 33% versus three of 323; 0.9%; P=0.003), and kidney transplant recipients (six of 43; 14.0% versus ten of 3247; 0.3%; P<0.001). Male gender, diabetic nephropathy as underlying kidney disease, and diabetes as comorbidity were significantly associated with higher death rates in patients on hemodialysis with versus those without SARS-CoV-2 infection (Supplemental Table 4).
Treating physicians attributed death directly to SARS-CoV-2 infection in 77% of patients on hemodialysis and 100% of transplant recipients (Table 5).
Table 5.
Causes of death in adult RRT with and without SARS-CoV-2 infection
| Cause of Death | Patients on Hemodialysis | Kidney Transplant Recipients | ||||
|---|---|---|---|---|---|---|
| COVID-19 Positive, No. (%) | COVID-19 Negative, No. (%) | P Value | COVID-19 Positive, No. (%) | COVID-19 Negative, No. (%) | P Value | |
| Infection | 49/64 (76.5) | 22/144 (15.3) | P<0.001 | 6/6 (100.0) | 1/10 (10.0) | P<0.001 |
| Stop therapy | 4/64 (6.3) | 29/144 (19.1) | P=0.01 | 0/6 (0.0) | 0/10 (0.0) | — |
| Failure to thrive | 1/64 (1.6) | 6/144 (4.2) | P=0.44 | 0/6 (0.0) | 1/10 (10.0) | P>0.99 |
| Sudden death | 2/64 (3.1) | 24/144 (16.7) | P=0.006 | 0/6 (0.0) | 2/10 (20.0) | P=0.50 |
| Cardiovascular | 3/64 (4.7)a | 19/144 (13.2) | P=0.09 | 0/6 (0.0) | 1/10 (10.0) | P>0.99 |
| Malignancy | 1/64 (1.6) | 18/144 (12.5) | P=0.009 | 0/6 (0.0) | 1/10 (10.0) | P>0.99 |
| Miscellaneous | 1/64 (1.6) | 10/144 (6.9) | P=0.18 | 0/6 (0.0) | 2/10 (20.0) | P=0.50 |
| Unknown | 3/64 (4.7) | 16/144 (11.1) | P=0.19 | 0/6 (0.0) | 2/10 (20.0) | P=0.50 |
—, not applicable.
Of which two fatal strokes.
Excess Mortality
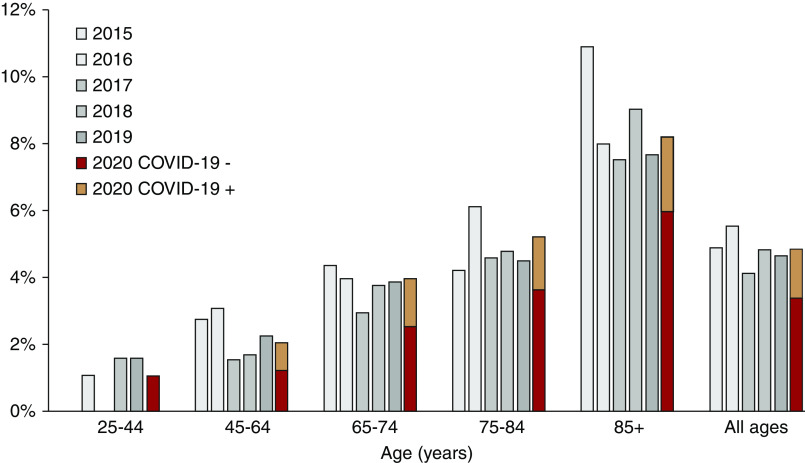
During the observation period of March 2 to May 25 (week 10 to week 21), all-cause mortality in the hemodialysis population was not higher than expected on the basis of mean mortality during the same period of 2015–2019: SMR=1.02; 95% CI, 0.88 to 1.16; P=0.82. In patients on hemodialysis who were not infected with SARS-CoV-2, mortality was 26% lower than expected: SMR=0.74; 95% CI, 0.63 to 0.87; P<0.001 (Supplemental Table 5). This effect was seen across all age categories (Figure 2).
Figure 2.
All-cause mortality in adult patients on hemodialysis. Periodic all-cause mortality stratified by age group in adult patients on hemodialysis, expressed as percentage of total number of patients on hemodialysis at risk. Mortality in patients with (orange bars) and without (red bars) diagnosis of SARS-CoV-2 infection during the study period (March 2 to May 25; week 10 to week 21) in 2020 was compared with mortality during the same period in 2015–2019 (gray bars).
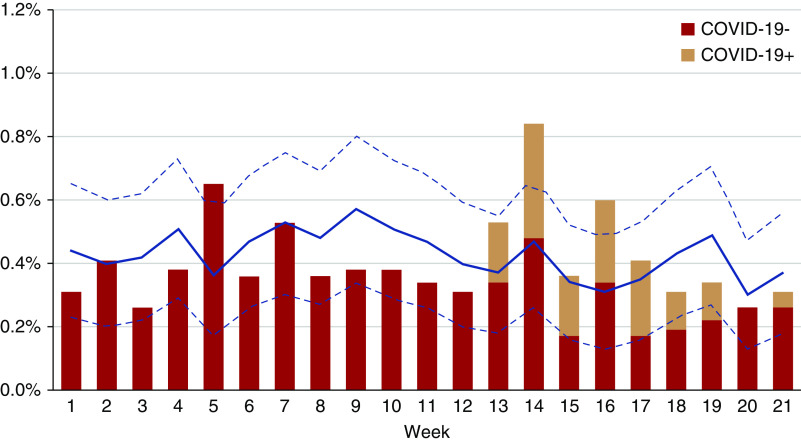
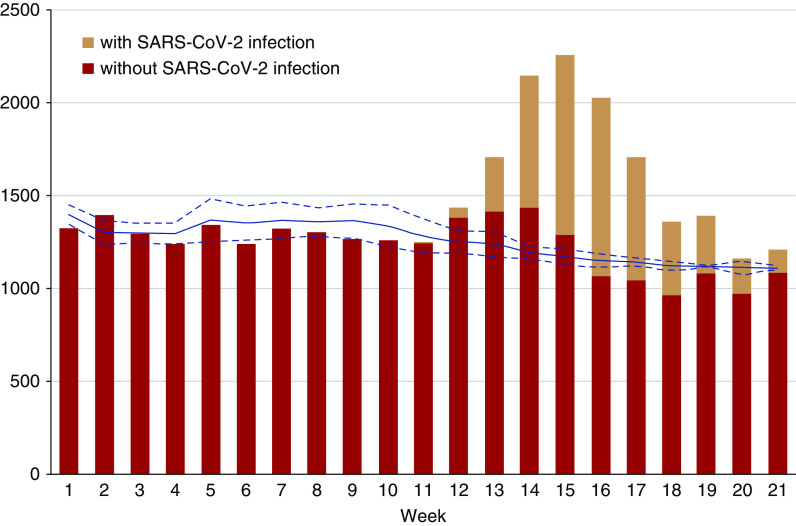
Weekly mortality during the first 21 weeks of 2020 was compared with mean weekly mortality in 2015–2019 for the entire hemodialysis population (Figure 3). During the first 12 weeks of 2020, observed mortality was generally lower than the mean of previous years. From week 13 onward, deaths occurred in patients with SARS-CoV-2 infection, but only in week 14 and week 16, excess mortality compared with previous years was observed. In the other weeks, mortality remained within the boundaries of the 95% CI for expected mortality.
Figure 3.
Excess all-cause mortality in adult patients on hemodialysis. All-cause mortality, expressed as percentage of deaths per week from week 1 to week 21, in adult patients on hemodialysis without (red bars) and with (orange bars) diagnosis of SARS-CoV-2 infection in 2020 compared with the mean and 95% confidence limits (blue line and dotted blue lines, respectively) for that week during the period 2015–2019.
In the adult general population, a substantial excess mortality was observed from week 13 onward compared with mean mortality observed in 2015–2019 (Figure 4). The calculated number of excess deaths (4759) corresponded well with the officially reported number of COVID-19–associated deaths (4673) during the observation period (weeks 10–21).
Figure 4.
Excess all-cause mortality in the adult general population. All-cause mortality, expressed as absolute number of deaths per week from week 1 to week 21, in the adult general population without (green bars) and with (orange bars) diagnosis of SARS-CoV-2 infection in 2020 compared with the mean and 95% confidence limits (blue line and dotted blue lines, respectively) of the period 2015–2019.
Discussion
The first SARS-CoV-2 epidemic wave swept through Flanders during a 12-week period extending from March 2 to May 25, 2020, and it officially affected 0.64% of the adult population. By the end of this period, 5.31% of patients on hemodialysis, 1.82% of patients on peritoneal dialysis, and 1.40% of kidney transplant recipients had been diagnosed with SARS-CoV-2 infection. After standardization for age, cumulative incidence remained four times greater in patients on hemodialysis (2.54%) and 2.5 times greater in kidney transplant recipients (1.60%) than in the general population. The geographic distribution across Flanders in patients on RRT was a virtual carbon copy of that in the general population, commensurate with community and nosocomial transmission rather than dialysis unit–specific practices or intrinsic susceptibility for infection as main determining factors of infection. Although the initial epidemic trajectory in the hemodialysis population closely matched that in the general population, the flattening of the curve was achieved more rapidly. These findings underscore that proactive implementation of containment measures in dialysis units, including maximization of individual transport, universal use of surgical masks, strict entrance screening, and swift quarantining of infected patients,25 can effectively curtail the spread of the epidemic.
The higher incidence in patients on hemodialysis compared with the general population may have resulted from a better recognition of infected patients. Patients on hemodialysis have been repeatedly instructed to report suggestive symptoms and were universally tested in case they developed symptoms even during the early stages of the epidemic. Later on, mandatory screening of nursing home residents and new hospital admissions, as well as screening policies in some dialysis units, has led to detection of presymptomatic and asymptomatic patients. The sizeable proportion of patients on hemodialysis detected through screening in our study (61 of 228; 26.7%) is in line with the high percentage of asymptomatic patients in a cohort of patients on dialysis from the United Kingdom (52 of 131; 39.6%)6 and supports systematic screening of patients as an additional containment practice.
The cumulative incidence of infection was also high in kidney transplant recipients, despite generally excellent compliance with social distancing measures in a population trained to be risk averse.26 Although immunosuppressive treatment likely increases the susceptibility to infection,27 a low threshold for testing may also have led to better identification of infected transplant recipients. Conversely, many oligosymptomatic individuals in the general population were not tested and thus, do not appear in the official statistics. As an example, a large seroprevalence study conducted between May 18 and 25, 2020, revealed a prevalence of anti–SARS-CoV-2 IgG in the general population of 6.9% (Herzog S, De Bie J, Abrams S, Wouters I, Ekinci E, Patteet L, et al.: Seroprevalence of IgG antibodies against SARS coronavirus 2 in Belgium: A prospective cross-sectional nationwide study of residual samples. medRxiv 2020.06.08.20125179, 2020), which is more than ten times greater than the officially reported cumulative incidence of infection at that point.
As already reported for the general population,28 obesity and diabetes were associated with SARS-CoV-2 infection in patients on dialysis. The absence of a strong effect of age on the incidence of SARS-CoV-2 infection in the RRT population, unlike in the general population, is interesting. It is well known that older age enhances the severity of the disease course of respiratory virus infections in general and SARS-CoV-2 in particular,29 resulting in an under-representation of younger people in the population statistics due to detection bias. The susceptibility to SARS-CoV-2 infection may also be intrinsically lower in younger people due to variations in angiotensin-converting enzyme-2 expression with age and in the innate immune response controlling the infection at the site of entry.30,31 That these protective mechanisms seem to be lost in a uremic environment is noteworthy but not entirely surprising.
Unexpectedly, women appeared to be more at risk of contracting SARS-CoV-2 infection. The preponderance of women became even more pronounced after standardization for age. These results are at odds with a higher proportion of men reported in case series of patients on hemodialysis8 and of hospitalized patients from the general population,1,2 but in none of these studies was the confounding role of age accounted for. The disproportionately low representation of women in these case series may also have resulted from a lower risk of exposure to the virus,32 a mechanism that does not apply to the collectivity of dialysis units. Many more elderly women on hemodialysis reside in a nursing home compared with elderly men, providing a further explanation for this unexpected sex distribution. Finally, as male gender is a risk factor for a more severe disease course, the dominance of men in early case series may have resulted from a prioritization of testing in patients with a more severe clinical presentation.
Cumulative mortality rates in SARS-CoV-2–infected Flemish patients on hemodialysis were 29.6% (crude) and 19.9% (age standardized), corresponding well with figures reported from Spain (seven of 25; 28%)12 and northern Italy (27 of 94; 29%).11 In accordance with reports from the general population, male gender and diabetes increased mortality risk in SARS-CoV-2–infected patients on hemodialysis. The unfavorable prognostic effects of male gender and metabolic syndrome in SARS-CoV-2 infection have been attributed to a state of chronic low-grade inflammation and dysregulated immune response, setting the stage for the feared “cytokine storm” that makes SARS-CoV-2 infection a deadly disease.28 Old age is similarly associated with a proinflammatory and procoagulant condition termed “inflammaging,” providing an explanation for the strong correlation between age and COVID-19 mortality in the general population. In contrast, we did not find a clear effect of age on mortality in patients on hemodialysis. It is tempting to speculate that premature senescence in patients on hemodialysis caused by the uremic state, gut dysbiosis, and oxidative stress, to name a few,33 over-rules the effects of chronological age.
Treating physicians attributed death in patients on hemodialysis with SARS-CoV-2 infection to COVID-19 in 77% of cases. In a German consecutive series of 80 autopsies of persons who tested positive for SARS-CoV-2 ante- or postmortem, 95% were ultimately classified as COVID-19 deaths, either directly (pneumonia with or without sepsis) or indirectly (mainly fatal pulmonary artery embolism).34 In our cohort, several cases of sudden death, fatal cardiovascular events (including fatal stroke), or unknown cause of death occurred, which in retrospect, may have been directly or indirectly caused by COVID-19. Additionally, advanced care directives had been given by several patients or surrogate medical decision makers to establish palliative care in the event of clinical deterioration, in particular in nursing home residents. As such, not all measures to treat COVID-19 were exhausted in these patients.
Although the limited number of affected kidney transplant recipients in our cohort does not allow us to draw definitive conclusions, cumulative mortality was similar with respect to the general population. Our results are at odds with initial reports of high patient fatality rates,13,15,17 potentially related to selective recruitment of the most severely ill patients during the early stages of the epidemic. Subsequent to these preliminary reports, decisions to rapidly lower immunosuppression upon diagnosis of SARS-CoV-2 infection35 may also have mitigated disease course in our cohort. In our opinion, the low absolute mortality risk in kidney transplant recipients (1.8 of 1000 in our cohort) does not justify empirical preventive lowering of immunosuppression or pre-exposure prophylaxis with antiviral agents,36 as it may result in rejection, drug interactions, or side effects.
Judging the effect of the SARS-CoV-2 epidemic with crude mortality or patient-fatality ratios can be misleading because surveillance has been biased toward detecting severe cases, especially at the beginning of the epidemic when test capacity was low.37 Counting only confirmed and probable COVID-19–associated deaths does not include deaths in patients who had no access to diagnostic testing, tested falsely negative, or died outside of a health care setting. Estimation of excess all-cause mortality, defined as the number of deaths above the expected seasonal baseline levels regardless of the reported cause, may be a more reliable measure of the effect of an outbreak.
At first sight and mainly judging by the high crude cumulative mortality, the hemodialysis population has been hit hard by the SARS-CoV-2 epidemic. However, when estimated by excess mortality, the overall effect is remarkably limited. One possible explanation is that the most vulnerable patients were afflicted and that these would have died from alternative causes had they not developed COVID-19. Alternatively, strict adoption of droplet infection–control measures prompted by the emerging SARS-CoV-2 epidemic may have protected against other respiratory infections, an important cause of death in patients on dialysis.38 Finally, a lower than usual virulence of the seasonal influenza strains may also have played a role. Interestingly, a French study reported that the mortality associated with respiratory viruses (mainly influenza A, respiratory syncytial virus, and SARS-CoV-2) was numerically but not statistically significantly higher during the colder months overlapping 2019–2020 than during the same period in 2018–2019.39 In fact, a lower number of deaths due to seasonal influenza and other respiratory viruses in 2019–2020 partially compensated for the SARS-CoV-2–associated deaths.39 A similar mechanism may be at play in the hemodialysis population and explain why there was already a lower than expected mortality in the weeks preceding the SARS-CoV-2 epidemic. In the general population, on the other hand, the SARS-CoV-2 epidemic was responsible for a substantial excess mortality.
The main strength of our study is the completeness of our RRT data and a well-delineated referral population, allowing reliable calculation of incidence and mortality, a robust estimation of risk factors for infection and death, and assessment of the overall effect of the SARS-CoV-2 epidemic. Our study also has several limitations. No information was gathered on laboratory data and maintenance medication (including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) at the time of diagnosis or on specific therapy for SARS-CoV-2 infection. Testing policies and volumes varied among and within dialysis units over time. Finally, strategies to discontinue quarantine in infected patients on dialysis varied among dialysis centers from test based40 to time based, although there was no evidence to suggest that early lifting of quarantine measures may have contributed to nosocomial spreading within specific dialysis units.
In conclusion, a substantial proportion of the RRT population became infected during the course of the SARS-CoV-2 epidemic. However, after adjusting for age and taking into account the under-reporting in the general population, the risk of contracting SARS-CoV-2 infection does not seem to be severely increased in patients on RRT. Conversely, mortality rates in the hemodialysis population are high even after adjustment for age, suggesting that the uremic environment creates a background for a more severe disease course. Nevertheless, there was no excess mortality in the hemodialysis population during the epidemic, which provides a more reliable perspective on the ultimate mortality burden of SARS-CoV-2 infection. We did not observe significantly increased mortality in kidney transplant recipients, despite the use of immunosuppression. Our findings underline the unassailable importance of correctly delineating the referral population and appropriately adjusting for sources of bias when reporting incidence and mortality of SARS-CoV-2 infection.
Disclosures
J. De Meester reports personal fees from Menarini, outside the submitted work. A. De Vriese reports personal fees from Ablynx, Achillion, Alexion, Amgen, and Baxter and grants from Amgen, outside the submitted work. B. Meijers reports personal fees from Astrazeneca, Baxter, Bayer, Fresenius, Medtronic (and its previous subsidiary Bellco), Menarini, and Vifor and grants from Baxter, BMS, Ionis, Nipro, and Pfizer, outside the submitted work. B. Meijers also reports editorial board membership of BMC Nephrology, editorial board membership of Toxins, and speakers bureau of Vifor. All remaining authors have nothing to disclose.
Funding
Financial support for this work was provided by the Belgian Society of Nephrology (NBVN). M. Naesens is a senior clinical investigator of Fonds Wetenschappelijk Onderzoek through grant 1844019N and is supported by Fonds Wetenschappelijk Onderzoek TBM grant T004417N, Fonds Wetenschappelijk Onderzoek junior project grant G087620N, H2020 European Institute of Innovation and Technology grant JTC2_29, and KU Leuven C3 internal grant C32/17/049. B. Meijers is a senior clinical investigator of Fonds Wetenschappelijk Onderzoek (1800820N) and is supported by the Horizon 2020-Marie Skłodowska Curie-Innovative Training Network-2019 (860329) Strategy-CKD.
Supplementary Material
Acknowledgments
The authors are indebted to all administrators of the NBVN database for their invaluable help in collecting the patient data.
J. De Meester and A. De Vriese designed the study; M. Couttenye, J. De Meester, A. De Vriese, B. Meijers, and M. Naesens collected the data; D. De Bacquer analyzed the data; D. De Bacquer and J. De Meester made the figures; J. De Meester and A. De Vriese drafted the manuscript; and all authors revised the manuscript and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: NBVN Kidney Registry Group, Eric Gheuens, Hylke de Jonge, Vicky De Meyer, Bart De Moor, Pieter Evenepoel, Manu Henckes, Wim Lemahieu, Koen Stas, Wim Van Biesen, and Karl Martin Wissing
Supplementary Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060875/-/DCSupplemental.
Supplemental Figure 1. Regional distribution of cumulative incidence of SARS-CoV-2 infection (A) in the adult general population and (B) of adult patients on hemodialysis in the Flemish region.
Supplemental Table 1. List of nephrology divisions in Flanders.
Supplemental Table 2. Outcome of patients with SARS-CoV-2 infection according to diagnostic indication.
Supplemental Table 3. Outcome of patients with SARS-CoV-2 infection according to diagnostic procedure.
Supplemental Table 4. Cumulative all-cause mortality in adult patients on RRT who are COVID-19 positive and negative.
Supplemental Table 5. Actual versus expected number of deaths from March 2 to May 25, 2020 (week 10 to week 21) in adult patients on hemodialysis.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al.; the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area [published correction appears in JAMA 323: 2098, 2020 10.1001/jama.2020.7681]. JAMA 323: 2052–2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S: Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published correction appears in JAMA 323: 1619, 2020 10.1001/jama.2020.6122]. JAMA 323: 1775–1776, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Van Praet JT, Claeys B, Coene AS, Floré K, Reynders M: Prevention of nosocomial COVID-19: Another challenge of the pandemic [published online ahead of print April 23, 2020]. Infect Control Hosp Epidemiol 10.1017/ice.2020.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al.; Public Health–Seattle and King County and CDC COVID-19 Investigation Team: Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 382: 2081–2090, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, et al.: High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 31: 1969–1975, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Li J, Zhu G, Zhang Y, Bi Z, Yu Y, et al.: Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol 15: 1139–1145, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, et al.: Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol 31: 1387–1397, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berlin DA, Gulick RM, Martinez FJ: Severe COVID-19 [published online ahead of print May 15, 2020]. N Engl J Med 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Liao C, He H, Hu C, Wei Z, Hong Z, et al.: COVID-19 in hemodialysis patients: A report of 5 cases. Am J Kidney Dis 76: 141–143, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al.: A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 98: 20–26, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trujillo H, Caravaca-Fontán F, Sevillano Á, Gutiérrez E, Caro J, Gutiérrez E, et al.: SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep 5: 905–909, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al.: A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int 97: 1083–1088, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manganaro M, Baldovino S; Working group of the Piedmont and Aosta Valley Section of the SIN: First considerations on the SARS-CoV-2 epidemic in the dialysis units of Piedmont and Aosta Valley, northern Italy. J Nephrol 33: 393–395, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, et al.: Covid-19 and kidney transplantation. N Engl J Med 382: 2475–2477, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschopp J, L’Huillier AG, Mombelli M, Mueller NJ, Khanna N, Garzoni C, et al.; Swiss Transplant Cohort Study (STCS): First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant 20: 2876–2882, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Ruiz M, Andrés A, Loinaz C, Delgado JF, López-Medrano F, San Juan R, et al.: COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am J Transplant 20: 1849–1858, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M: COVID-19 infection in kidney transplant recipients. Kidney Int 97: 1076–1082, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistiek Vlaanderen: Bevolking: Omvang en groei, 2020. Available at: https://www.statistiekvlaanderen.be/nl/bevolking-omvang-en-groei. Accessed May 25, 2020
- 20.Sciensano: COVID-19, 2020. Available at: https://epistat.wiv-isp.be/covid. Accessed May 25, 2020
- 21.Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, et al.; COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society: CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology 296: E97–E104, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sciensano: Gevalsdefinitie, indicaties voor testen en verplichte melding van COVID-19, 2020. Available at: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Case%20definition_Testing_NL.pdf. Accessed July 22, 2020
- 23.Belgian Federal Authorities: Coronavirus COVID-19, 2020. Available at: https://www.info-coronavirus.be/en/. Accessed May 25, 2020
- 24.Rothman KJ, Boice JD Jr: Epidemiologic Analysis with a Programmable Calculator, Bethesda, MD, National Institutes of Health, 1979 [Google Scholar]
- 25.Meijers B, Messa P, Ronco C: Safeguarding the maintenance hemodialysis patient population during the coronavirus disease 19 pandemic. Blood Purif 49: 259–264, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascale MM, Bianco G, Ferri L, Agnes S: COVID-19 health restrictions in a transplanted Italian cohort [published online ahead of print May 27, 2020]. Transpl Int 10.1111/tri.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishman JA: The immunocompromised transplant recipient and SARS-CoV-2 infection. J Am Soc Nephrol 31: 1147–1149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauvais-Jarvis F: Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes 69: 1857–1863, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Kelley WJ, Goldstein DR: Role of aging and the immune response to respiratory viral infections: Potential implications for COVID-19. J Immunol 205: 313–320, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peron JPS, Nakaya H: Susceptibility of the elderly to SARS-CoV-2 infection: ACE-2 overexpression, shedding, and antibody-dependent enhancement (ADE). Clinics (São Paulo) 75: e1912, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhochak N, Singhal T, Kabra SK, Lodha R: Pathophysiology of COVID-19: Why children fare better than adults? Indian J Pediatr 87: 537–546, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al.: Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 382: 2302–2315, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losappio V, Franzin R, Infante B, Godeas G, Gesualdo L, Fersini A, et al.: Molecular mechanisms of premature aging in hemodialysis: The complex interplay between innate and adaptive immune dysfunction. Int J Mol Sci 21: 3422, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al.: Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany [published correction appears in Int J Legal Med 134: 1977, 2020 10.1007/s00414-020-02336-7]. Int J Legal Med 134: 1275–1284, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kronbichler A, Gauckler P, Windpessl M, Il Shin J, Jha V, Rovin BH, et al.: COVID-19: Implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol 16: 365–367, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritschl PV, Nevermann N, Wiering L, Wu HH, Moroder P, Brandl A, et al.: Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: A by-proxy society recommendation consensus approach. Am J Transplant 20: 1826–1836, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan S: Likelihood of survival of coronavirus disease 2019 [published correction appears in Lancet Infect Dis 20: e116, 2020 10.1016/S1473-3099(20)30283-8]. Lancet Infect Dis 20: 630–631, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbertson DT, Rothman KJ, Chertow GM, Bradbury BD, Brookhart MA, Liu J, et al.: Excess deaths attributable to influenza-like illness in the ESRD population. J Am Soc Nephrol 30: 346–353, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giraud-Gatineau A, Colson P, Jimeno MT, Zandotti C, Ninove L, Boschi C, et al.: Comparison of mortality associated with respiratory viral infections between December 2019 and March 2020 with that of the previous year in southeastern France. Int J Infect Dis 96: 154–156, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vriese AS, Reynders M: IgG antibody response to SARS-CoV-2 infection and viral RNA persistence in patients on maintenance hemodialysis. Am J Kidney Dis 76: 440–441, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.