Significance Statement
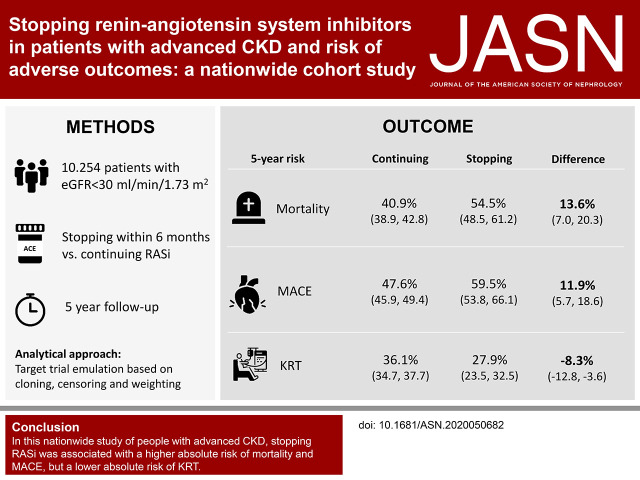
Whether renin-angiotensin system (RAS) inhibition is safe and effective in patients with advanced CKD is unknown. Single-center studies suggest there is improved kidney function after stopping RAS inhibition and possible delay in initiating kidney replacement therapy (KRT), but large prospective studies assessing cardiovascular and kidney outcomes are lacking. In this nationwide study of 10,254 Swedish patients with advanced CKD on RAS inhibitor therapy and under routine care by nephrologists, discontinuing this treatment associated with increases in the 5-year absolute risks of mortality and major adverse cardiovascular events—13.6% and 11.9%, respectively—but an 8.3% decrease in absolute risk of initiating KRT. These findings caution against routine discontinuation of RAS inhibitor therapy in such patients and suggest cardiovascular risk and risk of KRT be considered in decisions about stopping RAS inhibitor therapy.
Keywords: ACE inhibitors, mortality risk, dialysis, cardiovascular events, kidney disease, renin-angiotensin system
Visual Abstract
Abstract
Background
It is unknown whether stopping renin-angiotensin system (RAS) inhibitor therapy in patients with advanced CKD affects outcomes.
Methods
We studied patients referred to nephrologist care, listed on the Swedish Renal Registry during 2007–2017, who developed advanced CKD (eGFR<30 ml/min per 1.73 m2) while on RAS inhibitor therapy. Using target trial emulation techniques on the basis of cloning, censoring, and weighting, we compared the risks of stopping within 6 months and remaining off treatment versus continuing RAS inhibitor therapy. These included risks of subsequent 5-year all-cause mortality, major adverse cardiovascular events, and initiation of kidney replacement therapy (KRT).
Results
Of 10,254 prevalent RAS inhibitor users (median age 72 years, 36% female) with new-onset eGFR <30 ml/min per 1.73 m2, 1553 (15%) stopped RAS inhibitor therapy within 6 months. Median eGFR was 23 ml/min per 1.73 m2. Compared with continuing RAS inhibition, stopping this therapy was associated with a higher absolute 5-year risk of death (40.9% versus 54.5%) and major adverse cardiovascular events (47.6% versus 59.5%), but with a lower risk of KRT (36.1% versus 27.9%); these corresponded to absolute risk differences of 13.6 events per 100 patients, 11.9 events per 100 patients, and −8.3 events per 100 patients, respectively. Results were consistent whether patients stopped RAS inhibition at higher or lower eGFR, across prespecified subgroups, after adjustment and stratification for albuminuria and potassium, and when modeling RAS inhibition as a time-dependent exposure using a marginal structural model.
Conclusions
In this nationwide observational study of people with advanced CKD, stopping RAS inhibition was associated with higher absolute risks of mortality and major adverse cardiovascular events, but also with a lower absolute risk of initiating KRT.
Renin-angiotensin system inhibitors (RASi), that is, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, are a cornerstone in the treatment of proteinuric CKD, supported by trials showing their effectiveness in delaying the progression of CKD.1–7 However, evidence for the efficacy and safety of RASi in individuals with advanced CKD is limited to a small single-center trial8 and post-hoc analyses of the few patients with advanced CKD who were included in the pivotal RASi trials.9,10
A small observational study, showing improved GFR after stopping RASi,11 led to the hypothesis that continuing RASi in patients with advanced CKD might accelerate the need for kidney replacement therapy (KRT).12 Together with the concern that the persistent hemodynamic effects of RASi, which are manifested by an acute change in GFR at initiation,13,14 may cause harm by chronically lowering the GFR, this has led to frequently stopping RASi among patients with advanced CKD in routine clinical practice.15,16 However, stopping RASi may also harm patients by increasing cardiovascular risk and mortality.17
This clinical equipoise is being addressed by an ongoing randomized trial that evaluates the difference in 3-year eGFR change in patients with advanced CKD at baseline, randomized to continue or discontinue RASi, with publication anticipated in 2022.18,19 Recently, an observational study from a private health care provider in the United States suggested that stopping RASi in patients with advanced CKD was associated with an increased risk of major cardiovascular events (MACE) and death, but not with the risk of KRT.17 Although this study has generated considerable attention, confirmation of such findings in independent and geographically diverse health systems is needed to increase generalizability and provide the strength of evidence needed to inform clinical practice.
We used routine-care data from patients referred to nephrologist care in Sweden, to compare the outcomes of long-term users of RASi who stopped or continued treatment after developing advanced CKD (eGFR <30 ml/min per 1.73 m2). Our primary objective was to evaluate the risks of death, MACE, and commencement of KRT by this treatment decision. As a secondary objective, we investigated whether observed risks and benefits differed in individuals who stopped earlier (eGFR 20–30 ml/min per 1.73 m2) or later (eGFR <20 ml/min per 1.73 m2) in the course of their disease progression.
Methods
Swedish Renal Registry
We used data from the Swedish Renal Registry (SRR), a nationwide registry of patients with CKD G3–5 attending routine nephrologist-specialist care in Sweden,20,21 during the period 2007–2017. The SRR collects routine information from outpatient nephrologist visits, including CKD etiology, laboratory tests, BP, and other results obtained from routine clinical examination. The registry has a mandatory enrolment policy for patients with an eGFR <30 ml/min per 1.73 m2, but it also encourages the inclusion of patients earlier in the course of the disease (eGFR <45 ml/min per 1.73 m2) provided it is done systematically by the nephrology clinic (i.e., all or none are registered from each specific clinic with eGFR <45 ml/min per 1.73 m2). Registrations of subsequent outpatient visits to nephrology care (on average 2–3 per year per patient) are thereafter recorded until death, emigration from the country, or start of KRT. Nearly all nephrology clinics in Sweden (96%) report to the SRR-CKD, and the estimated national coverage is >75% for patients referred to nephrologist care with G4–5 CKD.20
Via each citizen’s unique personal identification number, the SRR was linked to other national registries; the Swedish Prescribed Drug Registry provided complete information on all prescribed drugs dispensed at Swedish pharmacies,22 and this was used to define RASi use and changes in RASi therapy; the Swedish Patient Registry added information on all outpatient specialist consultations and hospitalizations occurring in Swedish healthcare since 1997 until the end of follow-up, and this was used to obtain information on comorbidities and outcomes23; the Swedish Death Registry added information on date and causes of death.24 All these registries are run by the Swedish National Board of Welfare, a government institution, and are considered to have no, or minimal, loss to follow-up. All patients are informed about their participation in the registry and have the possibility to opt out at any time. We used data linked and de-identified by the Swedish government, and were judged not to require informed consent, being approved by the regional ethical review boards and the Swedish National Board of Welfare.
Patient Selection and Study Design
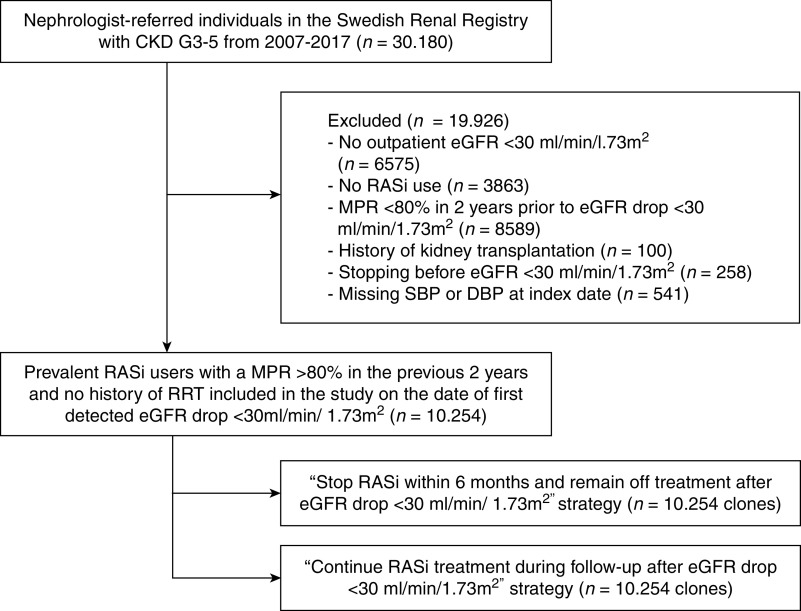
This observational study emulated a pragmatic clinical trial25 comparing the effect of stopping versus continuing RASi on cardiovascular and renal outcomes in people with advanced CKD.19 Supplemental Table 1 outlines the protocol of such a trial, which would randomize prevalent RASi users reaching incident CKD G4–5 to either stop RASi within 6 months or continue with the treatment.
We created a cohort of all adult (≥18 years) patients registered in the SRR after January 1, 2007, who experienced new CKD G4 (i.e., whose GFR decreased to <30 ml/min per 1.73 m2), and who had taken RASi for more than 80% of the 2 years before that date. We defined this using a medication possession ratio >80%, the proportion of the number of days of medication dispensed to total number of days of observation. Baseline (T0) was defined as the day on which the first recorded eGFR <30 ml/min per 1.73 m2 was identified. We chose to include only patients apparently adherent to RASi therapy to decrease the possibility of confounding bias due to nonadherence. We excluded patients with a history of kidney transplantation, patients with missing BP measurements at the time of eGFR decrease to <30 ml/min per 1.73 m2, or those who stopped RASi before the decrease in eGFR. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration26 from routine plasma creatinine measurements performed by enzymatic or corrected Jaffe methods traceable to isotope dilution mass-spectroscopy standards. As information on race is not available in Sweden by law, we did not use the variable for Black ethnicity.
Treatment Strategies
We compared the strategies “stop RASi within 6 months and remain off treatment after eGFR decrease <30 ml/min per 1.73 m2” versus “continue RASi for the whole follow-up.” We chose to examine the effect of stopping and remaining off treatment because a significant proportion of individuals who discontinued RASi restarted during follow-up (57.1%). The stopping of RASi was defined as the absence of a dispensation of RASi within 60 days (lag phase) after the estimated last day of pill supply from the previous dispensation, assuming the most common prescription pattern of one pill per day. When a prescription was filled before the expected end of the previous dispensation, we added the remaining pills to the next period, for the first occurrence, but did not carry this forward. In the case of hospitalization, we added as many additional pills as days spent in the hospital.
Study Outcomes
Each patient was followed until the first of: the occurrence of an event, 5 years after baseline, or administrative censoring (June 1, 2017). The primary outcome was 5-year all-cause mortality. Secondary outcomes included MACE (defined as a composite endpoint of mortality, myocardial infarction, and cerebrovascular events) and KRT (defined as undergoing kidney transplantation or initiating maintenance dialysis). International Classification of Diseases, Tenth Revision, codes for ascertainment of cardiovascular outcomes are listed in Supplemental Table 2. Information on the date of initiation of KRT was obtained from the SRR.
Emulation of the Target Trial
We used the method of cloning, censoring, and weighting25,27–29 to emulate a target trial comparing the effects of “stopping RASi within 6 months after eGFR dropped <30 ml/min per 1.73 m2 and remaining off treatment” versus “continuing RASi” (see Supplemental Methods and Supplemental Figure 1 for a detailed discussion on target trial emulation). Briefly, we created a dataset with two copies of each eligible individual (cloning or replicating), and assigned each of the replicates to one of the treatment strategies at the start of follow-up. Thereafter, at monthly intervals we assessed whether replicates adhered to their assigned treatment strategy; replicates were censored if and when their actual treatment deviated from their assigned treatment strategy, thereby ensuring that replicates followed their assigned strategy. For example, if a replicate was assigned to continuing RASi, but actually stopped RASi treatment on day 90, they would be censored at that point. A replicate that was assigned to the discontinuation arm and discontinued within 6 months, but subsequently restarted treatment would also be censored at the date of treatment restart. To adjust for the potential selection bias induced by this artificial censoring, each individual received a time-varying inverse probability weight.30 Informally, the denominator of the weights was the probability that a replicate remained uncensored (i.e., remained on the assigned treatment strategy) conditional on baseline and time-varying variables (Supplemental Table 3). The weights created two pseudopopulations in which treatment was independent of measured prognostic factors. We estimated the time-varying weights by fitting a pooled logistic model for the monthly probability of remaining uncensored, including variables for time and the baseline and time-varying covariates listed in Table 1. Models were fitted separately in both treatment arms to allow for treatment-covariate interaction.29 The variables for each model and their regression coefficients are reported in Supplemental Tables 4 and 5. To avoid undue influence of outliers, weights were truncated at the 99.5th percentile.31
Table 1.
Baseline characteristics of prevalent RASi users with eGFR <30 ml/min per 1.73 m2 registered in the Swedish Renal Registry during 2007–2017
| Characteristics | eGFR <30 ml/min Per 1.73 m2 Cohort (n=10,254) |
|---|---|
| Median age (IQR), yr | 72 (63–79) |
| Age category, n (%) | |
| <50 | 848 (8.3) |
| 50–59 | 1046 (10.2) |
| 60–69 | 2400 (23.4) |
| 70–79 | 3471 (33.9) |
| ≥80 | 2489 (24.3) |
| Women | 3662 (35.7) |
| Median eGFR (IQR), ml/min per 1.73 m2 | 23 (18–27) |
| eGFR category, n (%) | |
| <15 ml/min per 1.73 m2, n (%) | 1557 (15.2) |
| ≥15 ml/min per 1.73 m2, n (%) | 8697 (84.8) |
| Primary kidney disease, n (%) | |
| Diabetes | 2878 (28.1) |
| Hypertension | 2512 (24.5) |
| GN | 1096 (10.7) |
| Polycystic kidney disease | 574 (5.6) |
| Pyelonephritis | 171 (1.7) |
| Other | 1753 (17.1) |
| Missing | 1270 (12.4) |
| Mean SBP (SD), mm Hg | 139 (22) |
| SBP category, n (%) | |
| <120 | 1430 (13.9) |
| 120–139 | 3670 (35.8) |
| 140–159 | 3224 (31.4) |
| >160 | 1930 (18.8) |
| Mean DBP (SD), mm Hg | 76 (12) |
| DBP category, n (%) | |
| <80 | 5502 (53.7) |
| 80–89 | 3340 (32.6) |
| 90–99 | 1066 (10.4) |
| >100 | 346 (3.4) |
| Median urinary ACR (IQR), mg/mmol | 35 (6–156) |
| ACR category, n (%) | |
| A1 (<3) | 785 (7.7) |
| A2 (3–29) | 1445 (14.1) |
| A3 (30–69) | 614 (6.0) |
| A3 (≥70) | 1835 (17.9) |
| Missing | 5575 (54.4) |
| Mean serum potassium (SD), mmol/La | 4.5 (0.6) |
| Comorbidities, n (%) | |
| Hypertension | 9099 (88.7) |
| Myocardial infarction | 2212 (21.6) |
| Ischemic heart disease | 3390 (33.1) |
| Arrhythmia | 2302 (22.4) |
| Heart failure | 2868 (28.0) |
| Peripheral vascular disease | 1269 (12.4) |
| Cerebrovascular disease | 1620 (15.8) |
| Diabetes mellitus | 5079 (49.5) |
| Chronic obstructive pulmonary disease | 1811 (17.7) |
| Cancer diagnosis in previous 2 yr | 1018 (9.9) |
| Medication, n (%) | |
| Beta blockers | 6928 (67.6) |
| Calcium channel blockers | 6202 (60.5) |
| Diuretics | 8128 (79.3) |
| Statins | 6312 (61.6) |
| Antiplatelets | 4736 (46.2) |
| Potassium binder | 941 (9.2) |
| Calendar year | |
| 2007–2010 | 3431 (33.5) |
| 2011–2013 | 3399 (33.1) |
| 2014–2016 | 3424 (33.4) |
| Hospitalizations | |
| Any hospitalization in previous yr, n (%) | 4325 (42.2) |
| Hyperkalemia hospitalization, n (%) | 415 (4.0) |
| AKI hospitalization in previous yr, n (%) | 481 (4.7) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; ACR, albumin-to-creatinine ratio.
Potassium was missing in 37% of individuals.
We estimated the effect of stopping RASi on 5-year all-cause mortality, MACE, and KRT using weighted pooled logistic regression, including an indicator for treatment strategy, month, and its quadratic term, and their interactions to allow for nonproportional hazards. The predicted probabilities from this logistic model were used to estimate the adjusted 5-year predicted probability of mortality, MACE, and KRT under each treatment strategy and produce weighted cumulative-incidence curves.32,33 For the KRT curves, the competing risk of death was taken into account. Pointwise 95% confidence intervals (95% CIs) were calculated using a nonparametric bootstrap on the basis of 500 full samples. In addition to absolute risks and risk differences, we estimated the 5-year restricted mean survival time (RMST) under each treatment strategy and the 5-year RMST difference between both strategies. The RMST is interpreted as the average survival time over a fixed follow-up period, and graphically it corresponds to the area under the survival curve.34,35 The 5-year RMST difference compares the areas under the two survival curves for the intervention and control group. It is interpreted as the mean postponement of the outcome in one group compared with the other. For example, if the 5-year RMST difference equals 6 months, then on average, patients on one strategy survive 6 months longer compared with patients on another strategy, over a 5-year follow-up period. We used nonparametric bootstrapping to obtain 95% CIs using the SD of the bootstrap estimations as an estimation of the standard error of the RMST.36 We did not calculate hazard ratios because the proportionality of hazard assumptions was not met, and hazard ratios were thus difficult to interpret.29,37,38 R version 3.6.2 was used for all statistical analyses.
Secondary Objective: Stopping RASi at Different eGFRs
To evaluate whether observed associations differed in individuals who stopped earlier or later in the course of their disease progression, we created two additional cohorts using the same methodology: we evaluated separately the outcomes associated with stopping versus continuing RASi in a cohort of individuals on their first detected eGFR decrease to between 20 and 30 ml/min per 1.73 m2 (higher eGFR cohort) and another cohort of individuals on their first detected eGFR <20 ml/min per 1.73 m2 (lower eGFR cohort). Note there is some overlap of patients in these cohorts as they progress to a lower eGFR during observation.
Supporting and Sensitivity Analyses
We prespecified several analyses to test the robustness and consistency of our main results. First, we compared results when using nontruncated weights. Second, we performed stratified analyses by age (≥70 versus <70 years), sex, presence of diabetes, and presence of heart failure, and investigated the interaction of each of these variables with treatment on an additive scale by calculating the absolute excess risk due to interaction. Third, as a negative control analysis, we examined the association between stopping or continuing RASi and the long-term diagnosis of cancer.39 We did not expect stopping RASi to cause or prevent cancer. If we found stopping RASi to be associated with an increased risk of cancer, this would suggest the observed effect estimate suffers from residual confounding by unmeasured clinical conditions that are associated with stopping RASi, and which are likely to be associated with the risk of cancer, such as smoking and body mass index. For this analysis, patients with a recent cancer diagnosis (within 2 years from the index date) were excluded from this analysis to minimize the effects of reverse causality, because people may have stopped RASi because they had been diagnosed with cancer. Fourth, we compared results from our trial emulation design with an analysis handling RASi as a time-varying covariate.40 The effect of “always using RASi” versus “immediately stopping and not restarting RASi” after eGFR dropped <30 ml/min per 1.73 m2 was estimated using inverse probability of treatment and censoring weighted estimation of a marginal structural model (see Supplemental Methods for detailed explanation).30,41 Fifth, we additionally adjusted our analyses for time-dependent measures of urinary albumin-to-creatinine ratio (ACR) and plasma potassium. This analysis was restricted to the 3049 individuals with these data available and evaluated consistency across baseline albuminuria (≥70 versus <70 mg/mmol) and potassium (≥5.0 versus <5.0 mmol/L) strata. Finally, after reviewing the results of the work above, we conducted a nonprespecified analysis, in which we examined the associations of stopping versus continuing RASi on the combined outcome of death and KRT, as a surrogate of “net clinical benefit.”
RESULTS
Of 30,180 individuals registered in the SRR during the study period, 10,254 prevalent RASi users with a medication possession ratio >80% and no history of kidney transplantation were included from the day of their first recorded eGFR <30 ml/min per 1.73 m2. Figure 1 displays the patient selection flow chart, and Table 1 describes their baseline characteristics. At baseline, patients had a median (interquartile range, IQR) age of 72 (63–79) years and 35.7% were women. Median eGFR was 23 (18–27) ml/min per 1.73 m2, median ACR 35 (6–156) mg/mmol, mean (±SD) systolic BP 139 (SD 22) mm Hg, and mean diastolic BP 76 (SD 12) mm Hg. Hypertension (88.7%), diabetes (49.5%), ischemic heart disease (33.1%), and heart failure (28.0%) were the most common comorbidities. Concurrent use of diuretics (79.3%), beta blockers (67.6%), statins (61.6%), and calcium channel blockers (60.5%) was also prevalent. During the first 6 months of observation, 1553 (15.1%) individuals stopped RASi. Of these, 887 (57.1%) patients restarted RASi during follow-up.
Figure 1.
Selection of study participants. RASi, renin-angiotensin-system inhibitor; SBP, systolic blood pressure; DBP, diastolic blood pressure; MPR, medication possession ratio.
Stopping RASi and Outcomes
After cloning, 10,254 individuals were assigned to each treatment strategy. The mean of the truncated inverse probability weights was 2.2 (maximum 35.0). The characteristics in each treatment arm at the end of the grace period (6 months after baseline) before and after weighting are shown in Supplemental Table 6. The inverse probability weighting showed a good ability to remove covariate imbalance. The estimated 5-year mortality risk was 40.9% (95% CI, 38.9 to 42.8) among those who continued RASi, and 54.5% (95% CI, 48.5 to 61.2) among those who stopped RASi, corresponding to an absolute risk difference of 13.6 (95% CI, 7.0 to 20.3) deaths per 100 individuals and a 5-year RMST difference of −3.6 months (95% CI, −5.4 to −1.8) (Table 2). The 5-year risk of MACE was 47.6% (95% CI, 45.9 to 49.4) in the RASi continuation arm and 59.5% (95% CI, 53.8 to 66.1) in the stopping RASi arm, with an estimated 5-year absolute risk difference of 11.9 (95% CI, 5.7 to 18.6) events per 100 individuals, and a 5-year RMST difference of −3.3 months (95% CI, −5.3 to −1.4) (Figure 2, Table 2).
Table 2.
The 5-year RMST, RMST differences, absolute risks, and risk differences associated with stopping RASi and continuation on mortality, MACE, and KRT in advanced CKD patients with eGFR <30 ml/min per 1.73 m2
| Outcome and Treatment Strategy | Weighted Persons, n | Weighted Events, n | 5-yr RMST, mo (95% CI) | 5-yr RMST Difference, mo (95% CI) | 5-yr Absolute Risk, % (95% CI) | 5-yr Risk Difference, % (95% CI) |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Continuing RASi | 7971 | 3258 | 47.9 (46.2 to 49.7) | Reference | 40.9 (38.9 to 42.8) | Reference |
| Stopping RASi | 7078 | 3852 | 44.3 (43.8 to 44.8) | −3.6 (−5.4 to −1.8) | 54.5 (48.5 to 61.2) | 13.6 (7.0 to 20.3) |
| MACE | ||||||
| Continuing RASi | 8127 | 3870 | 44.7 (42.8 to 46.5) | Reference | 47.6 (45.9 to 49.4) | Reference |
| Stopping RASi | 7623 | 4543 | 41.4 (40.8 to 41.9) | −3.3 (−5.3 to −1.4) | 59.5 (53.8 to 66.1) | 11.9 (5.7 to 18.6) |
| KRT | ||||||
| Continuing RASi | 8329 | 3007 | 48.1 (46.5 to 49.7) | Reference | 36.1 (34.7 to 37.7) | Reference |
| Stopping RASi | 8808 | 2458 | 48.9 (48.3 to 49.5) | 0.8 (−0.8 to 2.5) | 27.9 (23.5 to 32.5) | −8.3 (−12.8 to −3.6) |
Analyses were adjusted through inverse probability weighting for age, sex, calendar yr, eGFR, systolic and diastolic BP, comorbidities (ischemic heart disease, myocardial infarction, arrhythmia, heart failure, peripheral vascular disease, cerebrovascular disease, diabetes, chronic pulmonary disease, cancer), medication use (beta blockers, calcium channel blockers, diuretic, statins, antiplatelet), and hospitalizations (total number of hospitalizations in previous yr, AKI hospitalization in previous yr, hyperkalemia hospitalization). Valid 95% CIs were derived using a nonparametric bootstrap on the basis of 500 samples to account for the within-subject correlation induced by weighting. Weights were truncated at the 99.5th percentile.
Figure 2.
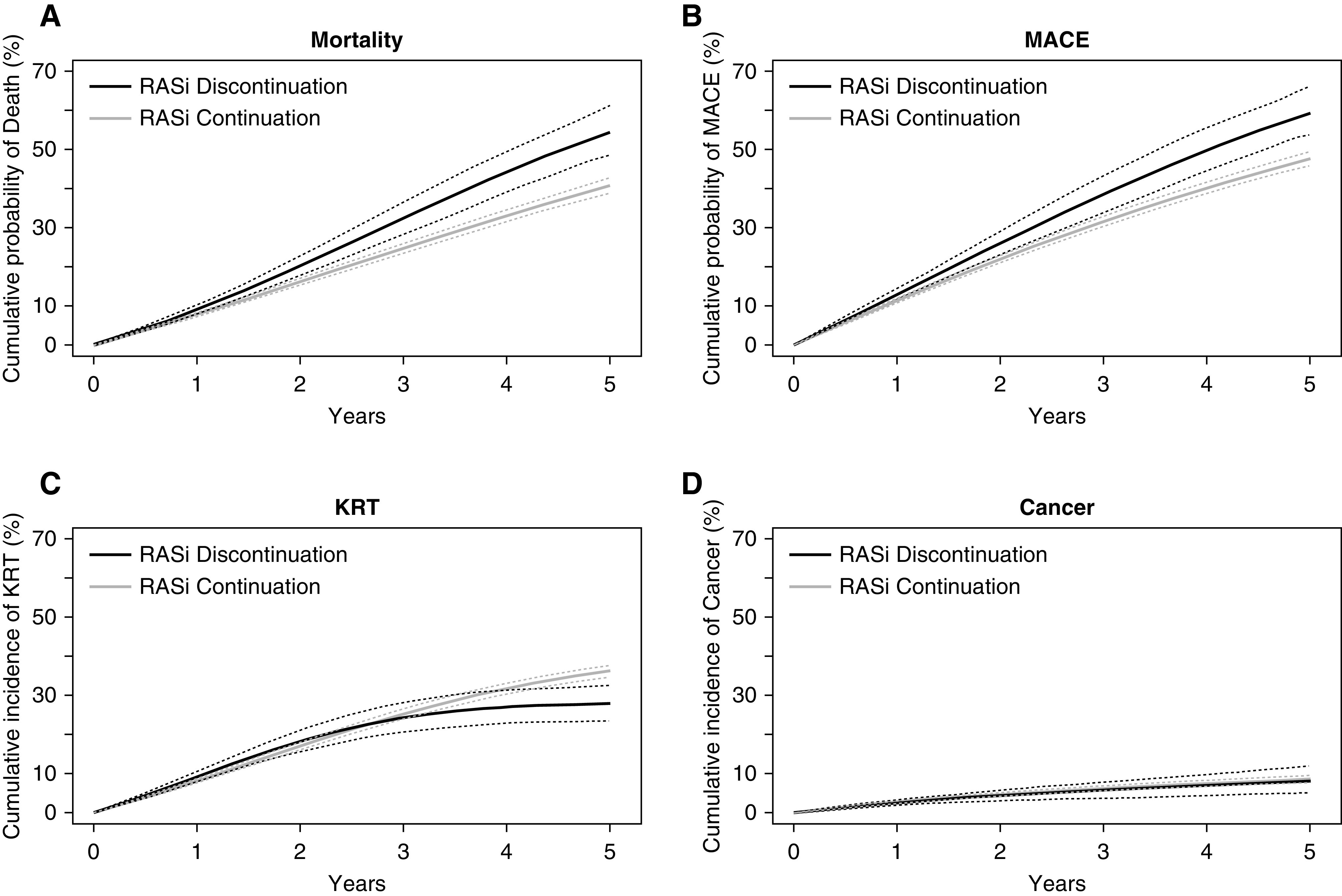
Weighted cumulative probability curves for stratified by RASi use strategy. (A) mortality, (B) MACE, (C) KRT, and (D) cancer (negative control outcome). Thinner dotted lines represent 95% CIs.
The 5-year estimated risk of KRT was 36.1% (95% CI, 34.7 to 37.7) for patients that continued with RASi and 27.9% (95% CI, 23.5 to 32.5) for those who stopped RASi. This corresponds to an absolute risk reduction of −8.3 (95% CI, −12.8 to −3.6) KRT events per 100 individuals among patients stopping RASi, and a 5-year RMST difference of 0.8 months (95% CI, −0.8 to 2.5). Figure 2 shows the weighted cumulative incidence curves for study outcomes stratified according to treatment strategy. The curves for mortality and MACE progressively diverged after a few months, whereas the curves for KRT crossed, and diverged after 3 years.
Stopping RASi and Outcomes at Different eGFR
The higher eGFR cohort included 7277 individuals whose first-observed eGFR was between 20 and 30 ml/min per 1.73 m2 (median eGFR 25; IQR, 23–28), and the lower eGFR cohort included 6907 individuals whose first observed eGFR was below 20 ml/min per 1.73 m2 (median eGFR 17; IQR 14–19). Baseline characteristics for both cohorts are displayed in Supplemental Table 7. In both cohorts, an increased risk for mortality and MACE was observed when RASi was stopped (Supplemental Figures 2 and 3, Tables 3 and 4). For instance, in the lower eGFR cohort, stopping RASi was associated with an increased absolute risk for mortality (17.1 per 100 individuals; 95% CI, 9.9 to 23.8) and MACE (12.6 per 100 individuals; 95% CI 5.8 to 19.3). In both cohorts, there also was a lower absolute risk of KRT among patients stopping RASi. For instance, in the low eGFR cohort there was an absolute risk reduction of −9.6 (95% CI, −15.0 to −3.8) KRT events per 100 individuals among patients stopping RASi. The cumulative incidence curve showed the risk for KRT was slightly higher in the stopping arm during the first 2 years of follow-up, crossed at 2 years, and diverged gradually (Supplemental Figures 2 and 3). Supporting and Sensitivity Analyses
Table 3.
The 5-year RMST, RMST differences, absolute risks, and risk differences associated with stopping RASi and continuation on mortality, MACE, and KRT in advanced CKD patients with eGFR 20–30 ml/min per 1.73 m2
| Outcome and Treatment Strategy | Weighted Persons, n | Weighted Events, n | 5-yr RMST, mo (95% CI) | 5-yr RMST Difference, mo (95% CI) | 5-yr Absolute Risk, % (95% CI) | 5-yr Risk Difference, % (95% CI) |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Continuing RASi | 5471 | 2114 | 48.7 (46.4 to 50.9) | Reference | 38.6 (36.3 to 40.9) | Reference |
| Stopping RASi | 4594 | 2340 | 46.1 (45.4 to 46.8) | −2.6 (−4.9 to −0.2) | 50.9 (42.4 to 60.1) | 12.3 (3.3 to 21.4) |
| MACE | ||||||
| Continuing RASi | 5634 | 2525 | 45.7 (43.3 to 48.1) | Reference | 44.8 (42.7 to 46.9) | Reference |
| Stopping RASi | 5005 | 2950 | 42.7 (42.0 to 43.4) | −3.0 (−5.5 to −0.5) | 58.9 (49.2 to 67.8) | 14.1 (4.6 to 23.5) |
| KRT | ||||||
| Continuing RASi | 5376 | 1360 | 53.3 (51.5 to 55.0) | Reference | 25.3 (23.4 to 27.3) | Reference |
| Stopping RASi | 5312 | 681 | 55.4 (54.9 to 55.9) | 2.1 (−0.3 to 3.9) | 12.8 (7.6 to 18.6) | −12.5 (−17.8 to −6.6) |
Analyses were adjusted through inverse probability weighting for age, sex, calendar yr, eGFR, systolic and diastolic BP, comorbidities (ischemic heart disease, myocardial infarction, arrhythmia, heart failure, peripheral vascular disease, cerebrovascular disease, diabetes, chronic pulmonary disease, cancer), medication use (beta blockers, calcium channel blockers, diuretic, statins, antiplatelet), and hospitalizations (total number of hospitalizations in previous yr, AKI hospitalization in previous yr, hyperkalemia hospitalization). Valid 95% CIs were derived using a nonparametric bootstrap on the basis of 500 samples to account for the within-subject correlation induced by weighting. Weights were truncated at the 99.5th percentile.
Table 4.
The 5-yr RMST, RMST differences, absolute risks and risk differences associated with stopping RASi and continuation on mortality, MACE, and KRT in advanced CKD patients with eGFR <20 ml/min per 1.73 m2
| Outcome and Treatment Strategy | Weighted Persons, n | Weighted Events, n | 5-yr RMST, mo (95% CI) | 5-yr RMST Difference, mo (95% CI) | 5-yr Absolute Risk, % (95% CI) | 5-yr Risk Difference, % (95% CI) |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Continuing RASi | 5470 | 2401 | 46.4 (44.7 to 48.2) | Reference | 43.9 (41.3 to 46.6) | Reference |
| Stopping RASi | 5423 | 3309 | 42.0 (41.3 to 42.7) | −4.4 (−6.3 to −2.5) | 61.0 (54.0 to 67.3) | 17.1 (9.9 to 23.8) |
| MACE | ||||||
| Continuing RASi | 5547 | 2845 | 43.0 (41.2 to 44.8) | Reference | 51.3 (48.9 to 53.9) | Reference |
| Stopping RASi | 5734 | 3663 | 39.9 (39.2 to 40.7) | −3.1 (−5.0 to −1.1) | 63.9 (57.0 to 70.0) | 12.6 (5.8 to 19.3) |
| KRT | ||||||
| Continuing RASi | 5914 | 3131 | 40.6 (38.6 to 42.6) | Reference | 52.9 (50.8 to 54.8) | Reference |
| Stopping RASi | 6872 | 2981 | 42.0 (41.3 to 42.7) | 1.4 (−0.7 to 3.5) | 43.4 (38.3 to 48.8) | −9.6 (−15.0 to −3.8) |
Analyses were adjusted through inverse probability weighting for age, sex, calendar yr, eGFR, systolic and diastolic BP, comorbidities (ischemic heart disease, myocardial infarction, arrhythmia, heart failure, peripheral vascular disease, cerebrovascular disease, diabetes, chronic pulmonary disease, cancer), medication use (beta blockers, calcium channel blockers, diuretic, statins, antiplatelet) and hospitalizations (total number of hospitalizations in previous yr, AKI hospitalization in previous yr, hyperkalemia hospitalization). Valid 95% CIs were derived using a nonparametric bootstrap on the basis of 500 samples to account for the within-subject correlation induced by weighting. Weights were truncated at the 99.5th percentile.
Using untruncated weights had no major influence on the point estimates (Supplemental Table 8). Subgroup analyses within strata of age, sex, diabetes, heart failure, and ischemic heart disease showed no suggestion of heterogeneity, with higher risks for mortality and MACE, and a lower risk for KRT observed across all subgroups for the stopping strategy (Supplemental Figure 4). We did not observe an association between continuing or stopping RASi and the risk of cancer in any of the studied cohorts (Supplemental Table 9). In the sensitivity analysis, using RASi as a time-dependent exposure through inverse probability of treatment and censoring weighted estimation of a marginal structural model, immediately stopping and not restarting RASi compared with always using RASi was associated with an 11.3% (95% CI, 8.1 to 14.5) higher risk for mortality, an 8.8% (95% CI, 5.5 to 12.5) higher risk for MACE, and a −7.1% (95%, CI −11.8 to −3.4) lower risk for KRT (Supplemental Figure 5, Supplemental Table 10). In patients with available measures of ACR and potassium, additional adjustment for these covariates showed results consistent with our main analysis, although with wider 95% CIs: compared with patients continuing RASi, stopping was associated with a 9.3% (95% CI, −1.1 to 23.7) higher absolute risk for mortality, 7.6% (95% CI, −23.6 to 21.2) higher risk for MACE, but −8.2% (95% CI, −15.8 to 5.8) lower risk for KRT (Supplemental Table 11). Stratified analyses by baseline ACR and potassium categories were largely consistent with the main results (Supplemental Figure 5). There was an increase in the magnitude of the association of stopping RASi on KRT events: risk difference of −11.4 (95% CI, −19.5 and −2.6) KRT events per 100 patients in patients with baseline potassium <5.0 mmol/L, and −33.3 (95% CI, −41.9 to −25.5) in patients with potassium ≥5.0 mmol/L over a 5-year follow-up period (interaction P<0.001). Finally, evaluating the composite outcome of death plus KRT favored the strategy of continuing with RASi versus stopping, although 95% CIs were wide, with an absolute 5-year risk difference of 5.1% (95% CI, −0.2 to 11.3) (Supplemental Figure 6, Supplemental Table 12).
DISCUSSION
Deciding whether and when to stop RASi in patients with advanced CKD is a frequent issue in clinical practice.15,16 A single-center observational study of 52 individuals (mean eGFR 16 ml/min per 1.73 m2) from the United Kingdom reported eGFR increased significantly after stopping RASi, leading to the idea that stopping RASi may prolong the time to KRT.11 Stopping RASi, in contrast, may also potentially harm patients by increasing cardiovascular risk and mortality, on the basis of generalizations from cardiovascular trials largely conducted in people with a higher GFR.17 We addressed this problem by modeling the consequences of this decision in a nationwide observational study of over 10,000 individuals with advanced CKD under routine nephrological care. We found that compared with continuing RASi, stopping treatment was associated with a higher 5-year risk of mortality and MACE, but a lower absolute KRT risk. These results appeared robust in various sensitivity and subgroup analyses, including the evaluation of stopping at a higher or lower eGFR.
Our findings of a higher absolute risk of death and MACE among patients stopping RASi confirm and expand a recent observational study of 3909 persons with advanced CKD from a single health care provider in the United States.17 Expansion of this evidence to a large, nationwide, and geographically diverse cohort of patients receiving universal government-subsidized health care increases generalizability. Collectively, this agrees with trial evidence on the cardioprotection that RASi confers to patients with CKD,42 and with observational evidence of lower cardiovascular risk associated with RASi use at all levels of eGFR.43,44 Our finding of a lower absolute KRT risk among patients stopping RASi differs from the previous US study. Qiao et al.17 observed that continuing RASi was not associated with increased risk of KRT (hazard ratio, 1.19; 95% CI, 0.86 to 1.65) and they summarized this as “KRT harms may not be excessive.” Because the assumption of proportional hazards was not met in our study, we reported absolute risk differences, and observed an association of stopping RASi therapy with reduced risk of KRT (8.3 KRT events could have been prevented per 100 patients who continued with RASi therapy over 5 years). The composite outcome of death plus KRT, which could be considered as the overall “net-clinical benefit” of the decision strategy, favored continuing with RASi. However, this analysis assumes death and dialysis are of equal importance, which is not the case in aggregate; individual patients may attribute different importance to these outcomes and their priorities should also be considered in decision making. Finally, individual patients may respond differently to RASi, and individualization of treatment and drug dosing are other important aspects not considered in our modeling.
We used comparable designs and analytical strategies to those used in the US study,17 with one exception: we censored patients when their initial strategy was changed, in acknowledgment that patients who stopped their therapy were frequently re-started during follow-up, and thus ensuring no crossovers; we think this is a strength of this current work. However, the source and type of data differ: whereas our cohort is representative of the CKD population under nephrologist care in Sweden, Geisinger is a large, predominantly rural, private, health care system in Pennsylvania that included both patients who were nephrologist referred or nonreferred. We believe our selection of patients who were nephrologist referred is a strength for evaluating KRT outcomes, because patients receive and stop or continue RASi for reasons and indications that may differ between primary care and specialist nephrology care. Both studies have a similar duration of follow-up, but a larger proportion of patients initiated KRT in our study, 35%, compared with 8% in the United States cohort. Between-country differences and differences between nephrologists and primary health care practitioners in clinical practice may additionally explain the divergent findings: for example, 15% of patients stopped RASi in our study versus 32% in the United States cohort.
Our study is the largest to date investigating the clinical consequences associated with this common clinical issue, whether to continue or stop RASi in patients with GFR <30 ml/min per 1.73 m2. Additional strengths are: (1) the application of two complementary state-of-the-art analytic approaches (i.e., target trial emulation and marginal structural modeling) to account for time-dependent confounding of a rich range of confounders; (2) confirmation of results across risk subgroups, including those with albuminuria or elevated potassium, which might explain why drugs were stopped or continued; (3) modeling a negative control outcome to evaluate the impact of reverse causation and unknown confounding; (4) evaluation of RASi use by pharmacy dispensations, which may be a better indicator for medication intake than prescriptions. Exclusion of patients with long-term use of RASi who did not have a high medication-possession ratio reduces the likelihood that medication nonadherence was the cause of drug cessation. We acknowledge a number of limitations. We did not have information on ethnic origin. Results apply to Swedish practice and extrapolation to other populations and countries should be done with caution. Initiation of KRT is itself a treatment decision that varies by practitioner, and variations in physician behavior were not captured in our study. Furthermore, the decision to stop RASi is not a random one, but the consequence of complex factors that likely herald worse outcomes. Frail patients where RASi may have been more likely to be stopped may also be more likely to be treated conservatively. Despite our sophisticated analytical design, residual confounding cannot be excluded from any observational analysis, and the precise reasons for stopping RASi remain unknown. Our conclusions remain observational in nature, and therefore do not substitute for randomized trials. However, until these trials are conducted, our findings may assist in informing clinical decisions.
To conclude, in this nationwide study, stopping RASi among patients referred to nephrologists with advanced CKD was associated with an increased absolute risk of mortality and MACE, but a lower absolute risk of KRT. To date, there is no trial evidence to inform the decision of stopping RASi therapy in these patients. Until the ongoing STOP-ACEi trial is completed,19 our analyses support current Kidney Disease Improving Global Outcomes’ recommendations of not routinely stopping RASi in people with advanced CKD.45,46
Disclosures
C. Clase has received consultation, advisory board membership, or research funding from Amgen, Astellas, Baxter, Boehringer-Ingelheim, Janssen, Johnson and Johnson, Leo Pharma, Ontario Ministry of Health, Pfizer, and Sanofi, all outside the submitted work; reports using the ONTARGET and TRANSCEND databases, which were funded by Boerhinger Ingelheim; is a coinvestigator on the REPORT study, funded by Astellas, and on the FLUID study, funded by Baxter; reports receiving Honoraria from Astellas, Janssen, Sanofi, and the University of Alberta; reports being a Scientific Advisor or a Member of the ACP Journal Club as associate editor, Canadian Journal of Kidney Health and Disease as editor-in-chief, Kidney Disease Improving Global Outcomes Potassium Controversies Conference co-chair, sponsored by AstraZeneca, Bayer HealthCare, Boehringer Ingelheim, Fresenius Medical Care, Relypsa, and Vifor Fresenius Medical Care; and reports being a speaker at an event organized by Sanofi in May 2019. F. Dekker reports receiving research funding from Astellas and Chiesi; being a Scientific Advisor or Member of the NDT editorial board; and other interests/relationships in collaborations with the Dutch Kidney Patients Association and the Dutch Quality Institute for Renal Care (Nefrovisie). M. Evans reports receiving Honoraria for lectures by Astellas, AstraZeneca, and Vifor Pharma; being a Scientific Advisor or a Member of Astellas, AstraZeneca, and the Vifor Pharma Advisory board; and being a Member of the Steering Committee of the SRR and the ERA-EDTA Registry Committee. J. Carrero acknowledges a consultancy for AstraZeneca and Baxter; reports receiving grant support to the Karolinska Institute from Astellas, AstraZeneca, and Vifor Pharma, and research funding from the Swedish Research Council and the Swedish Heart and Lung Foundation; reports receiving Honoraria from Baxter; was a Scientific Advisor or Member of the Advisory Committee of AstraZeneca, the Editorial Board of the Journal of Nephrology, Nephrology, Dialysis and Transplantation; was part of the Speakers Bureau from Abbott Laboratories, Astellas, AstraZeneca, and Vifor Pharma; and has other interests and relationships with the European Renal Nutrition working group at the ERA-EDTA and the International Society of Renal Nutrition and Metabolism. All remaining authors have nothing to disclose.
Funding
Research reported in this article was supported by the Swedish Research Council (2019-01059), the Swedish Heart and Lung Foundation (20190587), and the Westman Charitable Foundation. M. Evans is supported by a grant from Karolinska University Hospital and Stockholm City Council.
Supplementary Material
Acknowledgments
We thank A.C. Kemmeren for insightful discussions and help with the programming. The funders of this study had no role in the study design, data collection, data analysis, or interpretation, writing of the report, or the decision to submit the report for publication. Edouard L. Fu, Dr. Marie Evans, and Dr. Juan J. Carrero were responsible for the research idea and study design. Dr. Juan J. Carrero and Dr. Marie Evans were responsible for data acquisition. Edouard L. Fu, Dr. Marie Evans, Dr. Catherine M. Clase, Dr. Laurie A. Tomlinson, Dr. Merel van Diepen, Dr. Friedo W. Dekker, and Dr. Juan J. Carrero were responsible for data analysis and interpretation. Edouard L. Fu was responsible for the statistical analysis. Dr. Merel van Diepen and Dr. Juan J. Carrero were responsible for supervision or mentorship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050682/-/DCSupplemental.
Supplemental Table 1. Brief protocol of the pragmatic target trial and its emulation using data from the Swedish Renal Registry 2007–2017.
Supplemental Table 2. Definition of study outcomes and covariates.
Supplemental Table 3. Contribution to the weights at each time point by RASi treatment strategy.
Supplemental Table 4. Model coefficients for remaining uncensored in the continuation arm.
Supplemental Table 5. Model coefficients for remaining uncensored in the discontinuation arm.
Supplemental Table 6. Characteristics at 6 months after follow-up (end of grace period) on the cloned data while accounting, or not, for informative censoring (before and after weighting, respectively).
Supplemental Table 7. Baseline characteristics of RASi users across two subcohorts defined on their first detected eGFR drop between 20 and 30 ml/min and 1.73 m2 or below 20 ml/min per 1.73 m2.
Supplemental Table 8. Influence of weight truncation on the point estimates of risk differences comparing stopping versus continuing (reference) RASi.
Supplemental Table 9. Sensitivity analysis: 5-year absolute risks and risk differences associated with stopping versus continuing RASi on the negative control outcome of cancer diagnosis.
Supplemental Table 10. Sensitivity analysis: 5-year absolute risks and risk differences for always using versus immediately stopping and not restarting RASi. RASi was modelled as a time-dependent exposure using inverse probability of treatment and censoring weighted estimation of a marginal structural model.
Supplemental Table 11. Sensitivity analysis: 5-year absolute risks and risk differences associated with stopping versus continuing RASi among patients with ACR and potassium available (n=3049).
Supplemental Table 12. Sensitivity analysis: 5-year absolute risks and risk differences associated with stopping versus continuing RASi on the composite outcome of death and KRT.
Supplemental Figure 1. Schematic representation of cloning, censoring, and weighting algorithm.
Supplemental Figure 2. Weighted cumulative incidence curves for mortality (A), MACE (B), KRT (C), and cancer (D) stratified by RASi use strategy in the cohort with first detected eGFR drop between 20 and 30 ml/min per 1.73 m2. Thinner dotted lines represent 95% confidence intervals.
Supplemental Figure 3. Weighted cumulative incidence curves for mortality (A), MACE (B), KRT (C), and cancer (D) stratified by RASi use strategy in the cohort with first detected eGFR drop <20 ml/min per 1.73 m2. Thinner dotted lines represent 95% confidence intervals.
Supplemental Figure 4. Weighted cumulative incidence curves for mortality (A), MACE (B), and KRT (C) standardized to the baseline distribution of confounders using a time-dependent exposure. The effect of always using versus immediately stopping and not restarting RASi was estimated using inverse probability of treatment and censoring weighted estimation of a marginal structural model.
Supplemental Figure 5. Effect of stopping RASi on mortality (A), MACE (B), and KRT (C) across categories of age, sex, diabetes, heart failure, ischemic heart disease, ACR, and potassium. Subgroup analyses for ACR and potassium were performed on the subset of individuals with these measurements available.
Supplemental Figure 6. Weighted cumulative incidence curves for the composite outcome of death or KRT by RASi strategy for the main cohort (A), cohort of individuals with first detected eGFR drop between 20 and30 ml/min per1.73 m2 (B), and cohort of individuals with first detected eGFR drop <20 ml/min per 1.73 m2 (C). Thinner dotted lines represent 95% confidence intervals.
References
- 1.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al.; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, et al.: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Kent DM, Jafar TH, Hayward RA, Tighiouart H, Landa M, de Jong P, et al.: Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol 18: 1959–1965, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al.; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al.; The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G: Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril efficacy in nephropathy. Lancet 352: 1252–1256, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, et al.: Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perna A, Remuzzi G; Gruppo Italiano di Studi Epidemiologici in Nefrologia: ACE inhibitors to prevent end-stage renal disease: When to start and why possibly never to stop: A post hoc analysis of the REIN trial results. Ramipril efficacy in nephropathy. J Am Soc Nephrol 12: 2832–2837, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Remuzzi G, Ruggenenti P, Perna A, Dimitrov BD, de Zeeuw D, Hille DA, et al.; RENAAL Study Group: Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: A post hoc analysis of the RENAAL trial results. J Am Soc Nephrol 15: 3117–3125, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed AK, Kamath NS, El Kossi M, El Nahas AM: The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant 25: 3977–3982, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A, Jorna T, Bhandari S: Should we STOP angiotensin converting enzyme inhibitors/angiotensin receptor blockers in advanced kidney disease? Nephron 133: 147–158, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Fu EL, Trevisan M, Clase CM, Evans M, Lindholm B, Rotmans JI, et al.: Association of acute increases in plasma creatinine after renin-angiotensin blockade with subsequent outcomes. Clin J Am Soc Nephrol 14: 1336–1345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson LA, Abel GA, Chaudhry AN, Tomson CR, Wilkinson IB, Roland MO, et al.: ACE inhibitor and angiotensin receptor-II antagonist prescribing and hospital admissions with acute kidney injury: A longitudinal ecological study. PLoS One 8: e78465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku E, McCulloch CE, Vittinghoff E, Lin F, Johansen KL: Use of antihypertensive agents and association with risk of adverse outcomes in chronic kidney disease: Focus on angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. J Am Heart Assoc 7: e009992, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao Y, Shin JI, Sang Y, Inker LA, Secora A, Luo S, et al.: Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin Proc 94: 2220–2229, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao Y, Shin JI, Chen TK, Inker LA, Coresh J, Alexander GC, et al.: Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med 180: 718–726, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angiotensin converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) withdrawal in advanced renal disease. Available at: http://www.isrctn.com/ISRCTN62869767. Accessed November 8, 2020
- 19.Bhandari S, Ives N, Brettell EA, Valente M, Cockwell P, Topham PS, et al.: Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: The STOP-ACEi trial. Nephrol Dial Transplant 31: 255–261, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans M, Carrero JJ, Bellocco R, Barany P, Qureshi AR, Seeberger A, et al.: Initiation of erythropoiesis-stimulating agents and outcomes: A nationwide observational cohort study in anaemic chronic kidney disease patients. Nephrol Dial Transplant 32: 1892–1901, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Evans M, Suttorp MM, Bellocco R, Hoekstra T, Qureshi AR, Dekker FW, et al.: Trends in haemoglobin, erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant 31: 628–635, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al.: The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 16: 726–735, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al.: External review and validation of the Swedish national inpatient register. BMC Public Health 11: 450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al.: The Swedish cause of death register. Eur J Epidemiol 32: 765–773, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernán MA, Robins JM: Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183: 758–764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey Andrew S, Stevens Lesley A, Schmid Christopher H, Zhang Yaping Lucy, Castro Alejandro F 3rd, Feldman Harold I, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150[9]: 604–612, 2009. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernán MA, Lanoy E, Costagliola D, Robins JM: Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol 98: 237–242, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernán MA: When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat 6: 18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maringe Camille, Benitez Majano Sara, Exarchakou Aimilia, Smith Matthew, Rachet Bernard, Belot Aurélien, et al.: Reflections on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int J Epidemiol, 2020. 10.1093/ije/dyaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu EL, Groenwold RHH, Zoccali C, Jager KJ, van Diepen M, Dekker FW: Merits and caveats of propensity scores to adjust for confounding. Nephrol Dial Transplant 34: 1629–1635, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Cole SR, Hernán MA: Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168: 656–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA: Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 75: 45–49, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Buchanan AL, Hudgens MG, Cole SR, Lau B, Adimora AA; Women’s Interagency HIV Study: Worth the weight: Using inverse probability weighted Cox models in AIDS research. AIDS Res Hum Retroviruses 30: 1170–1177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloecker DE, Davies MJ, Khunti K, Zaccardi F: Uses and limitations of the restricted mean survival time: Illustrative examples from cardiovascular outcomes and mortality trials in type 2 diabetes. Ann Intern Med 172: 541–552, 2020 [DOI] [PubMed] [Google Scholar]
- 35.McCaw ZR, Yin G, Wei LJ: Using the restricted mean survival time difference as an alternative to the hazard ratio for analyzing clinical cardiovascular studies. Circulation 140: 1366–1368, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Carpenter J, Bithell J: Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat Med 19: 1141–1164, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Young JG, Stensrud MJ, Tchetgen Tchetgen EJ, Hernán MA: A causal framework for classical statistical estimands in failure-time settings with competing events. Stat Med 39: 1199–1236, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernán MA: The hazards of hazard ratios. Epidemiology 21: 13–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsitch M, Tchetgen Tchetgen E, Cohen T: Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 21: 383–388, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson T, Ravani P: Marginal structural models in clinical research: When and how to use them? Nephrol Dial Transplant 32[Suppl 2]: ii84–ii90, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Robins JM, Hernán MA, Brumback B: Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al.: Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: A bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis 67: 728–741, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Evans M, Carrero JJ, Szummer K, Åkerblom A, Edfors R, Spaak J, et al.: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in myocardial infarction patients with renal dysfunction. J Am Coll Cardiol 67: 1687–1697, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Molnar MZ, Kalantar-Zadeh K, Lott EH, Lu JL, Malakauskas SM, Ma JZ, et al.: Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol 63: 650–658, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group: KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney inter. Suppl 2: 337–414, 2012. Available at: https://kdigo.org/guidelines/blood-pressure-in-ckd/. Accessed May 18, 2020
- 46.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney inter. Suppl 3: 1–150, 2013. Available at: https://kdigo.org/guidelines/ckd-evaluation-and-management/. Accessed May 18, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.