Abstract
Objective
Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) is a severe immune-mediated disorder. We aim to report the neurologic features of children with PIMS-TS.
Methods
We identified children presenting to a large children's hospital with PIMS-TS from March to June 2020 and performed a retrospective medical note review, identifying clinical and investigative features alongside short-term outcome of children presenting with neurologic symptoms.
Results
Seventy-five patients with PIMS-TS were identified, 9 (12%) had neurologic involvement: altered conciseness (3), behavioral changes (3), focal neurology deficits (2), persistent headaches (2), hallucinations (2), excessive sleepiness (1), and new-onset focal seizures (1). Four patients had cranial images abnormalities. At 3-month follow-up, 1 child had died, 1 had hemiparesis, 3 had behavioral changes, and 4 completely recovered. Systemic inflammatory and prothrombotic markers were higher in patients with neurologic involvement (mean highest CRP 267 vs 202 mg/L, p = 0.05; procalcitonin 30.65 vs 13.11 μg/L, p = 0.04; fibrinogen 7.04 vs 6.17 g/L, p = 0.07; d-dimers 19.68 vs 7.35 mg/L, p = 0.005). Among patients with neurologic involvement, these markers were higher in those without full recovery at 3 months (ferritin 2284 vs 283 μg/L, p = 0.05; d-dimers 30.34 vs 6.37 mg/L, p = 0.04). Patients with and without neurologic involvement shared similar risk factors for PIMS-TS (Black, Asian and Minority Ethnic ethnicity 78% vs 70%, obese/overweight 56% vs 42%).
Conclusions
Broad neurologic features were found in 12% patients with PIMS-TS. By 3-month follow-up, half of these surviving children had recovered fully without neurologic impairment. Significantly higher systemic inflammatory markers were identified in children with neurologic involvement and in those who had not recovered fully.
SARS-CoV-2 is a novel and highly pathogenic respiratory coronavirus causing the global COVID-19 pandemic. Neurologic syndromes have been reported within the acute phase of disease, and these may arise from presumed direct viral invasion or result from a para or postinfectious inflammation.1 Although children present with a milder course of disease and case fatality rates are lower, a severe multisystem hyperinflammatory response has been reported in children during the pandemic, clinically defined as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS)2 or multisystem inflammatory syndrome in children.3 Children present with fever, abdominal pain, diarrhea, shock, myocardial injury, and development of coronary artery aneurysms.4 Central and peripheral neurologic involvement was also noted in PIMS-TS.5 Here, we characterize the spectrum of neurologic features among a cohort of 75 children with PIMS-TS and aim to identify differences to those presenting without neurologic symptoms.
Methods
A retrospective cross-sectional review of patients with PIMS-TS admitted to Evelina London Children's Hospital was performed to identify the clinical and investigative features alongside short-term outcome of children presenting with neurologic symptoms. Patients younger than 18 years and admitted between March and June 2020 who met the following criteria were included in our cohort:
Prospective diagnosis of PIMS-TS according to the Royal College of Paediatrics and Child Health case definition2 (persistent fever, inflammation, and evidence of single or multiorgan dysfunction and exclusion of other microbial causes) following review by a multidisciplinary team comprising of at least 1 each of infectious diseases, intensive care, cardiology, rheumatology, and general pediatric specialists.
New onset of at least one of the following: features of encephalopathy6 (altered consciousness that persisted for longer than 24 hours, including lethargy, irritability, or a change in personality and behavior), seizures, movement disorder, psychiatric, and/or any neurologic deficit.
All inflammatory and prothrombotic investigations (table e-1, links.lww.com/NXI/A474) were taken before therapy (including aspirin); the highest value was used for analysis. The modified Rankin Scale (mRS)7 at last clinical review was used to document clinical outcome. Demographic features and inflammatory markers were compared with the cohort of patients with PIMS-TS admitted during the same period with no neurologic symptoms. Statistical analysis was performed using SPSS 24.0 IBM (Chicago, IL). A p value less than 0.05 was considered statistically significant, and care was given to limiting multiple comparison.
Standard Protocol Approvals, Registrations, and Patient Consents
As only data required for standard medical care were collected, a full ethics review under the terms of the Governance Arrangements of Research Ethics Committees in the United Kingdom was deemed not necessary by the institutional PIMS-TS Study Group.
Data Availability
Deidentified participant data not included in the article are available on request by any qualified investigator to the corresponding author.
Results
Clinical and Investigative Features
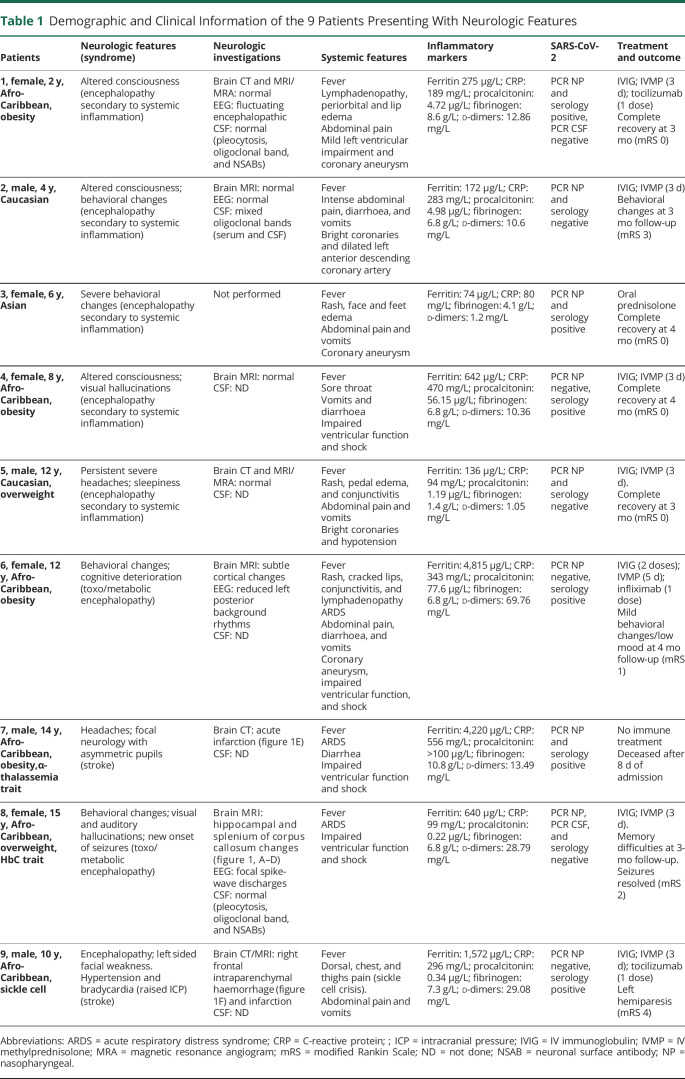
A total of 75 children (33% female; median 10 years, interquartile range 7.9 years) were diagnosed with PIMS-TS. At the time of testing availability (June–July 2020), 51 (68%) had detectable immunoglobulin (Ig) G to SARS-CoV-2. Of the 75 children, 9 (12%) had neurologic features (table 1; table e-2, links.lww.com/NXI/A475), altered consciousness (3/9; 33%), acute behavioral changes (3/9; 33%), focal neurologic deficits (2/9, 22%), severe persistent headaches (2/9; 22%), visual/auditory hallucinations (2/9, 22%), excessive sleepiness (1/9, 11%), and new-onset of focal seizures (1/9, 11%). All patients had fever at symptom onset and the range of systemic features include had gastrointestinal symptom (8/9; 89%), cardiovascular instability (5/9; 56%), and respiratory symptoms (4/9; 44%). Four patients (44%) had left ventricular dysfunction and 5 (56%) coronary artery abnormalities.
Table 1.
Demographic and Clinical Information of the 9 Patients Presenting With Neurologic Features
Four patients had abnormal imaging: focal diffusion restriction involving the splenium of the corpus callosum and mild signal changes in hippocampal regions (patient 8, figure 1, A–D); acute infarction (patient 7, figure 1E); intraparenchymal hemorrhage and infarction (patient 9, figure 1F); and subtle cortical changes as a possible sequelae of hypoxic event (patient 6). EEG showed abnormalities in the 3 of the 4 patients tested. These were reduced amplitude of background rhythms in 2 (patients 1 and 6; features of encephalopathy) and sharpened slow waves in 1 (patient 8; seizure liability). Three children had CSF analysis, and this was normal in 2 and neither had SARS-CoV-2 identified in CSF. One child had matched oligoclonal bands in serum and CSF (patient 2). Mean highest measured inflammatory markers were raised (table 2). Evidence of active infection (positive nasopharyngeal swab PCR) was found in 3 (33%) patients, and a further 3 (33%) had evidence of recent infection (negative PCR and positive IgG serology).
Figure 1. Brain MRI/CT Images.
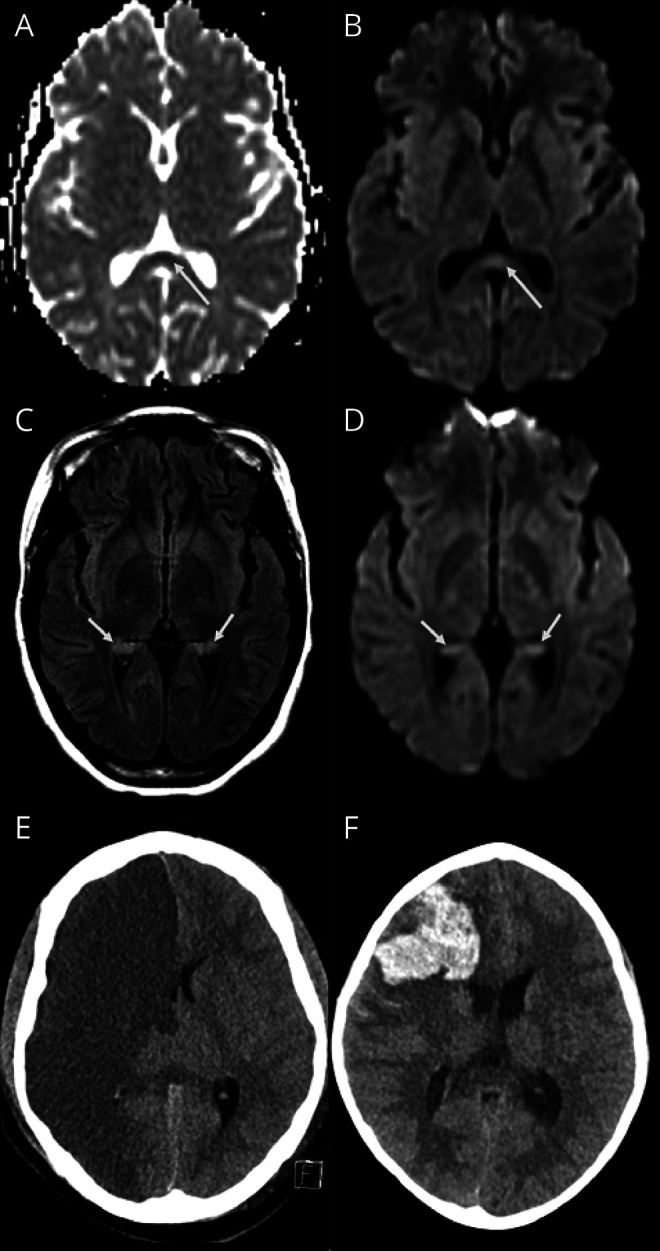
(A–F) MRI findings. The top row (A and B) showing ADC and DWI images of patient 8 shows mild diffusion restriction in the splenium of the corpus callosum (long arrows). The middle row (C and D) in the same patient also shows mild bilateral hippocampal changes on FLAIR with diffusion restriction on DWI. The bottom row shows CT images in 2 different patients; image E from patient 7 shows an acute right anterior circulation infarct, and image F from patient 9 shows an acute right frontal intraparenchymal hemorrhage. DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery.
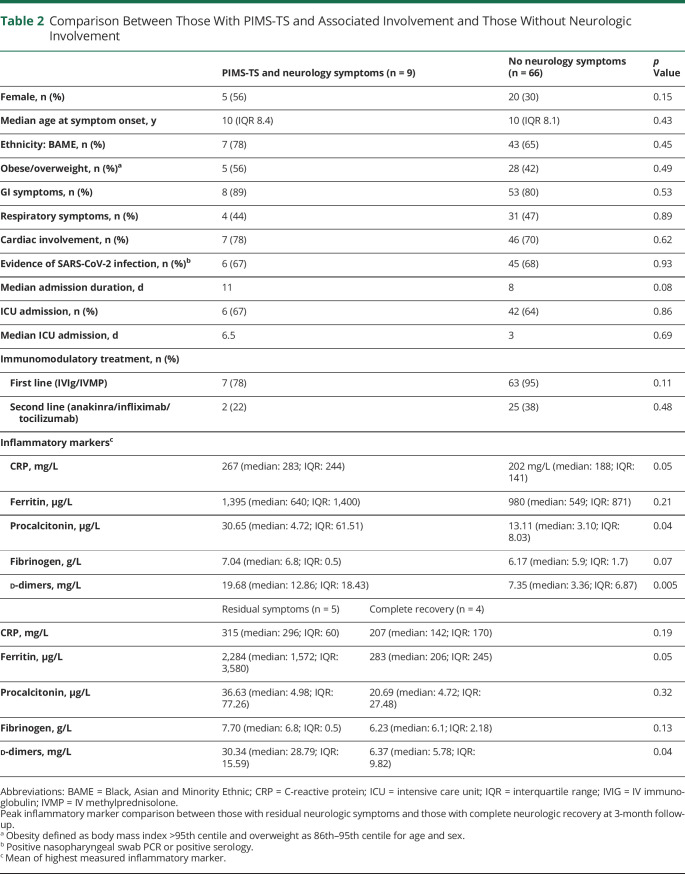
Table 2.
Comparison Between Those With PIMS-TS and Associated Involvement and Those Without Neurologic Involvement
Early Neurologic and Behavioral Outcome
One patient died after extensive brain infarction (patient 7, mRS 6), and another was transferred to a neurosurgical center for management of intraparenchymal hemorrhage (patient 9, mRS 4). The other 7 patients were discharged home after a median period of 11 (range 6–25) days of admission. Four (57%) patients recovered completely (mRS 0) at 3-month follow-up review. In the other 3 children, the following outcomes were seen: reported persistent significant behavioral and social interaction difficulties (mRS 3), mild behavioral changes with low mood (mRS 1), and memory difficulties (mRS 2).
Comparison With Children Who Did Not Exhibit Neurologic Symptoms
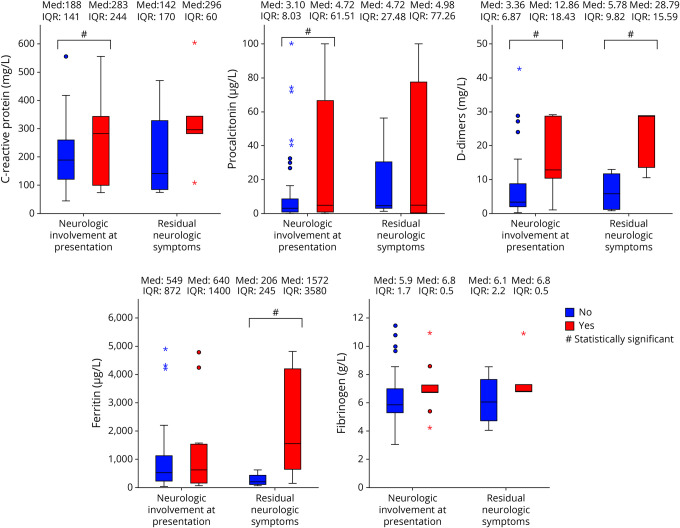
Comparison was made between those with PIMS-TS and associated neurologic involvement (n = 9) and those without neurologic involvement (n = 66) (table 2). Risk factors for PIMS-TS were similar: Black, Asian and Minority Ethnic ethnicity 78% vs 70%, obesity (body mass index >95th centile)/overweight (86th–95th centile) 56% vs 42%. Both groups had comparable number and duration of intensive care unit (ICU) admission and cardiac involvement. Notably, systemic inflammatory markers were higher in patients with neurologic involvement (figure 2). Considering patients with neurologic involvement (n = 9), peak inflammatory and prothrombotic markers during acute disease were higher in children with sequelae at 3-month follow-up (n = 5) in comparison with children with complete neurologic recovery (n = 4) (table 2, figure 2).
Figure 2. Box Plot Graphs Comparing Highest Pretreatment Mean Inflammatory Markers.
Box plot graphs comparing highest mean inflammatory markers. Left side of each plot: comparison of PIMS-TS patients (n = 75) without (blue, n = 66) and with (red, n = 9) neurologic involvement during acute illness. Right side of each plot: comparison of PIMS-TS patients with neurologic involvement at presentation (n = 9) without (blue, n = 4) and with (red, n = 5) neurologic residual symptoms at 3-month follow-up. Asterisks denote outliers >3 times the interquartile range (IQR). PIMS-TS = pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2.
Discussion
In this study, we describe the spectrum of neurologic manifestations in 9 children with PIMS-TS. The frequency of neurologic symptoms was relatively low (12%), and this was comparable to a previous smaller cohort.5 Two children developed extensive stroke. Stroke in adult patients has been extensively reported and is thought to be due to inflammation driven hypercoagulability with thrombotic microangiopathy playing a major role in COVID-19 syndromes.8 A characteristic radiologic feature noted in our cohort is the splenial lesion. Among other neuroradiologic features, the same splenial findings had been previously reported and also with a favorable clinical outcome, during the acute SARS-CoV-2 infection5 and delayed hyperinflammatory syndrome.9
Of interest, SARS-CoV-2 IgG was undetected in 24 (32%) of children. One plausible explanation for this is that antibody concentrations may have decayed to below the threshold of detection by the time testing was clinically available in our patients.10 Alternatively, antibody specificity and concentrations are known to differ in children with PIMS-TS in comparison to adults with COVID-19,11 explaining an incomplete seroprevalence in this PIMS-TS cohort. Seronegative patients with PIMS-TS were phenotypically similar to seropositive patients with PIMS-TS, presented in the period of the first surge of cases in the UK COVID-19 pandemic, and were negative by PCR and relevant serology to extensive virologic testing.
The key finding of our study is that children with neurologic symptoms as part of their PIMS-TS presentation have significantly higher systemic inflammatory markers than children without neurologic features. These findings support the hypothesis that PIMS-TS–associated neurologic involvement comprises a systemic para or postinfectious immune-mediated phenomenon. A study from our institution has demonstrated that there is a distinct immunophenotype with high levels of interleukin activation in children with PIMS-TS.12 The key question of how systemic inflammation might lead to CNS symptoms often implicates blood-brain barrier integrity or direct transfer via the circumventricular organs and regions devoid of a barrier.13
Of the surviving 8 children with PIMS-TS and neurologic symptoms, only half have returned to their baseline at 3-month follow-up. Similar difficulties are described short-term complications of ICU admissions in children.14 However, the important observation in our study is that those with residual reported symptoms had higher inflammatory markers at presentation. Systemic hyperinflammation may acutely influence brain structural connections and could lead to long-term neurocognitive sequelae, as seen in some patients following autoimmune encephalitis.15
This single-center retrospective observational study spans a short period where the case definition and management of a newly evolving disease entity was being established and has inherent limitations. The full spectrum of the neurologic symptoms would have been underestimated in children in critical care and may result in inaccurate cross-sectional comparison. In addition, treatment effects cannot be systematically evaluated, as within this cohort, virtually all have been given first-line immune therapy (70/75, 93%), and that therapy was variably escalated to second line in 28/75 (37%).
Nevertheless, we were able to demonstrate that children with neurologic features during acute PIMS-TS illness have a higher burden of inflammation, which may in turn influence their recovery, raising the question as to the utility of these biomarkers in directing inflammation treatments. Presence of neurologic features should alert the clinician to the potential for more systemic inflammation. Further larger scale multicenter collaborative studies are already being initiated16 to map the fuller spectrum of acute and long-term neurologic features of PIMS-TS and to evaluate the treatment effects of attenuating inflammation on neurologic outcome.
Acknowledgment
The authors are thankful to all members of the clinical teams caring for the complex needs of the patients described in this article (detailed in appendix 2, links.lww.com/NXI/A473).
Glossary
- ICU
intensive care unit
- Ig
immunoglobulin
- mRS
modified Rankin Scale
- PIMS-TS
pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2
Appendix 1. Authors

Appendix 2. Coinvestgators

Contributor Information
Mario Sa, Email: mario.sa@nhs.net.
Luwaiza Mirza, Email: luwaiza.mirza@kcl.ac.uk.
Michael Carter, Email: michael.carter@kcl.ac.uk.
Lalani Carlton Jones, Email: lalani.carltonjones@gstt.nhs.uk.
Vasantha Gowda, Email: vasantha.gowda@gstt.nhs.uk.
Jennifer Handforth, Email: jennifer.handforth@gstt.nhs.uk.
Tammy Hedderly, Email: tammy.hedderly@gstt.nhs.uk.
Julia Kenny, Email: julia.kenny@gstt.nhs.uk.
Karine Lascelles, Email: karine.lascelles@gstt.nhs.uk.
Jean-Pierre Lin, Email: jean-pierre.lin@gstt.nhs.uk.
Daniel Lumsden, Email: daniel.lumsden@gstt.nhs.uk.
Marilyn McDougall, Email: marilyn.mcdougall@gstt.nhs.uk.
Owen Miller, Email: owen.miller@gstt.nhs.uk.
Thomas Rossor, Email: thomas.rossor@gstt.nhs.uk.
Vinay Shivamurthy, Email: vinay.shivamurthy@gstt.nhs.uk.
Ata Siddiqui, Email: ata.siddiqui@gstt.nhs.uk.
Rahul Singh, Email: rahul.singh@gstt.nhs.uk.
Shan Tang, Email: shan-shan.tang@gstt.nhs.uk.
Marie White, Email: marie.white@gstt.nhs.uk.
Susan Byrne, Email: susan.byrne@gstt.nhs.uk.
Study Funding
No targeted funding reported.
Disclosure
M. Lim receives research grants from Action Medical Research, the DES society, GOSH charity, National Institute for Health Research, MS Society, and SPARKS charity; receives research support grants from the London Clinical Research Network and Evelina Appeal; has received consultation fees from CSL Behring, Novartis, and Octapharma; received travel grants from Merck Serono; and was awarded educational grants to organize meetings by Novartis, Biogen Idec, Merck Serono, and Bayer. V. Gowda receives research grants from PTC Pharma; receives personal fees from Roche; has received sponsoring for conference attendance from PTC Pharma and Biogen; participates in commercial clinical trials from Wave Life Sciences and Catabasis. All other authors have no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol 2020;19:767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf. Accessed January 11, 2021.
- 3.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). emergency.cdc.gov/han/2020/han00432.asp. Accessed January 11, 2021.
- 4.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Mannan O, Eyre M, Lobel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol 2020;77(11):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 2010;10(12):835–844. [DOI] [PubMed] [Google Scholar]
- 7.Bigi S, Fischer U, Wehrli E, et al. Acute ischemic stroke in children versus young adults. Ann Neurol 2011;70(2):245–254. [DOI] [PubMed] [Google Scholar]
- 8.Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol 2020;16(10):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindan CE, Mankad K, Ram D, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health 2020;5(3):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med 2020;383(11):1085–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter MJ, Shankar-Hari M, Tibby SM. Paediatric inflammatory multisystem syndrome temporally-associated with SARS-CoV-2 infection: an overview. Intensive Care Med 2021;47(1):90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020;26(11):1701–1707. [DOI] [PubMed] [Google Scholar]
- 13.Obermeier B, Verma A, Ransohoff RM. The blood-brain barrier. Handb Clin Neurol 2016;133:39–59. [DOI] [PubMed] [Google Scholar]
- 14.Als LC, Picouto MD, Hau SM, et al. Mental and physical well-being following admission to pediatric intensive care. Pediatr Crit Care Med 2015;16(5):e141–e149. [DOI] [PubMed] [Google Scholar]
- 15.Peer M, Pruss H, Ben-Dayan I, Paul F, Arzy S, Finke C. Functional connectivity of large-scale brain networks in patients with anti-NMDA receptor encephalitis: an observational study. Lancet Psychiatry 2017;4(10):768–774. [DOI] [PubMed] [Google Scholar]
- 16.The CoroNerve Studies Group. CoroNerve. coronerve.com. Accessed January 11, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data not included in the article are available on request by any qualified investigator to the corresponding author.