Abstract
This review article highlights selected advances in triphosgene-enabled organic synthetic reactions that were reported in the decade of 2010–2019. Triphosgene is a versatile reagent in organic synthesis. It serves as a convenient substitute for the toxic phosgene gas. Despite its first known preparation in the late 19th interestingly began only three decades ago. Despite the relatively short history, triphosgene has been proven to be very useful in facilitating the preparation of a vast scope of value-added compounds, such as organohalides, acid chlorides, isocyanates, carbonyl addition adducts, heterocycles, among others. Furthermore, applications of triphosgene in complex molecules synthesis, polymer synthesis, and other techniques, such as flow chemistry and solid phase synthesis, have also emerged in the literature.
Keywords: Triphosgene, Bis(trichloromethyl) carbonate, Organohalides, Carbonyl compounds, Heterocycles
1. Introduction
This review article describes recent advancement in organic synthetic reactions that utilize triphosgene as the key reagent. It is comprised of brief summaries on selected chemistries that were published in the literature between the decade of 2010–2019. This review is intended to provide updates to existing surveys published by Eckert [1], Sennyey [2], Imagawa [3], Bracher [4], Su [5], Ghorbani-Chogaramani [6], and Cotarca [7], as well as to further supplement the Encyclopedia of Reagents for Organic Synthesis entries initially reported by Mobashery, followed by Banerjee on the first update and Kartika on the second update [8]. The contents in this review are arranged primarily based on groups of reactions that are enabled by triphosgene. Each topic is then expanded further to include the various structural motifs that are produced and isolated from these reactions.
2. Triphosgene
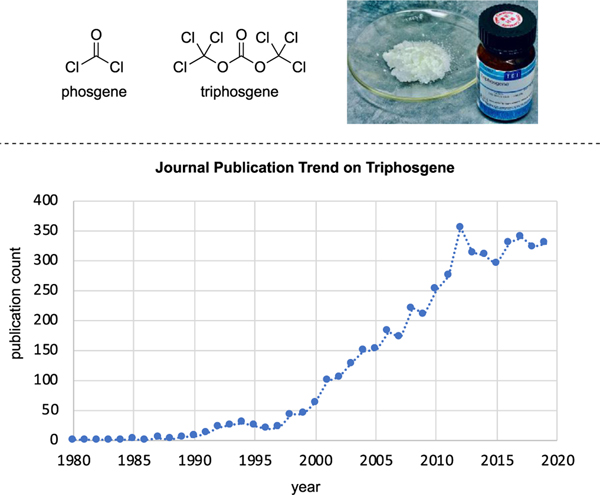
Triphosgene, also known as bis(trichloromethyl) carbonate or BTC, is a convenient substitute for the extremely toxic phosgene gas (Fig. 1). It exists as a stable crystalline solid with a melting point of 80 °C, but it decomposes at temperature above 200 °C. The first preparation of triphosgene was reported by Councler in 1880 [9], in which its synthesis was accomplished through liquid-phase photochlorination of dimethyl carbonate. The physical and chemical properties of triphosgene were documented by Hentschel in 1887 [10–12]. Its solid-state X-ray characterization data was then reported by Sorensen in 1971 [13]. Interestingly, the utility of triphosgene in synthetic reactions remained relatively unexplored until it was “rediscovered” in the late 1980s [14,15]. Since then, triphosgene has proven to be a convenient replacement of phosgene. It has been widely used in many laboratory and industrial reactions [16–19]. Because of its stable crystalline form, triphosgene provides safety benefits that consequently enable its transportation, storage, and handling that is more practicable than phosgene gas. Furthermore, triphosgene offers operational simplicity for typical laboratory scale reactions since the exact amounts of this reagent can be measured under standard safety protocols.
Fig. 1.
Triphosgene and journal publication trend.
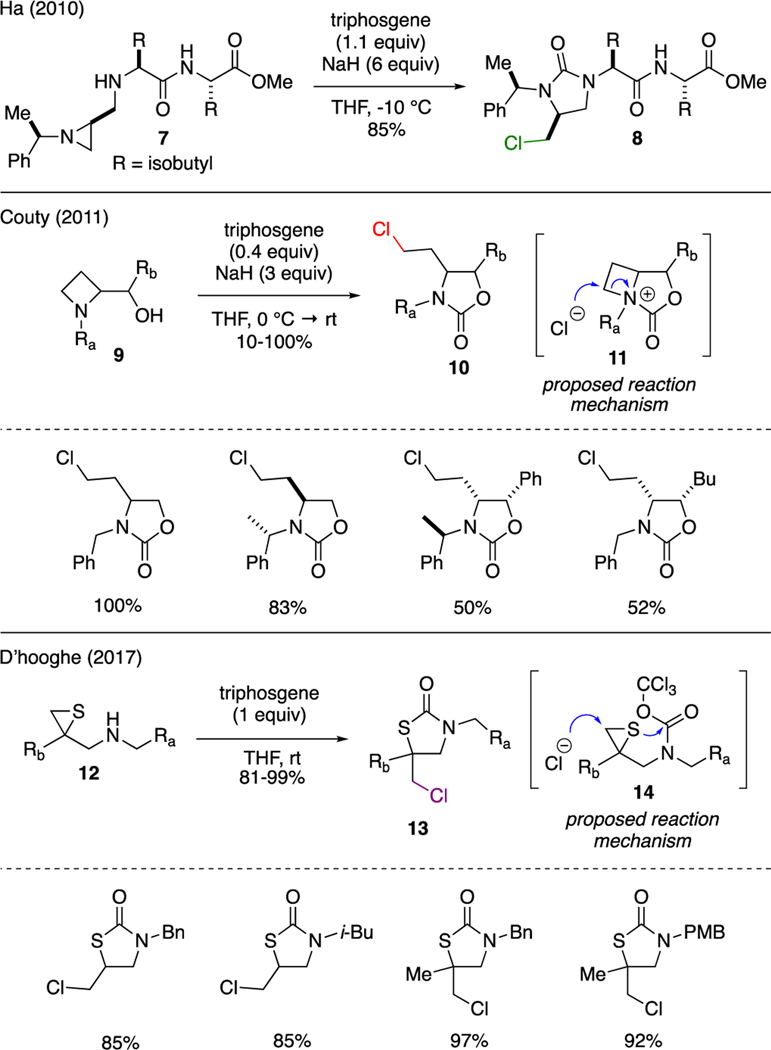
Between the years of 2010–2019, a tremendous growth on literature precedents that describe applications of triphosgene in numerous synthetic transformations was noted [20]. In this decade review, we wish to examine a selection of organic reactions that have been made possible by this extremely versatile reagent. The proposed mechanism for some examples will also be presented.
3. Synthesis of organohalides
There has been a growing number of reports that demonstrate the use of triphosgene to synthesize organohalides. In the past 10 years, a significant advancement was particularly realized in the development of reaction protocols that facilitated Halogenation of a much broader scope of substrates and functional groups, such as aliphatic alcohols and diols, epoxides, ketones, arenes, etc.
3.1. Alkyl chlorides
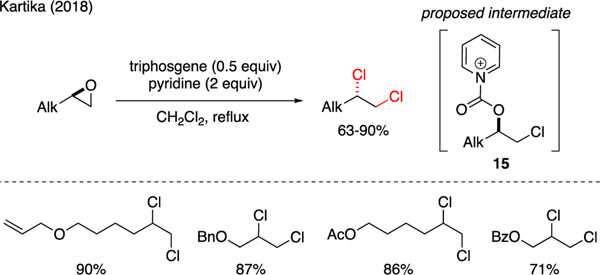
Scheme 1 conveys representative chemistries on the utility of triphosgene in the synthesis of various alkyl chlorides. For example, chlorination of aliphatic primary alcohols using a mixture of triphosgene and triethylamine was reported by Kartika [21]. This method was limited to linear primary alcohols as α-branched secondary alcohols predominantly formed N,N-diethylcarbamates, instead of alkyl chlorides. Interestingly, tertiary alcohols remained unaffected by the reaction conditions. Nonetheless, selectivity toward chlorination was restored simply by switching the amine base to pyridine [22]. The mechanism of these reactions was proposed to involve an intermediacy of triethylammonium carbamate 1 or pyridinium carbamate 2, which underwent decarboxylative SN2 displacement by chloride ions with complete inversion of stereochemistry. Vaidya reported a conversion of Baylis-Hillman adducts 3 into allylic chlorides 4 with (Z)-stereoselectivity using a mixture of triphosgene and pyridine [23]. Interestingly, substrates bearing a nitrile functionality 5 reversed the double bond selectivity to the (E)-geometry in products 6.
Scheme 1.
Synthesis of Alkyl Chlorides.
Triphosgene has been employed in the synthesis of heterocycles containing alkyl halides via chemistries involving a sequential addition of chloride ions, followed by ring expansion. As shown in Scheme 2, Ha reported the synthesis of 2-imidazolidinone-containing peptide 8 that was accomplished via exposure of azidirine-containing peptide 7 to NaH and triphosgene [24]. This reaction successfully installed the 2-imidazolidinone unit in compound 8 while simultaneously introducing alkyl chloride at the exo position in 85% yield. Another example was accounted by Couty who demonstrated that 2-(1-hydroxyalkyl) azetidines 9 could be transformed to chlorine-containing oxazolidinone adducts 10 upon treatment with NaH and triphosgene [25]. The mechanism of this reaction was proposed through the carbonyl addition between the azetidine and hydroxy groups, followed by ring opening of the resulting azetidinium ion 11 by chloride ions. D’hooghe expanded the utility of triphosgene in the synthesis of analogous S,N-heterocycles, i.e. thiazolidinones 13, which was achieved upon treatment of aminothiiranes 12 with triphosgene [26]. The mechanism of this reaction was proposed via chloroformylation of the amino group to produce trichloromethyl carbamate 14. The subsequent regioselective ring opening of thiirane at the terminal position by chloride ions then gave way to 5-membered thiocarbamate cyclization to furnish the product.
Scheme 2.
Synthesis of Alkyl Chlorides via Addition - Ring Expansion Reactions.
3.2. Alkyl 1,2-dichlorides
The conversion of terminal aliphatic epoxides to alkyl 1,2-dichlorides using triphosgene and pyridine was reported by Kartika (Scheme 3) [27]. It was proposed that the reaction mechanism proceeded via epoxide opening by chloride ions, followed by chloroformylation of the resulting chlorohydrin en route to pyridinium carbamate intermediate 15. The ensuing decarboxylative SN2 displacement with chloride ions then furnished the alkyl 1,2-dichloride motif with complete inversion of stereochemistry.
Scheme 3.
Synthesis of Alkyl 1,2-Dichlorides.
3.3. Alkyl 1,3-dichlorides and 1,3,5-trichlorides
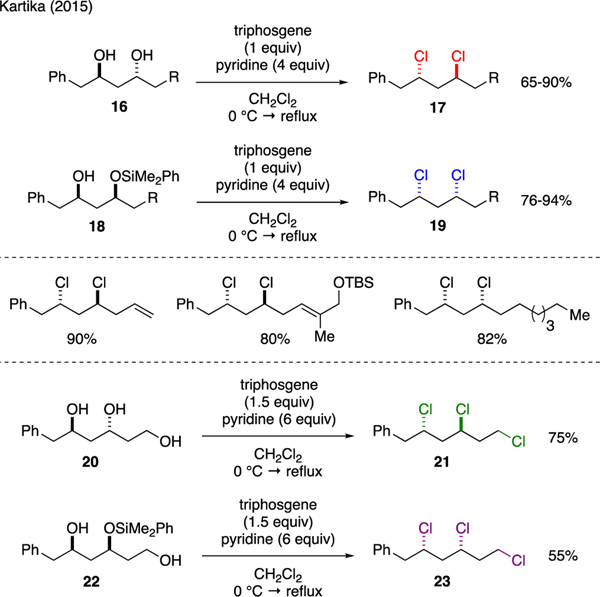
Kartika reported that chlorination of acylic aliphatic 1,3-diols could be performed using a mixture of triphosgene and pyridine in a stereoselective manner (Scheme 4) [28]. It was noted that while 1,3-anti diols 16 were straightforwardly converted to 1,3-anti dichlorides 17 under these conditions, chlorination of 1,3-syn diols to 1,3-syn dichlorides 19 required substrate modification to monosilylethers 18 to circumvent the competitive carbonate cyclization. This method was also applicable for the construction of stereocomplimentary alkyl 1,3,5-trichlorides 21 and 23 from their respective 1,3,5-triols 20 and 22.
Scheme 4.
Synthesis of Alkyl 1,3-Dichlorides and 1,3,5-Trichlorides.
3.4. Vinyl chlorides
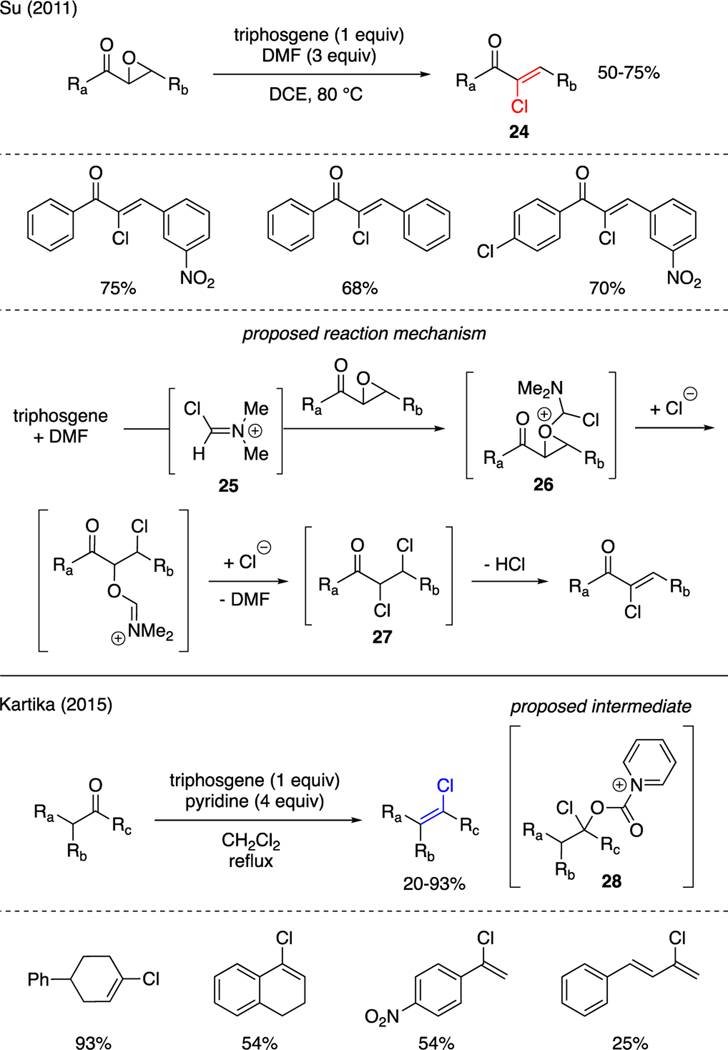
There are some examples on the synthesis of vinyl chlorides mediated by triphosgene (Scheme 5); one of which was disclosed by Su [29]. In this work, treatment of 2-epoxyketone with triphosgene and DMF readily produced vinyl chlorides 24. The proposed mechanism commenced with activation of the 2-epoxyketone by Vilsmeier reagent 25 that was generated in situ from triphosgene and DMF. The ensuing ring opening of 26 by chloride ions facilitated nucleophilic attack at the α-position by chloride ions to form carbonyl α,β-dichloride 27. Finally, elimination of the β-chloride under the reaction conditions generated the vinyl chloride moiety. Another example was reported by Kartika, who disclosed that the synthesis of vinyl chlorides from ketones could be achieved using a mixture of triphosgene-pyridine [30]. These reactions were proposed to involve the formation of α-chloro pyridinium carbamate intermediate 28, which then proceeded to decarboxylative elimination in the presence of excess pyridine.
Scheme 5.
Synthesis of Vinyl Chlorides.
3.5. Aryl chlorides
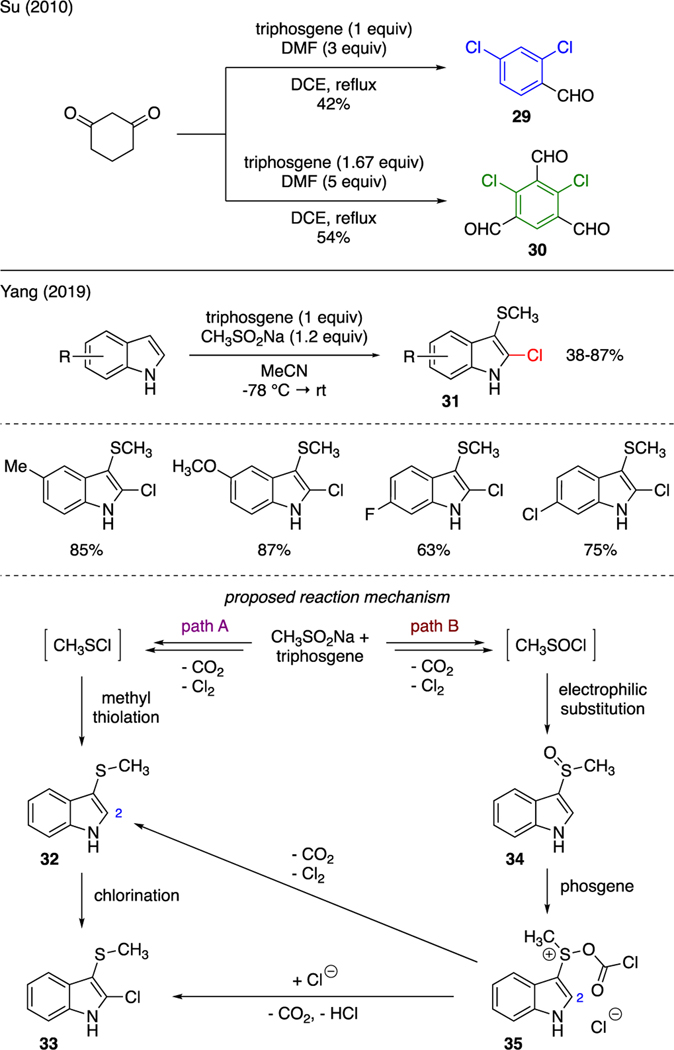
As depicted in Scheme 6, triphosgene could be utilized to prepare aryl chlorides. For instance, Su demonstrated that the Vilsmeier reagent produced from triphosgene and DMF could effectively react with 1,3-cyclohexanedione to produce dichlorobenzene derivatives [31]. More specifically, while a mixture of 1 equivalent of triphosgene and 3 equivalents of DMF afforded 2,4-dichlorobenzaldehyde 29 in 42% yield, increasing the amount of triphosgene and DMF to 1.67 and 5 equivalents, respectively produced 2,4-dichlorobenzene-1,3,5-tricarbaldehyde 30 in 54% yield.
Scheme 6.
Synthesis of Aryl Chlorides.
Yang reported an example of vicinal chloroalkylthiolation reaction to produce compounds 31 by exposing substituted indoles to triphosgene and sodium sulfinate [32]. In this report, triphosgene was believed to convert sodium sulfinate to two possible sulfur electrophilic intermediates, i.e. CH3SCl through pathway A or CH3SOCl through pathway B; both of which were captured by indoles. Following methylthiolation in pathway A, chlorination of adduct 32 was then achieved at the C2 position to furnish the observed product 33. In pathway B, it was proposed that the resulting methylsulfone 34, generated upon electrophilic substitution of indoles with CH3SOCl, was further activated with in situ generated phosgene to sulfonium intermediate 35. This species then proceeded directly to decarboxylative substitution by chloride ions at the C2 position and thereby produce the isolated product 33. Alternatively, a reaction mechanism involving reduction of the sulfone moiety in 35 to the methylthiolation adduct 32, en route to chlorination, could not be ruled out.
3.6. Chlorocarbonates
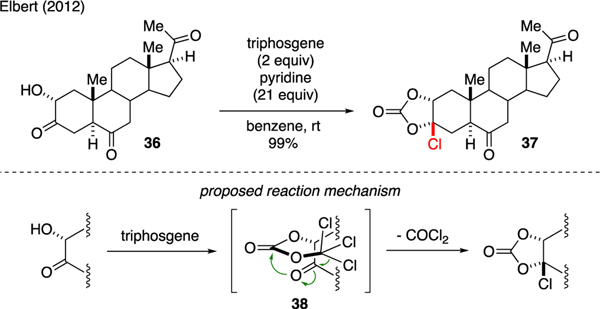
Elbert and Marek developed chemistries on the stereospecific chlorination of α-hydroxy ketone in steroid skeleton 36 to chlorocarbonate 37 (Scheme 7) [33,34]. The reaction mechanism was proposed through activation of α-hydroxy ketone to produce trichloromethyl carbonate intermediate 38, of which the ensuing chloride addition to the ketone moiety was believed to have occurred via a six-membered transition state. This step enabled intramolecular carbonate cyclization to furnish the chlorocarbonate adduct as a single diastereomer while liberating phosgene as byproduct.
Scheme 7.
Synthesis of Chlorocarbonates.
3.7. Aryl Bromides
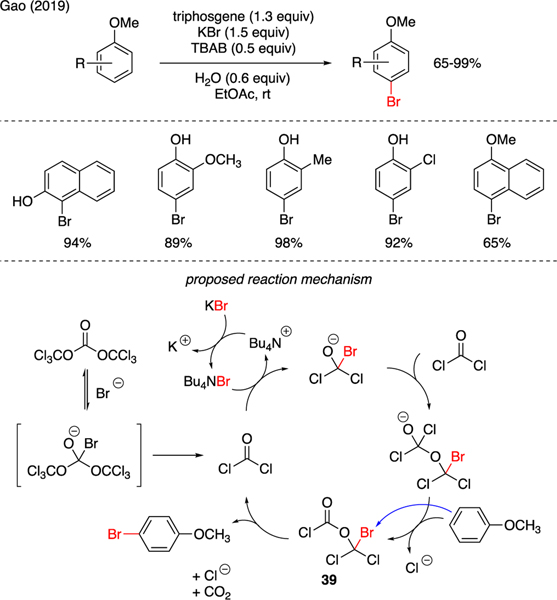
In addition to chlorination reactions, triphosgene has found a unique application in the aryl bromination reactions. As illustrated in Scheme 8, Gao described regioselective bromination of substituted phenols, naphthols, and anisoles, using a mixture of triphosgene, KBr, and phase-transfer catalyst t-butyl ammonium bromide (TBAB) [35]. Interestingly, this method readily suppressed polybromination that was typically observed using highly electronrich arenes. A plausible mechanism was proposed through decomposition of triphosgene to phosgene by bromide ions. Bromide ions then promoted nucleophilic dimerization of two phosgene molecules to bromo-derived diphosgene 39, which ultimately served as a source of electrophilic bromine. The ensuing electrophilic aromatic substitution furnished the observed aryl bromination products.
Scheme 8.
Synthesis of Aryl Bromides.
4. Functional group interconversions
The versatility of triphosgene was showcased in the interconversion of several functional groups, such as amines and alcohols, to other valuable synthetic intermediates.
4.1. Isocyanates and isothiocyanates
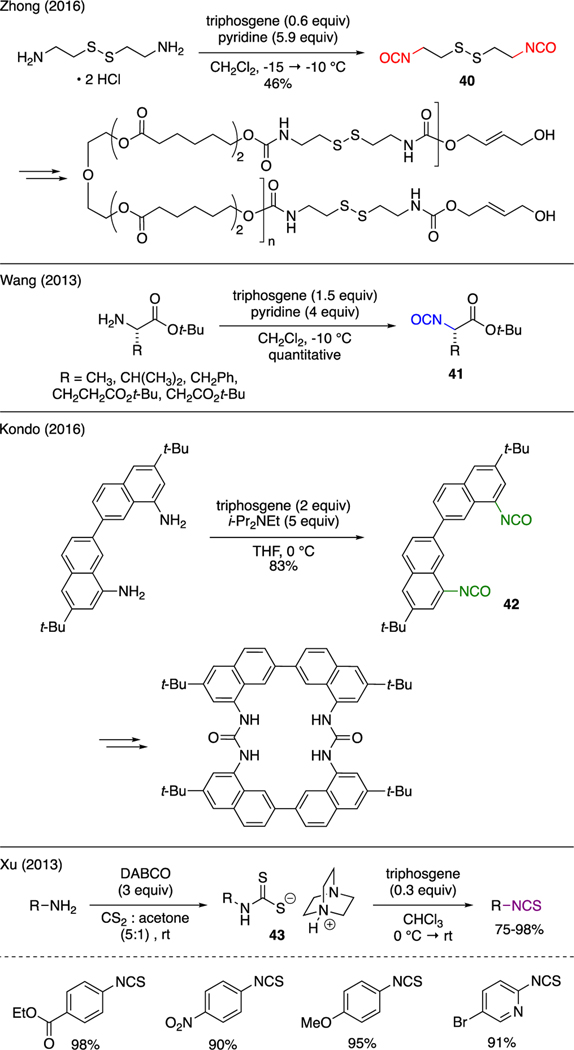
As detailed in the upcoming Section 5 and onward, the reaction of amines with triphosgene, depending upon reaction conditions, generated either carbamoyl chlorides or isocyanates that could be directly captured in situ by nucleophiles. In some instances, the resulting isocyanates could be isolated (Scheme 9). Zhong demonstrated the conversion of cystamine dihydrochloride to diisocyanate 40 in the presence of triphosgene and pyridine [36]. This compound was then used as a coupling agent in the synthesis of degradable biopolymers. Another example was conveyed by Wang [37], who prepared isocyanates 41 from L-amino acid t-butyl esters using a mixture of triphosgene and pyridine. Kondo reported the synthesis of bisisocyanate 42 from the corresponding diamine upon treatment with triphosgene and Hunig’s base. This compound served as a synthetic precursor to macrocyclic bisurea that exhibited selective binding affinity toward chloride ions [38]. Xu reported that isothiocyanates could be produced using triphosgene in a two-step procotol [39], commencing with the reaction of primary amines, CS2, and DABCO. The resulting isolable carbamodithioate salts 43 was subsequently treated with triphosgene to promote dehydrosulfurization, furnishing the isothiocyanate functionality.
Scheme 9.
Synthesis of Isocyanates and Isothiocyanates.
4.2. Trichloromethyl carbamates
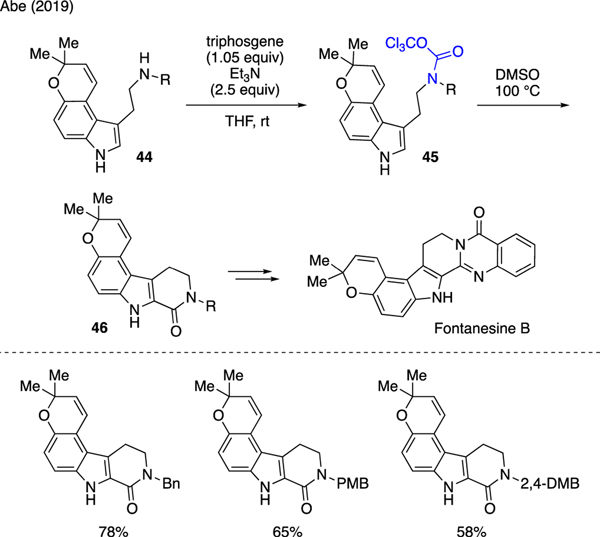
Abe reported an elegant total synthesis of indole alkaloid Fontanesine B (Scheme 10) [40]. In this work, a mixture of triphosgene and triethylamine in THF was employed to transform aminoindoles 44 to trichloromethyl carbamates 45. Upon removal of THF, treatment of the resulting crude materials with DMSO and heat led to δ-lactam cyclization to produce tetracyclic compounds 46. Several amine protecting groups were subjected in the studies, and the products were generally isolated in good yields.
Scheme 10.
Synthesis of Trichloromethyl Carbamates.
4.3. Alkenes
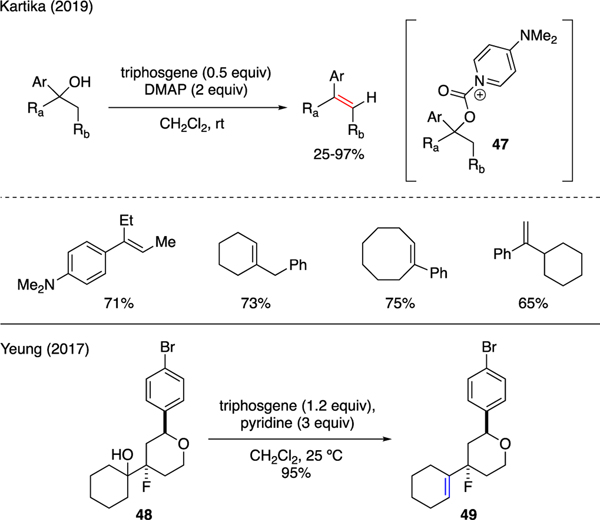
A recent report from Kartika highlights the synthetic utility of triphosgene and DMAP in the dehydration of tertiary alcohols (Scheme 11) [41]. The resulting alkenes generally showed a preference for the (E)-stereoselectivity. Interestingly, while formation of the Hoffman products was favored, a notable change in selectivity toward the Zaitsev products was noted by modulating the reaction temperature. The reaction mechanism was proposed to involve an intermediacy of pyridinium carbamate ion 47 that subsequently proceeded to decarboxylative elimination. Yeung also conveyed an example on the use of triphosgene and pyridine to promote dehydration of substituted cyclohexanol 48 to cyclohexene 49 [42].
Scheme 11.
Dehydration of Alcohols.
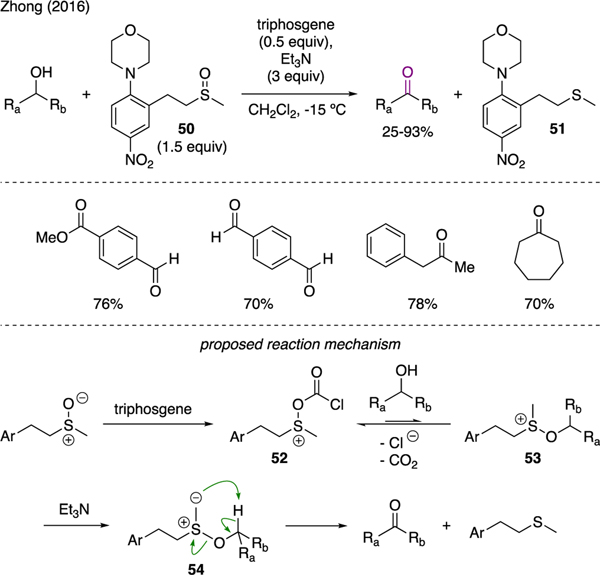
4.4. Aldehydes and ketones
As depicted in Scheme 12, Zhong reported a modified version of the Swern oxidation using triphosgene, sulfoxide 50, and triethylamine [43]. Using these conditions, oxidation of primary and secondary alcohols could be performed at increased temperature, i.e. between −15 and −10 °C, as compared to the colder temperature required for the traditional Swern reaction. Furthermore, the odorless sulfide byproduct 51 could be easily recovered and recycled. The proposed reaction mechanism bore a resemblance to the Swern oxidation, which commenced with activation of sulfoxide with triphosgene to yield sulfonium ion 52. The ensuing nucleophilic substitution by alcohols, followed by deprotonation of the resulting alkoxysulfonium intermediate 53 generated sulfur ylide 54. The final intramolecular deprotonation then furnished the carbonyl product, alongside the sulfide byproduct.
Scheme 12.
Oxidation of Alcohols.
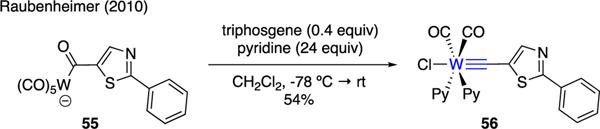
4.5. Metal-carbyne complex
Raubenheimer reported an unusual synthetic application of triphosgene and pyridine in the preparation of metal-carbyne complex 56 from the corresponding carbonyl starting material 55 (Scheme 13) [44]. Although this compound was found to be stable at low temperature, it underwent slow decomposition at room temperature.
Scheme 13.
Synthesis of Metal-Carbyne Complex.
5. Preparation of carbonyl chlorides
A series of carbonyl chloride compounds could be effectively synthesized through the use of triphosgene. Despite their high reactivity, these electrophiles, particularly those discussed below, could be isolated for further use.
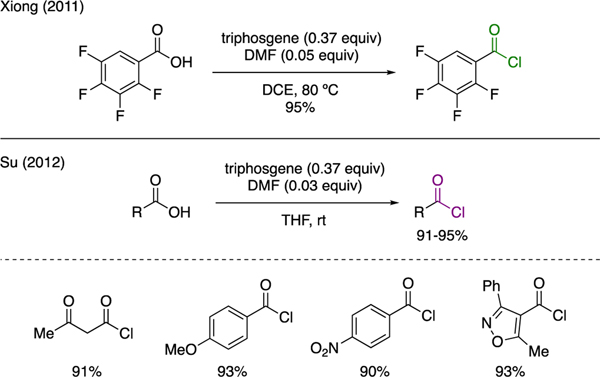
5.1. Acyl chlorides
As independently reported by Xiong and Su (Scheme 14), the Vilsmeier reagent generated upon the mixing of triphosgene and DMF could be exploited for the convenient preparation of acyl chlorides from various carboxylic acids [45,46]. In general, these acyl chlorides were produced in excellent yields.
Scheme 14.
Synthesis of Acid Chlorides.
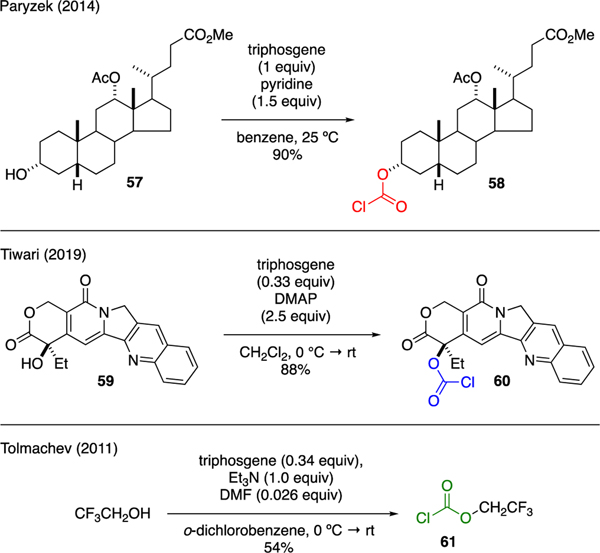
5.2. Chloroformates
Scheme 15 describes the utility of triphosgene in chloroformylation of complex alcohols. For instance, Paryzek reported the use of triphosgene in the synthesis of chloroformate 58 from steroid scaffold 57 [47]. It was found that the best yield and purity of the chloroformate was obtained when the reaction was carried out in benzene at room temperature with slight excess of pyridine. Tiwari also employed triphosgene to convert the C20 hydroxy group of Campothecin 59 to afford chloroformate 60. This transformation ultimately enabled conjugation of this natural product with functionalized cyclic peptides [48]. Tolmachev reported the reaction of 2,2,2-trifluoroethanol with triphosgene in the presence of triethylamine and catalytic DMF to prepare trifluoroethyl-chlorocarbonate 61, a new carbonyl linchpin that was useful for the synthesis of unsymmetrical ureas [49].
Scheme 15.
Synthesis of Chloroformates.
5.3. Carbamoyl chlorides
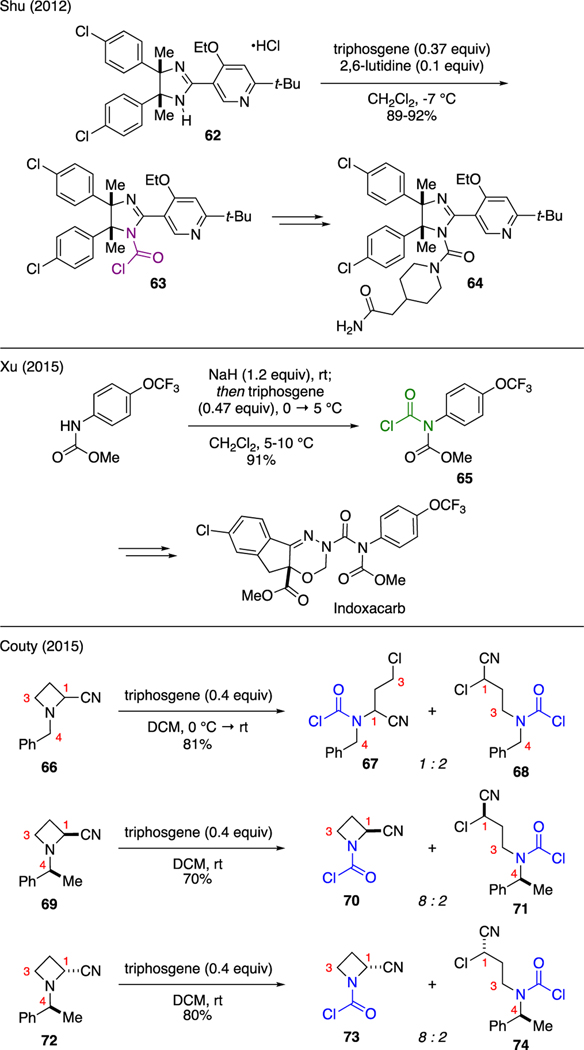
Carbamoyl chlorides could be prepared by subjecting certain nitrogen nucleophiles to triphosgene (Scheme 16). For instance, Shu reported the kilogram-scale synthesis of triarylimidazoline MDM2 antagonist 64 [50]. One of the important steps in this endeavor was realized through the reaction of dihydroimidazole 62 with triphosgene and catalytic 2,6-lutidine to selectively functionalize the dihydroimidazole core to furnish carbamoyl chloride 63 in high yields. Carbamoyl chlorides could be also produced from the reaction of triphosgene with protected aniline derivatives. As demonstrated by Xu [51], deprotonation of methoxycarbonyl protected p-OCF3 aniline with NaH gave rise to an anilide anion that could be captured by triphosgene to generate isolable carbamoyl chloride 65. This compound was then carried forward in several steps to oxadiazine insecticide Indoxacarb.
Scheme 16.
Synthesis of Carbamoyl Chlorides.
Couty noted interesting reactivities when N-alkyl azetidines was treated with triphosgene [52]. Two possible mechanistic pathways were noted: 1) azetidine ring cleavage to give acyclic N-carbamoyl chlorides or 2) N-alkyl scission to give cyclic N-carbamoyl chlorides. The steric size in the N-alkyl moiety on the azetidine ring was crucial in determining the reaction selectivity. For instance, treatment of N-benzyl azetidine 66 with triphosgene in dichloromethane produced a mixture of acyclic carbamoyl chlorides 67 and 68 upon azetidine ring opening at the C3 and C1 positions, respectively. In contrast, exposure of either diastereomers of the N-(1-phenylethyl) analogues 69 and 72 to the reaction conditions predominantly cleaved the N-alkyl group to afford cyclic carbamoyl chlorides 70 and 73 over the respective ring cleavage products 71 and 74 in 8:2 ratios.
6. Activation of carboxylic acids
Carboxylic acids could be readily activated by triphosgene to form reactive intermediates, such acid chlorides, acid anhydrides, or even N-succinimidyl esters. In the examples below, these activated carboxylic acid species could be directly subjected to in situ nucleophilic acyl substitution reactions.
6.1. Acid anhydrides
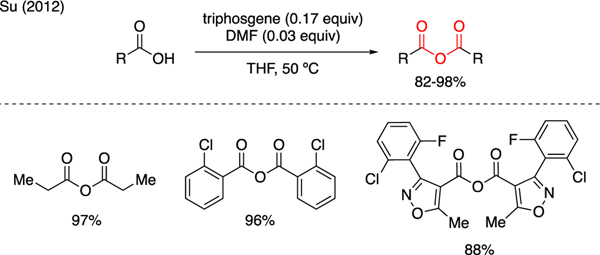
Triphosgene serves as a convenient reagent to prepare acid anhydrides from carboxylic acids. As illustrated in Scheme 17, Su reported the conversion of various carboxylic acids to acid anhydrides upon treatment with triphosgene and catalytic DMF [46].
Scheme 17.
Synthesis of Acid Anhydrides.
6.2. Amides
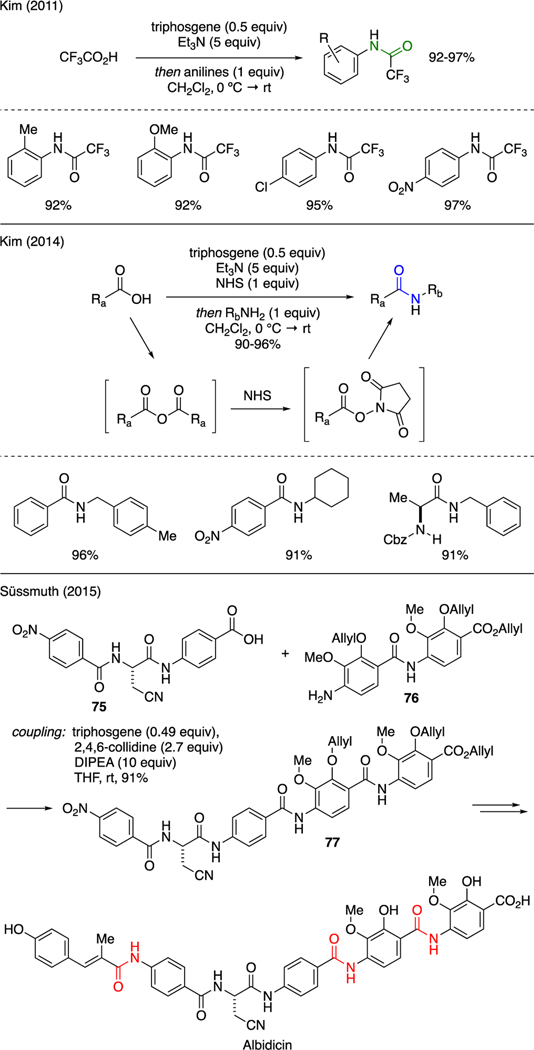
As depicted in Scheme 18, Kim reported the trifluoroacetylation of substituted anilines in the presence of triphosgene, trifluoroacetic acid, and triethylamine [53]. It was proposed that triphosgene enabled the in-situ generation of trifluoroacetic anhydride, which then acylated the arylamines to generate trifluoroacetamides. In a separate account, Kim demonstrated a new approach to synthesize amides and peptides via an intermediacy of N-succinimidyl esters, which were readily generated in situ upon activation of carboxylic acids with triphosgene, triethylamine, and N-hydroxysuccinimide [54]. The mechanism of this reaction was believed to involve the formation of carboxylic acid anhydride, followed by its conversion to the more reactive N-succinimidyl ester, which was then captured by amine to furnish the amide connectivity.
Scheme 18.
Synthesis of Amides.
Süssmuth reported total synthesis of an acyl pentapeptide antibiotic Albicidin, in which three of the aromatic amide bonds were forged using a mixture of triphosgene, 2,4,6-collidine, and DIPEA [55]. For instance, treatment of aromatic carboxylic acid segment 75 and highly functionalized aniline 76 readily produced tetramide framework 77 in 91% yield. This method was developed to circumvent the classical amide-coupling reactions that had failed due to the low reactivity of aromatic amines.
6.3. Pyrrolizinones
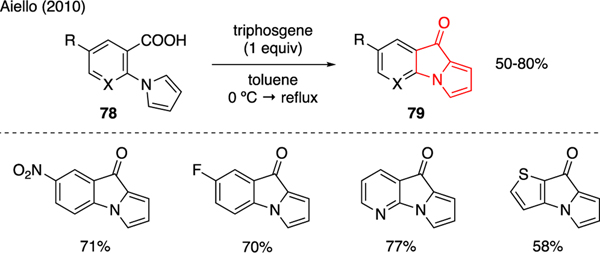
The use of triphosgene to promote intramolecular Friedel-Crafts reaction using arylcarboxylic acids was reported by Aiello (Scheme 19) [56]. In this instance, exposure of aryl carboxylic acids 78 to triphosgene in toluene at reflux furnished pyrrolizinones 79 in good yields. These conditions, interestingly, did not produce any measurable amounts of chlorinated pyrrole byproducts. The efficacy of this reaction was putatively attributed by triphosgene in selectively activating the carboxylic acid moiety, giving rise to electrophilic carbonyl that was directly captured by the pendant pyrrole ring.
Scheme 19.
Synthesis of Pyrrolizinones.
7. Addition of carbonyl groups
Triphosgene is a unique electrophile. It is a source of a carbonyl group that can link two heteroatoms through double nucleophilic acyl substitution. This carbonyl connectivity could be forged intramolecularly, therefore providing opportunities for the assembly of structurally diverse carbonyl heterocycles. As discussed below, other noteworthy advancements in the past decade included the strategic application of triphosgene to deduce relative stereochemistry of functional groups, such as 1,2-epoxyalcohols and 1,2-aminoalcohols, through their derivatization to the respective cyclic motifs.
7.1. Carbonates and thiocarbonates
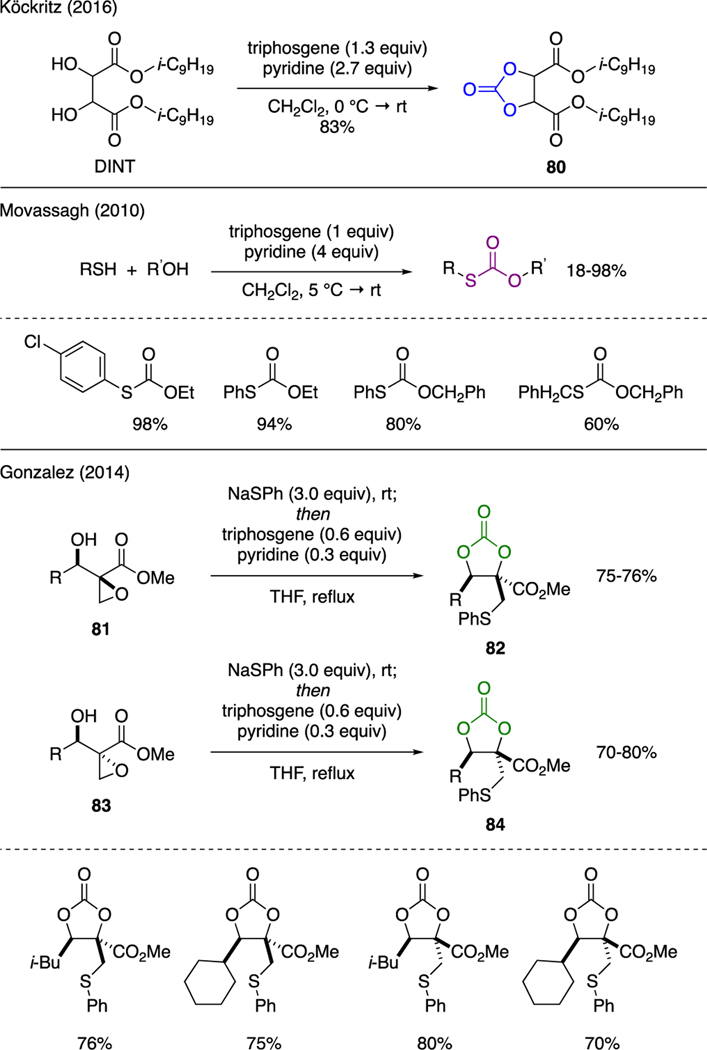
Several examples on the preparation of carbonates and thiocarbonates using triphosgene were reported (Scheme 20). For example, Köckritz synthesized cyclic carbonate 80 from diisononyl tartarate (DINT) using triphosgene and pyridine [57]. The mechanical properties of this compound, such as hardness, gelation and volatility, as a phthalate-free plastisizer were found to be comparable to those of commercial plasticizers. Movassagh reported a multicomponent reaction between thiols and alcohols or phenols with triphosgene in presence of pyridine to afford various thiocarbonates in good yields [58]. A wide scope of substrates were demonstrated.
Scheme 20.
Synthesis of Carbonates and Thiocarbonates.
Synthesis of cyclic carbonates could be used as an effective strategy to deduce relative stereochemistry in molecules. As exemplified by Gonzales [59], the conversion of diastereomeric 3-hydroxy-2-epoxy esters 81 and 83 to their corresponding cyclic carbonates 82 and 84 using a mixture of NaSPh, triphosgene, and pyridine enabled stereochemical assignment of the starting materials. This was achieved upon determination of the relative stereochemistry of the products by NOE. The mechanism of these reactions was proposed through an opening of the oxirane ring at the terminal carbon by NaSPh. The emerging diol intermediate was then captured in situ by triphosgene, leading to intramolecular carbonate cyclization.
7.2. Carbamates and related structures
7.2.1. Carbamates
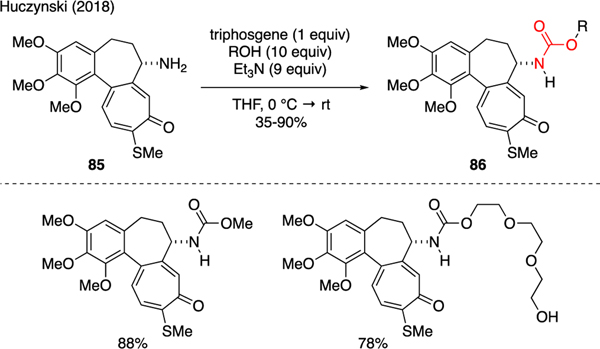
Triphosgene reacts with alcohols and amines to produce carbamates (Scheme 21). Huczynski prepared a series of 7-deacetyl-10-thiocolchichine carbamates 86 for the evaluation of anti-proliferative activity against human cancer cell lines [60]. These compounds were synthesized from colchichine-derived substrates 85, of which the amino group formed a carbamate with various primary and secondary alcohols in presence of triphosgene and triethylamine. It was found that these acyclic carbamates could be more potent than the parent colchichine in their antiproliferative properties as well as in tubulin depolymerization.
Scheme 21.
Synthesis of Carbamates.
7.2.2. Oxazolidinones
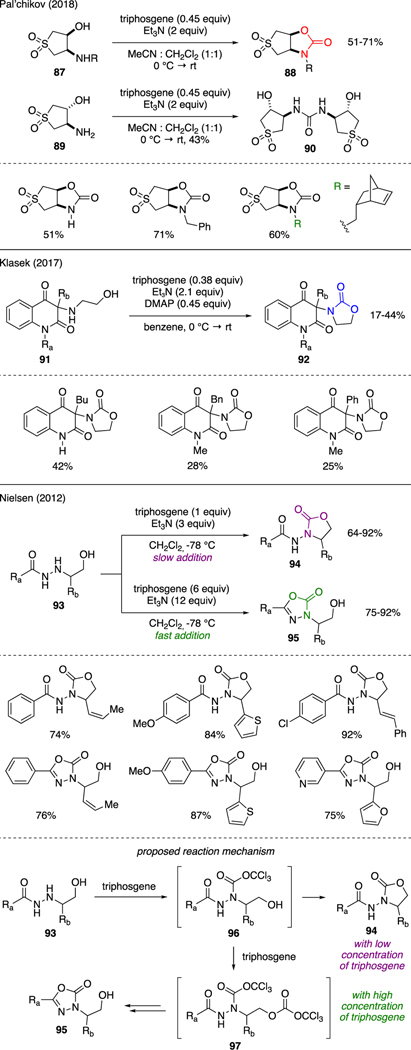
As depicted in Scheme 22, several new accounts on the synthesis of oxazolidinone via intramolecular carbamate cyclization of 1,2-aminoalcohols using triphosgene has been documented. For instance, Pal’chikov reported the effects of cis- and trans-relative stereochemistry in 1,2-aminoalcohols toward cyclization using diastereomeric sulfolanes 87 and 89 [61]. While reaction of the cis-isomer 87 with triphosgene and triethylamine afforded oxazolidinones 88, subjection of trans-isomer 89 to the same reaction conditions generated symmetrical urea 90. Another example of this chemistry can be found in the work by Klasek [62], in which 1,2-aminoalcohols 91 were converted to oxazolidinones 92 using a mixture of triphosgene, DMAP, and triethylamine.
Scheme 22.
Synthesis of Oxazolidinones from 1,2-aminoalcohols.
Another interesting reactivity to synthesize oxazolidinone using triphosgene was reported by Nielsen [63]. It was found that exposure of hydrazidoalcohols 93 to a slow addition of triphosgene in presence of triethylamine at −78 °C selectively produced oxazolidinones 94 in good yields. In contrast, a fast addition of triphosgene in an excess quantity led to competitive cyclization to oxadiazolones 95. The mechanism of these reactions was believed to have proceeded through common intermediate 96. The ensuing cyclization event to either oxazolidinone 93 or oxadiazolone 94 (via intermediate 97) was governed by reactivity of the hydroxy group, which could be altered by the effective concentration of triphosgene in the reaction mixture. Interestingly, itwas reported that oxadiazolones 95 could be easily converted to oxazolidinones 94 in near quantitative yields simply via treatment with aqueous NaOH solutions.
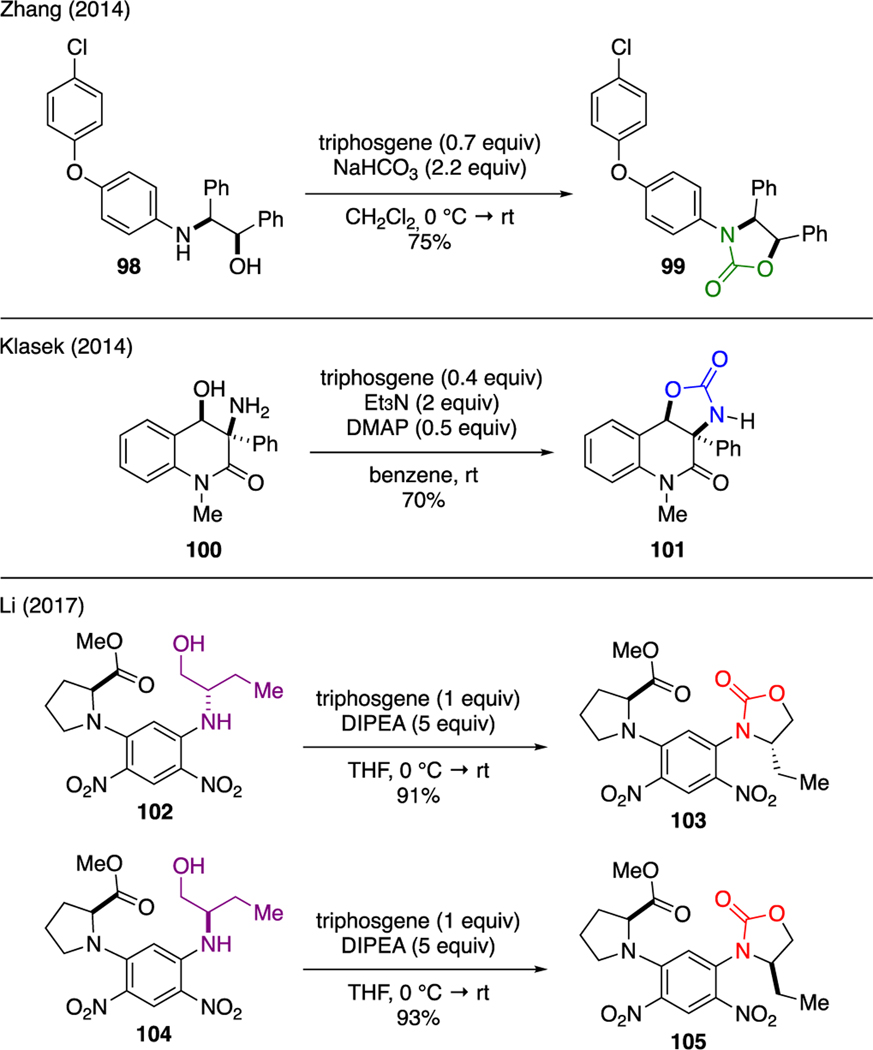
Analogous to carbonate cyclization described in Section 7.1, triphosgene could be also effectively utilized to determine the relative stereochemistry of 1,2-aminoalcohols upon their conversion to oxazolidinone (Scheme 23. For instance, Zhang derivatized 1,2-aminoalcohol 98 to oxazolidinone 99 using a mixture of triphosgene and sodium bicarbonate to deduce the relative stereochemistry of the 1,2-aminoalcohol motif [64]. A similar strategy was also employed by Klasek in the attempt to elucidate the relative stereochemistry of 1,2-aminoalcohol 100 by subjecting this compound to triphosgene, DMAP, and triethylamine, which furnished oxazolidinone 101 [65]. Another examplewas reported by Li [66]. In this study, the diastereomeric pairs of Marfey’s coupling adducts 102 and 104 were subjected to cyclization with triphosgene and DIPEA. The resulting oxazolidinones 103 and 105 were then analyzed using various analytical techniques to determine the absolute configuration of the parent 2-amino-1-butanol moiety.
Scheme 23.
Synthesis of Oxazolidinones to Deduce Stereochemistry.
7.2.3. Benzoxazolones
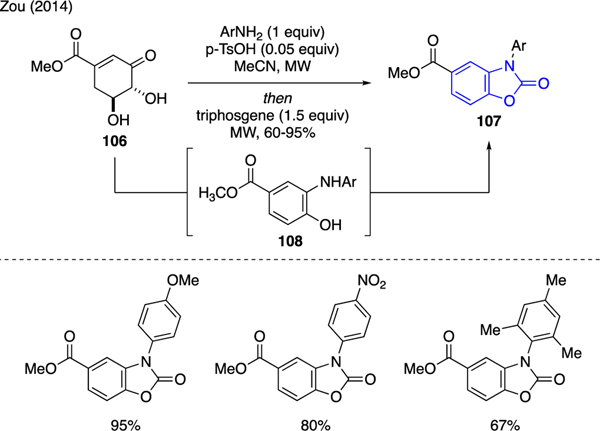
As shown in Scheme 24, Zou reported a microwave-assisted one-pot protocol for the synthesis of benzoxazolone derivatives 107 from the reaction of renewable biomass-derived methyl 3-dehydroshikimate 106 with anilines and catalytic tosic acid, followed by addition of triphosgene [67]. The mechanism of this reaction was proposed via acid-catalyzed condensation to form N-aryl-2-aminophenol 108, followed by in situ carbamate annulation with triphosgene to yield the benzoxazolone motif.
Scheme 24.
Synthesis of Benzoxazolones.
7.2.4. Benzoxazinones
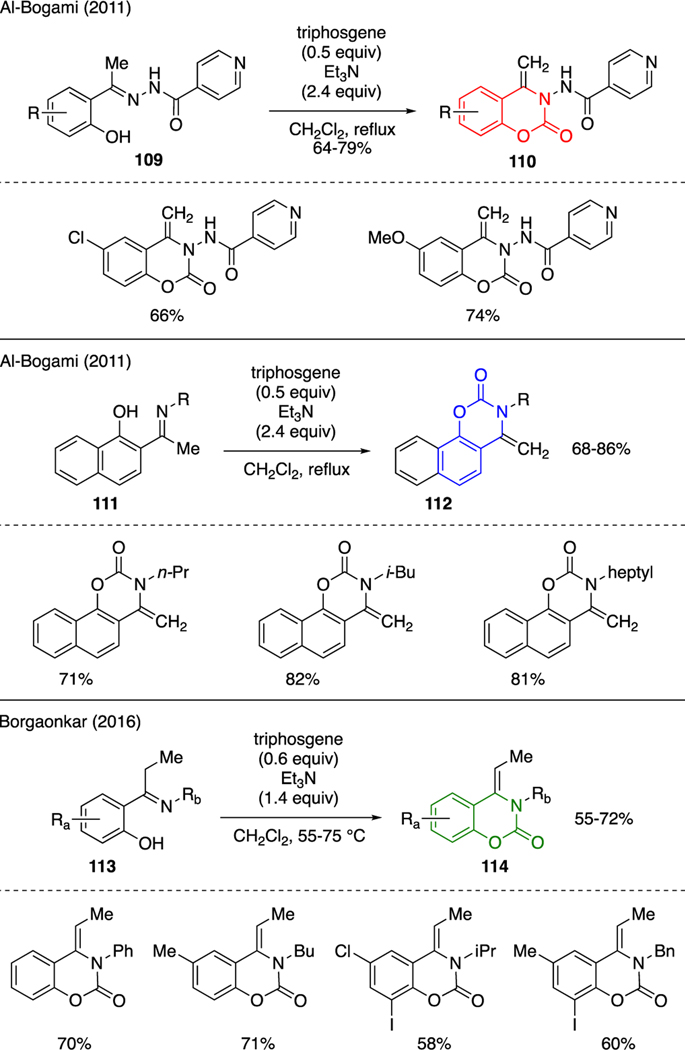
Benzoxazinones represent yet another class of N,O-heterocyclic compounds that could be prepared using triphosgene. Scheme 25 depicts several new reports, in which phenolic-derived ketimines were generally employed as substrates. For instance, Al-Bogami showed that carbamate-type cyclization of ketimines 109 could be performed with triphosgene and triethylamine to produce benzoxazinones 110 in good yields [68]. In a separate account, AlBogami showcased that naphthol-derived ketimines 111 could be subjected to the same conditions to generate products 112 [69]. Borgaonkar disclosed similar transformation of ketimines 113 to benzoxazinones 114 using a mixture of triphosgene and triethylamine [70].
Scheme 25.
Synthesis of Benzoxazinones from Phenol-Derived Ketimines.
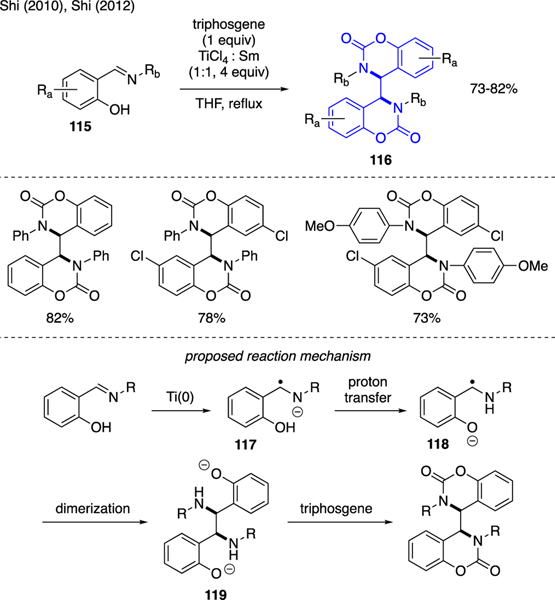
Scheme 26 depicts an example by which triphosgene was utilized in the synthesis of benzoxazinone dimer upon reductive cyclization of phenol-derived benzimine. More specifically, Shi demonstrated that treatment of benzimines 115 with a mixture of TiCl4, Sm powder, and triphosgene furnished benzoxazinone dimers 116 in good yields [71,72]. The proposed reaction mechanism commenced with the reduction of TiCl4 with Sm(0). The resulting low valent titanium species then delivered single electron to the benzimine moiety to yield radical anion 117. Following proton transfer, the new radical anion 118 then proceeded to self coupling, thereby creating the C–C bond in bisphenoxide intermediate 119. The ensuing in situ carbamate cyclization then installed the benzoxazinone core.
Scheme 26.
Synthesis of Benzoxazinone Dimers.
7.2.5. Oxadiazolones
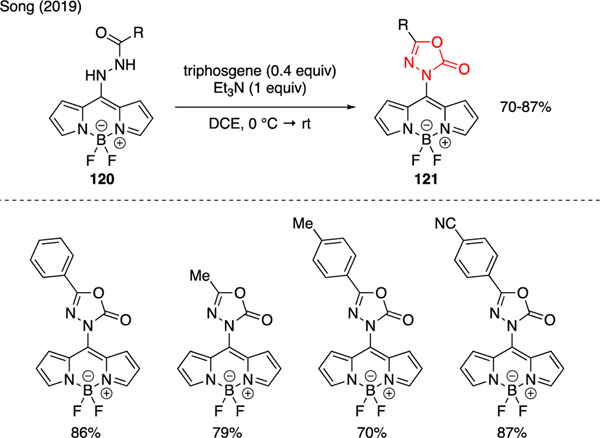
Song prepared various oxadiazolones 121 in high yields by reacting BODIPY hydrazides 120 with triphosgene and triethylamine in their studies toward developing BODIPY-based sensors to detect phosgene gas (Scheme 27) [73]. The photophysical properties of oxadiazolones 121 as well as other sensing parameters were evaluated using various colorimetric and fluorogenic methods.
Scheme 27.
Synthesis of Oxadiazolones.
7.3. Isatoic Anhydrides
Scheme 28 demonstrates a synthetic utility of triphosgene toward the construction of isatoic anyhydrides, which were generally approached via treatment of substituted anthranilic acids with triphosgene. Isatoic anhydrides are valuable synthetic building blocks, especially for the preparation of quinoline-derived compounds. Three examples of this annulation chemistry were independently conveyed by Parameshwar [74], Chen [75], and Donahue [76].
Scheme 28.
Synthesis of Isatoic Anhydrides.
7.4. Ureas and related compounds
7.4.1. Ureas
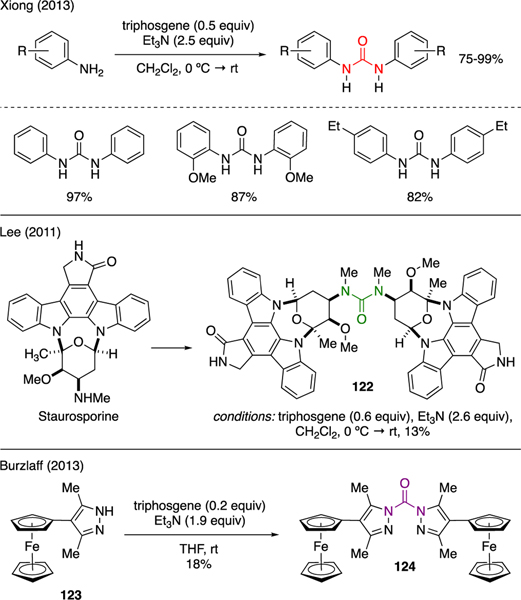
Urea and related compounds in both acyclic and cyclic forms could be prepared by combining two nitrogen nucleophiles in the presence of triphosgene. As shown in Scheme 29, Xiong developed a convenient protocol for the synthesis of symmetrical diaryl ureas by combining substituted anilines with triphosgene and triethyamine [77]. Lee reported the synthesis of a potential kinase inhibitor derived from Staurosporine [78]. In this work, high complex Staurosporine was linked into symmetrical urea 122 upon treatment with triphosgene and triethylamine in anhydrous dichloromethane albeit in 13% yield. Burzlaff also demonstrated the utility of triphosgene to synthesize symmetrical dipyrazole urea 124 from 4-ferrocenyl-3,5-dimethylpyrazole 123 [79]. This urea served as N,N-ligand that exhibited a reversible redox capability.
Scheme 29.
Synthesis of Symmetrical Ureas.
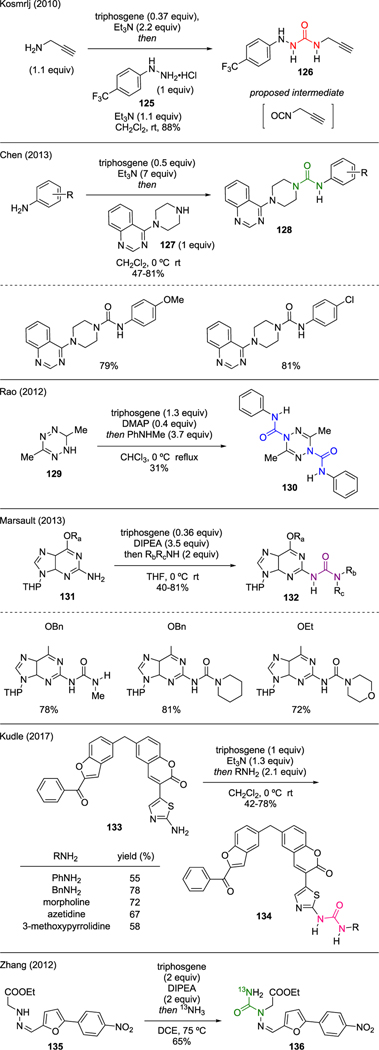
Triphosgene could be employed in a one-pot procedure for the synthesis of unsymmetrical ureas upon its reaction with sequentially added two different nitrogen nucleophiles. Multiple examples are depicted in Scheme 30. Kosmrlj reported the synthesis of N-(propargyl)diazenecarboxamide 126 by exposing propargyl amine to triphosgene and triethylamine, followed by a slow addition of aryl hydrazine 125 to capture the forming isocyanate intermediate [80]. Chen reported a similar approach, in which unsymmetrical 4-quinazolinyl piperazine aryl ureas 128 were generated from the reaction of various arylamines and triphosgene, followed by addition of substituted piperazine 127 [81].
Scheme 30.
Synthesis of Unsymmetrical Ureas.
Other complex nitrogen nucleophiles were also amenable in this chemistry. For instance, Rao conveyed that tetrazine-1,4-dicarboxamide 130 could be prepared by reacting 3,6-dimethyl-1,6-dihydro-1,2,4,5-tetrazine 129 with triphosgene in presence of catalytic DMAP, followed by addition of N-methylaniline [82]. Interestingly, it was observed from X-ray structure that the central tetrazine ring of product 130 exists in a boat conformation. Marsault also reported the usage of triphosgene in the synthesis of 2-ureaguanines 132, which was prepared from 2-isocyanatopurine 131 and a diverse range of amines [83]. Another example can be found in the work of Kudle, who reported the synthesis of coumarin-derived unsymmetrical ureas 134, a class of promising antimicrobial compounds against several Gram-positive and Gramnegative bacteria [84]. These ureas were synthesized by subjecting 2-aminothiozole 133 to a mixture of triphosgene and triethylamine, followed by a sequential addition of various primary amines. Lastly, Zhang demonstrated the utility of triphosgene to prepare 13N radio-labeled dantrolene 136, which was utilized as a no-carrier-added [13N]ammonia labeling agent. Triphosgene was critical for the in situ preparation of carbamoyl chloride intermediate from hydrazone 135. The ensuing treatment with 13NH3 then furnished the radio-labeled agent without purification [85].
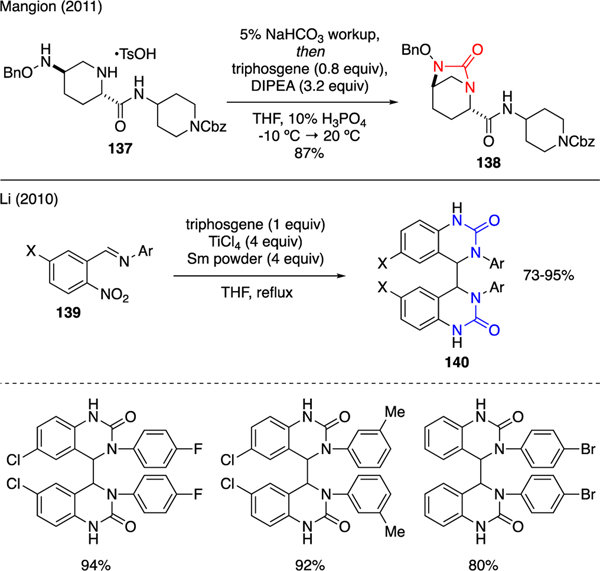
Cylic ureas could be prepared via intramolecular cyclization of two tethered nitrogen nucleophiles with triphosgene. Two methods are presented in Scheme 31. One of which was accounted by Mangion [86], who described a successful preparation of strained bicyclo-[3.2.1]urea 138 upon treatment of aminopiperizine 137 with triphosgene and DIPEA, interestingly under acidic reaction media. This cyclic urea served as a synthetic intermediate for compounds that showed activities as β-lactamase inhibitors. Another example was published by Li [87]. In this work, a facile synthesis of 2-quinazolinone dimer 140 was achieved by reductive cyclization of 2-nitrobenzylidene aniline 139 in the presence of triphosgene, TiCl4, and Sm dust. The reaction mechanism was proposed through similar single electron transfer sequences as depicted in Scheme 26 [72]. Notably, a number of compounds 140 exhibited remarkable anticancer activities.
Scheme 31.
Synthesis of Cyclic Ureas.
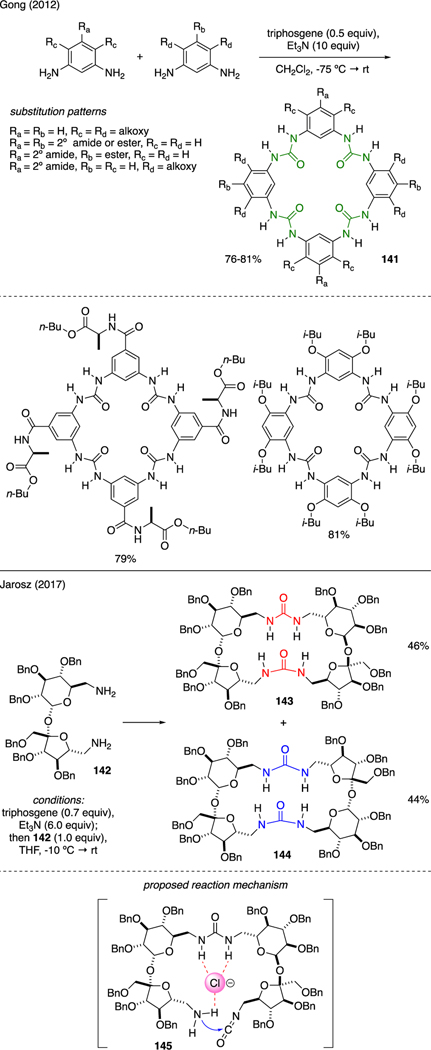
As shown in Scheme 32, Gong demonstrated that aromatic tetraurea macrocycles 141 could be readily prepared from the reaction between various substituted aromatic diamines with triphosgene and triethylamine [88]. This one-pot protocol allowed for the preparation of shape-persistent aromatic tetraurea macrocycles that shared the same flat backbone and non-collapsible internal cavity, but varying side chains led to differing electronic properties. Jarosz reported formation of macrocyclic ureas from sucrose derivative 142 upon exposure to triphosgene and triethylamine under dilute conditions [89]. The method afforded an equimolar mixture of two chromatographically separable, regioisomeric urea macrocycles 143 and 144. A plausible reason to account for the preference of macrocyclization over oligomerization was proposed to have originated from a chloride ion-templated stabilization of the linear intermediate 145, leading to intramolecular cyclization.
Scheme 32.
Synthesis of Macrocyclic Ureas.
7.4.2. Uracils
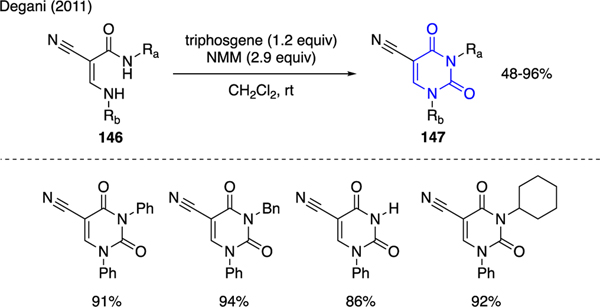
Triphosgene has been used as an effective cyclocarbonyl addition reagent for the synthesis of uracils (Scheme 33). For instance, Degani demonstrated the generation of 1,3-disubstituted-5-cyano uracils 147 via treatment of various 2-cyano-3-acrylamides 146 with triphosgene and N-methyl morpholine [90].
Scheme 33.
Synthesis of Uracils.
7.4.3. Quinazolidinones
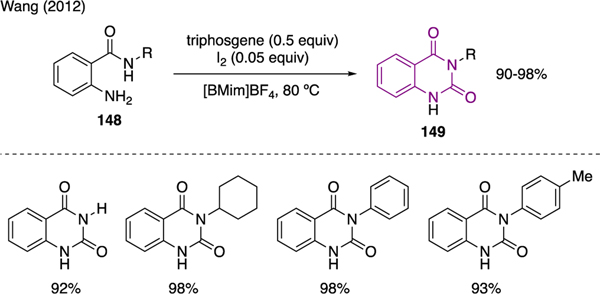
Quinazolidinone is another class of N-hetereocycles that could be successfully prepared using triphosgene. As depicted in Scheme 34, Wang developed a new method for the synthesis of quinazolinones 149 from 2-aminobenzamides 148 using a mixture of triphosgene and molecular iodine. The reaction was performed in ionic liquid [BMim]BF4 [91].
Scheme 34.
Synthesis of Quinazolidinones.
7.4.4. Imidazolidinones
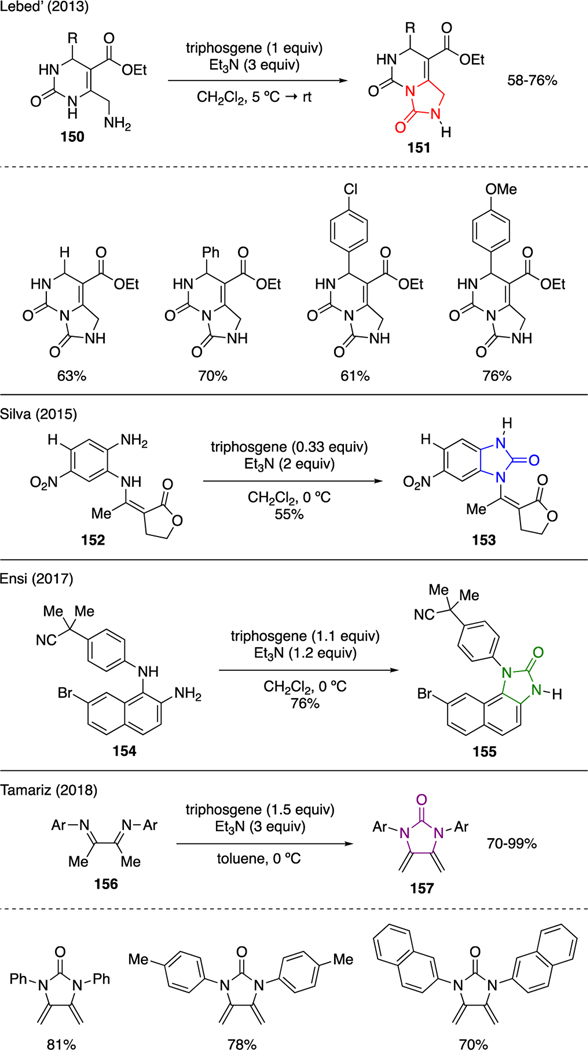
Scheme 35 conveys several examples by which triphosgene was utilized to afford imidazolidinone cores. Lebed’ prepared a series of tetrahydroimidazo[1,5-c] pyrimidinediones 151 upon annulation of 6-aminomethylpyrimidines 150 with triphosgene and triethylamine [92]. In a similar approach, Silva and Ensi independently reported the synthesis of benzimidazolone derivatives 153 and 155 starting from functionalized aromatic-1,2-diamines 152 and 154, respectively, which were subjected to a mixture of triphosgene and triethylamine [93,94]. A recent report by Tamariz showed the utility of triphosgene and triethylamine in the carbonylation of symmetrical α-bis-imines 156 to furnish exo-2-imidazolidinone dienes 157 in excellent yields [95].
Scheme 35.
Synthesis of Imidazolidinones.
7.4.5. Imidazopyridines
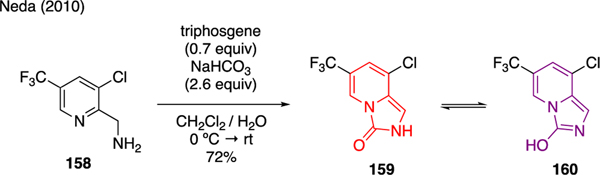
Neda described the synthesis of imidazopyridine 159 by which 3-chloro-5-(trifluoromethyl)pyridine-2-yl)methanamine 158 was subjected to triphosgene and sodium bicarbonate (Scheme 36). The product of this reaction exhibited an interesting keto-enol behavior. While the keto form existed in the solid state as confirmed by single X-ray crystal analysis, NMR studies indicated the predominance of the enol form 160 in solution [96].
Scheme 36.
Synthesis of Imidazopyridines.
7.4.6. Triazolidine-3,5-diones
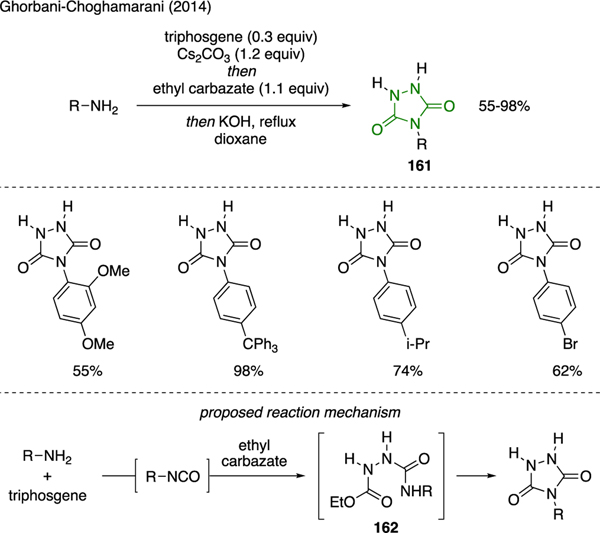
Ghorbani-Choghamarani described a one-pot synthesis of 4-substituted-1,2,4- triazolidin-3,5-diones 161 (Scheme 37) [97]. This multicomponent reaction was carried out by combining primary amines, triphosgene, and Cs2CO3, followed by addition of ethyl carbazate in a single synthetic operation. The proposed mechanism for this reaction began with the generation of an isocyanate intermediate upon nucleophilic addition of primary amine to triphosgene. The subsequent addition of ethyl carbazate to the isocyanate, followed by intramolecular cyclization of emerging urea adduct 162 afforded the triazolidine-3,5-dione core.
Scheme 37.
Synthesis of Triazolidine-3,5-Diones.
7.5. Selenodiazolones
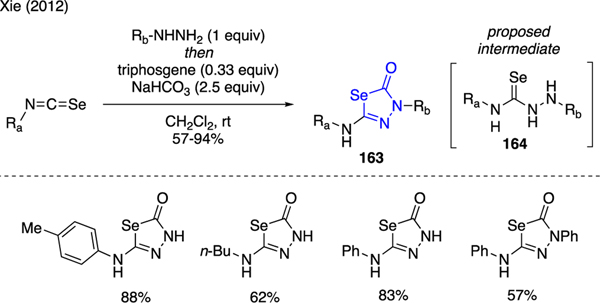
Another example of miscellaneous N-heterocycles that was prepared using triphosgene can be found in the work by Xie, who developed a one-pot protocol to produce selenadiazolones 163 [98]. As shown in Scheme 38, this unique heterocyclic motif was assembled by combining hydrazines and isoselenocyanates, followed by addition of triphosgene and NaHCO3. The mechanism of this reaction was proposed to involve carbonyl cyclization of selenourea intermediate 164 in the presence of triphosgene.
Scheme 38.
Synthesis of Selenodiazolones.
8. Electrophilic heterocyclization reactions
While triphosgene readily serves as an electrophilic carbonyl donor for various heterocyclization reactions, this versatile reagent can be employed to generate useful electrophilic promoters, such as the Vilsmeier reagent from DMF and chlorotriphenylphosphonium ion from triphenylphosphine oxide. As shown below, these reactive species can be strategically exploited for the preparation of various N-heterocyclic motifs.
8.1. Pyrimidinones
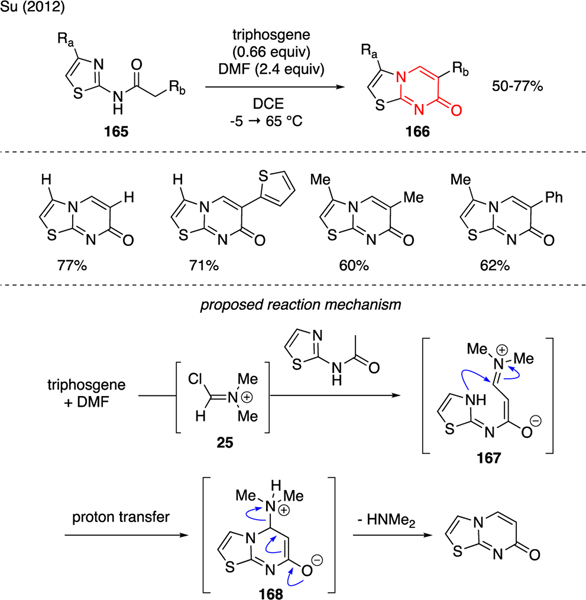
Su reported the synthesis of thiazolo[3,2-a]pyrimidin-7-ones 166 from 2-aminothiazoles 165 using a mixture of triphosgene and DMF (Scheme 39) [99]. The proposed mechanism involved the generation of electrophilic Vilsmeier reagent 25 from triphosgene and DMF, enabling formylation of the starting material at the α-carbon and proton transfer to generate fully conjugated enolate 167. The subsequent intramolecular cyclization, followed by proton transfer led to intermediate 168, which furnished the pyrimidinone core after dimethylamine extrusion.
Scheme 39.
Synthesis of Pyrimidinones.
8.2. Pyrazoles
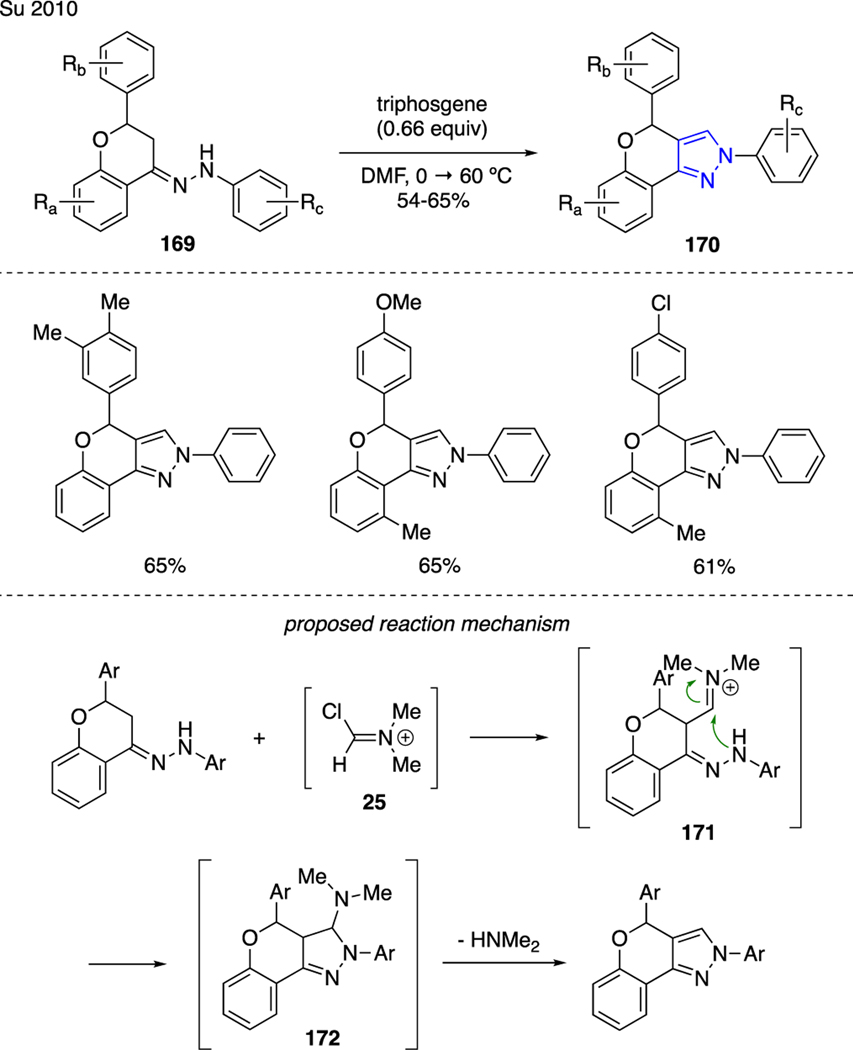
As an expansion to this methodology, Su developed the synthesis of dihydrochromino[4,3,c]pyrazoles 170 from flavanone-4-arylhydrazones 169 using triphosgene and DMF (Scheme 40) [100]. The mechanism of this reaction was similarly proposed via α-formylation of the ketimine moiety with the in situ generated Vilsmeier reagent 25. This carbon-carbon bond forming process was then followed by intramolecular N-heterocyclization of adduct 171 and elimination of dimethylamine in intermediate 172, therefore creating the pyrazole ring.
Scheme 40.
Synthesis of Pyrazoles.
8.3. Oxazoles
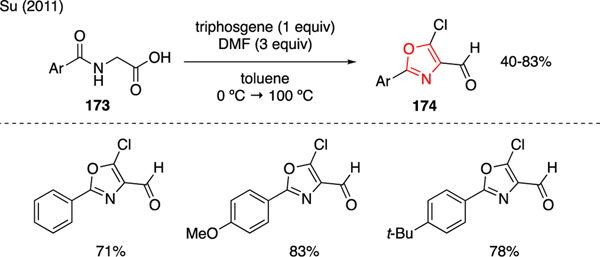
Oxazole is another example of N,O-heterocyclic compounds that could be prepared using the Vilsmeier reagent derived from triphosgene and DMF. As depicted in Scheme 41, Su reported the synthesis of chloroaryloxazole 174 from N-arylglycines 173 simply by exposing the starting material to a mixture of triphosgene and DMF [101].
Scheme 41.
Synthesis of Oxazoles.
8.4. 2,4-Dichloroquinazolines
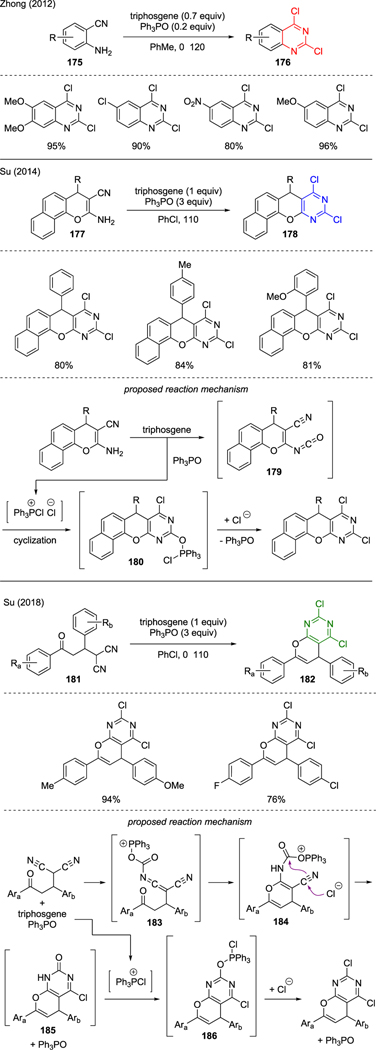
Scheme 42 describes precedence on the synthesis of 2,4-dichloroquinazoline motifs using triphosgene. Zhong showcased the successful synthesis of 2,4-dichloroquinazolines 176 via cyclization of anthranilonitriles 175 in the presence of triphosgene and catalytic triphenylphosphine oxide [102]. Su expanded this work by subjecting chromenocarbonitriles 177 to triphosgene and triphenylphosphine oxide, which furnished benzochromeno-[2,3-d]pyrimidines 178 in good yields [103]. The mechanism of this cyclization reaction was believed to proceed via in situ formation of isocyanate 179. O-activation of the isocyanate moiety by chlorotriphenylphosphonium ion induced intramolecular cyclization by the pendant cyano group, followed by addition of chloride ions at the C4 position to generate intermediate 180. Finally, nucleophilic substitution by chloride ions at the C2 position formed the 2,4-dichloroquinazoline core.
Scheme 42.
Synthesis of 2,4-Dichloroquinazolines.
Su further exploited this methodology toward the construction of 2,4-dichloro-substituted pyrano[2,3-d]pyrimidines 182 from γ-ketomalononitriles 181 using a mixture of triphosgene and triphenylphosphine oxide [104]. In this reaction, the mechanism was proposed through cascade cyclization events, starting with an intramolecular capture of the emerging acylcarbodiimide 183 by the pendant keto moiety. The ensuing addition of chloride ions to intermediate 184 consequently initiated the second intramolecular cyclization, thus creating the fused ring structure in 185. Finally, deoxychlorination of the remaining carbonyl group by in situ generated chlorotriphenylphosphonium ion furnished 2,4-dichloroquinazoline via intermediate 186.
9. Application to polymer synthesis
In recent years, the synthetic utility of triphosgene in polymerization reactions, in which it serves as a monomeric linchpin, has been exemplified. As depicted in Scheme 43, Jeon reported interfacial polymerization of bisphenol A and eugenol in the presence of triphosgene, triethylamine, and a phase-transfer catayst tetrabutylammonium chloride to produce eugenol polycarbonate polymer 187 [105]. Kim demonstrated a complementary reaction by employing 4,4′-bis(4-hydroxyphenyl)-valeric acid (BHPV) and bisphenol A [106]. Using a mixture of triphosgene, triethylamine, and tetrabutylammonium chloride, polymerization of these two monomeric species afforded polycarbonate copolymer 188.
Scheme 43.
Synthesis of Polycarbonates.
10. Flow chemistry
Adaption of triphosgene-promoted reactions in flow synthesis has been documented in the past decade. This advancement has garnered attention due to several key advantages. For instance, reactive intermediates generated upon substrate activation by triphosgene can be captured to furnish the target products in a single continuous operation. The smaller reaction volume and residence time that accompany flow synthesis can potentially minimize risk of unwanted side reactions, such as epimerization of labile stereocenters or even the inadvertent evolution of toxic phosgene gas from decomposition of triphosgene. As discussed below, flow synthesis is also amenable to scale-up processes.
10.1. Chloroformates
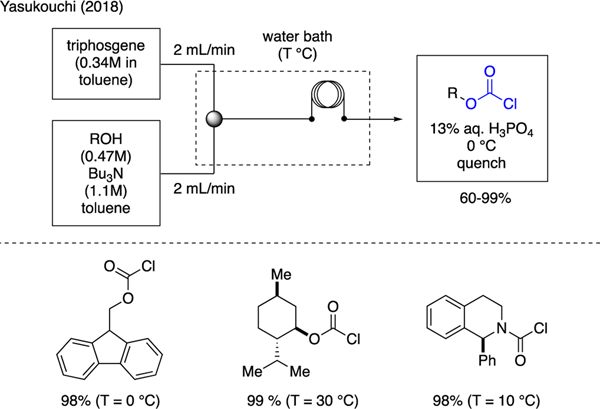
Yasukouchi presented an efficient phosgenation of alcohols to produce chloroformates in a continuous flow reactor (Scheme 44) [16]. In this example, a solution of triphosgene in one syringe and a solution of alcohol and tributylamine in another syringe were each injected at a flow rate of 2 mL per minute to a T-shaped mixer, and the reaction mixture was moved through a residence line in a water bath. This method allowed for the preparation of various chloroformates in high yields.
Scheme 45.
Flow Synthesis of Amides.
10.2. Amides
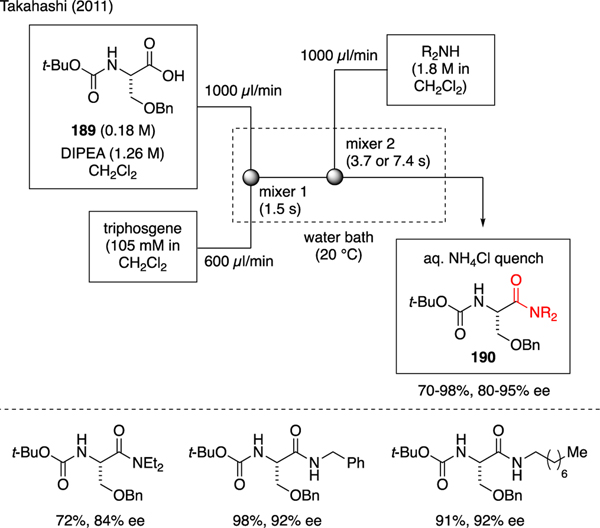
To the best of our knowledge, Takahashi conveyed one of the earliest accounts on the use of triphosgene in flow chemistry toward the preparation of chiral amides 190 [107]. As illustrated in Scheme 45, a solution of N-Boc-O-Bn-L-serine 189 and DIPEA was combined with a solution of triphosgene through a T-shaped mixer. The formed acid chloride was then allowed to react with a solution of amines through a second T-shaped mixer, therefore producing the desired amide products. In comparison to batch syntheses, this flow technology afforded better yields while minimizing epimerization of the α-carbon.
10.3. Peptides
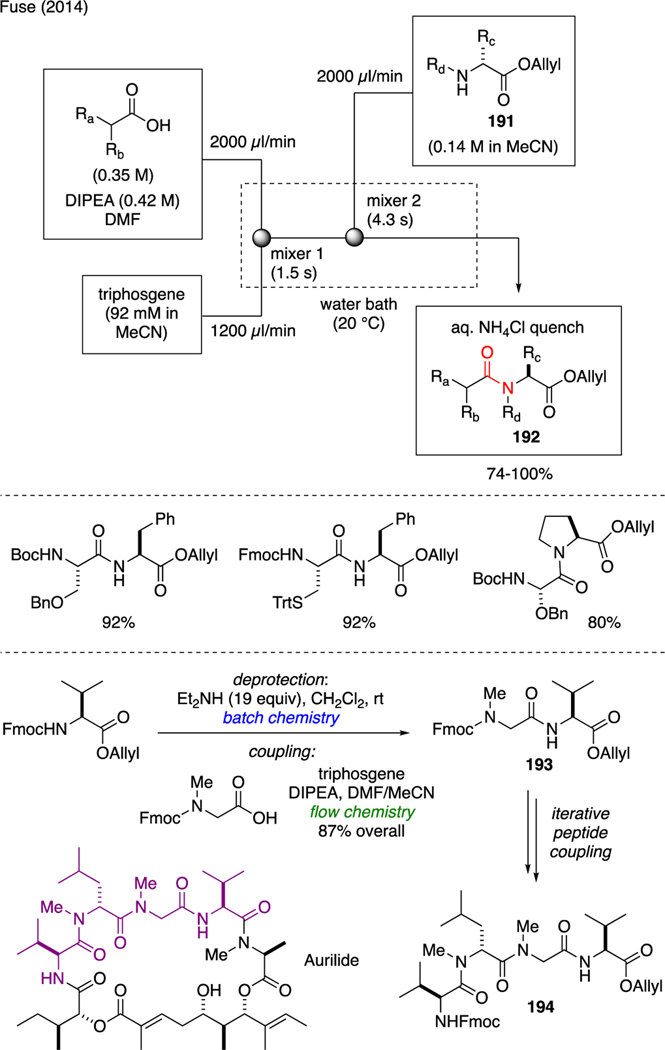
The use of flow chemistry to assemble peptides has been reported by Fuse (Scheme 46) [108]. In this endeavor, the peptide bond was forged upon the mixing of a solution containing N-protected amino acids and DIPEA with a solution of triphosgene using a T-shaped continuous flow reactor. The residence time in the generation of acid chloride was minimized to avoid epimerization. The ensuing addition of O-allyl amino acid esters 191 using a second T-shaped mixer then furnished dipeptides 192. Applicability of this technique in multistep synthesis was showcased in the preparation of tetrapeptide 194, a moiety of the natural product Aurilide.
Scheme 46.
Flow Synthesis of Peptides.
As depicted in Scheme 47, Fuse also demonstrated an interesting example on the use of flow chemistry to synthesize cyclic arginylglycylaspartic acid (RGD) pentapeptide, in which the acyclic precursor 198 was assembled in a microflow reactor [109]. This endeavor began with the mixing of triphosgene with a solution of Nα-Boc-Nω-nitro-L-arginine 195 and DIPEA in a T-shaped mixer. The ensuing addition of a photoactivable linker in glycine 196 using a second T-shaped mixer then produced dipeptide 197. Upon N-Boc deprotection, the resulting amine was then subjected to iterative amidation sequence with the appropriately protected L-lysine, D-phenylalanine, and L-aspartic acid residues to ultimately afford acyclic pentapeptide 198 in 7% yield over 8 steps.
Scheme 47.
Flow Synthesis of Cyclic RGD Pentapeptides.
10.4. Ureas
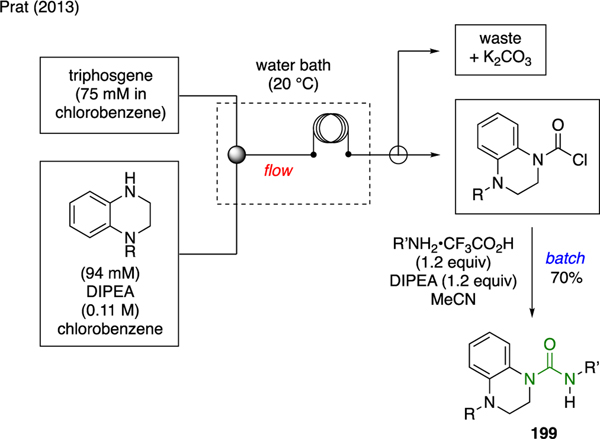
Prat developed scalable flow reactions to produce unsymmetrical ureas 199 in scales ranging from mg/h to kg/h (Scheme 48) [19]. The kilogram-size flow reactor was set up as follows. A solution of 1,2,3,4-tetrahydroquinoxaline and DIPEA and a solution of triphosgene was delivered using two separate pumps and combined in a 20-m reactor coil that was submerged in a water bath. Using a three-way valve, the reactor output, which contained the carbamoyl chloride intermediate, was then redirected to a batch solution of alkyl ammonium salt and DIPEA to furnish urea 199. In a separate line, the waste byproducts were captured by a solution of K2CO3.
Scheme 48.
Flow Synthesis of Ureas.
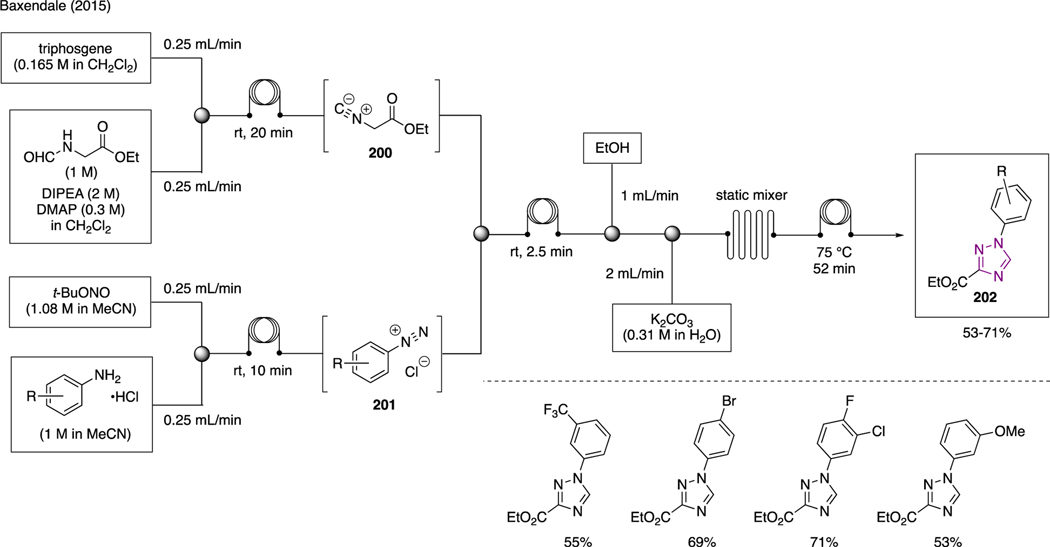
10.5. Triazoles
As illustrated in Scheme 49, Baxendale showcased an elegant multicomponent flow synthesis for the preparation of triazole 202 [110]. In this example, in situ preparation of isocyanate 200 was accomplished by combining a solution of ethylformyl glycinate, DIPEA, and DMAP with a solution of triphosgene in a reaction coil. Simultaneously in a separate reaction coil, aryldiazonium chloride salt 201 was generated upon mixing a solution of substituted aniline hydrochloride with a solution of tert-butyl nitrite. The two solutions of isocyanate and aryldiazonium chloride salt were then combined, at which ethanol and a solution of K2CO3 were sequentially introduced. The overall reaction mixtures were then allowed to pass through a static mixer and reaction coil at 75 °C to generate triazoles 202.
Scheme 49.
Flow Synthesis of Triazoles.
11. Solid phase synthesis
The compatibility of triphosgene in the solid phase synthesis methodology has been displayed in the preparation of oligoamides and oligopeptides. In general, triphosgene was used as a reagent to activate the carboxylic acid residues, thereby enabling amide bond coupling with the resin-bound amines. As discussed below, the successful utility of this emerging technology in the past decade has been realized in several elegant total syntheses of complex natural products.
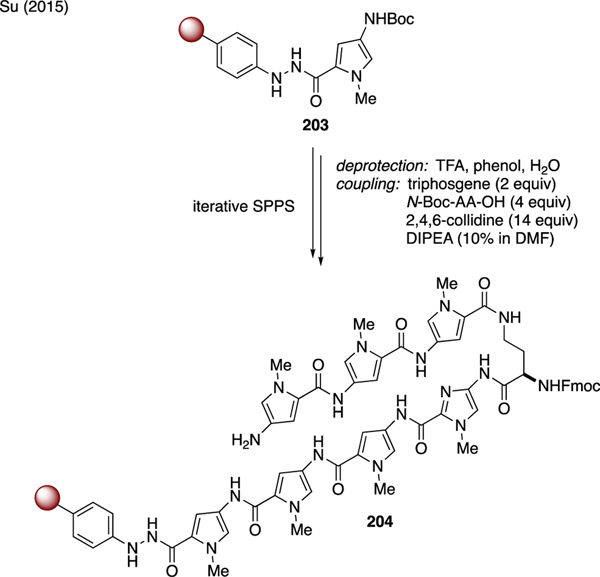
11.1. Oligoamides
As depicted in Scheme 50, Su showcased a solid phase synthesis of hairpin oligoamide 204 using triphosgene as a coupling agent [111]. Starting with resin-bound 4-aminopyrrole carboxamide 203, the pyrrole and imidazole backbone was elongated through an iterative sequence of N-Boc deprotection with TFA, followed by treatment of the unmasked amines with a premixed solution of triphosgene, N-Boc carboxylic acids, 2,4,6-collidine, and DIPEA.
Scheme 50.
Solid Phase Synthesis of Oligoamides.
11.2. Oligopeptides
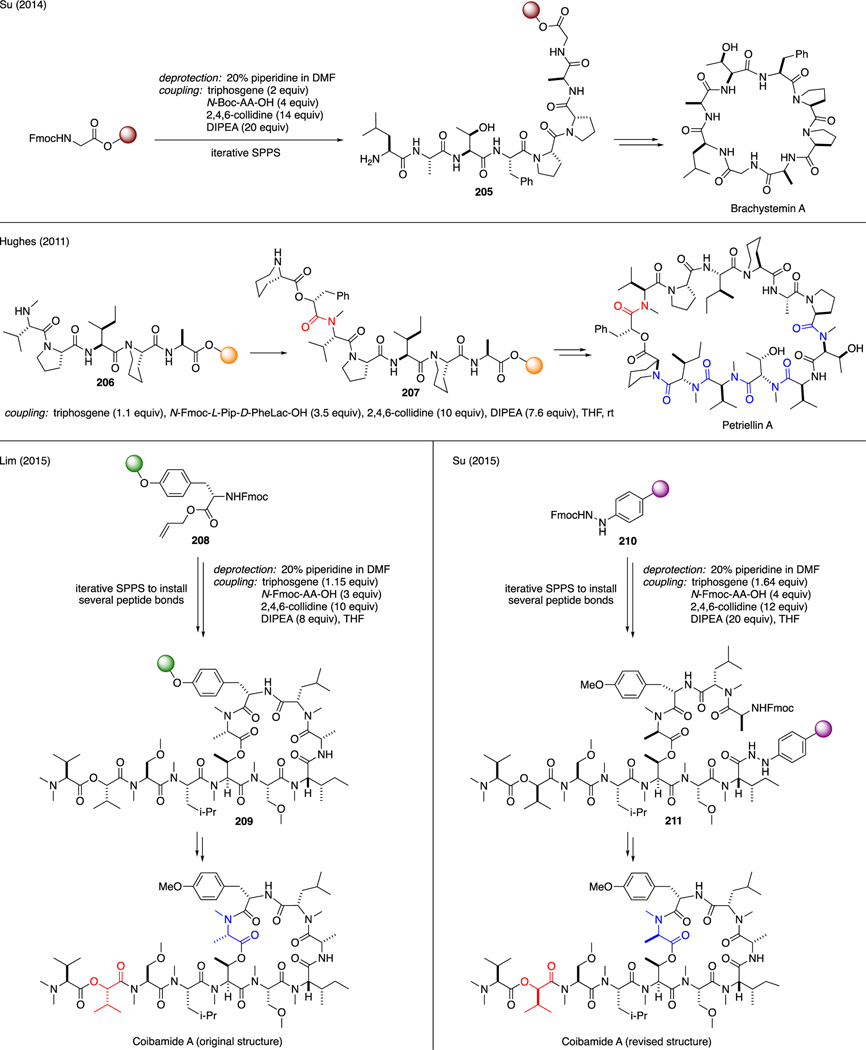
Several examples on triphosgene-promoted Fmoc solid phase peptide synthesis (SPPS) have been also reported. A typical protocol to this strategy generally involves a sequence of N-Fmoc deprotection using piperidine, followed by treatment of the free amines with a premixed coupling solution containing triphosgene, N-Fmoc amino acids, and hindered organic bases (Scheme 51). For instance, Su employed this approach in the total synthesis of cyclooctapeptide Brachystemin A [112], in which triphosgene along with collidine and DIPEA was employed to activate the various proteinogenic amino acid residues, thereby establishing the oligopeptide sequence 205. Hughes reported the total synthesis of antifungal Petriellin A on 2-chlorotrityl chloride (CTC) resin [113]. In this work, the coupling of hindered amines 206 with the next residue was realized upon activation with triphosgene, collidine, and DIPEA. Six different amide bonds were ultimately forged using this technology en route to the final macrocyclic natural product in 5% overall yield.
Scheme 51.
Solid Phase Peptide Synthesis of Natural Products.
Lim and Su independently published the total synthesis of cyclodepsipeptide Coibamide A using the SPPS technology [114,115]. Starting with the CTC resin 208 and the Fmochydrazinobenzoyl AM resin 210 respectively, Lim and Su employed triphosgene as a coupling reagent to install several of the peptide linkages 209 and 211 for the natural product. Lim reported that NMR signals of the synthetic compound were inconsistent with those of natural material, suggesting either the two samples existed in different conformations or the structure of the natural product had been inaccurately assigned. Ultimately, Su confirmed that the stereochemistry of the [L-HIV [2]] and [L-MeAla [11]] residues were indeed incorrect. The stereochemical assignment of Coibamide A was then subsequently revised to [D-HIV [2]] and [D-MeAla [11]].
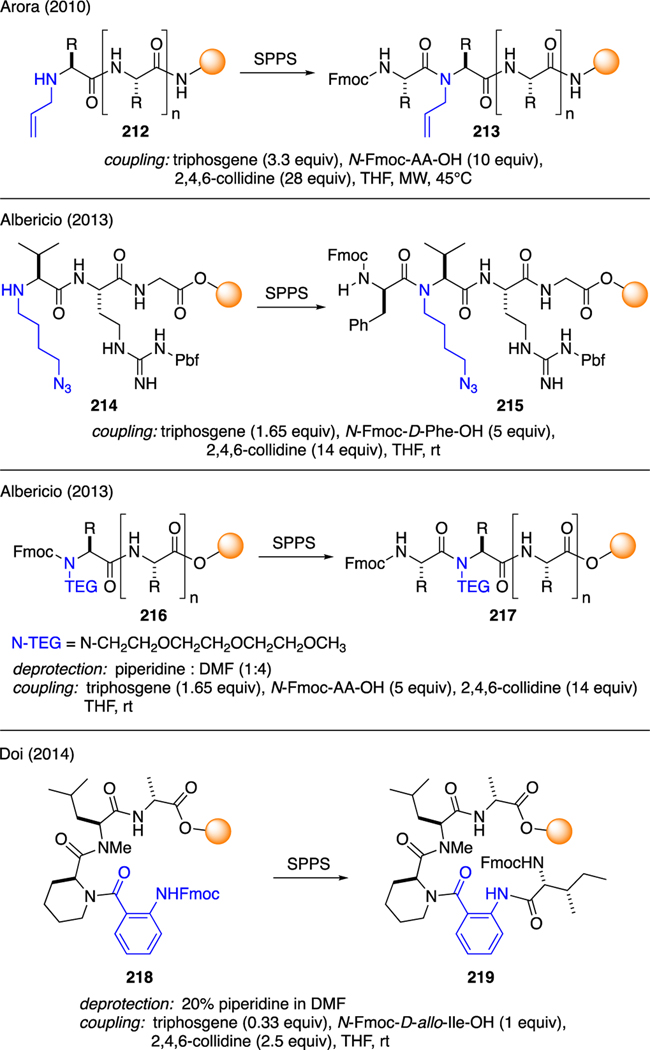
As shown in Scheme 52, Arora reported the coupling of sterically hindered N-allyl amino acid residues 212 with an N-Fmoc amino acid using a mixture of triphosgene and 2,4,6-collidine under microwave irradiation [116]. Another example was reported by Alberico in the synthesis of a cyclic RGD peptide 215 with a 4-azidobutyl linker 214 [117]. In this case, triphosgene was utilized to assist the coupling of the sterically cumbersome N-alkyl valine residue in the parent chain to Fmoc-protected D-phenylalanine. These coupling conditions were found to be superior than those of the classical methods, such as HATU/HOAt or PyBOP/HOAt. Albericio also identified the utility of triphosgene to forge peptide bonds from sterically congested N-triethylene glycol (TEG) amino acid residues 216 [118]. As reported by Doi [119], less nucleophilic anthranilic acid residue 218 was also found suitable for peptide coupling using the Fmoc-SPPS methodology. Similarly, the conversion of Fmoc-amino acids to the corresponding acid chlorides was performed in situ via treatment with triphosgene and collidine.
Scheme 52.
Solid Phase Peptide Synthesis Using Unreactive Amines.
12. Summary
This review article gleans various organic synthetic reactions that are enabled by the use of triphosgene in the decade of 2010–2019. Triphosgene has been effectively employed as a convenient surrogate to the highly toxic phosgene gas. Owing to its perceived safety and ease of handling, a surge of new chemistries based on this reagent, including its applications in the syntheses of complex molecular architectures, has been reported. Equally significant, the utility of triphosgene in emerging technologies and automation, such as flow chemistry and solid phase synthesis, has been also showcased. Triphosgene will continue to flourish as a versatile reagent to empower new synthetic transformations. Nonetheless, we must be cognizant that despite its well-known reputation as a seemingly safer substitute to phosgene gas, recent studies have revealed that triphosgene itself is highly toxic [120]. Safe handling of this reagent, especially in typical laboratory settings, is feasible with proper safety protocols in place, but further studies to better understand the safety profile of triphosgene may become necessary considering its potential for large-scale industrial applications.
Scheme 44.
Flow Synthesis of Chloroformates.
Acknowledgments
Generous financial supports from the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM127649 and Louisiana State University are gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography
Moshood Ganiu was born in Lagos, Nigeria. He obtained his B.S in Chemistry from Lagos State University in 2012 under the tutelage of Dr. M.S. Owolabi. Moshood joined the Department of Chemistry at LSU in the Fall of 2016 and is currently a 5th year Ph.D. candidate in the Kartika Lab. His projects encompass synthetic reactions development enabled by triphosgene.

Binod Nepal was born in Dhading district in Nepal. He completed undergraduate studies from the Amrit Campus in 2011 and the Master’s Degree in Organic Chemistry from the Central Department of Chemistry, Tribhuvan University in 2015 under the supervision of Prof. Susan Joshi. Binod then began his graduate studies at LSU in Fall 2016. He is currently a 5th year graduate student under the guidance of Prof. Rendy Kartika. Binod is working on synthetic reactions based on oxyallyl and amidoallyl cations.

Joshua P. Van Houten is a 5th year graduate student from Louisiana. Upon receiving his B.S. in Chemistry from Southwestern University in 2016, Joshua then continued with his graduate studies at LSU in Prof. Rendy Kartika’s laboratory. Presently, he is working on a one-pot synthetic reaction to access complex δ-valerolactones.

Rendy Kartika is an Associate Professor in the Department of Chemistry at Louisiana State University (LSU). Rendy was born in Malang, Indonesia and immigrated to the US in 1998. He earned his B.S. in Chemistry from California State Polytechnic University, Pomona in 2003 and his Ph.D. in Organic Chemistry from the University of Notre Dame in 2008 under the direction of Prof. Richard Taylor. After a postdoctoral training with Prof. David Spiegel at Yale University, Rendy began his independent academic career as an Assistant Professor at LSU in 2011 and rose to the rank of Associate Professor with tenure in 2017. Research in the Kartika lab centers in the development of organic synthetic reactions to assemble complex molecules of biological, pharmaceutical, and industrial importance.

Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Eckert H, Forster B, Angew Chem. Int. Ed. Engl 26 (1987) 894–895. [Google Scholar]
- [2].Sennyey G, Spec. Chem 10 (1990) 364. [Google Scholar]
- [3].Imagawa T, J. Syn. Org. Chem. Jpn 48 (1990) 1058–1059. [Google Scholar]
- [4].Bracher F, Litz T, Prakt J. Chem. Chem. Ztg 337 (1995) 516–518. [Google Scholar]
- [5].Su WK, Zhong WH, Bian GF, Shi XJ, Zhang JP, Org. Prep. Proced. Int 36 (2004) 499–547. [Google Scholar]
- [6].Ghorbani-Choghamarani A, Azadi G, Curr. Org. Chem 20 (2016) 2881–2893. [Google Scholar]
- [7].Cotarca L, Delogu P, Nardelli A, Sunjic V, Synthesis-Stuttgart 5 (1996) 553. [Google Scholar]
- [8].Roestamadji J, Mobashery S, Banerjee A, Saputra MA, Malone JA, Cleveland AH, Van Houten JP, Kartika R, Encyclopedia of Reagents for Organic Synthesis, Wiley Online Library, 2018, pp. 1–23. [Google Scholar]
- [9].Councler C, Ber. Dtsch. Chem. Ges 13 (1880) 1697–1699. [Google Scholar]
- [10].Hentschel W, J. Prakt. Chem 36 (1887) 99–113. [Google Scholar]
- [11].Hentschel W, J. Prakt. Chem 36 (1887) 305–317. [Google Scholar]
- [12].Hentschel W, J. Prakt. Chem 36 (1887) 468–480. [Google Scholar]
- [13].Sorensen AM, Acta Chem. Scand 25 (1971) 169. [Google Scholar]
- [14].Cotarca L, Bacaloglu R, Marcu N, Tarnaveanu A, J. Prakt. Chem 327 (1985) 881–886. [Google Scholar]
- [15].Cotarca L, Bacaloglu R, Csunderlik C, Marcu N, Tarnaveanu A, J. Prakt. Chem 329 (1987) 1052–1062. [Google Scholar]
- [16].Yasukouchi H, Nishiyama A, Mitsuda M, Org. Process Res. Dev 22 (2018) 247–251. [Google Scholar]
- [17].Movsisyan M, Delbeke EIP, Berton JKET, Battilocchio C, Ley SV, Stevens CV, Chem. Soc. Rev 45 (2016) 4892–4928. [DOI] [PubMed] [Google Scholar]
- [18].Eckert H, Auerweck J, Org. Process Res. Dev 14 (2010) 1509–1513. [Google Scholar]
- [19].Leroyer L, Prat L, Cabassud M, Gourdon C, Dechy-Cabaret O, Barthes M, Camus P, Hattou S, Green Process. Synth 2 (2013) 239–250. [Google Scholar]
- [20].Data was retrieved from Scifinder on June 22, To Obtain These Data, We Entered the Chemical Structure of Triphosgene in the “Structure Editor” Window. The Search Option of “Sources Other than Patents” Was Selected, 2020. [Google Scholar]
- [21].Ayala CE, Villalpando A, Nguyen AL, McCandless GT, Kartika R, Org. Lett 14 (2012) 3676–3679. [DOI] [PubMed] [Google Scholar]
- [22].Villalpando A, Ayala CE, Watson CB, Kartika R, J. Org. Chem 78 (2013) 3989–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thatikonda NR, Chebolu N, Budde M, Vaidya JR, J. Chem. Sci 124 (2012) 513–519. [Google Scholar]
- [24].Eum H, Lee Y, Kim S, Baek A, Son M, Lee KW, Ko SW, Kim S, Yun SY, Lee WK, Ha H, Bull J. Korean Chem. Soc 31 (2010) 611–614. [Google Scholar]
- [25].Couty F, Drouillat B, Lemee F, Eur. J. Org Chem 2011 (2011) 794–801. [Google Scholar]
- [26].Dolfen J, Van Hecke K, D’Hooghe M, Eur. J. Org Chem 2017 (2017) 3229–3233. [Google Scholar]
- [27].Cleveland AH, Fronczek FR, Kartika R, J. Org. Chem 83 (2018) 3367–3377. [DOI] [PubMed] [Google Scholar]
- [28].Villalpando A, Saputra MA, Tugwell TH, Kartika R, Chem. Commun 51 (2015) 15075–15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weng YY, Li JJ, Su WK, Chin. Chem. Lett 22 (2011) 1395–1398. [Google Scholar]
- [30].Saputra MA, Ngo L, Kartika R, J. Org. Chem 80 (2015) 8815–8820. [DOI] [PubMed] [Google Scholar]
- [31].Liang XR, Shi F, Chen RE, Su WK, Org. Prep. Proced. Int 42 (2010) 379–385. [Google Scholar]
- [32].He XL, Majumder S, Wu J, Jin CD, Guo SR, Guo ZP, Yang MH, Org. Chem. Front 6 (2019) 2435–2440. [Google Scholar]
- [33].Marek A, Patil MR, Klepetarova B, Kohout L, Elbert T, Tetrahedron Lett. 53 (2012) 2048–2050. [Google Scholar]
- [34].Marek A, Klepetarova B, Elbert T, Tetrahedron 71 (2015) 4874–4882. [Google Scholar]
- [35].Xu YZ, Hu DF, Zheng H, Mei D, Gao ZB, Tetrahedron 75 (2019) 130539. [Google Scholar]
- [36].Wang XX, Zhang J, Cheng R, Meng FH, Deng C, Zhong ZY, Biomacromolecules 17 (2016) 882–890. [DOI] [PubMed] [Google Scholar]
- [37].Jiang FJ, Fu XZ, Wang SW, Huang Y, Zhou W, Wang AM, Wang YL, Chin. Chem. Lett 24 (2013) 338–340. [Google Scholar]
- [38].Satake A, Ishizawa Y, Katagiri H, Kondo SI, J. Org. Chem 81 (2016) 9848–9857. [DOI] [PubMed] [Google Scholar]
- [39].Liu PF, Li CY, Zhang JW, Xu XY, Synth. Commun 43 (2013) 3342–3351. [Google Scholar]
- [40].Itoh T, Chiba Y, Kawaguchi S, Koitaya Y, Yoneta Y, Yamada K, Abe T, RSC Adv. 9 (2019) 10420–10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ganiu MO, Cleveland AH, Paul JL, Kartika R, Org. Lett 21 (2019) 5611–5615. [DOI] [PubMed] [Google Scholar]
- [42].Liu Y, Yeung YY, Org. Biomol. Chem 15 (2017) 6478–6482. [DOI] [PubMed] [Google Scholar]
- [43].Ye XJ, Fu HL, Ma JH, Zhong WH, Synth. Commun 46 (2016) 885–892. [Google Scholar]
- [44].Strasser CE, Cronje S, Raubenheimer HG, New J. Chem. 34 (2010) 458–469. [Google Scholar]
- [45].Yang JH, Han XX, Zhou LX, Xiong CH, Asian J. Chem. 23 (2011) 1615–1617. [Google Scholar]
- [46].Chen ZW, Jiang L, Su WK, Xu ZJ, J. Chem. Soc. Pakistan 34 (2012) 1003–1006. [Google Scholar]
- [47].Skiera I, Paryzek Z, J. Chem. Res 10 (2014) 597–599. [Google Scholar]
- [48].El-Sayed NS, Shirazi AN, Sajid MI, Park SE, Parang K, Tiwari RK, Molecules 24 (2019) 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bogolubsky AV, Ryabukhin SV, Pipko SE, Lukin O, Shivanyuk A, Mykytenko D, Tolmachev A, Tetrahedron 67 (2011) 3619–3623. [Google Scholar]
- [50].Shu LH, Gu C, Dong Y, Brinkman R, Org. Process Res. Dev 16 (2012) 1940–1946. [Google Scholar]
- [51].Xu DF, Guan J, Xu X, Gong SZ, Xu H, J. Heterocycl. Chem 53 (2016) 1469–1473. [Google Scholar]
- [52].Menguy L, Drouillat B, Couty F, Tetrahedron Lett. 56 (2015) 6625–6628. [Google Scholar]
- [53].Han KJ, Kim M, Lett. Org. Chem 8 (2011) 559–561. [Google Scholar]
- [54].Han KJ, Kim M, Org. Prep. Proced. Int 46 (2014) 370–375. [Google Scholar]
- [55].Kretz J, Kerwat D, Schubert V, Gratz S, Pesic A, Semsary S, Cociancich S, Royer M, Sussmuth RD, Angew. Chem. Int. Ed 54 (2015) 1969–1973. [DOI] [PubMed] [Google Scholar]
- [56].Aiello F, Garofalo A, Grande F, Tetrahedron Lett. 51 (2010) 6635–6636. [Google Scholar]
- [57].Polyakov M, Schaffner B, Kruse D, Martin A, Kockritz A, Tetrahedron Lett. 57 (2016) 964–968. [Google Scholar]
- [58].Movassagh B, Soleiman-Beigi M, Synth. Commun 40 (2010) 3467–3471. [Google Scholar]
- [59].Latorre A, Saez JA, Rodriguez S, Gonzalez FV, Tetrahedron 70 (2014) 97–102. [Google Scholar]
- [60].Majcher U, Urbaniak A, Maj E, Moshari M, Delgado M, Wietrzyk J, Bartl F, Chambers TC, Tuszynski JA, Huczynski A, Bioorg. Chem 81 (2018) 553–566. [DOI] [PubMed] [Google Scholar]
- [61].Pal’chikov VA, Zarovnaya IS, Dul’nev PG, Russ. J. Org. Chem 54 (2018) 1061–1070. [Google Scholar]
- [62].Klasek A, Kremen F, Kremenova H, Lycka A, Rouchal M, Tetrahedron 73 (2017) 1583–1593. [Google Scholar]
- [63].Le Quement ST, Flagstad T, Mikkelsen RJT, Hansen MR, Givskov MC, Nielsen TE, Org. Lett 14 (2012) 640–643. [DOI] [PubMed] [Google Scholar]
- [64].Liu LX, Zheng YS, Hu XY, Lian CX, Yuan WC, Zhang XM, Chem. Res. Chin. Univ 30 (2014) 235–241. [Google Scholar]
- [65].Klasek A, Lycka A, Rouchal M, Rudolf O, Ruzicka A, Helv. Chim. Acta 97 (2014) 595–612. [Google Scholar]
- [66].Li B, Zhang J, Yang BB, Li L, Yang XX, RSC Adv. 7 (2017) 45714–45720. [Google Scholar]
- [67].Zhang ES, Zhang XJ, Cai YC, Wang DJ, Xu TL, Li J, Yan M, Zou Y, RSC Adv. 4 (2014) 39020–39029. [Google Scholar]
- [68].Al-Bogami AS, Asian J. Chem. 23 (2011) 3045–3049. [Google Scholar]
- [69].Al-Bogami AS, Synth. Commun 41 (2011) 2952–2958. [Google Scholar]
- [70].Borgaonkar VV, Patil BR, J. Heterocycl. Chem 53 (2016) 1897–1901. [Google Scholar]
- [71].Shi DQ, Rong SF, Dou GL, Wang MM, J. Comb. Chem 12 (2010) 25–30. [DOI] [PubMed] [Google Scholar]
- [72].Dou GL, Sun F, Shi DQ, Tetrahedron 68 (2012) 4852–4859. [Google Scholar]
- [73].Wei XZ, Fu YL, Xue MJ, Song QH, Org. Lett 21 (2019) 9497–9501. [DOI] [PubMed] [Google Scholar]
- [74].Srinivas B, Devi NS, Sreenivasulu GK, Parameshwar R, Heterocycl. Lett 5 (2015) 459–466. [Google Scholar]
- [75].Huang YY, Feng Y, Gao WS, Zhang C, Chen LG, J. Heterocycl. Chem 53 (2016) 437–440. [Google Scholar]
- [76].Jentsch NG, Hume JD, Crull EB, Beauti SM, Pham AH, Pigza JA, Kessl JJ, Donahue MG, Beilstein J Org. Chem 14 (2018) 2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zhou SG, Yao T, Vi JC, Li DS, Xiong J, J. Chem. Res 5 (2013) 315–319. [Google Scholar]
- [78].El-Deeb IM, Jung SJ, Park BS, Yoo YJ, Choi K, Yang YM, Lee SW, Kim IT, Han DK, Lee SHB, Korean Chem. Soc 32 (2011) 1709–1714. [Google Scholar]
- [79].Tampier S, Bleifuss SM, Abd-Elzaher MM, Sutter J, Heinemann FW, Burzlaff N, Organometallics 32 (2013) 5935–5945. [Google Scholar]
- [80].Urankar D, Steinbucher M, Kosjek J, Kosmrlj J, Tetrahedron 66 (2010) 2602–2613. [Google Scholar]
- [81].Zhang GJ, Fu XB, Peng XM, Li XL, Chen JN, J. Chem. Res 12 (2013) 730–732. [Google Scholar]
- [82].Rao GW, Li Q, Zhao ZG, J. Chem. Res 3 (2012) 178–180. [Google Scholar]
- [83].Le Roux A, Marsault E, Synthesis-Stuttgart vol. 45, 2013, pp. 1983–1990. [Google Scholar]
- [84].Shankar B, Jalapathi P, Nagamani M, Gandu B, Kudle KR, Monatsh. Chem 148 (2017) 999–1009. [Google Scholar]
- [85].Kumata K, Ogawa M, Takei M, Fujinaga M, Yoshida Y, Nengaki N, Fukumura T, Suzuki K, Zhang MR, Bioorg. Med. Chem 20 (2012) 305–310. [DOI] [PubMed] [Google Scholar]
- [86].Mangion IK, Ruck RT, Rivera N, Huffman MA, Shevlin M, Org. Lett 13 (2011) 5480–5483. [DOI] [PubMed] [Google Scholar]
- [87].Dou GL, Shi DQ, Li YH, J. Comb. Chem 12 (2010) 195–199. [DOI] [PubMed] [Google Scholar]
- [88].Wu ZH, Hu T, He L, Gong B, Org. Lett 14 (2012) 2504–2507. [DOI] [PubMed] [Google Scholar]
- [89].Leczycka-Wilk K, Dabrowa K, Cmoch P, Jarosz S, Org. Lett 19 (2017) 4596–4599. [DOI] [PubMed] [Google Scholar]
- [90].Pedgaonkar YY, Degani MS, Iyer RP, Mol. Divers 15 (2011) 263–267. [DOI] [PubMed] [Google Scholar]
- [91].Wang SL, Yang K, Yao CS, Wang XS, Synth. Commun 42 (2012) 341–349. [Google Scholar]
- [92].Lebed PS, Kos PO, Tolmachev A, Vovk MV, Boyko AN, Chekotylo A, Synth. Commun 43 (2013) 2343–2348. [Google Scholar]
- [93].Bouaziz O, Amari M, Bachar R, Khier N, Fodili M, Paz FAA, Talhi O, Silva AMS, Tetrahedron Lett 56 (2015) 1020–1024. [Google Scholar]
- [94].Li YJ, Zhang XM, Niu SM, Zhao YP, Yang LJ, Shao XW, Wang ES, Chem. Res. Chin. Univ 33 (2017) 895–902. [Google Scholar]
- [95].Espinoza-Hicks C, Montoya P, Bautista R, Jimenez-Vazquez HA, Rodriguez-Valdez LM, Camacho-Davila AA, Cossio FP, Delgado F, Tamariz J, J. Org. Chem 83 (2018) 5347–5364. [DOI] [PubMed] [Google Scholar]
- [96].Mihorianu M, Mangalagiu I, Jones PG, Daniliuc CG, Franz MH, Neda I, Rev. Roum. Chem 55 (2010) 689–695. [Google Scholar]
- [97].Ghorbani-Choghamarani A, Nikoorazm M, Azadi G, Chin. Chem. Lett 25 (2014) 451–454. [Google Scholar]
- [98].Xie YY, Yang P, Chen XD, J. Chem. Res 7 (2012) 421–424. [Google Scholar]
- [99].Weng YY, Ying LM, Chen QX, Su WK, Chin. Chem. Lett 23 (2012) 911–914. [Google Scholar]
- [100].Chen ZW, Yang YY, Su WK, J. Chem. Res 11 (2010) 661–664. [Google Scholar]
- [101].Jin C, Chen J, Su WK, Heterocycles 83 (2011) 153–161. [Google Scholar]
- [102].Li ZH, Wu DL, Zhong WH, Heterocycles 85 (2012) 1417–1426. [Google Scholar]
- [103].Zou Y, Li ZH, Su WK, J. Chem. Res 3 (2014) 143–146. [Google Scholar]
- [104].Li ZH, Jiang ZJ, Shao QL, Qin JJ, Shu QF, Lu WH, Su WK, J. Org. Chem 83 (2018) 6423–6431. [DOI] [PubMed] [Google Scholar]
- [105].Jang H, Ahmed F, Joo H, Ryu T, Yang H, Yoon S, Kim W, Jeon HS, J. Nanosci. Nanotechnol 17 (2017) 7381–7386. [Google Scholar]
- [106].Jang H, Sutradhar SC, Ryu T, Choi K, Yoon S, Lee S, Kim WJ, Nanosci. Nanotechnol 17 (2017) 7454–7459. [Google Scholar]
- [107].Fuse S, Tanabe N, Takahashi T, Chem. Commun 47 (2011) 12661–12663. [DOI] [PubMed] [Google Scholar]
- [108].Fuse S, Mifune Y, Takahashi T, Angew. Chem. Int. Ed 53 (2014) 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mifune Y, Nakamura H, Fuse S, Org. Biomol. Chem 14 (2016) 11244–11249. [DOI] [PubMed] [Google Scholar]
- [110].Baumann M, Garcia AMR, Baxendale IR, Org. Biomol. Chem 13 (2015) 4231–4239. [DOI] [PubMed] [Google Scholar]
- [111].Fang LJ, Yao GY, Pan ZY, Wu CL, Wang HS, Burley GA, Su W, Org. Lett 17 (2015) 158–161. [DOI] [PubMed] [Google Scholar]
- [112].Fang LJ, Wu CL, Yu ZD, Shang P, Cheng YX, Peng YF, Su W, Eur. J. Org Chem 2014 (2014) 7572–7576. [Google Scholar]
- [113].Sleebs MM, Scanlon D, Karas J, Maharani R, Hughes AB, J. Org. Chem 76 (2011) 6686–6693. [DOI] [PubMed] [Google Scholar]
- [114].Sable GA, Park J, Kim H, Lim SJ, Jang S, Lim D, Eur. J. Org. Chem 2015 (2015) 7043–7052. [Google Scholar]
- [115].Yao GY, Pan ZY, Wu CL, Wang W, Fang LJ, Su W, J. Am. Chem. Soc 137 (2015) 13488–13491. [DOI] [PubMed] [Google Scholar]
- [116].Patgiri A, Witten MR, Arora PS, Org. Biomol. Chem 8 (2010) 1773–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fernandez-Llamazares AI, Garcia J, Adan J, Meunier D, Mitjans F, Spengler J, Albericio F, Org. Lett 15 (2013) 4572–4575. [DOI] [PubMed] [Google Scholar]
- [118].Fernandez-Llamazares AI, Garcia J, Soto-Cerrato V, Perez-Tomas R, Spengler J, Albericio F, Chem. Commun 49 (2013) 6430–6432. [DOI] [PubMed] [Google Scholar]
- [119].Masuda Y, Tanaka R, Kai K, Ganesan A, Doi TJ, Org. Chem 79 (2014) 7844–7853. [DOI] [PubMed] [Google Scholar]
- [120].Cotarca L, Geller T, Repasi J, Org. Process Res. Dev 21 (2017) 1439–1446. [Google Scholar]